Abstract
Objective
To examine the mortality risk presented by normal-weight central obesity, to identify a clinical measure to aid in the identification of this phenotype, and to explore the means for mitigation of this risk.
Quality of evidence
Only prospective cohort studies (level II) comparing participants with central obesity at normal weight with those at higher levels of body mass index (BMI) were found. Good level I studies were available to demonstrate the effect of diet and exercise interventions on central obesity and mortality.
Main message
Participants with atherogenic dyslipidemia who are centrally obese at normal BMI are at similar, and possibly higher, mortality risk compared with those who are centrally obese and overweight or obese according to their BMI. Waist-to-height ratio might be the most pragmatic clinical measure of central obesity. The Mediterranean diet is an effective intervention to prevent ongoing weight gain while reducing abdominal girth. Low levels of exercise can also reduce waist circumference. Weight loss need not be an objective.
Conclusion
A waist-to-height ratio exceeding 0.5 at normal BMI identifies elevated mortality risk for cardiometabolic disease. This risk might equal or exceed that of centrally obese patients who are overweight or obese. Modest dietary and exercise interventions can be effective in mitigation of this risk.
The World Health Organization defines obesity as “abnormal or excessive fat accumulation that may impair health.”1 This document concedes that body mass index (BMI) can only be a rough guide to the degree of adiposity. People with normal BMI can have a proportion of body fat exceeding 30%.2 If this fat is distributed primarily as central or visceral fat, it is strongly associated with cardiometabolic risk.3 Such people have abnormal adipose tissue distribution and function, with increased risk of diabetes and cardiovascular disease. These abnormally functioning fat deposits can produce atherosclerotic, dysmetabolic, and mechanical challenges leading to deteriorating health,4 thus fulfilling the World Health Organization criteria for obesity.
Rates of obesity in North America have continued to rise since the 1980s. As of 2015, 38.2% of the adult population in the United States (US) and 25.8% of the adult population in Canada is obese.5 Between 1990 and 2015, rates of ischemic heart disease fell 55% in the US and 60% in Canada5; however, while Canadian rates continue to fall, decline in the US has leveled off since 2011.6,7 Uniquely among Organisation for Economic Co-operation and Development countries, US life expectancy has begun to fall in the past few years,7 driven in part by rising obesity and social inequality.8
Prevalence of the normal-weight metabolically obese phenotype by direct measurement of fat distribution or metabolic characteristics might vary between 13% and 38%.9,10 Truncal fat, as estimated by waist circumference (WC), waist-to-hip ratio, or waist-to-height ratio (WHtR) (Table 1),11–15 is positively correlated with metabolic abnormalities, while fat in the lower body has a negative correlation. Subcutaneous fat, particularly in the femorogluteal area, might provide a depot helping to prevent lipid deposition at intra-abdominal and visceral sites, where it might be more damaging.16–18 Prevalence of abdominal obesity as measured by WC is currently rising faster than general obesity as measured by BMI.19,20
Table 1.
Anthropometric measures of body fat distribution: A) Mass-based and B) distribution-based measures.
| A) | ||
|---|---|---|
| MASS-BASED MEASURE | DEFINITION | COMMENTS |
| Body mass index11 | Weight in kg divided by the square of the height in m | Does not distinguish between lean and fat tissue mass |
| • Underweight | < 18.5 kg/m2 | Associated with higher mortality |
| • Normal weight • Overweight |
18.5–24.9 kg/m2 25.0–29.9 kg/m2 |
Lowest mortality associated with these categories |
| • Obesity class 1 | 30.0–34.9 kg/m2 | No consistent association with increased mortality |
| • Obesity class 2 • Obesity class 3 |
35.0–39.9 kg/m2 ≥ 40.0 kg/m2 |
Direct association with increased mortality |
| B) | ||
|---|---|---|
| DISTRIBUTION-BASED MEASURES | VALUES REPRESENTING INCREASED RISK | SURROGATE MEASURES OF CENTRAL OR VISCERAL ADIPOSITY |
| Waist circumference | Females ≥ 80 cm Males ≥ 95 cm |
Cut points vary according to ethnicity, sex, and age12 |
| Waist-to-hip ratio | Females ≥ 0.85 Males ≥ 0.95 |
Cut points not well established for ethnicity13 |
| Waist-to-height ratio | Increased risk 0.50–0.60 Substantial risk > 0.60 |
Cut points the same for ethnicity, sex, and age12 Best predicts visceral fat mass14,15 |
Objectives for this review are as follows:
to quantify the mortality risk posed by normal-weight central obesity;
to identify a pragmatic clinical measure to aid in identifying those at risk; and
to explore mitigation of this risk.
