Abstract
The prediction of clinical outcome for patients with infiltrative gliomas is challenging. Although preoperative hematological markers have been proposed as predictors of survival in glioma and other cancers, systematic investigations that combine these data with other relevant clinical variables are needed to improve prognostic accuracy and patient outcomes. We investigated the prognostic value of preoperative hematological markers, alone and in combination with molecular pathology, for the survival of 592 patients with Grade II-IV diffuse gliomas. On univariate analysis, increased neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR), and decreased albumin-to-globulin ratio (AGR), all predicted poor prognosis in Grade II/III gliomas. Multivariate analysis incorporating tumor status based on the presence of IDH mutations, TERT promoter mutations, and 1p/19q codeletion showed that in lower-grade gliomas, high NLR predicted poorer survival for the triple-negative, IDH mutation only, TERT mutation only, and IDH and TERT mutation groups. NLR was an independent prognostic factor in Grade IV glioma. We therefore propose a prognostic model for diffuse gliomas based on the presence of IDH and TERT promoter mutations, 1p/19q codeletion, and NLR. This model classifies lower-grade gliomas into nine subgroups that can be combined into four main risk groups based on survival projections.
Keywords: glioma, hematological marker, inflammation, molecular group, prognosis
INTRODUCTION
Gliomas are the most common malignant primary brain tumors, accounting for 27% of all central nervous system (CNS) tumors [1]. According to the World Health Organization (WHO) classification of CNS tumors, gliomas are pathologically categorized into four grades, of which Grade II to IV are considered diffusely infiltrating gliomas [2, 3].
Research on molecular alterations in gliomas has revealed three noteworthy biomarkers, namely codeletion of chromosome arms 1p and 19q (1p/19q codeletion), and mutations in IDH and the TERT promoter, that can be used to classify Grade II-IV gliomas into five principal molecular groups (triple-positive, IDH and TERT mutations, IDH mutation only, triple-negative, and TERT mutation only). These groups are associated with distinct prognosis, germline variants, and median age at diagnosis, highlighting different pathogenic mechanisms [3]. Although most glioma patients receive standard treatments, significant variations in clinical outcomes are often seen due to the heterogeneity of the tumors [4]. Therefore, it is necessary to identify more appropriate and effective biomarkers for predicting prognosis in glioma patients.
Inflammation and immunity are critically involved in glioma initiation and progression [5, 6], and several studies demonstrated that inflammatory response cells such as neutrophils [7], lymphocytes [8] and platelets [9] are associated with the prognosis of cancer patients. In recent years, the prognostic value of preoperative hematological markers, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), median platelet volume (MPV), platelet distribution width (PDW), and albumin-to-globulin ratio (AGR), has been investigated in several cancers, including gliomas [10–17]. However, there is a lack of studies systematically investigating the prognostic value of hematological markers in a large cohort of gliomas, particularly in relation to the different molecular subtypes.
Therefore, we investigated the prognostic value of preoperative hematological markers (NLR, PLR, MLR, MPV, PDW, and AGR), alone and in combination with the 5 glioma molecular groups, on the clinical outcomes of a relatively large cohort (n = 592) of Grade II-IV glioma patients. Based on these findings, we propose a prognostic model for Grade II-IV infiltrative gliomas based on molecular pathology and NLR, and identify for lower-grade (WHO Grade II and III) gliomas, four risk groups with distinct overall survival. Further validation of the model in more extensive cohorts should confirm its usefulness and possibly open the way to new therapeutic strategies.
RESULTS
Clinico-pathological characteristics of the cohort
A total of 592 cases (adult patients, age ≥ 16) of WHO Grade II-IV supratentorial gliomas were analyzed. The median age of the cohort was 42 years (interquartile range = 39–58 years). There were 335 male patients (56.6%) and 257 female patients (43.4%). The cohort included 404 patients (68.2%) with Grade II-III glioma and 188 patients (31.8%) with Grade IV glioma. Median duration of follow-up was 32.0 months. Complete resection was achieved in 456 patients (77%), and incomplete resection was performed in 136 patients (23%). Four hundred and fifty-nine patients (77.5%) received postoperative primary radiation therapy (RT) and 342 patients (57.8%) received postoperative primary chemotherapy (CHT). In patients with astrocytoma, 14 (9.0%) received postoperative primary RT, 10 (6.5%) received postoperative primary CHT, 113 (72.9%) received postoperative primary RT and CHT, and 18 (11.6%) received no postoperative treatment. Among patients with oligodendroglioma or oligoastrocytomas, 31 (12.5%) received postoperative primary RT, 24 (9.7%) received postoperative primary CHT, 166 (66.9%) received postoperative primary RT and CHT, and 27 (10.9%) received no postoperative treatment (Supplementary Table 3). Molecular pathology analyses were available for 573 cases. IDH mutations were found in 246 cases (42.9%), mutations in TERT promoter were detected in 286 cases (49.9%), and chromosome 1p/19q codeletion was detected in 139 cases (34.4%). Hematological markers were defined in 528/592 cases, as 64 cases were excluded due to conditions that could influence peripheral blood counts. Detailed information on the clinico-pathological features of the cohort is listed in Supplementary Table 1.
