Abstract
Objective
To explore whether there are observable physician characteristics associated with antibiotic prescribing for pediatric respiratory tract infections (RTIs).
Design
Population-based cohort study using a hierarchical generalized linear mixed-model analysis.
Setting
British Columbia.
Participants
All pediatric visits for RTIs between 2005 and 2011.
Main outcome measures
The association between an antibiotic prescription being dispensed within 5 days after each visit and patient, physician, and regional characteristics.
Results
Overall, 27.9% of RTI visits were followed by an antibiotic prescription. After accounting for observed patient, physician, and regional factors, median 2-fold variation was found across physicians in their odds of prescribing. Observable physician characteristics explained nearly half of the variation between them. Higher prescribing was evident among physicians with more years of clinical experience (odds ratio [OR] of 1.46, 95% CI 1.33 to 1.61), international medical graduates (OR = 1.73, 95% CI 1.63 to 1.83), and physicians with proportionally fewer recent visits for RTIs (OR = 1.45, 95% CI 1.38 to 1.52). Female physicians prescribed less often than male physicians did (OR 0.91, 95% CI 0.86 to 0.96).
Conclusion
Substantial variations were found among physicians in prescribing antibiotics for pediatric RTIs. Observable characteristics accounted for a meaningful proportion of this variation; however, some physicians have a higher propensity to prescribe than others do, which remains unexplained. Patient and regional characteristics did not explain much of the variation across physicians. In future, behavioural interventions should be designed and evaluated to target physicians with higher propensity to prescribe.
Résumé
Objectif
Examiner s’il existe, chez les médecins, des caractéristiques observables associées à la prescription d’antibiotiques pour des infections pédiatriques des voies respiratoires (IVR).
Type d’étude
Études de cohortes dans la population au moyen d’une analyse des modèles mixtes linéaires généralisés et log-linéaires hiérarchiques.
Contexte
Colombie-Britannique.
Participants
Toutes les visites pédiatriques pour une IVR entre 2005 et 2011.
Principaux paramètres à l’étude
L’association entre une ordonnance d’antibiotiques exécutée dans les 5 jours suivant chaque visite, et les caractéristiques du patient, du médecin et de la région.
Résultats
Dans l’ensemble, une ordonnance d’antibiotiques était prescrite à la suite de 27,9 % des visites pour une IVR. Après avoir tenu compte des facteurs observés chez le patient, le médecin et la région, une variation médiane du double a été cernée chez les médecins dans la probabilité qu’ils rédigent une ordonnance. Des caractéristiques observables des médecins ont expliqué près de la moitié de la variation entre eux. Un taux plus élevé de prescription était évident chez les médecins comptant plus d’années d’expérience clinique (rapport de cotes [RC] de 1,46, IC à 95 % de 1,33 à 1,61), chez les diplômés en médecine de l’étranger (RC = 1,73, IC à 95 % de 1,63 à 1,83), de même que chez les médecins qui avaient reçu proportionnellement moins de visites récentes pour une IVR (RC = 1,45, IC à 95 % de 1,38 à 1,52). Les femmes médecins prescrivaient moins souvent que leurs homologues masculins (RC = 0,91, IC à 95 % de 0,86 à 0,96).
Conclusion
Des variations considérables ont été observées entre les médecins quant à laprescription d’antibiotiques pour une IVR pédiatrique. Une proportion significative de cette variation est attribuable à des caractéristiques observables; toutefois, certains médecins ont une plus forte propension à prescrire que d’autres, ce qui demeure inexpliqué. Les caractéristiques des patients et des régions n’ont pas permis d’expliquer cette variation entre les médecins. À l’avenir, des interventions comportementales devraient être élaborées et évaluées pour cibler les médecins les plus enclins à prescrire.
Antibiotic resistance is an important and growing public health issue. Antibiotic use is one of the most important drivers of antibiotic resistance.1 Recent estimates suggest that up to 50% of antibiotic use for respiratory tract infections (RTIs) in children2 and adults3 is likely unnecessary or inappropriate.
Prescribing decisions are complex, and there are documented variations in antibiotic prescribing both across medical practice networks4 and across individual physicians5,6 that cannot be fully explained by patients’ clinical presentations. Patient,7 physician,8–10 and geographic11–13 characteristics have been previously associated with antibiotic prescribing, both for RTIs and more generally. However, investigations of these factors with consideration of patient and physician characteristics simultaneously are sparse,5,6 as are studies specifically of children. Given the high rate of potentially inappropriate prescriptions for this common set of syndromes,2 understanding antibiotic prescribing for pediatric RTIs is important for improvements in practice and policy to support the judicious use of these medicines.
Therefore, we used population-based data to study variations in antibiotic prescribing for children with RTIs to assess which observable physician characteristics are associated with prescribing after controlling for relevant patient and regional variables. In particular, we sought to identify demographic and practice-related characteristics that would help inform the design and targeting of future interventions.
METHODS
Data sources
We used administrative data for all residents of British Columbia (BC).14 Population Data BC provided de-identified data sets with common unique study identifiers attached. We linked data on physician visits,15 prescription drug dispensing,16 hospitalizations,17 and patient14 and physician demographic characteristics.18
Study cohort
We identified all pediatric visits for RTIs between 2005 and 2011 using existing methods.19 We then identified a subset of visits for acute syndromes less likely to require an antibiotic prescription: nasopharyngitis, sinusitis, pharyngitis, tonsillitis, laryngitis and tracheitis, upper respiratory infections of multiple or unspecified sites, bronchitis or bronchiolitis, viral pneumonia, or influenza (ICD-9 codes 460 to 466, 480, or 487).20 To ensure stability and adequate follow-up time, we restricted our cohort to individuals registered with the provincial public insurance program for 275 or more days in each studied year.21
Measurements
Outcome.
To determine whether an encounter resulted in prescribing, we identified the closest prescription matching both patient and prescriber for an antibiotic (ATC [Anatomic Therapeutic Chemical] code J01) filled within 5 days of a physician visit.
Factors.
