ABSTRACT
Long non-coding RNAs (lncRNAs) are key mediators of cancer. The dysregulation of a lncRNA, CASC15, has been linked to several cancers, except lung cancer. Here, the aim of the study was to explore the role and mechanism of CASC15 in lung cancer regulation, with the focus on its interaction with a potential target, microRNA-766-5p (miR-766-5p) and an oncogene, kallikrein-related peptidase 12 (KLK12). Quantitative real-time PCR (qRT-PCR) was used to assess levels of CASC15, miR-766-5p and KLK12 in lung cancer tissues or cells. Western blot analysis was used to detect KLK12 protein expression. Ectopic expression of CASC15 was induced by a lentiviral system. CCK-8 and transwell assays were used to evaluate lung cancer cell proliferation and invasion, respectively. The interaction among CASC15, miR-766-5p and KLK12 was investigated by bioinformatical analysis and luciferase assay. In lung cancer tissue and cells, CASC15 was upregulated, while miR-766-5p was downregulated. Overexpression of CASC15 promoted lung cancer cell proliferation and invasion. A negative correlation was found between CASC15 and miR-766-5p levels. Overexpression of miR-766-6p reversed the cancer-promoting role of CASC15 in lung cancer cells, which was mediated by KLK12. The tumor-promoting role of CASC15 and tumor-suppressing role of miR-766-5p were also validated in vivo in tumor bearing mice, and KLK12 was also shown as an important mediator. CASC15 promotes lung cancer through the miR-766-5p/KLK12 axis, indicating that CASC15 is a potential therapeutic in lung cancer.
KEYWORDS: Lung cancer, CASC15, miR-766-5p/KLK12
Introduction
Lung cancer is a leading cause of cancer-related mortality worldwide with a 5-year survival rate of less than 15%, despite the advances in both diagnostic and therapeutic approaches [1]. Lung cancer arises from the cells of the respiratory epithelium and can be divided into two broad categories: Small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC). SCLC accounts for 15% of lung cancer cases. It is a highly malignant tumor derived from cells exhibiting neuroendocrine characteristics. NSCLC accounts for the remaining 85% of cases, and is further divided into three major pathologic subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [2]. A combination of genomic and transcriptomic sequence have identified uncovered the widespread expression of “noncoding RNAs” including long non-coding RNAs (lncRNAs), which impact biologic responses through the regulation of mRNA transcription or translation [3].
LncRNAs form part of the human genome and are non-protein coding RNA transcripts longer than 200 nucleotides, characterized by capped, polyadenylated, and spliced transcripts that lack an open reading frame [4]. Although they show great similarities in their genetic organization, they play various roles at the cellular level, including regulation of transcription and translation, leading to alterations in gene expression and regulation of expression of chromosomally adjacent genes [3]. LncRNAs are involved in many cellular processes such as genomic imprinting, chromatin modification, protein transport and trafficking and RNA alternative splicing [5,6]. Based on functions, lncRNAs are classified as signaling, decoy, guide, and scaffold lncRNAs [7]. Unlike protein-coding genes, which are usually conserved across species, most lncRNAs are poorly conserved and hence have been taken for transcriptional noise [8]. Recent focus has been on the plausible role of the lncRNAs in progression of human diseases, especially polygenic disorders [9–11].
Currently, many studies have focused on the role of lncRNAs in lung cancer [12,13]. For example, Xu et al. found that lncRNA DB327252 was up-regulated in lung cancer, with its overexpression being correlated to various biological activities [14]. Park et al. found that lncRNA EPEL promoted lung cancer cell proliferation through e2f target activation [15], while Cheng et al. found that lncRNA BC087858 could stimulate acquired resistance to EGFR-TKIs in NSCLC and might contribute to a shorter progression-free survival (PFS). Another study by Pan et al. found that lncRNA BC087858 could induce non-T790M mutation acquired resistance to EGFR-TKIs by activating PI3K/AKT and MEK/ERK pathways [16].
