Many cancers have upregulated MET expression. Merestinib, an oral kinase inhibitor with antitumor proliferative and antiangiogenic activity in MET‐amplified and MET autocrine xenograft tumor models, was initially developed to target the MET kinase. This article evaluates the safety and tolerability of merestinib in patients with advanced cancer.
Keywords: Merestinib, LY2801653, Colorectal cancer, Head and neck squamous cell cancer, Cholangiocarcinoma
Abstract
Background.
The purpose of this nonrandomized, open‐label, phase I study (NCT01285037) was to evaluate the safety and tolerability of merestinib, an oral antiproliferative and antiangiogenic kinase inhibitor, and to determine a recommended phase II dose and schedule for patients with advanced cancer.
Materials and Methods.
This was a multicenter, nonrandomized, open‐label, phase I study of oral merestinib consisting of six parts: dose escalation (part A), followed by a four‐cohort dose‐confirmation study (part B) and subsequently a four‐part dose expansion and combination safety testing of merestinib with standard doses of cetuximab (part C), cisplatin (part D), gemcitabine and cisplatin (part E), and ramucirumab (part F) in patients with specific types of advanced cancers. Safety, tolerability, antitumor activity, and pharmacokinetics were evaluated in all cohorts.
Results.
The dose escalation, confirmation, and expansion results support the dosing of merestinib at 120 mg once daily, based on acceptable exposure and safety at this dose. One complete response was observed in a patient with cholangiocarcinoma, and three patients with cholangiocarcinoma achieved a partial response. Overall, 60 (32%) of the 186 patients enrolled in the study had a best response of stable disease.
Conclusion.
This study demonstrates that merestinib has a tolerable safety profile and potential anticancer activity and warrants further clinical investigation.
Implications for Practice.
Merestinib treatment in patients with advanced cancer demonstrated an acceptable safety profile and potential antitumor activity, supporting its future development in specific disease populations as a monotherapy and/or in combination with other therapies.
Introduction
The tyrosine kinase receptor MET, also known as the hepatocyte growth factor (HGF) receptor, promotes a metastatic and invasive tumor phenotype [1], [2]. The MET signaling pathway regulates normal cellular functions that can be subverted to support neoplasia, including cell proliferation, survival, apoptosis, invasion, and angiogenesis [1], [3]. Both MET amplification and MET exon 14 skipping mutations of the juxtamembrane receptor domain result in upregulation of MET expression and activity [4]. Aberrant MET signaling plays a key role in tumorigenesis and angiogenesis [3] and, in many cases, correlates with poor prognosis [5], [6]. Amplification of MET is also present in tumor cells with acquired resistance to treatments such as erlotinib and gefitinib [7], [8].
Merestinib (LY2801653), developed initially to target the MET kinase, is an oral kinase inhibitor with antitumor proliferative and antiangiogenic activity in MET‐amplified and MET autocrine xenograft tumor models [9], [10]. Merestinib is also active against other receptor tyrosine kinases (MST1R [RON], AXL, ROS1, PDGFRA, FLT3, TEK, DDR1, DDR2, MERTK, TYR03, TRKA, TRKB, and TRKC) [9], [11] and serine/threonine kinases (MKNK1 and MKNK2) [9]. Recent studies demonstrated merestinib inhibits MKNK1‐ and MKNK2‐induced phosphorylation of eukaryotic translation initiation factor 4E [12], [13] and may represent a unique approach to treatment of acute myeloid leukemia or tumors with mitogen‐activated protein kinase pathway activation [12].
Many human cancers have upregulated MET expression [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. In colorectal cancer (CRC), MET overexpression has been linked to metastatic progression [14]. In head and neck squamous cell cancer (HNSCC), the MET pathway was expressed or overexpressed in 52%–68% of cases; 11%–27% of HNSCC cases may have activating mutations in MET [18]. Patients with CRC were treated with merestinib monotherapy; patients with HNSCC were treated with merestinib monotherapy or in combination with cetuximab in this study.
MET receptor expression is a common feature of intrahepatic or extrahepatic cholangiocarcinoma (CCA), with approximately 50%–70% of cases demonstrating MET expression by immunohistochemistry (IHC) [26], [31]. c‐Met expression was correlated with epidermal growth factor receptor (EGFR) overexpression in CCA and was also a significant prognostic factor in intrahepatic CCA [31]. Given the high rate of MET expression in CCA and evidence that MET expression may confer resistance to cisplatin across tumor types [[32]; Lilly data on file], the combinations of merestinib with gemcitabine and/or cisplatin were tested in CCA in this study.
Uveal melanoma (UM) is the most common primary intraocular malignancy and represents approximately 5% of all melanoma diagnoses [27]. Approximately 30%–50% of UM will metastasize [28], usually to the liver [27], [28], [29], [30]. Expression of MET is a frequent finding in this disease, occurring in almost 60%–90% of primary UMs [29]. MET expression is associated with greater tumor invasiveness and worse overall survival (OS). Existing chemotherapy, targeted therapy, and immunotherapy regimens lack efficacy [29], [33]. Patients with UM were treated with merestinib monotherapy in this study.
Gastric cancer remains a significant health problem and is the third leading cause of cancer‐related death worldwide [34]. In some preclinical tumor models, selective inhibition of vascular endothelial growth factor (VEGF) signaling by anti‐VEGF antibody reduced tumor burden but increased invasion, metastasis, tumor hypoxia, and c‐Met activation [35]. However, concurrent inhibition of c‐Met reversed these effects. Given these observations and the upregulation of c‐Met and/or elevated HGF expression in gastric cancer, merestinib with a VEGF inhibitor was tested in patients with gastric cancer.
This phase I study (I3O‐MC‐JSBA [JSBA]) evaluating the safety and tolerability of merestinib in patients with advanced cancer opened to enrollment in November 2009. We report the results of all cohorts, including dose‐escalation and dose‐confirmation cohorts and the multiple phase Ib combination arms in specific tumor types.
Materials and Methods
Objectives and Study Design
A six‐part, multicenter, nonrandomized, open‐label, dose‐escalation (part A), phase I study (NCT01285037) of merestinib was performed, followed by a dose‐confirmation study (part B) with four cohorts to confirm the dose of merestinib as monotherapy, followed by a dose‐expansion evaluation with standard doses of concomitant cetuximab (part C), cisplatin (part D), gemcitabine and cisplatin (part E), and ramucirumab (part F) in patients with advanced cancer (supplemental online Table 1).
The primary objective of this study was to determine a recommended phase II dose and schedule of merestinib that could be safely administered alone or in combination to patients with advanced cancer. Secondary objectives included documentation of antitumor activity, characterization of the safety and toxicity profile, and estimation of pharmacokinetic (PK) parameters.
Eligibility Criteria
Patients aged ≥18 years with advanced cancer (supplemental online Table 1) refractory to prior or standard therapies, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, and an estimated life expectancy of ≥12 weeks were eligible. Patients were excluded if they were pregnant or had decompensated liver cirrhosis (≥Child‐Pugh stage B), hepatocellular cancer (HCC), or symptomatic central nervous system malignancy or metastasis. In the dose‐escalation (part A) and dose‐confirmation (part B) portions of the study, patients with adenocarcinoma of the colon or rectum were eligible if they received ≥2 prior chemotherapy regimens. Patients with HNSCC (part C) who were previously treated with cetuximab, as well as cetuximab‐naïve patients, were eligible if they received ≥1 and ≤3 prior systemic therapies. Patients with CCA were eligible if they had adequate biliary drainage prior to enrollment. Patients with UM were required to have liver metastasis. Part D patients with CCA were required to have one prior line of therapy, whereas part E patients with CCA were not eligible if they received prior therapy for metastatic or advanced disease. Part E also enrolled patients with gallbladder or ampulla of Vater cancer. Patients with histologically or cytologically confirmed gastric carcinoma, including gastric adenocarcinoma or gastroesophageal junction (GEJ) adenocarcinoma, who were ramucirumab naïve were included in part F.
