Abstract
BACKGROUND
Ultrasound-guided interscalene block (ISB) is the reference technique for pain control after ambulatory upper limb surgery, but supraclavicular block (SCB) is an alternative.
OBJECTIVES
The aim of this study was to compare the efficacy of SCB vs. ISB in patients undergoing ambulatory arthroscopic rotator cuff repair (ARCR), with the hypothesis of noninferiority of SCB analgesia compared with ISB.
DESIGN
A randomised, single-blind, noninferiority study.
SETTING
Hôpital Privé Jean Mermoz, Centre Paul Santy, Lyon, France.
PATIENTS
Ambulatory ARCR patients.
INTERVENTION
Patients were randomly allocated (1 : 1) to receive a single injection SCB or ISB, as well as general anaesthesia. All patients received a postoperative analgesic prescription for home use before leaving hospital (including fast-acting oral morphine sulphate). Patients completed a telephone questionnaire on days 1 and 2 postsurgery.
MAIN OUTCOME MEASURES
Primary endpoint was oral morphine consumption (mg) during the first 2 days postsurgery. If the difference between mean morphine consumption in the SCB vs. ISB group was less than 30 mg, noninferiority of SCB compared with ISB would be demonstrated. Secondary evaluation criteria included pain scores (numerical rating scale), duration of motor and sensory blockade, and satisfaction with treatment.
RESULTS
The per-protocol cohort included 103 patients (SCB = 52, ISB = 51) (57% men, median age 58 years). Mean morphine consumption in the 48 h postsurgery was 9.4 vs. 14.7 mg in the SCB and ISB groups, respectively (difference −5.3, P < 0.001). The upper limit of the 95% CI was less than 30 mg, demonstrating noninferiority of SCB compared with ISB. No difference was observed between the two groups in terms of pain scores or the duration of motor or sensory blockade. Overall, 98% of patients in the SCB group vs. 90% in the ISB group were satisfied with their treatment.
CONCLUSION
SCB is as effective as ISB in terms of postoperative analgesia based on oral morphine consumption in patients undergoing ambulatory ARCR.
TRIAL REGISTRATION
EudraCT number: 2016-A00747-47.
Introduction
The number of patients undergoing rotator cuff repair [arthroscopic rotator cuff repair (ARCR)] by arthroscopy as a day procedure is increasing annually.1 The procedure is associated with significant postoperative pain and effective analgesia is required for successful day-case surgery.2,3 Although ISB,4 and more particularly continuous interscalene catheter block, is considered to be the most effective analgesic technique for ARCR,5 it is frequently associated with phrenic nerve block,6,7 even with the use of ultrasound and low volumes of local anaesthetic.8 The difficulties in implementing and monitoring a perineural catheter at home have led to many anaesthesiologists to choose a single injection technique so that patients can be discharged on the same day as surgery with a satisfactory level of pain control.9
Phrenic nerve block is a concern to many anaesthesiologists and surgeons, as it may lead to respiratory complications after hospital discharge, preventing discharge from hospital on the same day.10 There are little data on the postoperative clinical consequences in the home, but it is known that changes in spirometry variables have been associated with ISB,11 whatever the site of injection around the roots, anterior or posterior. Nevertheless, effective regional anaesthesia that persists for as long as possible and that is compatible with local organisational constraints, is essential for this procedure because multimodal analgesia alone is insufficient.12
Several alternatives to ISB exist that are associated with a lower incidence of phrenic nerve paresis.8 Supraclavicular block (SCB) decreases the risk of phrenic nerve involvement, particularly when guided by ultrasound,13 but nevertheless the associated level of phrenic nerve paresis is not negligible.14 This technique, which has been linked to a risk of pneumothorax when carried out by neurostimulation only,15 has now been revived and is included among the regional anaesthesia techniques considered to be well tolerated in terms of respiratory risk, especially when guided by ultrasound.16–18 Many studies have demonstrated a lower risk of phrenic paresis with ultrasound-guided SCB,19,20 even when using low volumes of local anesthesic.21 Published studies have demonstrated that SCB is an effective alternative to ISB,22 and many studies have shown that ultrasound-guided SCB is a safe technique for ambulatory shoulder surgery in terms of respiratory complications.23–25 SCB is therefore a real alternative to ISB for ambulatory ARCR,26 but comparative studies are necessary to evaluate the analgesic results more than 24 h after surgery, particularly in terms of oral morphine consumption at home, given the fact that in our centre, patients are discharged less than 12 h after hospital admission. Most studies published to date have only shown similar intra-operative opioid analgesic requirements between ISB and SCB,27 or equivalent postoperative analgesia in the first 24 h only,28 and all findings are based on a small number of patients.
We carried out a single-centre randomised study to determine whether SCB is noninferior to ISB in terms of postoperative analgesia following ambulatory ARCR. Analgesic efficacy was determined by oral morphine use and/or pain scores assessed using a numerical rating scale (NRS) after hospital discharge on the evening of surgery. Our hypothesis was that SCB would provide similar or better analgesia to ISB in patients returning home after surgery.
Materials and methods
Study design
This randomised, single-blind, noninferiority study was carried out in the Hôpital Privé Jean Mermoz, Centre Paul Santy, Lyon, France, between September 2016 and October 2017.
The study was approved by the local ethics committee (CPP) South-East III (France) on 3 June 2016 prior to patient enrolment (CPP number 2016-023 B) and was carried out in accordance with the Declaration of Helsinki. The trial was registered prior to patient enrolment on www.clinicaltrialsregister.eu (EU Clinical Trials Register): ID-RCB clinical trial registration EudraCT number 2016-A00747-47; principal investigator: Julien Cabaton; date of registration: 3 June 2016.
