Abstract
The carotid body is the largest collection of paraganglia in the head and neck and is found on the medial aspect of the carotid bifurcation bilaterally. Carotid body tumors are rare neoplasms arising from the chemoreceptor cells of the carotid bulb. We report a case of carotid body tumor in a 42-year-old female, who presented with painless, pulsatile, gradually progressive lateral neck swelling. The diagnosis is suspected on the basis of history, clinical and radiological examination findings and a successful surgical excision of the tumor is performed. Histopathological examination confirms the diagnosis of carotid body tumor. We also present brief literature about carotid body tumors in terms of its clinical and imaging presentation, evaluation, and management.
Keywords: Carotid body tumor, Carotid space, Chemodectomas, Paraganglioma, Neck tumor
CASE REPORT
Carotid body tumors (CBTs), also known as paragangliomas or chemodectomas, are rare neuroendocrine neoplasms which arise near the carotid bifurcation within glomus cells derived from the embryonic neural crest. The reported incidence of CBTs is 1–2 per 100,000 [1]. CBTs are rare chemical receptor tumors which accounts for 0.6% of the head and neck tumors in humans. The CBT is usually benign with the incidence of malignant tumors below 10% [2]. The majority of these tumors are asymptomatic and initially noticed by inspection and palpation of neck swelling during the physical examination, or more commonly as incidental findings on radiological imaging studies. Nonetheless, the most observed symptoms are a pain, dysphagia, and autonomic dysfunction in symptomatic cases [3], [4].
Clinical presentation of the tumor is an asymptomatic slow growing mass in neck. However, they can produce symptoms due to pressure and local invasion of the surrounding tissue. Most commonly involved are hypoglossal nerve, glossopharyngeal nerve, vagus nerve and sympathetic chain [5]. Clinical history, examination, and radiological diagnosis are the keystones to diagnosis and management. Ultrasound, Computed tomography (CT) and Magnetic resonance imaging (MRI) are useful radiological tools in diagnosis. Yet Angiography is essential to study about the vascular anatomy [6]. Thus, in order to prevent local invasion and metastasis, early surgical excision is considered a primer curative treatment option for the treatment of CBTs. In this paper, we report a case of CBT which was successfully treated with complete surgical excision and review the literature.
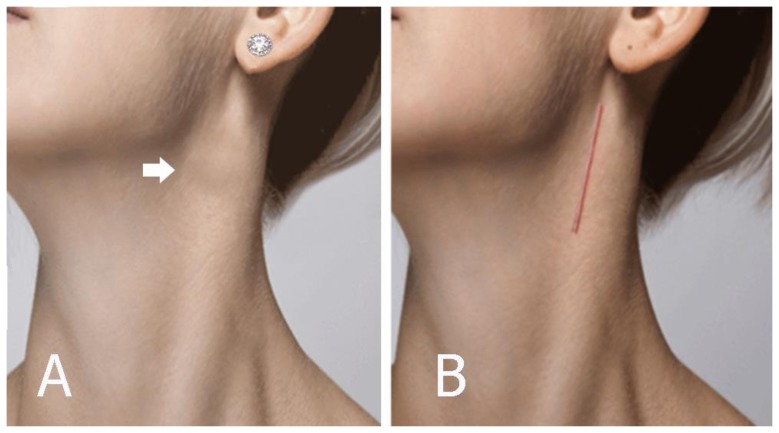
A 42-year-old female patient presented with a neck swelling that persisted for 1 year. On physical examination, a pulsating firm painless mass measuring about 4 × 3 cm in size was found on the left side of her neck, near the angle of the mandible in the left jugulo carotid region (Figure 1A). There was no pressure symptom and it was more mobile transversely than vertically. Pulsations were felt on deep palpation and a faint bruit was heard on auscultation.
Figure 1.
42-year-old female with a left carotid body tumor.
FINDINGS: (A) Patient photograph showing the upper lateral neck swelling due to left carotid body tumor (arrow). (B) Five months after surgery with a scar that can be obscured by hair.
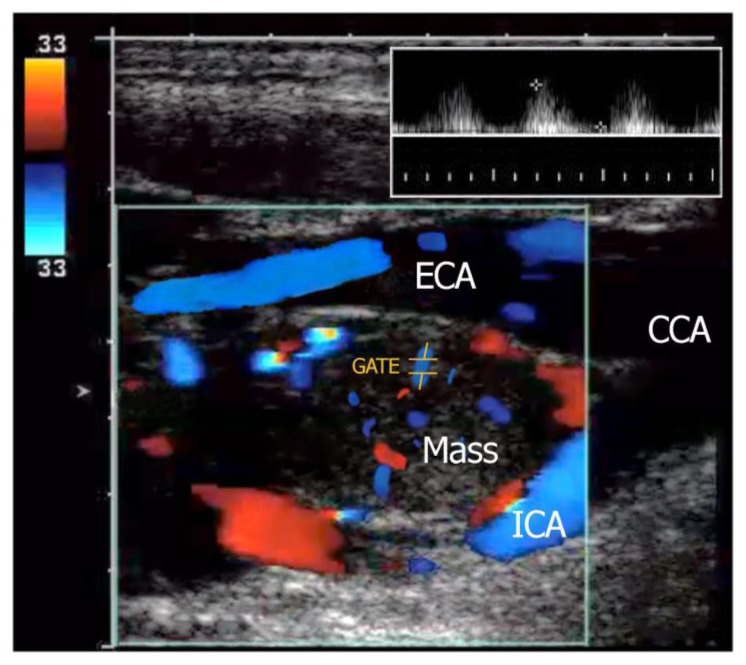
Doppler ultrasonography showed a well-defined hypoechoic mass with increased vascularity at the bifurcation of the common carotid artery causing splaying of the left external and internal carotid artery with no luminal compromise (Figure 2).