Treatment
Eligible patients received oral merestinib once daily in 28‐day (parts A, B, C, and F) or 21‐day (parts D and E) cycles (supplemental online Table 1). This study included multiple parts evaluating a range of doses of merestinib, with or without concomitant therapies (supplemental online Table 1).
The study commenced with a drug‐in‐capsule formulation of merestinib. PK evaluation at the end of the second dose level showed negligible systemic exposure and prompted a pause in enrollment to allow the development of an enabled (solid dispersion) formulation. Enrollment resumed with the solid dispersion formulation at a starting daily dose of 5 mg, and dose escalation continued to 240 mg daily.
Safety
The safety population included all patients who received ≥1 dose of merestinib. Safety measures included adverse events (AEs), dose‐limiting toxicities (DLTs), and electrocardiograms (ECGs). All AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Patients with a corrected QT (QTc) >470 ms were excluded. An ECG was performed at baseline, at the beginning of cycle 1 (predose and 2, 5–8, and 24 hours after dose), and at the beginning of cycle 2 (predose and 2 and 5–8 hours after dose). Absolute QT interval (Fridericia correction [QTcF]) and QTcF changes from baseline were evaluated by the investigator.
A DLT was defined as an AE occurring in cycle 1 that was possibly related to the study drug and fulfilled any of the following criteria: (a) grade ≥ 3 nonhematologic toxicity (except manageable nausea, vomiting, anorexia, fatigue, constipation, and diarrhea), (b) grade ≥ 3 thrombocytopenia with bleeding, (c) grade 4 hematologic toxicity of >7 days duration, (d) febrile neutropenia, or (e) other significant toxicity deemed by the primary investigator and clinical research physician to be dose limiting (e.g., grade 2 seizures, symptomatic or asymptomatic arrhythmias, or severe tremors).
Antitumor Activity
Best overall response, progression‐free survival (PFS), and duration of response (DoR) were documented in all parts of the study. Response analyses were based on the Response Evaluation Criteria in Solid Tumors version 1.1 [36] for patients with solid tumors and were performed every 56 days (±5 days), starting at the end of cycle 2 or sooner as clinically indicated. To better understand tumor growth kinetics at the time of study enrollment, information was collected on size of target lesions obtained on the radiological assessment done prior to this study's baseline measurement. A radiological assessment performed ≥3 weeks prior to the baseline study and using the same imaging modality as the baseline was used as the reference.
PFS analysis was defined as the elapsed time from the date of enrollment to the date of either objectively determined progressive disease (PD) or death. DoR analyses identified responding patients not known to have died as of the data cutoff date of November 13, 2017, and who did not have objective PD, or responders who received subsequent systemic anticancer therapy (after discontinuation from the study chemotherapy) before objectively determined PD.
Pharmacokinetics
PK parameters of merestinib were analyzed on plasma samples from patients who received ≥1 dose of the enabled formulation and had appropriate sampling to allow calculation of noncompartmental PK parameters using Phoenix WinNonlin, Certara USA Inc, Princeton, NJ, USA version 6.4. Maximum observed postdose concentration (Cmax) and time to Cmax (tmax) were reported directly from the raw data. Other calculated parameters included area under the concentration‐time curve from time 0 through 24 hours (AUC0–24), apparent clearance (CL/F), apparent volume of distribution (VZ/F), and elimination half‐life (t1/2). Descriptive analyses were performed to assess plasma concentrations over time and exposure by dose and cohort. In addition, Cmax and AUC0–24 obtained after the first dose (cycle 1, day 1) during dose escalation were evaluated to assess dose proportionality by the method of Smith et al. [37].
Statistical Analysis
All analyses were descriptive; no p values were calculated. For continuous variables, summary statistics included number of patients, mean, median, standard deviation, minimum, and maximum. Categorical endpoints were summarized using the number of patients, frequency, percentages, and standard errors. Missing data were not imputed.
Results
Baseline Characteristics
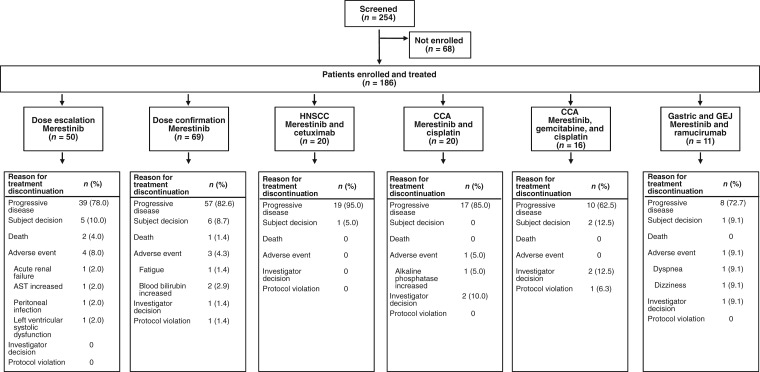
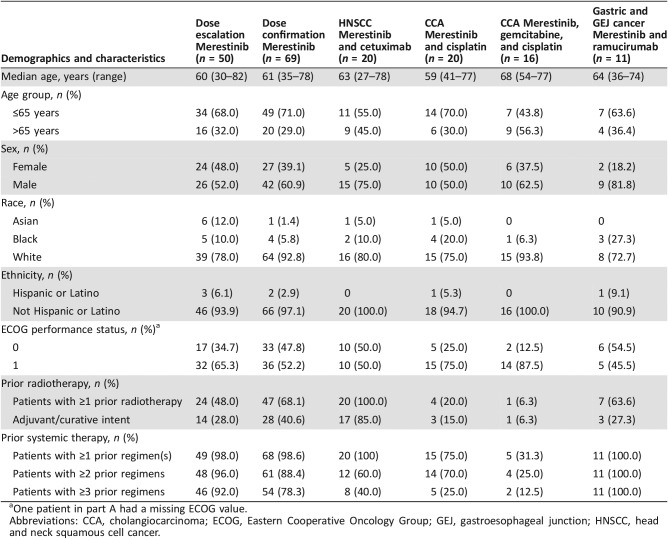
All patients were enrolled in the U.S. between November 10, 2009, and February 20, 2017. Of the 254 patients screened, 186 were subsequently enrolled and received ≥1 dose of merestinib (Fig. 1). In each cohort, most patients were white (73%–94%), male (50%–82%), and over age 59 (range, 27–82 years). All patients had a baseline ECOG PS of 0 (73 of 185 patients; 39.5%) or 1 (112 of 185 patients; 60.5%). Baseline patient demographics and disease characteristics are summarized in Table 1.
Figure 1.
Consolidated Standards of Reporting Trials diagram.
Abbreviations: AST, aspartate aminotransferase; CCA, cholangiocarcinoma; GEJ, gastroesophageal junction; HNSCC, head and neck squamous cell cancer.