All participants in the trial gave their written informed consent before taking part.
Patients
Adults undergoing ARCR performed by a single surgeon (NJ), under regional anaesthesia and general anaesthesia, and returning home on the evening of their surgery (hospital stay <12 h) were recruited. Exclusion criteria included the inability to return home on the evening of surgery (for a medical or other reason); administration of oral morphine derivatives before their surgery; contraindications for regional anaesthesia or regional anaesthesia not performed; contraindication to oral morphine derivatives; refusal to participate in the study or failure to record consent; and development of a complication during implementation of regional anaesthesia. Patients who were due to undergo ambulatory ARCR were asked to participate in the study during their surgical consultation or during their meeting with the anaesthesiologist. All were provided with specific information on the study and a consent form to sign after being given time to reflect on the procedures.
On the day of admission to the clinic, patients were randomly allocated to one of two parallel groups. Patients in both SCB and ISB groups received a single injection block. Randomisation was 1: 1 using the SAS 9.4 software (SAS Institute Inc., Cary, North Carolina, USA), and no stratification variable was taken into account. Two anaesthesiologists only took part in the study and performed the allocated block (no blinding at this stage), but neither the patient, nor the surgeon, nor the clinical research associate carrying out the follow-up telephone call knew which regional anaesthesia technique had been used.
Pre-operative management
In the anaesthesia consultation, the process was explained; all would receive a standard general anaesthesia preceded by ISB or SCB according to group allocation. Successful management would allow return home on the same evening as their procedure (ambulatory criteria).
During this consultation, the patients received written information, consent forms for participation in the study and also a standardised prescription for oral multimodal analgesia29 to be taken systematically after returning home. These prescriptions included codeine-paracetamol 1 g x four times per day, ketoprofen 100 mg x two times per day and nefopam 20 mg x four times per day. Fast-acting oral morphine sulphate, 10 mg x four times per day if necessary, was prescribed on a different prescription, to be taken only if pain relief proved inadequate. The dose was based on the maximum dose required to treat severe pain.
Peri-operative management
SCB and ISB were both performed on conscious sedated patients before general anaesthesia in a standardised manner by one of the two anaesthesiologists in our team: after oral premedication with sublingual midazolam 0.1 mg kg−1 conscious monitoring was applied; sedation with 0.2 mg kg−1 intravenously (i.v.) ketamine was given before ultrasonography with a Imagic Agile - Esaote Medical (Genova, Italy) or Logiq V2 - General Electric (Boston, USA) ultrasound machine; ultrasound guidance was associated with nerve stimulation performed with a Stimuplex HNS 12 - B Braun (Bethlehem, USA) set at 0.1 ms, 1 Hz and 1 mA stimulation, in sentinel mode, with the aim of securing the approach of the needle; a single perineural injection was performed with a 50 mm needle Vygon (Swindon, UK) containing a mixture of 100 mg levobupivacaine (20 ml, 0.5%) and clonidine (1 μg kg body weight−1) as is the common local protocol and in the absence of contraindications; the site of injection for ISB was the C6 plexus nerve root with a posterior in-plane approach, with neurostimulation control and ultrasound-controlled extra-plexus injection of the mixture posterior to the C6 root (Fig. 1a); and the site of injection for SCB was superficial and lateral to the trunks of the brachial plexus and not directly deep inside the ‘corner pocket’ zone, with neurostimulation control and visualisation of the lung (Fig. 1b).
Fig. 1.
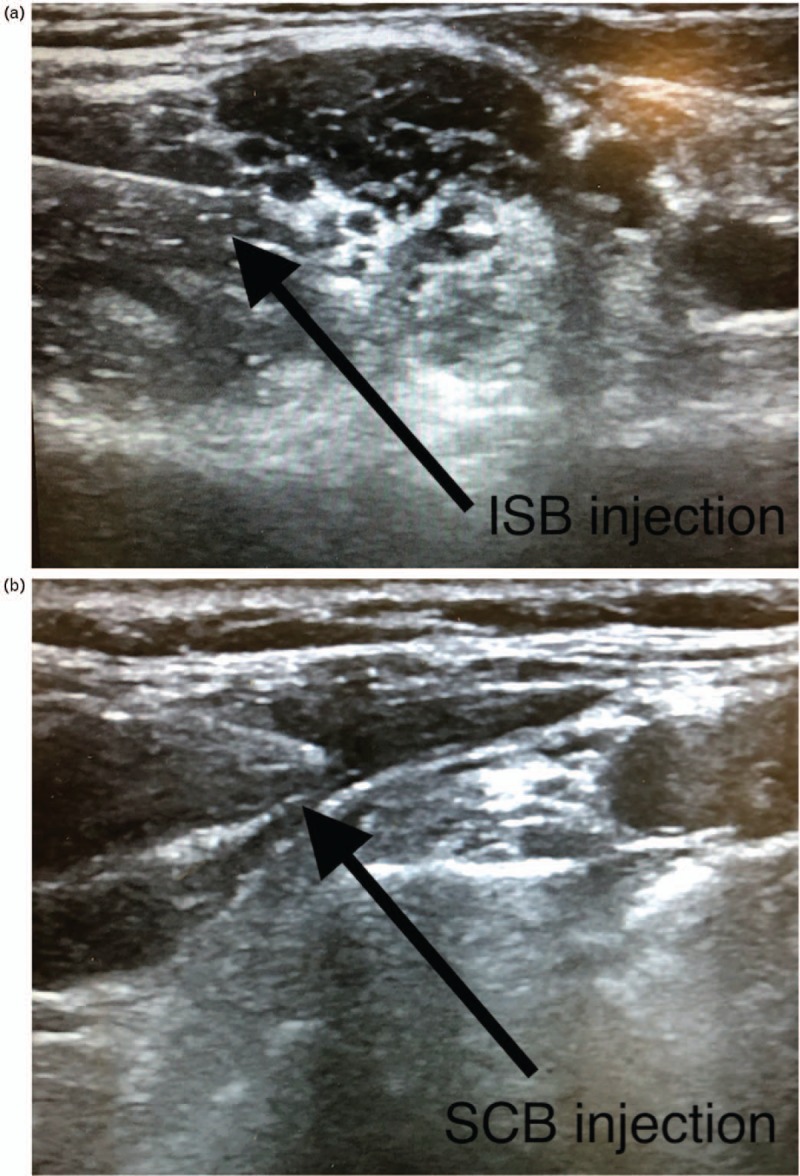
Sites of injection of anaesthesia for arthroscopic rotator cuff repair. (a) The site of injection for ultrasound-guided interscalene block was the C6 plexus nerve root with a posterior in-plane approach, with neurostimulation control, and ultrasound-controlled extraneural injection of the mixture. (b) The site of injection for supraclavicular block was superficial and lateral to the trunks of the brachial plexus, and not directly deep in the ‘corner pocket’ zone, with neurostimulation control and visualisation of the lung.
