Abstract
Background
Survival outcome disparities among esophageal cancer patients exist, but are not fully understood.
Aims
We used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to determine whether survival differences among racial/ethnic patient populations persist after adjusting for demographic and clinical characteristics.
Methods
Our study included T1-3N0M0 adenocarcinoma and squamous cell cancer patients diagnosed between 2003 and 2011. We compared survival among two racial/ethnic patient subgroups using Cox proportional hazards methods, adjusting for age, sex, histology, marital status, socioeconomics, SEER region, comorbidities, T stage, tumor location, diagnosis year, and treatment received.
Results
Among 2025 patients, 87.9% were White and 12.1% were Nonwhite. Median survival was 18.7 months for Whites vs 13.8 months for Nonwhites (p = 0.01). In the unadjusted model, Nonwhite patients had higher risk of mortality (HR = 1.29, 95% CI 1.11–1.49, p < 0.0001) when compared to White patients; however, in the Cox regression adjusted model there was no significant difference (HR = 0.94, 95% CI 0.80–1.10, p = 0.44). Surgery, chemotherapy, younger age, lower T stage, and lower Charlson comorbidity score were significant predictors in the full adjusted model.
Conclusions
Differences in mortality risk by race/ethnicity appear to be largely explained by additional factors. In particular, associations were seen in surgery and T stage. Further research is needed to understand potential mechanisms underlying the differences and to better target patients who can benefit from treatment options.
Keywords: Esophageal cancer, Disparities, SEER-Medicare, Outcomes, Survival
Introduction
Esophageal cancer incidence in the USA has risen over the past 20 years, with an estimated 16,940 new cases and 15,690 deaths expected in 2017 [1, 2]. The incidence of esophageal adenocarcinoma, which is predominately diagnosed in White patients, has risen dramatically, while squamous cell carcinoma, which is more commonly diagnosed in Black patients, has decreased [3, 4].
Despite advancements in treatment options for patients with esophageal cancer, overall survival remains poor, with a five-year survival of less than 20% [1]. Approximately 20% of esophageal cancer is found at the localized stage (T1–3, N0, M0), and five-year survival among this population is 43% [5]. However, Black patients have a five-year survival rate of 23%, a substantially smaller proportion compared with White patients, who have a five-year survival rate of 45%. Thus, this suggests a clear disparity in survival outcomes by race. Although differences in biology may play a role in these racial survival disparities, variation in the receipt of certain treatment options and access to health care is thought to explain these differences [6, 7]. Prior studies involving patients with esophageal and other cancer types have shown that race/ethnicity are predictors of whether patients receive cancer-directed surgery [7–10]. Potential explanations for the lower likelihood that racial minorities receive cancer-directed surgery have included theories suggesting that these patients’ lower socioeconomic status, higher comorbidities, and decreased access to care are involved [11].
While earlier studies have suggested that disparities no longer persist after adjusting for treatment receipt [6, 12, 13], they largely focused on registry data, which do not always contain important variables, such as comorbid medical conditions and complete treatment information. Therefore, in the current study, we used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to determine whether these racial/ethnic differences in overall and cancer-specific survival outcomes persist after controlling for important confounders, such as patients’ sociodemo-graphic and clinical characteristics.