The selection of factors was informed by socioecologic frameworks of behaviour,22 health promotion models,23 a health services research approach to understanding variations in care,24 and a previous systematic review of factors associated with antibiotic prescribing decisions for RTIs.25
Patient level: Patient demographic characteristics included age, sex, and neighbourhood income quintile as an indicator of socioeconomic status. As a proxy for patient attachment with the GP, we calculated the proportion of GP visits in the previous 3 years with the same physician as the RTI visit,26 and dichotomized this variable at the median (0.1). We used 2 measures of comorbidity. First, we summed the number of Johns Hopkins Adjusted Clinical Groups System aggregated diagnosis groups as a measure of general comorbidity.27 Second, we identified individuals with 1 of 8 high-risk indications within the past 2 years (≥ 2 physician visits or ≥ 1 hospitalization): asthma, chronic kidney disease, chronic liver disease, chronic obstructive pulmonary disease, congestive heart failure, cystic fibrosis, diabetes, and immunosuppression. Finally, we defined recent antibiotic users as those with any antibiotic prescription in the 6 months before the RTI visit, and follow-up visits as any visit within 14 days of another RTI visit.
Physician level: Physician demographic variables included sex, place of medical school graduation (Canada vs elsewhere), specialty, and years since medical school graduation. We calculated daily volume as the total number of claims billed on the day of the RTI visit, and frequency of RTI management as the proportion and number of visits in the past 30 days and the past year, respectively, with an RTI diagnosis.
Regional level: There are 89 local health areas (LHAs) nested within 5 geographic health authorities in BC. We calculated annual measures of LHA population demographic characteristics, and used meteorologic temperature readings assigned to each LHA28 to calculate a 28-day moving average for each visit day.
Statistical analysis
We used generalized linear mixed models to estimate variance parameters for the physician clustering level, and log-odds parameters for the covariates. We randomly selected 1 visit per child to overcome issues of modeling patient-level variation, in line with previous analyses.6
We first estimated an empty random intercept model. We then estimated a series of successive models by adding blocks of variables in the following order: year and quarter of the visit to address seasonality; patient-level variables to assess how physician-level effects resulted from differences in patient composition29; physician-level variables; and finally regional-level variables. All variables were retained in subsequent models, regardless of statistical significance.
We describe the variation in several ways. First, we calculated the intraclass correlation (ICC) according to the latent variable method.30 We also report the median odds ratio (OR), which can be interpreted as the median of the distribution of ORs that could theoretically be obtained by comparing 2 randomly chosen patients (with the same covariate values) from 2 different physicians.29,31,32 Finally, we report the proportion change in the variance as the relative change in the physician-level variance parameter between different models.33
RESULTS
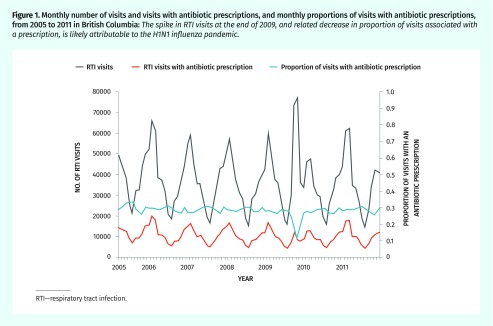
Between 2005 and 2011, we identified about 3 million pediatric RTI visits. Randomly selecting 1 visit per person left 671 342 observations, of which 27.3% were associated with an antibiotic dispensing. Table 1 shows descriptive statistics for both the complete data set and the analytic subset. Half of the visits in the complete data set were with female patients (47.5%), and the median patient age was 4 years (interquartile range [IQR] of 2 to 9). Figure 1 shows overall monthly trends in RTI visits.
Table 1.
Distribution of variables by antibiotic prescription for RTI visits in children
