Background:
Peripheral nerve compression and entrapment can be debilitating. Using a validated animal model of peripheral nerve compression, we examined the utility of 2 drugs approved for other uses in humans, 4-aminopyridine (4-AP) and erythropoietin (EPO), as treatments for surgically induced ischemia and as adjuvants to surgical decompression.
Methods:
Peripheral nerve compression was induced in wild-type mice by placing an inert silicone sleeve around the sciatic nerve. Decompression surgery was performed at 6 weeks with mice receiving 4-AP, EPO, or saline solution either during and after compression or only after decompression. A nerve conduction study and morphometric analyses were performed to compare the extent of the injury and the efficacy of the therapies, and the findings were subjected to statistical analysis.
Results:
During peripheral nerve compression, there was a progressive decline in nerve conduction velocity compared with that in sham-treatment animals, in which nerve conduction velocity remained normal (∼55 m/s). Mice treated with 4-AP or EPO during the compression phase had significantly smaller declines in nerve conduction velocity and increased plateau nerve conduction velocities compared with untreated controls (animals that received saline solution). Histomorphometric analyses of newly decompressed nerves (i.e., nerves that underwent decompression on the day that the mouse was sacrificed) revealed that both treated groups had significantly greater proportions of large (>5-µm) axons than the untreated controls. Following surgical decompression, all animals recovered to a normal baseline nerve conduction velocity by day 15; however, treatment significantly accelerated improvement (in both the 4-AP and the EPO group), even when it was only started after decompression. Histomorphometric analyses at 7 and 15 days following surgical decompression revealed significantly increased myelin thickness and significantly greater proportions of large axons among the treated animals.
Conclusions:
Both the 4-AP and the EPO-treated group demonstrated improvements in tissue architectural and electrodiagnostic measurements, both during and after peripheral nerve compression, compared with untreated mice.
Clinical Relevance:
Peripheral nerve decompression is one of the most commonly performed procedures in orthopaedic surgery. We believe that there is reason for some optimism about the translation of our findings to the clinical setting. Our findings in this murine model suggest that 4-AP and EPO may lessen the effects of nerve entrapment and that the use of these agents after decompression may speed and perhaps otherwise optimize recovery after surgery.
Peripheral nerve compression, or compression neuropathy, includes many types of peripheral nerves, such as spinal nerves compressed as they exit their intervertebral foramina and peripheral nerves in carpal, cubital, and tarsal tunnel syndrome, among others1,2. These syndromes are ubiquitous, with carpal tunnel syndrome alone accounting for an estimated 450,000 surgical procedures at a cost of billions annually3. There are no universally accepted pharmacological adjuvant therapies for compression neuropathies that improve objective histomorphometric and electrodiagnostic effects of compression. In this study, we propose 2 novel applications of therapeutics—previously used for other purposes—for the treatment of compression neuropathy both before and after decompression.
The selection of 4-aminopyridine (4-AP) and erythropoietin (EPO) as trial agents was based on previous literature suggesting their possible role in ameliorating the effects of peripheral nerve compression4-9. The ability of 4-AP to restore neurological function following chronic nervous system degeneration has been studied since 1977, and it is thought that 4-AP might promote remyelination after peripheral nerve injury through a mechanism related to electrical stimulation4,10-20. EPO, a hematopoietic factor used to treat anemia, also has neuroprotective21-23 and neuromodulatory effects in the central and peripheral nervous systems6,24-27. On the basis of our previous work and encouraging results with both EPO and 4-AP, we hypothesized that these agents would (1) improve electrical and histological parameters of compression during active compression and (2) contribute to improved recovery after surgical decompression in a standard model of compression neuropathy28.
Materials and Methods
Murine Model of Peripheral Nerve Compression
We obtained approval from our institutional animal care and use committee for these experiments.
Six-week-old male C57BL/6 mice (n = 90, 20 to 25 g) underwent peripheral nerve compression surgery in which a compressive sleeve was placed on 1 hindlimb (n = 75) or underwent sham surgery in which no compression was performed (n = 15), as previously described28-30. Briefly, the mice were anesthetized with ketamine (60 mg/kg) and xylazine (4 mg/kg) and a dorsal gluteal-splitting approach allowed mobilization of the sciatic nerve, which was then encircled atraumatically with a 3-mm inert silicone tube distal to the sciatic notch. Preoperatively, the tubes were prepared in 70% ethanol for 12 hours in a sterile ventilation unit. The incision was closed with 5-0 nylon sutures. The sham-operated mice underwent a similar operation in which the sciatic nerve was exposed and isolated but not encircled with a sleeve. All surgery was unilateral. Buprenorphine (0.05 mg/kg) was given for postoperative analgesia immediately following surgery and every 12 hours thereafter for 3 days. No mouse exhibited signs of pain after this period.
Surgical Decompression
The compression sleeves were left in place for a compression phase of 6 weeks, after which decompression surgery was performed to alleviate the compressive lesion30. Micro Adson tissue forceps (Miltex) and 18-G thin-wall needles (BD) were used for surgical removal of the compression sleeve.