Quality of evidence
The initial PubMed search included the following MeSH headings and key words: normal weight and central obesity (title and abstract) or visceral obesity (title and abstract) or visceral fat (title and abstract) or ectopic fat (title and abstract) or hypertriglyceridemic waist (title and abstract) or metabolically obese (title and abstract) or abdominal adiposity (title and abstract) or waist (title and abstract) and mortality not cancer. References from appropriate retrieved papers were also scanned. Key word searches were also done in Google Scholar and the Cochrane database. No level I studies were found. All included studies were observational, and pertinent prospective cohort studies or systematic reviews were selected.
Main message
Visceral obesity is uniquely atherogenic.
Central or abdominal obesity is contained in discrete compartments. Subcutaneous fat can be considerable over the abdominal area. Visceral intra-abdominal fat collects in the omentum, mesentery, liver, and pancreas. This visceral fat can be found at extra-abdominal sites such as the pericardium, myocardium, and skeletal muscle as well.21 Retroperitoneal fat can also contribute to abdominal girth. The intra-abdominal compartment, together with ectopic fat in other organs of the body and in skeletal muscle, constitutes metabolically active visceral fat, which behaves quite differently from subcutaneous fat.22 Their differing characteristics are summarized in Table 2.15–18,22–28
Table 2.
Characteristics of subcutaneous and visceral fat
| VARIABLES | CHARACTERISTICS | |
|---|---|---|
|
| ||
| SUBCUTANEOUS FAT | VISCERAL FAT | |
| Clinical measurement | Body mass index | Waist circumference, waist-to-height ratio, waist-to-hip ratio |
| Association with cardiometabolic disease16 | Association with mortality is inconsistent | Direct linear association with mortality |
| Function15,18 | Metabolic sink and longer-term energy storage | Short-term energy source |
| Cardiac risk23 | Moderate | High |
| Metabolic risk24 | Moderate | High |
| Inflammation22 | Moderate | High |
| Catecholamine response22 | Moderate | Rapid |
| Insulin sensitivity17 | Moderate | Low |
| Metabolic flux18 | Low | High |
| Trend with age25 | Increased to age 65 y, then reduced | Gradual increase |
| Storage duration26 | Long | Short |
| Effect of exercise27 | High levels needed for weight change | Low levels effective for cardiometabolic benefit |
| Adverse effects of refined carbohydrate28 | Moderate | High |
It is still not clear whether a population trend to increasing hyperinsulinemia is instrumental as a cause of obesity or whether established obesity is a cause of hyperinsulinemia.29 A central factor is the rising sugar content of the food supply. Apart from sugar added in home preparation, 66% of Canadian packaged foods and beverages now contain added sugars.30 This triggers insulin release, which drives circulating lipid into adipocytes to be stored as fat. Increasing fat in turn induces insulin resistance, and even higher levels of insulin are required to drive glucose into cells. Some individuals can store more fat peripherally than others, and as these deposits become replete, fat begins to be deposited in visceral sites as well, appearing in the liver, omentum, skeletal muscle, and peripheral organs. The insulin-resistant state tends to accelerate lipolysis, releasing free fatty acids (FFAs) into circulation. Visceral adipocytes are also very sensitive to catecholamine-induced lipolysis, releasing FFAs into the portal circulation and presenting the liver with increased lipid for processing.28 Levels of these FFAs in circulation are high in the abdominally obese. Although they can be used as a substrate for energy production, they also contribute to insulin resistance, inhibiting glucose uptake by muscle and other organs, further contributing to hyperglycemia.18
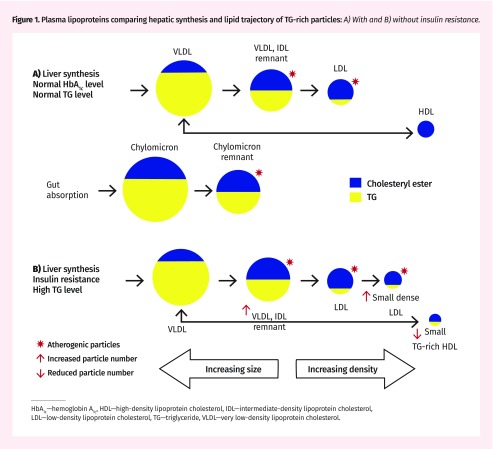
Free fatty acid lipid is combined with glycerol to form triglyceride (TG) and then packaged by the liver in water-soluble form as very low-density lipoprotein (VLDL) particles. These particles are large and contain predominantly TG, along with some cholesterol. Triglyceride, being an energy substrate, is hydrolyzed in various tissues, and these particles gradually become smaller and more dense, forming intermediate-density lipoprotein (IDL) and low-density lipoprotein (LDL) cholesterol particles.31 As particles become smaller they contain an increasing proportion of cholesterol, which is not an energy substrate (Figure 1A).
Figure 1.