| VARIABLE | FULL DATA SET* | SAMPLE OF 1 VISIT PER CHILD* | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| ALL VISITS (N = 2 996 186)† | ASSOCIATED WITH AN ANTIBIOTIC PRESCRIPTION (N = 834 673)‡ | NOT ASSOCIATED WITH AN ANTIBIOTIC PRESCRIPTION (N = 2 161 513)‡ | ALL VISITS (N = 671 342)† | ASSOCIATED WITH AN ANTIBIOTIC PRESCRIPTION (N = 183 118)‡ | NOT ASSOCIATED WITH AN ANTIBIOTIC PRESCRIPTION (N = 488 224)‡ | |
| Overall population, % | 100.0 | 27.9 | 72.1 | 100.0 | 27.3 | 72.7 |
| Year, % | ||||||
| • 2005 | 11.3 | 30.3 | 69.7 | 13.2 | 31.6 | 68.4 |
| • 2006 | 15.4 | 29.4 | 70.6 | 16.2 | 30.6 | 69.4 |
| • 2007 | 14.7 | 28.5 | 71.6 | 13.7 | 28.9 | 71.1 |
| • 2008 | 14.1 | 28.8 | 71.2 | 12.6 | 28.4 | 71.6 |
| • 2009 | 16.9 | 22.9 | 77.2 | 16.1 | 21.2 | 78.9 |
| • 2010 | 13.1 | 28.0 | 72.0 | 12.2 | 25.8 | 74.3 |
| • 2011 | 14.6 | 28.6 | 71.5 | 16.1 | 25.4 | 74.6 |
| Patient-level factors | ||||||
| Patient sex | ||||||
| • Female, % | 47.5 | 27.9 | 72.1 | 48.9 | 27.3 | 72.7 |
| • Male, % | 52.5 | 27.8 | 72.2 | 51.2 | 27.3 | 72.7 |
| • Missing, n | 2 | NA | NA | 1 | NA | NA |
| Median (IQR) patient age, y | 4 (2–9) | 6 (3–10) | 4 (1–8) | 6 (2–11) | 8 (4–12) | 5 (1–10) |
| • Missing, n | 2 | NA | NA | 2 | NA | NA |
| Follow-up RTI visit,§ % | 14.0 | 24.6 | 75.5 | 7.9 | 24.3 | 75.8 |
| QAIPPE | ||||||
| • 1 (lowest), % | 22.2 | 28.6 | 71.4 | 20.4 | 27.6 | 72.4 |
| • 2, % | 22.7 | 28.7 | 71.3 | 20.9 | 27.8 | 72.2 |
| • 3, % | 20.0 | 27.6 | 72.4 | 20.5 | 27.3 | 72.7 |
| • 4, % | 18.2 | 26.9 | 73.1 | 20 | 26.9 | 73.1 |
| • 5 (highest), % | 15.7 | 27.1 | 72.9 | 18.2 | 26.7 | 73.3 |
| • Missing, n | 38 224 | NA | NA | 9157 | NA | NA |
| Diabetes, % | 0.2 | 33.1 | 66.9 | 0.2 | 33.3 | 66.7 |
| Asthma, % | 12.4 | 31.5 | 68.5 | 8.1 | 31.1 | 68.9 |
| Cystic fibrosis, % | 0.2 | 18.6 | 81.4 | 0.1 | 17.7 | 82.3 |
| Immune deficiency, % | 0.2 | 30.2 | 69.8 | 0.2 | 28.7 | 71.3 |
| Chronic liver disease, % | < 0.1 | 27.8 | 72.2 | 0.0 | 26.4 | 73.6 |
| Congestive heart failure, % | < 0.1 | 27.2 | 72.8 | 0.0 | 22.9 | 77.1 |
| COPD, % | 0.5 | 31.5 | 68.5 | 0.3 | 31.4 | 68.7 |
| Chronic kidney disease, % | 0.1 | 28.7 | 71.4 | 0.1 | 28.5 | 71.6 |
| Recent antibiotic use,‖ % | 9.1 | 24.5 | 75.5 | 9.4 | 24.1 | 75.9 |
| Median (IQR) ADG sum | 4 (2–5) | 4 (2–5) | 4 (2–5) | 3 (2–5) | 3 (2–4) | 3 (2–5) |
| • Missing, n | 9 | NA | NA | 2 | NA | NA |
| Visits in past 3 y with this physician, % | ||||||
| • 0 | 36.6 | 28.6 | 71.4 | 46.8 | 28.7 | 71.3 |
| • ≤ 30 (but > 0) | 30.2 | 28.6 | 71.4 | 25.5 | 27.9 | 72.1 |
| • > 30 | 33.2 | 26.3 | 73.7 | 27.8 | 24.4 | 75.6 |
| Median (IQR) no. of different GPs seen in past 3 y | 5 (3–7) | 5 (3–8) | 5 (3–7) | 4 (2–6) | 4 (2–6) | 4 (2–6) |
| Season, % | ||||||
| • Mar–May | 24.2 | 29.1 | 70.9 | 24.5 | 29.0 | 71.0 |
| • Jun–Aug | 15.0 | 29.5 | 70.5 | 14.5 | 29.7 | 70.3 |
| • Sep–Nov | 29.3 | 25.4 | 74.6 | 28.8 | 24.0 | 76.0 |
| • Dec–Feb | 31.5 | 28.4 | 71.6 | 32.3 | 27.8 | 72.3 |
| Physician-level factors | ||||||
| Physicians, n | 6404 | NA | NA | 6148 | NA | NA |
| Physician sex | ||||||
| • Female, % | 27.7 | 23.5 | 76.6 | 28.3 | 23.0 | 77.0 |
| • Male, % | 72.3 | 29.5 | 70.5 | 71.7 | 28.9 | 71.1 |
| • Missing, n | 10 398 | NA | NA | 1910 | NA | NA |
| Specialty, % | ||||||
| • Emergency medicine physician | 0.6 | 25.1 | 74.9 | 0.7 | 25.2 | 74.8 |
| • Pediatrician | 2.9 | 11.2 | 88.8 | 2.4 | 12.8 | 87.2 |
| • Other | 0.8 | 41.8 | 58.2 | 0.7 | 40.7 | 59.3 |
| • GP | 95.7 | 28.3 | 71.7 | 96.2 | 27.6 | 72.5 |
| Visits for RTIs in the past 30 d, % | ||||||
| • None | 0.6 | 20.4 | 79.6 | 0.7 | 21.0 | 79.0 |
| • ≤ 2.5 | 5.7 | 21.7 | 78.3 | 6.9 | 22.1 | 77.9 |
| • > 2.5–5 | 14.7 | 24.4 | 75.6 | 16.8 | 24.7 | 75.3 |
| • > 5–7.5 | 16.5 | 26.0 | 74.0 | 17.8 | 26.2 | 73.8 |
| • > 7.5–10 | 14.8 | 27.9 | 72.2 | 14.8 | 27.4 | 72.6 |
| • > 10–15 | 22.1 | 29.9 | 70.1 | 20.6 | 29.3 | 70.7 |
| • > 15–20 | 14.0 | 31.9 | 68.1 | 12.2 | 30.9 | 69.1 |
| • > 20 | 11.7 | 29.4 | 70.6 | 10.2 | 28.7 | 71.3 |
| Medical school graduation location | ||||||
| • Canada, % | 62.1 | 24.3 | 75.8 | 64.1 | 23.8 | 76.2 |
| • International, % | 37.9 | 33.8 | 66.2 | 36.0 | 33.4 | 66.6 |
| • Missing, n | 70 648 | NA | NA | 15 683 | NA | NA |
| Daily patient volume,¶ % | ||||||
| • 1st quartile (< 28 visits) | 22.8 | 23.2 | 76.9 | 25.3 | 23.3 | 76.7 |