Experiment Design
There were 2 phases in this experiment, compression and post-decompression, and the mice were treated in 1 of 3 ways: (1) untreated throughout both phases, (2) treated only after decompression but not during the compression phase, or (3) treated both during the compression phase and following decompression. Treatment was with either 4-AP or EPO.
For treatment with 4-AP, active drug solubilized in sterile saline solution was administered via intraperitoneal injection at the dose equivalent (for mass) to currently approved human dosing (0.5 mg/kg/day), as in previous murine studies of 4-AP as a therapeutic agent4,31. Recombinant human EPO (PROCRIT; Amgen) was administered systemically via intraperitoneal injection at a 500-U/kg/day dosage, analogous to that used in previous murine studies as well as selected human trials32-34.
Mice were randomized to 1 of the following groups: (1) the saline/saline group received saline solution throughout the experiment and served as the untreated control (n = 15); (2) the saline/4-AP group received saline solution throughout the compression phase followed by administration of 4-AP immediately postoperatively and daily thereafter during the decompression phase (n = 15); (3) the saline/EPO group received saline solution throughout the compression phase followed by administration of EPO immediately postoperatively and daily thereafter during the decompression phase (n = 15); (4) the 4-AP/4-AP group received 4-AP throughout the duration of the experiment (n = 15); (5) the EPO/EPO group received EPO throughout the duration of the experiment (n = 15); and (6) a sham-operation group (n = 15).
For the histomorphometric analyses, 3 mice from each group were randomly killed at day 0, day 7, and day 15 after nerve decompression.
Electrodiagnostic Studies
Nerve conduction studies were employed as the primary outcome measure because of their clinical utility in assessment and diagnosis of peripheral nerve injuries35. It was not possible to use electromyography for repeated measurements because it would have required repeated injury to the small mouse muscles (at least 10 times per mouse). The nerve conduction study was performed preoperatively and every week during the compression phase as well as immediately after the decompression surgery (every 2 days starting at day 3 post-decompression). Nerve conduction velocity and distal/proximal latency were measured at all time points. Compound muscle action potentials (CMAPs) were measured at the aforementioned time points except in animals receiving 4-AP, as 4-AP is an electrically active drug and thus renders CMAP measurements useless immediately following administration. However, animals randomized to receive 4-AP were tested before and after placement of the compressive sleeve to ensure minimal damage to the nerve during placement of the sleeve. Recordings for both hindlimbs were obtained using a Viasys Viking Select Neurodiagnostic System (CareFusion) in anesthetized mice. A referencing jig was used to fix the distance between electrodes. The recording electrode was placed into the tibial-nerve-innervated tibialis anterior muscle, and the reference-recording electrode was inserted into the dorsal aspect of the foot. The reference-stimulating lead was placed in the ipsilateral paraspinal muscle. Two sites were stimulated to assess the motor conduction of the sciatic/tibial nerve: the proximal site at the sciatic notch and the distal site proximal to the knee. Disposable stainless-steel electroencephalography (EEG) needle electrodes were used to measure proximal and distal latency, CMAPs, and nerve conduction velocity.
Histomorphometric Analysis
Images of cross-sectioned sciatic nerves were obtained with light microscopy and were processed by ImageJ software (U.S. National Institutes of Health) to determine axon diameter, fiber diameter, myelin thickness, G ratio (axon diameter/fiber diameter on cross-section), and number of myelinated fibers. Each parameter was measured using 40 randomly selected axons in each image, all images were analyzed, and all myelinated axons were counted in every image.
Statistical Analyses
The sample size of mice needed for the study was based on a standard pre-hoc power analysis. The primary outcome measure during experimental planning was the electrodiagnostic measurement of nerve conduction velocity because of the wide use of this measure in the clinical setting. With an α level of 0.05 and a β level of 0.8, we found that 12 animals per treatment group would provide sufficient power to perform parametric statistical tests (analysis of variance [ANOVA] for multiple comparisons and the Student t test for paired comparisons) while accounting for attrition and scheduled sacrifice. Each group was therefore slated to include 15 animals to ensure that this experiment would have sufficient power should there be a need for early sacrifice.
For the axon morphometry, 1-way ANOVA was used to compare axon diameter, fiber diameter, myelin thickness, and G ratio between the different treatment groups. Z tests were used to compare axonal diameter distribution between the treatment groups. Axon diameter was binned into small (<3-µm), medium (3 to 5-µm), and large (>5-µm) size groups, and significant differences between distributions of axon diameter were calculated, as was done in a previous study30. Because there is no specific literature on the compression neuropathy model in terms of the expected size of axons during and after compression, the sizing of these bins was based on our data and data that we found in the literature on those of healthy and diseased axons in other models30. We then used these bin sizes as hard criteria for measurement of changes attributable to compression neuropathy and treatment.
