Abstract
BACKGROUND
Pre-operative anxiety in children is very common and is associated with adverse outcomes.
OBJECTIVE
The aim of this study was to investigate if virtual reality exposure (VRE) as a preparation tool for elective day care surgery in children is associated with lower levels of anxiety, pain and emergence delirium compared with a control group receiving care as usual (CAU).
DESIGN
A randomised controlled single-blind trial.
SETTING
A single university children's hospital in the Netherlands from March 2017 to October 2018.
PATIENTS
Two-hundred children, 4 to 12 years old, undergoing elective day care surgery under general anaesthesia.
INTERVENTION
On the day of surgery, children receiving VRE were exposed to a realistic child-friendly immersive virtual version of the operating theatre, so that they could get accustomed to the environment and general anaesthesia procedures.
MAIN OUTCOME MEASURES
The primary outcome was anxiety during induction of anaesthesia (modified Yale Preoperative Anxiety Scale, mYPAS). Secondary outcomes were self-reported anxiety, self-reported and observed pain, emergence delirium, need for rescue analgesia (morphine) and parental anxiety.
RESULTS
A total of 191 children were included in the analysis. During induction of anaesthesia, mYPAS levels (median [IQR] were similar in VRE, 40.0 [28.3 to 58.3] and CAU, 38.3 [28.3 to 53.3]; P = 0.862). No differences between groups were found in self-reported anxiety, pain, emergence delirium or parental anxiety. However, after adenoidectomy/tonsillectomy, children in the VRE condition needed rescue analgesia significantly less often (55.0%) than in the CAU condition (95.7%) (P = 0.002).
CONCLUSION
In children undergoing elective day care surgery, VRE did not have a beneficial effect on anxiety, pain, emergence delirium or parental anxiety. However, after more painful surgery, children in the VRE group needed rescue analgesia significantly less often, a clinically important finding because of the side effects associated with analgesic drugs. Options for future research are to include children with higher levels of anxiety and pain and to examine the timing and duration of VRE.
TRIAL REGISTRATION
Netherlands Trial Registry: NTR6116.
Introduction
Pre-operative anxiety is very common in children. On the day of surgery, 50 to 70% of children experience anxiety that usually peaks during induction of anaesthesia.1,2 Pre-operative anxiety is associated with problematic induction of anaesthesia,3 risk of emergence delirium,3,4 increased pain and poorer recovery.5,6 Anxious children undergoing surgery, and their parents, are also at risk of posttraumatic stress symptoms.7,8 These adverse outcomes underscore the urgent need for effective interventions to reduce pre-operative anxiety.
A promising innovative intervention is virtual reality (VR). VR is especially engaging for children, as they often become truly captivated by imaginative play.9 In our recent meta-analysis on VR interventions in children undergoing medical procedures,10 we found that VR is effective in reducing anxiety and pain. In most studies, VR was used as a distraction tool during medical procedures.10 However, research has demonstrated that exposure is more effective than distraction in reducing anxiety.11 Virtual reality exposure (VRE) has already been proven effective in treating anxiety disorders, such as specific phobias (fear of spiders),12,13 but very limited research has been conducted on the effect of VRE as preparation for medical procedures.
VRE offers the chance to reduce pre-operative anxiety by exposing children to a realistic virtual version of the operating theatre, in which they can get accustomed to the environment and procedures associated with anaesthesia. Until now, only two studies14,15 have applied VRE prior to surgery. In these studies, both including 69 children, the intervention took place on the day of surgery and consisted of either a 360° VR tour of the operating theatre14 or a VR game in which patients experienced the pre-operative process.15 Children in the control group received conventional education about the pre-operative process. Both studies were limited to pre-operative outcomes and found that children were significantly less anxious and more compliant in the VRE than in the control group.14,15 However, as pre-operative anxiety is associated with negative postoperative outcomes, such as increased pain and emergence delirium,3–6 it is also important to investigate postoperative effects of VRE.
This is the first randomised controlled trial (RCT) to study the effects of VRE on pre-operative anxiety, in addition to postoperative outcomes. The objective of the current study was to compare levels of anxiety during induction of anaesthesia (primary outcome), postoperative anxiety, pain, emergence delirium, rescue analgesia and parental anxiety (secondary outcomes) in children receiving VRE, with controls, and to identify predictors of VRE efficacy in children 4 to 12 years old undergoing elective maxillofacial, dental or ear-nose-throat (ENT) day surgery.
Materials and methods
The PREoperative VR Intervention to Enhance Wellbeing (PREVIEW) study16 was approved by the Medical Ethics Committee of the Erasmus Medical Centre (MEC-2016-626) on 30 November 2016 and registered at the Netherlands Trial Registry (NTR6116). This single-centre, single-blinded RCT was conducted in accordance with the CONSORT guidelines17 at the Erasmus MC-Sophia Children's Hospital in the Netherlands, by the Departments of Child and Adolescent Psychiatry/Psychology, Paediatric Anaesthesiology, Maxillofacial, Dental and ENT Surgery. Written informed consent was obtained from all parents and from all children aged 12 years. Children aged 11 years and under gave permission orally.
Participants
Eligible participants were consecutive children aged 4 to 12 years undergoing elective maxillofacial, dental or ENT day care surgery between March 2017 and October 2018. Exclusion criteria were mental retardation, inability of parents to read or write Dutch, epilepsy, visual impairment, an American Society of Anaesthesiologists (ASA) physical status at least III and need for pre-operative anxiolytic medication.
Procedure
Eligible children and their parents were informed about the study by paediatric anaesthesiologists during pre-operative screening. Those interested received a patient information folder via e-mail. During this screening, the anaesthesiologists recommended that all children and parents should watch an informative online film at home about general anaesthesia according to the standard hospital protocol.