Figure 2.
42-year-old female with a left carotid body tumor.
FINDINGS: Doppler ultrasound shows a well-defined, hypoechoic mass locates at the carotid bifurcation between the internal (ICA) and external carotid artery (ECA). Duplex imaging in the same patient shows the highly vascular nature of the paraganglioma located in the crux of the bifurcation. Waveform analysis (inset with sampling location is GATE) shows the internal arterial flow of this lesion which demonstrates low-resistance artery characteristics.
TECHNIQUE: Aloka F37, 7.5 MHz linear probe, Doppler ultrasonography.
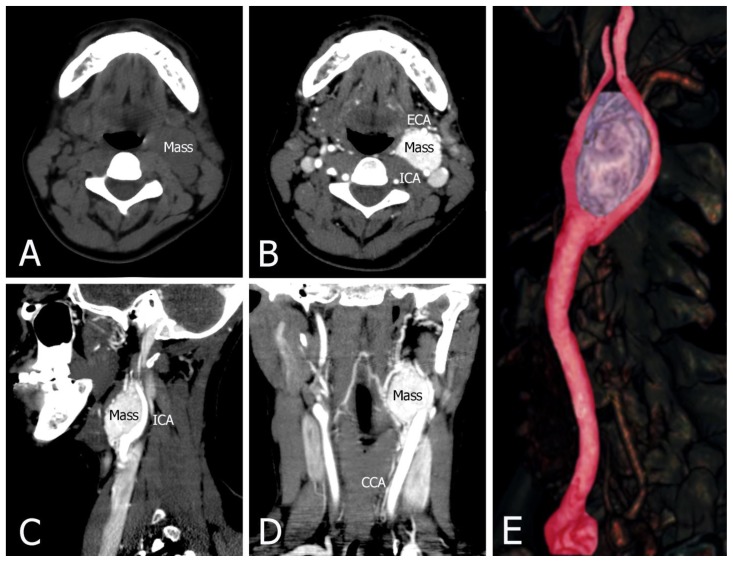
This lesion had soft tissue density on non-contrast CT. Contrast-enhanced CT demonstrated a well-circumscribed lobulated heterogeneously intensely enhancing soft tissue density mass measuring 4.5 cm × 4 cm × 3 cm. It was located at the bifurcation of the left carotid artery and causing splaying of external and internal carotid artery with normal calibre of these arteries. Laterally it abutted the anterior wall of the left internal jugular vein. No infiltration into adjacent structures was seen (Figure 3).
Figure 3.
42-year-old female with a left carotid body tumor.
FINDINGS: (A) Non-contrast CT shows a mass with soft tissue density. (B, C, and D) Contrast-enhanced CT with early arterial phase contrast shows a heterogeneously enhancing mass at the left carotid bifurcation. This lesion has bright and rapid enhancement. It demonstrates a carotid body tumor splaying the bifurcation of the internal (ICA) and external (ECA) carotid arteries. (E) CT angiography 3D reformat image of left carotid body tumor.
TECHNIQUE: Philips 64 CT scanner with non-contrast and contrast, 500 mA, 120 kVp, Slice thickness = 1.25 mm, a total of 60 mls of Omnipaque 350 was administered intravenously.
a) Axial soft tissue window, 1.25 mm slice thickness, non-contrast CT
b) Axial soft tissue window, 1.25 mm slice thickness, contrast CT
b) Sagittal soft tissue window, 1.25 mm slice thickness, contrast CT
c) Coronal soft tissue widow, 1.25 mm slice thickness, contrast CT
e) CT angiography 3D reformatted image, slice thickness = 15 mm.
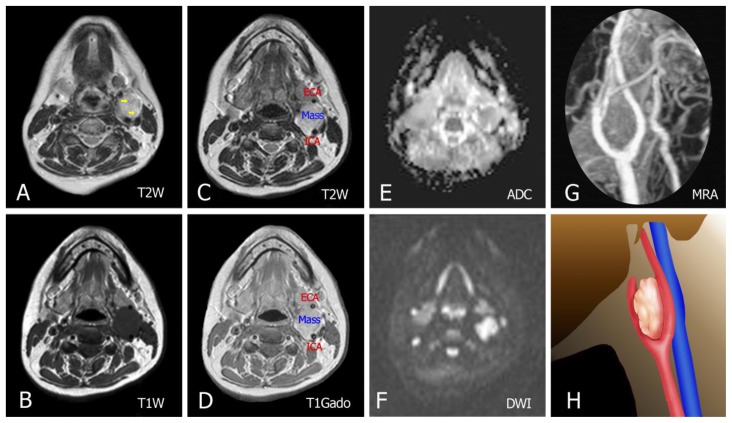
MRI showed a 4 × 3 cm lobulated oval well-defined lesion mass in the left carotid space. The mass signal intensity was nearly similar to that of muscle on T1-weighted, and of increased signal on T2-weighted, with well-defined flow voids on T2-weighted images. Diffusion-weighted imaging showed evidence of restricted diffusion along with low Apparent diffusion coefficient. Splaying of the external and internal carotid artery was clearly seen on enhanced MR angiography maximum-intensity projection image (MIP) (Figure 4).
Figure 4.