Results
Electrodiagnostic Measures of Nerves During Peripheral Nerve Compression with and without 4-AP and EPO Treatment
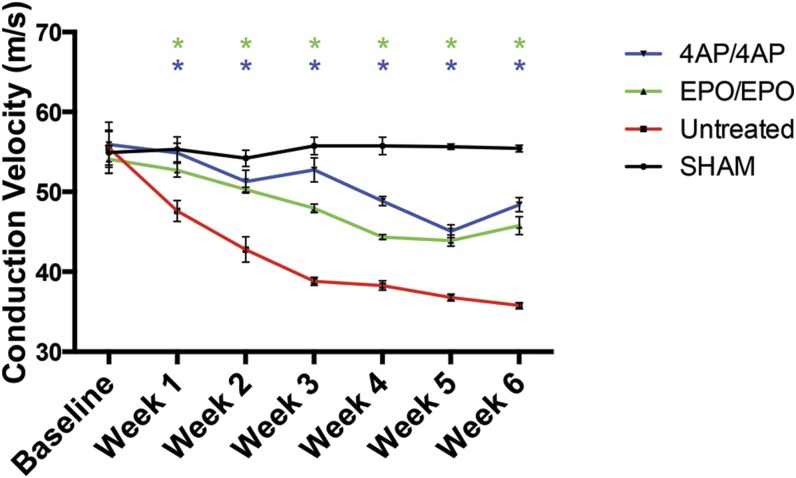
Treatment with 4-AP lessened the decline in nerve conduction velocity during the 6-week compression phase as compared with the untreated (control) animals—i.e., the nerve conduction velocity measurements for the 4-AP-treated mice were significantly higher than those for the untreated mice from week 1 through the end of the compression phase (Fig. 1; see Appendix Table E-1 for statistical comparisons). The nerve conduction velocity in the 4-AP-treated mice reached a plateau of 48.39 ± 0.89 m/s (mean and standard error of the mean [SEM]) during peripheral nerve compression compared with 35.77 ± 0.38 m/s in the untreated mice
Fig. 1.
Comparison of nerve conduction velocity (NCV) among the sham-operation, untreated (control), EPO/EPO, and 4-AP/4-AP groups during 6 weeks of sciatic nerve compression. The values are given as the mean and SEM. *A significant difference (p < 0.05) when compared with the untreated (control) group. See Appendix for specific significant p values.
The EPO-treated mice also had significantly smaller declines in nerve conduction velocity during the peripheral nerve compression phase compared with the untreated mice—i.e., the nerve conduction velocities of the EPO-treated mice were significantly higher than those of the untreated mice from week 1 through the end the compression phase (Fig. 1). The nerve conduction velocity in the EPO-treated mice reached a plateau of 45.77 ± 1.12 m/s during peripheral nerve compression compared with 35.77 ± 0.38 m/s for the untreated mice.
Histomorphometric Measures of Nerves During Peripheral Nerve Compression with and without 4-AP and EPO Treatment
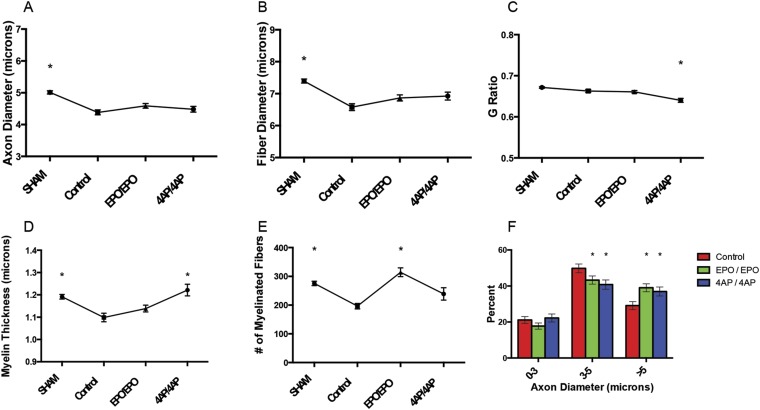
The 4-AP-treated mice had a significantly lower G ratio and a significantly thicker myelin sheath than the control mice (Figs. 2-C and 2-D). The EPO-treated mice had significantly more myelinated fibers than the untreated mice (Fig. 2-E). Additionally, both treated groups had a significantly higher proportion of large axons compared with the untreated mice (Fig. 2-F).
Fig. 2.
Comparison of axon diameter (Fig. 2-A), fiber diameter (Fig. 2-B), G ratio (Fig. 2-C), myelin thickness (Fig. 2-D), number of myelinated fibers (Fig. 2-E), and axon diameter histogram (Fig. 2-F) among the sham-operation, untreated (control), EPO/EPO, and 4-AP/4-AP groups after the 6-week compression phase. The values are given as the mean and SEM. *A significant difference (p < 0.05) when compared with the untreated (control) group. See Appendix for specific significant p values.