Molecular groups
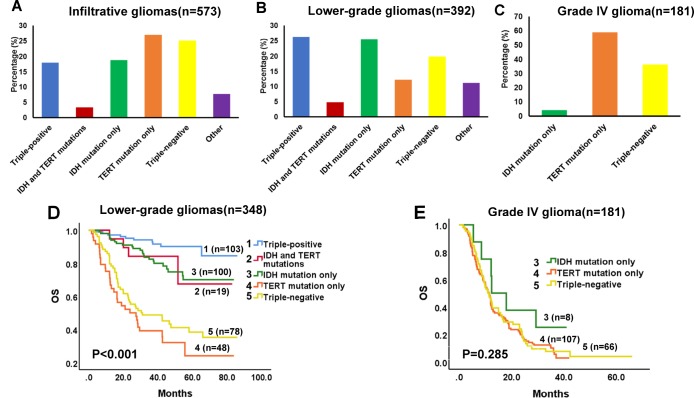
Among the 573 cases of Grade II-IV gliomas, 103 (18.0%) were triple-positive, 19 (3.3%) had mutations in both IDH and TERT, 108 (18.8%) had IDH mutation only, 144 (25.1%) were triple-negative, 155 (27.1%) had TERT mutation only, and 44 (7.7%) had other combinations of the three biomarkers (Figure 1A). For lower-grade glioma cases (n = 392), 103 (26.3%) were triple-positive, 19 (4.8%) had both IDH and TERT mutations, 100 (25.5%) had IDH mutation only, 48 (12.24%) had TERT mutation only, 78 (19.9%) were triple-negative, and 44 (11.2%) had other combinations (Figure 1B). For Grade IV glioma cases (n = 181), 8 (4.4%) had IDH mutation only, 107 (59.1%) had TERT mutation only, and 66 (36.5%) were triple-negative (Figure 1C). Univariate survival analysis demonstrated that molecular groups significantly influenced the OS of patients with lower-grade gliomas. The triple-positive group had favorable prognosis, whereas the TERT mutation group had a dismal survival expectancy (Figure 1D, univariate analysis in Supplementary Table 2), although this relationship was not found for Grade IV gliomas (Figure 1E, Supplementary Table 5). In subsequent multivariate analysis, molecular group was revealed as an independent prognostic factor in lower-grade gliomas (Table 1).
Figure 1.
Proportion and Kaplan-Meier survival analyses of molecular groups in diffuse infiltrative gliomas. Survival proportions in infiltrative (Grade II-IV) gliomas (A), lower-grade (Grade II-III) gliomas (B), and Grade IV glioma (C). (D) Kaplan-Meier OS curves in lower-grade gliomas. OS estimates for the 5 molecular groups are significantly different (P < 0.001). (E) Kaplan-Meier OS curves in Grade IV glioma. No differences in OS were detected for the 3 molecular groups (P = 0.285).
Table 1. Multivariate analysis of adjusting putative prognostic factors for molecular group (n=348a) and risk group (n=348a) in lower-grade gliomas.
| Factors | OS | |
| HR (95%CI) | P-value | |
| Molecular group | 1.578 (1.366–1.822) | <0.001 |
| Age | 1.883 (1.220–2.908) | 0.004 |
| Extent of resection | 1.262 (0.813–1.960) | 0.300 |
| RT (Yes or No) | 1.770 (1.147–2.732) | 0.010 |
| Grade (II or III) | 3.408 (2.300–5.049) | <0.001 |
| KPS (≤80 or >80) | 0.693 (0.478–1.006) | 0.054 |
| Risk group | 1.214 (1.149–1.283) | <0.001 |
| Age (≤40 or >40) | 1.630 (1.061–2.504) | 0.026 |
| Extent of resection | 1.122 (0.721–1.745) | 0.610 |
| RT (Yes or No) | 1.772 (1.153–2.724) | 0.009 |
| KPS (≤80 or >80) | 0.742 (0.510–1.079) | 0.118 |
| Grade (II or III) | 3.112 (2.091–4.634) | <0.001 |
a12 cases were excluded due to unavailability of FFPE tissues of the tumors, and 44 cases of other combinations of the three molecular markers were excluded.
Molecular group: the five molecular groups based on the statuses of IDH mutations, TERT promoter mutations and 1p/19q co-deletion, which include triple positive, IDH and TERT mutations, IDH mutation only, TERT mutation only and triple negative).