42-year-old female with a left carotid body tumor.
FINDINGS: (A, C) Axial T2-weighted images show a lesion with increased signal and well-defined flow voids (yellow arrows in A). (B, D) Pre- and post-contrast T1-weighted show a mass centered in the left carotid bifurcation with avid enhancement (E, F) DWI shows evidence of restricted diffusion along with the ADC map. (G) Enhanced MR angiography maximum-intensity projection image shows tumoral enhancement and proximal splaying of the internal (ICA) and external carotid artery (ECA). (H) Illustration demonstrating the carotid body tumor in this case.
TECHNIQUE: Siemens Magnetom Amira MRI scanner, magnetic strength = 1.5 Tesla, intravenous contrast was administered.
A: Axial T2W. TR = 4000 ms. TE = 110 ms. Slice thickness = 4 mm.
B: Axial T1W pre-contrast. TR 600ms. TE 15ms. Slice thickness = 4 mm.
C: Axial T2W. TR = 4000 ms. TE = 110 ms. Slice thickness = 4 mm.
D: Axial T1W post-contrast with IV contrast in venous phase, Gadovist 15mL. TR 670 ms. TE 10ms. Slice thickness = 4 mm.
E: Axial ADC. TR = 3500 ms. TE = 90 ms. Slice thickness = 4 mm
F: Axial DWI. TR = 3500 ms. TE = 90 ms. Slice thickness = 4 mm
G: Maximum intensity projection (MIP) of a contrast enhanced magnetic resonance angiography (CE-MRA)
H: Illustration
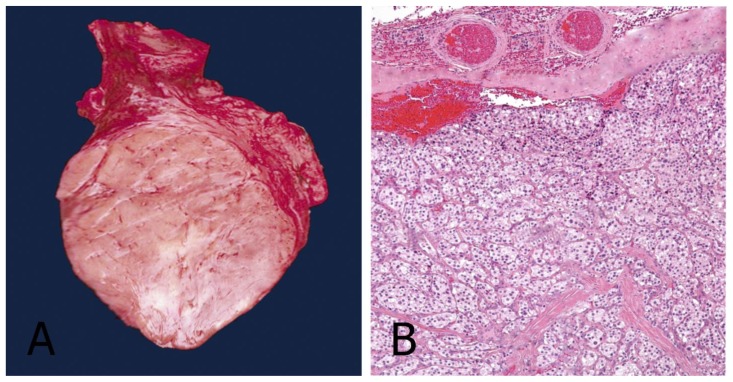
The neck was explored by a vertical incision under general anesthesia. A homogeneous tumor with a smooth contour and approximately 4 × 3 cm long was observed at the carotid bifurcation. It was classified as a grade-2 tumor according to the Shamblin classification system. The common, internal, and external carotid arteries were isolated. Excision of a fairly vascular tumor at the bifurcation was possible. Small arterial branches arising from the bifurcation had to be ligated. The postoperative recovery was uneventful. Gross section of the mass resected from the left neck was dense and flexible as rubber with grooves on either side representing impressions left by internal and external carotid artery branches. Histologically, the tumor was composed of nests of fairly uniform epithelioid cells with finely granular pale eosinophilic cytoplasm, with round nuclei seen surrounded by several vascular stroma (Figure 5). The patient is alive and asymptomatic with a scar that can be obscured by hair (Figure 1B).
Figure 5.
42-year-old female with a left carotid body tumor.
FINDINGS: (A) The operative specimen of the carotid body tumor. It is dark-purple in color and fairly well circumscribed. (B) Hematoxylin and eosin (H&E) stain showing a well-developed Zellballen (“cell balls” in German) growth pattern. The neoplastic cells demonstrate an eosinophilic granular cytoplasm and round hyperchromatic nuclei.
TECHNIQUE:
A: Operative specimen.
B: Light microscopy, magnification 10x, hematoxylin-eosin stain.
DISCUSSION
Etiology & Demographics
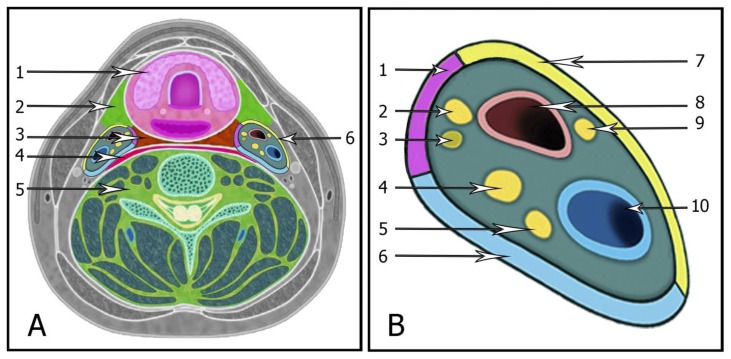
The carotid space (Figure 6) extends from the lower border of the jugular foramen-carotid canal all the way to the aortic arch and is delimited by the three layers of the deep cervical fascia. The carotid space includes suprahyoid and infrahyoid regions. Lesions involving the carotid space may arise from any of these constituents. Lesions of the carotid space may arise from asymmetry of normal vasculature, inflammatory or infectious processes, and benign or malignant tumors, including metastatic disease processes [7], [8].
Figure 6.
A: The carotid space related to the neck cavities. (1) Pharyngeal mucosal space, (2) Anterior cervical and masticator spaces, (3) Retropharyngeal space, (4) Lateral retropharyngeal and Danger spaces, (5) Perivertebral space, (6) Carotid space.
