Abstract
Uterine intravenous leiomyomatosis is an uncommon tumor, usually arising from the uterus, with nodular masses which extend intravascularly over variable distances and may reach the inferior vena cava, right atrium, and pulmonary arteries. Early diagnosis and surgical intervention are crucial as intracardiac leiomyomatosis not only causes cardiac symptoms but may result in pulmonary embolism and sudden death. Complete tumor resection is key in disease management, thus rendering cardiac-extending uterine intravenous leiomyomatosis one of the most challenging conditions for surgical treatment. The use of interventional radiology procedures can facilitate the surgical approach. We report the case of a massive pelvic recurrence of uterine leiomyomatosis with intracardiac extension and pulmonary embolism, analyzing management and surgical outcomes, highlighting the role of interventional radiology during the therapeutic pathway. Nonetheless, there are currently very few data available concerning the use of interventional radiology procedures in the therapeutic strategy of uterine intravenous leiomyomatosis with intracardiac extension.
Keywords: Uterine intravenous leiomyomatosis, intracardiac extension, Computed Tomography angiography, Percutaneous embolizazion, inferior vena cava filter
CASE REPORT
A Caucasian 48-year-old woman, overweight (BMI 29.7 kg/m2), was referred to the cardiovascular medicine unit of our hospital three weeks after the onset of worsening dyspnea.
The patient’s symptoms started with progressive dyspnea, palpitations, metrorrhagia, generalized pelvic pain, and bilateral leg edema, which had all worsened during the last 48 hours.
Her relevant clinical history included uterine leiomyomatosis for which she had undergone multiple myomectomies. As result of pelvic recurrence of uterine leiomyomatosis the patient subsequently underwent subtotal hysterectomy with preservation of the cervix and adnexae, approximately one year before the present scenario. No risk factors for cardiovascular disease were noted in her history.
The patient was awake and oriented in time, person, and place, with no evidence of distress at that moment. Physical examination showed tachypnea, bilateral mild pitting edema of the lower legs, and jugular venous distension. Upon admission the vital parameters were: low blood pressure (90/60 mmHg), sinus tachycardia (100 bpm), hypoxemia (PaO2 65 mmHg), respiratory rate 30 breaths per minute, and temperature 36 °C. No other abnormalities were found upon physical examination.
Laboratory tests revealed moderate anemia and slightly increased d-dimers (0.80 μg/mL), but were normal for kidney and liver function, tumor markers and prothrombin and partial thromboplastin times.
Electrocardiogram revealed no arrhythmias however bi-atrial enlargement and prolonged QT interval were noted.
An initial imaging evaluation with chest X-ray was performed showing no significant findings (Figure 1).
Figure 1.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: x-ray performed in postero-anterior projection, 2mAs, 120kV. GE Connexity.
Findings: No significant abnormalities
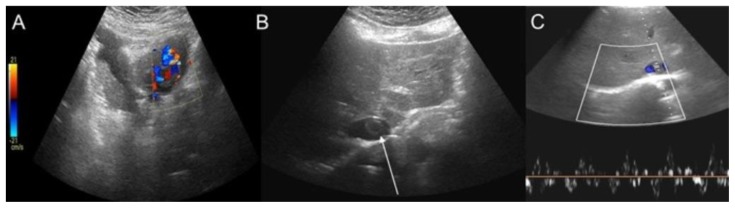
Subsequently a two-dimensional transthoracic Doppler echocardiography was carried out. It showed the presence of a fluctuating solid, thrombus-like mass extending into the right atrium from the inferior vena cava (IVC). The color-Doppler Ultrasound (CDUS) of the abdomen confirmed the presence of a tubular lesion inside the retro-hepatic IVC, with intraluminal CDUS triphasic waveform; the lesion looked like a vessel inside the IVC extending into the right atrium (Figure 2).
Figure 2.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Ultrasound performed on GE LOGIC E9 with a 4C-RS transducer at a frequency of 4.0 MHz.
Findings: A) The color-Doppler-ultrasound (CDUS) examination showed a mass in the lower abdomen. B, C) the mass was characterized by intralesional vascularization and a tubular lesion inside the inferior vena cava (IVC), with a triphasic CDUS waveform (see the thin white arrow). CTA is mandatory for further evaluation.