Electrodiagnostic Measures of Nerves After Decompression with and without 4-AP and EPO Treatment
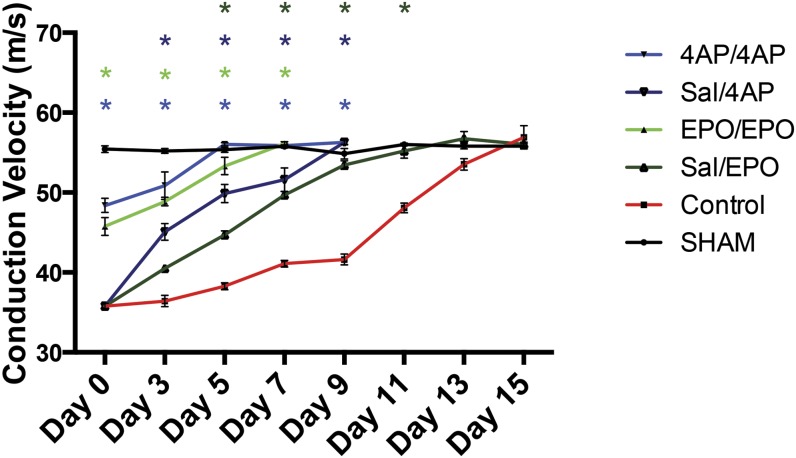
Following decompression, the 4-AP and EPO-treated mice had faster recovery to baseline nerve conduction velocity (∼55 m/s) than the control mice (Fig. 3; see Appendix Table E-3 for statistical comparisons). The 4-AP/4-AP-treated mice (4-AP treatment during both the compression and the decompression phase) recovered normal nerve conduction velocity at day 5 after decompression, and the EPO/EPO-treated mice (i.e., those receiving EPO during both phases) recovered at day 7 after decompression. The saline/4-AP and saline/EPO-treated mice both had a return to baseline nerve conduction velocity at day 9 after decompression. The control mice did not achieve this recovery until day 15 after decompression (Fig. 3). The saline/4-AP-treated mice had significantly higher nerve conduction velocity than the control mice from day 3 until day 9 after decompression, at which time measurements were stopped for the saline/4-AP-treated group. Similarly, the saline/EPO-treated mice had a significantly higher nerve conduction velocity than the control mice from day 5 until day 11 after decompression.
Fig. 3.
Comparison of nerve conduction velocity (NCV) among the sham-operation, untreated (control), saline/EPO, EPO/EPO, saline/4-AP, and 4-AP/4-AP groups following surgical decompression of the sciatic nerve. The values are given as the mean and SEM. *A significant difference (p < 0.05) when compared with the untreated (control) group. See Appendix for specific significant p values.
Histomorphometric Measures of Nerves After Decompression with and without 4-AP and EPO Treatment
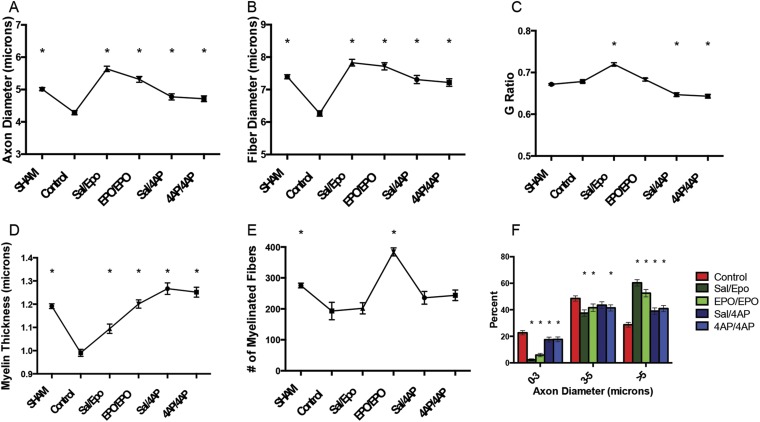
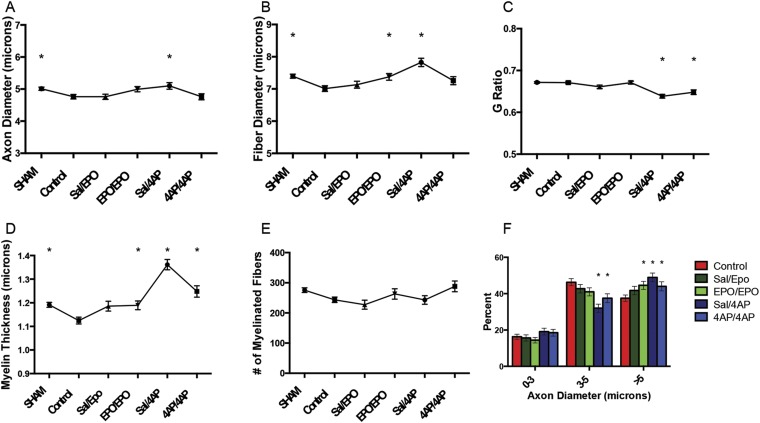
Figure 4 (see Appendix Table E-4 for statistical comparisons) demonstrates the protective/regenerative impact of 4-AP and EPO treatment at 7 days following surgical decompression. One week post-decompression, all 4 treated groups (saline/4-AP, 4-AP/4-AP, saline/EPO, and EPO/EPO) had a significantly greater axon diameter (Fig. 4-A), fiber diameter (Fig. 4-B), and myelin thickness (Fig. 4-D) compared with the control group. However, only the 4-AP-treated animals (saline/4-AP and 4-AP/4-AP groups) had significantly lower G ratios than the control mice (Fig. 4-C). Additionally, all treatment groups (saline/4-AP, 4-AP/4-AP, saline/EPO, and EPO/EPO) displayed significantly higher proportions of large axons compared with the control mice (Fig. 4-F).