General anaesthesia was performed after regional anaesthesia according to the same protocol for all patients, with endotracheal intubation following i.v. propofol, sufentanil and cis-atracurium. All patients were placed in the beach-chair position. Prophylactic postoperative nausea and vomiting prevention with i.v. 1.25 mg droperidol was given. Dexamethasone 8 mg i.v. was given to potentiate the duration of regional anaesthesia, and in the absence of contraindications, multimodal i.v. analgesia was given from induction with paracetamol 1 g, ketoprofen 100 mg and nefopam 20 mg. General anaesthesia was maintained with inhaled desflurane, end-tidal minimal alveolar concentration 0.8 to 1.0 MAC and additional injections of sufentanil if required.
Surgery was carried out on all patients by a single surgeon using the same arthroscopic technique (one, two or three tendons could be repaired) and the same beach-chair installation. No open repair was performed and an acromioplasty was performed at the end of the procedure.
Patients were excluded from the study if they underwent any nonarthroscopic repair (peri-operative conversion to open technique), or any other type of regional anaesthesia.
Outcomes and data collection
Data were collected by the anaesthesiologist responsible and by nurses involved in patient care during hospital admission, and by a clinical research assistant who telephoned patients at home on postoperative Day 1 and Day 2. Nurses and the research assistant were blinded to the type of anaesthesia.
The primary outcome was oral morphine consumption in mg at home during the 2 days postsurgery. The secondary outcomes were pain scores using a NRS (0 = no pain, 10 = worst imaginable pain); in the recovery room 1 h after surgery and in the day-surgery unit 8 h after surgery, and on Day 1 and Day 2 by phone call; the duration of motor blockade; the time in hours from regional anaesthesia to the time at which forearm and/or arm movement became possible; the duration of sensory blockade; the time in hours from regional anaesthesia to the time that sensation (pulling of the scar tissue, itching/tingling, pain) returned; side effects linked to morphine such as nausea, vomiting, constipation, generalised pruritus, excessive sedation visual hallucinations, spatio-temporal disorientation or confusion, coma, respiratory arrest with a need to call the emergency services; complications at home requiring a consultation with a doctor or persistent perineural block; and overall satisfaction with treatment on Day 2. Items (ii) to (vi) were ascertained by telephone questionnaire on Day 1 and Day 2. The data were then stored on a dedicated website created for the study.
Statistical analysis
Forty-eight patients were required per group to have an 80% power of demonstrating noninferiority of SCB compared with ISB (one-sided 2.5% level), with an anticipated loss to follow-up of 10%. This calculation was based on the hypothesis of an expected difference in mean morphine consumption between the two techniques (SCB vs. ISB) of 10 mg and on a difference in standard deviation (SD) of morphine consumption of 35 mg in the first 48 h postsurgery. This was based on the results of a retrospective study carried out in our centre in 2014 to 2015 on 323 ARCR patients and their mean oral morphine use.30 The primary analysis was based on the per-protocol cohort (all patients without any deviations from the protocol and who received the correct treatment allocated by randomisation). A robustness analysis was performed on the intent-to-treat (ITT) principle and involved all patients who were randomly assigned to the two groups, including observed data for all patients who started the assigned treatment, regardless of whether it was continued or not. The difference between mean morphine consumption in the SCB group and mean morphine consumption in the ISB group was calculated with 95% CIs. If the upper limit of the 95% CI was less than 30 mg, noninferiority of SCB compared with ISB would be demonstrated.
Intergroup differences were assessed using the Wilcoxon or Student's t-test for continuous variables and χ2 or Fisher's exact test for categorical variables. All statistical analyses were carried out using SAS version 9.4 (SAS Institute Inc.).
Results
One-hundred and ten patients were included in the study. One-hundred and seven patients were randomised and analysed in the ITT analysis: 54 in the SCB group and 53 in the ISB group. One patient was randomised to the SCB group but received ISB; the safety analysis therefore included 53 and 54 patients in the SCB and ISB groups, respectively. One patient did not receive the treatment allocated by randomisation, two patients did not receive the telephone call on Days 1 and 2, and one patient could not return home the same evening as surgery; hence, 103 patients were analysed in the per-protocol analysis (as shown in Fig. 2).
Fig. 2.