Methods
Cohort Inclusion/Exclusion Criteria
We identified adenocarcinoma and squamous cell cancer patients diagnosed between 2003 and 2011 from the 2015 release of the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute and Medicare linked data, using International Classification of Diseases for Oncology (ICD-O-3) codes as outlined in Table 1. The SEER database includes cancer incidence and survival data collected from cancer registries covering about 28% of the US population. The Medicare database includes information for approximately 97% of patients aged 65 and older who receive Medicare benefits in the USA. The SEER-Medicare data link SEER to Medicare enrollment and claim files maintained by the Center for Medicare and Medicaid Services, including Parts A and B claims for covered healthcare services. We included patients who were diagnosed with a non-metastatic primary cancer at age 66 or above between January 1, 2003, and December 31, 2011, that was pathologically confirmed. To ensure that we captured complete claims data for each patient, we included only those with continuous enrollment in Medicare Parts A and B from 13 months before diagnosis (for Charlson comorbidity score estimation) until death or December 31, 2013, whichever came first, and who were not enrolled in an HMO (for treatment information). We determined T, N, and M stage according to the AJCC 7th edition per the Collaborative Stage Data Collection System Version 02.05 using SEER variables for tumor extension, lymph nodes, and metastasis, respectively. We included patients who had T1–3, N0, M0 tumors to focus on early-stage cancer who are most likely to receive treatment, including surgery.
Table 1.
SEER-Medicare claims codes
| Variable | Source | Codes |
|---|---|---|
| Adenocarcinoma diagnosis | ICD-0–3 codes | 8050, 8140–8147, 8160–8162, 8180–8221, 8250–8507, 8514, 8520–8551, 8560, 8570–8574, 8576, 8940–8941 |
| Squamous cell carcinoma diagnosis | ICD-0–3 codes | 8070–8078, 8083, 8084 |
| Surgery | CPT or ICD-9 Procedure Codes | 43100, 43101, 43107, 43108, 43112, 43113, 43116, 43117, 43118, 43119, 43121, 43122, 43123, 43124, 43217, 43216, 43228, 43250, 43251, 43257, 43258, 96570, 96571, 42.33 |
| Radiation | CPT or ICD-9 Procedure Codes | ICD-9-CM: V58.0, 92.21–92.29 |
| CPT: 7331–7336, 73399, 77400–77499, 77750–77799 | ||
| Revenue Center: 0330, 0333, 0339 | ||
| Chemotherapy | CPT or ICD-9 Procedure Codes | ICD-9-CM: V58.1, 99.25 |
| HCPCS: C1166, C1168, C1179, C9110, C9205, C9207, C9213-C9216, C9411, C9414-C9419, C942x, C9430-C9438, G0345-G0363, J9000-J9999, Q0083-Q0085 | ||
| CPT: 9651x-9654x, 964xx | ||
| Revenue Center 0331, 0332, 0335 |
We estimated the comorbidity score by applying the Deyo et al. adaptation of the Charlson comorbidity index, which allows for index scores from ICD-9 diagnosis and procedure codes to Medicare inpatient, outpatient, and physician claims during the 13-month period prior to cancer diagnosis and classified patients into the groups based on the presence of 0, 1, or 2 + comorbid conditions [14–17]. We calculated an ecological socioeconomic status by using US Census data provided in SEER-Medicare to derive quintiles of ZIP code-level median household income. We categorized patients into two race/ethnicity groups (White, Nonwhite [Black, Hispanic, Asian]) using SEER variables; we excluded < 11 patients with other or unknown race. We defined treatment variables based on the CPT and ICD-9 codes listed in Table 1; we included local endoscopic treatment in the surgery variable. We determined the cause of death using SEER data.
Statistical Analysis
Our primary outcomes of interest were overall and cancer-specific survival among the racial/ethnic subgroups. We evaluated differences in the distribution of baseline characteristics between these groups using Chi-square tests. We plotted overall and cancer-specific survival using Kaplan–Meier curves. We constructed a Cox proportional hazard model to examine factors contributing to the survival differences across groups. We defined survival as the time from the date of diagnosis to the date of death or December 31, 2013, whichever came first. We estimated the hazard ratio before adjustment and then after adjustment for a select number of potential confounders: age at diagnosis (66–69, 70–74, 75–79, 80–84, 85 +); sex; race and ethnicity; year of diagnosis (2003–2005, 2006–2018, 2009–2011); SEER region; marital status; median income (census tract quintile); histology (adenocarcinoma, squamous cell); T stage; tumor location (lower, middle, upper); Charlson comorbidity score; and treatment (surgery/local therapy, radiation, chemotherapy). We analyzed the adjusted model without treatment and again with treatment (surgery/endoscopic therapy, radiation, or chemotherapy) included. We defined statistical significance as p value < 0.05 in a two-sided test. We performed all statistical analyses using SAS software, version 9.4 (SAS Institute, Inc).