Plasma lipoproteins comparing hepatic synthesis and lipid trajectory of TG-rich particles: A) With and B) without insulin resistance.
HbA1c—hemoglobin A1c, HDL—high-density lipoprotein cholesterol, IDL—intermediate-density lipoprotein cholesterol, LDL—low-density lipoprotein cholesterol, TG—triglyceride, VLDL—very low-density lipoprotein cholesterol.
In the presence of insulin resistance and hypertriglyceridemia the characteristics of these particles change. The VLDL and IDL remnant particle numbers increase, and LDL particles become even smaller, more dense, and much more numerous. All these particles easily penetrate the vascular endothelium, where they become intensely atherogenic. At the same time, high-density lipoprotein (HDL) particles, which are involved in reverse cholesterol transport, tend to become TG rich and smaller, to the extent that some are lost in the urine. The result is a smaller number of HDL particles, which cannot contain as much cholesterol for clearance from the vasculature (Figure 1B).21,32
A patient with this metabolic profile is likely to have high TG, low and dysfunctional HDL, and increased numbers of small LDL particles, characteristic of atherogenic dyslipidemia.33,34 Low-density lipoprotein is not reliable for risk evaluation, as much of the cholesterol might be hiding in VLDL, IDL, and other remnant lipoproteins, including chylomicron remnants in the nonfasting state. These particles are at least as potent as LDL in predicting cardiovascular events.35 The most common clinical characteristic is increased visceral fat as indicated by central obesity.36
Clinical evaluation of central obesity.
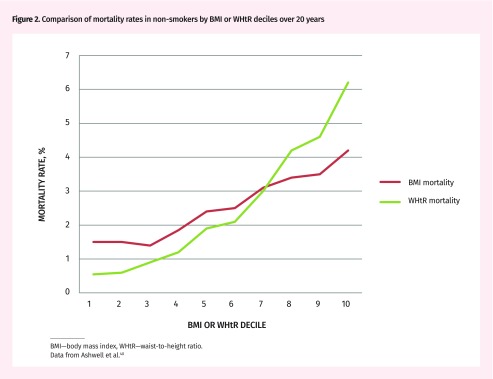
There is consensus that an anthropometric measure of abdominal or central obesity is a better predictor of cardiometabolic risk, diabetes risk, and all-cause mortality than BMI is, and that combining the 2 indices might be even better. Usually, mortality risk plotted against BMI is J shaped, with increased mortality only in underweight and high class 1 to class 3 obesity (Table 1).11–15 Being overweight carries consistently lower mortality risk than being normal weight does.37 Removal of competing causes of mortality (smoking, comorbid disease, advanced age) tends to remove the steep increase in the underweight category and move the nadir for mortality into the normal BMI range.38 The relation between BMI and mortality then becomes more progressive and linear. A similar plot of mortality against waist-to-hip ratio, WC, or WHtR typically shows a much more steeply progressive linear relation, allowing a more granular assessment of mortality risk (Figure 2).39,40 There are mixed opinions in the literature as to which of these central obesity measurements is best, but, of the systematic reviews and meta-analyses comparing the 3,12,14,23,39 WHtR is favoured. There are pragmatic reasons for use of WHtR as well:
It can be used without the aid of a scale or reference to BMI categories.
The cutoff value for WHtR is 0.5 and is the same regardless of sex and ethnic origin, although children might require further study.39
It allows simple home monitoring. Patients can be told, “Your waist should not exceed half your height.”
It is easily used in austere settings with minimal equipment.41 A piece of string representing patient height can be retained, folded in half, and periodically pulled around the waist.42
Figure 2.
Comparison of mortality rates in non-smokers by BMI or WHtR deciles over 20 years
BMI—body mass index, WHtR—waist-to-height ratio.
Data from Ashwell et al.40
Mortality risk presented by normal-weight central obesity.
Available prospective studies documenting all-cause mortality associated with increasing central obesity at any given BMI are presented in Table 3.39,43–57 The striking finding is that, with one exception,51 the mortality risk of those with central obesity at normal BMI is similar to or greater than the risk of those with central obesity who are overweight or obese. In most cases these normal-weight participants have the highest mortality of any group combination of body mass and central fat distribution. In some studies the magnitude of this difference is statistically significant.46,52,54 The implication of this is that normal-weight centrally obese people might be at uniquely high risk of all-cause mortality.
Table 3.
