Abstract
The primary cilium is a hair-like surface-exposed organelle of the eukaryotic cell that decodes a variety of signals — such as odorants, light and Hedgehog morphogens — by altering the local concentrations and activities of signalling proteins. Signalling within the cilium is conveyed through a diverse array of second messengers, including conventional signalling molecules (such as cAMP) and some unusual intermediates (such as sterols). Diffusion barriers at the ciliary base establish the unique composition of this signalling compartment and cilia adapt their proteome to signalling demands through regulated protein trafficking. Much progress has been made on the molecular understanding of regulated ciliary trafficking, which encompasses not only exchanges between the cilium and the rest of the cell but also the shedding of signalling factors into extracellular vesicles.
INTRODUCTION
Nearly every cell in our body assembles one primary, non-motile cilium that protrudes from the plasma membrane and functions as a key signalling organelle. The broad importance of ciliary signalling is highlighted by a group of diseases collectively named ciliopathies that span a wide range of symptoms, including retinal degeneration, skeletal malformations, obesity and polycystic kidneys1. Primary cilia have been known as signalling centres for the light and olfactory transduction pathways since the 1950’s2,3 and their signaling functions were greatly broadened in 2003 when a forward genetic screen in mice uncovered a requirement for primary cilia in Hedgehog signalling, one of the central developmental signalling pathways that patterns limbs and neural tube4. Since then, a number of signalling pathways have been associated with primary cilia, including Wnt5, Notch6, planar cell polarity7, receptor tyrosine kinase (PDGFRα8, IGF-19), G-protein coupled receptors [G] (GPCRs)10 and transforming growth factor β (TGFβ)11 although the physiological requirement for cilia in some of these signaling modalities remains to be conclusively established. The discovery of major cilia-associated signalling pathways is still ongoing: it was only in 2018 that the GPCR melanocortin receptor 4 (MC4R), which is the most frequently mutated gene in familial cases of obesity, was shown to utilize the primary cilium for its anorexigenic [G] function12; as another example, the ciliary signalling pathways associated with left–right asymmetry determination and with polycystic kidney disease are still elusive. In addition to primary cilia, motile cilia found on motile cells (where they are often termed flagella) and on the surface of specialized epithelia (e.g. in the airways) also serve signalling functions (reviewed in13,14). Given the common signalling roles of primary cilia and motile cilia, they will be jointly discussed as cilia.
The first paradigms of ciliary signalling emerged from the study of olfactory receptor neurons and photoreceptors [G] where specialized cilia organize the entirety of light and olfactory transduction cascades. To do so, these cilia statically concentrate the GPCRs that sense photons (rhodopsin [G]) and specific odorants (olfactory receptors) at concentrations sufficient to enable sensitivity to single photons15 or a handful of odorant molecules16. Activation of these GPCRs alters the levels of the cyclic nucleotides cGMP (vision) or cAMP (smell) inside cilia to regulate the opening of cyclic nucleotide-gated ion channels. The resulting change in membrane potential propagates through the photoreceptor and olfactory receptor neurons and regulates neurotransmitter release. Studies of Hedgehog transduction in vertebrates and of fertilization in the motile protist Chlamydomonas reinhardtii introduced a twist in the paradigm of ciliary signaling—rather than relying on static concentration of signalling receptors in cilia, these pathways rely on the dynamic redistribution of signalling proteins between cilia and the rest of the cell to appropriately transduce signals17–21.
Much progress has been made in recent years in our understanding of how protein trafficking in and out of cilia is regulated and how the dynamic re-localization of pathway components influences signal processing at the molecular level. Defects in the machineries that mediate the entry and exit of signalling receptors have been found to underlie various ciliopathies such as Bardet-Biedl Syndrome [G], several skeletal dysplasia syndromes and some cases of retinal degeneration1,22. The regulated ciliary accumulation and localized activation of signalling receptors, channels and enzymes is now thought to endow the cilium with the ability to dynamically adjust concentrations of signalling molecules, such as Ca2+, cAMP and cGMP independently from their concentrations in the cytoplasm23,24. Presumably, the ability to separately regulate second messenger levels in the cytoplasm and cilia enabled the last common eukaryotic ancestor to transduce multiple signalling inputs without undesired crosstalk. Furthermore, lipids of the ciliary membrane have emerged as signalling and trafficking regulators25. In particular ciliary phosphoinositides [G] function as signposts that may enforce the directionality of trafficking processes, while mounting evidence points to sterols as key signalling intermediates in Hedgehog signalling26–28. Finally, extracellular vesicles budding from cilia tips and packaged with activated signalling molecules represent a novel modality utilized to dispose excess signalling molecules and to regulate cilium length29–31.
This Review focuses on how cilia establish their unique composition of proteins, lipids and second messengers and how they dynamically regulate their composition to function as signalling compartments. After introducing how cilia establish their unique proteome and lipidome, we examine the mechanisms that regulate protein entry into and exit out of cilia and how they dynamically control cilia composition and shape ciliary signalling pathways.
Maintenance of ciliary composition
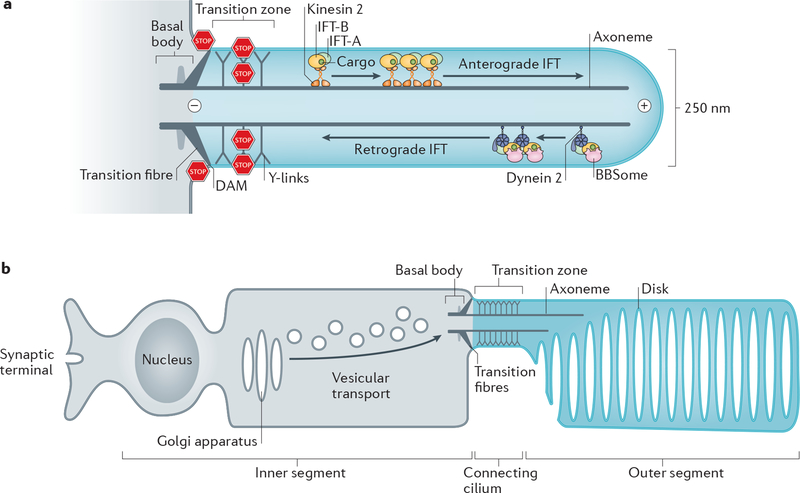
The cilium is a microtubule-based structure consisting of a basal body [G] with nine triplet microtubules that extend into an axoneme [G] with nine doublet microtubules ensheathed within a ciliary membrane. Typically 2 to 10 μm-long, cilia can reach exceptional lengths of 200 μm in olfactory neurons. The transition zone and the transition fibres [G] are two ultrastructural specializations that physically separate the ciliary shaft from the basal body (Fig. 1a). The transition fibres connect the basal body to the plasma membrane and are identical to the distal appendages of the oldest ‘mother’ centriole while the transition zone typically lines the proximal 0.5 μm of the cilium, where Y-links [G] connect the axoneme to the membrane32–34. Three distinct biological strategies are utilized to enrich molecules in cilia versus the rest of the cell: selective diffusion barriers, local production and diffusion to capture.
Figure 1|. Organization of primary cilia and photoreceptors.
a| Diagram of a primary cilium. The basal body consists of 9 triplets of microtubules and is connected to the plasma membrane by the transition fibres. The axoneme is formed by 9 doublets of microtubules (only 2 are shown). At the base of the axoneme is the transition zone, which is connected to the ciliary membrane by Y-links. Microtubule motors and three intraflagellar transport (IFT) complexes (Box 3) assemble into IFT trains that move ciliary cargoes move along the axoneme.. Kinesin-II mediates anterograde IFT and cytoplasmic dynein 2 retrograde IFT. The DAM (distal appendage matrix) –positioned between the transiton fibres together with the transition zone separate the cilium from the rest of the cell by blocking (STOP signs) lateral diffusion of membrane proteins and diffusion of soluble proteins. Plus (+) and minus (–) mark the orientation of the microtubules in the axoneme. b| Diagram of a photoreceptor neuron. The outer segment (equivalent of the cilium shaft) comprises hundreds of membrane disks that each concentrates about 1 million molecules of rhodopsin. All proteins required for phototransduction are produced in the inner segment, transported through the transition zone (termed ‘connecting cilium’), and are injected into the outer segment.
Selective barriers for proteins.
How cilia could maintain their unique protein composition despite a topological continuity between plasma and ciliary membranes and between ciliary interior and cytoplasm was long a conundrum. Bulk photokinetic studies, single molecule tracking and in vitro assays in mammalian cells resolved this problem by demonstrating that membrane proteins and large soluble proteins are prevented from freely diffusing in and out of cilia by the transition zone and the transition fibres35–43 (Fig. 1a). Nearly 30 proteins are known to constitute the transition zone and they assemble into at least four biochemical entities (MKS, NPHP and CEP290 modules, RPGRIP1L protein) encoded by genes mutated in ciliopathies: Meckel-Gruber (MKS) and Joubert syndromes, and nephronophtisis (NPHP)33. At time scales of tens of minutes, the MKS module is stably anchored at the transition zone of mammalian cells and nematodes44,45, whereas the localization of C. reinhardtii CEP290 at the transition zone is dynamic46. Intriguingly, localization of NPHP4 to the transition zone is dynamic in mammalian cells45 but static in C. reinhardtii47 and, at multi-hour time scales, even the MKS module may undergo dynamic exchange between the transition zone and the ciliary shaft in mammalian cells and nematodes48–50. It is thus conceivable that some ‘transition zone’ proteins actually function as trafficking factors whose rate-limiting transport step resides at the transition zone. In photoreceptors (Fig. 1b), the transition zone is augmented with a specific set of proteins that help this specialized cell type cope with the unique demands of transporting 1000 rhodopsin molecules per second from the cell body (the inner segment) into a highly specialized ciliary shaft (the outer segment)51. Compositional differences of the transition zone have also been reported among various fly and mouse tissues52–54 and may contribute to the morphological and functional diversity of cilia. The finding that mutations in transition zone components cause leakage of various proteins into and out of the cilium first established that the transition zone is a diffusion barrier for ciliary proteins37,46,47,55–58. Single molecule tracking directly demonstrated that the transition zone blocks the free diffusion of the 7 transmembrane proteins Smoothened (Hedgehog transducer) and GPR161 (negative regulator of Hedgehog signalling) out of cilia42,59.
To date, five structural components of the transition fibres have been identified and mapped by super-resolution microscopy41,60,61. Given the large gaps (50 nm) between neighboring transition fibres and the observation that nematode cilia can be maintained in the absence of transition fibres62–64, little attention had been given to the hypothesis that transition fibres hinder protein diffusion. This recently changed with the discovery of a distal appendage matrix (DAM) between the transition fibres. The protein FBF1 (Fas-binding factor 1) is currently the only known component of the DAM and is required to confine membrane proteins to cilia41. In addition, GPR161 molecules that exit cilia have to cross a periciliary barrier whose dimensions are very similar to the DAM42 (see below). Remarkably, FBF1 is also localized at the tight junctions [G] of epithelial cells and participates in forming the fence between apical and basolateral membranes65. Similarly, the NPHP module of the transition zone localizes to tight junctions and participates in epithelial polarity66,67. Given that FBF1 is present in nematodes where it localizes at the base of cilia and functionally interacts with HYLS1 (hydrolethalus 1)68 to maintain ciliary composition, it is conceivable that a DAM composed of FBF1 and HYLS1 assembles in the absence of transition fibres.
Local supply of second messengers.
Foundational electrophysiological and biosensor-based measurements demonstrated that the concentration of free Ca2+ ([Ca2+]) is three to five times higher in cilia than in the cytoplasm69,70. Most importantly, these studies found no evidence for a diffusion barrier that impedes the movement of Ca2+ between the cilium and the cytoplasm. Rather, [Ca2+]cilia is the product of Ca2+ influx through polycystin family ion channels that reside in the ciliary membrane (Box 1) and of passive Ca2+ efflux into the cytoplasm. 200 to 300 Ca2+ ions are sufficient to generate 1 μM [Ca2+]cilia and leakage of Ca2+ from the cilium into the cytoplasm is of negligible consequence to cellular calcium homeostasis due to the three to four orders of magnitude larger volume of the cytoplasm. Thus, the constitutive supply of a molecule to the interior of cilia is sufficient to maintain a high local concentration in the absence of a diffusion barrier (Fig. 2a).
BOX 1 |. Polycystic kidney disease — a case of disrupted ciliary signalling.
Autosomal dominant polycystic kidney disease (PKD), the most common monogenic disorder in humans, is caused by dysfunction of polycystin 1 and 2 (encoded by the PKD1 and PKD2 genes)217, two transmembrane proteins related to transient receptor potential channels that form a complex localized to cilia218,219. The existence of a PKD2-dependent cation-selective current within primary cilia of kidney epithelial cells220,221 and of a Ca2+ channel formed by the polycystin-related proteins PKD1L1 and PKD2L1 in cilia of retinal pigmented epithelial (RPE) cells and mouse embryonic fibroblasts69 suggest that polycystin 1 and polycystin 2 may function as a Ca2+ channel. It was initially proposed that the polycystin1–polycystin2 complex functions as a mechanosensitive Ca2+ channel that responds to urine flow inside kidney tubules222, but careful investigations have rejected this model223 and the specific stimulus that activates the polycystin channel remains elusive.