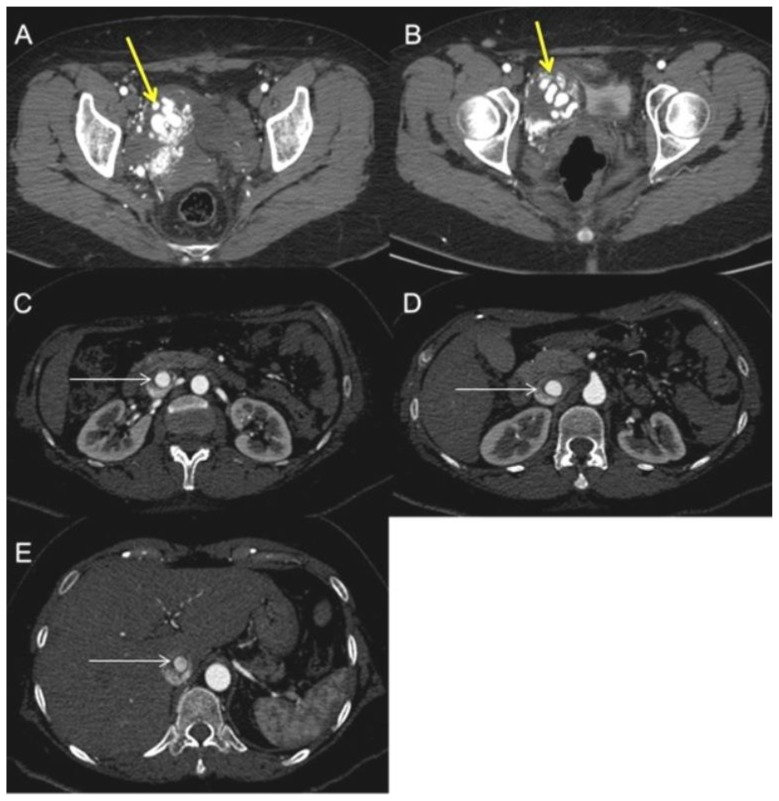
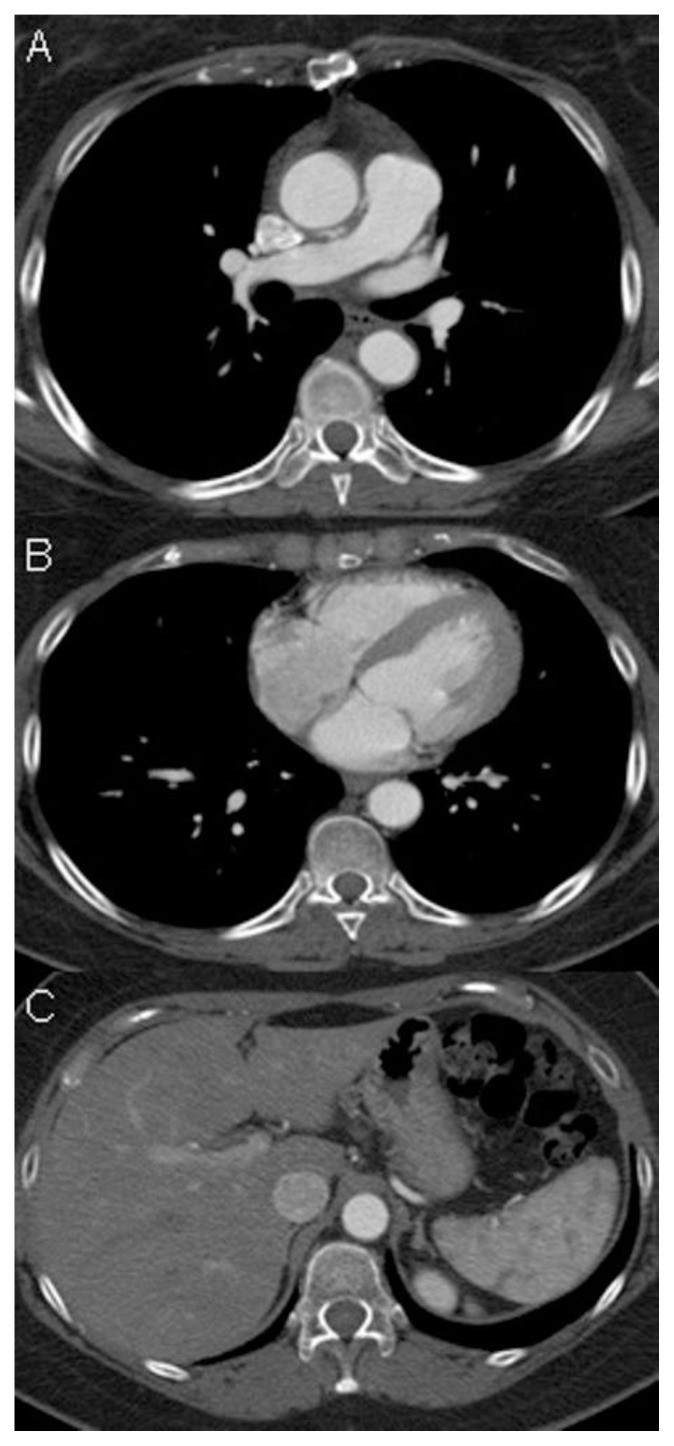
Subsequent chest-abdomen-pelvis Computed Tomography (CT) revealed a voluminous pelvic mass that well demonstrated to be a uterine mass, from which a vessel (characterized by early and marked contrast enhancement) arose, penetrating in the IVC and reaching the right atrium and ventricle (Figure 3, Figure 4), and the pulmonary trunk causing pulmonary embolism (Figure 5). Reconstructions on CT scans showed that the vessel’s origin was due to the uterine mass invading the IVC.
Figure 3.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Contrast enhanced CT, 249 mA, 120kV, 2.5 mm slice thickness, intravenous contrast: 130 mL of Ultravist (Iopromide). GE LightSpeed 64.
Findings: A, B) Axial contrast enhanced CT of the abdomen in the arterial phase demonstrated the presence of a voluminous pelvic mass with significant contrast enhancement due to hypervascularization (see the yellow arrow). C, D, E) Notice the presence of contrast-enhanced large vessels within the IVC (see the thin white arrow).
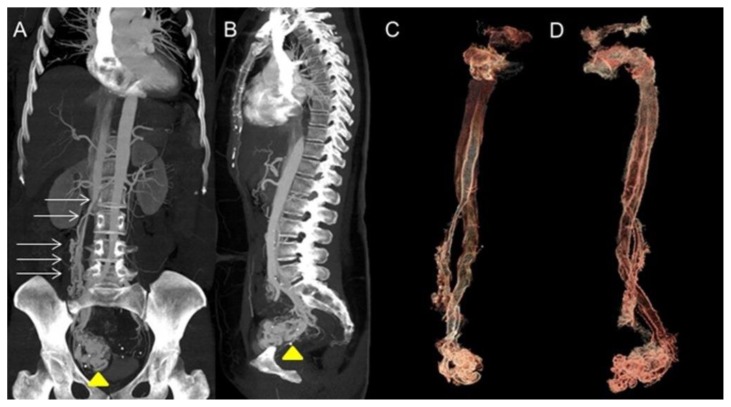
Figure 4.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Contrast enhanced CT, 249mA, 120kV, 2.5 mm slice thickness, intravenous contrast: 130 mL of Ultravist (Iopromide). GE LightSpeed 64.