CASC15 is a novel lncRNA that has been shown to be involved in different cancers. Yao et al. found that high expression of CASC15 was a high-risk factor for gastric cancer prognosis and promoted the proliferation of gastric cancer [17]. Wu et al. further showed that CASC15 regulated gastric cancer cell proliferation, migration and epithelial mesenchymal transition by targeting CDKN1A and ZEB1 [18]. Fenando et al., while working on leukemia, found that lncRNA CASC15 led to a myeloid bias in development, and overall, decreased engraftment and colony formation. At the cellular level, they showed that CASC15 regulated cellular survival, proliferation, and the expression of its chromosomally adjacent gene, SOX4 [19]. The role of CASC15 in the development of lung cancer has not been previously reported. The current study thus aims to unravel the expression and role of lncRNA CASC15 in lung cancer.
Methods
Clinical tissue specimens
This study was approved by the Ethics Committees of The First Affiliated Hospital of Zhengzhou University. Lung cancer tissues and paired adjacent non-tumor tissue were collected from The First Affiliated Hospital of Zhengzhou University between Jun 2012 and Sep 2014 from a total of 52 patients. Written informed consent was obtained from all patients. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C.
Cell lines and culture
Human lung cancer cell lines, including A549, H1299, H1975 and SPC-A-1 cells, and normal human lung epithelial cell, BEAS-2B, were acquired from American Type Culture Collection (ATCC) (Manassas, VA), which were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37°C.
Oligonucleotides transfection
Oligonucleotides, including siRNAs against CASC15 (si-CASC15), short-hairpin RNA plasmid directly targeting CASC15 (sh-CASC15), miR-766-5p inhibitor, miR-766-5p mimics, and their controls were synthesized by GenePharma (Shanghai, China). Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used to transfect oligonucleotides into cells according to the manufacturer’s protocol. The sequence of siRNA for CASC15 and Control were: Si-CASC15, sense 5′- CCCCATGATGGTTCCTCAGTT-3′, antisense 3′- GGAAAGCAAGTGCAGGTTAGTC-5′; Control: sense: 5ʹ- GGCCGTCACTCAATGATTCCG −3ʹ, antisence: 5ʹ-UUTTGGATGGCATACGCATGA-3ʹ;
BLAST alignment
Alignment searches to RNA sequences were performed with NCBI’s BLAST suite and the top search results with an e-value of <0.01 were reported. RNA transcripts were allowed to possess multiple exons aligning to different non-contiguous regions of a chromosome.
qRT-PCR
Total RNAs from tissues and cells were extracted using the miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) in accordance to the manufacturer’s instructions. RNAs were characterized for concentration and quality using NanoDrop 2000 (Thermo Fisher, Wilmington, DE, USA). cDNA synthesis was performed using the TransScript first-strand cDNA synthesis SuperMix (TransGen, Beijing, China). RT-PCR assay was performed in ABI prism 7500 sequence detection system (Applied Biosystems Life Technologies) using the SYBR green qPCR SuperMix (Applied Biosystems Life Technologies, Foster, CA, USA). Fold changes of each gene were calculated using the 2− ΔΔCt (cycle threshold) method, and expression levels of miRNA and lncRNA/target gene were normalized by U6 and GADPH, respectively. The following primers were used in this study: CASC15, forward: sense 5′- CCCCATGATGGTTCCTCAGTT-3′, reverse 3′-GGAAAGCAAGTGCAGGTTAGTC-5′; miR-766-5p, forward: 5′- TAAAATAGGAGTACTGTCTAA-3′, reverse, 5′- ATTAGTAAATTGGCTGCTGCAG-3ʹ; GAPDH, forward, 5′-TCGACAGTCA GCCGCATCTTCTTT-3′, reverse, 5′-ACCAAATCCGTTGACTCCGACCTT-3′.
Luciferase reporter assay
Oligonucleotides containing CASC15 cDNA fragment that encompasses the microRNA binding sites was amplified and cloned into the pmirGLO plasmids (Promega, Madison, WI, USA). Mutant CASC15 (pmirGLO-CASC15 -MUT) was generated by site-directed mutagenesis PCR with platinum pfx DNA polymerase, which served as negative control. Lipofectamine 2000 was used to transfect the luciferase reporter plasmids and target miR-766-5p mimics or miR-NC mimics into cells. At 48 h after transfection, relative luciferase activity was measured in a luminometer by Dual-Luciferase Reporter Assay System (Promega).