Results
Patient Characteristics
The final cohort included 2025 patients; 1779 (88%) were White, and 246 (12.1%) were Nonwhite. Among the Non-white group, 152 (61.8%) were Black, 68 (27.6%) were Asian, and 27 (11.0%) were Hispanic. Nonwhite patients were more likely to be female, unmarried, diagnosed in earlier years, from the South or West/Hawaii, have a lower SES (census tract quintile), or receive radiation compared to White patients. White patients were more likely to have adenocarcinoma or a tumor in the lower esophagus, while Nonwhite patients were more likely to have a squamous cell cancer or tumor in the middle esophagus. White patients were more likely to receive surgery compared to Nonwhites (46.4% vs 28.4%). We observed no statistically significant differences in age, AJCC stage, Charlson comorbidity score, or chemotherapy receipt between the two groups. The full list of patient characteristics is listed in Table 2.
Table 2.
Patient characteristics (T1-3N0M0)
| Characteristic | White (N = 1779) | Nonwhite (N = 246) | p value |
|---|---|---|---|
| Age | |||
| 66–69 | 320 (18.0%) | 40 (16.3%) | 0.25 |
| 70–74 | 407 (22.9%) | 67 (27.2%) | |
| 75–79 | 414 (23.3%) | 63 (25.6%) | |
| 80–84 | 348 (19.6%) | 47 (19.1%) | |
| 85+ | 290 (16.3%) | 29 (11.8%) | |
| Age (Mean, SD) | 76.9 (7.1) | 75.1 (6.5) | |
| Sex | |||
| Male | 1266 (72.2%) | 160 (65.0%) | 0.049 |
| Female | 513 (28.8%) | 86 (35.0%) | |
| Marital status | |||
| Unmarried | 648 (36.4%) | 133 (54.1%) | < 0.0001 |
| Married | 1038 (58.4%) | * | |
| Unknown | 93 (5.2%) | * | |
| Year of diagnosis | |||
| 2003–2005 | 611 (34.5%) | 103 (41.9%) | 0.03 |
| 2006–2008 | 608 (34.2%) | 83 (33.7%) | |
| 2009–2011 | 560 (31.5%) | 60 (24.4%) | |
| SEER region | |||
| Northeast | 406 (22.8%) | 32 (13.0%) | 0.0001 |
| South | 427 (24.0%) | 80 (32.5%) | |
| Midwest | 243 (13.7%) | 23 (9.4%) | |
| West/Hawaii | 703 (39.5%) | 111 (45.1%) | |
| SES** | |||
| 0 (lowest) | 240 (13.5%) | 104 (42.3%) | < 0.0001 |
| 1 | 337 (18.9%) | 48 (19.5%) | |
| 2 | 361 (20.3%) | 38 (15.5%) | |
| 3 | 415 (23.3%) | 34 (13.8%) | |
| 4 (highest) | 426 (24.0%) | 22 (8.9%) | |
| Histology | |||
| Adenocarcinoma | 1220 (68.6%) | 54 (22.0%) | < 0.0001 |
| Squamous cell carcinoma | 559 (31.4%) | 192 (78.15%) | |