Findings: A,B) Coronal and sagittal CT Maximum Intensity Projections (MIP) of the abdomen in the arterial phase showing the pelvic tumor (see the yellow arrowhead) directly extending into the IVC (see the thin white arrows).
C,D) 3-D reconstruction from the source data confirmed the origin of the thrombus from a uterine mass invading the right iliac vein and then the IVC.
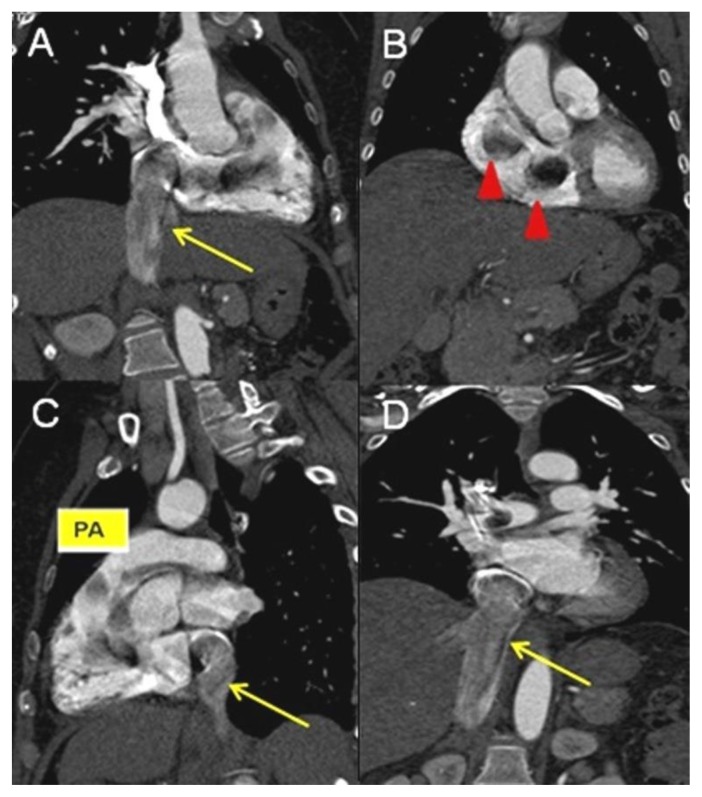
Figure 5.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Contrast enhanced CT, 249mA, 120 kV, 2.5 mm slice thickness, intravenous contrast: 130 mL of Ultravist (Iopromide). GE LightSpeed 64.
Findings: A, B, C, D) Coronal and sagittal CT projections showing the caval (see the yellow arrow) and cardiac extension of the tumor, up to the right atrium, right ventricle (see the red arrowheads), right ventricle outflow, to the pulmonary trunk (pa).
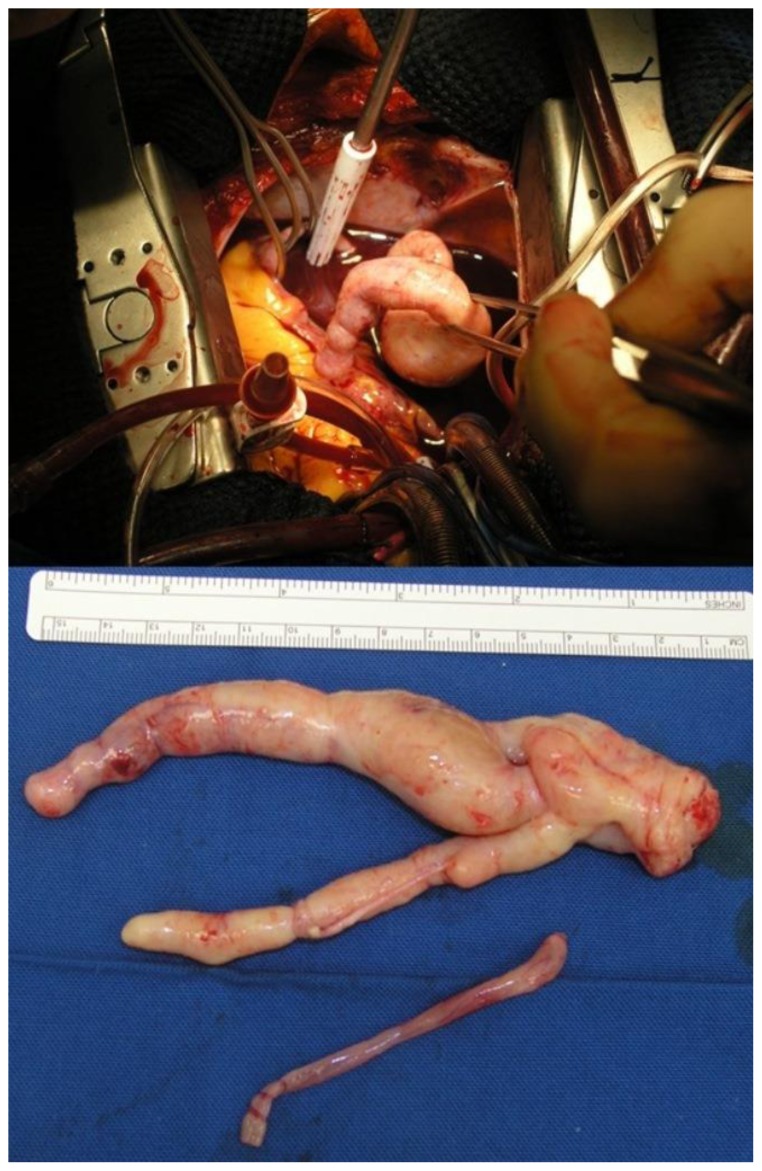
The patient underwent urgent surgical removal of the intracardiac mass and tricuspid valve repair (De Vega) through median sternotomy and right atrium and right pulmonary artery incision. The ascending aorta, superior vena cava, and right femoral vein (with a short cannula) were used for extracorporeal circulation, with moderate hypothermia (28 °C) and short intermittent circulatory arrest sessions to achieve visibility. The mass presented as a smooth tubular lesion obstructing the whole right atrium, and was cut a few centimeters deep into the IVC orifice; the distal end of the mass was attached to the right pulmonary artery wall and appeared as a nodule (Figure 6).