Prospective observational studies and related SRs and MAs of studies relating normal-weight central obesity to mortality
| STUDY AND YEAR | STUDY TYPE (DURATION) | STUDY OBJECTIVES | NO. AND AGE OF PARTICIPANTS | RESULTS | COMMENTS |
|---|---|---|---|---|---|
| Coutinho et al, 201343 | SR of prospective observational studies and collaborative analysis (0.5–7.4 y) | Relation of measures of central obesity to mortality | 15 923 patients with known CAD Mean (SD) age 65.7 (11.5) y | Highest mortality category combined lowest BMI with highest WHR or WC; HR = 1.7 | Central obesity associated with equal increase in mortality risk in lean and obese patients. Increased BMI was associated with reduced risk in these patients |
| Kramer et al, 201344 | MA of prospective observational studies (3–30 y) | Relation of cardiometabolic risk and BMI category to CVD events and all- cause mortality | 61 386 adults from the general population Mean age range 44–60 y |
Highest mortality or CVD event rate was similar in metabolically unhealthy normal-weight and obese participants; HR = 2.65 compared with those of normal weight and metabolic health | Did not specifically consider central obesity. Metabolic health was based on absence of metabolic syndrome components, insulin resistance, or inflammatory markers |
| Carmienke et al, 201339 | SR and regression MA of prospective cohorts (5–24 y) | Relation of measures of abdominal obesity parameters to mortality | 689 465 healthy adults Age ≥ 18 y |
Highest mortality category combined lowest BMI with highest WHR or WC | WHR, WC, or WHtR combined with BMI gives best mortality prediction. In participants > 65 y there was a non-significant or negative association with increasing BMI, WC, and WHR |
| Folsom et al, 200045 | Prospective observational study (11–12 y) | Relation of BMI, WC, and WHR to mortality, cancer, diabetes, hypertension, and fracture | 31 702 healthy US women Age 55–69 y |
Highest mortality category combined lowest BMI with highest WHR | WHR had the best mortality prediction |
| Pischon et al, 200846 | Prospective observational study (9.7 y) | Association of distribution of adiposity with risk of death | 359 387 participants from general European population Age 25–70 y |
Highest mortality associated with lowest BMI percentile having highest WC or WHR | Association of BMI with mortality is J shaped. Measures of central obesity showed positive linear association with mortality when adjusted for BMI |
| Zhang et al, 200847 | Prospective observational study (16 y) | Relation of abdominal adiposity to premature death | 44 636 US women from the Nurses’ Health Study Age 30–55 y |
Highest mortality category combined highest BMI and highest WC or WHR. Risk was only slightly lower for normal BMI with high central obesity | WC and WHR were both directly associated with mortality |
| Koster et al, 200848 | Prospective observational study (9 y) | Relation of WC to all-cause mortality | 245 533 US adults Age 51–72 y |
Normal weight with high WC increased mortality by 22%. Exceeded only by class 2 and 3 obesity with high WC |
WC used as measure of central obesity in mortality prediction |
| Reis et al, 200949 | Prospective observational study (12 y) | Relation of BMI, WHR, and WTR to mortality | 13 065 participants from the general US population Age 30–102 y |
Highest mortality category combined lowest BMI with highest WHR or WTR | WHR or WTR had the best mortality prediction. No association at > 65 y of age |
| Romero-Corral et al, 200950 | Prospective observational study (8.8 y) | Relation of body fat percentage to cardiovascular mortality and metabolic dysregulation in participants with normal BMI | 6171 US patients with normal BMI and CAD Age ≥ 20 y |
High body fat percentage and WC were associated with increased risk of metabolic syndrome. CVD mortality increased in normal-weight obese women (HR = 2.20) compared with nonobese women | Body fat measured by bioimpedance. Body fat percentage did not correlate with all-cause mortality in women or men at any BMI |
| Staiano et al, 201251 | Prospective observational study (13 y) | Relation of BMI, WC, and WHR to CVD and all-cause mortality | 8061 Canadian adults Age 18–74 y |
No mortality increase with WC in normal-weight adults. Highest mortality in highest WC terciles of obese adults | WC had the best association with CVD and all-cause mortality |
| Thomas et al, 201352 | Prospective observational study (mean [SD] 5.6 [2.4] y) | Relation of obesity measured by BMI or WC on mortality at differing ages | 119 010 French participants with BMI > 20 kg/m2 Age 17–85 y |
Mortality at BMI 20–25 kg/m2 and WC ≥ 102 cm ...