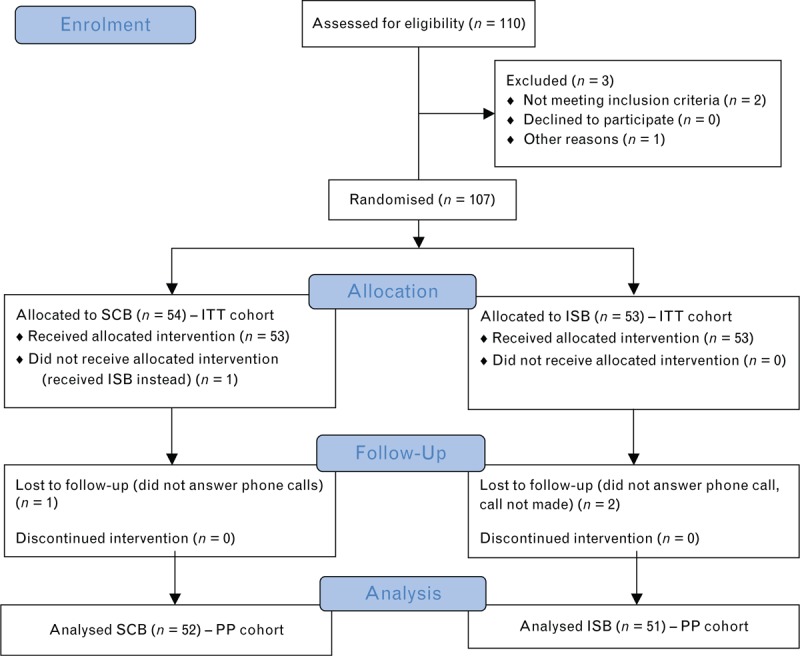
Consort Diagram.
The baseline characteristics of the study patients are summarised in Table 1. Overall, 57% of patients were men, median age was 58 years and 16% of patients had a BMI of more than 30 kg m−2. All except 10 had a history of shoulder surgery and the majority (77%) had difficulties with their left shoulder. Fifty-five percent of patients had one ruptured tendon and half had undergone the usual treatments before surgery. The baseline characteristics were similar in the two groups in the per-protocol and ITT analyses (Table 1).
Table 1.
Baseline characteristics
| PP | ITT | |||||
| SCB (n = 52) | ISB (N = 51) | Total (n = 103) | SCB (n = 54) | ISB (n = 53) | Total (n = 107) | |
| Male | 32 (62) | 27 (53) | 59 (57) | 33 (61) | 28 (53) | 61 (57) |
| Age (years) | 57 [51 to 65] | 58 [54 to 65] | 58 [52 to 65] | 57 [50 to 65] | 58 [54 to 65] | 58 [52 to 65] |
| BMI class | ||||||
| <18.5 | 2 (4) | 2 (2) | 2 (4) | 2 (2) | ||
| 18.5–25 | 20 (39) | 16 (31) | 36 (35) | 20 (37) | 17 (32) | 37 (35) |
| 25–30 | 23 (44) | 25 (49) | 48 (47) | 23 (43) | 26 (49) | 49 (46) |
| ≥30 | 9 (17) | 8 (16) | 17 (16) | 11 (20) | 8 (15) | 19 (18) |
| History of surgery | 49 (94) | 44 (86) | 93 (90) | 51 (94) | 46 (87) | 97 (91) |
| Shoulder | ||||||
| Right | 42 (81) | 37 (73) | 79 (77) | 43 (80) | 38 (72) | 81 (76) |
| Left | 10 (19) | 14 (27) | 24 (23) | 11 (20) | 15 (28) | 26 (24) |
| Number of tendons ruptured | ||||||
| 1 | 26 (50) | 31 (61) | 57 (55) | 27 (50) | 31 (59) | 58 (54) |
| 2 | 19 (36) | 19 (37) | 38 (37) | 20 (37) | 21 (40) | 41 (38) |
| 3 | 7 (14) | 1 (2) | 8 (8) | 7 (13) | 1 (2) | 8 (8) |
| Usual treatment | 23 (44) | 29 (57) | 52 (51) | 24 (44) | 30 (57) | 54 (51) |
All values shown are n (%) or median [IQR].
IQR, interquartile range; ISB, interscalene block; ITT, intent to treat; PP, per protocol; SCB, supraclavicular block.
Four patients discontinued the study prematurely, two in the SCB group and two in the ISB group.
Primary endpoint
In the per-protocol analysis, mean morphine consumption in the 48 h following surgery was 9.4 mg (95% CI: 5.4 to 13.5) and 14.7 mg (95% CI: 9.6 to 19.8) in the SCB and ISB groups (P < 0.001), respectively, yielding a difference of −5.3 (95% two-sided CI: −11.7 to 1.17). The upper limit of the 95% CI (1.17) was less than 30 mg, demonstrating noninferiority of SCB compared with ISB. As noninferiority had been demonstrated, we tested the superiority of SCB compared with ISB, but this was not significant.
In the ITT analysis, mean morphine consumption in the 48 h following surgery was 9.4 vs. 14.7 mg in the SCB and ISB groups, respectively, yielding a difference of −5.3 (upper limit of 95% one-sided CI: 1.17). The results for the ITT analysis were identical to those for per-protocol because the four patients excluded from the ITT analysis had no data collected concerning their morphine consumption.
Secondary endpoints
The results for morphine consumption on Day 1 and Day 2, pain scores and duration of motor and sensory blockade are summarised in Table 2. No statistically significant difference was observed between the groups for the pain scores in the recovery room (immediately after the end of the surgery) and in the ambulatory ward, just before discharge home (between 5 and 12 h after ISB or SCB was performed). In the per-protocol analysis, mean morphine consumption was 5.0 and 4.4 mg on Days 1 and 2, respectively, in the SCB group, and 9.2 mg and 5.5 mg, respectively, in the ISB group. In the SCB group, 40% of patients had intense pain (moderate or severe) on Day 1 and this decreased to 35% on Day 2. In the ISB group, 47% had intense pain on Day 1 and Day 2. No significant difference was observed between the two groups regarding the duration of motor or sensory blockade.