Risk group: the four groups based on the statuses of IDH mutations, TERT promoter mutations, 1p/19q codeletion and the levels of NLR in each molecular subgroup
OS: overall survival
RT: radiation therapy, indicating postoperative radiation therapy after first operation
KPS: Karnofsky Performance Status
HR: Hazard-ratio
Prognostic value of hematological markers in lower-grade and Grade IV gliomas
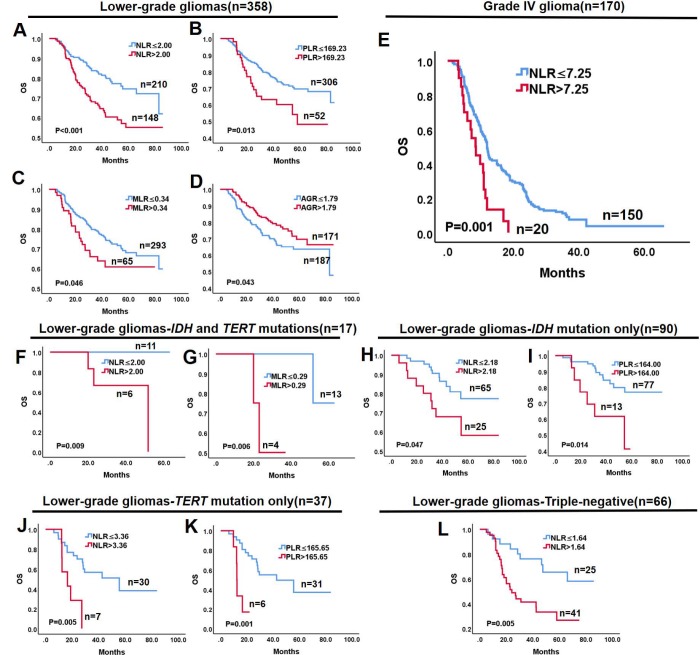
The prognostic value of the hematological markers was evaluated in lower-grade gliomas and Grade IV gliomas. Optimal cut-off values of NLR, PLR, MLR, MPV, PDW, and AGR were computed by X-tile software. Univariate analysis demonstrated that high NLR (P < 0.001), PLR (P = 0.013), and MLR (P = 0.046), and low AGR (P = 0.043) were associated with shorter survival in lower-grade gliomas (Figure 2A–2D), while MPV (P = 0.204) and PDW (P = 0.422) had no prognostic significance. Because hematological markers were strongly correlated and interfered with each other [14], they were separately analyzed with other potential prognostic factors in multivariate analysis. The latter revealed that NLR (P = 0.046, Table 2) is a prognostic factor for lower-grade gliomas independent of age, extent of resection, and adjuvant therapies. Conversely, neither PLR (P = 0.102), MLR (P = 0.188), nor AGR (P = 0.621) were independent prognostic factors (Supplementary Table 4). In Grade IV gliomas, only NLR emerged as a significant prognostic factor in univariate (P = 0.001, Figure 2E) and multivariate (P = 0.002, Table 3) analyses.
Figure 2.
Kaplan-Meier survival curves of glioma subgroups based on hematological markers. Lower-grade gliomas: (A) NLR > 2.00 is associated with worse OS (P < 0.001); (B) PLR > 169.23 is associated with worse OS (P = 0.013); (C) MLR > 0.34 is associated with worse OS (P = 0.046); and (D) AGR > 1.79 is associated with better OS (P = 0.043). (E) In Grade IV glioma, NLR > 7.25 is associated with better OS (P = 0.001). (F, G) In the IDH and TERT mutations group of lower-grade gliomas, NLR > 2.00 and MLR > 0.29 predict worse OS (P < 0.009 and P = 0.006, respectively). (H, I) In the IDH mutation only group of lower-grade gliomas, NLR > 2.18 and PLR > 164.00 predict worse OS (P = 0.047 and P = 0.014, respectively). (J, K) In the TERT mutation only group of lower-grade gliomas, NLR > 3.36 and PLR > 165.65 predict worse OS (P = 0.005 and P = 0.001, respectively). (L) In triple-negative lower-grade gliomas, NLR > 1.64 predicts worse OS.
Table 2. Multivariate analysis of adjusting putative prognostic factors for NLR (n=358a) in lower-grade gliomas.
| Factors | OS | |
| HR (95%CI) | P-value | |
| NLR | 1.502 (1.007–2.240) | 0.046 |
| Age | 1.042 (1.024–1.060) | <0.001 |
| Extent of resection | 0.907 (0.540–1.524) | 0.713 |
| RT (Yes or No) | 0.860 (0.514–1.440) | 0.567 |
| Grade (II or III) | 3.746 (2.499–5.618) | <0.001 |
| KPS (≤80 or >80) | 0.618 (0.416–0.916) | 0.017 |
a46 cases were excluded due to conditions that could influence hematological makers
OS: overall survival
RT: radiation therapy, indicating postoperative radiation therapy after first operation
KPS: Karnofsky Performance Status
HR: Hazard-ratio
Table 3. Multivariate analysis of adjusting putative prognostic factors for NLR in Grade IV glioma (n=170a).