Figure 6.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Image taken intraoperatively during cardiac surgery for intra-cardiac-intra-caval mass removal. It shows the bizarre morphology of the tumor resembling the intraluminal shape of the IVC in which it runs.
The intraoperative pathologist consult revealed intravascular leiomyomatosis.
After weaning from extracorporeal circulation, the tricuspid valve was repaired because of severe regurgitation due to annular dilation.
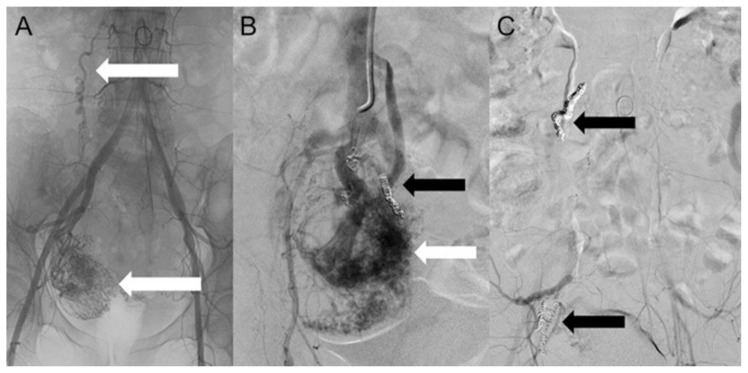
On post-operative day 18, uterine artery embolization was carried out in an attempt to arrest mass growth and minimize the risk of profuse intraoperative bleeding. Arteriography of the hypogastric arteries showed a right-sided hypervascular pelvic mass, with an anomalous peripheral new vessel, invading the IVC. The embolization of the uterine mass was performed using metallic coils. A final angiogram showed a drastic reduction of the vascularization (Figure 7, Figure 8).
Figure 7.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Selective embolization of the uterine mass performed using microcatheter Direxion (Direxion HI-FLO™ Torqueable Microcatheters Boston Scientific) and metallic coils.
Findings: A, B) The angiogram showed a hypervascular pelvic mass, with anomalous peripheral new vessels formation, invading the IVC (see the white and black arrows). C) A final angiogram showed a drastic reduction of the vascularization (see the black arrows).
Figure 8.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Contrast enhanced CT, 249mA, 120kV, 2.5 mm slice thickness, intravenous contrast: 130 mL of Ultravist (Iopromide). GE LightSpeed 64.
Findings: A, B) An axial contrast enhanced CT of the abdomen performed after percutaneous embolization procedure of the uterine mass showed a relevant reduction in tumor size and vascularization. Note the metallic coils (see the yellow arrows).
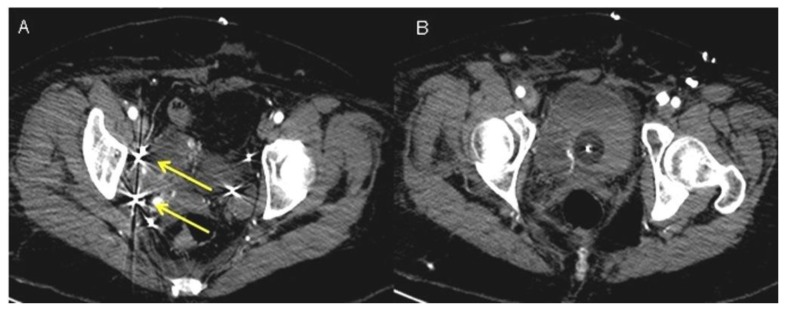
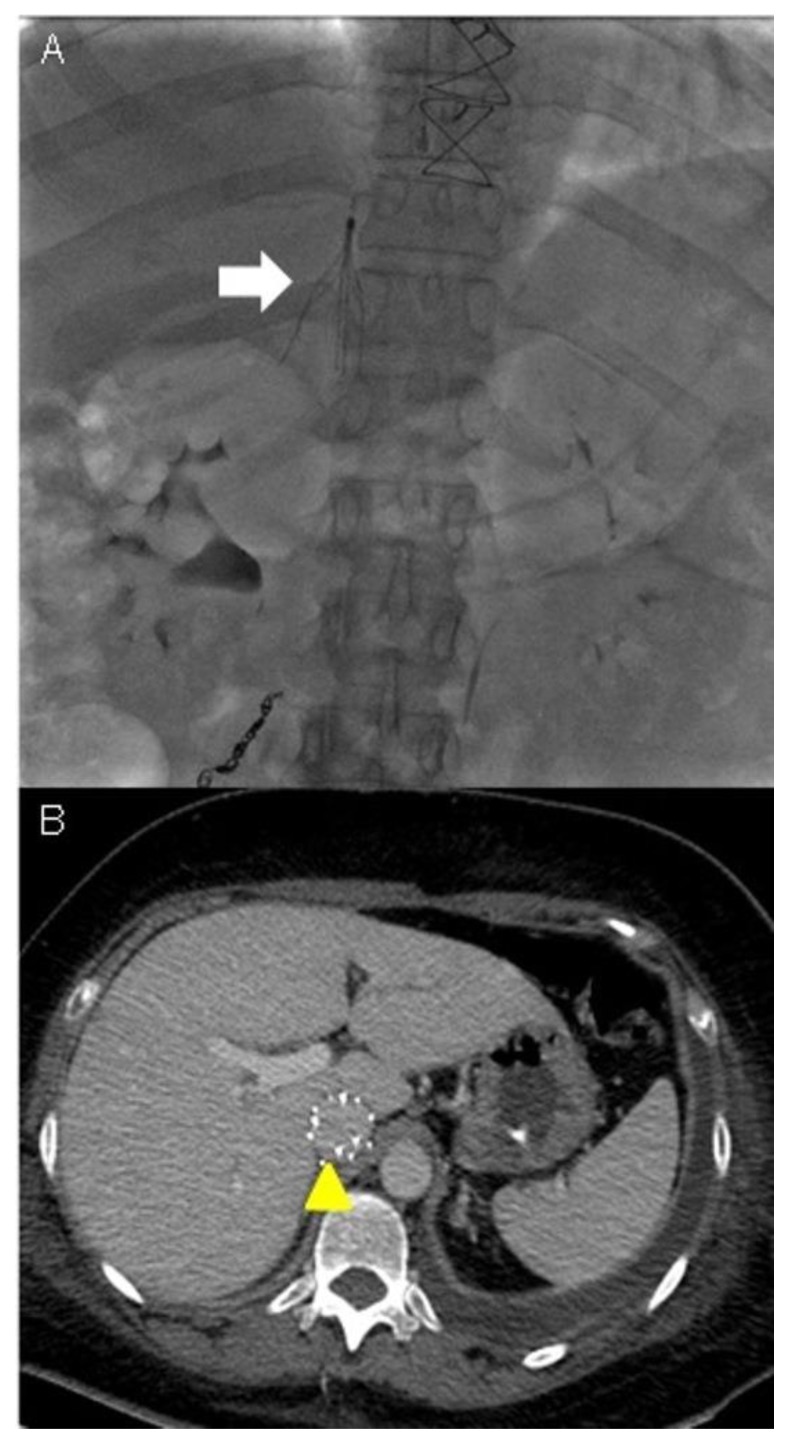
On post-operative day 22, an IVC filter was placed as an alternative therapy to anticoagulation, as the latter was contraindicated (due to the risk of hemorrhage). This aimed to limit both the extension of the thrombus along the IVC into the heart and the risk of recurrence of pulmonary embolism (Figure 9).
Figure 9.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Under radiologic guidance a catheter was inserted via jugular vein access and advanced to the inferior vena cava in the abdomen. Contrast material was then injected into the vein to assess for proper positioning of the IVC filter.
Findings: A) The filter was correctly expanded and attached itself to the walls of the IVC (see the white arrow). B) CTA was used to monitor the results of IVC caval filter. CTA venous phase showed the caval filter in the retrohepatic IVC, of note the anomalous position of the filter was necessary due to the extension of the thrombosis above the renal veins (see the yellow arrowhead).
Second-stage surgery was performed 35 days later. This consisted of a midline laparotomy, longitudinal cavotomy superiorly to the right iliac confluence, and extraction of the intravenous leiomyoma occupying the iliocaval bifurcation extending to the right iliac vein. An “en bloc” resection of the huge pelvic leiomyomatous mass was then performed.
The IVC filter was removed 20 days after the second-stage surgery. Following a three day stay in the intensive care unit, the patient had a recovery free of complications.