Table 1. Baseline patient demographics and disease characteristics.
One patient in part A had a missing ECOG value.
Abbreviations: CCA, cholangiocarcinoma; ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; HNSCC, head and neck squamous cell cancer.
Dose Escalation
With the enabled formulation, daily dosing began at 5 mg and doubled in each subsequent cohort until the 160 mg dose in cohort 8 (supplemental online Table 1). For the first three patients treated at 160 mg, no DLTs were observed, and the dose was increased to 240 mg in cohort 9. Two of the patients experienced a reversible and transient (<7 days) grade 3 increase in liver function tests (LFTs) at 240 mg. Therefore, the 160 mg dose level was reopened to enrollment, and a total of 11 evaluable patients were treated at this dose level, with two DLTs (reversible grade 3 increases in LFTs) observed. Thereafter, the starting daily dose was de‐escalated to 120 mg. This 120 mg cohort enrolled 10 patients, 6 of whom were evaluable. One patient in the 120 mg cohort had a reversible grade 3 LFT increase. Supplemental online Table 2 summarizes the dose escalation. The 120 mg daily dose was selected for subsequent study in the dose‐confirmation part of the study (part B), which enrolled four separate cohorts of patients with CRC, HNSCC, CCA, and metastatic UM. In the combination cohorts parts C and D, three patients observed DLTs at the 120 mg dose: grade 3 increased aspartate aminotransferase (AST) and grade 3 increased alanine aminotransferase (ALT; part C) and grade 3 hyperbilirubinemia (part D). These DLTs were reversible, and patients resumed and tolerated study treatment at the reduced dose of 80 mg.
Safety
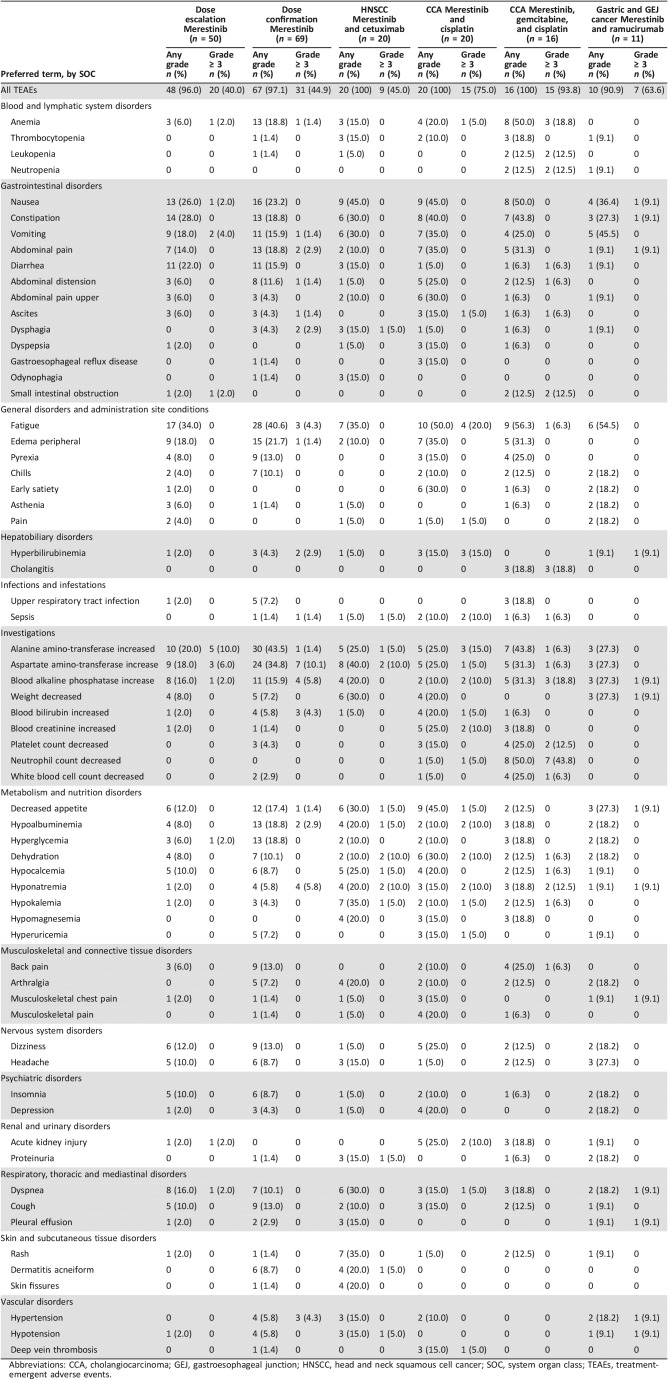
In the dose‐escalation part of the study, 48 patients (96%) experienced treatment‐emergent adverse events (TEAEs), regardless of causality, and 20 patients (40%) experienced grade ≥ 3 TEAEs (Table 2). The most common (≥5%) grade ≥ 3 TEAEs were ALT increased (five patients [10%]) and AST increased (three patients [6%]). Analysis of ECG results across cohorts showed no QTcF prolongation trends by dose or over time.
Table 2. Treatment‐emergent adverse events: any‐grade events ≥15% by study part, regardless of relatedness.
Abbreviations: CCA, cholangiocarcinoma; GEJ, gastroesophageal junction; HNSCC, head and neck squamous cell cancer; SOC, system organ class; TEAEs, treatment‐emergent adverse events.
In the dose‐confirmation part of the study, 67 patients (97%) experienced TEAEs, regardless of causality, and 31 patients (45%) experienced grade ≥ 3 TEAEs (Table 2). The most common (≥5%) grade ≥ 3 TEAEs were AST increased (seven patients [10%]), blood alkaline phosphatase increased (four patients [6%]), and hyponatremia (four patients [6%]).
Twenty patients (100%) with HNSCC treated with merestinib and cetuximab experienced TEAEs, regardless of causality, and nine patients (45.0%) experienced grade ≥ 3 TEAEs (Table 2). The most common (≥10%) grade ≥ 3 TEAEs, each experienced by two patients (10%), were AST increased, dehydration, and hyponatremia.
Twenty patients (100%) with CCA treated with merestinib and cisplatin experienced TEAEs, regardless of causality, and 15 patients (75%) experienced grade ≥ 3 TEAEs (Table 2). The most common (≥10%) grade ≥ 3 TEAEs were fatigue (four patients [20%]); hyperbilirubinemia (three patients [15%]); ALT increased (three patients [15%]); and sepsis, blood alkaline phosphatase increased, blood creatinine increased, hyponatremia, dehydration, hypoalbuminemia, and acute kidney injury, each experienced by two patients (10%).
Sixteen patients (100%) with CCA treated with merestinib, gemcitabine, and cisplatin experienced TEAEs, and 15 patients (94%) experienced grade ≥ 3 TEAEs, regardless of causality (Table 2). The most common (≥10%) grade ≥ 3 TEAEs were neutrophil count decreased (seven patients [44%]); anemia (three patients [19%]); cholangitis (four patients [25%]); blood alkaline phosphate increased (three patients [19%]); and leukopenia, neutropenia, small intestinal obstruction, platelet count decreased, and hyponatremia, each experienced by two patients (13%).