| Factors | OS | |
| HR (95%CI) | P-value | |
| NLR | 2.228 (1.329–3.733) | 0.002 |
| Extent of resection | 2.815 (1.952–4.059) | <0.001 |
| RT (Yes or No) | 1.213 (0.772–1.907) | 0.402 |
| CHT (Yes or No) | 1.339 (0.871–2.061) | 0.184 |
| Age (≤62 or >62) | 1.587(1.103–2.284) | 0.013 |
a18 cases were excluded due to conditions that could influence hematological makers
OS: overall survival
RT: radiation therapy, indicating postoperative radiation therapy after first operation
CHT: chemotherapy, indicating postoperative chemotherapy after first operation
HR: Hazard-ratio
Prognostic value of hematological markers within molecular groups of lower-grade gliomas
Since molecular subtype is an independent factor influencing survival in lower-grade gliomas but not Grade IV glioma, we evaluated the prognostic value of hematological markers for each molecular group in lower-grade gliomas. Optimal cut-off values for NLR, PLR, MLR, and AGR in each group were computed by X-tile software. As shown in Figure 2F–2L, univariate analysis showed that high NLR predicted shorter OS in lower-grade glioma groups defined by IDH and TERT mutations (P = 0.009), IDH mutation only (P = 0.047), TERT mutation only (P = 0.005), and in the triple-negative group (P = 0.005). In turn, high PLR predicted shorter OS in the IDH mutation only (P = 0.014) and TERT mutation only (P = 0.001) groups, while high MLR was associated with shorter OS in gliomas with IDH and TERT mutations (P = 0.006). In contrast, none of the hematological markers impacted OS in the triple-positive group of lower-grade gliomas (Supplementary Figure 1A–1D). Likewise, no prognostic significance was found for PLR and AGR in the IDH and TERT mutation group (Supplementary Figure 1E, 1F), MLR and AGR in the IDH mutation only group (Supplementary Figure 1G, 1H), MLR and AGR in the TERT mutation only group (Supplementary Figure 2A, 2B), and PLR, MLR, and AGR in the triple-negative group (Supplementary Figure 2C–2E) of lower-grade gliomas.
A glioma prognostic model combining molecular pathology and hematological markers
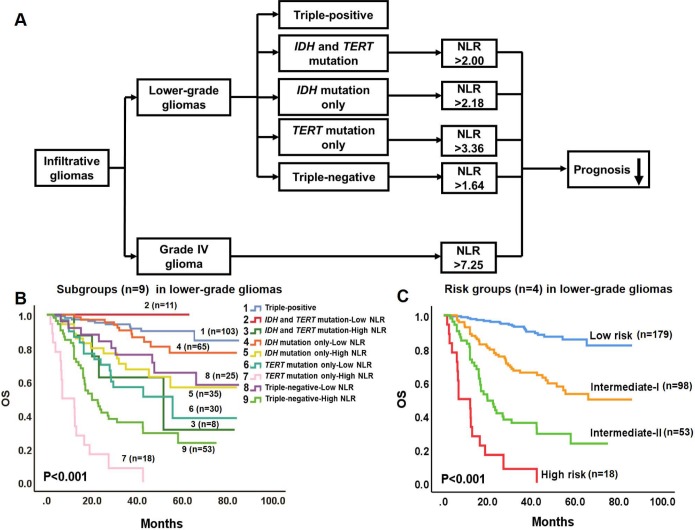
Based on combined data derived from survival analyses of molecular pathology and hematological markers, we propose a prognostic model to predict survival in glioma patients (Figure 3A). In the model, infiltrative gliomas include lower-grade gliomas and Grade IV glioma. Lower-grade gliomas were divided into 5 primary molecular groups associated with distinct OS [3]. Since NLR is a prognostic factor independent of putative clinical variables in lower-grade gliomas, and predicts survival in 4 tumor subtypes (triple-negative, IDH and TERT mutations, IDH mutation only, and TERT mutation only), this hematological marker is proposed to further stratify the prognosis of these 4 molecular groups. In contrast, high NLR arises as an independent predictor of worse survival for Grade IV glioma.
Figure 3.