The patient was discharged from our hospital on post-operative day 60. At two years follow-up, the patient showed no evidence of disease recurrence (Figure 10). Long-term surgical follow-up and monitoring was recommended nonetheless. The clinical timeline is reported in table 3.
Figure 10.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension.
Technique: Contrast enhanced CT, 249mA, 120kV, 2.5 mm slice thickness, intravenous contrast: 130 mL of Ultravist (Iopromide). GE LightSpeed 64.
Findings: A, B, C) Axial CT images showed complete regression of intravascular and intracardiac leiomyomatosis after the surgical excision. At two-year follow-up the patient showed no evidence of disease recurrence.
DISCUSSION
Etiology & Demographics
Uterine intravenous leiomyomatosis is an uncommon tumor, usually arising from the uterus, with nodular masses extending intravascularly over variable distances and may reach the inferior vena cava, right atrium, and pulmonary arteries. The tumor may arise from uterine leiomyomatosis, walls of uterine vessels, or myometrium [1,2].
There are two main theories which attempt to explain the etiology of uterine intravenous leiomyomatosis [2,3].
One theory suggests direct mural origin of the tumor from the venous walls, whereas the second states that it results from direct vascular invasion by a primary tumor arising from an uterine leiomyoma [2,3].
The second theory is favored in the literature for the following reasons: a) patients with uterine intravenous leiomyomatosis have a history of uterine myoma or have undergone myomectomy or hysterectomy; b) tumor bases were often connected to the uterine wall on pathological and imaging examination; c) tumor cells tested positive for estrogen- and progesterone-receptors on immunohistochemical analysis [3].
Since it is encountered so rarely, uterine intravenous leiomyomatosis is frequently misdiagnosed pre-operatively and not diagnosed on time, subsequently resulting in inappropriate treatment [4]. To date, less than 300 cases have been reported and fewer than 100 cases with cardiac involvement are encountered in the literature [4].
Patients are usually between 20 and 70 years of age, with an average age at presentation of 45 years [1]. Pre-menopausal and multiparous females are most commonly affected.
According to pathologic reports extra-uterine involvement is seen in 30% to 80% of cases of uterine intravenous leiomyomatosis, with cardiac involvement in 10% to 30% [2].
Clinical & Imaging findings
Patients may be asymptomatic even with tumor reaching the IVC, and only develop symptoms once intracardiac tumor extension results in cardiac insufficiency [1,5,6].
Clinical manifestation varies depending on tumor size and extension. Signs and symptoms include: a) syncopal attacks probably due to tricuspid orifice obstruction by the tumor; b) chest tightness, chest pain, and shortness of breathing caused by pulmonary embolism; c) pelvic pain, irregular menses, irregular vaginal bleeding due to pelvic mass; d) swelling in the lower extremities due to inferior vena cava occlusion; e) sudden death from obstruction of the tricuspid valve orifice or the right ventricular outflow tract; f) tachycardia; g) abnormalities in ECG possibly related to enlargement of the cardiac chambers, systolic dysfunction, and involvement of the cardiac valves [1,5,6,7].