The presence of numerous cystic lesions in ciliopathy patients and mouse models224 established the important role of cilia in cystogenesis (reviewed in1,217,225). Because cilia are required for rapid and widespread cystogenesis in mice lacking Pkd1 or Pkd2 (ref.226), polycystins are thought to repress a ciliary cystogenic pathway. The suppression of cyst formation in Pkd mutants by genetic ablation of adenylyl cyclase 5 (AC5) or AC6 placed these ciliary enzymes as positive regulators of the ciliary cystogenic pathway76,77. Based on these genetic interactions, a parsimonious yet speculative model emerges where an (as of yet unidentified) GPCR in kidney cilia, transducing the signal through Gαs, activates AC5 and AC6 to elevate ciliary cAMP and thus generates the cystogenic signal. This pathway is normally antagonized by the Ca2+-mediated inhibition of AC5 and AC6 made possible by Ca2+ entry through the ciliary polycystin 1–polycystin 2 channel (see figure). Currently, the sole approved PKD therapy relies on reduction of cellular cAMP levels217. A precise understanding of the ciliary cystogenic pathway promises to lead to more targeted therapies.
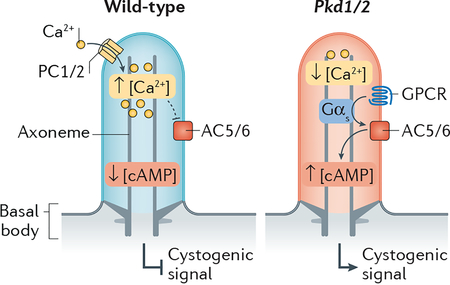
Figure 2|. Transport processes that shape the ciliary environment.
a| Local supply of ciliary molecules. Left: ciliary calcium channels allow local influx of Ca2+ into cilia, which then passively leaks into the cytoplasm (dashed arrows). Middle: adenylyl cyclases generate cAMP in cilia, which then passively leaks into the cytoplasm (dashed arrows). Right: phosphatidylinositol (PtdIns) 5-phosphatase INPP5E in the ciliary membrane converts PtdIns(4,5)P2 into PtdIns(4)P. Dashed arrows indicate lipid exchange between cilia and plasma membrane (assuming that there is no diffusion barrier for lipids). b| A diffusion-to-capture mechanism can enrich certain ciliary proteins. One example is microtubule plus-end binding protein EB1. c| Some membrane proteins, such as Chlamydomonas reinhardtii adhesion receptor SAG1 can enter cilia and accumulate at the ciliary base in the absence of intraflagellar transport (IFT) proteins. How this occurs or whether there are additional factors required is unknown (question mark). Similarly, tubulin dimers enter cilia by passive diffusion (dashed arrows) but require consumption of energy (solid arrow) to efficiently reach the growing ends of axonemal microtubules by IFT. d| Import of certain G-protein coupled receptors (GPCRs) by the TULP3–IFT-A complex. IFT-A recognizes ciliary targeting signals within GPCRs, and unloads its cargoes upon hydrolysis of PtdIns(4,5)P2 to PtdIns(4)P in the cilium. This ‘injection’ into the cilium may not require mechanochemical coupling of kinesin-II. e| Import of prenylated ciliary proteins is facilitated by specific chaperones that shield the hydrophobic prenyl groups. Exemplified here is INPP5E, whose farnesyl group (zigzag) is chaperoned by PDE6δ. Upon entry of the INPP5E–PDE6δ complex into cilia, the binding of ARL3–GTP to PDE6δ triggers the release of INPP5E, which becomes membrane-associated. GTP loading on ARL3 is promoted by ciliary protein ARL13B due to its guanine nucleotide exchange factor (GEF) activity, which ensures high levels of ARL3–GTP in cilia. RP2 at the transition zone functions as a GTPase-activating protein (GAP) for ARL3, ensuring that ARL3 is GDP-bound after exiting cilia. This creates a gradient of ARL3–GTP, with ARL3–GTP — and consequently INPP5E release — being restricted to the ciliary shaft. f| Hierarchy of ciliary localizations during protein targeting to cilia. Shown are factors required to allow for an enrichment of IFT-A cargos in cilia. ARL13B in cilia ensures GTP loading of ARL3, which is required for INPP5E localization to cilia. INPP5E hydrolyzes PtdIns(4,5)P2 to PtdIns(4)P in cilia, which allows cargo release from TULP3–IFT-A. How ARL13B is enriched in cilia is currently unknown. g| Passive influx of molecules can be overcome by active removal, exemplified by the BBSome-dependent retrieval of phospholipase D (PLD). h| Activated GPCRs are recognized by the β-arrestin 2, followed by capture by nascent BBSome-containing IFT trains at the ciliary tip, processive retrograde IFT and ultimately transition zone crossing. This may be followed by either GPCR endocytosis or lateral diffusion into the plasma membrane, both outcomes leading to receptor retrieval from the cilium into the cell.
Cyclic nucleotides are well-established as critical intermediates in ciliary signalling24. cGMP is the second messenger for phototransduction, and cAMP is the second messenger for olfactory signalling. Concentrations of free cAMP in olfactory cilia71 and in sperm flagella72 have been estimated at around 100 nM and one study reported a free cAMP concentration in fibroblast cilia of 800 nM73. Given the challenges posed with using biosensors to measure absolute concentrations when organelle dimensions approach the diffraction limit, multiple independent approaches will be required to accurately determine [cAMP]cilia in relevant cell types. The enzymes that generate cAMP inside cilia in vertebrates are the adenylyl cyclases 3 (AC3), AC5 and AC6. AC3 localizes to neuronal cilia and mutations in AC3 cause severe obesity — a cardinal symptom of several ciliopathies74,75. AC5 and AC6 localize to kidney cilia where they promote cystogenesis in polycystic kidney disease76,77 (Box 1). AC5 and AC6 are also the major adenylyl cyclase isoforms regulating Hedgehog signalling in primary cilia (Box 2) as shown in fibroblasts and neuroblasts78–80 and AC6 regulates cilium function in osteocytes81. An important functional difference between the ciliary adenylyl cyclases is that AC5 and AC6 are inhibited by sub-micromolar concentrations of free Ca2+ while AC3 is not82. Ciliary Ca2+ may thus feed into the regulation of ciliary cAMP in cell types where AC5 and AC6 are the major ciliary ACs (see Boxes 1 and 2). GPCRs are the major source of regulatory inputs for adenylyl cyclases, activating all ACs through the heterotrimeric G protein α subunit [G] Gαs and inhibiting AC5 and AC6 through Gαi. All olfactory GPCRs (~400 in human) are expected to be in olfactory cilia and out of the 400 non-olfactory GPCRs in the human genome, 30 have been localized to cilia83. Although the physiological importance of ciliary localization of these GPCRs is coming into focus—one example being ciliary signalling by MC4R in body weight homeostasis12 — downstream signalling pathways inside cilia remain uncharacterized for the vast majority of ciliary GPCRs. The regulation of cyclic nucleotide-gated ion channels in phototransduction and olfactory signalling and the regulation of the processing of Gli transcription factors [G] by ciliary protein kinase A (PKA) in Hedgehog signalling (see Box 2) provide conceptual framework for how ciliary GPCRs may regulate downstream targets.
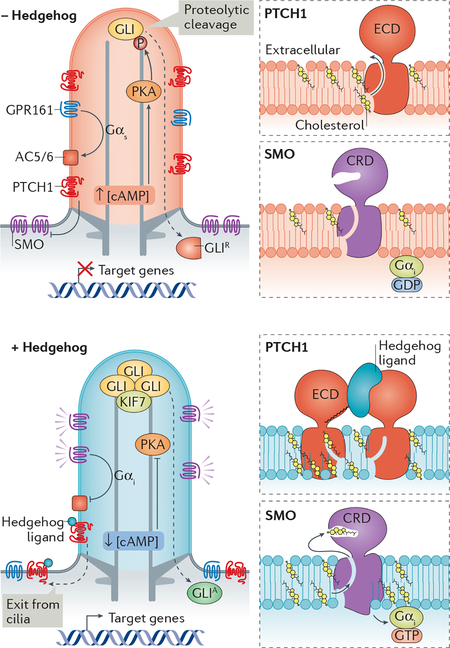
BOX 2 |. Hedgehog signalling at the cilium.
Hedgehog signalling patterns limbs and neural tube and its deregulation can cause cancer22. As the most intensely studied ciliary signalling pathway, Hedgehog signalling has established how signalling molecules interact with the ciliary environment to trigger a transcriptional response.
In cilia of unstimulated cells, activation of Gαs by the G protein-coupled receptor (GPCR) GPR161 leads to elevated cAMP concentration in cilia ([cAMP]cilia) and activation of protein kinase A (PKA). Phosphorylation (P) of the transcriptional effectors GLI2 and GLI3 by PKA inside cilia commits them for proteolytic processing into transcriptional repressors (GLIR), thus ensuring low expression of target genes. The main transducer of the Hedgehog signal is the 7 transmembrane protein Smoothened (SMO). Multiple lines of evidence suggest that SMO behaves as a Gαi-coupled GPCR (reviewed in227). Hedgehog-induced intraciliary activation of Gαi will inhibit adenylyl cyclase 5 (AC5) and AC6, thus decreasing [cAMP]cilia and PKA-mediated phosphorylation of GLI2 and GLI3. This inhibition ultimately shifts the processing of GLI2 and GLI3 from transcriptional repressors into activators (GLIA). Simultaneously, dephosphorylation of the kinesin KIF7 promotes its accumulation at the tip of cilia where it recruits GLI2, GLI3 and the BBSome42,228–231. GLI2 and GLI3 may undergo activating modifications at the ciliary tip and BBSome recruitment to the tip drives GPR161 retrieval to the cell body to further decrease [cAMP]cilia (see figure).
In the absence of Hedgehog, SMO is catalytically inhibited by Patched 1 (PTCH1), the Hedgehog receptor. Hedgehog binding to PTCH1 promotes PTCH1 exit form cilia21 and SMO accumulation in cilia to enable downstream signalling (see figure). The mechanism by which PTCH1 negatively regulates SMO remained an enigma until cholesterol was suggested to serve as the second messenger that links PTCH1 to SMO26,27. Consistent with its similarity to multidrug transporters, PTCH1 facilitates efflux of hydrophobic drug-like molecules out of the cell232,233. More specifically, PTCH1 can transport cholesterol out of the cytoplasmic leaflet of the plasma membrane (presumably into the exoplasmic leaflet) and binding of Hedgehog to PTCH1 leads to increased cholesterol concentrations in the cytoplasmic leaflet234. Congruently, structural studies uncovered a hydrophobic tunnel that courses from the cytoplasmic leaflet to the extracellular domain of PTCH1234–237 and the tunnel becomes obstructed upon binding of Hedgehog to PTCH1235 (see figure). In a striking parallel, a structure of active SMO identified a hydrophobic tunnel that may allow cholesterol to travel from the inner leaflet to its cysteine rich extracellular domain (CRD)238. As cholesterol binding to the CRD of SMO is sufficient to activate SMO26,27, high cholesterol levels in the inner leaflet of the ciliary membrane may gate SMO activation (see figure). In addition, ciliary oxysterols may participate in SMO activation28. Constant trafficking of SMO in and out of cilia (Fig. 3a) ensures that increased cholesterol levels in the inner leaflet of the ciliary membrane are rapidly sensed by SMO, causing its activation and trapping within cilia.
While several parts of this model are still speculative, it showcases how the cilium integrates second messenger levels, protein trafficking and membrane lipids with the signalling status to generate a transcriptional response.
Passive diffusion and capture.
Moderate size proteins (molecular weight≲100kDa) can freely diffuse into cilia and become enriched within cilia by binding to partner proteins or structures (Fig. 2b). One example is the microtubule +end-binding protein EB1 in C. reinhardtii, which localizes to cilia independently from the ciliary import machinery (see below and Box 3)84 and diffuses inside cilia before becoming enriched at the tip owing to binding to the +end of the microtubule axoneme85. The EB1 example illustrates how diffusion is sufficient to rapidly explore the volume of a femtoliter-scale compartment and how binding partners determine the localization of proteins.
BOX 3 |. Intraflagellar transport dynamics.
Much progress in understanding intraciliary trafficking has been made since the coat-like complexes intraflagellar transport (IFT) IFT-A, IFT-B and BBSome were discovered two decades ago239,240. IFT-B, a 16 subunit complex, polymerizes into IFT trains that move processively from base to tip (anterograde) and tip to base (retrograde) while IFT-A, a 6 subunit complex, and the BBSome, an 8 subunit complex, appear to function as adaptors between IFT-B polymers and membranous cargoes (reviewed in106,241). At the base of cilia, the microtubule motor kinesin 2 directly associates with IFT-B242 to move IFT-A, inactive cytoplasmic dynein 2 (also known as dynein 1b)243, as well as cargoes and BBSome on tracks provided by the microtubule axoneme. Once they arrive at the tip, IFT trains must be remodelled to associate with active dynein 2 and mediate retrograde transport. The purpose of retrograde IFT is two-fold: it delivers IFT-B complexes back to the base122 and it represents the first step in BBSome-mediated retrieval of cargoes back into the cell42. Single-molecule and sophisticated imaging of the turnaround step at the tip in Chlamydomonas reinhardtii and Caenorhabditis elegans have revealed that IFT-A complexes and IFT motors rapidly detach from anterograde trains and immediately re-attach to retrograde trains while IFT-B complexes spend several seconds confined at the tip before assembling into retrograde trains135,244,245. This delay in reassembly of IFT-B trains is likely to reflect a step of molecular rearrangement of the IFT-B complexes that is consistent with the drastically altered nanoscale morphology of retrograde trains compared to anterograde trains246. The serine/threonine kinase ICK (also known as intestinal cell kinase) is likely to be a driver of the tip remodelling events as it phosphorylates kinesin 2, localizes to the tip, and IFT-B, IFT-A, BBSome and kinesin 2 accumulate at the ciliary tip in its absence247. Furthermore, mutants for the counterparts of ICK in C. reinhardtii, Leishmania mexicana and C. elegans all present with longer cilia248–250, suggesting impaired ciliary retrieval.