On the day of surgery, after informed consent was obtained, personal and medical data were collected by the research assistant and baseline anxiety and problem behaviour were assessed (T1) (Fig. 1). Next, the research assistant randomly allocated children to the VRE intervention, which the children received together with usual care, or to the control group, in which children only received care as usual (CAU). Block randomisation was performed, stratified by type of surgery: adenoidectomy and/or tonsillectomy, insertion of tympanostomy tubes, maxillofacial and dental procedures or other ENT procedures. After randomisation, the VRE intervention took place in a separate room, under the guidance of the research assistant. Afterwards, children were admitted to the day care unit. Children in the CAU group were admitted to the day care unit directly after randomisation. Assessments after randomisation were performed by the blinded researcher (RE) in the holding area (T2) and during induction of anaesthesia (T3), and by a blinded recovery nurse, postoperatively, in the recovery room (T4). One assessment per time point was performed. In the recovery room, assessments took place during later phases of awakening. Parents were present from early phases of awakening (one parent per child). Anaesthesiologists were blinded to group allocation. Postoperatively parents were encouraged and reminded by phone and e-mail to complete online questionnaires at home, via a secure website, on the third day after surgery, but were allowed to do so until 2 weeks after surgery (T5). An overview of the process can be found in Fig. 1.
Fig. 1.
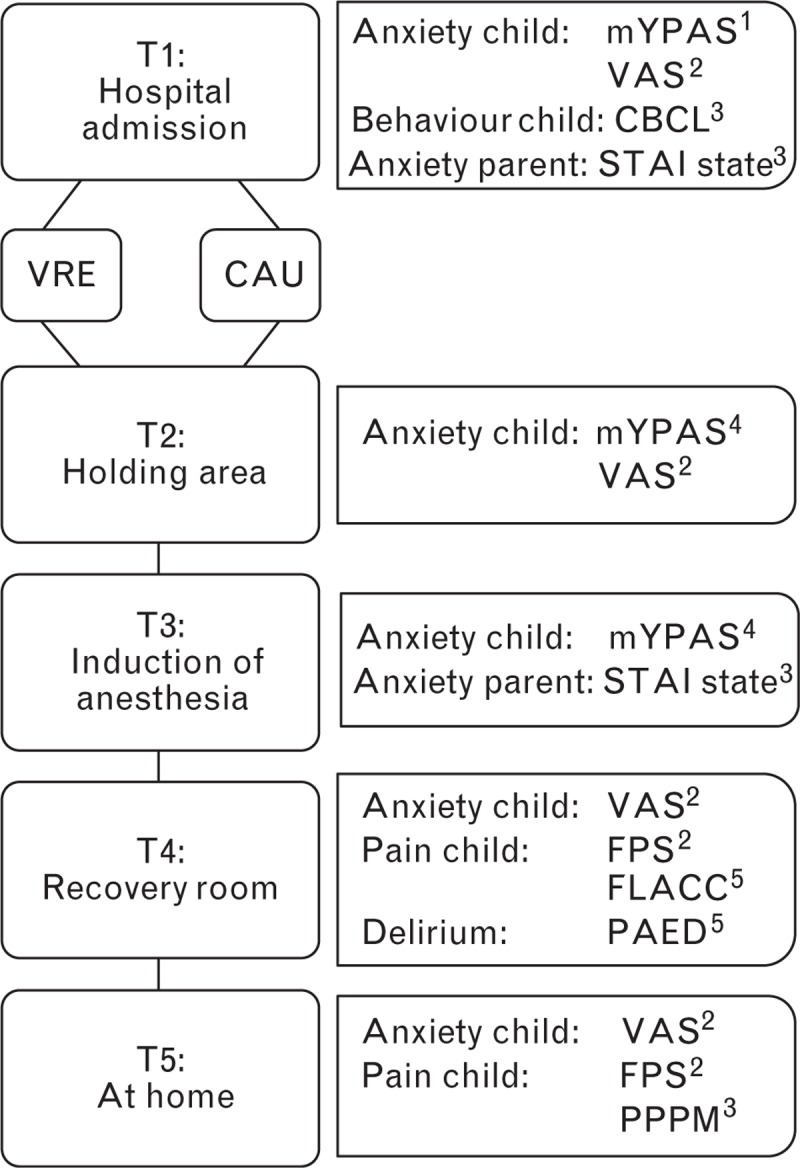
Flowchart of the study design with outcomes and instruments at each time point. Informants are denoted in superscript: 1. Research assistant, 2. Child, 3. Parent, 4. Researcher, 5. Recovery nurse. CAU, care as usual; VRE, virtual reality exposure.
Virtual reality intervention
The VRE tool encompasses a highly realistic virtual environment that is modelled according to the real operating theatre and medical staff. The virtual environment is computer-generated, interactive and child-friendly. It was presented to the child for approximately 15 min via an HTC Vive (HTC Corporation, Xindian, New Taipei, Taiwan) head-mounted display and was also displayed on a personal computer monitor, so the accompanying parent could see what the child was viewing. We developed two versions, for children aged 4 to 7 and 8 to 12 years, in order to attune explanations to a child's developmental level. The storyline begins in the holding area (Fig. 2a). A receptionist welcomes the child and shows a video on a virtual tablet that explains that one of the child's parents will stay with him/her until the child is anaesthetised and shows the hospital gowns they will be wearing to the operating room. Next, the child is transported, in a hospital bed, into the corridor of the operating theatre by an anaesthesiologist and a nurse anaesthetist (Fig. 2b). After arrival in the operating room, the child can point at different instruments with a motion tracked controller so that the nurse anaesthetist can explain what these are used for (Fig. 2c). Then, the child moves onto the operating table and preparation for anaesthesia takes place. The programme is able to show both intravenous (i.v.) and inhalational induction. After induction, the operating room fades out and the recovery room fades in (Fig. 2d). Here, the nurse anaesthetist shows another video that explains what kind of feelings the child might experience after surgery, for example nausea. For a more detailed overview of the storyline, as well as the technical hardware and software specifications, we refer to the trial article.16
Fig. 2.