B: Illustration demonstrating the contents and configuration of the left carotid space. (1) Middle layer fascia, (2) Cranial nerve XII, (3) Sympathetic trunk, (4) Cranial nerve X, (5) Cranial nerve XI, (6) Deep layer fascia, (7) Superficial layer fascia, (8) Carotid artery, (9) Cranial nerve IX, (10) Jugular vein.
The carotid body is the largest collection of paraganglia in the head and neck and is found in the carotid space. Carotid body was firstly described by von Haller in the year 1743 [9]. It is a reddish-brown, well circumscribed, highly specialized round organ, located in the adventitia of the carotid bifurcation, supplied by the feeding vessels run primarily from the ascending pharyngeal branch of the external carotid artery, and innervated through the glossopharyngeal and vagus nerves. The normal carotid body measures 2–6 mm in diameter but is often larger in people living at higher altitudes. It functions as a chemoreceptor organ which is stimulated by acidosis, hypoxia and hypercapnea, and plays a role in the autonomous control of blood pressure, heart rate, respiration, and blood temperature in response to changes in these parameters by increasing sympathetic flow [10].
CBT can be found at any age and is frequently seen in those between 50 and 70 years old (range, 18–94 years), with slightly higher prevalence in women than men (male-to-female ratio of 1:1.9). Bilateral disease is significantly more frequent in familial (31.8% of cases) than in non-familial CBT (4.4%) [11]. In this case, the patient is female with the tumor on one side and has a slightly younger age than the frequently seen age. The blood supply of CBT is abundant, which is mainly from external carotid artery and branches. Blood supplies from internal carotid artery, vertebral artery, ascending pharyngeal artery and superior thyroid artery have also been reported [12]. CBTs are slow-growing hypervascular tumors which represent approximately 0.03% of all neoplasms [10]. More tumors (57%) were on the right side, whereas 25% were on the left, 17% were bilateral, and 10% were malignant [4], [13]. Notably, the lesion in the present case is in the left neck and does not have malignant characteristics.
Clinical & Imaging findings
CBTs usually manifests as an asymptomatic anterior neck mass. In larger tumors, they can be associated with the myriad of presenting symptoms of a space-occupying lesion in this location, such as fullness, pain, dysphagia, odynophagia, hoarseness, and stridor. The tissue is often rubbery, firm, and noncompressible. The mass may be displaced laterally but not vertically. Generally, a palpable thrill or bruit may be absent if the tumor is small. Historically, cranial nerve deficits were present in approximately 10% of patients secondary to nerve compression; however with improved imaging modalities, CBTs are detected earlier at smaller sizes, and thereby cranial nerve compromise is rare currently. Lastly, a rare functional CBT may produce neuroendocrine secretions causing catecholamine-related symptoms, such as palpitations, headaches, hypertension, tachycardia, or flushing [13]. The lesion in our case showed an asymptomatic neck mass with rubbery, firm, noncompressible, and more mobile transversely than vertically.
CBTs are usually identified by clinical examination or found incidentally on imaging studies. Final diagnosis mainly depends on image examination and pathological anatomy. Doppler ultrasound, CT, MRI, and angiography play important roles in the clinical diagnosis of CBT. Color Doppler ultrasound, a simple and non-invasive examination, has relatively high specificity and sensitivity for CBT. Color-flow carotid duplex is the ideal screening test for CBTs, and these tumors are characteristically a well-defined hypoechoic mass that splays the carotid bifurcation. With color Doppler imaging, a hypervascularity with low-resistance flow pattern is evident. Historically, angiography was the “gold standard” diagnostic procedure for CBTs to confirm the diagnosis and provide accurate delineation of the vascular supply. However, with improvements in high-resolution imaging, cross-sectional imaging (such as CT angiography or MR angiography) is the preferred modality for surgical planning of tumor resection because it best defines the relationship of the tumor with the artery bifurcation and the likely location of the cranial nerves. MRI does not use ionizing radiation, and the accuracy of MRI is higher in comparison to that of CT in many cases. However, CTA is able to define the size of the tumor and its relation to bone landmarks, which can modify the surgical approach. It can also easily identify contralateral tumors and other head/neck paragangliomas. Head and neck paragangliomas demonstrate distinctive features on DWI and dynamic contrast-enhanced MRI than other benign tumors. In addition to the morphological features, a relatively lower ADC value and washout time-intensity curves would support the diagnosis, which is otherwise more supportive for a malignant nature [14]. On DSA, the splaying of the carotid vessels (lyre sign) is identified with an intense blush in tumor with an “early vein” seen due to arteriovenous shunting. The ascending pharyngeal artery is the main contributing supply. The tumor in the present case was correctly diagnosed with ultrasound and CT. MRI was taken for preoperative evaluation. Ultrasound showed a well-defined hypoechoic mass with increased vascularity at the bifurcation of the common carotid artery causing splaying of the left external and internal carotid artery. This lesion had a soft tissue density and vividly enhanced on CT. On MRI, the mass had a slightly high signal on T2W and slightly low signal on non-contrast-enhanced T1W, and strong enhancement on contrast-enhanced T1W. DWI showed evidence of restricted diffusion along with low ADC. Our case is similar to the characteristics of the research of Ying Yuan et al [14]. That is carotid body tumors demonstrate as well-circumscribed and intensely enhancing masses with low ADC value.