| • 2nd quartile (28–37 visits) | 24.9 | 26.4 | 73.6 | 26.6 | 26.2 | 73.8 |
| • 3rd quartile (38–48 visits) | 26.0 | 28.9 | 71.1 | 25.7 | 28.7 | 71.3 |
| • 4th quartile (> 48 visits) | 26.3 | 32.3 | 67.7 | 22.4 | 31.4 | 68.6 |
| Years since medical school graduation | ||||||
| • 0–5, % | 4.6 | 21.8 | 78.2 | 5.2 | 20.8 | 79.3 |
| • 6–10, % | 9.3 | 22.1 | 77.9 | 9.8 | 22.4 | 77.6 |
| • 11–15, % | 13.0 | 24.0 | 76.0 | 13.4 | 24.3 | 75.7 |
| • 16–20, % | 18.5 | 26.9 | 73.1 | 18.2 | 27.0 | 73.1 |
| • 21–25, % | 16.8 | 28.7 | 71.3 | 16.5 | 27.7 | 72.3 |
| • 26–30, % | 13.9 | 30.0 | 70.0 | 13.7 | 29.3 | 70.7 |
| • 31–35, % | 11.9 | 30.7 | 69.3 | 11.6 | 29.7 | 70.3 |
| • 36–40, % | 7.4 | 30.7 | 69.3 | 7.2 | 29.7 | 70.3 |
| • > 40, % | 4.7 | 39.1 | 60.9 | 4.5 | 37.9 | 62.1 |
| • Missing, n | 4032 | NA | NA | 1050 | NA | NA |
| No. of RTIs seen in the past y, % | ||||||
| • 1st quartile (≤ 248) | 20.4 | 20.8 | 79.2 | 24.8 | 21.5 | 78.5 |
| • 2nd quartile (249–490) | 22.2 | 25.6 | 74.5 | 25.1 | 25.9 | 74.1 |
| • 3rd quartile (491–972) | 25.2 | 28.2 | 71.8 | 25.0 | 28.5 | 71.5 |
| • 4th quartile (≥ 973) | 32.2 | 33.7 | 66.4 | 25.0 | 33.2 | 66.8 |
| Regional-level factors | ||||||
| Median (IQR) proportion of LHA population aged > 65 y | 0.13 (0.10–10.15) | 0.13 (0.10–0.15) | 0.13 (0.11–0.15) | 0.13 (0.11–0.16) | 0.13 (0.10–0.16) | 0.13 (0.11–0.16) |
| Median (IQR) proportion of LHA population aged < 15 y | 0.18 (0.16–0.21) | 0.19 (0.17–0.22) | 0.18 (0.16–0.21) | 0.18 (0.16–0.21) | 0.19 (0.16–0.21) | 0.18 (0.16–0.21) |
| Median (IQR) age of LHA population, y | 39 (36–41) | 38 (35–41) | 39 (37–41) | 39 (37–42) | 39 (37–41) | 39 (37–42) |
| Health authority | ||||||
| • Interior, % | 11.8 | 27.8 | 72.2 | 14.5 | 28.0 | 72.1 |
| • Fraser, % | 46.3 | 29.6 | 70.4 | 41.3 | 28.5 | 71.5 |
| • Vancouver Coastal, % | 23.2 | 22.4 | 77.6 | 22.3 | 21.9 | 78.1 |
| • Vancouver Island, % | 12.2 | 26.4 | 73.6 | 14.3 | 26.5 | 73.5 |
| • Northern, % | 6.5 | 37.5 | 62.5 | 7.5 | 36.6 | 63.4 |
| • Missing, n | 4689 | NA | NA | 1251 | NA | NA |
| Median (IQR) 28-d moving average apparent temperature, °C | 4.73 (0.57–11.76) | 4.62 (0.33–12.14) | 4.77 (0.66–11.57) | 4.49 (0.36–11.50) | 4.42 (0.17–12.14) | 4.52 (0.43–11.24) |
ADG—aggregated diagnosis group, COPD—chronic obstructive pulmonary disease, IQR—interquartile range, LHA—local health area, NA—not applicable, QAIPPE—Quintile of Annual Income Per Person Equivalent, RTI—respiratory tract infection.
Not all percentages add to 100 owing to rounding.
Overall values.
Within-category values.
A follow-up visit is any visit within 14 d of another RTI visit.
Recent antibiotic use is any antibiotic prescription within the past 6 mo.
Daily patient volume is the total number of claims billed on the RTI visit day.
Figure 1.
Monthly number of visits and visits with antibiotic prescriptions, and monthly proportions of visits with antibiotic prescriptions, from 2005 to 2011 in British Columbia: The spike in RTI visits at the end of 2009, and related decrease in proportion of visits associated with a prescription, is likely attributable to the H1N1 influenza pandemic.
RTI—respiratory tract infection.
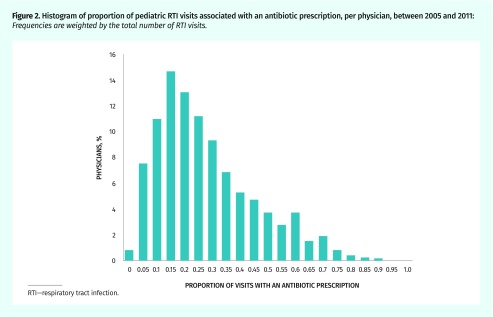
These visits were conducted by 6404 unique physicians. In 27.7% of visits the physician was female, 95.7% of visits were with a GP, and across visits, physicians had an average of 22.5 years of experience. In the complete data set, 8.2% of practitioners (527 of 6404) never prescribed antibiotics. These practitioners had fewer visits overall (median [IQR] 3 [1 to 8]). Figure 2 shows the variation in prescribing rates by physician; the median proportion of visits associated with a prescription was 19.2% (IQR 10.0% to 33.1%).
Figure 2.
Histogram of proportion of pediatric RTI visits associated with an antibiotic prescription, per physician, between 2005 and 2011: Frequencies are weighted by the total number of RTI visits.
RTI—respiratory tract infection.
Model results
Results of the series of models are presented in Table 2. The final model, with all covariates, was based on 645 094 observations owing to missing data (3.9% of the observations were excluded).
Table 2.