No association at age > 65 y |
Mortality risk higher for central obesity in normal BMI range than in class 2 and 3 obesity. WC gives best mortality prediction at < 65 y of age. Neither WC nor BMI are useful in the elderly |
| Cerhan et al, 201453 | Pooled data from 11 prospective observational studies (median 9 y, maximum 21 y) | Relation of WC to mortality across entire range of BMI categories | 650 386 non-Hispanic white adults Age 20–83 y |
Highest mortality associated with BMI ranges < 20 and ≥ 35 kg/m2 in those with highest WC. Highest WC in those with BMI 20–22.5 kg/m2 had higher mortality than those with class 1 obesity at highest WC | WC should be assessed in combination with BMI even in those in normal BMI range. WC is directly associated with mortality at all levels of BMI |
| Sahakyan et al, 201554 | Prospective observational study (14.3 y) | Relation of central obesity and survival in adults of normal body weight | 16 124 US adults with BMI ≥ 18.5 kg/m2 Age 18–90 y |
Men with normal-weight central obesity had higher mortality risk than any other BMI and WHR combination. Similar women had 40% and 32% relative risk increase compared with overweight and obese women without central obesity |
WHR used as measure of central obesity in mortality prediction |
| Klingberg et al, 201555 | Prospective observational study (average 6 y) | Relation of baseline WC and change in WC to morality and CVD | 2492 healthy Danish and Swedish women Age 44–74 y at baseline |
Association of mortality with both high baseline WC and large increase in WC over time; particularly high in those with BMI < 25 kg/m2 | WC used in mortality prediction. Hip circumference was unrelated to mortality |
| Sharma et al, 201656 | Prospective observational study (average 7.1 y) | Relation of WC or WHR and BMI to mortality in elderly patients with CVD | 7057 elderly patients with CAD Mean age 73 y |
Highest mortality in patients with normal BMI and central obesity | Highlights importance of including WC or WHR along with BMI when making adiposity-related mortality assessment |
| Hamer et al, 201757 | Prospective observational study (average 9 y) | Determine whether WHR is more predictive of mortality than BMI is | 42 702 UK adults Mean age 57.7 y |
Normal weight with central obesity showed highest mortality; HR = 1.22 for death | WHR used as measure of central obesity in mortality prediction |
BMI—body mass index, CAD—coronary artery disease, CVD—cardiovascular disease, HR—hazard ratio, MA—meta-analysis, SR—systematic review, UK—United Kingdom, US—United States, WC—waist circumference, WHR—waist-to-hip ratio, WHtR—waist-to-height ratio, WTR—waist-to-thigh ratio.
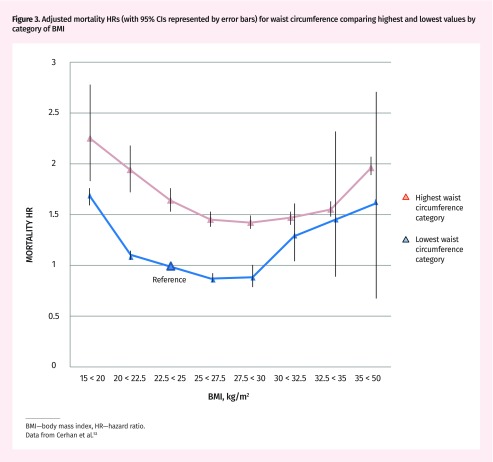
The other consistent finding from these studies is that, when central obesity is present, the existence of subcutaneous fat seems to offer protection. Centrally obese participants in the overweight and class 1 obesity BMI range actually have lower mortality risk than their normal-weight centrally obese counterparts (Figure 3).46–49,51–54,57 The limited capacity to store lipid subcutaneously might lead to a mortality disadvantage, particularly in those with the highest levels of visceral adipose tissue.58
Figure 3.
Adjusted mortality HRs (with 95% CIs represented by error bars) for waist circumference comparing highest and lowest values by category of BMI
BMI—body mass index, HR—hazard ratio. Data from Cerhan et al.53
Mitigating risk without weight loss.
It seems reasonable that interventions targeting visceral fat should target causation—primarily the environment of processed, calorie-dense foods and sedentary lifestyle.59 Weight loss need not be an objective. In a review of randomized and non-randomized trials primarily using exercise for weight control, 11 of 29 studies showed a significant reduction in visceral fat despite no clinically relevant weight loss.60 Other studies61 suggest that with exercise-induced weight management, visceral fat is preferentially lost in those with minimal weight loss, whereas higher weight-loss categories showed predominant loss of subcutaneous fat.
In addition to reduction of both visceral fat and some cardiometabolic risk factors, exercise is effective in preservation of muscle mass and facilitation of mobility.60 This is particularly important in the elderly, who might have difficulty maintaining weight, but tend to accumulate visceral fat and lose muscle mass and subcutaneous fat mass.62,63 Exercise is an essential component in minimizing central obesity and maintaining muscle mass. A meta-analysis of studies lasting 4 to 52 weeks27 showed that, with weight held stable, visceral adipose tissue is reduced 6.1% by exercise and only 1.1% by diet.
Each 5-cm reduction in WC is associated with reduction in mortality by as much as 9% over 6.7 years at any level of BMI.64 The risk of disability and mortality can be mitigated by exercise interventions well below targets currently recommended.19 Large cohort studies now exist demonstrating mortality reduction associated with walking 15 minutes a day65 or jogging 5 to 10 minutes a day.66 The focus in this population should be on reduction in central obesity and prevention of weight gain. Emphasis should be on small and achievable changes in behaviour. More intensive physical activity can remain an option, as the mechanical disadvantage of obesity in these patients is not an issue.