Prognostic model combining molecular pathology and hematological markers for gliomas. (A) In the model, infiltrative gliomas include lower-grade gliomas and Grade IV glioma. Lower-grade gliomas are divided into 5 molecular groups with different OS. NLR is proposed to further stratify the OS of 4 of these groups (triple-positive tumors are excepted). In Grade IV glioma, high NLR independently predicts worse OS. (B) Kaplan-Meier OS curves of the 9 subgroups of lower-grade gliomas. The OS of the 9 subgroups are significantly different (P < 0.001). (C) Kaplan-Meier OS curves of the 4 risk groups in lower-grade gliomas: Subgroups 1, 2, and 4 were integrated into a Low risk group; Subgroups 3, 5, 6, and 8 were integrated into an Intermediate-I risk group; Subgroup 9 comprises the Intermediate-II risk group; and Subgroup 7 constitutes the High risk group. The OS of the 4 risk groups differs significantly (P < 0.001).
Molecular pathology and NLR stratify lower-grade gliomas into four risk groups
According to the prognostic model proposed in Figure 3A, lower-grade gliomas were categorized into nine subgroups based on the status of IDH and TERT promoter mutations, 1p/19q codeletion, and NLR. Survival analyses revealed significantly different OS for these nine subgroups (P < 0.001, Figure 3B, Supplementary Table 6). Furthermore, subgroups with non-significant differences in OS between them were integrated into individual risk groups: Subgroups 1, 2, and 4 conformed the Low risk group, Subgroups 3, 5, 6, and 8 conformed the Intermediate-I risk group, Subgroup 9 was defined as the Intermediate-II risk group, and Subgroup 7 represented the High risk group (Figure 3B, 3C). Univariate (P < 0.001, Figure 3C, Supplementary Table 7) and multivariate (P < 0.001, Table 1) analyses yielded significantly different OS for these four risk groups.
DISCUSSION
In the present study, data from a large cohort of gliomas (n = 592) were used to corroborate previous findings on the 5 glioma molecular groups defined by three robust markers, 1p/19q codeletion, IDH mutations, and TERT promoter mutations [2] and to demonstrate, for lower-grade gliomas, the differential prognostic value of hematological markers in each molecular group. Based on these findings, we propose a prognostic model for infiltrative gliomas that combines molecular and hematological markers.
The involvement of tumor-associated inflammatory cells in carcinogenesis has been firmly established [5, 7]. Cancer cells secrete chemokines and cytokines that attract host inflammatory cells such as neutrophils and lymphocytes, and these cells produce in turn proinflammatory cytokines, growth factors, and chemokines that contribute to tumor progression [18–20]. Unlike genetic biomarkers, preoperative hematological markers can be easily calculated from routine blood tests and may have important clinical significance for cancer prognosis. In recent years the prognostic value of NLR, PLR, MLR, and AGR has been investigated and corroborated in several cancers, such as hepatocellular carcinoma [21], pancreatic carcinoma [22], renal carcinoma [11], esophageal cancer [23], gastric carcinoma [24], colorectal cancer [25], lung cancer [26] and gliomas [14–17, 27, 28].
Our study demonstrated that NLR, PLR, MLR, and AGR are prognostic factors in univariate analysis for lower-grade gliomas. Also, for these tumors, multivariate analysis revealed that NLR is an independent prognostic factor after adjusting for age, grade, histology, extent of resection, and adjuvant therapies.
Studies demonstrated that neutrophil-induced immunosuppression can promote glioma progression, and that certain subsets of T-lymphocytes can instead inhibit it via induction of cytotoxic cell death and cytokine production [20, 29, 30]. Accordingly, Han et al. reported that high neutrophil and low CD3+ T-cell infiltration (elevated NLR) in glioblastomas was correlated with poorer outcomes [15]. Evidence for the importance of PLR in oncogenesis comes from studies showing that platelet activation contributes to tumor angiogenesis, disruption of the extracellular matrix and release of adhesion molecules to promote cancer cell proliferation and metastasis [31, 32]. As for AGR, its relevance in cancer may be related to the antioxidative effects of albumin against carcinogens such as nitrosamines and aflatoxins [33], and the association of elevated globulin levels with the progression and metastasis of some cancers [34].
Although predicting the clinical outcome of infiltrative gliomas is challenging, considerable progress in the classification of gliomas based on molecular markers has been made in the past several years [2, 3, 35–39]. Particularly, three robust molecular alterations, namely 1p/19q codeletion and IDH and TERT promoter mutations, were used to categorize five principal molecular groups of gliomas with distinct clinical traits and mechanisms of carcinogenesis [2]. Chromosome 1p/19q codeletion is associated with oligodendrogliomas, sensitivity to adjuvant therapies, and favorable survival [35, 40]. Mutations in IDH genes (IDH1 and IDH2) have been revealed in the majority of lower-grade gliomas and in secondary glioblastoma multiforme, and predict better survival [36, 41]. In a previous study we demonstrated that TERT promoter mutations could identify among lower-grade gliomas a group of IDH-mutated-1p/19q-intact tumors with better survival and a subset of IDH wild-type tumors with worse prognosis [42]. At present, the classification of infiltrative gliomas based on these three molecular markers is routinely conducted and of vital significance in clinical practice [3]. Based on this scheme, through multivariate survival analysis on 573 adult infiltrative gliomas we confirmed in lower-grade tumors the prognostic significance of the principal molecular groups independent of age, histology, and clinical variables. Our results further corroborate the findings reported by Eckel-Passow et al. [3] while adding several key clinical variables omitted in their research. Meanwhile, consistent with Eckel-Passow et al., for WHO Grade IV gliomas the molecular groups lacked independent prognostic significance.