In the presence of an abnormal uterine bleeding laboratory tests may detect anemia.
Pre-operative imaging is also useful as it can delineate the full extent of the tumor and may provide accurate information on tumor relationship with the surrounding tissues.
Typical imaging investigation for uterine intravenous leiomyomatosis includes different methods: ultrasonography (US), magnetic resonance imaging (MRI), computed tomography angiography (CTA) [3,7,8,9].
US, especially cardiac ultrasound conducted by an experienced operator, is useful to evaluate intravascular and intra-cardiac lesions, but does require highly specialized training. US may show a floating mass inside the IVC, right atrium, and right ventricle [9].
The importance of MRI in modern diagnostic imaging is on the rise, however this imaging modality is limited by poor spatial resolution and increased number of artifacts. It is time-consuming and not suitable for all patients such as those with cardiac pacemaker or metal intrauterine devices [3,7].
Advanced spatial resolution in modern CTA allows excellent three-dimensional image reconstruction with image detail which rivals that of conventional angiography. It also confers the advantage of rapid acquisition of images from a large field of view (chest, abdomen and pelvis) using just a single bolus of contrast [3]. CTA is superior to US and MRI in mapping the full path of tumor extension and has a shorter examination time compared to MRI [3]. A total body CT scan could clearly display a UIL extension pathway [3].
Patients presenting with cardiac symptoms (chest tightness, dyspnea, palpitations, lower limb swelling, syncope) in addition to abdominal/pelvic mass, should undergo investigation with CTA if the US demonstrates a space-occupying lesion in the lower abdomen or inside the inferior vena cava and heart.
The investigation is performed prior to and following administration of iodinated contrast media at high flow rate (5ml/sec), followed by saline. The amount of contrast media is established based on patient weight: 1.8 ml/kg up to 30 ml. Three dynamic contrast phases are acquired: a) the pulmonary angiographic phase of thorax (acquired around 15–20 seconds after contrast media administration) to study the pulmonary arteries, the right heart chambers and the right ventricular outflow tract; b) the venous phase of the abdomen (acquired around 60–70 seconds after contrast media administration) to evaluate the inferior vena cava and iliac veins; c) the late phase of thorax and abdomen (acquired around 180 seconds after contrast media administration) to investigate thoracic and abdominal organs. Data sets are processed using a workstation that allows interactive post-processing of imaging data, including volume rendering (VR), maximum intensity projection (MIP), and multi-planar reconstruction (MPR).
CTA provides information on location, dimension, morphology, density, blood supply, contrast, extension pathway, and adhesion of the tumor. On CT images, the uterus may be enlarged and myomatous, with heterogeneous enhancing mass or masses, possibly of different size, may calcify to different degrees and show cystic degeneration/necrosis. Enhancing tumors may extend to iliac, or gonadal veins, as well as to the IVC, heart, and pulmonary arteries [1,11].
In the multiplanar reconstruction images, tumor lesions in the pelvis and IVC are continuous, heterogeneously enhancing, and “sausage-shaped”. Typical “walking stick head” or “snake head” tumor masses are observed when the right heart is also involved [2,3,11].
The surface of the tumors protruding into the venous lumen is covered by smooth intima or endothelium, do not demonstrate mural adhesion, and characteristically present as free mass within the venous system.
Cross-sectional imaging generally reveals the tumor to be a circular or semi-circular filling defect in the vascular system, at times with intra-tumoral calcification.
There are several pathways by which UIL can spread intravenously with possible intra-cardiac extension. Two such routes of spread were described by Lam et al. The first involves invasion of an ovarian vein with extension into the infra-diaphragmatic segment of the IVC, bypassing the iliac veins. The second possible pathway is via the uterine vein, with progressive extension to the IVC via the internal and common iliac veins [10,12].
Intra-cardiac propagation of the tumor may result in it reaching the right atrium (45.6% of cases), right ventricle (45.6%), or pulmonary vasculature (8.8%) [12].
Differential Diagnoses
Space-occupying lesions in the venous system or right heart and pulmonary system are considered in the differential diagnosis. Examples of such conditions include intravenous thrombus, Budd-Chiari syndrome, right atrial myxoma, primary leiomyosarcoma, endometrial stromal sarcoma [2,3,12].
These conditions do not usually correlate with a history of uterine leiomyoma.
As opposed to tumor thrombus, intravenous thrombus is not vascularized and therefore does not demonstrate any enhancement on dynamic CT scan.
Patients with Budd-Chiari syndrome present with symptoms resulting from severe ascites, cutaneous portosystemic shunts in the lower limbs and thoracic and abdominal walls, hepatomegaly and splenomegaly. These symptoms which result from partial or complete obstruction of the hepatic veins or intrahepatic IVC are not present in uterine intravenous leiomyomatosis [3].
Right atrium myxoma is mainly limited to the cardiac chambers and may protrude into the pulmonary arteries. The IVC is usually spared. [3,13,14]
Primary leiomyosarcoma is a malignant condition that originates from inferior vena cava.
The various non-specific symptoms common to both conditions make differentiation between primary leiomyosarcoma and uterine intravenous leiomyomatosis challenging at an early stage; metastases to lung, liver and retroperitoneal lymph nodes favor a diagnosis of primary leiomyosarcoma. In this disease heterogeneous enhancement of tumor lesions, invasion of vessel walls and adjacent tissues and formation of collateral circulation may be seen on CTA, features which are absent in UIL. UIL tumors usually have clear boundaries and do not adhere to vascular walls [15].
Endometrial stromal sarcoma is a tumor with low malignant potential; it can invade small veins and lymphatic vessels and can have a lot of spiral arteries seen on CTA images [16].
Treatment & Prognosis
Treatment of UIL is almost always surgical. This includes a combined multidisciplinary thoraco-abdominal surgical approach with excision of the tumor in the right heart chambers and pulmonary trunk (when UIL presents an intracardiac extension), followed by the removal of the uterine mass [13,14,15,16,17].