Establishing ciliary membrane identity.
Despite their topological continuity, the ciliary and plasma membranes appear to have distinct lipid compositions. Models of local lipid generation in the cilium are sufficient to explain some of the compositional biases of ciliary lipids. Thus, in contrast to proteins, it is conceivable that there is no ciliary diffusion barrier for lipids. However, careful studies will be needed to determine if this is the case. An example of local lipid generation is provided by phosphatidylinositol (PtdIns) conversion in the cilium. Ciliary membranes contain high levels of PtdIns(4)P and low levels of PtdIns(4,5)P2 and the converse compositional bias is found in the plasma membrane86,87. When the ciliary PtdIns5-phosphatase INPP5E is defective, PtdIns(4,5)P2 is drastically elevated in cilia86,87. Thus, a compelling model posits that PtdIns(4,5)P2 diffuses from the plasma membrane into the cilium where it becomes hydrolyzed into PtdIns(4)P by INPP5E (Fig. 2a).
Besides the well-established enrichment of PtdIns(4)P in cilia, anecdotal evidence suggest high levels of long chain polyunsaturated lipids88–90 and a general enrichment in sphingolipids [G], sterols and short chain saturated fatty acids91–98. Lipidomics of isolated sea urchin embryo cilia found that cilia are enriched in at least four distinct oxysterols [G], two of which were found to activate Smoothened28. The existence of cilia-enriched lipid activators of Smoothened may provide an answer to the enigma of why cilia organize Hedgehog signalling in vertebrates (see also Box 2). Given that no cholesterol or sphingolipid metabolism enzyme has been reported in the cilium, the enrichment of cholesterol and sphingolipids in the ciliary membrane compared to the plasma membrane99–101 is harder to explain by the local generation model. In C. elegans, a GFP modified with the unsaturated lipid anchor geranylgeranyl is excluded from the cilium102, suggesting that cilia may exclude certain lipids. The findings that caveolin-1 and flotillin-2, which mark condensed membrane microdomains [G], localize to the transition zone in a sterol-dependent manner103 and that the transition zone membrane is the most detergent-resistant of all surface membranes in C. reinhardtii104 collectively suggest that a liquid-ordered lipid phase [G] exists at the transition zone. Densely packed lipids at the transition zone may thus interfere with lateral diffusion of membrane proteins and lipids between ciliary and plasma membrane. Understanding the mechanisms that establish and maintain the unique ‘raft [G]-like’ composition of the ciliary membrane remains a major challenge in cilia biology.
Ciliary protein transport
The accumulation of molecules against a concentration gradient — which is a paradigm for establishing the cilium as a unique signalling compartment — requires energy and a machinery that transforms chemical energy into directional transport. In the simplest embodiment of this principle, chemical energy is transformed into the directed movement of molecules across lipid bilayers by transmembrane transporters. A more complex model of indirect energy consumption is the nucleocytoplasmic gradient of Ran–GTP, which drives the directionality of nuclear import by promoting the dissociation of importins from their cargoes in the nucleus. Besides the problem of directionality in ciliary entry and exit, the barriers at the base of cilia present a major obstacle on the way in and out of cilia. Moreover, part of the ciliary proteome is dynamic as certain proteins only enter and exit the cilium upon stimulation with a signal. The mechanisms driving dynamic protein transport into, within, and out of cilia are now coming into focus.
Intraciliary movements.
The processive movement of proteins within cilia is referred to as intraflagellar transport (IFT). Three major multi-subunit protein complexes: IFT-A, IFT-B and the BBSome [G] cooperate with the microtubule motors kinesin-II and cytoplasmic dynein 2 (also known as dynein 1b) to move cargoes within cilia105. These complexes assemble into arrays of a few hundred nm in size, known as IFT trains [G], which transport ciliary proteins along the axoneme in both directions: towards the tip (anterograde transport) and towards the base (retrograde transport) (Fig. 1a). In addition, the three IFT complexes function in entry into cilia and exit from cilia106,107 (Box 3).
After the seminal observation that the carboxy-terminal tail of tubulin is directly recognized by the IFT-B subcomplex IFT74–IFT81 (ref.108), persuasive work demonstrated that this interaction underlies the processive transport of tubulin from ciliary base to tip in C. reinhardtii109,110 (Fig. 2c). Axonemal dyneins are responsible for the motility of motile cilia and represent another class of validated IFT-B cargoes but, unlike tubulin, they use specialized adaptors to bind IFT-B111–113. Intriguingly, the loading of tubulin and of the inner dynein arm adaptor onto anterograde IFT is high in growing cilia and low in fully assembled cilia109,113, suggesting the existence of mechanisms for length-dependent IFT loading. Similarly, cargo loading onto retrograde IFT trains is regulated by demands in cargo transport as exemplified by the increased rates of retrograde IFT movement of activated GPCRs that undergo regulated exit from cilia42.
In contrast to the motor-driven transport of tubulin and axonemal proteins observed in growing cilia, diffusion is the primary movement modality for membrane proteins and even for tubulin within fully assembled cilia (Fig. 2c)114–116. These observations reflect the need of a continuous supply of tubulin subunits to support the growth of the axoneme. Membrane proteins on the other hand need not be delivered to the tip for their signalling functions and the demands on tubulin delivery to the tip are likely to decrease considerably once cilia have reached their steady-state length.
Protein entry into cilia.
Membrane proteins reach the base of the cilium either through vesicular trafficking from the Golgi apparatus or recycling endosomes, or through lateral diffusion from the plasma membrane117. Several factors function in delivery of membrane proteins to the base of cilia including the Rab GTPases RAB8, RAB11, RAB23 and RAB34, the vesicle tethering complexes exocyst, TRAPII and the Golgi-associated IFT-B subunit IFT20 in concert with its partner GMAP210 (reviewed in118,119). Yet, vesicles are too large to cross the ciliary diffusion barrier, hence, vesicular transport does not mediate protein entry into cilia.
The anterograde trafficking machinery offers an appealing candidate for the ciliary importer given that anterograde IFT trains assemble at transition fibres before entry into cilia120–122. However, recent data have cast doubt on this simple model. In C. reinhardtii, acute inactivation of kinesin-II interrupted IFT within minutes. Notably, even after complete cessation of IFT, the adhesion receptor SAG1, which is involved in sexual reproduction of the alga, was still able to translocate from the cell body to cilia upon activation of the fertilization cascade. The finding that SAG1 still translocates into cilia after kinesin-II has been inactivated demonstrates that motor-driven IFT is dispensable for SAG1 entry into cilia20 (Fig. 2c). Similar experiments have demonstrated that kinesin-II is dispensable for ciliary entry of polycystin 2 (ref.123), αβ-tubulin109 and EB1 (ref.85) in C. reinhardtii and of several GPCRs in nematodes124. Consistently, in nematode IFT-B mutants, polycystin-2 (ref.125) and an olfactory GPCR126 still localize to cilia and it has been proposed that IFT-B might be dispensable for the entry of Smoothened into cilia in mammalian cells127. Furthering the disconnect between entry and anterograde IFT, a small domain of C. reinhardtii phospholipase D (crPLD) confers both anterograde and retrograde IFT yet is not sufficient for entry128. This collection of results makes a persuasive case for separate factors carrying out ‘injection’ into cilia and intraciliary transport. What, then, are the import complexes?
Given that the cut-off for passive entry into cilia is around 100 kDa35,39, some proteins, including αβ-tubulin and EB1 may enter cilia by simple diffusion. Larger soluble proteins such as the Gli transcription factors (see Box 2) or the dynein arms of motile cilia will need specific import factors that remain to be identified. For transmembrane proteins, evidence is emerging that the IFT-A complex mediates entry into cilia42,129–134 (Fig. 2d). In this process IFT-A is accompanied by Tubby family proteins Tubby and TULP3, which are absolutely required for the entry of nearly all known ciliary GPCRs and of the polycystic kidney disease proteins polycystin 1 and polycystin 2 as well as polycystic kidney and hepatic disease 1 [G] protein (PKHD1, also known as fibrocystin) into mammalian cilia42,129,132,134. The role of IFT-A–Tubby proteins in ciliary import is supported by observations that most of the known ciliary GPCRs fail to accumulate in cilia of mammalian cells deleted of IFT-A components130,131. Similarly, Tubby and IFT-A are required for ciliary accumulation of GPCRs in nematodes124 and a number of transmembrane proteins are absent from cilia in a C. reinhardtii IFT-A mutant133. Furthermore, the purified IFT-A complex directly recognizes the ciliary targeting signal [G] (CTS) of somatostatin receptor 3 [G] (SSTR3)42, and TULP3 interacts –most likely through IFT-A– with the CTSs of PKHD1, GPR161 and melanocortin concentrating hormone receptor 1 [G] (MCHR1)129.
An unexpected paradox emerges when one considers the view that kinesin-II-powered IFT-B trains inject IFT-A into cilia120,122. How then can entry of transmembrane proteins into cilia require IFT-A but not kinesin-II? One possible model to answer this paradox posits that trains consisting of IFT-B, IFT-A and kinesin-II assemble at the transition fibres and are injected into cilia without utilizing the mechanochemical coupling of kinesin-II. While provocative, this hypothesis is consistent with the very slow anterograde movement of IFT-B through the transition zone measured by single molecule imaging in C.elegans135 and in mammalian cells136. Anterograde IFT trains accelerate considerably once they have passed the transition zone, suggesting that kinesin-II may become active once trains have entered the ciliary shaft. Instead of relying on kinesin-II, the injection of IFT-A–IFT-B into cilia may rely on RABL2, a Rab family GTPase localized to transition fibres that selectively associates with IFT-B when bound to GTP and is required for IFT-B injection into cilia137,138.
If injection of IFT-A–cargo complexes does not rely on mechanochemical coupling, then how do IFT-A cargoes prominently accumulate inside cilia? Recent studies suggest that the directionality of transport by the IFT-A–TULP3 importer may be driven by a PtdIns switch between plasma membrane and cilium. In mammalian cells, TULP3, IFT-A and its cargoes GPR161 and PC2 hyperaccumulate inside cilia when ciliary PtdIns(4,5)P2 is no longer hydrolyzed into PtdIns(4)P86,87,129. In Drosophila melanogaster, the sole Tubby family protein dTULP is required for the ciliary import of the transient receptor potential channel Inactive and Inpp5e mutants accumulate PtdIns(4,5)P2, dTULP and Inactive inside cilia139,140. As TULP3 specifically binds to PtdIns(4,5)P2 but not to PtdIns(4)P, a model can be proposed where cargoes stay bound to IFT-A/TULP3 as long as membranes are rich in PtdIns(4,5)P2. Hydrolysis of PtdIns(4,5)P2 into PtdIns(4)P by ciliary INPP5E triggers unloading of cargoes from the IFT-A–TULP3 import complex (Fig. 2d). Once unloaded from IFT-A–TULP3, cargoes such as GPR161 exit cilia at a modest, constitutive rate because they can be recognized by the ciliary export machinery42 (see below). Meanwhile, cargoes bound to the IFT-A–TULP3 complex inside cilia —as happens in response to elevated levels of ciliary PtdIns(4,5)P2 — remain stuck and accumulate inside cilia because they are sheltered from the ciliary exit machinery.
Direct mechanochemical coupling appears similarly dispensable for the import of lipidated proteins into cilia. Foundational structural and biochemical work has established that the targeting of N-myristoylated (e.g. Gα) and prenylated (e.g. INPP5E) proteins to cilia relies on the ciliary shuttles UNC119 and PDE6δ, which directly recognize lipid groups in the cytoplasm and unload their cargoes inside cilia upon binding to small Arf-like GTPase ARL3 [G] bound to GTP (141–144, reviewed in ref.145,146) (Fig. 2e). ARL3 is converted into its active, GTP-bound state inside cilia because its guanine nucleotide exchange factor (GEF) ARL13B constitutively resides inside cilia. Furthermore, the localization of RP2, the GTPase activating protein (GAP) for ARL3, at the base of cilia in most mammalian cells, in nematode and in trypanosomes147–149 is predicted to restrict ARL3–GTP to the ciliary shaft and keep ARL3 in its GDP-bound state in the rest of the cell150–152. Of note, grafting the minimal PDE6δ-binding determinants of INPP5E onto a non-ciliary protein only confers equilibration between cilia and cytoplasm in kidney cells153. Thus, the cilia-to-cytoplasm ARL3–GTP gradient may be too shallow to promote sufficient enrichment of lipidated cargoes. This hypothesis is in line with the very weak ARL3 GEF activity detected on ARL13B150 and the possible presence of some RP2 inside cilia of kidney cells154. UNC119 and PDE6δ may thus render lipidated proteins competent for passive diffusion through the transition zone by shielding their lipid moiety and then rely on capture by a ciliary partner (see Fig. 2b) to drive cargo enrichment in cilia. For example, INPP5E needs to directly bind to ARL13B to achieve enrichment in cilia155.