| Stage | |||
| I | 1098 (61.7%) | 154 (62.6%) | 0.79 |
| II | 681 (38.3%) | 92 (37.4%) | |
| T stage | |||
| T1a | 369 (20.7%) | 29 (11.8%) | < 0.0001 |
| T1b | 166 (9.3%) | 14 (6.7%) | |
| T1NOS | 563 (31.7%) | 111 (45.1%) | |
| T2 | 286 (16.1%) | 41 (16.7%) | |
| T3 | 395 (22.2%) | 51 (20.7%) | |
| Charlson score | |||
| 0 | 745 (41.9%) | 105 (42.7%) | 0.83 |
| 1 | 502 (28.2%) | 65 (26.4%) | |
| 2+ | 532 (29.9%) | 76 (26.4%) | |
| Esophagus location | |||
| Lower | 1092 (61.4%) | 83 (33.7%) | <0.0001 |
| Middle | 375 (21.1%) | 107 (43.5%) | |
| Upper | 123 (6.9%) | 23 (9.4%) | |
| Unknown | 189 (10.6%) | 33 (13.4%) | |
| Surgery | |||
| No | 953 (53.6%) | 176 (71.5%) | < 0.0001 |
| Yes | 826 (46.4%) | 70 (28.4%) | |
| Radiation | |||
| No | 724 (40.7%) | 79 (32.1%) | 0.01 |
| Yes | 1055 (59.3%) | 167 (67.9%) | |
| Chemotherapy | |||
| No | 938 (52.7%) | 146 (59.4%) | 0.051 |
| Yes | 841 (47.3%) | 100 (40.7%) |
Values suppressed in accordance with SEER-Medicare guidelines to mask cell sizes that may be < 11 and ensure patient confidentiality. Percentages may not add to 100 due to rounding
SES: quintiles based on median income by census tract ZIP code
Survival Trends and Outcomes
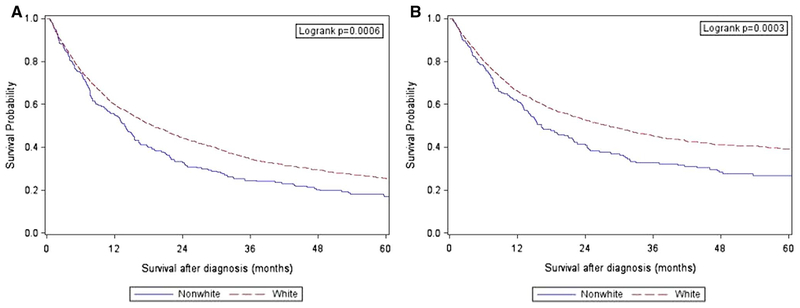
Figure 1 displays the Kaplan–Meier curves for overall and cancer-specific survival, stratified by race/ethnicity. The median (25th, 75th percentile) survival for the entire cohort was 17.6 months (6.2, 42.1). The median (25th, 75th percentile) survival was 18.7 months (6.2, 43.6) and 13.8 months (5.9, 32.0) for White and Nonwhite patients, respectively (p = 0.01).
Fig. 1.
Overall (a) and cancer-specific (b) survival by race/ethnicity
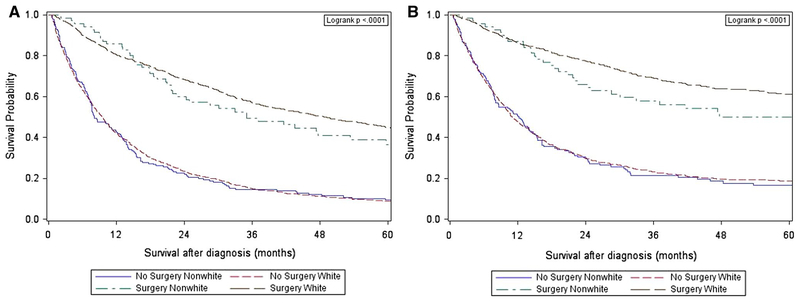
Figure 2 displays the Kaplan–Meier curves for overall and cancer-specific survival, stratified by surgery receipt and race/ethnicity. Among patients who received surgery, the median (25th, 75th percentile) survival was 36.8 months (17.8, 66.8) and 31.8 months (16.4, 53.6) for White and Nonwhite patients, respectively (p = 0.06). Among patients who did not receive surgery, the median (25th, 75th percentile) survival was 9.5 months (3.8, 22.8) and 8.1 months (4.0, 21.2) for White and Nonwhite patients, respectively (p = 0.80).