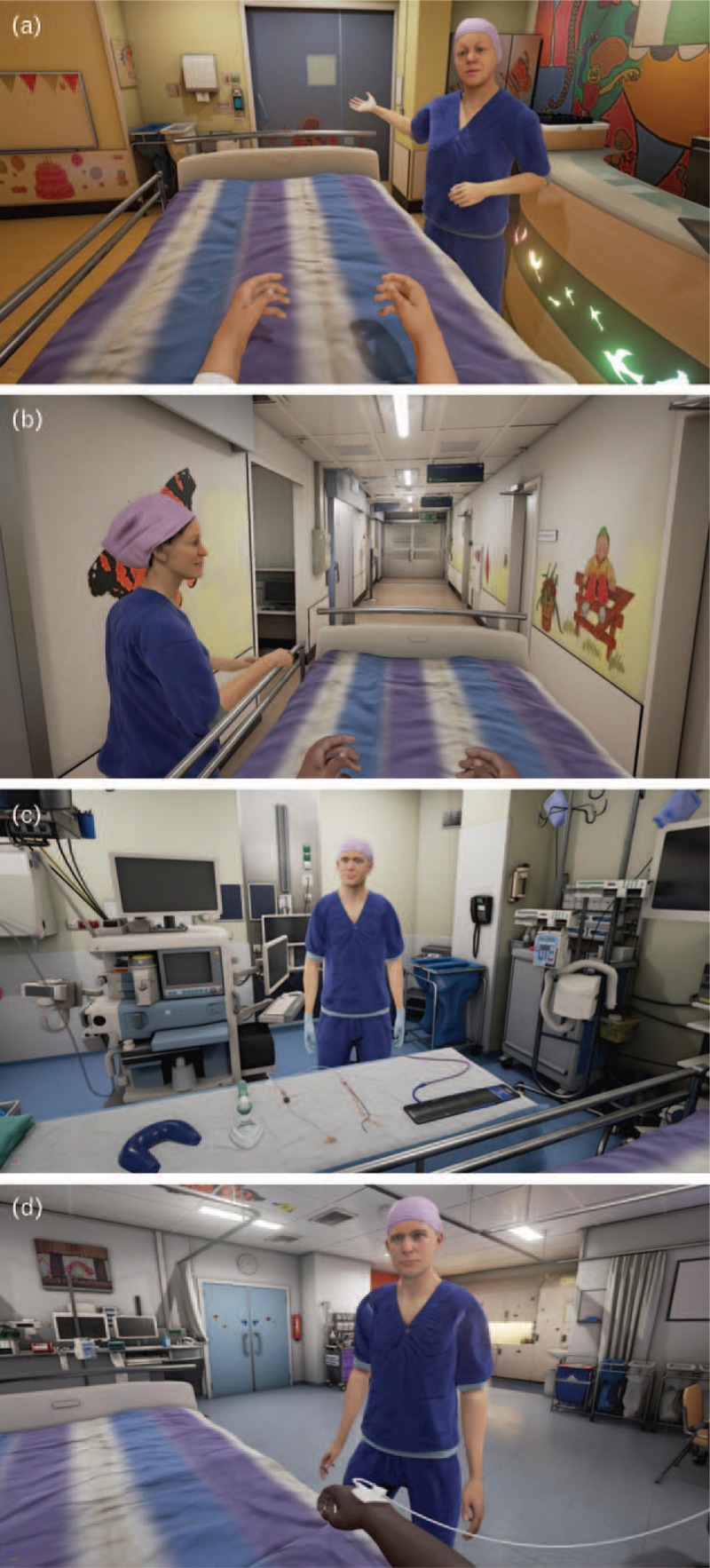
VR environment. (a) The receptionist welcomes the child to the holding area. (b) The operating room, where the child receives information about different instruments (pulse oximeter, blood pressure cuff and anaesthesia mask). (c) The child wakes up in the recovery room. VR, virtual reality.
Anaesthesia protocol
None of the children received pre-operative anxiolytic premedication. EMLA cream (lidocaine/prilocaine) or Rapydan (lidocaine/tetracaine) plasters were applied on the back of the hands, 30 to 60 min before going to the operating room. Induction of anaesthesia took place in the operating room in the presence of a parent or a guardian. Children were lying down or sitting on the operating table but were also permitted to sit on the parent's lap. After placement of the electrocardiography electrodes, pulse oximeter and blood pressure cuff, anaesthesia was induced, i.v. or by inhalation if i.v. cannulation was declined or i.v. access was unsuccessful. For i.v. induction, a peripheral i.v. catheter was placed in the back of the hand, and i.v. propofol 2 to 4 mg kg−1 and fentanyl 1 to 2 μg kg−1 were administered. For inhalation induction, sevoflurane in a mixture of oxygen and air was administered by mask. In these cases, i.v. cannulation took place after induction, after which i.v. fentanyl 1 to 2 μg kg−1 was administered. Depending on the surgical procedure, a laryngeal mask airway (LMA) or an endotracheal tube (ETT) was inserted. Before intubation, the child received a muscle relaxant. Anaesthesia was maintained with sevoflurane 0.7 to 1.0 minimal alveolar concentration (MAC) in air and oxygen. During surgery, i.v. fentanyl was administered at the discretion of the anaesthesiologist. At the end of the procedure, first doses of i.v. paracetamol 20 mg kg−1 and diclofenac 1 mg kg−1 were administered. If needed, i.v. morphine 0.1 mg kg−1 was also administered. After extubation, children were brought to the recovery area. Rescue analgesia, extra morphine, could be administered by the recovery nurse according to perceived clinical need. Standard postoperative analgesics were prescribed: paracetamol 90 mg kg−1 per day orally or rectally and diclofenac 3 mg kg−1 per day orally or rectally.
Assessment instruments
An overview of the well validated assessment instruments at each time point is provided in Fig. 1.
Child anxiety
The primary outcome was child anxiety during induction of anaesthesia (T3) assessed with the modified Yale Preoperative Anxiety Scale (mYPAS).18 The mYPAS is considered the gold standard in observational instruments to assess pre-operative anxiety in children18 and was completed at three timepoints (T1, T2 and T3). The mYPAS consists of 27 items divided into five domains: activity, emotional expressivity, state of arousal, vocalisation and use of parents. Scores range from 23.33 to 100, with higher scores indicating higher levels of anxiety. The domains have good to excellent interobserver and intra-observer reliability.18 The research assistant (T1) and blinded researcher (T2 & T3) were trained in administering the mYPAS with standardised instructions. Children indicated their own anxiety level on a visual analogue scale (VAS)19 prior to anaesthesia and after surgery (T1, T2, T4 and T5).