Although not specific, it shows uptake with metaiodobenzylguanidine (MIBG) and octreoscan scintigraphy and can be useful for assessing multiple lesions on Scintigraphy [12], [13]. CBTs can show uptake with metaiodobenzylguanidine (MIBG) on octreoscan scintigraphy specific in patients with familial, multiple, or functional paragangliomas. Peptide receptor radionuclide therapy (PRRT) can be a therapeutic option in selected cases of head and neck paragangliomas, including tumors that cannot be removed, occult, recurrently, malignant, or metastatic lesions. It is administered with peptide receptor radionuclide therapy using Lu-177 DOTATATE. This therapy is a very promising treatment option in neuroendocrine tumors, with good results. Currently, there are only a few reports regarding its use in paragangliomas [15].
Treatment & Prognosis
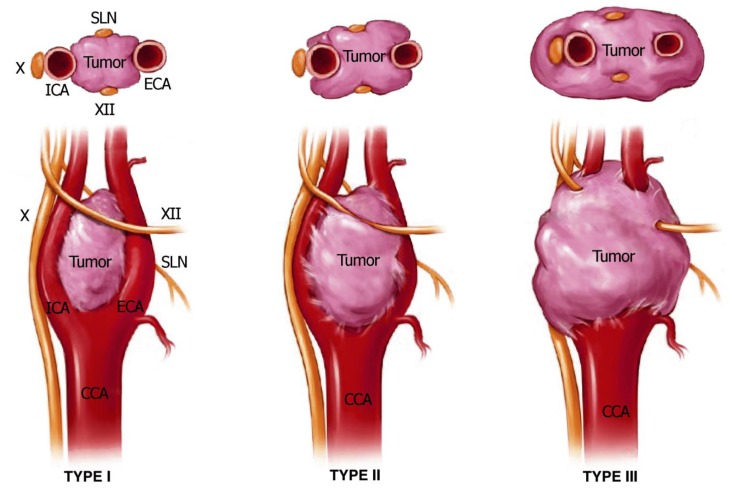
In the year 1971, Shamblin research group introduced a classification system according to the relationship with the carotid arteries in order to determine the respectability of these tumors. Shamblin type I tumors are small lesions at the carotid bifurcation and can generally be removed without difficulty. Shamblin type II tumors are larger and significantly splay the carotid bifurcation, but do not circumferentially encase the carotid arteries. Shamblin type III tumors are large, encase the vessels and thus the most difficult type to attempt resection. According to the Shamblin classification, type III tumors are associated with more perioperative neurovascular complications and complex surgical procedure (Figure 7) [13], [16].
Figure 7.
Shamblin classification of carotid body tumors.
Type I tumors are small lesions, that do not splay the carotid bifurcation.
Type II tumors are larger, significantly splay the carotid bifurcation, but do not circumferentially encase the carotid arteries.
Type III tumors are large, encapsulate the internal or external carotid arteries, and often adhere or incorporate the adjacent cranial nerves.
CN cranial nerve, ECA external carotid artery, ICA internal carotid artery, SLN superior laryngeal nerve.
CBTs have diagnostic and management difficulties since there is a lack of guidelines in the literature for their diagnosis and treatment. If a diagnosis of CBT is suspected following a detailed physical examination, the diagnosis is almost always established by radiological imaging methods such as duplex ultrasonography, CT angiography, MR angiography, and digital subtraction angiography [6]. Nowadays, ultrasonographic examination is widely used for screening because it is an easily available and non-invasive imaging modality. CT and MRI help to assess the size, degree, and invasiveness of the tumor. Angiographic methods allow the evaluation of the vessels supplying the tumor and preoperative embolization. On account of the hypervascularization and proximity to various vascular and nervous structures of these tumors, biopsy as a diagnostic method is contraindicated since it presents a risk of massive hemorrhage and dissemination and can lead to pseudoaneurysm formation and carotid thrombosis as well [8], [10].
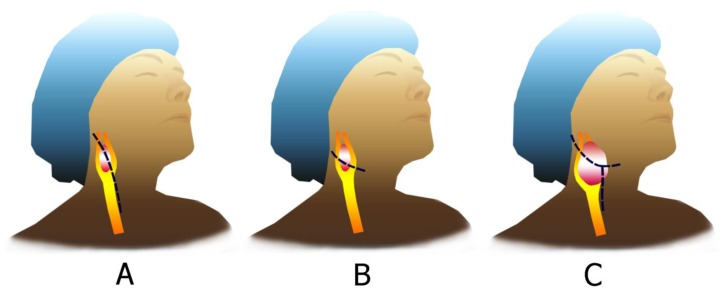
Surgical excision still remains the gold standard therapeutic modality for the treatment of CBTs. Radiotherapy is an alternative treatment modality which may decrease the tumor size or stop its growth. It is recommended for patients who cannot undergo surgery on account of extensive involvement, multiple tumors, and high operative and anesthetic risk. Additional considerations specific to CBT resection include routine vein mapping, and preparation of one thigh into the operative field for potential saphenous vein harvest should be needed for reconstruction. Additionally, the choice of the incision may vary depending upon the extent of the tumor. Incision choices include a curvilinear incision along the midportion of the tumor, a “hockey stick” incision along the anterior border of the sternocleidomastoid, and in the case of a very high tumor, a modified radical neck T incision (Figure 8) [13]. Regardless of patient age and tumor size, early surgical removal of CBTs is advised in order to prevent the development of larger and more advanced tumors, which are related to higher morbidity and mortality. If the size of CBT is more than 5 cm, mortality is 1% – 3% after surgical intervention [17]. Surgical excision of CBTs is a very effective and safe procedure with a low rate of major neurovascular complication and mortality. Early detection and complete surgical removal of CBTs improve the outcome [3], [12], [13]. The tumor in the present case is classified as a grade-2 tumor according to the Shamblin classification system and it is removed by a vertical incision under general anesthesia. Patients with bilateral carotid body tumors require special attention. In these patients the recommended procedure is staged excision, removing the largest tumor first, and re-operating the second tumor later. Doing it simultaneously carries the risk of experience labile blood pressure postoperatively, which is difficult to control medically due to bilateral Hering nerves excisions [18].