Table 2.
Morphine consumption and pain scores on days 1 (D1) and 2 (D2) and block duration
| Per-protocol population | ||||
| SCB group (n = 52) | ISB group (n = 51) | Total (n = 103) | P | |
| Morphine consumption at 24 h (mg) | 5.0 ± 9 | 9.2 ± 13 | 7.1 ± 11 | |
| Morphine consumption at 24 h | ||||
| No | 38 (73) | 27 (53) | 65 (63) | 0.0342 |
| Yes | 14 (27) | 24 (47) | 38 (37) | |
| Morphine consumption at 48 h (mg) | 4.4 ± 7 | 5.5 ± 8 | 5.0 ± 7 | |
| Morphine consumption at 48 h | ||||
| No | 36 (69) | 31 (61) | 67 (65) | 0.3687 |
| Yes | 16 (31) | 20 (39) | 36 (35) | |
| Pain score in recovery room | 0.5 ± 1 | 0.5 ± 2 | 0.5 ± 2 | 0.5906 |
| Pain score before leaving the hospital | 0.2 ± 1 | 0.5 ± 1 | 0.4 ± 1 | |
| Pain score at 24 h | 3.3 ± 3 | 3.4 ± 3 | 3.3 ± 3 | 0.3574 |
| Pain score at 24 h | ||||
| No pain or mild pain (<4) | 31 (60) | 27 (53) | 58 (56) | |
| Intense pain (≥4) | 21 (40) | 24 (47) | 45 (44) | |
| Pain score at 48 h | 2.8 ± 2 | 3.4 ± 3 | 3.1 ± 2 | |
| Pain score at 48 h | ||||
| No pain or mild pain (<4) | 34 (65) | 27 (53) | 61 (59) | |
| Intense pain (≥4) | 18 (35) | 24 (47) | 42 (41) | |
| Duration of motor block (h) | 20.8 [14 to 24] | 20.3 [18 to 24] | 20.6 [15 to 26] | |
| Duration of sensory block (h)Data are given as mean ± SD, n (%) or median [IQR] | 19.7 [14 to 22] | 20.3 [14 to 23] | 19.9 [14 to 23] | |
ISB, interscalene block; SCB, supraclavicular block.
Safety
Adverse events are summarised in Tables 3 and 4. The most frequent adverse events in the whole cohort were somnolence (19%) and nausea (14%). Five events were considered to be possibly related to treatment: one patient had heartburn, one patient had hot flushes and vomiting, one had nausea and two patients had episodes of somnolence. No patient suffered a serious adverse event and only one in the ISB group had a severe adverse event (heartburn) possibly related to treatment. The treatments were well tolerated and few adverse events occurred during the study.
Table 3.
Adverse events
| BMI <30 kg m−2 | BMI ≥30 kg m−2 | Safety cohorts | |||||||
| Group | SCB (n = 43) | ISB (n = 45) | Total (n = 88) | SCB (n = 10) | ISB (n = 9) | Total (n = 19) | SCB (n = 53) | ISB (n = 54) | Total (n = 107) |
| Patient with at least one AE | 5 (12) | 10 (22) | 15 (17) | 2 (20) | 2 (22) | 4 (21) | 7 (13) | 12 (22) | 19 (18) |
| Patient with at least one mild AE | 4 (9) | 7 (16) | 11 (13) | 1 (10) | 1 (11) | 2 (11) | 5 (9) | 8 (15) | 13 (12) |
| Patient with at least one moderate AE | 1 (2) | 2 (4) | 3 (3) | 1 (10) | 1 (11) | 2 (11) | 2 (4) | 3 (6) | 5 (5) |
| Patient with at least one severe AE | 1 (2) | 1 (1) | – | 1 (2) | 1 (1) | ||||
| Patient with at least one ongoing AE | 2 (5) | 2 (4) | 4 (5) | 2 (4) | 2 (4) | 4 (4) | |||
| Patient with at least one AE possibly related to treatment | 4 (9) | 4 (5) | 1 (10) | 1 (5) | 1 (2) | 4 (7) | 5 (5) | ||
Data are shown as n (%).
AE, adverse event; ISB, interscalene block; SCB, supraclavicular block.
Table 4.
Types of adverse events
| Patient | Safety arm | Adverse event | Place | Start date | End date | Intensity |
| 1 | SCB | Constipation and stomach pain | Home | 17 May 2017 | Mild | |
| 2 | SCB | Dryness of the mouth and stress | Home | 12 May 2017 | 12 May 2017 | Mild |
| 3 | ISB | Redness of thorax | Home | 04 May 2017 | 04 May 2017 | Moderate |
| 4 | ISB | Sensation of not standing upright | Home | 04 July 2017 | 04 July 2017 | Mild |
| 5 | SCB | Hot flushes | Home | 16 May 2017 | 16 May 2017 | Mild |
| 5 | SCB | Constipation | Home | 16 May 2017 | 17 May 2017 | Mile |
| 6 | SCB | Somnolence | Home | 15 November 2016 | 16 November 2016 | Mild |
| 7 | ISB | Dizziness | Home | 30 November 2016 | Mild | |
| 8 | ISB | Heartburn | Home | 19 September 2017 | 19 September 2017 | Severe |
| 9 | ISB | Fall | Other | 24 November 2016 | 24 November 2016 | Mild |
| 10 | ISB | Somnolence | Home | 26 October 2016 | Mild | |
| 11 | ISB | Redness and shortage of breath | Home | 11 May 2017 | 11 May 2017 | Moderate |
| 12 | SCB | Stomach pain | Home | 11 May 2017 | 11 May 2017 | Moderate |
| 13 | ISB | Nausea | Home | 30 March 2017 | 30 March 2017 | Mild |
| 14 | ISB | Somnolence | Home | 25 October 2016 | 26 November 2016 | Mild |
| 15 | ISB | Vomiting | Home | 04 September 2017 | 04 September 2017 | Moderate |
| 15 | ISB | Feeling hot and vomiting | Home | 06 September 2017 | 06 September 2017 | Moderate |
| 16 | SCB | Stomach pain and diarrhoea | Home | 16 May 2017 | 17 May 2017 | Moderate |
| 17 | SCB | Excessive somnolence | Home | 28 October 2016 | Mild | |
| 18 | ISB | Nausea | Outpatients | 24 October 2016 | 24 October 2016 | Mild |
| 19 | ISB | Nausea | Home | 17 November 2016 | 17 November 2016 | Mild |
ISB, interscalene block; SCB, supraclavicular block.