Child pain and emergence delirium
Postoperative pain was reported by three informants. Children reported their pain intensity (T4 and T5) with the six-faces revised Faces Pain Scale (FPS-r): range 0 to 10.20 A blinded recovery nurse assessed pain intensity (T4) with the Face, Legs, Activity, Cry and Consolability (FLACC) scale: range 0 to 10.21 Parents assessed their child's pain (T5) by completing the Parents’ Postoperative Pain Measure (PPPM): range 0 to 15.22 Emergence delirium was assessed (T4) with the Paediatric Anaesthesia Emergency Delirium (PAED) scale by a blinded recovery nurse: range 0 to 20.23
Child behaviour problems
At T1, parents completed the Child Behaviour Checklist (CBCL) to assess pre-operative emotional and behavioural problems during the past 6 months.24,25 Either the 1.5 to 5 years of age version with 100 items (for 4 to 5-year-old participants) or the 6 to 18 years of age version with 113 items (for 6 to 12-year-old participants) was used. T-scores for total scores were computed.
Parental anxiety
The State–Trait Anxiety Inventory (STAI) is a self-reporting instrument that contains two separate scales for trait and state anxiety.26 Scores on both Likert-type scales range from 20 to 80. Parents completed the state form directly after induction of anaesthesia (T3).
Statistical analyses
Intention-to-treat (ITT) analyses were performed for all randomised participants. We used two-way imputation to adjust for missing item data. The Shapiro–Wilk test was used to test the assumption of normal distribution. Nonnormally distributed continuous variables were compared between conditions using the Mann–Whitney U test. Categorical variables were analysed with the χ2 test. Continuous nonnormally distributed data were reported as median [interquartile range]. Categorical nonnormally distributed data were presented as frequency (percentage).
For children in the VRE condition, linear regression analyses were performed with children's state anxiety during induction of anaesthesia (mYPAS at T3) and self-reported pain (FPS-r at T4) as outcomes. The following predictor variables were entered simultaneously in the model: sex, age, type of surgery, pre-operative state anxiety (mYPAS T1), pre-operative problem behaviour (CBCL T1) and pre-operative parental state anxiety (STAI state T1). All data were analysed with IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp. Armonk, NY). A P value less than 0.05 was considered statistically significant.
Sample size calculation
A sample size of 100 patients per group was sufficient to compare the primary outcome, anxiety during induction of anaesthesia (mYPAS) between the intervention and control groups, with a Cohen's d of 0.4 (small to medium effect size), an alpha of 0.05 (two-tailed) and a power of 0.85. A sample size of 100 patients in the intervention group was sufficient to perform regression analyses with six predictor variables, a small to medium effect size and a power of 0.85.
Results
No significant differences were found between groups in patient and surgical characteristics (all Ps > 0.05).
Between March 2017 and October 2018, 393 children were assessed for eligibility, of whom 193 children did not participate. Reasons for non participation were did not meet the inclusion criteria (n = 35), did not want to participate (n = 109) or for other reasons such as inability to contact, postponement or cancellation of surgery before data collection (n = 49) (Fig. 3). Two hundred children were enrolled in the study (VRE: n = 100, CAU: n = 100). Nine children were excluded because of accidental unblinding (n = 5), noncompliance with the anaesthetic protocol (n = 2), no data collection at T2 and T3, due to logistical reasons (n = 1), or cancelled surgery (n = 1). Therefore, 191 participants were included in the data analyses (VRE: n = 94, CAU: n = 97). Baseline characteristics of all participants are given in Table 1. Twenty-one children in the VRE condition discontinued the intervention by taking off the VR headset. Ad-hoc analyses showed that this group consisted of an equal number of boys (n = 11, 52.4%) and girls (n = 10, 47.6%), with a median age of 5.0 years [4.5 to 6.3]. More specifically, 71.4% of these children were 4 or 5 years old.
Fig. 3.
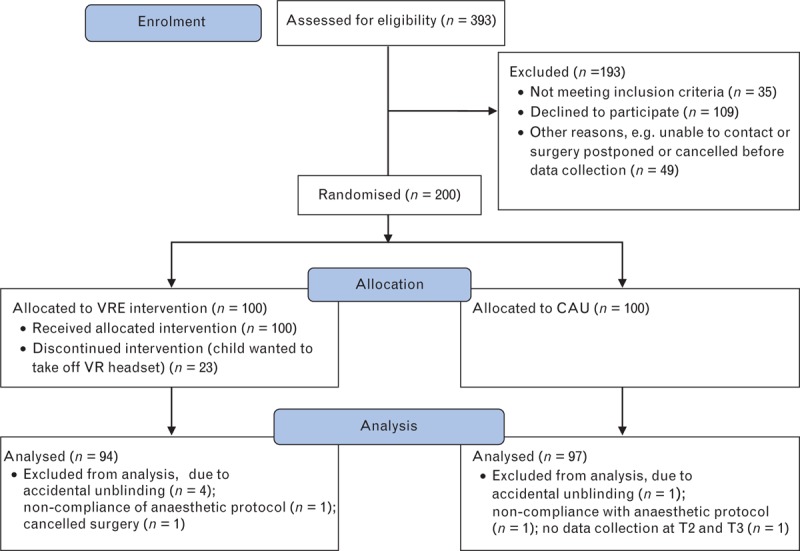
CONSORT study flowchart. CAU, care as usual; T2, in the holding are; T3, during induction of anaesthesia; VRE, virtual reality exposure.
Table 1.