We investigated for the first time, to the best of our knowledge, the prognostic value of hematological markers within the 5 primary glioma molecular groups and found that for lower-grade gliomas, high NLR and MLR predicted worse survival in the IDH and TERT mutations group, high NLR and PLR predicted worse survival in the IDH mutation only and TERT mutation only groups, and high NLR was associated with shorter survival in the triple-negative group. Interestingly, no predictive value was found for any hematological marker in triple-positive tumors. We speculate that any potential contribution to prognosis may be masked by the favorable survival characteristic of lower-grade gliomas within this molecular group. The differential prognostic values found for these hematological markers may be related to distinct immune microenvironments associated with specific molecular groups. For example, Qian et al. reported that immune responses in lower-grade gliomas are regulated by IDH mutations [43]. In Grade IV glioma, NLR was revealed as an independent prognostic factor in multivariate analysis, while the predictive values of PLR, MLR, and AGR were not significant in univariate analyses. We therefore developed a prognostic model for infiltrative gliomas by combining molecular and hematological markers. The model identified four risk groups based on molecular pathology and NLR in lower-grade gliomas, and two risk groups based on NLR in Grade IV glioma. The model only requires information of routine preoperative blood tests and molecular analysis of 1p/19q codeletion and IDH and TERT promoter mutations, which is also available in most medical centers. We think the model can be used readily and easily in the clinic, after corroboration from a multi-center, prospective clinical trial.
The present study has some limitations. First, due to the retrospective nature of the study, systematic bias might influence the accuracy of the results. Second, although the current study enrolled a relatively large sample size, it was carried out in a single research center. Thus, multi-center, prospective studies are necessary to corroborate our findings. Lastly, more extensive research is needed to clarify the detailed mechanisms through which hematological markers influence the prognosis of molecular groups in gliomas.
In summary, our study corroborates the prognostic significance of glioma subtypes based on 1p/19q codeletion and IDH and TERT promoter mutations in a large Chinese cohort. Moreover, we propose a novel prognostic model for diffuse infiltrative gliomas that combines molecular pathology and hematological markers, and may increase prognostic accuracy and improve patient outcomes.
MATERIALS AND METHODS
Study cohort
This study was approved by the Human Scientific Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Five hundred and ninety-two cases of infiltrative gliomas (WHO II, III and IV) with complete follow-up data were enrolled in the study. Patients in the cohort were surgically treated in the First Affiliated Hospital of Zhengzhou University from 2011 to 2016. The diagnosis was made by pathological examination and centrally reviewed by two pathologists according to the 2016 WHO classification of tumors of the CNS [2]. All patients enrolled in the current study were treatment-naïve (i.e neither surgical resection, chemotherapy, nor radiotherapy were administered before the first operation). For survival analysis of hematological markers, patients with hematological diseases, serious infections, surgery, trauma, and anti-coagulant therapy were excluded. All clinical data, including gender, age, preoperative Karnofsky Performance Status (KPS) score, extent of resection, histological grade, and adjuvant therapies were collected from the medical record system. Follow-up data were acquired by telephone or out-patient follow-up. Overall survival (OS) was calculated as the time interval between the date of surgery and the date of death or the end of follow-up.
Molecular classification
Formalin-fixed, paraffin embedded (FFPE) tissues were available in 573 cases. The detection of molecular markers was centrally conducted with standardized protocols. Mutational hotspots in IDH1, IDH2, and the TERT promoter were detected by Sanger sequencing. Chromosome 1p/19q status was evaluated by fluorescence in situ hybridization in all WHO Grade II and Grade III gliomas. Detailed protocols are described in the Supplementary Materials. According to the status of the three molecular markers, infiltrative gliomas were categorized into five principal groups: triple-positive (mutations in TERT promoter and IDH, plus 1p/19q codeletion); mutations in both TERT and IDH; mutation in IDH only; mutation in TERT only; and triple- negative [3].
Hematological markers
Routine preoperative blood and hepatic function tests prior to the first surgical resection were centrally performed at the Department of Clinical Laboratory within 2 hours of blood sample collection. Blood test results included neutrophil, lymphocyte, mononuclear cell, and platelet counts, as well as mean platelet volume and platelet distribution width. Results of the hepatic function test included albumin and globulin levels to calculate AGR. Hematological markers included: NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, MLR = monocyte-to- lymphocyte ratio, MPV = median platelet volume, PDW = platelet distribution width, and AGR = albumin-to-globulin ratio.