Generalized linear mixed model of antibiotic prescription for pediatric RTIs: A) Effect estimates and B) measures of model fit and variance.
| A) | |||||
|---|---|---|---|---|---|
| EFFECT | EMPTY MODEL | BASIC MODEL WITH YEAR ONLY | WITH PATIENT COVARIATES | WITH PATIENT AND PHYSICIAN COVARIATES | WITH PATIENT, PHYSICIAN, AND REGIONAL COVARIATES |
| Random effect, variance (SE) | |||||
| Physician | 0.96 (0.023) | 0.95 (0.022) | 0.91 (0.011) | 0.78 (0.019) | 0.76 (0.019) |
| Fixed effects, OR (95% CI) | |||||
| Constant term | 0.27 (0.26–0.27) | 0.35 (0.34–0.36) | 0.18 (0.17–0.18) | 0.10 (0.09–0.11) | 0.10 (0.08–0.12) |
| Year (reference: 2005) | |||||
| • 2006 | 0.93 (0.91–0.95) | 0.94 (0.92–0.96) | 0.91 (0.88–0.93) | 0.90 (0.88–0.92) | |
| • 2007 | 0.89 (0.87–0.91) | 0.93 (0.91–0.95) | 0.88 (0.86–0.90) | 0.87 (0.85–0.89) | |
| • 2008 | 0.85 (0.83–0.87) | 0.92 (0.90–0.94) | 0.87 (0.85–0.89) | 0.86 (0.84–0.88) | |
| • 2009 | 0.58 (0.57–0.60) | 0.61 (0.60–0.63) | 0.58 (0.56–0.59) | 0.57 (0.56–0.59) | |
| • 2010 | 0.76 (0.74–0.78) | 0.92 (0.90–0.94) | 0.83 (0.81–0.86) | 0.83 (0.81–0.85) | |
| • 2011 | 0.75 (0.73–0.77) | 0.94 (0.92–0.96) | 0.86 (0.83–0.88) | 0.85 (0.82–0.87) | |
| Season (reference: Jun–Aug) | |||||
| • Mar–May | 0.98 (0.97–1.00) | 0.99 (0.97–1.00) | 1.05 (1.03–1.07) | 0.99 (0.97–1.02) | |
| • Sep–Nov | 0.80 (0.79–0.82) | 0.80 (0.79–0.82) | 0.86 (0.85–0.88) | 0.80 (0.77–0.82) | |
| • Dec–Feb | 0.99 (0.97–1.00) | 0.96 (0.94–0.98) | 1.06 (1.04–1.09) | 0.95 (0.92–0.98) | |
| Patient sex (reference: male) | |||||
| • Female | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | ||
| Patient age (per y increase) | 1.08 (1.08–1.08) | 1.08 (1.08–1.08) | 1.08 (1.08–1.08) | ||
| Follow-up RTI visit | 0.98 (0.96–1.01) | 0.97 (0.95–1.00) | 0.97 (0.95–1.00) | ||
| QAIPPE (reference: 1) | |||||
| • 2 | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | ||
| • 3 | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) | ||
| • 4 | 1.02 (1.00–1.04) | 1.02 (1.00–1.05) | 1.02 (1.00–1.04) | ||
| • 5 | 1.03 (1.01–1.05) | 1.04 (1.02–1.06) | 1.04 (1.02–1.06) | ||
| Diabetes | 1.08 (0.95–1.22) | 1.05 (0.93–1.19) | 1.05 (0.93–1.19) | ||
| Asthma | 1.06 (1.04–1.08) | 1.06 (1.04–1.08) | 1.06 (1.04–1.08) | ||
| Cystic fibrosis | 0.96 (0.80–1.16) | 0.97 (0.80–1.18) | 0.97 (0.80–1.18) | ||
| Immune deficiency | 0.92 (0.80–1.07) | 0.92 (0.79–1.06) | 0.92 (0.79–1.06) | ||
| Chronic liver disease | 1.07 (0.74–1.56) | 1.03 (0.70–1.52) | 1.00 (0.68–1.48) | ||
| Congestive heart failure | 1.30 (0.88–1.92) | 1.12 (0.75–1.69) | 1.12 (0.74–1.68) | ||
| COPD | 0.87 (0.78–0.98) | 1.14 (1.02–1.29) | 1.15 (1.02–1.29) | ||
| Chronic kidney disease | 0.90 (0.72–1.13) | 1.12 (0.89–1.40) | 1.12 (0.90–1.40) | ||
| Recent antibiotic use | 0.85 (0.83–0.87) | 0.86 (0.84–0.88) | 0.86 (0.84–0.88) | ||
| ADG sum | 0.97 (0.97–0.97) | 0.97 (0.97–0.97) | 0.97 (0.97–0.97) | ||
| Visits in past 3 y with this physician, % (reference: > 30) | |||||
| • 0 | 1.20 (1.18–1.22) | 1.21 (1.19–1.24) | 1.21 (1.19–1.24) | ||
| • ≤ 30 (but > 0) | 1.07 (1.05–1.09) | 1.07 (1.05–1.09) | 1.07 (1.05–1.09) | ||
| No. of different GPs seen in past 3 y | 1.02 (1.02–1.02) | 1.02 (1.02–1.02) | 1.02 (1.02–1.02) | ||
| Physician sex (reference: male) | |||||
| • Female | 0.89 (0.84–0.94) | 0.91 (0.86–0.96) | |||
| Specialty (reference: GP) | |||||
| • Emergency medicine physician | 0.79 (0.61–1.02) | 0.79 (0.62–1.02) | |||
| • Pediatrician | 0.47 (0.41–0.55) | 0.48 (0.42–0.56) | |||
| • Other | 0.88 (0.72–1.06) | 0.89 (0.73–1.07) | |||
| Visits for RTIs in the past 30 d, % (reference: > 20) | |||||
| • None | 1.34 (1.21–1.49) | 1.36 (1.23–1.51) | |||
| • ≤ 2.5 | 1.42 (1.35–1.49) | 1.45 (1.38–1.52) | |||
| • > 2.5–5 | 1.40 (1.34–1.45) | 1.42 (1.36–1.48) | |||
| • > 5–7.5 | 1.32 (1.27–1.37) | 1.33 (1.29–1.38) | |||
| • > 7.5–10 | 1.25 (1.21–1.30) | 1.26 (1.22–1.31) | |||
| • > 10–15 | 1.21 (1.17–1.25) | 1.22 (1.18–1.25) | |||
| • > 15–20 | 1.13 (1.10–1.16) | 1.13 (1.10–1.16) | |||
| Medical school graduation location (reference: Canada) | |||||
| • International | 1.77 (1.68–1.88) | 1.73 (1.63–1.83) | |||
| Daily patient volume (reference: 1st quartile [< 28 visits]) | |||||
| • 2nd quartile (28–37 visits) | 0.99 (0.97–1.01) | 0.99 (0.98–1.01) | |||
| • 3rd quartile (38–48 visits) | 0.97 (0.95–0.99) | 0.97 (0.95–0.99) | |||
| • 4th quartile (> 48 visits) | 0.94 (0.92–0.96) | 0.94 (0.92–0.97) | |||
| Years since medical school graduation (reference: 0–5) | |||||
| • 6–10 | 0.98 (0.93–1.03) | 0.99 (0.94–1.04) | |||
| • 11–15 | 1.07 (1.01–1.14) | 1.08 (1.02–1.15) | |||
| • 16–20 | 1.15 (1.08–1.22) | 1.16 (1.09–1.24) | |||
| • 21–25 | 1.21 (1.13–1.29) | 1.23 (1.15–1.31) | |||
| • 26–30 | 1.29 (1.21–1.39) | 1.32 (1.23–1.41) | |||
| • 31–35 | 1.32 (1.23–1.42) | 1.35 (1.26–1.46) | |||
| • 36–40 | 1.36 (1.25–1.47) | 1.39 (1.29–1.51) | |||