Dietary constituents can affect visceral fat. The PREDIMED (Prevención con Dieta Mediterránea) study was a randomized controlled trial comparing the Mediterranean diet with a low-fat diet over 4.8 years67; it showed statistically significant mortality reduction with the Mediterranean diet. Despite unrestricted intake, no weight gain was seen with the intervention. A secondary analysis68 revealed that those assigned to the Mediterranean diet were significantly more likely to no longer meet the criterion of central obesity compared with those in the control group (P < .001). As avoidance of weight gain is an objective along with reduction in visceral fat, the Mediterranean eating pattern could be a preferred option for those with normal-weight central obesity, particularly as it tends to be low in refined carbohydrate. It is important to recognize that the benefits of exercise might be completely negated by poor dietary choices.69
Increased WC can be an indication for statin therapy according to Canadian guidelines in men older than 50 years and women older than 60 years who remain at intermediate Framingham risk despite optimized individual uptake of lifestyle recommendations.70
Conclusion
Visceral obesity is increasing faster in the North American population than generalized obesity is and it has a more profound effect on morbidity and mortality. The simplest and most valid measure of central obesity is WHtR. This phenotype is closely linked to atherogenic dyslipidemia, which predisposes one to the deposition of cholesterol in the vascular endothelium and resultant atherosclerosis. Individuals with normal-weight central obesity are at equivalent, and possibly higher, risk than people with central obesity who are overweight or obese by BMI.
Reduction in central obesity and weight stabilization can be achieved with a Mediterranean-style eating pattern. Exercise with minimal weight loss can reduce visceral fat. Small changes in exercise and eating patterns can produce cardiometabolic and mortality benefit.
Editor’s key points
▸ Visceral obesity is increasing faster in the North American population than generalized obesity is and it has a more profound effect on morbidity and mortality. Individuals with a normal-weight body mass index and central obesity are at equivalent, and possibly higher, risk than people with central obesity who are overweight or obese by body mass index.
▸ The simplest and most valid measure of central obesity is waist-to-height ratio. The cutoff value is 0.5 and is the same regardless of sex and ethnic origin. It can be easily measured with minimal equipment. A piece of string representing patient height can be retained, folded in half, and periodically pulled around the waist.
▸ Reduction in central obesity and weight stabilization can be achieved with a Mediterranean-style eating pattern. Exercise with minimal weight loss can reduce visceral fat. Small changes in exercise and eating patterns can produce cardiometabolic and mortality benefit.
Footnotes
Competing interests
None declared
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de juin 2019 à la page e251.
References
- 1.World Health Organization . Obesity and overweight. Geneva, Switz: World Health Organization; 2018. Available from: www.wpro.who.int/mediacentre/factsheets/obesity/en/. Accessed 2018 Jan 22. [Google Scholar]
- 2.Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56(4):426–33. doi: 10.1016/j.pcad.2013.10.003. Epub 2013 Oct 5. [DOI] [PubMed] [Google Scholar]
- 3.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 4.Jensen M. Normal weight obesity. CMR eJournal. 2008;2(1):22–30. Available from: www.myhealthywaist.org/cmrejournal/pdf/vol2/v2i1a5.pdf. Accessed 2018 Jan 22. [Google Scholar]
- 5.OECD. Health at a glance 2017. Overweight and obesity among adults: OECD indicators. Paris, Fr: OECD Publishing; 2017. Available from: . Accessed 2018 Jan 22. [DOI] [Google Scholar]
- 6.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132(11):997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. Epub 2015 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Case A, Deaton A. Mortality and morbidity in the 21st century. Brookings Pap Econ Act. 2017;2017:397–476. doi: 10.1353/eca.2017.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323–35. doi: 10.1016/S0140-6736(16)32381-9. Epub 2017 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89(6):2569–75. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 10.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58(13):1343–50. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Kit BK, Orpana H, Graubard B. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–86. doi: 10.1111/j.1467-789X.2011.00952.x. Epub 2011 Nov 23. [DOI] [PubMed] [Google Scholar]
- 13.Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. 2010;64(1):42–61. doi: 10.1038/ejcn.2009.70. Epub 2009 Aug 12. [DOI] [PubMed] [Google Scholar]