Patient and surgical characteristics
| VREc (n = 94) | CAU (n = 97) | Pd | VREe (n = 73) | P f | |
| Age | 8.3 [5.7 to 10.2] | 7.5 [5.6 to 10.7] | 0.938a | 9.0 [6.4 to 10.7] | 0.064a |
| Sex | 0.172b | 0.149b | |||
| Male | 45 (47.9) | 56 (57.7) | 34 (46.6) | ||
| Female | 49 (52.1) | 41 (42.3) | 39 (53.4) | ||
| ASA physical status | 0.141b | 0.073b | |||
| I | 72 (76.6) | 65 (67.0) | 58 (79.5) | ||
| II | 22 (23.4) | 32 (33.0) | 15 (20.5) | ||
| Type of surgery | 0.915b | 0.477b | |||
| Adenoidectomy and/or tonsillectomy | 20 (21.3) | 23 (23.7) | 12 (16.4) | ||
| Tympanostomy tubes | 23 (24.5) | 23 (23.7) | 17 (23.3) | ||
| Maxillofacial and dental procedures | 23 (24.5) | 26 (26.8) | 18 (24.7) | ||
| Other ENT procedures | 28 (29.8) | 25 (25.8) | 26 (35.6) | ||
| Induction method | 0.499b | 0.674b | |||
| Inhalation | 54 (57.4) | 51 (52.6) | 36 (49.3) | ||
| Intravenously | 40 (42.6) | 46 (47.4) | 37 (50.7) | ||
| Total problem behaviour (CBCL) | 47.0 [41.0 to 56.0] | 46.0 [39.0 to 53.0] | 0.251a | 47.0 [41.0 to 55.0] | 0.268a |
| Parental education level | 0.442b | 0.728b | |||
| Low | 5 (5.3) | 2 (2.1) | 3 (4.1) | ||
| Medium | 30 (31.9) | 35 (36.1) | 25 (34.2) | ||
| High | 59 (62.8) | 60 (61.9) | 45 (61.6) | ||
Values are median [interquartile range] or frequency (percentage).
ASA, American Society of Anaesthesiologists; CAU, care as usual; CBCL, Child Behaviour Checklist; VRE, virtual reality exposure.
aMann–Whitney U-test.
bχ2 test.
cChildren who were allocated to the VRE condition (n = 94).
dIntention-to-treat analyses.
eChildren who completed the VRE intervention.
fPer-protocol analyses (ad-hoc).
For the data collection at T5, most parents (56.0%) had completed the online questionnaires on the fourth day after surgery. On the eighth day after surgery, almost all parents (82.7%) had completed the questionnaires. The final percentage of completed questionnaires after 14 days was 91.6%. No significant correlations (Spearman's ρ) were found between day of completion and postoperative outcomes (P = 0.228, P = 0.577, and P = 0.721, for VAS, FPS-r and PPPM, respectively). Therefore, T5 data from all postoperative days (3 to 14) were combined and included in the analysis.
Child anxiety
At baseline (T1), in the holding area (T2) and during induction of anaesthesia (T3), mYPAS scores were similar in CAU and VRE (P = 0.697, P = 0.765 and P = 0.862, respectively). Self-reported VAS scores were also comparable between conditions at different time points (P = 0.407 at T1, P = 0.753 at T2, P = 0.735 at T4 and P = 0.727 at T5) (Table 2).
Table 2.
Anxiety, pain, rescue analgesia and emergence delirium levels, and parental anxiety levels in both groups
| VREc (n = 94) | CAU (n = 97) | Pd | VREe (n = 73) | Pf | |
| Anxiety | |||||
| mYPAS (observed) | |||||
| T1 | 28.3 [23.3 to 31.7] | 26.7 [23.3 to 32.5] | 0.697a | 28.3 [23.3 to 30.0] | 0.636a |
| T2 | 28.3 [23.3 to 36.7] | 28.3 [23.3 to 41.7] | 0.765a | 26.7 [23.3 to 36.7] | 0.129a |
| T3 | 40.0 [28.3 to 58.3] | 38.3 [28.3 to 53.3] | 0.862a | 36.7 [27.5 to 48.3] | 0.266a |
| VAS (self-reported) | |||||
| T1 | 3.0 [0.1 to 5.0] | 1.5 [0.0 to 5.0] | 0.407a | 3.0 [0.5 to 5.0] | 0.209a |
| T2 | 3.0 [1.0 to 5.5] | 3.5 [0.0 to 6.0] | 0.753a | 3.5 [1.0 to 6.0] | 0.997a |
| T4 | 0.0 [0.0 to 2.0] | 0.0 [0.0 to 2.0] | 0.735a | 0.3 [0.0 to 2.0] | 0.466a |
| T5 | 0.5 [0.0 to 1.0] | 0.3 [0.0 to 2.0] | 0.727a | 0.5 [0.0 to 1.0] | 0.620a |
| Pain | |||||
| FPS-r (self-reported) | |||||
| T4 | 2.0 [0.0 to 4.0] | 2.0 [0.0 to 2.5] | 0.699a | 2.0 [0.0 to 4.0] | 0.763a |
| T5 | 0.0 [0.0 to 2.0] | 0.0 [0.0 to 2.0] | 0.454a | 0.0 [0.0 to 2.0] | 0.551a |
| FLACC (observed, T4) | 0.0 [0.0 to 0.0] | 0.0 [0.0 to 0.0] | 0.669a | 0.0 [0.0 to 0.0] | 0.735a |
| PPPM (observed, T5) | 3.0 [0.0 to 5.0] | 3.0 [1.0 to 8.0] | 0.410a | 3.0 [0.0 to 5.8] | 0.502a |
| Rescue analgesiag (T4) | |||||
| Overall | 28 (29.8) | 39 (40.2) | 0.131b | 22 (30.1) | 0.175b |
| Adenoidectomy and tonsillectomy | 11 (55.0) | 22 (95.7) | 0.002b | 6 (50.0) | 0.001b |
| Tympanostomy tubes | 0 (0.0) | 1 (4.3) | 0.312b | 0 (0.0) | 0.384b |
| Maxillofacial and dental procedures | 4 (17.4) | 6 (23.1) | 0.622b | 4 (22.2) | 0.947b |
| Other ENT procedures | 13 (46.4) | 10 (40.0) | 0.637b | 12 (22.2) | 0.657b |
| Emergence delirium | |||||
| PAED (observed, T4) | 7.0 [5.0 to 9.0] | 6.0 [5.0 to 9.0] | 0.266a | 7.5 [5.0 to 9.0] | 0.223a |
| Parental anxiety | |||||
| STAI-state (self-reported, T3) | 41.0 [34.5 to 48.5] | 40.5 [33.0 to 50.0] | 0.753a | 38.5 [34.0 to 45.75] | 0.579a |
| VAS (observed, T3) | 3.0 [2.0 to 5.0] | 3.5 [2.0 to 5.0] | 0.418a | 3.0 [2.0 to 4.0] | 0.171a |
Values are median [interquartile range] or frequency (percentage).