Fig. 4.
Comparison of axon diameter (Fig. 4-A), fiber diameter (Fig. 4-B), G ratio (Fig. 4-C), myelin thickness (Fig. 4-D), number of myelinated fibers (Fig. 4-E), and axon diameter histogram (Fig. 4-F) among the sham-operation, untreated (control), saline/EPO, EPO/EPO, saline/4-AP, and 4-AP/4-AP groups at 7 days after surgical decompression. The values are given as the mean and SEM. *A significant difference (p < 0.05) when compared with the untreated (control) group. See Appendix for specific significant p values.
Fifteen days following decompression, both of the 4-AP treatment groups (saline/4-AP and 4-AP/4-AP) and the EPO/EPO-treated mice maintained significantly thicker myelin than the untreated controls (Fig. 5-D; see Appendix Table E-5 for statistical comparisons). Only the 4-AP-treated mice (saline/4-AP and 4-AP/4-AP) had significantly lower G ratios than the control mice (Fig. 5-C). The saline/4-AP, 4-AP/4-AP, and EPO/EPO-treated groups also had a significantly higher proportion of large axons than the control mice (Fig. 5-F).
Fig. 5.
Comparison of axon diameter (Fig. 5-A), fiber diameter (Fig. 5-B), G ratio (Fig. 5-C), myelin thickness (Fig. 5-D), number of myelinated fibers (Fig. 5-E), and axon diameter histogram (Fig. 5-F) among the sham-operation, untreated (control), saline/EPO, EPO/EPO, saline/4-AP, and 4-AP/4-AP groups at 15 days after surgical decompression. The values are given as the mean and SEM. *A significant difference (p < 0.05) when compared with the untreated (control) group. See Appendix for specific significant p values.
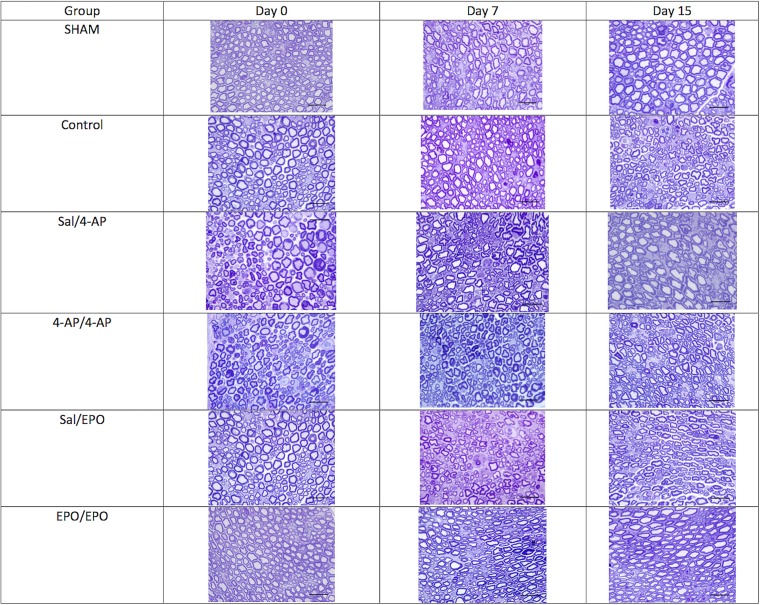
Representative tissue sections from each group are shown in Figure 6.
Fig. 6.
Representative light microscopy images (toluidine blue; 100×) of the sham-operation, untreated (control), saline/4-AP, 4-AP/4-AP, saline/EPO, and EPO/EPO groups at the end of 6 weeks of compression (here termed “day 0” relative to decompression surgery) and at day 7 and day 15 post-decompression.
Discussion
In both neurotrauma and compression neuropathy, a key element of dysfunction occurs secondary to the loss of myelin, and yet there is no treatment other than surgery to target demyelination. Our previous work has specifically shown that both EPO and 4-AP foster myeloprotection and promote remyelination after traumatic crush injury4-6. This previous work turned our attention to a clinical condition in which the primary dysfunction involves myelin. Within the scope of our clinical practice, patients with dysfunction from compression neuropathy often require surgical intervention36-38.