Cell proliferation assay
Proliferation of cells was detected using the Cell Counting Kit-8 (CCK-8; Dojindo, JPN). A549 and H1299 cells were transfected with si-NC, si-CASC15, miR-766-5p inhibitor or si- CASC15+miR-766-5p inhibitor, followed by harvestation and seeding into 96-well plates. After 24, 48 72 or 96 h, 10 ul of CCK-8 assay reagent was added to each well, incubated for 2 h, followed by measuring absorbance at 480 nm using an enzyme immunoassay analyser (Bio-rad, Hercules, CA, USA).
Cell migration and invasion assay
The migration and invasion of cancer cells were evaluated using scratch wound assay and transwell assay, respectively. In wound healing assay, A549 and H1299 cells were seeded in six-well plates and cultured to 90% confluence. After removal of the medium, the cell monolayer was manually scraped using a sterile pipette tip. After 24 h, the width of the wound gap was measured and expressed as relative percentage of the initial gap width at 0 hr using the following formula: migration rate = migration distance/original distance. For transwell assay, A549 and H1299 cells, seeded at the density of 5 × 104 per well, were suspended in 200 ml serum-free DMEM. The BioCoat Matrigel was used to coat the chambers (8 mm, BD Biosciences) according to the manufacturer’s protocol. After 24 h incubation, cells on the upper membrane surface were removed with a cotton tip and the member of lower chamber was fixed and stained by violet crystalline.
Western blot analysis
Cells were lyzed by RIPA buffer (Sigma–Aldrich, St. Louis, MO) supplemented with protease inhibitors cocktail (Roche, Diagnostics, Mannheim, Germany). The lysate was centrifuged with 12,000 g and total protein concentration in the supernatant was measured using BCA assay. Proteins of equal amounts were loaded into 10% gel and separated by SDS–PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), followed by transferring to PVDF membrane (Millipore, Bedford, MA). PVDF membrane was blocked with no-fat milk overnight at 4°C and incubated with primary antibodies, including anti-KLK12 (1:1,000, Abcam, Cambridge, MA) or anti-GADPH (1:1,000, Abcam) for 1 h at room temperature. Following this, PVDF membrane was washed with TBST and incubated with conjugated goat anti-rabbit IgG (Abcam) at room temperature for 2 h. Finally, protein bands were visualized using ECL detection kit (Beyotime Biotechnology, Shanghai, China).
Lentivirus construction and infection
A recombinant lentiviral vector expressing CASC15-shRNA was constructed by Shanghai Genechem. CASC15-shRNA was cloned into the pFU-GW-RNAi vector, which contained the green fluorescent protein (GFP) reporter gene driven by the U6 promoter. A549 cells were seeded onto 6-well plates at the density of 2 × 105 cells per well. After 12 h, A549 cells were infected with Lv-shRNA-NC or Lv-shRNA-CASC15 at 10 MOI, respectively. Virus-containing culture medium was replaced with fresh RPMI-1640 medium at 12 h postinfection.
In vivo animal experiments
Animal study was conducted in accordance to the experimental protocols approved by the Institutional Animal Care and Use Committee of The First Affiliated Hospital of Zhengzhou University. Briefly, 1 × 107 A549 cells stably transfected with lentiviruses carrying sh-CASC15 or sh-NC were injected subcutaneously into the flank of BALB/c nude mice (Jacksons labratories, USA, age of 6 weeks, weight of 20-25 g). Tumor growth was monitored by caliper measurements every 3 days and tumor volume were calculated according to the following formula: volume = 0.5 × length × width × width. The mice were sacrificed by injection of 120 μl 10% chloral hydrate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) at the experimental endpoint, and the tumors were harvested, weighed and photographed.
Immunohistochemical staining
Tumor tissue sections from nude mice were dried for 1 h at 60°C, dewaxed in xylene, and rehydrated by graded ethanol using standard methods. Antigen retrieval was achieved by autoclaving the slides for 90 s at 121°C in citrate buffer (pH 6.0). After PBS washing, goat serum (Boster, Wuhan, China) was used to block the sections for 30 min at room temperature. Subsequently, Ki67 antibody (Bioss Antibodies, Inc, 1:200) was used to incubate the sections overnight at 4°C or Colorimetric TUNEL Apoptosis Assay Kit (Beyotime, Shanghai, China) at 37°C for 60 min. Next, sections were incubated with Polink-1 HRP DAB Detection System One-step polymer detection system (ZSGB-BIO, Beijing, China) for 20 min at room temperature. Slides were counterstained with hematoxylin.