The current hierarchy of ciliary enrichment factors places ARL13B as the most upstream regulator (Fig. 2f). How then does this master regulator become enriched in cilia? Ciliary retention of ARL13B has been proposed to involve direct binding to axonemal tubulin156 or IFT-B36. However, the distance between membrane and axoneme renders microtubules unlikely as candidates for the ciliary anchor of ARL13B, and a mutant of ARL13B defective in IFT-B binding still localizes to cilia157. Further complicating the linear hierarchy of ciliary localization factors, the IFT-A complex is required for ciliary enrichment of ARL13B in C. reinhardtii, in mice and in mammalian cells130,133,158. Meanwhile, several studies have pointed to palmitoylation of ARL13B as the critical mechanism underlying its ciliary enrichment159–161. Interestingly, palmitoylation is a reversible modification (unlike prenylation and N-myristoylation) that is essential for ciliary targeting of several ciliary proteins, namely the pseudokinase STRADβ162,163, the calcium-binding protein calflagin164 and PKHD1 (ref.165), and it has been estimated that a third of all ciliary proteins are palmitoylated159. Yet, the mechanistic basis for ciliary enrichment of palmitoylated proteins is currently unknown. Considering that local palmitoylation of the small GTPase H-Ras on Golgi membranes traps H-Ras at the Golgi apparatus166,167, it is appealing to consider that intraciliary palmitoylation may trap ARL13B in cilia. However, none of the known palmitoyltransferases have been found in cilia thus far159. Even if a ciliary palmitoyltransferase were to be identified, the current hierarchy of ciliary localizations (Fig. 2f) would remain reliant on yet another protein factor. Ultimately, it will be important to define an overarching feature that initiates robust self-organization of the ciliary compartment from first principles.
Protein exit from cilia.
Proteins need to be returned from cilia back into the cell (retrieval) in several scenarios. First, ciliary components exit cilia in a non-discriminate manner when cilia shorten and axonemal proteins are thought to be returned into the cell for future reutilization168. Second, non-resident proteins that are not efficiently excluded from cilia must be constitutively removed by a quality control machinery, as exemplified by crPLD163 (Fig. 2g). Finally, some proteins exit cilia in an inducible manner when dictated by specific signalling pathways. For example, signalling receptors undergo signal-dependent retrieval into the cell and the Gli transcription factors need to exit cilia and return to the cytoplasm before they can regulate transcription in the nucleus (Box 2). Signal-dependent retrieval applies to nearly all ciliary signaling receptors examined to date as SSTR3, the IGF1 receptor, the TGF-β receptor, and Patched1 all exit cilia upon activation by their respective ligands9,11,21,169. Finally, GPR161 exits cilia when the Hedgehog pathway is engaged, possibly because it becomes activated under these conditions170 although no ligand is known for this GPCR. One notable exception to signal-dependent retrieval is Smoothened which accumulates in cilia when activated17.
A body of evidence has now emerged showing that the BBSome is the primary mediator of constitutive ciliary retrieval162,171–173 (Fig. 2g) and carries out the signal-dependent removal of GPCRs with the help of β-arrestin 2 [G], a sensor of GPCR activation42,169,170,174,175 (Fig. 2h). First, both SSTR3 and GPR161 fail to undergo signal-dependent exit in BBSome or β-arrestin 2 mutants42,57,169,170,174,175. Second, the BBSome directly recognizes specific motifs in SSTR3 and GPR161, and β-arrestin 2 directly recognizes the activated form of these GPCRs42,170,176,177. Congruently, the BBSome and β-arrestin 2 accumulate in cilia when SSTR3 or GPR161 become activated42,169. Finally, single-molecule and bulk imaging of SSTR3 and GPR161 revealed that they undergo co-movement with the BBSome on retrograde trains when activated and they cross the transition zone in a BBSome-dependent manner42. The finding that the same domain of crPLD that confers BBSome-dependent intraciliary movements is required for export from cilia but not for import strengthens the notion that the BBSome directs export from cilia128.
A role for the BBSome in ciliary exit is further supported by a proteomic analysis that found over 130 non-ciliary proteins accumulating in photoreceptor outer segments of Bbs mutant mice171. Here, the extreme rate of transport to the cilium of photoreceptors leads to the entry of inert bystanders lacking ciliary targeting signals into the outer segment178, and the BBSome is needed to remove these bystanders from the outer segment.
The clearing of proteins that constitutively enter cilia is likely to extend into signalling mechanisms. Smoothened is absent from cilia of unstimulated cells, but accumulates in cilia when dynein 2or BBSome function is compromised49,174,179,180. This suggests that Smoothened constitutively exits cilia at a higher rate than it enters cilia. A decreased rate of ciliary exit may then underlie ciliary accumulation of Smoothened in Hedgehog-treated cells42,59,106, but how this decrease in ciliary exit could be regulated in coordination with signalling cues is currently unknown. By continuously moving in and out of cilia, Smoothened can sample the ciliary membrane composition and rapidly activate in response to elevated levels of bioactive sterols (cholesterol or oxysterols) in the ciliary membrane, resulting from Hedgehog ligand binding to its receptor Patched 1 (Box 2). This concept of patrolling the cilium by specific sensors is particularly attractive as it allows for signalling molecules to rapidly respond to changes in the chemical environment of the cilium (here, sterol concentration and distribution) and to initiate a robust response upon stimulation (Fig. 3a). In this context, it is particularly intriguing that a central regulator of metabolism (AMP kinase, AMPK), a coxsackie- and adenovirus receptor (CAR) and crPLD become detectable in cilia when BBSome function is compromised162,173. One is left to wonder what ciliary stimuli may lead to the accumulation of AMPK, CAR and crPLD in cilia.
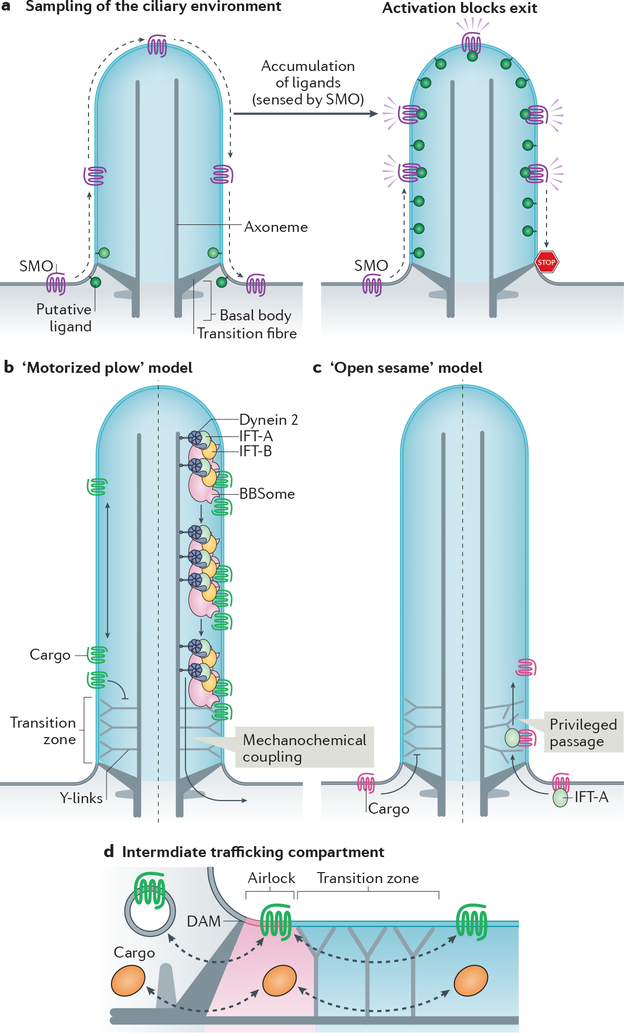
Figure 3|. Models of transition zone crossing and of the intermediate compartment.
a| Constitutive passage through the cilium enables Smoothened (SMO) to continuously sample the environment of the cilium. Elevation of inner leaflet cholesterol (see Box 2) or other second messengers leads to the activation of SMO and blockage of exit to result in ciliary accumulation of activated SMO and activation of downstream signalling. b|–c| Membrane proteins cannot cross the transition zone unassisted. According to the ‘motorized plow’ model (b), dynein 2 utilizes the energy of ATP to generate microtubule-based motion and drags retrograde intraflagellar transport (IFT) trains and their attached cargoes through the transition zone (right). According to the ‘open sesame’ model (c), cargo binding to import factors (exemplified by IFT-A) allows privileged passage of the transition zone in a motor-independent manner (right). In this model, the transport factors locally remodel the transition zone to allow passage. Note that the models may apply to either trafficking directions. d| Increasing evidence points towards the existence of an intermediate trafficking compartment at the ciliary base that functions as an ‘airlock’ proximal to the transition zone and may thus represent a checkpoint for ciliary import and export. The proximal boundary of the airlock is likely to correspond to the distal appendage matrix (DAM) located at the membrane between transition fibres.
Selective crossing of the barriers.
In contrast to the membrane diffusion barriers at the tight junctions of epithelia and the axon initial segment [G] of neurons, which hermetically block the passage of membrane proteins and must be crossed by vesicular intermediates181, the transition zone of cilia excludes non-resident proteins but allows privileged ones to pass through. While the formation of a picket fence of immobilized membrane proteins is sufficient to explain the properties of the tight junction and the axon initial segment, other mechanisms will need to be uncovered to account for the selective crossing of the transition zone by cargoes. Two models are conceivable for transition zone crossing. First, the observation that exiting GPR161 molecules cross the transition zone after undergoing processive retrograde IFT suggests that dynein 2 powers transition zone crossing of BBSome cargoes exiting cilia42. This ‘motorized plow’ model posits that cargoes are physically dragged across a zone that offers resistance to diffusion (Fig. 3b). The accumulation of BBSome cargoes inside cilia when dynein 2 function is compromised173,179 offers support for this model. Meanwhile, the kinesin-II-independent entry of a number of cargoes suggest an ‘open sesame’ model20,33, wherein the import complex (e.g. IFT-A–TULP3) instructs the transition zone to let its attached cargoes pass through (Fig. 3c). This privileged passage of cargoes bound to transport complexes through the transition zone is reminiscent of karyopherins [G] enabling passage through the nuclear pore182. Whether either model holds true remains to be tested experimentally.
After BBSome-dependent crossing of the transition zone, activated GPCRs undergo confinement in an intermediate compartment analogous to an airlock before finally crossing a periciliary barrier, which may correspond to the DAM42 (Fig. 3d). In addition to its function as a waystation for sorting between ciliary shaft and cell body, the airlock may function as a second ciliary signalling compartment where activated signalling receptors accumulate transiently and communicate to the downstream signalling machinery9,11. Finally, the airlock may serve as an intermediate compartment for both ciliary entry and exit because rhodopsin-containing vesicles destined to the outer segment fuse with a structure whose location corresponds to the suggested ciliary airlock183.
Molecules may exit the airlock either by lateral diffusion into the plasma membrane or by undergoing endocytosis. Support for molecules exiting cilia by endocytosis at the base has accumulated over the past decade (reviewed in184). An increased density of clathrin-coated pits is found at the ciliary pocket185, a structural invagination associated with the base of some cilia. Several regulators of endocytosis (clathrin, β-arrestin 2, dynamin) have been shown to be required for ciliary exit of GPR161170. Finally, the BBSome localizes to coated vesicles at the ciliary pocket of trypanosome and interacts with clathrin186. Yet, the example of β-arrestin 2 acting within cilia169 clearly shows that previously characterized regulators of endocytosis may be repurposed as regulators of ciliary trafficking acting within cilia. Moreover, single molecule imaging revealed that GPR161 is confined within the focal plane of imaging for more than 10s after exiting the airlock42, suggesting that GPR161 is diffusing within the plasma membrane after exiting cilia. However, the very small number of airlock exit events that were visualized makes this evidence far from definitive. Together, these observations highlight the need to follow signalling receptors exiting cilia by single molecule tracking to unequivocally determine the fate of exiting ciliary proteins. Whether signalling receptors undergo endocytosis immediately after exit from cilia or instead remain at the plasma membrane is an important question as signalling receptors can signal differently at the plasma membrane and at endosomes187.
Shedding of extracellular vesicles
An unexpected pathway for the removal of ciliary content from cilia besides retrieval into the cell body is ciliary ectocytosis, a process that describes the shedding of extracellular vesicles (ectosomes [G]) from the tip of cilia. Ciliary ectocytosis has been described in a variety of organisms and likely originated early in evolution. Mechanistically it has been shown to rapidly dispose of excess ciliary material, consistent with the disposal function of extracellular vesicles in cells (reticulocytes) or cellular locations (axons) that do not possess lysosomes188,189. Alternatively, ciliary ectocytosis may package ciliary signalling molecules for non-cell-autonomous signalling.
Functions of ciliary membrane shedding in disposal.