The pathophysiology of neuronal injury due to compression is still a matter of inquiry. The pathology is initially localized to the myelin sheath, with axonal involvement occurring much later in the disease course39. Studies have suggested that the pathogenesis of compression is likely secondary to ischemia and mechanical forces that induce a stress response in Schwann cells40-44. The initial degenerative change observed is the loss of Schwann cells via apoptosis45, followed by focal segmental demyelination of axons46. This begins a prolonged cycle of demyelination-remyelination and Schwann cell turnover. Electrodiagnostic studies remain the clinical mainstay for both diagnosis and staging of compression and entrapment neuropathies47. The cellular and molecular effects of compression are believed to manifest as a slowing of nerve conduction velocity as measured across a population of thousands of myelinated fibers.
We chose an established murine model of nerve compression for experiments to test our hypotheses regarding 4-AP and EPO28-30, with the belief that electrodiagnostic parameters may serve as a correlate for important histological measures of nerve function that are difficult to measure (axon diameter, myelin thickness, internodal length, etc.). We found that EPO and 4-AP had significant effects on axon diameter and myelin thickness and believe that this may underlie improvements in nerve conduction velocity. Untreated (control) animals demonstrated a progressive decline in nerve conduction velocity characteristic of compression injury, which continued during the compression period until reaching a plateau before week 6. As clinical sensory symptoms cannot be recreated in an animal model, the outcome measure most often used to establish the diagnosis of compression neuropathy in mice is nerve conduction velocity, a key correlate to the clinical situation, where patients are currently routinely examined with the same modality48.
Our results support the idea that 4-AP and EPO have a potential neuroprotective effect on the electrodiagnostic parameters of compressed nerves, an idea that is based on our previous work demonstrating effects of both of these agents on myelination4-6,49. Our results show that 4-AP and EPO attenuate electrophysiologic impairment caused by compression, with the untreated mice demonstrating significantly greater losses in nerve conduction velocity than the 4-AP and EPO-treated mice. Furthermore, our results demonstrate a possible role for both of these agents as an adjuvant to surgical decompression. Both 4-AP and EPO treatment accelerated recovery of nerve conduction velocity following decompression, and both agents promoted remyelination in this scenario as well. We believe that there is reason for some optimism about the clinical translation of these findings given that both of these agents are currently approved for other uses in humans by the U.S. Food and Drug Administration.
In order to gain a preliminary glimpse into the method by which these agents may be acting, we assayed nerves after treatment with either 4-AP or EPO. Durable and statistically significant improvements in the number of myelinated fibers as well as other measures, such as the G ratio (demonstrating increased remyelination after decompression), suggest that pharmacological agents may mitigate the effects of nerve entrapment. The additional benefit of pharmacological treatment after decompression supports the use of these agents as therapeutics to speed and perhaps otherwise optimize recovery after surgery.
There are limitations to our work. First, we have offered no information about the potential improvements in sensory function afforded by these treatments. Although sensory function has been studied in different rodent models50,51, this function has not been translated to the setting of compression neuropathy in humans and requires further study. Moreover, the limitations of this study mirror the limitations of this model, which include the fact that animals seem to retain their gait parameters no matter how long the compression is left in place. We know that chronic compression in humans leads to irreversible motor dysfunction. We cannot predict, on the basis of this work, what would happen in the treatment of patients with nerve dysfunction who demonstrated motor impairment. Although we know that treatment seems to affect the histomorphometric appearance of the nerve tissue, we cannot know if improvements in measures such as the size of axons (binned into size categories), G ratio, or even axon diameters will translate into clear functional improvements in these animals. A measure that is more sensitive than standard sciatic function indices may reveal such a deficit in the future. Finally, we are proposing the use of pharmacological agents for conditions well served by surgery today. Given the side-effect profiles of these drugs, we must wait for clinical trials to see if future patients are better served by traditional approaches. It may prove that the side effects of this type of pharmaco-adjuvant therapy are a poor trade-off for the benefits in actual patients.
Appendix
A description of the tissue harvest for the histological analysis as well as tables showing significant p values for the data presented in the figures are available with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/F109).
Acknowledgments
Note: The authors thank Karen Bentley and the URMC Electron Microscope Shared Resource Laboratory for their role in preparing and imaging the samples.
Footnotes
Investigation performed at the University of Rochester, Rochester, New York, and the Center for Orthopaedic Research and Translational Science, The Pennsylvania State University College of Medicine, Hershey, Pennsylvania
Disclosure: This work was supported by grants from the National Institutes of Health (NIH) (K08 AR060164-01A) and the Department of Defense (DOD) (W81XWH-16-1-0725), an American Society for Surgery of the Hand (ASSH) Hand Surgeon Scientist Award grant, and University of Rochester Medical Center Clinical & Translational Science Institute grants (TR000042 and TR000096). Additionally, institutional support was provided by the University of Rochester and Pennsylvania State University. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a patent (4-aminopyridine [4-AP] for peripheral nerve injury) and/or copyright, planned, pending, or issued, broadly relevant to this work (http://links.lww.com/JBJS/F108).