Overall, 98% in the SCB group and 90% in the ISB group were satisfied with their treatment.
Discussion
Our results demonstrate that SCB is noninferior to ISB in terms of oral morphine consumption given as part of multimodal analgesia, in the 48 h following ambulatory ARCR, in a large number of patients. In addition, no significant difference was found between ISB and SCB in terms of the duration of sensory and motor blockade (mean 20 h), with a similar duration to previous reports for ISB with the addition of i.v. dexamethasone.9 The absence of a significant difference in the duration of sensory blockade and in the pain scores in the first hours after surgery is in agreement with the results of our study, which showed no inferiority of SCB in terms of analgesia.
Similar studies published recently have shown comparable results to ours, but with a smaller number of patients14 and other types of surgery including arthroplasty,31 forearm surgery10 and open-sky nonarthroscopic surgery,32 but did not investigate oral morphine use after the return of the patients to their homes.33
Our study is a noninferiority study, but the difference in oral morphine consumption observed seems to indicate a difference in favour of SCB, especially as all of our patients received multimodal analgesia in addition to the morphine given as rescue analgesia. Although this difference was statistically significant, the design of the study did not allow use to conclude that SCB is superior to ISB, because the difference was observed only on day 1 postsurgery and concerned a binary nonquantitative criterion. Moreover, the morphine consumption found in our study was lower than expected from local data.29
Combining axillary nerve block with suprascapular nerve block34 might be a way to avoid phrenic nerve block. Studies comparing this combination have recently been published, but the results vary, often with inferior analgesic efficacy to ISB,35 or limited early postoperative efficacy.36
Our study did not investigate the impact of the two blocks on respiratory outcomes and in particular did not include ultrasound assessment of a possible phrenic palsy. This is a major limitation of the design of our study that could not be modified after we started to recruit patients. However, a recent meta-analysis demonstrated no great difference between ISB and SCB in the development of dyspnea.37 This limits the conclusions of our study for clinical practice, but confirms the equivalence in analgesic efficacy between ISB and SCB. It should be noted that no respiratory event occurred among the adverse events reported by any of the patients in the two groups in our study (Table 4), and there was no important difference in adverse events between patients with a BMI less than 30 kg m−2 and those with a BMI at least 30 kg m−2. No patient had a contraindication to phrenic paresis, and this reassured us about the feasibility of safely managing these ambulatory patients, even the obese ones. However, the exclusion of patients who were hospitalised on the evening of their surgery is a methodological limitation of our study, because this could have been due to complications of adverse event. Similarly, despite the prescription of morphine, which is often feared for its side effects, only one patient among the 107 randomised presented with vomiting and vomiting. Nevertheless, almost 20% of our patients were somnolent, which could be explained by the systematic prescription of codeine.
Concerning possible confounding factors, it should be noted that our patients were all operated on by the same surgeon and that a subgroup analysis by number of tendons repaired did not demonstrate any significant difference.
However certain methodological choices in our study limit the conclusions for clinical practice. The timescale of our study may be different to other reported studies, as a patient is described as ambulatory in France if they are discharged from hospital within 12 h, whereas the time period in other countries is less than 23 h. We chose to use higher concentrations of levobupivacaine than in recent studies38–40 in order to obtain a longer block despite our concern that the phrenic nerve should be spared.41,42 Perineural catheters are rarely inserted in our practice due to a high rate of accidental removals. Reducing the volume of local anaesthetic may not help because some studies have not shown any reduction in phrenic paresis by decreasing the volume injected from 20 to 10 ml.43
The difference in consumption of oral morphine considered to be clinically significant is debatable, as is the observation period of 48 h: a longer period would have been preferred and another model of statistical analysis chosen, although our model seems to be the best adapted.31 Finally, our results only show pain scores at 24 and at 48 h despite intensive multimodal analgesia, and we should remember that arthroscopic shoulder surgery can result in severe postoperative pain, even after the second day postsurgery. Analgesia via a perineural catheter would have been a more effective method, taking into account the fact that around 40% of our patients described intense pain after the wearing off of their block, but the use of morphine after removal of the catheter is necessary whatever the choice of technique used for regional anaesthesia.
Conclusion
Despite the limitations of our study, especially the absence of evaluation of the phrenic nerve palsy, no important difference was found between SCB and ISB in terms of postoperative analgesia with oral morphine in the 48 h following ambulatory ARCR. Further studies are required to confirm these results, with an evaluation of the respiratory consequences of these techniques when used in ambulatory shoulder surgery. Taking into account the recent literature, a superiority study would now be the way to compare these two types of nerve block.