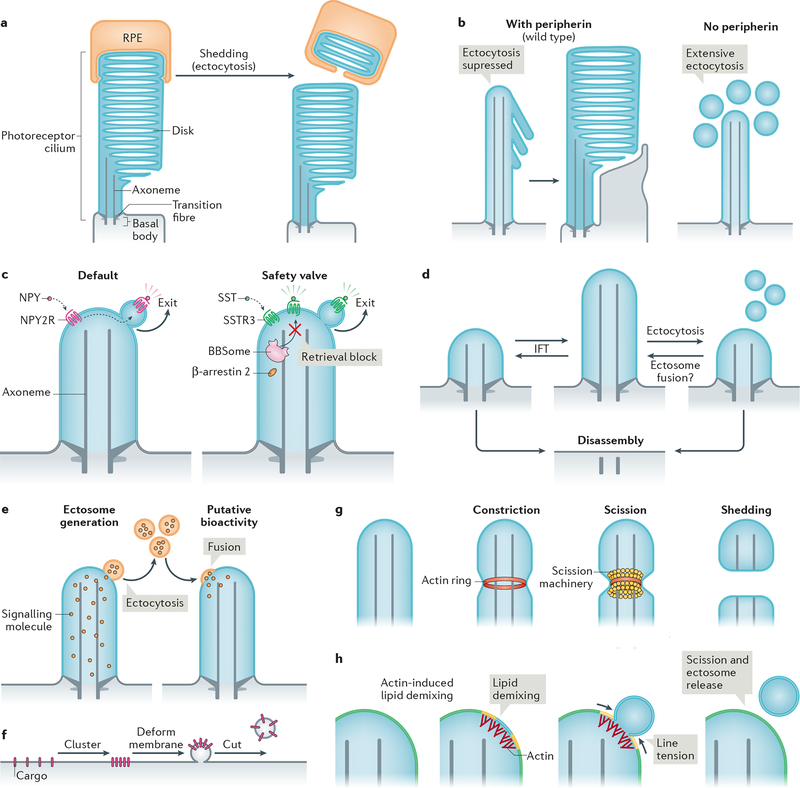
Vertebrate photoreceptors package up to one billion molecules of the light-sensing GPCR rhodopsin into the outer segment. Newly synthesized rhodopsin molecules are transported from the cell body (inner segment) and incorporated into a nascent disk at the base of the outer segment (Fig. 1b), thus creating a gradient of rhodopsin age from base to tip of the outer segment. The obstacle-laden distance between inner segment and oldest disks poses a considerable logistical problem for the photoreceptor to recycle its oldest, potentially photodamaged, rhodopsin. To solve this problem, photoreceptors have established a symbiotic relationship with the closely apposed retinal pigmented epithelium, which phagocytoses a packet of ~100 disks shed daily from the tip of each photoreceptor190,191 (Fig. 4a). Described 50 years ago, this process illustrates how ectocytosis enables the rapid and effective disposal of ciliary material when retrieval into the cell body is no longer feasible. The morphogenesis of photoreceptor disks of mice offers further in vivo support of the importance of ectocytosis in photoreceptor biology192. Disks of the outer segment normally form as evaginations at the base of cilia, but in the absence of the disk protein peripherin nascent discs are shed as small vesicles. This shedding occurs at a large scale and precludes the disks from forming. This suggests that ciliary ectocytosis must be repressed by peripherin in the nascent disks to enable disk morphogenesis (Fig. 4a).
Figure 4|. Modalities and mechanisms of ciliary ectocytosis.
a| The tips of photoreceptor outer segments are shed daily to remove aged rhodopsin molecules. Shed material is phagocytosed by retinal pigmented epithelial (RPE) cells (and recycled back to photoreceptors; not shown) (left). Ectocytosis also negatively regulates outer segment morphogenesis: the presence of disk protein peripherin blocks ectocytosis and enables retention of ciliary components to form disks (right). b| Upon activation, some G protein-coupled receptors (GPCRs) are removed from cilia by ectocytosis. For example, neuropeptide Y receptor type 2 (NPY2R) uses ectocytosis as a primary mechanism for cilia exit (left). Activated somatostatin receptor 3 (SSTR3) becomes ectocytosed when its BBSome-based retrieval (see Fig. 3b) is blocked (right). NPY, neuropeptide Y, SST, somatostatin. c| Intraflagellar transport (IFT) elongates or shrinks the cilium by exchanging material with the rest of the cell. Ectocytosis may serve as an alternative mechanism of ciliary length homeostasis: shedding of fragments of cilia from the tip by ectocytosis reduces ciliary length, whereas the hypothetical fusion of extracellular vesicles with the cilium may increase cilia length. d| Cilia package signalling molecules into ectosomes. These may be received by other cells to confer putative bioactivity. As exemplified in Chlamydomonas reinhardtii, recipient cells may receive extracellular vesicles via their cilia; incorporation via the plasma membrane is also conceivable. e| Basic steps of extracellular vesicle formation: clustering of cargoes is followed by membrane deformation and finally, a forming vesicle is released from the donor membrane by scission. f| Actin may serve to constrict the diameter of the cilium by forming a contractile ring prior to scission driven by an additional scission machinery, such as ESCRT-III-based complexes. g| Model of how actin may function in ectosome budding and scission. For simplicity, the ciliary membrane is depicted as consisting of two lipid species (yellow and blue, which blend to green). Actin polymerization in cilia causes local lipid demixing. Line tension at the interface between lipid phases ensues, which is resolved by budding and scission of the de-mixed lipids, releasing a vesicle.
Shedding of GPCRs by cilia is not limited to rhodopsin and photoreceptors. Upon activation, the GPCR neuropeptide Y receptor type 2 [G] (NPY2R) is ectocytosed from the tip of cilia instead of being retrieved back into the cell29 (Fig. 4b). Signal-dependent ectocytosis also applies to the adhesion receptor SAG1 in C. reinhardtii, which traffics to the cilium tip and is shed into ectosomes during ciliary adhesion and signalling in gametes193. Even ciliary GPCRs for which retrieval has been directly observed42, such as GPR161 and SSTR3 (see Fig. 2g), undergo signal-dependent ectocytosis when the retrieval machinery (BBSome, β-arrestin 2) is compromised or when retrieval motifs are lost from the GPCR29 (Fig. 4b). The observation that NPY2R lacks the determinants required for recognition by BBSome or β-arrestin 2 (ref.194,195) (Fig. 2h) rationalizes the exclusive use of ectocytosis for ciliary exit of this GPCR and it is likely that other ciliary signalling receptors besides NPY2R and SAG1 utilize signal-dependent ectocytosis as their default exit route.
By providing a safety valve when retrieval fails, ectocytosis may have acted as an evolutionary buffer that allowed for multiple instances of BBSome loss in organisms (fungi, moss and diatoms) that only use cilia for gamete motility and no longer need to retrieve signalling molecules196,197. Considering the paucity of direct reports of retrieval42, the limited capacity of BBSome-mediated retrieval (less than 10 molecules per minute in mammalian cells42) and the tremendous efficiency of ectocytosis (which can consume an entire C. reinhardtii cilium in 6 hrs198), it is conceivable that ectocytosis constitutes the major route for disposal of excess ciliary material. In support of this hypothesis, dynein 2 ablation (which should block all retrieval activity; see above) results in very mild ciliary phenotypes in Tetrahymena199 and in select cilia of nematodes200, and can be tolerated in mice when combined with IFT-A mutations201. In the latter case, the decreased rate of ciliary import brought about by IFT-A mutations may compensate for the decreased rate of retrieval in dynein 2 mutants. Finally, ciliary ectocytosis serves as the dominant mechanism for cell cycle-dependent cilium shortening in C. reinhardtii31,198 and in several mammalian cell types30,202,203(Fig. 4c).
As ectocytosis results in irreversible loss of proteins from cilia, it can lead to global losses of signalling receptors from cilia of retrieval mutants. Such mass depletion of ciliary proteins has been reported for Smoothened in Arrb2 mutants (loss of β-arrestin) and for SSTR3, MCHR1, Smoothened and Polycystin 1 in Bbs mutants106, and was previously interpreted as a ciliary import defect. This cautionary note emphasizes the need for monitoring the dynamics of signalling receptors in real time and at near-endogenous expression levels to properly deconvolve how specific perturbations impact the rates of delivery, retrieval and ectocytosis.
Bioactivity of ectosomes.
Exosomes [G] — which are the best studied example of extracellular vesicles —are known to carry a wide variety of signalling molecules including signalling receptors, enzymes, second messengers and RNA molecules, and they have the ability to transfer these molecules between cells204. Similarly, bioactivity has been reported for ciliary ectosomes (Fig. 4d). For example, in C. reinhardtii, the cilium is the sole site of extracellular vesicle release as the plasma membrane is covered by a cell wall205. Here, ciliary ectosomes have been shown to carry proteolytic enzymes that digest the cell wall to release the daughter cells following mitosis206. Furthermore, ciliary fertilization in C. reinhardtii was associated with a release of SAG1-containing ectosomes, which were biologically active, as shown by the ability to induce cilia-based signalling when applied to gametes193. In the sleeping sickness parasite Trypanosoma brucei, ciliary ectosomes can transfer virulence factors to other parasites or fuse with host blood cells to promote lysis and eventually cause anaemia207. Finally, extracellular vesicles released from male nematodes in a cilium-dependent manner are sufficient to promote mating behaviors in recipient worms208.
Yet, despite these exciting advances, it is important to note the current literature only indicates that ciliary ectosomes are sufficient to transfer signals outside of a given cell. It remains possible that minute amounts of ciliary material disposed into ciliary ectosomes exert a biological effect because these vesicles were experimentally concentrated before addition to recipient cells. In this context, the demonstration of a physiological role for ciliary ectocytosis in gamete fertilization, gamete release, transfer of virulence factors or mating behaviour as discussed above awaits the development of specific means to interfere with ciliary ectocytosis.
Molecular mechanisms of ciliary ectocytosis.
Proteomics of ciliary ectosomes from C. reinhardtii and mammalian cells has demonstrated their unique protein composition30,31 and quantitative assessments of receptor concentration have estimated that activated SAG1 and GPCRs are enriched 5-fold in ectosomes compared to cilia29,193. This indicates that ectosomal cargoes are actively packaged — a process that generally entails cargo sorting into the forming vesicle, local membrane deformation and scission of the vesicle from the donor membrane (Fig. 4e). Mechanistic insights into ectosomal release are just beginning to emerge.
Ectocytosis is a ‘reverse topology’ vesicular budding pathway akin to budding of intralumenal vesicles into the lumen of late endosomes or of viruses at the plasma membrane. Like other examples of reverse topology membrane budding and scission, ciliary ectocytosis involves endosomal sorting complex required for transport [G] (ESCRT) complexes31,209. Interestingly, in C. reinhardtii the entire transition zone becomes ectocytosed at the terminal step of flagellar shortening and the transition zone-containing ectosomes are enriched for several ESCRT components210.
In unusual fashion within the realm of reverse topology budding, actin polymerization within cilia participates in the release of ectosomes29,30. F-actin and several actin regulators — drebrin, myosin 6, cofilin, fascin and SNX9 — are specifically enriched at the presumptive site of ciliary ectosome release29,30. Accordingly, interference with these regulators or with actin polymerization blocks ectosome release from cilia, results in increased cilium length and inhibits cilium resorption upon cell cycle reentry29,30. Interestingly, a targeted increase in ciliary PtdIns(4,5)P2 is sufficient to promote branched actin polymerization inside cilia and subsequent ectocytosis30. Thus, a local elevation of PtdIns(4,5)P2 levels may constitute the trigger that couples actin polymerization to ciliary ectocytosis.
Collectively, these data build a persuasive case for actin and an associated cast of regulators participating in the scission of ectocytic buds, although the specific mechanism by which actin polymerization promotes scission remains to be determined. One way by which F-actin functions in ectocytosis is by constricting the diameter of the cilium (similar to the activity of actin rings in cytokinesis, for example)211. However, it should be noted that purse-string actomyosin-based constriction does not accomplish membrane scission209. In this context, actin may cooperate with ESCRT-III (Fig. 4f), consistent with the universally conserved function of ESCRT-III from archaea to man in severing reverse topology tubes212,213. As another mechanism, actin could drive membrane scission by inducing lipid phase separation at the ciliary membrane tip. Reconstituted systems have shown that raft-like membranes rich in cholesterol, sphingolipids and PtdIns(4,5)P2 undergo phase separation into liquid ordered and liquid disordered phases when juxta-membraneous branched actin networks form in apposition with PtdIns(4,5)P2 headgroups214,215. The energetically costly mismatch at the boundary between the two lipid phases produces line tension [G]216, which minimizes energy by decreasing the length of the boundary line, first by constriction of a bud and ultimately by scission (Fig. 4g). While it remains to be tested whether actin mediates the scission of ectosomes through line tension, the raft-like lipid composition of the cilium100 is consistent with this mechanism. Future studies will need to deconvolve mechanisms and the roles of actin in various cilia-related processes using targeted and acute interference with actin polymerization at specific locations.
Conclusions and perspective
The explosive progress of the ciliary research field in the past 10 years has taken us from a list of proteins to mechanistic models for ciliary trafficking and signalling. Although some questions have now reached a sophisticated level of understanding, major unknowns remain in the field. First, the molecular mechanisms of selective permeability of the transition zone and of the periciliary barrier remain unknown. Genetic interactions and biochemistry have defined proteins that constitute these barriers, but little is known about the biophysical nature of gating. Testing for the molecular and energetic requirements of barrier crossing will likely require acute inactivation of IFT complexes, BBSome and motors together with innovatory imaging of cargoes, trafficking complexes and transition zone markers. Second, the extent of functions for ciliary ectocytosis is an open question. To determine whether ciliary ectosomes convey signals or merely serve as a disposal modality will necessitate the interference with specific factors responsible for packaging receptors into ciliary ectosomes. How cargoes are selected for packaging into ciliary ectosomes is currently unknown and is an interesting avenue for future studies. The functional dissection of actin, lipid, and ESCRT interactions in the cilium promises to enrich our understanding of cilia as well as ESCRT biology. Third, the emerging role of lipids in cilia paints a fascinating picture of a unique membrane compartment that harbours unprecedented second messengers — cholesterol and oxysterols. How cilia maintain their lipid identity is currently an open question. In this regard, single-molecule tracking of specific lipids will be necessary to test the existence of a diffusion barrier for lipids at the base of cilia. The extent of roles ciliary lipids play in ciliary signalling pathways also remains to be fully tested.