- 14.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr Res Rev. 2010;23(2):247–69. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 15.Swainson MG, Batterham AM, Tsakirides C, Rutherford ZH, Hind K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS One. 2017;12(5):e0177175. doi: 10.1371/journal.pone.0177175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91(11):4459–66. doi: 10.1210/jc.2006-0814. Epub 2006 Aug 22. [DOI] [PubMed] [Google Scholar]
- 17.McCarty MF. A paradox resolved: the postprandial model of insulin resistance explains why gynoid adiposity appears to be protective. Med Hypotheses. 2003;61(2):173–6. doi: 10.1016/s0306-9877(02)00238-4. [DOI] [PubMed] [Google Scholar]
- 18.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35(1):83–92. doi: 10.1093/ije/dyi253. Epub 2005 Dec 8. [DOI] [PubMed] [Google Scholar]
- 19.Ross R, Blair S, de Lannoy L, Després JP, Lavie CJ. Changing the endpoints for determining effective obesity management. Prog Cardiovasc Dis. 2015;57(4):330–6. doi: 10.1016/j.pcad.2014.10.002. Epub 2014 Oct 25. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA. 2014;312(11):1151–3. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 22.Bays H, Blonde L, Rosenson R. Adiposopathy: how do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expert Rev Cardiovasc Ther. 2006;4(6):871–95. doi: 10.1586/14779072.4.6.871. [DOI] [PubMed] [Google Scholar]
- 23.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–53. doi: 10.1016/j.jclinepi.2007.08.012. Epub 2008 Mar 21. [DOI] [PubMed] [Google Scholar]
- 24.Lemieux I, Poirier P, Bergeron J, Alméras N, Lamarche B, Cantin B, et al. Hypertriglyceridemic waist: a useful screening phenotype in preventive cardiology? Can J Cardiol. 2007;23(Suppl B):23B–31B. doi: 10.1016/s0828-282x(07)71007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thibault R, Pichard C. The evaluation of body composition: a useful tool for clinical practice. Ann Nutr Metab. 2012;60(1):6–16. doi: 10.1159/000334879. Epub 2011 Dec 16. [DOI] [PubMed] [Google Scholar]
- 26.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 27.Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17(8):664–90. doi: 10.1111/obr.12406. Epub 2016 May 23. [DOI] [PubMed] [Google Scholar]
- 28.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129(3):557–70. doi: 10.1542/peds.2011-2912. Epub 2012 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lustig RH. Which comes first? The obesity or the insulin? The behavior or the biochemistry? J Pediatr. 2008;152:601–2. doi: 10.1016/j.jpeds.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Acton RB, Vanderlee L, Hobin EP, Hammond D. Added sugar in the packaged foods and beverages available at a major Canadian retailer in 2015: a descriptive analysis. CMAJ Open. 2017;5(1):E1–6. doi: 10.9778/cmajo.20160076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sniderman AD, Bergeron J, Frohlich J. Apolipoprotein B versus lipoprotein lipids: vital lessons from the AFCAPS/TexCAPS trial. CMAJ. 2001;164(1):44–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–30. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 33.Chapman MJ, Ginsberg HN, Amarenco P, Anderotti F, Borén J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–61. doi: 10.1093/eurheartj/ehr112. Epub 2011 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenenbaum A, Fisman EZ. “The metabolic syndrome…is dead”; these reports are an exaggeration. Cardiovasc Diabetol. 2011;10(1):11. doi: 10.1186/1475-2840-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varbo A, Freiberg JJ, Nordestgaard BG. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem. 2015;61(3):533–43. doi: 10.1373/clinchem.2014.234146. Epub 2015 Jan 20. [DOI] [PubMed] [Google Scholar]
- 36.Ross R, Després JP. Abdominal obesity, insulin resistance, and the metabolic syndrome: contribution of physical activity/exercise. Obesity (Silver Spring) 2009;17(Suppl 3):S1–2. doi: 10.1038/oby.2009.381. [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Childers DK, Allison DB. The ‘obesity paradox’: a parsimonious explanation for relations among obesity, mortality rate and aging? Int J Obes (Lond) 2010;34(8):1231–8. doi: 10.1038/ijo.2010.71. Epub 2010 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmienke S, Freitag MH, Pischon T, Schlattmann P, Fankhaenel T, Goebel H, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr. 2013;67(6):573–85. doi: 10.1038/ejcn.2013.61. Epub 2013 Mar 20. [DOI] [PubMed] [Google Scholar]