Statistical analysis
All the statistical data are presented as the means SD. Two-tailed Student’s t-test or one-way ANOVA followed by the LSD post hoc test was performed for comparisons between groups. Expression correlation assays were analyzed using Pearson’s coefficient correlation. Differences in patient survival were performed using the Kaplan-Meier method and analyzed by log-rank test. A value of P < 0.05 was considered to be statistically significant.
Results
CASC15 upregulation is a characteristic of lung cancer
To explore the role of CASC15 in lung cancer, the tumor tissue and adjacent normal tissue were collected from 52 patients with lung cancers of stage I-II. qRT-PCR was used to quantify CASC15 levels. As shown in Figure 1(a), compared to normal tissue, tumor tissue exhibited markedly upregulated CASC15 expression (p < 0.05, n = 52). Based on median expression of CASC15, patients were divided to low expression group (n = 24) and high expression group (n = 28) (Figure 1(b)). Survival analysis showed that patients with a high CASC15 demonstrated much poorer survival than those with a low CASC15 expression (Figure 1(c)). These data implicated that CASC15 upregulation is a characteristic of lung cancer and may play an important role in lung cancer progression.
Figure 1.
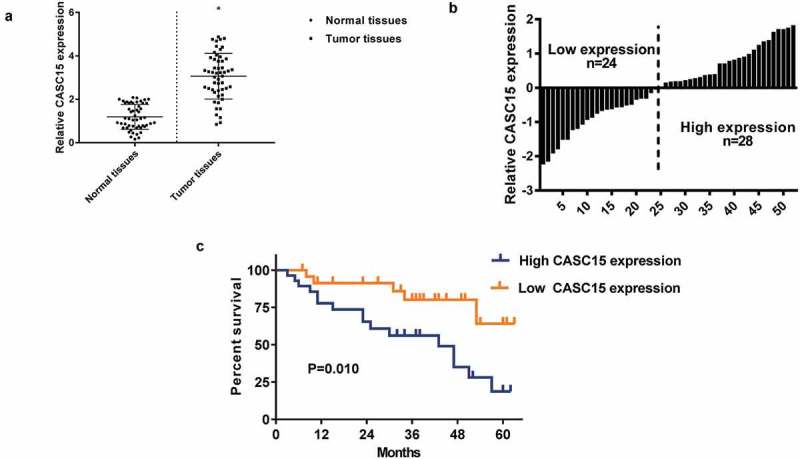
CASC15 upregulation is a characteristic of lung cancer.
(a). qRT-PCR analysis of CASC15 mRNA levels in normal and tumor tissues. Levels of CASC15 were normalized to GAPDH expression. (b) Grouping of patients based on the median level of CASC15 expression in tumor tissues. (c) Survival curves of patients with high or low CASC15 expression.
CASC15 upregulatin in lung cancer cells promotes cell proliferation and invasion
A number of lung cancer cells, including A549, H1299, H1975 and SPC-A-1 were investigated for CASC15 expression. The CASC15 levels in the normal lung cell, BEAS-2B were used as control. As shown in Figure 2(a), the upregulation of CASC15 in the lung cancer cells was quite prominent (p < 0.05), and because A549 and H1299 showed the strongest upregulation, these two cell lines were used in subsequent studies. Three siRNAs specific to CASC15 were designed, namely si-CASC15-1, si-CASC15-2, si-CASC15-3, and si-CASC1-3 demonstrated the most efficacious silencing of CASC1 in both A549 and H1299 cells (Figure 2(b)). Using si-CASC15-3, we showed that cells transfected with si-CASC15-3 exhibited a diminished cell proliferation (Figure 2(c)), colony formation (Figure 2(d)), migration (Figure 3(e)) and invasion (Figure 3(f)).
Figure 2.