ACKNOWLEDGMENTS
We thank Bill Snell, George Witman, Jeremy Reiter, Markus Delling, David Breslow, Irene Ojeda Naharros and Swapnil Shinde for comments on the manuscript and Drew Nager for help with drafting the manuscript. We apologize to our colleagues whose publications we could not cite due to length restrictions. Research in the Nachury lab is supported by NIGMS grant GM089933, a Stein Innovation Award from Research to Prevent Blindness and, in part, by NEI core grant EY002162 and by an RPB Unrestricted Grant. Research in the Mick lab is supported by the Deutsche Forschungsgemeinschaft (DFG) SFB894/TPA-22.
GLOSSARY TERMS
- G protein-coupled receptors (GPCRs)
membrane proteins with seven transmembrane domains that sense various external signals (such as drugs, odorants, etc.) and relay them into the cell through heterotrimeric G proteins.
- Anorexigenic
effect related to the loss of appetite (anorexia), thus resulting in lower food consumption and weight loss.
- Photoreceptor
A sensory neuron that detects light using rhodopsin localized inside its specialized cilium.
- Rhodopsin
A photosensory GPCR that transduces light using a tightly bound molecule of retinal.
- Bardet-Biedl Syndrome (BBS)
A ciliopathy (with mutations in 21 different genes identified to date) characterized by retinal degeneration, obesity, kidney abnormalities and polydactyly.
- Phosphoinositides
hallmark lipids that mark the cytoplasmic leaflets of cellular membranes.
- Basal Body
A specialized mother centriole that templates the ciliary axoneme.
- Axoneme
The microtubule core of cilia, composed of nine microtubule doublets.
- Transition fibres
Identical to the distal appendages of mother centrioles in unciliated cells, the transition fibres physically connect the basal body to the membrane in ciliated cells.
- Y-links
Y-shaped electron dense structures that connect the ciliary axoneme (bottom of the Y) to the membrane (top of the Y) at the transition zone.
- Tight junctions
The junctions between adjacent epithelial cells that seal the epithelium and function as a barrier for lipid and integral membrane protein diffusion between the apical and basolateral membranes of cells.
- heterotrimeric G protein α subunit
The GTPase subunit of heterotrimeric G-proteins. It becomes loaded with GTP after encountering an active GPCR and modulates downstream activities, such as adenylyl cyclases. Gαs–GTP stimulates adenylyl cyclases while Gαi–GTP inhibits them.
- Gli transcription factors
The Gli1 transcriptional activator was first identified in glioblastoma (a Hedgehog-driven tumour). The paralogues Gli2 and Gli3 are processed into transcriptional activators or repressors depending upon regulatory inputs from the Hedgehog pathway.
- Sphingolipids
Lipids formed by a backbone that consists of a sphingosine N-acylated by a fatty acid. Named after the Sphinx because of their once enigmatic functions.
- Oxysterols
products of cholesterol oxydation with hydroxyl, carbonyl, or epoxide groups. Their best-established roles are in regulation of cholesterol metabolism.
- BBSome
A complex of eight Bardet-Biedl-Syndrome proteins that removes proteins from cilia.
- IFT trains
High-order oligomers of IFT complexes that transport cargo along the axoneme.
- Condensed membrane microdomains
Tightly packed and viscous regions of the membrane that can form through clustering of lipid rafts.
- Liquid-ordered lipid phase
Biological membranes are two-dimensional liquids that can phase separate into liquid-ordered (e.g. rafts) and liquid-disordered (e.g. fluid mosaic) phases.
- Raft
Relatively ordered membrane domains formed by interactions between proteins and lipids. Rafts typically present as cholesterol- and sphingolipid-enriched membrane nanodomains less than 200 nm in size that are thought to facilitate interactions between signalling molecules.
- Polycystic Kidney and Hepatic Disease 1 (PKHD1)
a large single-pass membrane protein of unknown function mutated in autosomal recessive polycystic kidney disease.
- Ciliary targeting signal (CTS)
In the strictest sense, a short stretch of amino acids that is necessary and sufficient for targeting a protein to cilia. Some CTS (e.g. in SSTR3) have only been shown to be sufficient for ciliary targeting.
- Somatostatin receptor 3 (SSTR3)
a ciliary GPCR expressed in hippocampal neurons and required for novelty recognition. It couples to Gαi.
- Melanocortin concentrating hormone receptor 1 (MCHR1)
a ciliary GPCR that has a modest role in body weight homeostasis.
- ARL3
a small GTPase of the Arf-like family, most members of which regulate vesicular trafficking.
- β-Arrestin 2
recognizes activated GPCRs, blocks GPCR-to-Gα communication and promotes GPCR endocytosis, thereby typically driving termination of GPCR signalling.
- Axon initial segment
In neurons, the first few microns of the axon. It is an area rich in actin that functionally separates the axonal plasma membrane from the soma plasma membrane.
- Karyopherins
Nuclear transport receptors that ferry proteins across the nuclear pore complex.
- Ectosomes
Extracellular vesicles that directly bud from the limiting membrane of the cell. Also termed microvesicles.
- Neuropeptide Y receptor 2 (NPY2R)
a Gαi-coupled GPCR that regulates appetite under stress conditions.
- Exosomes
Extracellular vesicles that originate as vesicles inside the lumen of the multivesicular body and become extracellular upon fusion of the multivesicular body with the plasma membrane.
- Endosomal sorting complex required for transport (ESCRT)
A cascade of protein complexes (0, I, II and III in order of action) discovered initially in yeast as factors involved in budding transmembrane proteins from the endosomal membrane into intraluminal vesicles. ESCRTs have since been demonstrated to be necessary for many cellular processes involving a bud-like topology.
- Line tension
the two-dimensional version of surface tension, which reflects the energy minimization of a system where an energetically costly discontinuity exists between two neighbouring phases.
Footnotes
Competing interests
The authors declare no competing interests.
REFERENCES
- 1.Reiter JF & Leroux MR Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol 18, 533–547 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Robertis E Electron microscope observations on the submicroscopic organization of the retinal rods. The Journal of biophysical and biochemical cytology 2, 319–330 (1956). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom G Studies on the olfactory epithelium of the frog and the toad with the aid of light and electron microscopy. Zeitschrift für Zellforschung und Mikroskopische Anatomie 41, 89–100 (1954). [DOI] [PubMed] [Google Scholar]
- 4.Huangfu D et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–7 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Gerdes JM et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nature Genetics 39, 1350–1360 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Ezratty EJ et al. A Role for the Primary Cilium in Notch Signaling and Epidermal Differentiation during Skin Development. Cell 145, 1129–1141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross AJ et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nature Genetics 37, 1135–1140 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Schneider L et al. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Current biology : CB 15, 1861–1866 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Yeh C et al. IGF-1 activates a cilium-localized noncanonical Gβγ signaling pathway that regulates cell-cycle progression. Developmental Cell 26, 358–368 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schou KB, Pedersen LB & Christensen ST Ins and outs of GPCR signaling in primary cilia. EMBO Reports 16, 1099–1113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement CA et al. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell reports 3, 1806–1814 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Siljee JE et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat. Genet 50, 180–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloodgood RA Sensory reception is an attribute of both primary cilia and motile cilia. Journal of Cell Science 123, 505–509 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Wachten D, Jikeli JF & Kaupp UB Sperm Sensory Signaling. Cold Spring Harbor Perspectives in Biology 9, a028225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvert PD, Schiesser WE & Pugh EN Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J. Gen. Physiol 135, 173–196 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhandawat V, Reisert J & Yau K-W Signaling by olfactory receptor neurons near threshold. PNAS 107, 18682–18687 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbit KC et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Haycraft CJ et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1, e53 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May SR et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Developmental Biology 287, 378–389 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Belzile O, Hernandez-Lara CI, Wang Q & Snell WJ Regulated Membrane Protein Entry into Flagella Is Facilitated by Cytoplasmic Microtubules and Does Not Require IFT. Current Biology 23, 1460–1465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohatgi R, Milenkovic L & Scott MP Patched1 regulates hedgehog signaling at the primary cilium. Science (New York, NY) 317, 372–376 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Bangs F & Anderson KV Primary cilia and mammalian Hedgehog signaling. Cold Spring Harbor perspectives in biology 9, a028175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pablo JL, DeCaen PG & Clapham DE Progress in ciliary ion channel physiology. The Journal of General Physiology 149, 37–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson J-LF & Leroux MR cAMP and cGMP signaling: sensory systems with prokaryotic roots adopted by eukaryotic cilia. Trends in cell biology 20, 435–444 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Garcia G, Raleigh DR & Reiter JF How the Ciliary Membrane Is Organized Inside-Out to Communicate Outside-In. Current Biology 28, R421–R434 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luchetti G et al. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. eLife 5, e20304 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang P et al. Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell 166, 1176–1187.e14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raleigh DR et al. Cilia-Associated Oxysterols Activate Smoothened. Molecular Cell 72, 316–327.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies the oxysterols 24,25-epoxycholesterol and 7β,27-dihydroxycholesterol as ciliary lipids that bind and activate Smoothened.
- 29.Nager AR et al. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 168, 252–263.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper uncovers signal-dependent ectocytosis as a means for ciliary exit of activated GPCRs in mammalian cells, see also 193. It also discovered a role for actin in ectosome scission, see also 30.
- 30.Phua SC et al. Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell 168, 264–279.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of a role for actin in ectosome release, see also 29. This study also suggests that cilia shorten by shedding ectosomes; see also 31.
- 31.Long H et al. Comparative Analysis of Ciliary Membranes and Ectosomes. Curr. Biol 26, 3327–3335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that cilia shorten, at least in part, by shedding ectosomes, see also 30. It also suggests a role for ESCRT proteins ALG-2 and VPS-4 in ciliary ectocytosis.
- 32.Fisch C & Dupuis-Williams P Ultrastructure of cilia and flagella - back to the future! Biol Cell 103, 249–70 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Gonzalo FR & Reiter JF Open Sesame: How Transition Fibers and the Transition Zone Control Ciliary Composition. Cold Spring Harb Perspect Biol 9, a028134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonçalves J & Pelletier L The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate. Mol. Cells 40, 243–253 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breslow D, Koslover EF, Seydel F, Spakowitz AJ & Nachury MV An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. The Journal of Cell Biology 203, 129–147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cevik S et al. Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain. PLoS Genetics 9, e1003977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chih B et al. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol 14, 61–72 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Hu Q et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science (New York, NY) 329, 436–439 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kee HL et al. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol 14, 431–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y-C et al. Chemically inducible diffusion trap at cilia reveals molecular sieve-like barrier. Nat. Chem. Biol 9, 437–443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang TT et al. Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat Commun 9, 2023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Establishes the existence of the distal appendage matrix (DAM) and suggests that the DAM functions as a barrier for ciliary membrane proteins.
- 42.Ye F, Nager AR & Nachury MV BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol 217, 1847–1868 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using single molecule tracking, this study directly demonstrates that the transition zone is breached by exiting GPCRs in a BBSome-dependent manner.