References
- 1.Andreisek G, Crook DW, Burg D, Marincek B, Weishaupt D. Peripheral neuropathies of the median, radial, and ulnar nerves: MR imaging features. Radiographics. 2006. Sep-Oct;26(5):1267-87. [DOI] [PubMed] [Google Scholar]
- 2.Donovan A, Rosenberg ZS, Cavalcanti CF. MR imaging of entrapment neuropathies of the lower extremity. Part 2. The knee, leg, ankle, and foot. Radiographics. 2010. Jul-Aug;30(4):1001-19. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton MJ. Occupation and carpal tunnel syndrome. ANZ J Surg. 2006. June;76(6):494-6. [DOI] [PubMed] [Google Scholar]
- 4.Tseng KC, Li H, Clark A, Sundem L, Zuscik M, Noble M, Elfar J. 4-aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol Med. 2016. December 1;8(12):1409-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundem L, Chris Tseng KC, Li H, Ketz J, Noble M, Elfar J. Erythropoietin enhanced recovery after traumatic nerve injury: myelination and localized effects. J Hand Surg Am. 2016. October;41(10):999-1010. Epub 2016 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elfar JC, Jacobson JA, Puzas JE, Rosier RN, Zuscik MJ. Erythropoietin accelerates functional recovery after peripheral nerve injury. J Bone Joint Surg Am. 2008. August;90(8):1644-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundh H, Nilsson O, Rosén I. 4-aminopyridine—a new drug tested in the treatment of Eaton-Lambert syndrome. J Neurol Neurosurg Psychiatry. 1977. November;40(11):1109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefoski D, Davis FA, Faut M, Schauf CL. 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol. 1987. January;21(1):71-7. [DOI] [PubMed] [Google Scholar]
- 9.Strupp M, Feil K, Bardins S, Waidelich R. 4-aminopyridine improves lower urinary tract symptoms in a patient with benign prostatic hyperplasia and downbeat nystagmus syndrome. Int Neurourol J. 2014. December;18(4):221-5. Epub 2014 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nix WA, Hopf HC. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983. August 1;272(1):21-5. [DOI] [PubMed] [Google Scholar]
- 11.Pockett S, Gavin RM. Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci Lett. 1985. August 30;59(2):221-4. [DOI] [PubMed] [Google Scholar]
- 12.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000. April 1;20(7):2602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002. August 1;22(15):6631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol. 2007. November;208(1):137-44. Epub 2007 Aug 23. [DOI] [PubMed] [Google Scholar]
- 15.English AW, Schwartz G, Meador W, Sabatier MJ, Mulligan A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007. February 1;67(2):158-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007. June;205(2):347-59. Epub 2007 Feb 21. [DOI] [PubMed] [Google Scholar]
- 17.Vivó M, Puigdemasa A, Casals L, Asensio E, Udina E, Navarro X. Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp Neurol. 2008. May;211(1):180-93. Epub 2008 Feb 13. [DOI] [PubMed] [Google Scholar]
- 18.Haastert-Talini K, Schmitte R, Korte N, Klode D, Ratzka A, Grothe C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma. 2011. April;28(4):661-74. Epub 2011 Mar 24. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Lu L, Zhang J, Hu X, Zhang Y, Liang W, Wu S, Luo Z. Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect. PLoS One. 2012;7(6):e39526 Epub 2012 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh B, Xu QG, Franz CK, Zhang R, Dalton C, Gordon T, Verge VM, Midha R, Zochodne DW. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm. J Neurosurg. 2012. March;116(3):498-512. Epub 2011 Dec 9. [DOI] [PubMed] [Google Scholar]
- 21.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997. January;76(1):105-16. [DOI] [PubMed] [Google Scholar]
- 22.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998. April 14;95(8):4635-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dame C, Juul SE, Christensen RD. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate. 2001;79(3-4):228-35. [DOI] [PubMed] [Google Scholar]
- 24.Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001. March 27;98(7):4044-9. Epub 2001 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Gonias SL, Campana WM. Schwann cells express erythropoietin receptor and represent a major target for EPO in peripheral nerve injury. Glia. 2005. September;51(4):254-65. [DOI] [PubMed] [Google Scholar]
- 26.Lykissas MG, Sakellariou E, Vekris MD, Kontogeorgakos VA, Batistatou AK, Mitsionis GI, Beris AE. Axonal regeneration stimulated by erythropoietin: an experimental study in rats. J Neurosci Methods. 2007. August 15;164(1):107-15. Epub 2007 Apr 19. [DOI] [PubMed] [Google Scholar]