Fig. 2.
Overall (a) and cancer-specific (b) survival by race/ethnicity and surgery receipt
In the unadjusted Cox proportional hazards models, Non-white patients had higher hazards for mortality in the overall (HR 1.29, 95% CI 1.11–1.49, p = 0.0006) and cancer-specific models (HR 1.36, 95% CI 1.15–1.60, p = 0.0003) compared with White patients. These were no longer statistically significant when adjusted for all variables except treatment (HR 1.001, 95% CI 0.85–1.18, p = 0.99 and HR 1.00, 95% CI 0.83–1.20, p = 0.99) for overall and cancer-specific models, respectively). We found similar results with the addition of the treatment variables (surgery, radiation, chemotherapy) in the models comparing Nonwhite and White patients (HR 0.94 95% CI 0.80–1.10, p = 0.44 and HR 0.93, 95% CI 0.77–1.12, p = 0.45) for overall and cancer-specific models, respectively).
Surgery had a significant association in the full adjusted model, with patients who received surgery having lower hazards for mortality when compared to those who did not receive surgery, with HR 0.36 (95% CI 0.32–0.41, p < 0.0001) and HR 0.31 (95% CI 0.27–0.36, p < 0.0001) for overall and cancer-specific models, respectively. Chemotherapy receipt also was associated with a lower hazard for mortality in the overall (HR 0.82, 95% CI 0.72–0.93, p = 0.002) and cancer-specific models (HR 0.79, 95% CI 0.68–0.91, p = 0.002). A Charlson comorbidity score of 2 or higher predicted worse outcomes in both models, with HR 1.51 (95% CI 1.34–1.70, p < 0.0001) and HR 1.35 (95% CI 1.06–1.42, p = 0.006) for overall and cancer-specific models, respectively, when compared to a Charlson score of 0. Older patient subgroups had a higher hazard for mortality compared with ages 66–69 in the adjusted overall model (HR 1.29, 95% CI 1.09–1.53, p = 0.003 for 75–79; HR 1.37, 95% CI 1.15–163, p = 0.0005 for 80–84; HR 1.89, 95% CI 1.57–2.27, p < 0.0001 for 85 +); similar results were found in the cancer-specific model. Sex, SES, histology, and receipt of radiation were not significant in either model. Full results are found in Table 3.
Table 3.
Cox proportional hazard ratios for overall and cancer-specific mortality after adjustment for patient and tumor characteristics
| Characteristic | Overall | Cancer-specific | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Race/ethnicity | ||||
| Unadjusted model | ||||
| White | 1.0 (Ref) | 1.0 (Ref) | ||
| Nonwhite | 1.29 (1.11–1.49) | 0.0006 | 1.36 (1.15–1.60) | 0.0003 |
| Adjusted model (no treatment)* | ||||
| White | 1.0 (Ref) | 1.0 (Ref) | ||
| Nonwhite | 1.001 (0.85–1.18) | 0.99 | 1.00 (0.83–1.20) | 0.99 |
| Adjusted model (including treatment)** | ||||
| Race/ethnicity | ||||
| White | 1.0 (Ref) | 1.0 (Ref) | ||
| Nonwhite | 0.94 (0.80–1.10) | 0.44 | 0.93 (0.77–1.12) | 0.45 |
| Age at diagnosis | ||||
| 66–69 | 1.0 (Ref) | 1.0 (Ref) | ||
| 70–74 | 1.10 (0.93–1.30) | 0.29 | 1.08 (0.88–1.33) | 0.46 |
| 75–79 | 1.29 (1.09–1.53) | 0.003 | 1.29 (1.06–1.58) | 0.01 |
| 80–84 | 1.37 (1.15–1.63) | 0.0005 | 1.24 (1.01–1.53) | 0.04 |
| 85+ | 1.89 (1.57–2.27) | < 0.0001 | 1.76 (1.42–2.19) | < 0.0001 |
| Sex | ||||
| Male | 1.0 (Ref) | 1.0 (Ref) | ||
| Female | 0.54 (0.84–1.07) | 0.41 | 1.04 (0.90–1.19) | 0.62 |
| Marital status | ||||