In our case, the patient underwent a two-stage combined multidisciplinary thoraco-abdominal operation, with an urgent cardiac surgery, followed by abdominal surgery a few weeks later, after a complete recovery of the hemodynamic and respiratory functions.
Myomectomy in uterine leiomyomatosis is often technically challenging and carries a high risk of hemorrhage due to peri-tumoral hypervascularization. Various techniques have been developed to mitigate this risk and facilitate surgery. Preoperative temporary embolization of the uterine arteries using absorbable gelatin particles, and preoperative temporary surgical ligation of the same arteries may help to limit intraoperative blood loss [18, 19].
The most common indication for IVC filter placement is an absolute contraindication for anticoagulation during acute treatment of deep vein thrombosis. Absolute contraindication for anticoagulation would include active uncontrollable bleeding and high risk of major bleeding [20, 21].
There are currently numerous available models of IVC filter, most of which can be placed percutaneously and removed up to a few months after insertion [20, 21].
In the present case, anticoagulation therapy was contraindicated to manage the extensive IVC thrombosis because of the high risk of hemorrhage, due to the hypervascularization of the uterine leiomyomatosis and the clinical finding of metrorrhagia upon admission.
To the best of our knowledge, there is limited evidence in literature on the use of interventional radiology procedures to manage UIL with intra-cardiac extension.
In this case, the interventional radiology procedures (percutaneous embolization and IVC filter placement) were successfully included in the complex therapeutic pathway.
The percutaneous embolization was performed to reduce the size and vascularization of the uterine mass and subsequently the risk of tumor bleeding. The placement of the IVC filter was considered an alternative therapy to anticoagulation, as this was contraindicated, and its aim was to limit the extension of the thrombosis along the IVC into the heart, and the risk of pulmonary embolism.
The IVC filter was removed 30 days after its insertion.
In the post-operative follow-up, CTA was used to monitor response to embolization, the results of IVC filter, and the response to surgery.
Long-term prognosis was very good after complete surgical resection; the patient is alive and free of recurrence with a two-year clinical and imaging follow-up.
TEACHING POINT
Uterine intravenous leiomyomatosis (UIL) can extend into the IVC and right cardiac chambers causing right-sided heart failure and potentially massive pulmonary embolism; a woman with a history of uterine leiomyomas presenting with signs and symptoms of right-sided heart failure and evidence of pulmonary embolism should be assessed for uterine intravenous leiomyomatosis as a potential diagnosis. CTA is the best diagnostic investigative tool to assess the location and size, and highlight the vascular extension of UIL, and can be used as first-line imaging technique because the time required for examination is short.
Table 1.
Summary table for uterine intravenous leiomyomatosis with intracardiac extension.
| Age predilection | Patients are usually between 20 and 70 years of age, with an average age at presentation of 45 years |
| Gender | Female |
| Incidence |
|
| Risk factors | History of leiomyomatosis, myomectomy or hysterectomy |
| Treatment | Surgical: including a combined multidisciplinary thoraco-abdominal surgical approach |
| Prognosis | Usually benign |
| Findings on imaging |
|
Table 2.
Differential diagnosis table for uterine intravenous leiomyomatosis with intracardiac extension.
| Intravenous thrombus | Not vascularized and therefore does not demonstrate any enhancement on dynamic CT scan |
| Budd-Chiari syndrome | Thrombus is observed in the hepatic vein and hepatic segment of inferior vena cava, causing partial or complete venous obstruction; clinical setting includes liver and spleen enlargement, severe ascites, varicosity in the thoracic and abdominal walls as well as lower extremities, symptoms which lack in UIL |
| Right atrial myxoma | Mainly limited within the heart chamber, and generally does not affect the IVC |
| Primary leiomyosarcoma | A malignant condition that originates from inferior vena cava; metastases to liver, lung and retroperitoneal lymph nodes may be present |
| Endometrial stromal sarcoma | Have plenty of spiral arteries on CTA images |
Table 3.
A 48-year-old woman with uterine intravenous leiomyomatosis and intracardiac extension - Clinical case timeline.