- 43.Najafi M, Maza NA & Calvert PD Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc Natl Acad Sci U S A 109, 203–208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambacher NJ et al. TMEM107 recruits ciliopathy proteins to subdomains of the ciliary transition zone and causes Joubert syndrome. Nat. Cell Biol 18, 122–131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takao D, Wang L, Boss A & Verhey KJ Protein Interaction Analysis Provides a Map of the Spatial and Temporal Organization of the Ciliary Gating Zone. Current biology : CB 27, 2296–2306.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craige B et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol 190, 927–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Awata J et al. NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. Journal of Cell Science 127, 4714–4727 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheidel N & Blacque OE Intraflagellar Transport Complex A Genes Differentially Regulate Cilium Formation and Transition Zone Gating. Curr. Biol 28, 3279–3287.e2 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Vuolo L, Stevenson NL, Heesom KJ & Stephens DJ Dynein-2 intermediate chains play crucial but distinct roles in primary cilia formation and function. Elife 7, e39655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen VL et al. Role for intraflagellar transport in building a functional transition zone. EMBO reports 19, e45862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dharmat R et al. SPATA7 maintains a novel photoreceptor-specific zone in the distal connecting cilium. J Cell Biol 217, 2851–2865 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiegering A et al. Cell type‐specific regulation of ciliary transition zone assembly in vertebrates. The EMBO Journal 37, e97791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jana SC et al. Differential regulation of transition zone and centriole proteins contributes to ciliary base diversity. Nat. Cell Biol 20, 928–941 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Lewis WR et al. Mks6 mutations reveal tissue- and cell type-specific roles for the cilia transition zone. The FASEB Journal 33, 1440–1455 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen VL et al. Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance. The EMBO Journal 34, 2537–2556 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams CL et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol 192, 1023–1041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yee LE et al. Conserved Genetic Interactions between Ciliopathy Complexes Cooperatively Support Ciliogenesis and Ciliary Signaling. PLoS genetics 11, e1005627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberson EC et al. TMEM231, mutated in orofaciodigital and Meckel syndromes, organizes the ciliary transition zone. J. Cell Biol 209, 129–142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milenkovic L et al. Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proc Natl Acad Sci U S A 112, 8320–8325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi X et al. Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat. Cell Biol 19, 1178–1188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanos BE et al. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev 27, 163–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W et al. Centriole translocation and degeneration during ciliogenesis in Caenorhabditis elegans neurons. EMBO J 36, 2553–2566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nechipurenko IV, Berciu C, Sengupta P & Nicastro D Centriolar remodeling underlies basal body maturation during ciliogenesis in Caenorhabditis elegans. Elife 6, e25686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serwas D, Su TY, Roessler M, Wang S & Dammermann A Centrioles initiate cilia assembly but are dispensable for maturation and maintenance in C. elegans. J. Cell Biol 216, 1659–1671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugimoto M et al. The keratin-binding protein Albatross regulates polarization of epithelial cells. The Journal of Cell Biology 183, 19–28 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sang L et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145, 513–528 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delous M et al. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum Mol Genet 18, 4711–4723 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei Q et al. The hydrolethalus syndrome protein HYLS-1 regulates formation of the ciliary gate. Nature Communications 7, 12437 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeCaen PG, Delling M, Vien TN & Clapham DE Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315–318 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delling M, DeCaen PG, Doerner JF, Febvay S & Clapham DE Primary cilia are specialized calcium signalling organelles. Nature 504, 311–314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borisy FF et al. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J. Neurosci 12, 915–923 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mukherjee S et al. A novel biosensor to study cAMP dynamics in cilia and flagella. eLife 5, e14052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore BS et al. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc Natl Acad Sci U S A 113, 13069–13074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grarup N et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat. Genet 50, 172–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saeed S et al. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat. Genet 50, 175–179 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Rees S et al. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J. Am. Soc. Nephrol 25, 232–237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Q et al. Adenylyl cyclase 5 deficiency reduces renal cyclic AMP and cyst growth in an orthologous mouse model of polycystic kidney disease. Kidney Int 93, 403–415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Breslow DK et al. A CRISPR-based screen for Hedgehog signaling provides insights into ciliary function and ciliopathies. Nat. Genet 50, 460–471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pusapati GV et al. CRISPR Screens Uncover Genes that Regulate Target Cell Sensitivity to the Morphogen Sonic Hedgehog. Dev. Cell 44, 113–129.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vuolo L, Herrera A, Torroba B, Menendez A & Pons S Ciliary adenylyl cyclases control the Hedgehog pathway. Journal of Cell Science 128, 2928–2937 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Kwon RY, Temiyasathit S, Tummala P, Quah CC & Jacobs CR Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology 24, 2859–2868 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sadana R & Dessauer CW Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neuro-Signals 17, 5–22 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hilgendorf KI, Johnson CT & Jackson PK The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Current Opinion in Cell Biology 39, 84–92 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedersen LB, Geimer S, Sloboda RD & Rosenbaum JL The Microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Current biology : CB 13, 1969–1974 (2003). [DOI] [PubMed] [Google Scholar]
- 85.Harris JA, Liu Y, Yang P, Kner P & Lechtreck KF Single-particle imaging reveals intraflagellar transport-independent transport and accumulation of EB1 in Chlamydomonas flagella. Molecular Biology of the Cell 27, 295–307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chávez M et al. Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output. Dev. Cell 34, 338–350 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Gonzalo FR et al. Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev. Cell 34, 400–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; References 86–87 demonstrate that the lipid PtdIns(4)P is enriched in the ciliary membrane thanks to the phosphatase INPP5E, which converts PtdIns(4,5)P2 into PtdIns(4)P
- 88.Poo M & Cone RA Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature 247, 438–441 (1974). [DOI] [PubMed] [Google Scholar]
- 89.Aveldaño MI & Bazán NG Molecular species of phosphatidylcholine, -ethanolamine, -serine, and -inositol in microsomal and photoreceptor membranes of bovine retina. J Lipid Res 24, 620–627 (1983). [PubMed] [Google Scholar]
- 90.Boesze-Battaglia K & Schimmel R Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J. Exp. Biol 200, 2927–2936 (1997). [DOI] [PubMed] [Google Scholar]
- 91.Andrews D & Nelson DL Biochemical studies of the excitable membrane of Paramecium tetraurelia. II. Phospholipids of ciliary and other membranes. Biochimica et biophysica acta 550, 174–187 (1979). [DOI] [PubMed] [Google Scholar]
- 92.Chailley B & Boisvieux-Ulrich E Detection of plasma membrane cholesterol by filipin during microvillogenesis and ciliogenesis in quail oviduct. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 33, 1–10 (1985). [DOI] [PubMed] [Google Scholar]
- 93.Lobasso S et al. Lipidomic analysis of porcine olfactory epithelial membranes and cilia. Lipids 45, 593–602 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Montesano R Inhomogeneous distribution of filipin-sterol complexes in the ciliary membrane of rat tracheal epithelium. Am. J. Anat 156, 139–145 (1979). [DOI] [PubMed] [Google Scholar]
- 95.Morris RJ & Bone Q Metazoan lipids: An unusual association of saturated sterols with relatively saturated fatty acids in the cilia of Ciona intestinalis. Lipids 18, 900–901 (1983). [Google Scholar]
- 96.Morris RJ & Bone Q Highly saturated lipid composition of ctenophore cilia: Possible indication of low membrane permeability. Lipids 20, 933–935 (1985). [Google Scholar]
- 97.Souto-Padrón T & de Souza W Freeze-fracture localization of filipin-cholesterol complexes in the plasma membrane of Trypanosoma cruzi. The Journal of parasitology 69, 129–137 (1983). [PubMed] [Google Scholar]
- 98.Tyler KM et al. Flagellar membrane localization via association with lipid rafts. Journal of Cell Science 122, 859–866 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Emmer BT, Maric D & Engman DM Molecular mechanisms of protein and lipid targeting to ciliary membranes. Journal of Cell Science 123, 529–536 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nachury MV, Seeley ES & Jin H Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annual review of cell and developmental biology 26, 59–87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serricchio M et al. Flagellar membranes are rich in raft-forming phospholipids. Biology Open 4, 1143–1153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen PAT, Liou W, Hall DH & Leroux MR Ciliopathy proteins establish a bipartite signaling compartment in a C. elegans thermosensory neuron. Journal of Cell Science 127, 5317–5330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schou KB et al. KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling. Nature Communications 8, 14177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates cholesterol-dependent localization of the cholesterol-binding protein caveolin to the transition zone, which suggests a specialized lipid organization at the transition zone.
- 104.Kamiya R & Witman GB Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol 98, 97–107 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lechtreck KF IFT–Cargo Interactions and Protein Transport in Cilia. Trends in Biochemical Sciences 40, 765–778 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nachury MV The molecular machines that traffic signaling receptors into and out of cilia. Current Opinion in Cell Biology 51, 124–131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakayama K & Katoh Y Ciliary protein trafficking mediated by IFT and BBSome complexes with the aid of kinesin-2 and dynein-2 motors. J. Biochem 163, 155–164 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Bhogaraju S et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science (New York, NY) 341, 1009–1012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Craft JM, Harris JA, Hyman S, Kner P & Lechtreck KF Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. The Journal of Cell Biology 208, 223–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kubo T et al. Together, the IFT81 and IFT74 N-termini form the main module for intraflagellar transport of tubulin. Journal of Cell Science 129, 2106–2119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taschner M, Mourão A, Awasthi M, Basquin J & Lorentzen E Structural basis of outer dynein arm intraflagellar transport by the transport adaptor protein ODA16 and the intraflagellar transport protein IFT46. J. Biol. Chem 292, 7462–7473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hou Y, Witman GB & Marshall W The N-terminus of IFT46 mediates intraflagellar transport of outer arm dynein and its cargo-adaptor ODA16. MBoC 28, 2420–2433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hunter EL et al. The IDA3 adapter, required for intraflagellar transport of I1 dynein, is regulated by ciliary length. Molecular Biology of the Cell 29, 886–896 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ye F et al. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife 2, e00654 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Craft JM, Harris JA, Hyman S, Kner P & Lechtreck KF Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. The Journal of Cell Biology 208, 223–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Milenkovic L et al. Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proc Natl Acad Sci U S A 112, 8320–8325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Monis WJ, Faundez V & Pazour GJ BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J Cell Biol 216, 2131–2150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morthorst SK, Christensen ST & Pedersen LB Regulation of ciliary membrane protein trafficking and signalling by kinesin motor proteins. The FEBS Journal 285, 4535–4564 (2018). [DOI] [PubMed] [Google Scholar]
- 119.Blacque OE, Scheidel N & Kuhns S Rab GTPases in cilium formation and function. Small GTPases 9, 76–94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown JM, Cochran DA, Craige B, Kubo T & Witman GB Assembly of IFT Trains at the Ciliary Base Depends on IFT74. Current Biology 25, 1583–1593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deane JA, Cole DG, Seeley ES, Diener DR & Rosenbaum JL Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Current biology : CB 11, 1586–1590 (2001). [DOI] [PubMed] [Google Scholar]
- 122.Wingfield JL et al. IFT trains in different stages of assembly queue at the ciliary base for consecutive release into the cilium. eLife 6, e26609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang K, Diener DR, Mitchell A & Rosenbaum JL Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. The Journal of Cell Biology 179, 501–514 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brear AG, Yoon J, Wojtyniak M & Sengupta P Diverse Cell Type-Specific Mechanisms Localize G Protein-Coupled Receptors to Caenorhabditis elegans Sensory Cilia. Genetics 197, 667–684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bae YK et al. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development 133, 3859–70 (2006). [DOI] [PubMed] [Google Scholar]
- 126.Dwyer ND, Adler CE, Crump JG, L’Etoile ND & Bargmann CI Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron 31, 277–87 (2001). [DOI] [PubMed] [Google Scholar]
- 127.Eguether T, Cordelieres FP & Pazour GJ Intraflagellar transport is deeply integrated in hedgehog signaling. Mol. Biol. Cell 29, 1178–1189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu P & Lechtreck KF The Bardet–Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export. PNAS 115, E934–E943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Badgandi HB, Hwang S, Shimada IS, Loriot E & Mukhopadhyay S Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. The Journal of Cell Biology 216, 743–760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fu W, Wang L, Kim S, Li J & Dynlacht BD Role for the IFT-A Complex in Selective Transport to the Primary Cilium. Cell reports 17, 1505–1517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hirano T, Katoh Y & Nakayama K Intraflagellar transport-A complex mediates ciliary entry and retrograde trafficking of ciliary G protein-coupled receptors. Molecular Biology of the Cell 28, 429–439 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mukhopadhyay S et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes & development 24, 2180–2193 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Picariello T et al. A global analysis of IFT-A function reveals specialization for transport of membrane-associated proteins into cilia. J Cell Sci jcs.220749 (2019). doi: 10.1242/jcs.220749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun X et al. Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia 1, 21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oswald F, Prevo B, Acar S & Peterman EJG Interplay between Ciliary Ultrastructure and IFT-Train Dynamics Revealed by Single-Molecule Super-resolution Imaging. Cell Rep 25, 224–235 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Yang T, Tran MNT, Chong WM, Huang C-E & Liao J-C Intraflagellar transport proteins undergo nonaxonemal staged hindrance between the recruiting distal appendages and the cilium. bioRxiv 227587 (2017). doi: 10.1101/227587 [DOI] [Google Scholar]
- 137.Kanie T et al. The CEP19-RABL2 GTPase Complex Binds IFT-B to Initiate Intraflagellar Transport at the Ciliary Base. Dev. Cell 42, 22–36.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nishijima Y et al. RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Molecular Biology of the Cell 28, 1652–1666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park J et al. dTULP, the Drosophila melanogaster Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia. PLoS Genetics 9, e1003814 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park J et al. Ciliary Phosphoinositide Regulates Ciliary Protein Trafficking in Drosophila. Cell Reports 13, 2808–2816 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Ismail SA et al. Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat. Chem. Biol 7, 942–949 (2011). [DOI] [PubMed] [Google Scholar]
- 142.Ismail SA et al. Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. The EMBO Journal 31, 4085–4094 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wright KJ et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes & development 25, 2347–2360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang H et al. UNC119 is required for G protein trafficking in sensory neurons. Nature neuroscience 14, 874–880 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; References 141–144 described the myristoyl shuttle UNC119 and the farnesyl shuttle PDE6δ and the release of their cargoes by ARL3–GTP inside cilia as a cogent mechanism of ciliary import of lipidated proteins.