Ten patients (90.9%) with gastric or GEJ cancer experienced TEAEs, regardless of causality, and seven patients (63.6%) experienced grade ≥ 3 TEAEs (Table 2). No grade ≥ 3 TEAEs were experienced by more than one patient (Table 2).
Reasons for treatment discontinuation are summarized in Figure 1. Overall, nine patients discontinued from the study because of AEs. Serious AEs, regardless of causality (supplemental online Table 3), and serious AEs possibly related to merestinib (supplemental online Table 4) are detailed by study part in the supplemental online data.
Thirty‐six patients died during the study. Twelve patients died during the dose‐escalation part of the study, 10 from PD. The remaining two patient deaths (cardiac arrest, peritoneal infection) were determined by the investigator to be unrelated to merestinib. Eleven patients died during the dose‐confirmation part of the study, all from PD. Two patients with HNSCC died, both from PD. Six patients with CCA treated with merestinib and cisplatin died during the study, all from PD. One patient with CCA treated with merestinib, gemcitabine, and cisplatin died from PD during the study. Four patients with gastric or GEJ cancer died during the study, three (75.0%) from PD. The remaining patient death was an AE of dyspnea.
Antitumor Activity
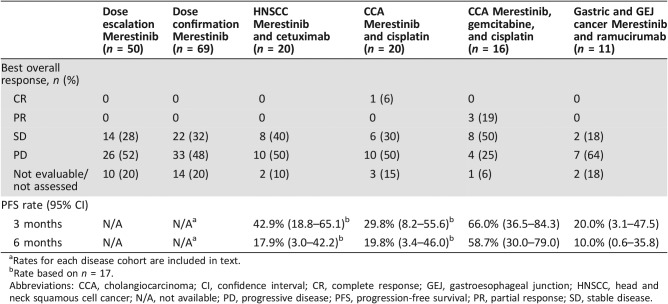
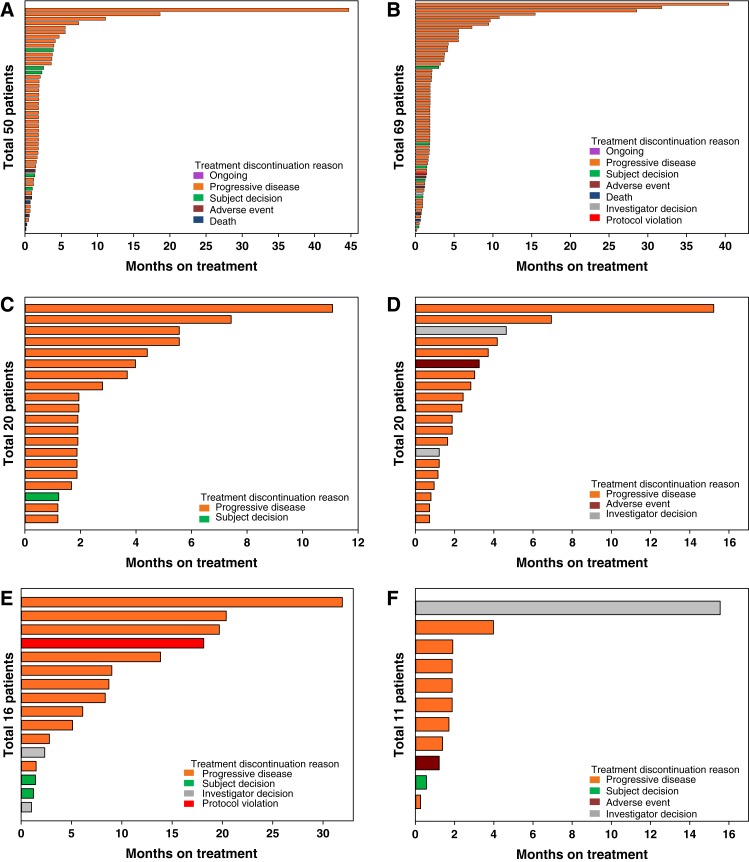
Of the 50 patients enrolled in the dose‐escalation part of the study, 14 (28%) had a best overall response of stable disease (SD), and 26 patients (52%) had a best overall response of PD (Table 3). PFS for patients across all dose levels ranged from 0.3 to >40 months. Duration on study ranged from 4 days to 45 months (Fig. 2A).
Table 3. Best overall response.
Rates for each disease cohort are included in text.
Rate based on n = 17.
Abbreviations: CCA, cholangiocarcinoma; CI, confidence interval; CR, complete response; GEJ, gastroesophageal junction; HNSCC, head and neck squamous cell cancer; N/A, not available; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Figure 2.
Duration of treatment by study part. (A): Dose escalation. (B): Dose confirmation. (C): Head and neck squamous cell cancer. (D): Cholangiocarcinoma (CCA) treated with merestinib and cisplatin. (E): CCA treated with merestinib, gemcitabine, and cisplatin. (F): Gastric and gastroesophageal junction cancer.
Of the 69 patients enrolled in the dose‐confirmation part of the study, 22 (32%) had a best response of SD (Table 3). The estimated median PFS by cohort was 1.8 months (CRC), 1.7 months (HNSCC), 1.9 months (CCA), and 1.8 months (UM). The PFS rate at 3 months was 43.1% (95% confidence interval [CI] 17.9–66.2) for the CRC cohort, 24.5% (95% CI 6.1–49.3) for the HNSCC cohort, 26.9% (95% CI 8.4–50.0) for the CCA cohort, and 40.0% (95% CI 16.5–62.8) for the UM cohort. The PFS rate at 6 months was 7.2% (95% CI 0.5–27.6) for the CRC cohort, 16.3% (95% CI 2.7–40.4) for the HNSCC cohort, 20.2% (95% CI 4.9–42.7) for the CCA cohort, and 20.0% (95% CI 4.9–42.4) for the UM cohort. Duration on study ranged from 6 days to 40 months (Fig. 2B).
Of the 20 patients with HNSCC, a combined total of 8 patients (40%) had a best response of SD (Table 3; Fig. 3A). The estimated median PFS by dose cohort was 2.8 months (120 mg) and 2.3 months (80 mg). The PFS rates at 3 months and 6 months are in Table 3. Duration on study ranged from 1 to 11 months (Fig. 2C).