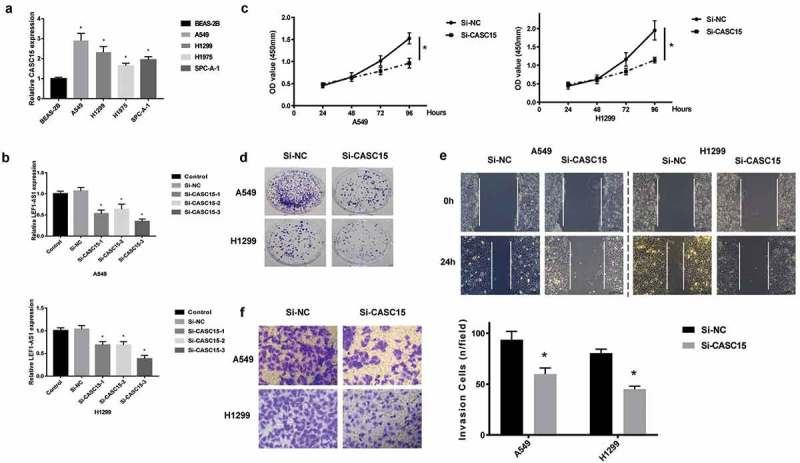
CASC15 upregulation in lung cancer cells promotes cell proliferation and invasion.
(a). qRT-PCR analysis of CASC15 levels in lung cancer cells, including A549, H1299, H1975 and SPC-A-1. Normal lung cell, BEAS-2B was used as control. (b). Evaluation of CASC15 silencing using three siRNA specific to CASC15, namely si-CASC15-1, siCASC15-2, si-CASC15-3. Non-transfected cell (control) and cells transfected with si-NC were used as controls. (c). CCK-8 assay of cell proliferation. (d). Colony formation assay. (e). Scratch wound assay of cell migration. (f). Transwell assay of cell invasion.
Figure 3.
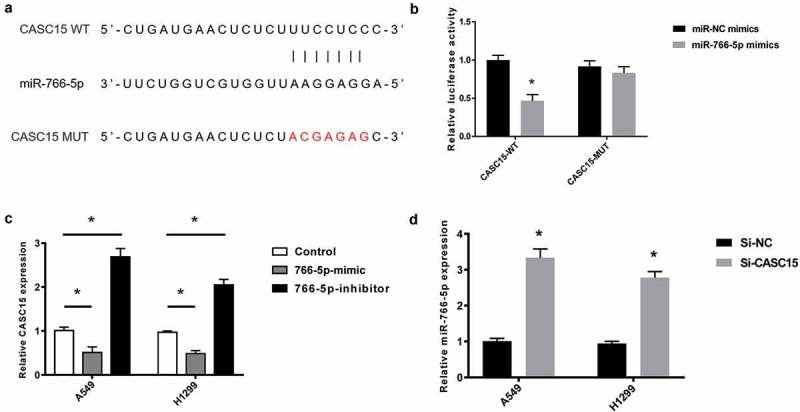
miR-766-5p is a target of CASC15.
(a). Bioinformatical analysis of the binding between CASC15 WT, miR-766-5p and CASC15 MUT. (b). Dual-luciferase assay of the interaction between miR-766-5p and CASC15-WT or CASC15-MUT. (c). qRT-PCR analysis of CASC15 levels in AS549 and H1299 transfected with miR-766-5p mimic or miR-766-5p inhibitor. Non-transfected cells were used as controls. (d). qRT-PCR analysis of miR-766 levels in A549 and H1299 cells transfected with si-NC or si-CASC15.
miR-766-5p is a target of CASC15
We next proceeded to explore the mechanism of CASC15 regulation in lung cancer. Bioinformatical analysis indicated that a binding site exists between CASC15 and miR-766-5p (Figure 3(a)). The mutant CASC15 was constructed by mutating the binding sequences. Dual-luciferase assay showed that transfection of miR-766-5p induced significant decrease in CASC5-WT activity. Whereas, miR-766-5p transfection did not led to significant changes in CASC15-MUT activity (Figure 3(b)). qRT-PCR also suggested that miR-766-5p overexpression led to inhibition of CASC15 expression, while miR-766-5p inhibition resulted in an increase in CASC15 expression, in both A549 and H1299 cells (Figure 3(c)). Consistently, transfection of si-CASC15 also induced upregulation of miR-766-5p (Figure 3(d)), suggesting that miR-766-5p and CASC15 reciprocally inhibited each other.