Acknowledgements relating to this article
Assistance with the study: we thank Sebastien Durochat and Damien De Paulis, clinical research assistants, Hôpital Privé Jean Mermoz, Centre Paul Santy, Lyon, France, for their help in this study, and Ramsay Générale de Santé Research Department, for their support.
Financial support and sponsorship: none.
Conflicts of interest: none
Presentation: none
Footnotes
Published online 29 July 2019
References
- 1.Data Base PMSI HPJM 2016-2017/Shoulder Surgeons HPJM/GHM 08C40 and 08C48 Shoulder Arthroscopy. Medical Datas Department - Hôpital Privé Jean Mermoz - 3 March 2019. [Google Scholar]
- 2.Uquillas CA, Capogna BM, Rossy WH, et al. Postoperative pain control after arthroscopic rotator cuff repair. J Shoulder Elbow Surg 2016; 25:1204–1213. [DOI] [PubMed] [Google Scholar]
- 3.Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia 2010; 65:608–624. [DOI] [PubMed] [Google Scholar]
- 4.Ullah H, Samad K, Khan FA. Continuous interscalene brachial plexus block versus parenteral analgesia for postoperative pain relief after major shoulder surgery. Cochrane Database Syst Rev 2014; 2:CD007080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warrender WJ, Syed UAM, Hammoud S, et al. Pain management after outpatient shoulder arthroscopy: a systematic review of randomized controlled trials. Am J Sports Med 2017; 45:1676–1686. [DOI] [PubMed] [Google Scholar]
- 6.Hortense A, Perez MV, Amaral JL, et al. Interscalene brachial plexus block. Effects on pulmonary function. Rev Bras Anestesiol 2010; 60:130–137. 74–78. [DOI] [PubMed] [Google Scholar]
- 7.Cangiani LH, Rezende LA, Giancoli Neto A. Phrenic nerve block after interscalene brachial plexus block. Case report. Rev Bras Anestesiol 2008; 58:152–159. [DOI] [PubMed] [Google Scholar]
- 8.Stunder O, Meissnitzer M, Brummett CM, et al. Comparison of tissue distribution, phrenic nerve involvement, and epidural spread in standard- vs. low-volume ultrasound-guided interscalene plexus block using contrast magnetic resonance imaging: a randomized, controlled study. Br J Anaesth 2016; 116:405–412. [DOI] [PubMed] [Google Scholar]
- 9.Desmet M, Vanneste B, Reynvoet M, et al. A randomised controlled trial of intravenous dexamethasone combined with interscalene brachial plexus blockade for shoulder surgery. Anaesthesia 2015; 70:1180–1185. [DOI] [PubMed] [Google Scholar]
- 10.Bharti N, Bhardawaj N, Wig J. Comparison of ultrasound-guided supraclavicular, infraclavicular and below-C6 interscalene brachial plexus block for upper limb surgery: a randomised, observer-blinded study. Anaesth Intensive Care 2015; 43:468–472. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann L, Martini S, Kesselmeier M, et al. Phrenic nerve block caused by interscalene brachial plexus block: breathing effects of different sites of injection. BMC Anesthesiol 2016; 16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verelst P, Van Zundert A. Respiratory impact of analgesic strategies for shoulder surgery. Reg Anesth Pain Med 2013; 38:50–53. [DOI] [PubMed] [Google Scholar]
- 13.Renes SH, Spoormans HH, Gielen MJ, et al. Hemidiaphragmatic paresis can be avoided in ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2009; 34:595–599. [DOI] [PubMed] [Google Scholar]
- 14.Kim BG, Han JU, Song JH, et al. A comparison of ultrasound-guided interscalene and supraclavicular blocks for postoperative analgesia after shoulder surgery. Acta Anaesthesiol Scand 2017; 61:427–435. [DOI] [PubMed] [Google Scholar]
- 15.Abell DJ, Barrington MJ. Pneumothorax after ultrasound-guided supraclavicular block: presenting features, risk, and related training. Reg Anesth Pain Med 2014; 39:164–167. [DOI] [PubMed] [Google Scholar]
- 16.Sadowski M, Tułaza B, Lysenko L. Renaissance of supraclavicular brachial plexus block. Anaesthesiol Intensive Ther 2014; 46:37–41. [DOI] [PubMed] [Google Scholar]
- 17.Kakazu C, Tokhner V, Li J, et al. In the new era of ultrasound guidance: is pneumothorax from supraclavicular block a rare complication of the past? Br J Anaesth 2014; 113:190–191. [DOI] [PubMed] [Google Scholar]
- 18.Vermeylen K, Engelen S, Sermeus L, et al. Supraclavicular brachial plexus blocks: review and current practice. Acta Anaesthesiol Belg 2012; 63:15–21. [PubMed] [Google Scholar]
- 19.Neal JM, Moore JM, Kopacz DJ, et al. Quantitative analysis of respiratory, motor, and sensory function after supraclavicular block. Anesth Analg 1998; 86:1239–1244. [DOI] [PubMed] [Google Scholar]
- 20.Wiesmann T, Feldmann C, Muller HH, et al. Phrenic palsy and analgesic quality of continuous supraclavicular vs. interscalene plexus blocks after shoulder surgery. Acta Anaesthesiol Scand 2016; 60:1142–1151. [DOI] [PubMed] [Google Scholar]
- 21.Pearson LT, Lowry BP, Culp WC, Jr, et al. Effect of adding tetracaine to bupivacaine on duration of analgesia in supraclavicular brachial plexus nerve blocks for ambulatory shoulder surgery. Proc (Bayl Univ Med Cent) 2015; 28:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SS, Gordon MA, Shaw PM, et al. A prospective clinical registry of ultrasound-guided regional anesthesia for ambulatory shoulder surgery. Anesth Analg 2010; 111:617–623. [DOI] [PubMed] [Google Scholar]