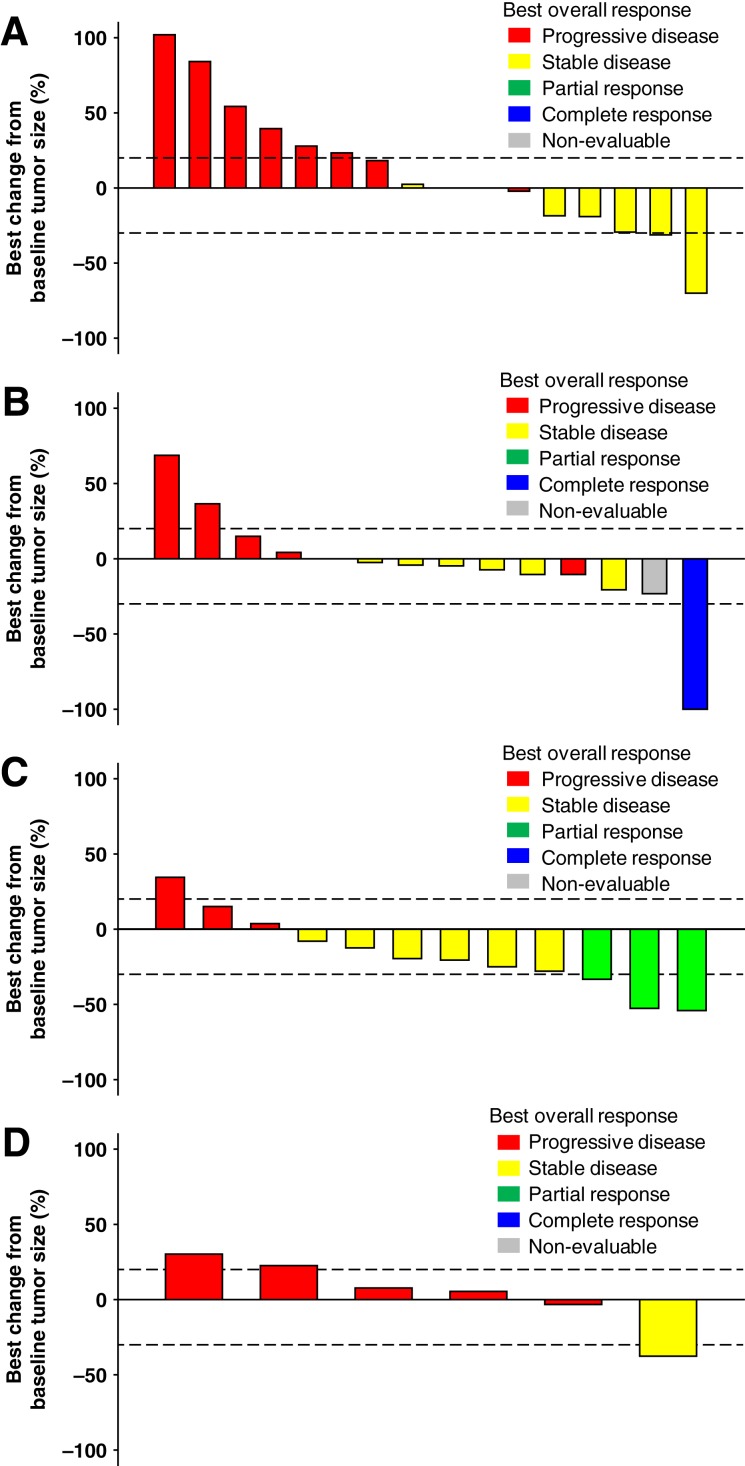
Figure 3.
Tumor size percent change from baseline by study part. (A): Part C: Head and neck squamous cell cancer. (B): Part D: Cholangiocarcinoma (CCA), treated with merestinib and cisplatin. (C): Part E: CCA, treated with merestinib, gemcitabine, and cisplatin. (D): Part F: Gastric and gastroesophageal junction cancer. Patients who had more or fewer target lesions at postbaseline than baseline, or whose target lesions that were assessed at baseline were not all assessed at postbaseline, are excluded from the waterfall plot.
Of the 20 patients with CCA who were treated with merestinib and cisplatin (all dose cohorts), 1 (6%) had a complete response (CR) and 6 (30%) had a best response of SD (Table 3; Fig. 3B). The estimated median PFS by dose cohort was 1.8 months (80 mg) and 3.5 months (120 mg). The PFS rates at 3 months and 6 months are in Table 3. Duration on study ranged from 22 days to 15 months (Fig. 2D).
Of the 16 patients with treatment‐naïve CCA treated with merestinib, gemcitabine, and cisplatin (all dose cohorts), 3 (19%) had a partial response (PR). A total of eight patients (50%) had a best response of SD (Table 3; Fig. 3C). The estimated median PFS was 8.57 months. The PFS rates at 3 months and 6 months are in Table 3. Duration on study ranged from 1 to 20 months (Fig. 2E).
Of the 11 patients with gastric and GEJ cancer, 2 (18.2%) had a best response of SD (Table 3; Fig. 3D). The estimated median PFS in part F was 1.7 months. The PFS rates at 3 months and 6 months are in Table 3. Duration on study ranged from 8 days to 16 months (Fig. 2F).
Supplemental online Figures 1 and 2 show the estimated tumor growth kinetics per patient from 100 days prior to start of treatment through the end of treatment with merestinib monotherapy or merestinib in combination with other therapies, respectively.
Pharmacokinetics Analysis
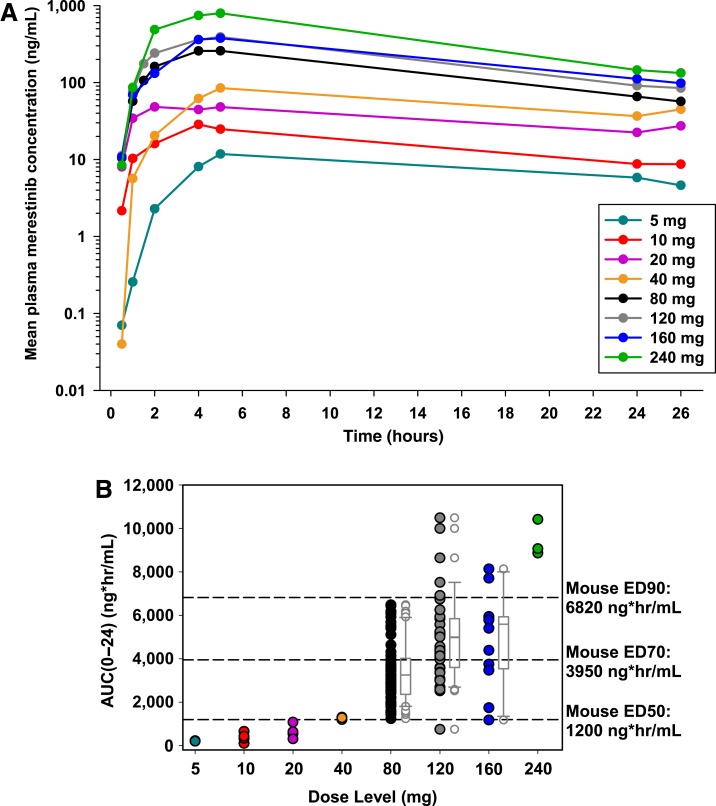
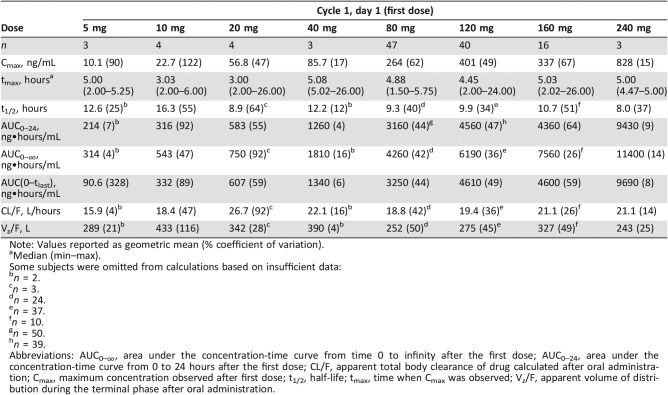
Data from patients who received the enabled formulation exhibited a notable increase in exposure compared with cohorts receiving the drug‐in‐capsule formulation, which was minimally absorbed and hence exhibited negligible exposure (data not shown). Figure 4A illustrates the mean concentration‐time profiles following the first dose of merestinib on day 1 of cycle 1 in patients receiving the enabled formulation. In a noncompartmental analysis, Cmax and AUC0–24 following the first dose of merestinib (cycle 1, day 1) indicated exposures increased with dose in an approximately proportional manner from 5 to 240 mg of the enabled formulation in part A (supplemental online Fig. 3). Individual AUC0–24 values following the first dose across all study parts, plotted by dose level, likewise increased with dose (Fig. 4B). Exposure thresholds (AUC0–24) associated with efficacy in a mouse (U87MG) xenograft model were overlaid for comparison. Across all dose levels, the geometric mean of oral volume of distribution (Vz/F) was 284 L (49% coefficient of variation [CV]), and CL/F was 19.6 L/hour (37% CV), leading to a typical t1/2 of 10.0 hours (41% CV). The wide range of tmax suggests variability in the rate of absorption, which in part may account for differences in exposure over the postdose sampling period. Table 4 provides a summary of PK parameters by treatment following the first dose of merestinib calculated for each cohort receiving the enabled formulation.