CASC15 promotes lung cancer through the inhibition of miR-766-5p
To elucidate the role of CASC15 in affecting lung cancer cell phenotypes, the proliferation, migration and invasion of cells transfected with miR-766 inhibitor, si-CASC15 or both were analyzed. It was clear that transfection with si-CASC15 markedly attenuated cell proliferation (Figure 4(a)), colony formation (Figure 4(b)), migration (Figure 4(c)) and invasion (Figure 4(d)). On the contrary, transfection with miR-766-5p inhibitor exerted the opposite effects. Cells transfected with both CASC15 and miR-766-5p inhibitor exhibited proliferation, colony formation, migration and invasion similar to those of the cells transfected with si-NC. Further, we show that the cancer proliferation and invasion marker, kallikrein-related peptidase 12 (KLK12) [20–22], was inhibited by si-CASC15 but promoted by miR-766-5p inhibitor, while transfection of both did not alter KLK12 expression significantly (Figure 4(e)). These evidences corroborated the antagonizing effects of miR-766-5p inhibitor and si-CASC15.
Figure 4.
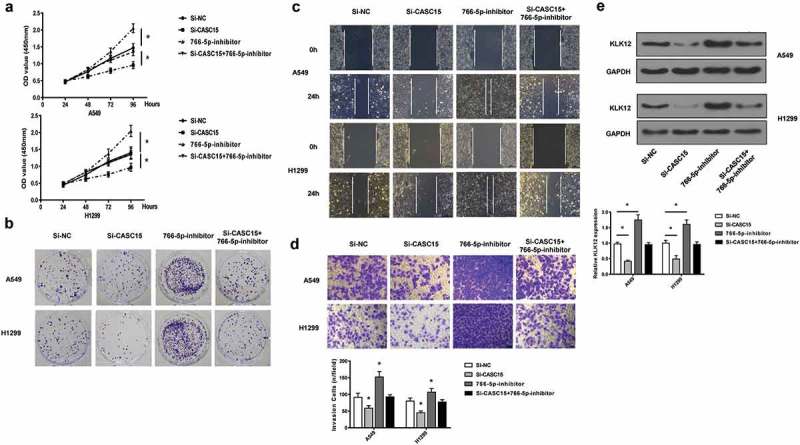
CASC15 promotes lung cancer through the inhibition of miR-766-5p.
(a). CCK-assay of proliferation of A549 and H1299. (b). Colony formation assay. (c). scratch wound assay. (d). Transwell assay. €. Western blot fo KLK-12 in lung cancer cells. GAPDH was used as a loading control.
CASC15 knockdown attenuates tumor growth in vivo
Given that CASC15 silencing was able to inhibit lung cancer cell proliferation, migration and invasion in vitro, we next examine if CASC15 knockdown would exert anti-tumor effects in vivo. To this end, A549 cells, transfected with Lv-sh-CASC15 or Lv-sh-control were inoculated in mice. After 8 weeks, mice were sacrificed and tumors were harvested. As shown in Figure 5(a), tumors with Lv-sh-CASC15 were observably smaller. A slower tumor growth was also observed for tumors with Lv-sh-CASC15. Staining of Ki-67 also showed a reduction of Ki-67 expression in tumors with Lv-sh-CASC15 transfection (Figure 5(b)). CASC15 and miR-766 levels were also attenuated and increased, respectively, in tumor with Lv-sh-CASC15 transfection, as revealed by qRT-PCR (Figure 5(c)). Western blot analysis of KLK-12 also confirmed that CASC15 knockdown reduced KLK12 expression. Together, these data validated the anti-tumor role of CASC15 silencing in lung cancer.
Figure 5.
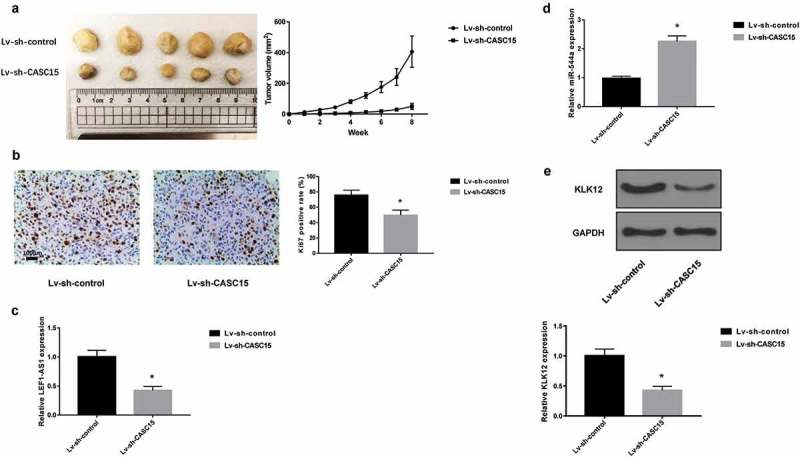
CASC15 knockdown attenuates tumor growth in vivo.