- 40.Ashwell M, Mayhew L, Richardson J, Rickayzen B. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS One. 2014;9(9):e103483. doi: 10.1371/journal.pone.0103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thaikruea L, Thammasarot J. Prevalence of normal weight central obesity among Thai healthcare providers and their association with CVD risk: a cross-sectional study. Sci Rep. 2016;6:37100. doi: 10.1038/srep37100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashwell M, Gibson S. A proposal for a primary screening tool: ‘keep your waist circumference to less than half your height.’. BMC Med. 2014;12:207. doi: 10.1186/s12916-014-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coutinho T, Goel K, Corrêa de Sá D, Carter RE, Hodge DO, Kragelund C, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity.”. J Am Coll Cardiol. 2013;61(5):553–60. doi: 10.1016/j.jacc.2012.10.035. Erratum in: J Am Coll Cardiol 2013;62(3):261. [DOI] [PubMed] [Google Scholar]
- 44.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–69. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 45.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160(14):2117–28. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 46.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714. Epub 2008 Mar 24. [DOI] [PubMed] [Google Scholar]
- 48.Koster A, Leitzmann MF, Schatzkin A, Mouw T, Adams KF, van Eijk JT, et al. Waist circumference and mortality. Am J Epidemiol. 2008;167(12):1465–75. doi: 10.1093/aje/kwn079. Epub 2008 Apr 15. [DOI] [PubMed] [Google Scholar]
- 49.Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity (Silver Spring) 2009;17(6):1232–9. doi: 10.1038/oby.2008.664. Epub 2009 Feb 5. [DOI] [PubMed] [Google Scholar]
- 50.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31(6):737–46. doi: 10.1093/eurheartj/ehp487. Epub 2009 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staiano AE, Reeder BA, Elliott S, Joffres MR, Pahwa P, Kirkland SA, et al. Body mass index versus waist circumference as predictors of mortality in Canadian adults. Int J Obes (Lond) 2012;36(11):1450–4. doi: 10.1038/ijo.2011.268. Epub 2012 Jan 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas F, Pannier B, Benetos A, Vischer UM. Visceral obesity is not an independent risk factor of mortality in subjects over 65 years. Vasc Health Risk Manag. 2013;9:739–45. doi: 10.2147/VHRM.S49922. Epub 2013 Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335–45. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. 2015;163(11):827–35. doi: 10.7326/M14-2525. Epub 2015 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klingberg S, Mehlig K, Lanfer A, Björkelund C, Heitmann BL, Lissner L. Increase in waist circumference over 6 years predicts subsequent cardiovascular disease and total mortality in Nordic women. Obesity (Silver Spring) 2015;23(10):2123–30. doi: 10.1002/oby.21203. Epub 2015 Sep 4. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S, Batsis JA, Coutinho T, Somers VK, Hodge DO, Carter RE, et al. Normal-weight central obesity and mortality risk in older adults with coronary artery disease. Mayo Clin Proc. 2016;91(3):343–51. doi: 10.1016/j.mayocp.2015.12.007. Epub 2016 Feb 6. [DOI] [PubMed] [Google Scholar]
- 57.Hamer M, O’Donovan G, Stensel D, Stamatakis E. Normal-weight central obesity and risk for mortality. Ann Intern Med. 2017;166(12):917–8. doi: 10.7326/L17-0022. Epub 2017 Apr 25. [DOI] [PubMed] [Google Scholar]
- 58.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32(6):1068–75. doi: 10.2337/dc08-2280. Epub 2009 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009;5(6):319–25. doi: 10.1038/nrendo.2009.78. [DOI] [PubMed] [Google Scholar]
- 60.Ross R, Janiszewski PM. Is weight loss the optimal target for obesity-related cardiovascular disease risk reduction? Can J Cardiol. 2008;24(Suppl D):25D–31D. doi: 10.1016/s0828-282x(08)71046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond) 2008;32(4):619–28. doi: 10.1038/sj.ijo.0803761. Epub 2008 Jan 8. [DOI] [PubMed] [Google Scholar]
- 62.Seidell JC, Pérusse L, Després JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74(3):315–21. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 63.Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull. 2011;97:169–96. doi: 10.1093/bmb/ldr002. Epub 2011 Feb 16. [DOI] [PubMed] [Google Scholar]
- 64.Berentzen TL, Jakobsen MU, Halkjaer J, Tjønneland A, Overvad K, Sørensen TI. Changes in waist circumference and mortality in middle-aged men and women. PLoS One. 2010;5(9):e13097. doi: 10.1371/journal.pone.0013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–53. doi: 10.1016/S0140-6736(11)60749-6. Epub 2011 Aug 16. [DOI] [PubMed] [Google Scholar]
- 66.Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64(5):472–81. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389. Epub 2018 Jun 13. [DOI] [PubMed] [Google Scholar]
- 68.Babio N, Toledo E, Estruch R, Ros E, Martínez-González MA, Castañer O, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186(17):E649–57. doi: 10.1503/cmaj.140764. Epub 2014 Oct 14. Expression of concern in: CMAJ 2018;190(26):E808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49(15):967–8. doi: 10.1136/bjsports-2015-094911. Epub 2015 Apr 22. [DOI] [PubMed] [Google Scholar]
- 70.Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263–82. doi: 10.1016/j.cjca.2016.07.510. Epub 2016 Jul 25. [DOI] [PubMed] [Google Scholar]