Figure 4.
Pharmacokinetics of merestinib. (A): Pharmacokinetics following the first dose of merestinib on day 1 of cycle 1 in patients receiving enabled formulation. (B): Exposure to merestinib following first dose, AUC0–24. Note: Dose levels on the x‐axis are categorical, not continuous. The axis is not drawn to scale.
Abbreviation: AUC0–24, area under the concentration‐time curve over 24 hours.
Table 4. Merestinib noncompartmental pharmacokinetic parameters for cycle 1, day 1 by dose.
Note: Values reported as geometric mean (% coefficient of variation).
Median (min–max).
Some subjects were omitted from calculations based on insufficient data:
n = 2.
n = 3.
n = 24.
n = 37.
n = 10.
n = 50.
n = 39.
Abbreviations: AUC0–∞, area under the concentration‐time curve from time 0 to infinity after the first dose; AUC0–24, area under the concentration‐time curve from 0 to 24 hours after the first dose; CL/F, apparent total body clearance of drug calculated after oral administration; Cmax, maximum concentration observed after first dose; t1/2, half‐life; tmax, time when Cmax was observed; Vz/F, apparent volume of distribution during the terminal phase after oral administration.
Discussion
Results of the dose‐escalation and dose‐confirmation parts of this study support dosing of merestinib as a single agent at 120 mg once daily, based on the acceptable exposure and safety at this dose level. During the dose‐escalation phase, DLTs of reversible and transient asymptomatic grade 3 increases in AST and/or ALT were observed. These increases were not accompanied by changes in bilirubin or other LFTs and reversed when dosing was interrupted. One CR was observed in a patient with CCA, and three patients with CCA achieved a PR. Overall, 60 (32%) of the 186 patients enrolled had a best response of SD.
Exposures of merestinib reflected in AUC or Cmax increased in an approximately dose‐proportional manner across a dose range of 5 to 240 mg following drug reformulation. Patients treated at the 120 mg daily proposed monotherapy dose exhibited similar exposures regardless of tumor type. Overall, exposures (AUC0–24) following single doses of 80 mg or higher reached or exceeded corresponding thresholds associated with efficacy in a preclinical (mouse) U87MG xenograft PK‐PD model, equal to or greater than the EC50 threshold associated with tumor growth inhibition in this model.
Several MET pathway inhibitors have been tested in clinical trials with disappointing results. Early results of rilotumumab and onartuzumab in patients with gastric cancer and non‐small cell lung cancer (NSCLC) were promising but were not confirmed in larger phase III studies in various cancers [38], [39], [40], [41], [42], [43]. Small‐molecule MET inhibitors have also been disappointing. The addition of tivantinib to cetuximab and irinotecan in patients with metastatic CRC failed to meet the primary endpoint of improved PFS [44]. The phase III MARQUEE trial study of erlotinib with or without tivantinib in unselected patients with previously treated nonsquamous NSCLC was halted when the interim analysis demonstrated no improvement in OS despite increased PFS [45]. A phase II placebo‐controlled trial of tivantinib as second‐line treatment for HCC demonstrated a prolonged time to disease progression, with a more pronounced effect in patients with MET‐high tumors [46]. Unfortunately, the phase III trial of tivantinib as second‐line treatment for HCC in patients with high MET expression did not meet its primary efficacy endpoint [47]. Originally designed as a MET inhibitor, crizotinib distinguished itself in clinical trials via tumor reduction treatment in patients with anaplastic lymphoma kinase‐positive NSCLC [48], although more recent evidence suggests that crizotinib can benefit patients with the MET exon 14 skipping mutation, which occurs in 3%–4% of patients with lung adenocarcinoma and less frequently in other tumor types [49], [50], [51]. Several other small‐molecule MET inhibitors are also under investigation for use as NSCLC treatments in patients with exon 14 skipping mutations [52]. These largely disappointing data underscore the challenge of realizing the clinical utility of MET inhibition.
Patient selection is likely key to improving outcomes; to date, there is no validated biomarker useful in selecting patients most or least likely to benefit from MET inhibition. MET positivity by IHC has been the criterion for most of the aforementioned negative studies. Results from a MET tyrosine kinase inhibitor (AMG 337) study in advanced solid tumors suggested greater antitumor effects in patients with MET amplification as determined by fluorescence in situ hybridization (FISH) analysis [53]. The FISH analysis threshold that defines MET positivity, however, can vary across trials, with more stringent criteria probably reducing inclusion of patients whose tumors have oncogenic drivers other than MET [54]. The proportion of patients with MET amplification and MET mutation in any tumor type is generally very low, which makes clinical trials aimed at such populations challenging to conduct. Nevertheless, given the high MET expression detected in different tumor types [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [55], targeting MET and related pathways may yet prove to be useful in the effective treatment of various cancers, provided patients most likely to respond can be identified.
Although designed to be an inhibitor of MET, merestinib also inhibits the receptor tyrosine kinases MST1R, AXL, ROS1, PDGFRA, FLT3, TEK, DDR1, DDR2, MERTK, TYR03, TRKA, TRKB, and TRKC [9], [11], as well as the serine/threonine kinases MKNK1 and MKNK2 [9]. This property may explain the occasional observed responses in the few patients with tumor regression in our study.
One limitation of this study was that the patient population enrolled in the dose‐confirmation part of the study included treatment‐refractory patients who were not selected for a particular MET phenotype or genotype. Although the search for candidate biomarkers has evolved since the trial began in 2009, archived tumor samples of suitable quality were not available from a sufficient number of patients to conduct a meaningful retrospective biomarker analysis as part of this study. However, it is interesting that among the tested populations, the only group to demonstrate an objective response was the patients with cholangiocarcinoma, an indication that has previously been associated with significant levels of MET overexpression [26], [31], [32]. This finding has triggered a follow‐on study (NCT02711553), which includes prospective sample collection for the interrogation of biomarkers that are related to the pathways inhibited by merestinib.
Conclusion
Our study suggests that merestinib has a tolerable safety profile and potential anticancer activity. On the basis of the results in this and other studies of merestinib, subsequent disease‐directed development of merestinib as monotherapy and as part of combination regimens is ongoing (NCT02711553).
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank the patients, their families, the study sites, and the study personnel who participated in this clinical trial. This study was sponsored by Eli Lilly and Company. Editorial support was provided by Sarah Becker‐Marrero of Syneos Health and funded by Eli Lilly and Company. Medical writing support was provided by Meghan Greenwood of Syneos Health and funded by Eli Lilly and Company. This work was presented in part at AACR 2014.