(a). Photograph of harvested tumor at 8 weeks and tumor growth curve. (b). Staining of Ki-67. qRT-PCR analysis of CASC15 (c) and miR-766 levels (d). E. Western blot analysis of KLK12.
Discussion
The current study is reporting for the first time an up-regulation of CASC15 expression in individuals who had lung cancer as compared to the healthy controls. This observation is in agreement with other previous studies that reported up-regulation of CASC15 for other types of cancers [17,18]. Survival analysis showed a poor prognosis for patients with a high CASC15 as compared to those with a low CASC15 expression. Poor prognosis has also been previously associated with other types of lncRNAs in relation to lung cancer. For example, Nie et al., demonstrated that increased levels of lncRNA MVIH was a poor prognostic biomarker in NSCLC [23]. Gao et al. on the other hand identified a novel lncRNA TCONS_00001798 in patients with non‑small cell lung cancer. They demonstrated that TCONS_00001798 was significantly down-regulated in NSCLC tumor tissues compared with adjacent non‑tumor tissues and that the decreased expression of TCONS_00001798 was negatively associated with lymph node metastasis and advanced pathological stage [24].
We demonstrated an increase in proliferation of lung cancer cells that had high CASC15 as compared to those with low expression. This was confirmed with use siRNA specific to CASC15 where the transfected cells showed diminished cell proliferation, colony formation, migration and invasion. This observation is similar to what has been previously observed in other cancers involving CASC15 [18,25]
We further explored the potential target for CASC15 in order to understand its contribution to lung cancer. Bioinformatical analysis indicated that a binding site exists between CASC15 and miR-766-5p. We found that transfection of miR-766-5p induced significant decrease in CASC5-WT activity, whereas transfection of miR-766-5p did not led to significant changes in CASC15-MUT activity. qRT-PCR also suggested that miR-766-5p overexpression led to inhibition of CASC15 expression, while miR-766-5p inhibition resulted in an increase in CASC15 expression. These data collectively suggest an antagonistic role of miR-766-5p and CASC15 on each other. A similar antagonistic role of miR-766-5p was observed by Jia et al. in colorectal cancer where they showed that miR-766-5p inhibitor repressed the process of colorectal cancer by targeting suppressor of cancer cell invasion (SCAI) [26]. Wang et al. found that miR-766 could serve as a novel p53 activator which functioned by targeting MDM4 and thereby enhancing the p53 signaling axis [27]. Thus that miR-766-5p can serve a potential marker for cancer therapies.
We also established that CASC15 promoted lung cancer through the inhibition of miR-766-5p. Cells transfected with si-CASC15 had marked attenuated cell proliferation, colony formation, migration and invasion. Conversely, transfection with miR-766-5p inhibitor exerted the opposite effects. Cells transfected with both CASC15 and miR-766-5p inhibitor exhibited proliferation, colony formation, migration and invasion similar to those of the cells transfected with si-NC. This is a novel finding with regards to lung cancer and CASC15 and supports the antagonistic effect previously demonstrated in this study.
Finally, we also demonstrated that CASC15 knockdown attenuates tumor growth in vivo. Slower growth of tumors and reduction in tumor size were observed in the CASC15 knockdown lung cells, showing that CASC15 plays a vital role in the progressive growth of lung tumors. A similar observation was made by Yao et al. while working on gastric cancer [17]. This observation has a potential implication on therapy of lung cancer as targeting CASC15 may reduce tumor size.
In conclusion, this study reports for the first time an over-expression of CASC15 that is associated with cell proliferation, colony formation, migration and invasion in lung cancer. We have established that CASC15 activities are moderated by miR-766-5p in an antagonistic manner. CASC15 is unique in lung cancer and has potential applications in diagnosis and therapy.
Funding Statement
this work was supported by Henan provincial Medical Science and Technology Research Program (Grant NO:2018020097).