FLACC, Face, Legs, Activity, Cry and Consolability; FPS-r, Faces Pain Scale revised; mYPAS, modified Yale Preoperative Anxiety Scale; PAED, Paediatric Anaesthesia Emergency Delirium; PPPM, Parents’ Postoperative Pain Measure; STAI, State-Trait Anxiety Inventory; T1, hospital admission (baseline); T2, holding area; T3, induction of anaesthesia; T4, recovery room, when children were fully awake, in the presence of a parent; T5, at home, within 2 weeks after surgery; VAS, Visual Analogue Scale.
aMann–Whitney U-test.
bχ2 test.
cChildren who were allocated to the VRE condition (n = 94).
dIntention-to-treat analyses.
eChildren who completed the VRE intervention.
fPer-protocol analyses (ad-hoc).
gNeed for rescue analgesia (yes or no) was also analysed for each type of surgery, separately.
The only significant predictor of anxiety during induction of anaesthesia was pre-operative parental state anxiety [F(1,85) = 5.05, P = 0.027]. Higher parental anxiety levels prior to surgery were related to higher child anxiety levels during induction, in the VRE group. The linear regression model accounted for 11.3% of the variance in anxiety during induction of anaesthesia [F(6,85) = 1.80, P = 0.109].
Child pain and emergence delirium
No differences in pain levels were found between VRE and CAU, neither when self-reported with FPS-r (P = 0.699 at T4, P = 0.454 at T5), nurse-observed with FLACC (P = 0.669) nor parent-observed with PPPM (P = 0.410). Further investigation of pain levels in the recovery room (T4) indicated that there were no differences between VRE and CAU in the proportion of children experiencing considerable levels of pain (FPS-r > 3 or FLACC > 3),27,28 neither when self-reported with the FPS-r (VRE: n = 24, CAU: n = 23, P = 0.211), nor when nurse-observed with the FLACC (VRE: n = 4, CAU: n = 5, P = 0.549). No differences were found in emergence delirium symptoms between conditions (P = 0.266), nor in proportion of children experiencing considerable levels of emergence delirium symptoms (PAED > 10)23 (VRE: n = 3, CAU: n = 1, P = 0.505). No significant predictors of postoperative pain were found in the model. The linear regression model accounted for 7.4% of the variance in self-reported pain in the recovery room, F(6,81) = 1.07, P = 0.386.
Rescue analgesia
Overall, there was no difference in need for rescue analgesia between VRE and CAU (P = 0.131). When analysing rescue analgesia for each type of surgery separately, 11 out of 20 (55%) children in the VRE group who underwent adenoidectomy and tonsillectomy needed significantly less frequent rescue analgesia than the 22 out of 23 (95.7%) children in the CAU group (χ2 = 9.91, P = 0.002). No differences in rescue analgesia were found for the other three types of surgery.
Parental anxiety
No differences in parental anxiety during induction of anaesthesia were found between groups, either when self-reported (STAI-state) (P = 0.753), or when observed (VAS) (P = 0.418).
Ad-hoc analyses
We did not replace the 21 children who discontinued the VRE intervention, in line with intention-to-treat principles. However, because this concerns a substantial number of children, we repeated the analyses per-protocol, in which we compared the children in the VRE group who completed the intervention (n = 73) with children in the CAU group (n = 97). These analyses did not produce significantly different results when compared with the intention-to-treat analyses (Tables 1 and 2; the two columns on the right).
Discussion
This single-blinded RCT, with a sample of 191 children, was designed to investigate the effect of fully immersive VRE in children undergoing elective day care surgery. No significant differences were found between VRE and CAU in child anxiety, pain, emergence delirium or parental anxiety. However, after VRE, children undergoing the most painful surgical procedure29 needed significantly less rescue analgesia compared with CAU. Lastly, levels of parental anxiety did not differ between VRE and CAU.
VR has previously been investigated as a means of improving health outcomes and previous studies have found that VR reduced pain and anxiety in children undergoing different medical procedures.10,14,15 Most of these studies showed VR being successfully used as a method of distraction.10 Because these studies were small, often not blinded and lacked standardised assessments, chance findings and a degree of bias could not be ruled out. Previously studied medical procedures that included oncological and burn wound care were more complex and painful10 compared with the procedures in our study. This is reflected by the relatively small proportion of our patients who experienced substantial levels of pain. It may be possible that VRE is more effective prior to more problematic surgery, with higher levels of anticipated anxiety and pain, compared with elective day care surgery. This is supported by our finding that only children who underwent the most painful type of surgery29 needed less rescue analgesia after VRE. This finding is of great clinical importance, because rescue analgesia such as morphine has several side effects, including nausea, vomiting and dizziness.30 Therefore, administering rescue analgesia may be associated with slower postoperative recovery. In our study, pain levels at T4 were similar in VRE and CAU groups. However, by that time, rescue analgesia would have already been administered if needed. It is possible that, despite substantial pain levels, no treatment effect was found because of adequate pain management. A final explanation for the absence of effects on anxiety and pain is that more time was needed between VRE and surgery for children to process the information. Children require up to 1 week for the processing of information about peri-operative processes.31 Therefore, VRE may be more effective if taken up no earlier than a week prior to surgery, perhaps even in multiple sessions, or via a mobile application for smartphones.32 This dispenses with an extra hospital visit and requires no hospital staff for the intervention, resulting in no extra healthcare costs. Considering the intervention only takes 15 min, it is achievable to implement VRE even in a busy clinical setting. However, it might be preferable to limit exposure to children who are most at risk for high levels of anxiety and pain, because these are the children who might benefit the most from VRE.