| • > 40 | 1.41 (1.28–1.56) | 1.46 (1.33–1.61) | |||
| No. of RTIs seen in the past y (reference: 1st quartile [≤ 248]) | |||||
| • 2nd quartile (249–490) | 1.10 (1.07–1.13) | 1.11 (1.08–1.14) | |||
| • 3rd quartile (491–972) | 1.17 (1.12–1.21) | 1.19 (1.15–1.23) | |||
| • 4th quartile (≥ 973) | 1.27 (1.21–1.33) | 1.31 (1.25–1.37) | |||
| LHA population aged > 65 y (per 10%) | 0.99 (0.98–1.00) | ||||
| LHA population aged < 15 y (per 10%) | 1.12 (1.06–1.17) | ||||
| Median age of LHA population (centred on mean) | 1.19 (1.11–1.27) | ||||
| Health authority (reference: Vancouver Coastal) | |||||
| • Interior | 1.07 (1.02–1.13) | ||||
| • Fraser | 1.00 (0.97–1.03) | ||||
| • Vancouver Island | 1.03 (0.98–1.08) | ||||
| • Northern | 1.20 (1.13–1.28) | ||||
| 28-d moving average apparent temperature | 0.99 (0.99–0.99) | ||||
| B) | |||||
|---|---|---|---|---|---|
| MEASURE | EMPTY MODEL | BASIC MODEL WITH YEAR ONLY | WITH PATIENT COVARIATES | WITH PATIENT AND PHYSICIAN COVARIATES | WITH PATIENT, PHYSICIAN, AND REGIONAL COVARIATES |
| Observations used, n | 671 342 | 671 342 | 662 202 | 645 815 | 645 094 |
| AIC | 616 216.2 | 692 274.7 | 666 759.5 | 648 692.3 | 647 788.8 |
| ICC,* % | 22.6 | 22.4 | 21.6 | 19.2 | 18.8 |
| MOR | 2.55 | 2.53 | 2.48 | 2.33 | 2.30 |
| PCV (relative to intercept-only model) at level of physician, % | NA | 1.49 | 5.86 | 18.55 | 20.82 |
| PCV (relative to previous model) at level of physician, % | NA | 1.49 | 4.44 | 13.48 | 2.78 |
ADG—aggregated diagnosis group, AIC—Akaike information criterion, COPD—chronic obstructive pulmonary disease, ICC—intraclass correlation, IQR—interquartile range, LHA—local health area, MOR—median odds ratio, NA—not applicable, OR—odds ratio, PCV—percent change in variance, QAIPPE—Quintile of Annual Income Per Person Equivalent, RTI—respiratory tract infection.
ICC is the proportion of total variance attributable to between-physician differences.
Model variation
Before accounting for other variables, the ICC was 22.6%, indicating that between-physician differences accounted for nearly one-quarter of the total variation in antibiotic prescribing. Patients seen by higher-prescribing physicians had a median 2.55 times higher odds of receiving a prescription compared with peers seen by a lower-prescribing physician.
After adjusting for patient, physician, and regional factors, the between-physician differences (ie, ICC) accounted for 18.8% of the total variation. Patients seen by higher-prescribing physicians had a median 2.30 times higher odds of receiving a prescription compared with peers seen by another physician. We observed the largest decrease in between-physician variation when the physician-level characteristics were included in the model. Controlling for these characteristics explained nearly one-fifth of the variation attributed to physicians (the proportional change in variance relative to the empty model is 18.55%), whereas controlling for patient-level characteristics only explained 5.96%. The inclusion of a few select regional-level variables, including gross-level geographic indicators, while statistically significant, did not explain much of the physician-level variation. Overall, this suggests that most variation was random (according to the characteristics that we measured), and thus more likely to be driven by individual physician practice styles.
Patient-level factors
As shown in our final model, having a recent outpatient antibiotic prescription (at any time in the past 6 months) was associated with a lower probability of prescribing. Older children, and children with asthma or chronic obstructive pulmonary disease, had higher odds of receiving a prescription. A lack of physician familiarity was also associated with more prescribing: patients who had less than 30% of their medical visits in the past 3 years with the RTI visit physician had a higher likelihood of prescribing (OR = 1.07, 95% CI 1.05 to 1.09), as did those with no documented visits with any provider in the past 3 years (OR = 1.21, 95% CI 1.19 to 1.24).
Clinician-level factors
Pediatricians had lower odds of prescribing compared with GPs (OR = 0.48, 95% CI 0.42 to 0.56). Physicians who graduated medical school outside of Canada had 1.73 times greater odds of prescribing (95% CI 1.63 to 1.83). The number of US-trained physicians was small, and grouping them with Canadian graduates did not change the results (data not shown). The number of years in practice was also associated with higher prescribing: physicians with more than 40 years of experience had odds of prescribing 1.46 times higher (95% CI 1.33 to 1.61) than newly graduated physicians had. Finally, physicians who had seen relatively fewer RTIs in the past 30 days were more likely to prescribe compared with those for whom 20% or more of their visits were coded as RTIs.