- 27.Sekiguchi Y, Kikuchi S, Myers RR, Campana WM. ISSLS prize winner: Erythropoietin inhibits spinal neuronal apoptosis and pain following nerve root crush. Spine (Phila Pa 1976). 2003. December 1;28(23):2577-84. [DOI] [PubMed] [Google Scholar]
- 28.Jung J, Hahn P, Choi B, Mozaffar T, Gupta R. Early surgical decompression restores neurovascular blood flow and ischemic parameters in an in vivo animal model of nerve compression injury. J Bone Joint Surg Am. 2014. June 4;96(11):897-906. Epub 2014 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung J, Frump D, Su J, Wang W, Mozaffar T, Gupta R. Desert hedgehog is a mediator of demyelination in compression neuropathies. Exp Neurol. 2015. September;271:84-94. Epub 2015 May 1. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, Nassiri N, Hazel A, Bathen M, Mozaffar T. Chronic nerve compression alters Schwann cell myelin architecture in a murine model. Muscle Nerve. 2012. February;45(2):231-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008. March;22(3):659-61. Epub 2007 Oct 17. [DOI] [PubMed] [Google Scholar]
- 32.Garcia P, Speidel V, Scheuer C, Laschke MW, Holstein JH, Histing T, Pohlemann T, Menger MD. Low dose erythropoietin stimulates bone healing in mice. J Orthop Res. 2011. February;29(2):165-72. Epub 2010 Aug 25. [DOI] [PubMed] [Google Scholar]
- 33.Holstein JH, Orth M, Scheuer C, Tami A, Becker SC, Garcia P, Histing T, Mörsdorf P, Klein M, Pohlemann T, Menger MD. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone. 2011. November;49(5):1037-45. Epub 2011 Aug 9. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Rüther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Sirén AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002. August;8(8):495-505. [PMC free article] [PubMed] [Google Scholar]
- 35.Lane LB, Starecki M, Olson A, Kohn N. Carpal tunnel syndrome diagnosis and treatment: a survey of members of the American Society for Surgery of the Hand. J Hand Surg Am. 2014. November;39(11):2181-87.e4. Epub 2014 Sep 13. [DOI] [PubMed] [Google Scholar]
- 36.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999. July 14;282(2):153-8. [DOI] [PubMed] [Google Scholar]
- 37.Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007. August;36(2):167-71. [DOI] [PubMed] [Google Scholar]
- 38.Carofino BC, Brogan DM, Kircher MF, Elhassan BT, Spinner RJ, Bishop AT, Shin AY. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013. September 18;95(18):1667-74. [DOI] [PubMed] [Google Scholar]
- 39.Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013. August;29(3):317-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham K, Gupta R. Understanding the mechanisms of entrapment neuropathies. Review article. Neurosurg Focus. 2009. February;26(2):E7. [DOI] [PubMed] [Google Scholar]
- 41.Tapadia M, Mozaffar T, Gupta R. Compressive neuropathies of the upper extremity: update on pathophysiology, classification, and electrodiagnostic findings. J Hand Surg Am. 2010. April;35(4):668-77. Epub 2010 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta R, Truong L, Bear D, Chafik D, Modafferi E, Hung CT. Shear stress alters the expression of myelin-associated glycoprotein (MAG) and myelin basic protein (MBP) in Schwann cells. J Orthop Res. 2005. September;23(5):1232-9. Epub 2005 Apr 25. [DOI] [PubMed] [Google Scholar]
- 43.Gupta R, Rummler L, Steward O. Understanding the biology of compressive neuropathies. Clin Orthop Relat Res. 2005. July;436:251-60. [DOI] [PubMed] [Google Scholar]
- 44.Frieboes LR, Gupta R. An in-vitro traumatic model to evaluate the response of myelinated cultures to sustained hydrostatic compression injury. J Neurotrauma. 2009. December;26(12):2245-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003. June 23;461(2):174-86. [DOI] [PubMed] [Google Scholar]
- 46.Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004. June;187(2):500-8. [DOI] [PubMed] [Google Scholar]
- 47.Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013. August;29(3):363-70. Epub 2013 Jun 12. [DOI] [PubMed] [Google Scholar]
- 48.Lee DH, Claussen GC, Oh S. Clinical nerve conduction and needle electromyography studies. J Am Acad Orthop Surg. 2004. Jul-Aug;12(4):276-87. [DOI] [PubMed] [Google Scholar]
- 49.Geary MB, Li H, Zingman A, Ketz J, Zuscik M, De Mesy Bentley KL, Noble M, Elfar JC. Erythropoietin accelerates functional recovery after moderate sciatic nerve crush injury. Muscle Nerve. 2017. July;56(1):143-151. Epub 2017 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009. April;89(2):707-58. [DOI] [PubMed] [Google Scholar]
- 51.Jeong Y, Holden JE. Commonly used preclinical models of pain. West J Nurs Res. 2008. April;30(3):350-64. Epub 2007 Nov 20. [DOI] [PubMed] [Google Scholar]