Figure 8.
Surgical incisions for exposure for a carotid body tumor depends on the characteristic of the tumor.
(A, B) For Shamblin type I or II, a straight incision along the anterior border of the sternocleidomastoid muscle or a curvilinear incision centered on the midportion of the tumor is advocated.
(C) For Shamblin type III, a modified radical neck T incision can facilitate exposure.
Differential Diagnoses
Differential diagnosis of CBTs should be distinguished from masses in the neck or the lesions originating from the carotid space. The most important differential diagnosis include aneurysm or pseudoaneurysm of the carotid artery, hematoma, glomus vagale tumor, and vagal schwannoma [8], [19]. In addition to these, carotid body hyperplasia should also be remembered. It may develop on account of chronic hypoxia and is primarily described in patients living at high altitudes [10], [20].
Carotid artery aneurysm
Carotid artery aneurysm is usually from either mechanical trauma or an underlying connective tissue disorder. This can result in an intramural hematoma leading to a thrombus, which causes narrowing of the lumen. A pseudoaneurysm or dissection of the carotid artery can occur. The diagnosis is confirmed by CT or MRI; the angiography can delineate extension, detect stenosis and help during surgical planning. CTA or MRA is considered the study of choice when carotid artery aneurysm or dissection is suspected, and will classically reveal a change in the caliber of the blood vessel. Other findings on CT or MRI indicative of dissection include an oval, irregular, or slit-like cross-section of the vessel lumen. On ultrasound, swirling blood in the sac itself can create the so-called “yin-yang” sign [8], [19]. The lesion in the present case is a solid tumor, without the characteristics of a blood vessel like an aneurysm.
Carotid artery pseudoaneurysm
Pseudoaneurysm occurs when there is an injury of the tunica intima and media, resulting in a hematoma that is contained only by the thin outer adventitial layer of the vessel. On ultrasound, a hypoechoic cystic structure can be demonstrated adjacent to the true vessel. Color duplex imaging can demonstrate a “to and fro” color flow and bidirectional Doppler waveform in the pseudoaneurysm neck. CT imaging can demonstrate the irregularity of a vessel wall and irregular outpouching. Hematoma with contrast extravasation seen on angiographic studies is compatible with rupture and active bleeding [17]. When a pseudoaneurysm is large enough, it is important to note that the entire sac may not enhance, as the pseudoaneurysm can partially thrombose. MRI with T1-weighted fat-suppressed sequences allows for evaluation of intraluminal thrombus and of pseudoaneurysm sac size. Digital subtraction angiography remains the gold standard for evaluation of pseudoaneurysm and simultaneously offers a therapeutic potential. Distinguishing between carotid artery pseudoaneurysm and carotid body tumor is usually simple because pseudoaneurysm usually contains blood [8].
Hematoma/Thrombus
Hematoma can form as a result of trauma, dissection, pseudoaneurysm, infection. Hematoma may be diagnosed easily with contrast-enhanced CT, MRI, or ultrasonography. On non-enhanced CT, hematoma appears as a high-density mass. On enhanced CT, it does not enhance with contrast agent. MRI produces greater soft-tissue contrast and can delineate altered rates of blood flow more sensitively than CT. On T2-weighted MR, acute thrombus of the arterial will have a bright lumen, whereas subacute arterial thrombus will have a low signal. Ultrasonography is an easy, safe, noninvasive, and widely available test. Findings on ultrasonography include a dilated and incompressible arterial, intraluminal clot, and no response to the arterial maneuver [12], [13]. The lesion in the present case has a strong enhancement on contrast-enhanced images, so it could be easily distinguished from a hematoma.
Glomus vagale tumor
Vagal paragangliomas are rare, accounting for fewer than 5% of all head and neck paragangliomas. These tumors are less common than carotid body tumors. Vagal paragangliomas arise from paraganglionic tissue associated with 1 of the 3 ganglia of the vagus nerve. Vagal paragangliomas tend to present similarly to carotid body tumors on radiology. MRI sometimes demonstrates the classic “salt-and-pepper” appearance on T1-weighted and T2-weighted images seen with all types of paragangliomas. Both glomus vagale and carotid body tumor have avid enhancement on MR and CT images. A glomus vagale tumor will anteriorly displace the carotid artery and laterally displace the internal jugular vein and do not widen the carotid bifurcation like carotid body tumors [19], [20].
Schwannoma
Schwannomas in the carotid space can involve any of the nerves found within this space, including the glossopharyngeal, vagus, accessory, hypoglossal, and sympathetic chain nerves. Schwannomas are usually better visualized on MR than CT imaging. They are enhancing lesions that will often demonstrate areas of cystic degeneration most evident on T2-weighted MR images. These tumors may be hypodense on CT, approaching the attenuation of simple fluid. Contrast-enhanced MRA may help distinguish a schwannoma from a hypervascular paraganglioma. Schwannomas involving the vagus or sympathetic chain will displace the internal and external carotid arteries anteriorly. Vagal schwannomas typically push the carotid artery anteromedially, and sympathetic chain schwannomas will push the carotid artery anterolaterally. These relationships are well preserved superiorly in the carotid space for both paragangliomas and schwannomas; however, lower in the neck, the direction of carotid displacement is a less reliable indicator of a tumor’s origin [8], [20]. In our case, there is no cystic degeneration as in schwannomas. Schwannomas often push the internal and external carotid arteries anteriorly. In contrast, the lesion in the present case splays the internal and external carotid arteries.