Author Contributions
Conception/design: Johan Wallin, Richard Walgren
Provision of study material or patients: Aiwu Ruth He, Roger B. Cohen, Crystal S. Denlinger, Ariel Birnbaum, Jimmy Hwang, Nancy Lewis, Elizabeth R. Plimack
Collection and/or assembly of data: Aiwu Ruth He, Crystal S. Denlinger, Ashwin Sama, Takami Sato, Nancy Lewis, Michele Niland, Jennifer Giles, Richard Walgren, Elizabeth R. Plimack
Data analysis and interpretation: Aiwu Ruth He, Roger B. Cohen, Jimmy Hwang, Michelle Mynderse, Johan Wallin, Brian Moser, Wei Zhang, Richard Walgren, Elizabeth R. Plimack
Manuscript writing: Ashwin Sama, Michelle Mynderse, Michele Niland, Jennifer Giles, Johan Wallin, Brian Moser, Wei Zhang, Richard Walgren
Final approval of manuscript: Aiwu Ruth He, Roger B. Cohen, Crystal S. Denlinger, Ashwin Sama, Ariel Birnbaum, Jimmy Hwang, Takami Sato, Nancy Lewis, Michelle Mynderse, Michele Niland, Jennifer Giles, Johan Wallin, Brian Moser, Wei Zhang, Richard Walgren, Elizabeth R. Plimack
Disclosures
Aiwu Ruth He: EMD Serono, Bristol‐Myers Squibb, Bayer, and ArQule (SAB), EMD Serono and Merck (RF); Roger B. Cohen: Eli Lilly and Company (RF, institutional); Crystal S. Denlinger: Merck, EMD Serono, and Eli Lilly and Company (SAB), Carevive (C/A), Eli Lilly and Company, Bristol‐Myers Squibb, Merrimack Pharmaceuticals, MedImmune, Advaxis, Genentech, Incyte, OncoMed Pharmaceuticals, and AstraZeneca (RF, institutional); Ashwin Sama: Bristol‐Myers Squibb (E, O/I), OncoPlex Diagnostics and Celgene (H), Merrimack Pharmaceuticals (C/F), Incyte, AstraZeneca, Eli Lilly and Company, and EMD Serono (RF, institutional); Jimmy Hwang: Amgen, Bayer/Onyx, Genentech/Roche, Eli Lilly and Company, Merrimack Pharmaceuticals, Sanofi, Taiho Pharmaceutical (SAB), and Roche (ET); Takami Sato: Immunocore Ltd (SAB), Eli Lilly and Company (RF, institutional); Nancy Lewis: Novartis Pharmaceuticals (E); Michelle Mynderse: Syneos Health (E); Michelle Niland: Eli Lilly and Company (E, O/I); Jennifer Giles: Eli Lilly and Company (E, O/I); Johan Wallin: Eli Lilly and Company (E, O/I); Brian Moser: Eli Lilly and Company (E, O/I); Wei Zhang: Eli Lilly and Company (E, O/I); Richard Walgren: Eli Lilly and Company (E, O/I); Elizabeth R. Plimack: AstraZeneca, Bristol‐Myers Squibb, Eli Lilly and Company, Exelixis, Genentech, Horizon Pharma, Inovio, Novartis, Pfizer, and Roche (SAB), Acceleron, Agensys, AstraZeneca, Bristol‐Myers Squibb, Merck, Peloton Pharmaceuticals, and Pfizer (RF, institutional). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Maulik G, Shrikhande A, Kijima T et al. Role of the hepatocyte growth factor receptor, c‐Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev 2002;13:41–59. [DOI] [PubMed] [Google Scholar]
- 2.Gherardi E, Birchmeier W, Birchmeier C et al. Targeting MET in cancer: Rationale and progress. Nat Rev Cancer 2012;12:89–103. [DOI] [PubMed] [Google Scholar]
- 3.Migliore C, Giordano S. Molecular cancer therapy: Can our expectation be MET? Eur J Cancer 2008;44:641–651. [DOI] [PubMed] [Google Scholar]
- 4.Ma PC, Jagadeeswaran R, Jagadeesh S et al. Functional expression and mutations of c‐Met and its therapeutic inhibition with SU11274 and small interfering RNA in non‐small cell lung cancer. Cancer Res 2005;65:1479–1488. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier C, Birchmeier W, Gherardi E et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915–925. [DOI] [PubMed] [Google Scholar]
- 6.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504–516. [DOI] [PubMed] [Google Scholar]
- 7.Bean J, Brennan C, Shih JY et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932–20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelman JA, Zejnullahu K, Mitsudomi T et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039–1043. [DOI] [PubMed] [Google Scholar]
- 9.Yan SB, Peek VL, Ajamie R et al. LY2801653 is an orally bioavailable multi‐kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti‐tumor activities in mouse xenograft models. Invest New Drugs 2013;31:833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu W, Bi C, Credille KM et al. Inhibition of tumor growth and metastasis in non‐small cell lung cancer by LY2801653, an inhibitor of several oncokinases, including MET. Clin Cancer Res 2013;19:5699–5710. [DOI] [PubMed] [Google Scholar]
- 11.Konicek BW, Capen AR, Credille KM et al. Merestinib (LY2801653) inhibits neurotrophic receptor kinase (NTRK) and suppresses growth of NTRK fusion bearing tumors. Oncotarget 2018;9:13796–13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosciuczuk EM, Saleiro D, Kroczynska B et al. Merestinib blocks MNK kinase activity in acute myeloid leukemia progenitors and exhibits antileukemic effects in vitro and in vivo. Blood 2016;128:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell JB, Eckerdt F, Alley K et al. MNK inhibition disrupts mesenchymal glioma stem cells and prolongs survival in a mouse model of glioblastoma. Mol Cancer Res 2016;14:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng ZS, Weiser MR, Kuntz E et al. c‐Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett 2008;265:258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Oliveira AT, Matos D, Logullo AF et al. MET is highly expressed in advanced stages of colorectal cancer and indicates worse prognosis and mortality. Anticancer Res 2009;29:4807–4811. [PubMed] [Google Scholar]
- 16.Liu Y, Yu XF, Zou J et al. Prognostic value of c‐Met in colorectal cancer: A meta‐analysis. World J Gastroenterol 2015;21:3706–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao H, Guan M, Sun Z et al. High c‐Met expression is a negative prognostic marker for colorectal cancer: A meta‐analysis. Tumour Biol 2015;36:515–520. [DOI] [PubMed] [Google Scholar]
- 18.Sattler M, Reddy MM, Hasina R et al. The role of the c‐Met pathway in lung cancer and the potential for targeted therapy. Ther Adv Med Oncol 2011;3:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S, Wang Z. Head neck squamous cell carcinoma c‐Met(+) cells display cancer stem cell properties and are responsible for cisplatin‐resistance and metastasis. Int J Cancer 2011;129:2337–2348. [DOI] [PubMed] [Google Scholar]
- 20.Casado V. Role of c‐MET pathway in the outcome prediction of cetuximab based therapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. ASCO Annual Meeting Proceedings (Post‐Meeting Edition) 2012;30:5520. [Google Scholar]
- 21.Lee CH, Yen CY, Liu SY et al. Axl is a prognostic marker in oral squamous cell carcinoma. Ann Surg Oncol 2012;19(suppl 3):S500–S508. [DOI] [PubMed] [Google Scholar]
- 22.Hong J, Peng D, Chen Z et al. ABL regulation by AXL promotes cisplatin resistance in esophageal cancer. Cancer Res 2013;73:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Stabile LP, Gubish CT et al. Dual blockade of EGFR and c‐Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin Cancer Res 2011;17:4425–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stabile LP, He G, Lui VW et al. c‐Src activation mediates erlotinib resistance in head and neck cancer by stimulating c‐Met. Clin Cancer Res 2013;19:380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dulak AM, Gubish CT, Stabile LP et al. HGF‐independent potentiation of EGFR action by c‐Met. Oncogene 2011;30:3625–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thobe MN, Yan SB, Credille KM et al. Prevalence of MET expression, activating mutations of KRAS and IDH1/2, and ROS1 fusions in cholangiocarcinoma patient tumor samples [abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; April 6–10, 2013; Washington, DC.