Two studies by Ryu et al.,14,15 who used VRE prior to elective day care surgery, found positive effects. These studies were methodologically sound and included an acceptable number of participants. We offer several reasons for the discrepancy in results compared with our study. First, during induction of anaesthesia, Ryu et al.14,15 considered compliance, whereas our study considered anxiety. Compliance and anxiety are known to be different concepts,33 and even though patients were more compliant,14,15 they might still have been anxious during induction of anaesthesia. This is in line with their finding that distress levels in the operating room were not affected by playing a VR game pre-operatively.15 Second, the VRE group actually consisted of VRE and CAU because all the children in our study, including those in the VRE group, received routine care in a hospital setting that places great emphasis on patient comfort, in line with patient-centred and family-centred care.34 More specifically, all children and their parents received a pre-operative visit from a paediatric anaesthesiologist, during which elaborate education was provided along with a suggestion that the child and parents should watch an informative online movie about general anaesthesia. In addition, according to routine practice, children were not separated from their parents during anaesthetic induction and all parents were with their children throughout the recovery room stay. Successful routine care, in both the VRE and CAU groups, might have resulted in relatively low anxiety levels. A cut-off score of mYPAS at least 30 indicates high anxiety.18 In the current study, median anxiety levels in the CAU condition were 26.7 at baseline and 28.3 in the holding area. In comparison, Ryu et al.14,15 found substantially higher median levels of anxiety, also measured with the mYPAS, during baseline (CAU: 51.715) and in the holding area (CAU: 51.714 and 46.715). Unfortunately, it is not possible to make the same comparison for anxiety during induction of anaesthesia, as Ryu et al.14,15 did not use the mYPAS during induction. However, the comparisons between studies at admission (baseline) and in the holding area indicate that, overall, anxiety scores in our study were low, making it potentially more difficult to detect treatment effects. Finally, the lack of strong game design elements in our VRE intervention may explain the absence of results. Patients in the most recent study by Ryu et al.15 faced different challenges and received rewards whilst playing the VR game. These game elements are associated with greater engagement and education,35,36 so possibly also with a greater anxiolytic effect.
Strengths and limitations
The strengths of this study include the large sample size, limited missing data, use of internationally established standardised assessment tools, blinding of the medical and research staff and the narrow range of surgical procedures (elective day care surgery).
This study also has some limitations. First, in the recovery room, only one assessment took place. Multiple assessments, on entering the recovery room, and again after 5, 10 and 15 min would have provided a more comprehensive insight into the postoperative effects of VRE.19 Second, we did not include a survey on the subjective experience of the VRE, such as satisfaction, in children or their parents. Third, 21 children discontinued the intervention by taking off the headset. We found that the majority (71.4%) of these children were 4 or 5 years old. Wearing the rather large and heavy headset may have been uncomfortable, or the intervention may have been too lengthy for younger children, who overall have a limited attention span.37 Finally, by excluding patients who received anxiolytic premedication, we excluded the most anxious children in our study. Hospital policy dictates that anxiolytic premedication is not given unless, for example, it is after a previous traumatic experience with anaesthetic induction. Therefore, these cases can be considered exceptions and excluding them probably did not influence our results.
Conclusion
No significant differences were found between VRE and CAU in child anxiety, pain, or emergence delirium, or parental anxiety. However, after VRE, less rescue analgesia was needed after painful surgery. Considering the side effects of rescue analgesia, this means that VRE could be associated with increased patient comfort and a decreased need for postoperative care. It is possible that we did not find an effect of VRE on the other outcomes because we only investigated relatively mild procedures, the VRE intervention and surgery were too close to each other in time, and anxiety levels prior to induction of anaesthesia were relatively low. This is in line with the fact that more compelling results have been found in previous studies that either applied VR to more complex procedures or to patient groups with higher levels of anxiety prior to induction.
Future research
On the basis of our results and conclusions, an option for future research is to investigate VRE in children with higher levels of pre-operative anxiety or to investigate VRE prior to more complex procedures with higher levels of expected postoperative pain. Second, when investigating postoperative effects of VRE, it would be valuable to make multiple assessments in the recovery room, as well as collecting information on nausea, vomiting and length of stay. Finally, more research is needed on the inclusion of game elements and the timing of VRE in relation to the day of surgery.
Acknowledgements relating to this article
Assistance with the study: we wish to thank child life specialists Marieke Bruseker and Birgitta Houtman for their input on the VR script and the contribution of Cyborn 3D Productions for developing the VR software. We would also like to thank the anaesthesiologists, nurse anaesthetists, recovery nurses, day-care nurses and the medical and psychology students for their cooperation and help with data collection.
Financial support and sponsorship: this research was funded by the Zilveren Kruis foundation (project number: 2015233) and the Coolsingel foundation (project number: 401).
Conflicts of interest: none.
Presentation: none.