| Unmarried | 1.0 (Ref) | 1.0 (Ref) | ||
| Married | 0.77 (0.69–0.86) | < 0.0001 | 0.79 (0.69–0.90) | 0.0004 |
| Unknown | 0.70 (0.55–0.90) | 0.005 | 0.66 (0.49–0.90) | 0.008 |
| Year of diagnosis | ||||
| 2003–2005 | 1.0 (Ref) | 1.0 (Ref) | ||
| 2006–2008 | 1.06 (0.94–1.19) | 0.37 | 0.99 (0.87–1.14) | 0.93 |
| 2009–2011 | 0.89 (0.78–1.01) | 0.09 | 0.79 (0.68–0.92) | 0.002 |
| SEER region | ||||
| Northeast | 1.0 (Ref) | 1.0 (Ref) | ||
| South | 1.45 (1.24–1.70) | < 0.0001 | 1.40 (1.16–1.69) | 0.0004 |
| Midwest | 1.18 (0.94–1.36) | 0.16 | 0.96 (0.77–1.20) | 0.73 |
| West/Hawaii | 1.11 (0.96–1.27) | 0.16 | 1.11 (0.94–1.31) | 0.22 |
| SES | ||||
| 0 (lowest) | 1.0 (Ref) | 1.0 (Ref) | ||
| 1 | 0.95 (0.80–1.11) | 0.50 | 0.93 (0.76–1.13) | 0.47 |
| 2 | 0.88 (0.75–1.04) | 0.13 | 0.88 (0.73–1.08) | 0.23 |
| 3 | 0.88 (0.75–1.03) | 0.12 | 0.92 (0.77–1.12) | 0.41 |
| 4 (highest) | 0.91 (0.77–1.08) | 0.27 | 0.90 (0.73–1.08) | 0.23 |
| Histology | ||||
| Adenocarcinoma | 1.0 (Ref) | 1.0 (Ref) | ||
| Squamous cell carcinoma | 0.96 (0.84–1.09) | 0.50 | 0.93 (0.79–1.09) | 0.35 |
| T stage | ||||
| 1a | 1.0 (Ref) | 1.0 (Ref) | ||
| 1b | 1.15 (0.83–1.31) | 0.21 | 1.21 (0.90–1.61) | 0.20 |
| 1NOS | 1.51 (1.22–1.69) | < 0.0001 | 1.70 (1.39–2.08) | < 0.0001 |
| 2 | 1.25 (0.97–1.41) | 0.02 | 1.39 (1.10–1.74) | 0.005 |
| 3 | 1.78 (1.35–1.92) | < 0.0001 | 1.97 (1.59–2.43) | < 0.0001 |
| Charlson score | ||||
| 0 | 1.0 (Ref) | 1.0 (Ref) | ||
| 1 | 1.09 (0.96–1.23) | 0.18 | 1.01 (0.87–1.16) | 0.93 |
| 2+ | 1.51 (1.34–1.70) | < 0.0001 | 1.35 (1.06–1.42) | 0.006 |
| Esophagus location | ||||
| Lower | 1.0 (Ref) | 1.0 (Ref) | ||
| Middle | 0.96 (0.83–1.10) | 0.52 | 0.91 (0.78–1.07) | 0.27 |
| Upper | 0.71 (0.57–0.88) | 0.002 | 0.71 (0.55–0.91) | 0.006 |
| Unknown | 1.10 (0.93–1.30) | 0.27 | 1.04 (0.86–1.27) | 0.67 |
| Surgery | ||||
| No | 1.0 (Ref) | 1.0 (Ref) | ||
| Yes | 0.36 (0.32–0.41) | < 0.0001 | 0.31 (0.27–0.36) | < 0.0001 |
| Radiation | ||||
| No | 1.0 (Ref) | 1.0 (Ref) | ||
| Yes | 0.89 (0.77–1.02) | 0.10 | 0.88 (0.75–1.04) | 0.10 |
| Chemotherapy | ||||
| No | 1.0 (Ref) | 1.0 (Ref) | ||
| Yes | 0.82 (0.72–0.93) | 0.002 | 0.79 (0.68–0.91) | 0.002 |
Race/ethnicity HRs based on overall and cancer-specific adjusted models without treatment
HRs for race/ethnicity and all other covariates based on full adjusted models, with treatment
Discussion
We analyzed data from the SEER-Medicare linked database and demonstrated the presence of racial/ethnic survival disparities among patients with localized esophageal cancer. Importantly, we found that these disparities no longer persisted after controlling for key demographic and clinical characteristics. Treatment received, such as surgery, and chemotherapy were strongly associated with differences in survival outcomes across races. Notably, White patients were more likely to receive surgery than Nonwhite patients (Table 2). While the Kaplan–Meier curve showed poorer survival among Nonwhites compared to Whites, when stratified into four groups, survival appeared to be largely driven by surgery rather than racial/ethnic group (Fig. 2). Thus, our findings suggest that racial disparities in survival among patients with localized esophageal cancer may be partially explained by the disparities in treatment received.