- 27.Wu X, Zhou J, Rogers AM et al. c‐Met, epidermal growth factor receptor, and insulin‐like growth factor‐1 receptor are important for growth in uveal melanoma and independently contribute to migration and metastatic potential. Melanoma Res 2012;22:123–132. [DOI] [PubMed] [Google Scholar]
- 28.Economou MA, All‐Ericsson C, Bykov V et al. Receptors for the liver synthesized growth factors IGF‐1 and HGF/SF in uveal melanoma: Intercorrelation and prognostic implications. Invest Ophthalmol Vis Sci 2005;46:4372–4375. [DOI] [PubMed] [Google Scholar]
- 29.Abdel‐Rahman MH, Boru G, Massengill J et al. MET oncogene inhibition as a potential target of therapy for uveal melanomas. Invest Ophthalmol Vis Sci 2010;51:3333–3339. [DOI] [PubMed] [Google Scholar]
- 30.Mallikarjuna K, Pushparaj V, Biswas J et al. Expression of epidermal growth factor receptor, ezrin, hepatocyte growth factor, and c‐Met in uveal melanoma: An immunohistochemical study. Curr Eye Res 2007;32:281–290. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto M, Ojima H, Iwasaki M et al. Prognostic significance of overexpression of c‐Met oncoprotein in cholangiocarcinoma. Br J Cancer 2011;105:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun S, Wang Z. Head neck squamous cell carcinoma c‐Met+ cells display cancer stem cell properties and are responsible for cisplatin‐resistance and metastasis. Int J Cancer 2011;129:2337–2348. [DOI] [PubMed] [Google Scholar]
- 33.Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol 2009;148:119–127. [DOI] [PubMed] [Google Scholar]
- 34.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 35.Sennino B, Ishiguro‐Oonuma T, Wei Y et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c‐Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2012;2:270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 37.Smith BP, Vandenhende FR, DeSante KA et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res 2000;17:1278–1283. [DOI] [PubMed] [Google Scholar]
- 38.Bendell JC, Hochster H, Hart LL et al. A phase II randomized trial (GO27827) of first‐line FOLFOX plus bevacizumab with or without the MET inhibitor onartuzumab in patients with metastatic colorectal cancer. The Oncologist 2017;22:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cloughesy T, Finocchiaro G, Belda‐Iniesta C et al. Randomized, double‐blind, placebo‐controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: Efficacy, safety, and hepatocyte growth factor and O(6)‐methylguanine‐DNA methyltransferase biomarker analyses. J Clin Oncol 2017;35:343–351. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch FR, Govindan R, Zvirbule Z et al. Efficacy and safety results from a phase II, placebo‐controlled study of onartuzumab plus first‐line platinum‐doublet chemotherapy for advanced squamous cell non‐small‐cell lung cancer. Clin Lung Cancer 2017;18:43–49. [DOI] [PubMed] [Google Scholar]
- 41.Iveson T, Donehower RC, Davidenko I et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first‐line treatment for gastric or oesophagogastric junction adenocarcinoma: An open‐label, dose de‐escalation phase 1b study and a double‐blind, randomised phase 2 study. Lancet Oncol 2014;15:1007–1018. [DOI] [PubMed] [Google Scholar]
- 42.Spigel DR, Edelman MJ, O'Byrne K et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non‐small‐cell lung cancer: METLung. J Clin Oncol 2017;35:412–420. [DOI] [PubMed] [Google Scholar]
- 43.Wakelee H, Zvirbule Z, De BF et al. Efficacy and safety of onartuzumab in combination with first‐line bevacizumab‐ or pemetrexed‐based chemotherapy regimens in advanced non‐squamous non‐small‐cell lung cancer. Clin Lung Cancer 2017;18:50–59. [DOI] [PubMed] [Google Scholar]
- 44.Eng C, Bessudo A, Hart LL et al. A randomized, placebo‐controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild‐type KRAS who have received first‐line systemic therapy. Int J Cancer 2016;139:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scagliotti G, von PJ, Novello S et al. Phase III multinational, randomized, double‐blind, placebo‐controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non‐small‐dell lung cancer. J Clin Oncol 2015;33:2667–2674. [DOI] [PubMed] [Google Scholar]
- 46.Santoro A, Rimassa L, Borbath I et al. Tivantinib for second‐line treatment of advanced hepatocellular carcinoma: A randomised, placebo‐controlled phase 2 study. Lancet Oncol 2013;14:55–63. [DOI] [PubMed] [Google Scholar]
- 47.Rimassa L, Assenat E, Peck‐Radosavljevic M et al. Tivantinib for second‐line treatment of MET‐high, advanced hepatocellular carcinoma (METIV‐HCC): A final analysis of a phase 3, randomised, placebo‐controlled study. Lancet Oncol 2018;19:682–693. [DOI] [PubMed] [Google Scholar]
- 48.Poon CC, Kelly JJ. Development of crizotinib, a rationally designed tyrosine kinase inhibitor for non‐small cell lung cancer. Int J Cancer 2017;140:1945–1954. [DOI] [PubMed] [Google Scholar]
- 49.Awad MM, Oxnard GR, Jackman DM et al. MET exon 14 mutations in non‐small‐cell lung cancer are associated with advanced age and stage‐dependent MET genomic amplification and c‐Met overexpression. J Clin Oncol 2016;34:721–730. [DOI] [PubMed] [Google Scholar]
- 50.Frampton GM, Ali SM, Rosenzweig M et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850–859. [DOI] [PubMed] [Google Scholar]
- 51.Paik PK, Drilon A, Fan PD et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reungwetwattana T, Liang Y, Zhu V et al. The race to target MET exon 14 skipping alterations in non‐small cell lung cancer: The why, the how, the who, the unknown, and the inevitable. Lung Cancer 2017;103:27–37. [DOI] [PubMed] [Google Scholar]
- 53.Kwak EL, LoRusso P, Hamid O et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET‐amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. J Clin Oncol 2015;33(suppl 3):1.25332246 [Google Scholar]
- 54.Noonan SA, Berry L, Lu X et al. Identifying the appropriate FISH criteria for defining MET copy number‐driven lung adenocarcinoma through oncogene overlap analysis. J Thorac Oncol 2016;11:1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyamoto M, Ojima H, Iwasaki M et al. Prognostic significance of overexpression of c‐Met oncoprotein in cholangiocarcinoma. Br J Cancer 2011;105:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]