Footnotes
Published online 25 July 2019
References
- 1.Kain ZN, Mayes LC, Caldwell-Andrews AA, et al. Predicting which children benefit most from parental presence during induction of anesthesia. Pediatr Anesth 2006; 16:627–634. [DOI] [PubMed] [Google Scholar]
- 2.Kain ZN, Mayes LC, O’Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med 1996; 150:1238–1245. [DOI] [PubMed] [Google Scholar]
- 3.Kain ZN, Mayes LC, Caldwell-Andrews AA, et al. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics 2006; 118:651–658. [DOI] [PubMed] [Google Scholar]
- 4.Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg 2004; 99:1648–1654. [DOI] [PubMed] [Google Scholar]
- 5.Chorney JM, Kain ZN. Behavioral analysis of children's response to induction of anesthesia. Anesth Analg 2009; 109:1434–1440. [DOI] [PubMed] [Google Scholar]
- 6.Kain ZN, Caldwell-Andrews AA, Mayes LC, et al. Family-centered preparation for surgery improves perioperative outcomes in children: a randomized controlled trial. Anesthesiology 2007; 106:65–74. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Amitay G, Kosov I, Reiss A, et al. Is elective surgery traumatic for children and their parents? J Paediatr Child Health 2006; 42:618–624. [DOI] [PubMed] [Google Scholar]
- 8.Meentken MG, van Beynum IM, Legerstee JS, et al. Medically related posttraumatic stress in children and adolescents with congenital heart defects. Front Pediatr 2017; 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lillard AS. Pretend play skills and the child's theory of mind. Child Dev 1993; 64:348–371. [PubMed] [Google Scholar]
- 10.Eijlers R, Utens EMWJ, Staals LM, et al. A systematic review and meta-analysis of virtual reality in pediatrics: effects on pain and anxiety. Anesth Analg 2019; doi: 10.1213/ANE.0000000000004165. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendall PC, Southam-Gerow MA. Long-term follow-up of a cognitive-behavioral therapy for anxiety-disordered youth. J Consult Clin Psychol 1996; 64:724–730. [DOI] [PubMed] [Google Scholar]
- 12.Powers MB, Emmelkamp PMG. Virtual reality exposure therapy for anxiety disorders: a meta-analysis. J Anxiety Disord 2008; 22:561–569. [DOI] [PubMed] [Google Scholar]
- 13.Carl E, Stein AT, Levihn-Coon A, et al. Virtual reality exposure therapy for anxiety and related disorders: a meta-analysis of randomized controlled trials. J Anxiety Disord 2019; 61:27–36. [DOI] [PubMed] [Google Scholar]
- 14.Ryu JH, Park SJ, Park JW, et al. Randomized clinical trial of immersive virtual reality tour of the operating theatre in children before anaesthesia. Br J Surg 2017; 104:1628–1633. [DOI] [PubMed] [Google Scholar]
- 15.Ryu J-H, Park J-W, Nahm F, et al. The effect of gamification through a virtual reality on preoperative anxiety in pediatric patients undergoing general anesthesia: a prospective, randomized, and controlled trial. J Clin Med 2018; 7:pii: E284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eijlers R, Legerstee JS, Dierckx B, et al. Development of a virtual reality exposure tool as psychological preparation for elective pediatric day care surgery: methodological approach for a randomized controlled trial. JMIR Res Protoc 2017; 6:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutron I, Altman DG, Moher D, et al. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017; 167:40–47. [DOI] [PubMed] [Google Scholar]
- 18.Kain ZN, Mayes LC, Cicchetti DV, et al. The Yale Preoperative Anxiety Scale: how does it compare with a ‘gold standard’? Anesth Analg 1997; 85:783–788. [DOI] [PubMed] [Google Scholar]
- 19.Berghmans JM, Poley M, Weber F, et al. Does the Child Behavior Checklist predict levels of preoperative anxiety at anesthetic induction and postoperative emergence delirium? A prospective cohort study. Minerva Anestesiol 2015; 81:145–156. [PubMed] [Google Scholar]
- 20.Hicks CL, von Baeyer CL, Spafford PA, et al. The Faces Pain Scale–Revised: toward a common metric in pediatric pain measurement. Pain 2001; 93:173–183. [DOI] [PubMed] [Google Scholar]
- 21.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 1997; 23:293–297. [PubMed] [Google Scholar]
- 22.Chambers CT, Reid GJ, McGrath PJ, Finley GA. Development and preliminary validation of a postoperative pain measure for parents. Pain 1996; 68:307–313. [DOI] [PubMed] [Google Scholar]
- 23.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 2004; 100:1138–1145. [DOI] [PubMed] [Google Scholar]
- 24.Achenbach TM, Rescorla LA. Manual for ASEBA preschool forms & profiles. Burlington, Vermont: University of Vermont, Research Center for Children, Youth and Families; 2000. [Google Scholar]
- 25.Achenbach TM, Rescorla L A. Manual for the ASEBA school-age forms and profiles. Burlington, Vermont: University of Vermont Research Center for Children, Youth & Families; 2001. [Google Scholar]
- 26.Spielberger C, Gorsuch R. Manual for the State-Trait Anxiety Inventory (“Self-Evaluation Questionnaire”). Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 27.Björkman B, Nilsson S, Sigstedt B, Enskär K. Children's pain and distress while undergoing an acute radiographic examination. Radiography 2012; 18:191–196. [Google Scholar]
- 28.Pagé MG, Katz J, Stinson J, et al. Validation of the numerical rating scale for pain intensity and unpleasantness in pediatric acute postoperative pain: sensitivity to change over time. J Pain 2012; 13:359–369. [DOI] [PubMed] [Google Scholar]
- 29.Elinder K, Söderman A-CH, Stalfors J, Knutsson J. Factors influencing morbidity after paediatric tonsillectomy: a study of 18,712 patients in the National Tonsil Surgery Register in Sweden. Eur Arch Otorhinolaryngol 2016; 273:2249–2256. [DOI] [PubMed] [Google Scholar]
- 30.Mather SJ, Peutrell JM. Postoperative morphine requirements, nausea and vomiting following anaesthesia for tonsillectomy. Comparison of intravenous morphine and nonopioid analgesic techniques. Pediatr Anesth 1995; 5:185–188. [DOI] [PubMed] [Google Scholar]
- 31.Kain ZN, Mayes LC, Caramico LA. Preoperative preparation in children: a cross-sectional study. J Clin Anesth 1996; 8:508–514. [DOI] [PubMed] [Google Scholar]
- 32.Liguori S, Stacchini M, Ciofi D, et al. Effectiveness of an app for reducing preoperative anxiety in children: a randomized clinical trial. JAMA Pediatr 2016; 170:e160533–e1160533. [DOI] [PubMed] [Google Scholar]
- 33.Sadhasivam S, Cohen LL, Hosu L, et al. Real-time assessment of perioperative behaviors in children and parents: development and validation of the perioperative adult child behavioral interaction scale. Anesth Analg 2010; 110:1109–1115. [DOI] [PubMed] [Google Scholar]
- 34.Kuhlthau KA, Bloom S, Van Cleave J, et al. Evidence for family-centered care for children with special healthcare needs: a systematic review. Acad Pediatr 2011; 11:136–143.e138. [DOI] [PubMed] [Google Scholar]
- 35.Theng Y-L, Lee JWY, Patinadan PV, Foo SSB. The use of videogames, gamification, and virtual environments in the self-management of diabetes: a systematic review of evidence. Games Health J 2015; 4:352–361. [DOI] [PubMed] [Google Scholar]
- 36.McCoy L, Lewis JH, Dalton D. Gamification and multimedia for medical education: a landscape review. J Am Osteopath Assoc 2016; 116:22–34. [DOI] [PubMed] [Google Scholar]
- 37.Gavens N, Barrouillet P. Delays of retention, processing efficiency, and attentional resources in working memory span development. J Memory Lang 2004; 51:644–657. [Google Scholar]