Statistical methods
SPSS 19.0 (IBM Corp., Armonk, NY, USA), Graph-Pad Prism 6.0 (Graph-pad Inc, La Jolla, USA) and X-tile 3.6.1 (http://medicine.yale.edu/lab/rimm/research/ software.aspx) were used to analyze the data. The Kaplan-Meier method and the log-rank test were used to calculate survival rates. Post-hoc Bonferroni test was used for multiple comparisons. Multivariate analysis using Cox regression was performed to evaluate independent prognostic factors. P < 0.05 was considered statistically significant.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: Research conception and design, manuscript revision and approval: Zhen-yu Zhang, Xian-zhi Liu, Wei-wei Wang; Material and data collection, statistical analysis, drafting of the manuscript: Zhen-yu Zhang, Yun-bo Zhan; Molecular pathology: Wei-wei Wang, Li Wang; Acquisition of tissue specimens and assessment of clinical outcomes: Feng-jiang Zhang, Bin-Yu, Yu-chen Ji, Jin-qiao Zhou, Ya-hui Bai, Yan-min Wang, Yan Jing, Wen-chao Duan, Chen Sun, Tao Sun, Hai-biao Zhao, Ke Li, Wen-qing Wang, Ruo-yan Li, Hong-wei Sun, Guang Zhai, Shu-kai Wang, Xin-ting Wei, Bo Yang, Dong-ming Yan.
CONFLICTS OF INTEREST: None of the authors have any conflicts of interest to declare.
FUNDING: This research was supported by the National Natural Science Foundation of China (No. 81702465 and U1804172), the Science and Technology Program of Henan Province (No. 182102310113 and 192102310050), the Youth Innovation Fund of The First Affiliated Hospital of Zhengzhou University to Zhen-yu Zhang, and the Key Research Projects of Henan Higher Education (No.18A310033).
REFERENCES
- 1.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro-oncol. 2015. (Suppl 4); 17:iv1–62. 10.1093/neuonc/nov189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumors of the Central Nervous System. Lyon: IARC Press; 2016. [DOI] [PubMed] [Google Scholar]
- 3.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015; 372:2499–508. 10.1056/NEJMoa1407279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aum DJ, Kim DH, Beaumont TL, Leuthardt EC, Dunn GP, Kim AH. Molecular and cellular heterogeneity: the hallmark of glioblastoma. Neurosurg Focus. 2014; 37:E11. 10.3171/2014.9.FOCUS14521 [DOI] [PubMed] [Google Scholar]
- 5.Mostofa AG, Punganuru SR, Madala HR, Al-Obaide M, Srivenugopal KS. The Process and Regulatory Components of Inflammation in Brain Oncogenesis. Biomolecules. 2017; 7:34. 10.3390/biom7020034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelson N, Rincon-Torroella J, Quiñones-Hinojosa A, Greenfield JP. Exploring the role of inflammation in the malignant transformation of low-grade gliomas. J Neuroimmunol. 2016; 297:132–40. 10.1016/j.jneuroim.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 7.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013; 218:1402–10. 10.1016/j.imbio.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 8.Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015; 16:807–20. 10.1080/15384047.2015.1040960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015; 126:582–88. 10.1182/blood-2014-08-531582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia WK, Liu ZL, Shen D, Lin QF, Su J, Mao WD. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol. 2016; 14:127. 10.1186/s12957-016-0889-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H, Yao X, Xie X, Wu X, Zheng C, Xia W, Ma S. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017; 35:261–70. 10.1007/s00345-016-1864-9 [DOI] [PubMed] [Google Scholar]
- 12.Song X, Zhu H, Pei Q, Tan F, Li C, Zhou Z, Zhou Y, Yu N, Li Y, Pei H. Significance of inflammation-based indices in the prognosis of patients with non-metastatic colorectal cancer. Oncotarget. 2017; 8:45178–89. 10.18632/oncotarget.16774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakacak M, Serin S, Ercan Ö, Köstü B, Bostancı MS, Bakacak Z, Kıran H, Kıran G. Utility of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios to distinguish malignant from benign ovarian masses. J Turk Ger Gynecol Assoc. 2016; 17:21–25. 10.5152/jtgga.2015.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang PF, Meng Z, Song HW, Yao K, Duan ZJ, Yu CJ, Li SW, Yan CX. Preoperative Changes in Hematological Markers and Predictors of Glioma Grade and Survival. Front Pharmacol. 2018; 9:886. 10.3389/fphar.2018.00886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015; 15:617. 10.1186/s12885-015-1629-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes M, Carvalho B, Vaz R, Linhares P. Influence of neutrophil-lymphocyte ratio in prognosis of glioblastoma multiforme. J Neurooncol. 2018; 136:173–80. 10.1007/s11060-017-2641-3 [DOI] [PubMed] [Google Scholar]