DISCUSSION
In the past 10 years, strong public health messages have promoted reductions in prescribing of antibiotics for RTIs. We found that 27.9% of visits for RTIs between 2005 and 2011 in BC were associated with an antibiotic prescription, which is similar to the proportions reported in other studies.3,34 After accounting for a number of relevant patient, physician, and regional factors, we found 2-fold variation in the odds of prescribing. Physician-level factors had the most influence, as observed factors decreased the between-physician variance by 18.55%, but more important, the rest of the variation remains unaccounted for. This suggests that individual physician practice style is driving a notable amount of the variation in antibiotic prescribing for RTIs. In comparison, patient and regional effects were relatively small.
Evidence of provider-level variation in prescribing has been previously reported5,6,35; the current study extends these findings with a population-based analysis of children across 7 years of data using a multilevel modeling framework. Additionally, we were able to include measures of health care usage and clinician practice characteristics.
Our findings suggest that a substantial amount of variability in antibiotic prescribing among physicians remains unexplained by observed factors, and might be more strongly related to individual practice styles. Efforts to measure prescriptions, and to target physicians with higher propensities to prescribe, might be the most promising interventions. Our findings also suggest a potential benefit from focusing on physicians who have been in practice longer and internationally trained physicians. Provision of individualized feedback via prescription database extracts, targeting of continuing education and academic detailing efforts, and tailoring our foreign-trained licensing requirements might be relevant approaches in these respects.
Limitations
Several limitations are worth bearing in mind as our results are interpreted. A previous systematic review of factors associated with antibiotic prescribing for RTI identified a number of patient-clinical and patient-physician communication factors as relevant.25 The present study was unable to include many of these measures owing to the nature of our data, and their inclusion could have explained more of the variation. However, we have addressed many known determinants in our model. A number of variables had missing data, as is common with administrative data. Physician demographic variables, in particular, had a higher rate of missing values. We did not specifically aim to assess the appropriateness of prescriptions. Rather, we considered any prescription for RTIs as potentially unnecessary, for the purpose of exploring factors associated with prescribing. In this regard, we caution against an interpretation of these findings as relating to inappropriate antibiotic prescriptions.
Conclusion
This population-level analysis demonstrated that variations exist among physicians in the antibiotic management of pediatric RTIs. Physician characteristics account for some, but not all, of the observed variations. The design of effective community-oriented programs and policies should aim to address variations in use of these essential drugs by focusing on physicians with higher-prescribing practices.
Acknowledgments
Dr McKay was supported by a Canadian Institutes of Health Research Doctoral Award (Frederick Banting and Charles Best Canada Graduate Scholarship). A University of British Columbia Centre for Disease Control Communal Fund Grant was awarded to Drs Patrick and McKay for this project. This study was also supported by a Canadian Institutes of Health Research Foundation Scheme Grant (Improving Access to Medicines in Canada and Abroad). Dr Law received salary support through a Canada Research Chair and a Michael Smith Foundation for Health Research Scholar Award. Population Data BC also granted a student waiver of some costs associated with the acquisition of the data. The British Columbia Ministry of Health approved access to and use of the data facilitated by Population Data BC for this study. All inferences, opinions, and conclusions drawn in this article are those of the authors and do not reflect the opinions or policies of the data stewards.
Editor’s key points
▸ Despite knowledge that reducing inappropriate antibiotic use is important to decrease resistance, meaningful physician behaviour change has remained difficult to achieve.
▸ The findings suggest that a substantial amount of variability in antibiotic prescribing among physicians remains unexplained by observed factors, and might be more strongly related to individual practice styles. Efforts to measure prescriptions, and to target physicians with higher propensities to prescribe, might be the most promising interventions.
▸ There might be a potential benefit from focusing on physicians who have been in practice longer and on internationally trained physicians, as they were more likely to prescribe antibiotics for pediatric respiratory tract infections.
Points de repère du rédacteur
▸ Même si limportance de réduire le recours inapproprié aux antibiotiques pour freiner la résistance est bien connue, il est demeuré difficile de changer le comportement des médecins de manière significative.
▸ Les constatations de létude font valoir que la variation considérable entre les médecins dans la prescription dantibiotiques demeure inexpliquée par les facteurs observés, et pourrait être plus fortement liée aux styles de pratique individuels. Des efforts pour mesurer le nombre de prescriptions et pour cibler les médecins plus enclins à prescrire des antibiotiques pourraient se révéler les interventions les plus prometteuses.
▸ Il y aurait peut-être lieu de cibler les médecins qui exercent depuis plus longtemps et ceux formés à létranger, car ils ont une plus forte propension à prescrire des antibiotiques pour une infection des voies respiratoires chez les enfants.
Footnotes
Contributors
Dr McKay, who takes responsibility for the integrity of the work as a whole, from inception to published article, led the conception and design of the study and acquisition of data; conducted the analysis and preliminary interpretation; and drafted the article and gave final approval of the version to be published. Drs Patrick, McGrail, and Law contributed to the conception and design of the study, acquisition of data, and interpretation of the results; and revised the article critically for important intellectual content and gave final approval of the version to be published.