- 23.Koscielniak-Nielsen ZJ. Supraclavicular catheter may be an alternative to interscalene catheter in patients at risk for respiratory failure after major shoulder surgery. Reg Anesth Pain Med 2013; 38:251. [DOI] [PubMed] [Google Scholar]
- 24.King R, Mariano ER, Yajnik M, et al. Outcomes of ambulatory upper extremity surgery patients discharged home with perineural catheters from a veterans Health administration medical center. Pain Med 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomised, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med 2015; 40:125–132. [DOI] [PubMed] [Google Scholar]
- 26.Lin E, Choi J, Hadzic A. Peripheral nerve blocks for outpatient surgery: evidence-based indications. Curr Opin Anaesthesiol 2013; 26:467–474. [DOI] [PubMed] [Google Scholar]
- 27.Ryu T, Kil BT, Kim JH. Comparison between ultrasound-guided supraclavicular and interscalene brachial plexus blocks in patients undergoing arthroscopic shoulder surgery: a prospective, randomized, parallel study. Medicine (Baltimore) 2015; 94:e1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aliste J, Bravo D, Fernandez D, et al. A randomized comparison between interscalene and small-volume supraclavicular blocks for athroscopic shoulder surgery. Reg Anesth Pain Med 2018; 43:590–595. [DOI] [PubMed] [Google Scholar]
- 29.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016; 17:131–157. [DOI] [PubMed] [Google Scholar]
- 30.Cabaton J. [[Accessed 11 July 2019]]. Pain management after shoulder surgery: a prospective study of the efficiency of single-injection interscalene block (ISB) for ambulatory procedures. ESRA Academy 2016; 138491. https://academy.esraeurope.org/esra/2016/35th/138491/julien.cabaton.pain.management.after.shoulder.surgery.a.prospective.study.of.html. [Google Scholar]
- 31.Auyong DB, Yuan SC, Choi DS, et al. A double-blind randomized comparison of continuous interscalene, supraclavicular, and suprascapular blocks for total shoulder arthroplasty. Reg Anesth Pain Med 2017; 42:302–309. [DOI] [PubMed] [Google Scholar]
- 32.Koh WU, Kim HJ, Park HS, et al. A randomised controlled trial comparing continuous supraclavicular and interscalene brachial plexus blockade for open rotator cuff surgery. Anaesthesia 2016; 71:692–699. [DOI] [PubMed] [Google Scholar]
- 33.Checcucci G, Allegra A, Bigazzi P, et al. A new technique for regional anesthesia for arthroscopic shoulder surgery based on a suprascapular nerve block and an axillary nerve block: an evaluation of the first results. Arthroscopy 2008; 24:686–696. [DOI] [PubMed] [Google Scholar]
- 34.Dhir S, Sondekoppam RV, Sharma R, et al. A comparison of combined suprascapular and axillary nerve blocks to interscalene nerve block for analgesia in arthroscopic shoulder surgery: an equivalence study. Reg Anesth Pain Med 2016; 41:564–571. [DOI] [PubMed] [Google Scholar]
- 35.Neal JM, McDonald SB, Larkin KL, Polissar NL. Suprascapular nerve block prolongs analgesia after nonarthroscopic shoulder surgery but does not improve outcome. Anesth Analg 2003; 96:982–986. [DOI] [PubMed] [Google Scholar]
- 36.Guo CW, Ma JX, Ma XL, et al. Supraclavicular block versus interscalene brachial plexus block for shoulder surgery: a meta-analysis of clinical control trials. Int J Surg 2017; 45:85–91. [DOI] [PubMed] [Google Scholar]
- 37.Renes SH, van Geffen GJ, Rettig HC, et al. Minimum effective volume of local anesthetic for shoulder analgesia by ultrasound-guided block at root C7 with assessment of pulmonary function. Reg Anesth Pain Med 2010; 35:529–534. [DOI] [PubMed] [Google Scholar]
- 38.Thackeray EM, Swenson JD, Gertsch MC, et al. Diaphragm function after interscalene brachial plexus block: a double-blind, randomized comparison of 0.25% and 0.125% bupivacaine. J Shoulder Elbow Surg 2013; 22:381–386. [DOI] [PubMed] [Google Scholar]
- 39.Falcão LF, Perez MV, deCastro AM, et al. Minimum effective volume of 0.5% bupivacaine with epinephrine in ultrasound-guided interscalene brachial plexus block. Br J Anaesth 2013; 110:450–455. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Cho SH, Kim SH, et al. Ropivacaine for ultrasound-guided interscalene block: 5 mL provides similar analgesia but less phrenic nerve paralysis than 10 mL. Can J Anesth 2011; 58:1001–1006. [DOI] [PubMed] [Google Scholar]
- 41.Wong AK, Keeney LG, Cheng L, et al. Effect of local anesthetic concentration (0.2% vs 0.1% ropivacaine) on pulmonary function, and analgesia after ultrasound-guided interscalene brachial plexus block: a randomized controlled study. Pain Med 2016; 17:2397–2403. [DOI] [PubMed] [Google Scholar]
- 42.Sinha SK, Abrams JH, Barnett JT, et al. Decreasing the local anesthetic volume from 20 to 10 mL for ultrasound-guided interscalene block at the cricoid level does not reduce the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med 2011; 36:17–20. [DOI] [PubMed] [Google Scholar]
- 43.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ. Altman DG; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012; 308:2594–2604. [DOI] [PubMed] [Google Scholar]