TEACHING POINT
Carotid body tumors are rare, slow-growing, hypervascular neuroendocrine tumors. They can undergo malignant transformation. Carotid body tumors can be preliminarily diagnosed according to history, physical examinations, and radiology. On imaging, they often splay the internal and external carotid arteries. Surgical resection is the preferred treatment for carotid body tumors.
Table 1.
Differential diagnosis table for carotid body tumor
| Diagnosis | Ultrasound findings | CT findings | MRI findings | Angiography/DSA findings |
|---|---|---|---|---|
| Carotid body tumor | Well-defined hypoechoic mass that splays the carotid bifurcation; hypervascular with low-resistance flow pattern | A mass with soft tissue density; heterogeneously enhancing at the carotid bifurcation in the early arterial phase; strong and rapid enhancement | Nestled between the external and internal carotid arteries; signal intensity is nearly similar to muscle on T1-weighted, and hyperintense signal on T2-weighted; salt and pepper sign appearance | Splaying of the carotid vessels; the ascending pharyngeal artery is the main contributing supply; conventional angiography can demonstrate vascular anatomy |
| Carotid artery aneurysm | “Yin-Yang” sign; pseudoaneurysm or dissection can occur | Change in the diameter of the blood vessel | An oval, irregular, or slit-like cross section of the vessel lumen | Delineate extension, detect stenosis |
| Carotid artery pseudoaneurysm | A hypoechoic cystic structure adjacent to the true vessel; color Doppler imaging present “to and fro” flow and bidirectional Doppler waveform in pseudoaneurysm neck | CT can demonstrate the vessel wall irregularity and irregular outpouching; compatible with rupture and active bleeding | MRI with T1-weighted fat-suppressed sequences allows for evaluation of intraluminal thrombus and pseudoaneurysm sac size | DSA remains the gold standard for evaluation of pseudoaneurysm |
| Neck hematoma/ thrombus | Dilated and incompressible arterial, intraluminal clot, and no response to arterial maneuver | On non-enhanced CT, hematoma appears as a high-density mass. On enhanced CT, it does not enhance with contrast agent | Hemorrhage on MRI has highly variable imaging characteristics that depend on the age of the blood. On T2-weighted, acute thrombus of the arterial will have a high intensity, whereas subacute arterial thrombus will have a low signal | May locate the active arterial bleed/source of hemorrhage |
| Glomus vagale tumor | May be seen as a solid heterogeneously hypoechoic lesion comprising of small vascular structures | A mass with soft tissue density; heterogeneously enhancing | Usually low signal on T1-weighted; high signal with multiple flow voids on T2-weighted; intense contrast enhancement on T1-gado | Same carotid body tumor but tends to displace the carotid artery anteriorly |
| Schwannoma | Permitted direct visualization of the vagus nerve, so its position relative to the schwannoma could be examined; can result in an increase of the distance between the artery and vein (separation) | A mass with soft tissue density; non-enhancement of the mass on contrast-enhanced | Well-encapsulated tumors appear as a round or ovoid mass that is isointense to muscle on T1-weighted, hyperintense on T2-weighted; nerve sheath tumors that may or may not enhance | Tends to displace both vessels together rather than splaying them |
Table 2.
Summary table for carotid body tumor
| Definition | Carotid body tumors, also known as paragangliomas or chemodectomas, are rare neuroendocrine neoplasms which arise near the carotid bifurcation within glomus cells. |
| Etiology | Derived from the embryonic neural crest. |
| Incidence | CBTs is 1–2 per 100,000. CBTs are rare chemical receptor tumors which accounts for 0.6% of the head and neck tumors in humans. The CBT is usually benign with the incidence of malignant tumors below 10%. |
| Gender ratio | Slightly higher prevalence in women than men (male-to-female ratio of 1:1.9). |
| Age predilection | CBT can be found at any age and is frequently seen in those between 50 and 70 years old. |
| Risk factors | Familial factor (31.8% of cases) accounts for a higher proportion non-familial factor (4.4% of cases). |
| Location | More tumors (57%) are on the right side, whereas 25% are on the left, and 17% are bilateral. |
| Treatment | In order to prevent local invasion and metastasis, early surgical excision is considered a primer curative treatment option for the treatment of CBTs. |
| Prognosis | While the majority of these tumors are benign, 2%–13% pursue a malignant course with metastases to regional lymph nodes, lungs, and bones. |