Competing interests
None declared
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–33. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronman MP, Zhou C, Mangione-Smith R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections. Pediatrics. 2014;134(4):e956–65. doi: 10.1542/peds.2014-0605. Epub 2014 Sep 15. [DOI] [PubMed] [Google Scholar]
- 3.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, Jr, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315(17):1864–73. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 4.Butler CC, Hood K, Verheij T, Little P, Melbye H, Nuttall J, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones BE, Sauer B, Jones MM, Campo J, Damal K, He T, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med. 2015;163(2):73–80. doi: 10.7326/M14-1933. [DOI] [PubMed] [Google Scholar]
- 6.Mousquès J, Renaud T, Scemama O. Is the “practice style” hypothesis relevant for general practitioners? An analysis of antibiotics prescription for acute rhinopharyngitis. Soc Sci Med. 2010;70(8):1176–84. doi: 10.1016/j.socscimed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Brookes-Howell L, Hood K, Cooper L, Coenen S, Little P, Verheij T, et al. Clinical influences on antibiotic prescribing decisions for lower respiratory tract infection: a nine country qualitative study of variation in care. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2011-000795. pii:e000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold SR, To T, McIsaac WJ, Wang EE. Antibiotic prescribing for upper respiratory tract infection: the importance of diagnostic uncertainty. J Pediatr. 2005;146(2):222–6. doi: 10.1016/j.jpeds.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Rutschmann OT, Domino ME. Antibiotics for upper respiratory tract infections in ambulatory practice in the United States, 1997–1999: does physician specialty matter? J Am Board Fam Pract. 2004;17(3):196–200. doi: 10.3122/jabfm.17.3.196. [DOI] [PubMed] [Google Scholar]
- 10.Petursson P. GPs’ reasons for “non-pharmacological” prescribing of antibiotics. A phenomenological study. Scand J Prim Health Care. 2005;23(2):120–5. doi: 10.1080/02813430510018491. [DOI] [PubMed] [Google Scholar]
- 11.Mueller T, Östergren PO. The correlation between regulatory conditions and antibiotic consumption within the WHO European Region. Health Policy. 2016;120(8):882–9. doi: 10.1016/j.healthpol.2016.07.004. Epub 2016 Jul 12. [DOI] [PubMed] [Google Scholar]
- 12.Koller D, Hoffmann F, Maier W, Tholen K, Windt R, Glaeske G. Variation in antibiotic prescriptions: is area deprivation an explanation? Analysis of 1.2 million children in Germany. Infection. 2013;41(1):121–7. doi: 10.1007/s15010-012-0302-1. Epub 2012 Jul 24. [DOI] [PubMed] [Google Scholar]
- 13.Marra F, Mak S, Chong M, Patrick DM. The relationship among antibiotic consumption, socioeconomic factors and climatic conditions. Can J Infect Dis Med Microbiol. 2010;21(3):e99–106. doi: 10.1155/2010/965268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.British Columbia Ministry of Health. Consolidation file [data extract]. Vancouver, BC: Population Data BC; 2013. Available from: www.popdata.bc.ca/data. Accessed 2019 May 10. [Google Scholar]
- 15.British Columbia Ministry of Health. Medical Services Plan (MSP) payment information file [data extract]. Vancouver, BC: Population Data BC; 2014. Available from: www.popdata.bc.ca/data. Accessed 2019 May 10. [Google Scholar]
- 16.British Columbia Ministry of Health. PharmaNet [data extract]. Vancouver, BC: British Columbia Ministry of Health; 2011. Available from: www.popdata.bc.ca/data. Accessed 2019 May 10. [Google Scholar]
- 17.Canadian Institute for Health Information. Discharge Abstract Database (hospital separations) [data extract]. Vancouver, BC: Population Data BC; 2014. Available from: www.popdata.bc.ca/data. Accessed 2017 Jul 25. [Google Scholar]
- 18.British Columbia Ministry of Health. Medical Services Plan (MSP) practitioner file. Vancouver, BC: Population Data BC; 2014. Available from: www.popdata.bc.ca/data. Accessed 2019 May 28. [Google Scholar]
- 19.Cadieux G, Tamblyn R. Accuracy of physician billing claims for identifying acute respiratory infections in primary care. Health Serv Res. 2008;43(6):2223–38. doi: 10.1111/j.1475-6773.2008.00873.x. Epub 2008 Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blondel-Hill E, Fryters S. Bugs & drugs: an antimicrobial/infectious diseases reference. Edmonton, AB: Alberta Health Services; 2012. [Google Scholar]
- 21.Morgan SG, Cunningham CM, Hanley GE. Individual and contextual determinants of regional variation in prescription drug use: an analysis of administrative data from British Columbia. PLoS One. 2010;5(12):e15883. doi: 10.1371/journal.pone.0015883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissman J, Besser RE. Promoting appropriate antibiotic use for pediatric patients: a social ecological framework. Semin Pediatr Infect Dis. 2004;15(1):41–51. doi: 10.1053/j.spid.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Green LW, Kreuter MW. Health promotion planning. An educational and environmental approach. 2nd ed. Mountainview, CA: Mayfield Publishing Company; 1991. [Google Scholar]
- 24.Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16(7):811–24. doi: 10.1016/0277-9536(82)90234-9. [DOI] [PubMed] [Google Scholar]
- 25.McKay R, Mah A, Law MR, McGrail K, Patrick DM. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrob Agents Chemother. 2016;60(7):4106–18. doi: 10.1128/AAC.00209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breslau N, Reeb KG. Continuity of care in a university-based practice. J Med Educ. 1975;50(10):965–9. doi: 10.1097/00001888-197510000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29(5):452–72. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Henderson SB, Wan V, Kosatsky T. Differences in heat-related mortality across four ecological regions with diverse urban, rural, and remote populations in British Columbia, Canada. Health Place. 2013;23:48–53. doi: 10.1016/j.healthplace.2013.04.005. Epub 2013 Apr 29. [DOI] [PubMed] [Google Scholar]
- 29.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–7. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models. Understanding Stat. 2002;1(4):223–31. [Google Scholar]
- 31.Larsen K, Petersen JH, Budtz-Jørgensen E, Endahl L. Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–14. doi: 10.1111/j.0006-341x.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 32.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–8. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa S, Schielzeth H. A general and simple method for obtaining R from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–42. Epub 2012 Dec 3. [Google Scholar]
- 34.Dekker AR, Verheij TJ, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patients. Fam Pract. 2015;32(4):401–7. doi: 10.1093/fampra/cmv019. Epub 2015 Apr 24. [DOI] [PubMed] [Google Scholar]
- 35.Cordoba G, Siersma V, Lopez-Valcarcel B, Bjerrum L, Llor C, Aabenhus R, et al. Prescribing style and variation in antibiotic prescriptions for sore throat: cross-sectional study across six countries. BMC Fam Pract. 2015;16:7. doi: 10.1186/s12875-015-0224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]