| Past medical history: multiple uterine myomectomies | Two years before |
| Presenting Symptoms: progressive dyspnea, palpitations, metrorrhagia, generalized pelvic pain, and bilateral leg edema) | Day 1 |
| Physical Examination: tachypnea, bilateral mild pitting edema of the lower legs, and jugular venous distension) | Day 1 |
| Diagnostic Evaluation: chest X-ray, Doppler transthoracic echocardiography, color-Doppler Ultrasound of the abdomen, chest-abdomen-pelvis Computed Tomography | Day 1 |
| Diagnosis: pelvic mass extending into the right iliac vein and ascending trough the inferior vena cava to the right heart and causing pulmonary embolism | Day 1 |
| First-stage surgical procedure | Day 2 |
| Uterine artery embolization | Day 18 |
| IVC filter placement | Day 22 |
| Second-stage surgery | Day 35 |
| IVC filter removal | Day 54 |
| Discharge | Day 59 |
| Follow up: the patient showed no evidence of disease recurrence | two years after |
ABBREVIATIONS
- CDUS
Color-Doppler Ultrasound
- CT
Computed Tomography
- CTA
Computed Tomography Angiography
- IVC
Inferior Vena Cava
- MIP
Maximum Intensity Projection
- MPR
Multi-planar Reconstruction
- MRI
Magnetic Resonance Imaging
- UIL
Uterine intravenous leiomyomatosis
- US
Ultrasonography
- VR
Volume Rendering
REFERENCES
- 1.Fornaris RJ, Rivera M, Jiménez L, Maldonado J. Multimodality Evaluation of Intravenous Leiomyomatosis: A Rare, Benign but Potentially Life-Threatening Tumor. Am J Case Rep. 2015;16:794–800. doi: 10.12659/AJCR.894939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakai G, Maeda K, Yamamoto K, Yamada T, Hirose Y, Terai Y, Ohmichi M, Katsumata T, Narumi Y. Uterine Intravenous Leiomyomatosis with Cardiac Extension: Radiologic Assessment with Surgical and Pathologic Correlation. Case Rep Obstet Gynecol. 2015;2015 doi: 10.1155/2015/576743. 576743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gui T, Qian Q, Cao D, Yang J, Peng P, Shen K. Computerized tomography angiography in preoperative assessment of intravenous leiomyomatosis extending to inferior vena cava and heart. BMC Cancer. 2016;16:73. doi: 10.1186/s12885-016-2112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu ZF, Yong F, Chen YY, Pan AZ. Uterine intravenous leiomyomatosis with cardiac extension: Imaging characteristics and literature review. World J Clin Oncol. 2013;4(1):25–28. doi: 10.5306/wjco.v4.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Kim DK, Narm KS, Cho SH. Pulmonary artery embolization of intravenous leiomyomatosis extending into the right atrium. Korean J Thorac Cardiovasc Surg. 2011;44(3):243–6. doi: 10.5090/kjtcs.2011.44.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HJ, Choi J, Kim KR. Pulmonary benign metastasizing leiomyoma associated with intravenous leiomyomatosis of the uterus: clinical behavior and genomic changes supporting a transportation theory. Int J Gynecol Pathol. 2008;27(3):340–5. doi: 10.1097/PGP.0b013e3181656dab. [DOI] [PubMed] [Google Scholar]
- 7.Kang LQ, Zhang B, Liu BG, Liu FH. Diagnosis of intravenous leiomyomatosis extending to heart with emphasis on magnetic resonance imaging. Chin Med J (Engl) 2012;125(1):33–7. [PubMed] [Google Scholar]
- 8.Li R, Shen Y, Sun Y, Zhang C, Yang Y, Yang J, Su R, Jiang B. Intravenous Leiomyomatosis with Intracardiac Extension: Echocardiographic Study and Literature Review. Tex Heart Inst J. 2014;41(5):502–506. doi: 10.14503/THIJ-13-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lou YF, Shi XP, Song ZZ. Intravenous leiomyomatosis of the uterus with extension to the right heart. Cardiovasc Ultrasound. 2011;9:25. doi: 10.1186/1476-7120-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peña A, Tamaña M. Intracardiac extension of intravenous leiomyoma, a rare phenomenon: A case report. Radiol Case Rep. 2018;13(2):427–430. doi: 10.1016/j.radcr.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun C, Wang XM, Liu C, Xv ZD, Wang D, Sun XL. Intravenous leiomyomatosis: diagnosis and follow-up with multislice computed tomography. Am J Surg. 2010;200(3):e41–e43. doi: 10.1016/j.amjsurg.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Lam P, Lo KW, Yu MY, Wong WS, Lau JY, Arifi AA. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg. 2004;39(2):465–469. doi: 10.1016/j.jvs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Clay TD, Dimitriou J, McNally OM, Russell PA, Newcomb AE, Wilson AM. Intravenous leiomyomatosis with intracardiac extension - A review of diagnosis and management with an illustrative case. Surgical Oncology. 2013;22(3):e44–e52. doi: 10.1016/j.suronc.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Shi E, Gu T, Xiu Z, Fang Q, Wang C. Intravenous leiomyomatosis with intracardiac extension: a report of two cases. J Card Surg. 2011;26(1):56–60. doi: 10.1111/j.1540-8191.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 15.Castagneto Gissey L, Mariano G, Musleh L, Lepiane P, Colasanti M, Meniconi RL, Ranocchi F, Musumeci F, Antonini M, Ettorre GM. Massive pelvic recurrence of uterine leiomyomatosis with intracaval-intracardiac extension: video case report and literature review. BMC Surg. 2017;17(1):118. doi: 10.1186/s12893-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalaguier-Coudray A, Allain-Nicolai J, Thomassin-Piana R, Villard-Mahjoub B, Delarbre B, Rua S, Lambaudie, Houvenaeghel G. Radio-surgical and pathologic correlations of pelvic intravenous leiomyomatosis. Abdominal Radiology. 2017;42(12):2927–2932. doi: 10.1007/s00261-017-1225-1. [DOI] [PubMed] [Google Scholar]
- 17.Du J, Zhao X, Guo D, Li H, Sun B. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol. 2011;42(9):1240–1246. doi: 10.1016/j.humpath.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Tixier H, Grevoul J, Loffroy R, Lauferon J, Guiu B, Mutamba W, Filipuzzi L, Cercueil JP, Douvier S, Krause D, Sagot P. Preoperative embolization or ligature of the uterine arteries in preparation for conservative uterine fibroma surgery. Acta Obstet Gynecol Scand. 2010;89(10):1310–5. doi: 10.3109/00016349.2010.512060. [DOI] [PubMed] [Google Scholar]
- 19.Butori N, Tixier H, Filipuzzi L, Mutamba W, Guiu B, Cercueil JP, Douvier S, Sagot P, Krausé D, Loffroy R. Interest of uterine artery embolization with gelatin sponge particles prior to myomectomy for large and/or multiple fibroids. Eur J Radiol. 2011;79(1):1–6. doi: 10.1016/j.ejrad.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Duffett L, Carrier M. Inferior vena cava filters. J Thromb Haemost. 2017;15(1):3–12. doi: 10.1111/jth.13564. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita Y, Unoki T, Takagi D, Hamatani Y, Ishii M, Iguchi M, Ogawa H, Masunaga N, Wada H, Hasegawa K, Abe M, Akao M. Indications, applications, and outcomes of inferior vena cava filters for venous thromboembolism in Japanese patients. Heart Vessels. 2016;31(7):1084–90. doi: 10.1007/s00380-015-0709-6. [DOI] [PubMed] [Google Scholar]