- 145.Jensen VL & Leroux MR Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Current Opinion in Cell Biology 47, 83–91 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Stephen LA & Ismail S Shuttling and sorting lipid-modified cargo into the cilia. Biochemical Society Transactions 44, 1273–1280 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Evans RJ et al. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum. Mol. Genet 19, 1358–1367 (2010). [DOI] [PubMed] [Google Scholar]
- 148.Blacque OE et al. Functional genomics of the cilium, a sensory organelle. Current biology : CB 15, 935–941 (2005). [DOI] [PubMed] [Google Scholar]
- 149.Stephan A, Vaughan S, Shaw MK, Gull K & McKean PG An essential quality control mechanism at the eukaryotic Basal body prior to intraflagellar transport. Traffic (Copenhagen, Denmark) 8, 1323–1330 (2007). [DOI] [PubMed] [Google Scholar]
- 150.Gotthardt K et al. A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. eLife 4, e11859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ivanova AA et al. Biochemical characterization of purified mammalian ARL13B protein indicates that it is an atypical GTPase and ARL3 guanine nucleotide exchange factor (GEF). J. Biol. Chem 292, 11091–11108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Q et al. GTP-binding of ARL-3 is activated by ARL-13 as a GEF and stabilized by UNC-119. Scientific reports 6, 24534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fansa EK, Kösling SK, Zent E, Wittinghofer A & Ismail S PDE6δ-mediated sorting of INPP5E into the cilium is determined by cargo-carrier affinity. Nature Communications 7, 11366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hurd T et al. The retinitis pigmentosa protein RP2 interacts with polycystin 2 and regulates cilia-mediated vertebrate development. Human Molecular Genetics 19, 4330–4344 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Humbert MC et al. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proceedings of the National Academy of Sciences 109, 19691–19696 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Revenkova E, Liu Q, Gusella GL & Iomini C The Joubert syndrome protein ARL13B binds tubulin to maintain uniform distribution of proteins along the ciliary membrane. Journal of Cell Science 131, jcs212324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nozaki S et al. Regulation of ciliary retrograde protein trafficking by the Joubert syndrome proteins ARL13B and INPP5E. Journal of Cell Science 130, 563–576 (2017). [DOI] [PubMed] [Google Scholar]
- 158.Liem KF et al. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. The Journal of Cell Biology 197, 789–800 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roy K et al. Palmitoylation of the ciliary GTPase ARL13b is necessary for its stability and its role in cilia formation. Journal of Biological Chemistry 292, 17703–17717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cevik S et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. The Journal of Cell Biology 188, 953–969 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li Y, Wei Q, Zhang Y, Ling K & Hu J The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. The Journal of Cell Biology 189, 1039–1051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mick DU et al. Proteomics of Primary Cilia by Proximity Labeling. Developmental Cell 35, 497–512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Viau A et al. Cilia-localized LKB1 regulates chemokine signaling, macrophage recruitment, and tissue homeostasis in the kidney. EMBO J 37, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Emmer BT et al. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. Journal of Cell Science 122, 867–874 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Follit JA, Li L, Vucica Y & Pazour GJ The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. The Journal of Cell Biology 188, 21–28 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goodwin JS et al. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol 170, 261–272 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rocks O et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307, 1746–1752 (2005). [DOI] [PubMed] [Google Scholar]
- 168.Huang K, Diener DR & Rosenbaum JL The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. The Journal of Cell Biology 186, 601–613 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Green JA et al. Recruitment of β-Arrestin into Neuronal Cilia Modulates Somatostatin Receptor Subtype 3 Ciliary Localization. Molecular and Cellular Biology 36, 223–235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pal K et al. Smoothened determines β-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. The Journal of Cell Biology 212, 861–875 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Datta P et al. Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A 112, E4400–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lechtreck KF et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. The Journal of Cell Biology 187, 1117–1132 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lechtreck KF et al. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. The Journal of Cell Biology 201, 249–261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eguether T et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Developmental Cell 31, 279–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liew GM et al. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Developmental Cell 31, 265–278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jin H et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141, 1208–1219 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Klink BU et al. A recombinant BBSome core complex and how it interacts with ciliary cargo. eLife 6, e27434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Baker SA et al. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. The Journal of Cell Biology 183, 485–498 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ocbina PJR & Anderson KV Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Developmental dynamics 237, 2030–2038 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang Q et al. Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc Natl Acad Sci U S A 108, 20678–20683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Trimble WS & Grinstein S Barriers to the free diffusion of proteins and lipids in the plasma membrane. The Journal of Cell Biology 208, 259–271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schmidt HB & Görlich D Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem. Sci 41, 46–61 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Papermaster DS, Schneider BG & Besharse JC Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Investigative ophthalmology & visual science 26, 1386–1404 (1985). [PubMed] [Google Scholar]
- 184.Pedersen LB, Mogensen JB & Christensen ST Endocytic Control of Cellular Signaling at the Primary Cilium. Trends in Biochemical Sciences 41, 784–797 (2016). [DOI] [PubMed] [Google Scholar]
- 185.Benmerah A The ciliary pocket. Current Opinion in Cell Biology 25, 78–84 (2013). [DOI] [PubMed] [Google Scholar]
- 186.Langousis G et al. Loss of the BBSome perturbs endocytic trafficking and disrupts virulence of Trypanosoma brucei. Proc Natl Acad Sci U S A 113, 632–637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lobingier BT & von Zastrow M When trafficking and signaling mix: How subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic 20, 130–136 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pan BT, Teng K, Wu C, Adam M & Johnstone RM Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. The Journal of Cell Biology 101, 942–948 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Von Bartheld CS & Altick AL Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Progress in neurobiology 93, 313–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Besharse JC, Hollyfield JG & Rayborn ME Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. The Journal of Cell Biology 75, 507–527 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Young RW & Bok D Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42, 392–403 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Salinas RY et al. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. The Journal of Cell Biology 216, 1489–1499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides an in vivo demonstration of a role for ciliary ectocytosis in disposing of excess membranes. Ectocytosis must hence be suppressed for the formation of fresh disks in photoreceptor outer segments.
- 193.Cao M et al. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. eLife 4, e05242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper uncovers signal-dependent ectocytosis as a means for ciliary exit of activated adhesion molecules in C. reinhardtii, see also 29.
- 194.Loktev AV & Jackson PK Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell reports 5, 1316–1329 (2013). [DOI] [PubMed] [Google Scholar]
- 195.Marion S, Oakley RH, Kim K-M, Caron MG & Barak LS A beta-arrestin binding determinant common to the second intracellular loops of rhodopsin family G protein-coupled receptors. The Journal of biological chemistry 281, 2932–2938 (2006). [DOI] [PubMed] [Google Scholar]
- 196.van Dam TJP et al. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc Natl Acad Sci U S A 110, 6943–6948 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shida T, Cueva JG, Xu Z, Goodman MB & Nachury MV The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A 107, 21517–21522 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dentler W A Role for the Membrane in Regulating Chlamydomonas Flagellar Length. PLoS ONE 8, e53366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rajagopalan V, Subramanian A, Wilkes DE, Pennock DG & Asai DJ Dynein-2 affects the regulation of ciliary length but is not required for ciliogenesis in Tetrahymena thermophila. Molecular Biology of the Cell 20, 708–720 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hao L, Efimenko E, Swoboda P & Scholey JM The retrograde IFT machinery of C. elegans cilia: two IFT dynein complexes? PLoS One 6, e20995 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ocbina PJR, Eggenschwiler JT, Moskowitz I & Anderson KV Complex interactions between genes controlling trafficking in primary cilia. Nat. Genet 43, 547–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Das RM & Storey KG Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science (New York, NY) 343, 200–204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mirvis M, Siemers KA, Nelson WJ & Stearns T Heterogeneous Dynamics and Mechanisms of Primary Cilium Disassembly in Mammalian Cells. bioRxiv 433144 (2018). doi: 10.1101/433144 [DOI] [Google Scholar]
- 204.van Niel G, D’Angelo G & Raposo G Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology 19, 213–228 (2018). [DOI] [PubMed] [Google Scholar]
- 205.Wood CR & Rosenbaum JL Ciliary ectosomes: transmissions from the cell’s antenna. Trends in cell biology 25, 276–285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wood CR, Huang K, Diener DR & Rosenbaum JL The cilium secretes bioactive ectosomes. Curr Biol 23, 906–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Szempruch AJ et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell 164, 246–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang J et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr. Biol 24, 519–525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hurley JH ESCRTs are everywhere. The EMBO Journal 34, 2398–2407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Diener DR, Lupetti P & Rosenbaum JL Proteomic analysis of isolated ciliary transition zones reveals the presence of ESCRT proteins. Current biology : CB 25, 379–384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mierzwa B & Gerlich DW Cytokinetic abscission: molecular mechanisms and temporal control. Dev. Cell 31, 525–538 (2014). [DOI] [PubMed] [Google Scholar]
- 212.Chiaruttini N et al. Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation. Cell 163, 866–879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wollert T, Wunder C, Lippincott-Schwartz J & Hurley JH Membrane scission by the ESCRT-III complex. Nature 458, 172–177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu AP & Fletcher DA Actin Polymerization Serves as a Membrane Domain Switch in Model Lipid Bilayers. Biophysical Journal 91, 4064–4070 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Römer W et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell 140, 540–553 (2010). [DOI] [PubMed] [Google Scholar]
- 216.Allain J-M, Storm C, Roux A, Amar MB & Joanny J-F Fission of a Multiphase Membrane Tube. Phys. Rev. Lett 93, 158104 (2004). [DOI] [PubMed] [Google Scholar]
- 217.Ma M, Gallagher A-R & Somlo S Ciliary Mechanisms of Cyst Formation in Polycystic Kidney Disease. Cold Spring Harbor perspectives in biology a028209 (2017). doi: 10.1101/cshperspect.a028209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pazour GJ et al. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current biology : CB 12, R378–80 (2002). [DOI] [PubMed] [Google Scholar]
- 219.Yoder BK, Hou X & Guay-Woodford LM The Polycystic Kidney Disease Proteins, Polycystin-1, Polycystin-2, Polaris, and Cystin, Are Co-Localized in Renal Cilia. JASN 13, 2508–2516 (2002). [DOI] [PubMed] [Google Scholar]
- 220.Kleene SJ & Kleene NK The native TRPP2-dependent channel of murine renal primary cilia. Am. J. Physiol. Renal Physiol 312, F96–F108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu X et al. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 7, e33183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nauli SM et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet 33, 129–137 (2003). [DOI] [PubMed] [Google Scholar]
- 223.Delling M et al. Primary cilia are not calcium-responsive mechanosensors. Nature 531, 656–660 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pazour GJ et al. Chlamydomonas IFT88 and Its Mouse Homologue, Polycystic Kidney Disease Gene Tg737, Are Required for Assembly of Cilia and Flagella. The Journal of Cell Biology 151, 709–718 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Braun DA & Hildebrandt F Ciliopathies. Cold Spring Harbor perspectives in biology 9, a028191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ma M, Tian X, Igarashi P, Pazour GJ & Somlo S Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet 45, 1004–1012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Riobo NA Canonical and Non-Canonical Hedgehog Signaling Pathways: Role of G Proteins in The Smoothened Receptor in Cancer and Regenerative Medicine 13–42 (Springer International Publishing, 2014). doi: 10.1007/7355_2014_63 [DOI] [Google Scholar]
- 228.Liu YC et al. The PPFIA1-PP2A protein complex promotes trafficking of Kif7 to the ciliary tip and Hedgehog signaling. Sci Signal 7, ra117 (2014). [DOI] [PubMed] [Google Scholar]
- 229.Cheung HO-L et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Science Signaling 2, ra29 (2009). [DOI] [PubMed] [Google Scholar]
- 230.Endoh-Yamagami S et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Current biology : CB 19, 1320–1326 (2009). [DOI] [PubMed] [Google Scholar]
- 231.He M et al. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nature Cell Biology 16, 663–672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bidet M et al. The hedgehog receptor patched is involved in cholesterol transport. PLoS ONE 6, e23834 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fiorini L et al. Natural paniceins from mediterranean sponge inhibit the multidrug resistance activity of Patched and increase chemotherapy efficiency on melanoma cells. Oncotarget 6, 22282–22297 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang Y et al. Structural Basis for Cholesterol Transport-like Activity of the Hedgehog Receptor Patched. Cell 175, 1352–1364.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gong X et al. Structural basis for the recognition of Sonic Hedgehog by human Patched1. Science 361, eaas8935 (2018). [DOI] [PubMed] [Google Scholar]
- 236.Qi X, Schmiege P, Coutavas E, Wang J & Li X Structures of human Patched and its complex with native palmitoylated sonic hedgehog. Nature 560, 128–132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Qi X, Schmiege P, Coutavas E & Li X Two Patched molecules engage distinct sites on Hedgehog yielding a signaling-competent complex. Science 362, eaas8843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; References 234–237 present structures of Hedgehog receptor Patched 1 that uncover a channel in Patched 1 through which cholesterol may be transported from inner leaflet to the ECD. Ref. 234 also provides direct evidence that Patched 1 transports cholesterol from the inner to the outer leaflet of the membrane.
- 238.Huang P et al. Structural Basis of Smoothened Activation in Hedgehog Signaling. Cell 174, 312–324.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; The structure of activated Smoothened presented in this paper uncovers a channel through which cholesterol may travel from the inner leaflet to the CRD, thereby promoting receptor activation.
- 239.Cole DG et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. The Journal of Cell Biology 141, 993–1008 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nachury MV et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007). [DOI] [PubMed] [Google Scholar]
- 241.Wingfield JL, Lechtreck K-F & Lorentzen E Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery. Essays In Biochemistry 62, 753–763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Funabashi T, Katoh Y, Okazaki M, Sugawa M & Nakayama K Interaction of heterotrimeric kinesin-II with IFT-B-connecting tetramer is crucial for ciliogenesis. J. Cell Biol 217, 2867–2876 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jordan MA, Diener DR, Stepanek L & Pigino G The cryo-EM structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. Nat. Cell Biol 20, 1250–1255 (2018). [DOI] [PubMed] [Google Scholar]
- 244.Chien A et al. Dynamics of the IFT machinery at the ciliary tip. Elife 6, e28606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mijalkovic J, van Krugten J, Oswald F, Acar S & Peterman EJG Single-Molecule Turnarounds of Intraflagellar Transport at the C. elegans Ciliary Tip. Cell Rep 25, 1701–1707.e2 (2018). [DOI] [PubMed] [Google Scholar]
- 246.Stepanek L & Pigino G Microtubule doublets are double-track railways for intraflagellar transport trains. Science (New York, NY) 352, 721–724 (2016). [DOI] [PubMed] [Google Scholar]
- 247.Chaya T, Omori Y, Kuwahara R & Furukawa T ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport. EMBO J 33, 1227–1242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Berman SA, Wilson NF, Haas NA & Lefebvre PA A Novel MAP Kinase Regulates Flagellar Length in Chlamydomonas. Current Biology 13, 1145–1149 (2003). [DOI] [PubMed] [Google Scholar]
- 249.Burghoorn J et al. Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proceedings of the National Academy of Sciences 104, 7157 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bengs F, Scholz A, Kuhn D & Wiese M LmxMPK9, a mitogen-activated protein kinase homologue affects flagellar length in Leishmania mexicana: MAP kinase and flagellar length. Molecular Microbiology 55, 1606–1615 (2005). [DOI] [PubMed] [Google Scholar]