Disclosure statement
The authors declare that they have no conflict of interests.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Dela Cruz CS, Tanoue LT, Matthay RA.. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cao J. The functional role of long non-coding RNAs and epigenetics. Biol Proced Online. 2014;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu C, Bai B, Skogerbo G, et al. NONCODE: an integrated knowledge database of non-coding RNAs. Nucleic Acids Res. 2005;33:D112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liao Q, Liu C, Yuan X, et al. Large-scale prediction of long non-coding RNA functions in a coding-non-coding gene co-expression network. Nucleic Acids Res. 2011;39:3864–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Zhang J, Zheng H, et al. Mouse transcriptome: neutral evolution of ‘non-coding’ complementary DNAs. Nature. 2004;431: 1 p following 757; discussion following 757 1–2. [PubMed] [Google Scholar]
- [9].Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. [DOI] [PubMed] [Google Scholar]
- [10].Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. [DOI] [PubMed] [Google Scholar]
- [11].Li J, Xuan Z, Liu C. Long non-coding RNAs and complex human diseases. Int J Mol Sci. 2013;14:18790–18808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mitra SA, Mitra AP, Triche TJ. A central role for long non-coding RNA in cancer. Front Genet. 2012;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. [DOI] [PubMed] [Google Scholar]
- [14].Xu E, Yu X, Zeng Q, et al. Functional role of lncRNA DB327252 in lung cancer. J Thorac Dis. 2016;8:2793–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park SM, Choi EY, Bae DH, et al. The LncRNA EPEL promotes lung cancer cell proliferation through E2F target activation. Cell Physiol Biochem. 2018;45:1270–1283. [DOI] [PubMed] [Google Scholar]
- [16].Pan H, Jiang T, Cheng N, et al. Long non-coding RNA BC087858 induces non-T790M mutation acquired resistance to EGFR-TKIs by activating PI3K/AKT and MEK/ERK pathways and EMT in non-small-cell lung cancer. Oncotarget. 2016;7:49948–49960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yao XM, Tang JH, Zhu H, et al. High expression of LncRNA CASC15 is a risk factor for gastric cancer prognosis and promote the proliferation of gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21:5661–5667. [DOI] [PubMed] [Google Scholar]
- [18].Wu Q, Xiang S, Ma J, et al. Long non-coding RNA CASC15 regulates gastric cancer cell proliferation, migration and epithelial mesenchymal transition by targeting CDKN1A and ZEB1. Mol Oncol. 2018;12:799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fernando TR, Contreras JR, Zampini M, et al. The lncRNA CASC15 regulates SOX4 expression in RUNX1-rearranged acute leukemia. Mol Cancer. 2017;16:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li XS, He XL. Kallikrein 12 downregulation reduces AGS gastric cancer cell proliferation and migration. Genet Mol Res. 2016;15. [DOI] [PubMed] [Google Scholar]
- [21].Lose F, Batra J, O’Mara T, et al. B. Australian prostate cancer, common variation in Kallikrein genes KLK5, KLK6, KLK12, and KLK13 and risk of prostate cancer and tumor aggressiveness. Urol Oncol. 2013;31:635–643. [DOI] [PubMed] [Google Scholar]
- [22].Papachristopoulou G, Tsapralis N, Michaelidou K, et al. Human kallikrein-related peptidase 12 (KLK12) splice variants discriminate benign from cancerous breast tumors. Clin Biochem. 2018;58:78–85. [DOI] [PubMed] [Google Scholar]
- [23].Nie FQ, Zhu Q, Xu TP, et al. Long non-coding RNA MVIH indicates a poor prognosis for non-small cell lung cancer and promotes cell proliferation and invasion. Tumour Biol. 2014;35:7587–7594. [DOI] [PubMed] [Google Scholar]
- [24].Gao L, Zhang H, Zhang B, et al. A novel long non-coding RNATCONS_00001798 is downregulated and predicts survival in patients with non-small cell lung cancer. Oncol Lett. 2018;15:6015–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].He T, Zhang L, Kong Y, et al. Long non-coding RNA CASC15 is upregulated in hepatocellular carcinoma and facilitates hepatocarcinogenesis. Int J Oncol. 2017;51:1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jia B, Xia L, Cao F. The role of miR-766-5p in cell migration and invasion in colorectal cancer. Exp Ther Med. 2018;15:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Q, Selth LA, Callen DF. MiR-766 induces p53 accumulation and G2/M arrest by directly targeting MDM4. Oncotarget. 2017;8:29914–29924. [DOI] [PMC free article] [PubMed] [Google Scholar]