- 17.Wang PF, Song HW, Cai HQ, Kong LW, Yao K, Jiang T, Li SW, Yan CX. Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget. 2017; 8:50117–23. 10.18632/oncotarget.15235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002; 16:217–26. [PubMed] [Google Scholar]
- 19.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001; 357:539–45. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 21.Peng W, Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res. 2014; 192:402–08. 10.1016/j.jss.2014.05.078 [DOI] [PubMed] [Google Scholar]
- 22.Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, Yamamoto K, Miyake M, Haraguchi N, Nishikawa K, Hirao M, Ikeda M, Sekimoto M, Nakamori S. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016; 16:434–40. 10.1016/j.pan.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 23.Lucas T, Abraham D, Aharinejad S. Modulation of tumor associated macrophages in solid tumors. Front Biosci. 2008; 13:5580–88. 10.2741/3101 [DOI] [PubMed] [Google Scholar]
- 24.Hsu JT, Wang CC, Le PH, Chen TH, Kuo CJ, Lin CJ, Chou WC, Yeh TS. Lymphocyte-to-monocyte ratios predict gastric cancer surgical outcomes. J Surg Res. 2016; 202:284–90. 10.1016/j.jss.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 25.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014; 31:305. 10.1007/s12032-014-0305-0 [DOI] [PubMed] [Google Scholar]
- 26.Go SI, Kim RB, Song HN, Kang MH, Lee US, Choi HJ, Lee SJ, Cho YJ, Jeong YY, Kim HC, Lee JD, Kim SH, Kang JH, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014; 31:323. 10.1007/s12032-014-0323-y [DOI] [PubMed] [Google Scholar]
- 27.Xu WZ, Li F, Xu ZK, Chen X, Sun B, Cao JW, Liu YG. Preoperative albumin-to-globulin ratio and prognostic nutrition index predict prognosis for glioblastoma. OncoTargets Ther. 2017; 10:725–33. 10.2147/OTT.S127441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao Y, Yang M, Jin C, Hou S, Shi B, Shi J, Lin N. Preoperative Hematologic Inflammatory Markers as Prognostic Factors in Patients with Glioma. World Neurosurg. 2018; 119:e710–16. 10.1016/j.wneu.2018.07.252 [DOI] [PubMed] [Google Scholar]
- 29.Massara M, Persico P, Bonavita O, Mollica Poeta V, Locati M, Simonelli M, Bonecchi R. Neutrophils in Gliomas. Front Immunol. 2017; 8:1349. 10.3389/fimmu.2017.01349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–67. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kono SA, Heasley LE, Doebele RC, Camidge DR. Adding to the mix: fibroblast growth factor and platelet-derived growth factor receptor pathways as targets in non-small cell lung cancer. Curr Cancer Drug Targets. 2012; 12:107–23. 10.2174/156800912799095144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, Varki A, McEver RP. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011; 118:4015–23. 10.1182/blood-2011-07-368514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seaton K. Albumin concentration controls cancer. J Natl Med Assoc. 2001; 93:490–93. [PMC free article] [PubMed] [Google Scholar]
- 34.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009; 30:1073–81. 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- 35.Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000; 18:636–45. 10.1200/JCO.2000.18.3.636 [DOI] [PubMed] [Google Scholar]
- 36.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360:765–73. 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013; 110:6021–26. 10.1073/pnas.1303607110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas BH, Wang Z, Greer PK, Zhu H, Wang CY, Carpenter AB, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014; 5:1515–25. 10.18632/oncotarget.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang ZY, Chan AK, Ding XJ, Qin ZY, Hong CS, Chen LC, Zhang X, Zhao FP, Wang Y, Wang Y, Zhou LF, Zhuang Z, Ng HK, et al. TERT promoter mutations contribute to IDH mutations in predicting differential responses to adjuvant therapies in WHO grade II and III diffuse gliomas. Oncotarget. 2015; 6:24871–83. 10.18632/oncotarget.4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013; 31:337–43. 10.1200/JCO.2012.43.2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009; 27:4150–54. 10.1200/JCO.2009.21.9832 [DOI] [PubMed] [Google Scholar]
- 42.Chan AK, Yao Y, Zhang Z, Chung NY, Liu JS, Li KK, Shi Z, Chan DT, Poon WS, Zhou L, Ng HK. TERT promoter mutations contribute to subset prognostication of lower-grade gliomas. Mod Pathol. 2015; 28:177–86. 10.1038/modpathol.2014.94 [DOI] [PubMed] [Google Scholar]
- 43.Qian Z, Li Y, Fan X, Zhang C, Wang Y, Jiang T, Liu X. Molecular and clinical characterization of IDH associated immune signature in lower-grade gliomas. OncoImmunology. 2018; 7:e1434466. 10.1080/2162402X.2018.1434466 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.