T stage, which represents tumor depth invasion into the esophagus, was also strongly associated with differences in survival outcomes. This is comparable to an earlier study, which demonstrated that patients with a higher T stage had poorer prognosis, independent of other factors [18]. White patients were more likely to have T1a cancers, while T3 cancers were comparable among Whites and Nonwhites, and Nonwhites were more likely to be T1NOS (Table 2). Previous studies have shown that Black patients present with esophageal cancer at later stages than White patients [6, 13, 19]. It is possible that among localized cancer patients, Nonwhites still present later than White patients. This result highlights the need for further research in this area.
Our results are consistent with several prior studies analyzing disparities among esophageal cancer patients within the SEER database [12, 20]. An earlier SEER-Medicare study also demonstrated that Black patients diagnosed with locoregional esophageal cancer in 1991–1999 had lower rates of surgical receipt when compared to White patients [21]. Survival rates in this study were lower among Blacks, but this difference did not persist when adjusting for treatment, suggesting that underuse of surgery is a major factor for worse survival in this population. Lower rates of surgery among Black patients may be explained by factors such as barriers to care, patient preferences, and low patient–physician interactions, which could explain the differences seen [9, 22]. Importantly, our study of more current data demonstrates that these treatment disparities still exist, and our findings underscore the need to better understand potential barriers to surgery.
Our study has several strengths. First, we used the SEER-Medicare linked database, which contains a large number of patients and allowed us to include additional variables, such as chemotherapy receipt, Charlson comorbidity score, and ecological SES when analyzing more recent years. Prior studies have been largely based on SEER and other registries that lacked the information available to study the potential importance of these variables. Our study also has several limitations. SEER-Medicare mainly includes patients 65 years or older, and thus, we cannot generalize our findings to younger populations. However, esophageal cancer is more common among older age-groups, with approximately 60% of patients diagnosed at age 65 or older. The number of Hispanic and Asian patients was too small to be included as their own subgroups. To date, few studies have investigated disparities among Hispanic and Asian patients with esophageal cancer [12, 13]. Thus, additional research that focused on disparities among Hispanic and Asian patients is warranted.
In addition, we lack information about access to treatment facilities and specialists, as well as data regarding patient–physician communication, and these are all factors that could influence treatment decision making for patients with esophageal cancer. Medicare claims data do not completely and accurately capture behavioral factors, such as smoking and alcohol use, which are known risk factors for squamous cell cancer and may influence both treatment decisions and survival outcomes [23–25].