| Findings on imaging | -Ultrasound: These tumors are characteristically a well-defined hypoechoic mass that splays the carotid bifurcation. With color Doppler imaging, a hypervascular, low-resistance flow pattern is evident. -CT-Scan: Mass with soft tissue density on non-contrast CT images. The lesion shows heterogeneous enhancement at the carotid bifurcation in the early arterial phase on contrast-enhanced CT images. It has bright and rapid enhancement. -MRI: On T1-weighted images, the lesions are iso to hypointense compared to muscle. They have intense enhancement following gadolinium administration. On T2-weighted images, they are hyperintense compare to muscle. DWI shows evidence of restricted diffusion along with the ADC map. Typical lesions show signs of “salt and pepper” appearance. -Angiography: Splaying of the carotid vessels is identified with an intense blush in tumor with an “early vein” seen due to arteriovenous shunting. The ascending pharyngeal artery is the main contributing supply. -Scintigraphy: Although not specific, shows uptake with metaiodobenzylguanidine and octreoscan scintigraphy and can be useful for assessing multiple lesions. |
ABBREVIATIONS
- ADC
Apparent diffusion coefficient
- CBTs
Carotid body tumors
- CT
Computed tomography
- CTA
Computed tomography angiography
- DSA
Digital subtraction angiography
- DWI
Diffusion-weighted imaging
- MIP
Maximum-intensity projection
- MRA
Magnetic resonance angiography
- MRI
Magnetic resonance imaging
REFERENCES
- 1.Sevilla GMA, Llorente PJL, Rodrigo TJP, Garcia RG, Suarez FV, Coca PA, et al. Head and neck paragangliomas: revision of 89 cases in 73 patients. Acta Otorrinolaringol Esp. 2007;58(3):94–100. [PubMed] [Google Scholar]
- 2.Bakoyiannis KC, Georgopoulos SE, Klonaris CN, Tsekouras NS, Felekouras ES, Pikoulis EA, et al. Surgical treatment of carotid body tumors without embolization. Int Angiol. 2006;25(1):40–45. [PubMed] [Google Scholar]
- 3.Dixon JL, Atkins MD, Bohannon WT, Buckley CJ, Lairmore TC. Surgical management of carotid body tumors: a 15-year single institution experience employing an interdisciplinary approach. Proceedings (Baylor University Medical Center) 2016;29(1):16–20. doi: 10.1080/08998280.2016.11929343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albsoul NM, Alsmady MM, Al-Aardah MI, Altaher RN. Carotid body paraganglioma management and outcome. Eur J Sci Res. 2009;37:567–574. [Google Scholar]
- 5.Goffredo C, Antignani PL, Gervasi F, Ricottini E. Update in carotid chemodectoma. The International Journal of Medicine. 2009;2(2):221–226. [Google Scholar]
- 6.Shahi S, Upadhyay AR, Devkota A, Pantha T, Gautam D, Paudel DR. Excision of rare carotid body tumour without preembolisation: Case report and literature review. International journal of surgery case reports. 2018;53:99–101. doi: 10.1016/j.ijscr.2018.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanekar S, Kyle M. Elsevier Health Sciences. 6. Vol. 45. 2012. Imaging of Head and Neck Spaces for Diagnosis and Treatment, E-Book. An Issue of Otolaryngologic Clinics; pp. 1273–1292. [Google Scholar]
- 8.Chengazi HU, Bhatt AA. Pathology of the carotid space. Insights into Imaging. 2019;10(21):1–16. doi: 10.1186/s13244-019-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gratiot JH. Carotid body tumors: collective review. Internat Abstr Surg. 1943;77:177–186. [Google Scholar]
- 10.Muduroglu A, Yuksel A. Carotid body tumors: A report of three cases and current literature review. Vascul Dis Ther. 2017;2:1–3. [Google Scholar]
- 11.Grufferman S, Gillman MW, Pasternak LR, Peterson CL, Young WG. Familial carotid body tumors: case report and epidemiologic review. Cancer. 1980;46(9):2116–2122. doi: 10.1002/1097-0142(19801101)46:9<2116::aid-cncr2820460934>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Luo T, Zhang C, Ning Y, Gu Y, Li J, Wang Z. Surgical treatment of carotid body tumor: case report and literature review. Journal of geriatric cardiology. 2013;10(1):116–118. doi: 10.3969/j.issn.1671-5411.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis FM, Obi A, Osborne N. Carotid Body Tumors. In: Hans S, editor. Extracranial Carotid and Vertebral Artery Disease. Springer; 2018. pp. 253–260. [Google Scholar]
- 14.Yuan Y, Shi H, Tao X. Head and neck paragangliomas: diffusion weighted and dynamic contrast enhanced magnetic resonance imaging characteristics. BMC medical imaging. 2016;16(12):1–6. doi: 10.1186/s12880-016-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta SK, Singla S, Karunanithi S, Damle N, Bal C. Peptide receptor radionuclide therapy with (177)Lu DOTATATE in a case of recurrent carotid body paraganglioma with spinal metastases. Clin Nucl Med. 2014;39(5):440–441. doi: 10.1097/RLU.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 16.Shamblin WR, ReMine WH, Sheps SG, Harrison EG. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122:732–739. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- 17.Sajid MS, Hamilton G, Baker DM. A Multicenter Review of Carotid Body Tumour Management. European Journal of Vascular and Endovascular Surgery. 2007;34(2):127–130. doi: 10.1016/j.ejvs.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Burgess A, Calderon M, Jafif-Cojab M, Jorge D, Balanza R. Bilateral carotid body tumor resection in a female patient. International journal of surgery case reports. 2017;41:387–391. doi: 10.1016/j.ijscr.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwada C, Mannion K, Aulino JM, Kanekar SG. Imaging of the carotid space. Otolaryngol Clin North Am. 2012;45(6):1273–1292. doi: 10.1016/j.otc.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Stambuk HE, Patel SG. Imaging of the parapharyngeal space. Otolaryngology Clinics North America. 2008;41(1):77–101. doi: 10.1016/j.otc.2007.10.012. [DOI] [PubMed] [Google Scholar]