In conclusion, our results suggest that race/ethnicity disparities in overall or cancer-specific survival in localized esophageal cancer may be explained by demographic, clinical, and treatment variables. Notably, T stage, surgery, and chemotherapy were strongly associated with survival differences, suggesting the presence of treatment disparities between Nonwhite and White patients confound their survival outcomes. Further research is needed to understand the causes of these differences and to better target patients who can benefit from specific treatment options, such as surgery.
Funding
NIH R01 CA140574
Footnotes
Conflict of interest There are no conflicts of interest to disclose.
References
- 1.Fast Stats (Esophageal Cancer): An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute; Accessed October 15, 2017. [Google Scholar]
- 2.Siegel R, Miller K, Jemal A. Cancer Statistics, 2017. CA A Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br J Cancer. 2009;101:855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thrift AP, Whiteman DC. The incidence of esophageal adeno-carcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. [DOI] [PubMed] [Google Scholar]
- 5.Howlader N, Noone A, Krapcho M, et al. , SEER Cancer Statistics Review, 1975–2014, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. Accessed October, 2017. [Google Scholar]
- 6.Greenstein AJ, Litle VR, Swanson SJ, et al. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Oncol. 2008;15:881–888. [DOI] [PubMed] [Google Scholar]
- 7.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–113. [DOI] [PubMed] [Google Scholar]
- 8.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. [DOI] [PubMed] [Google Scholar]
- 9.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–1163. [DOI] [PubMed] [Google Scholar]
- 10.Paulson EC, Ra J, Armstrong K, Wirtalla C, Spitz F, Kelz RR. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143:1198–1203. (discussion 1203). [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer NJ, Gu N, Baser O, Morris AM, Birkmeyer JD. Socioeconomic status and surgical mortality in the elderly. Med Care. 2008;46:893–899. [DOI] [PubMed] [Google Scholar]
- 12.Revels SL, Morris AM, Reddy RM, Akateh C, Wong SL. Racial disparities in esophageal cancer outcomes. Ann Surg Oncol. 2013;20:1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran PN, Taylor TH, Klempner SJ, Zell JA. The impact of gender, race, socioeconomic status, and treatment on outcomes in esophageal cancer: a population-based analysis. J Carcinog. 2017;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 17.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. (discussion 1081–1090). [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Yuan P, Wang L, et al. Clinical Nomogram for Predicting Survival of Esophageal Cancer Patients after Esophagectomy. Sci Rep. 2016;6:26684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald TL, Bradley CJ, Dahman B, Zervos EE. Gastrointestinal malignancies: when does race matter? J Am Coll Surg. 2009;209:645–652. [DOI] [PubMed] [Google Scholar]
- 20.Kim A, Ashman P, Ward-Peterson M, Lozano JM, Barengo NC. Racial disparities in cancer-related survival in patients with squamous cell carcinoma of the esophagus in the US between 1973 and 2013. PLoS ONE. 2017;12:e0183782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Earle CC, Neville BA, Weeks JC. Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2005;23:510–517. [DOI] [PubMed] [Google Scholar]
- 22.McCann J, Artinian V, Duhaime L, Lewis JW Jr, Kvale PA, DiGio-vine B. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–3446. [DOI] [PubMed] [Google Scholar]
- 23.Freedman ND, Abnet CC, Caporaso NE, et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol. 2016;45:846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islami F, Fedirko V, Tramacere I, et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. Int J Cancer. 2011;129:2473–2484. [DOI] [PubMed] [Google Scholar]
- 25.Pandeya N, Williams G, Green AC, Webb PM, Whiteman DC, Australian Cancer S. Alcohol consumption and the risks of adeno-carcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009;136:1215–1224. ( e1–2). [DOI] [PubMed] [Google Scholar]