Abstract
Diabetic gastroparesis (GP) is a clinical syndrome characterized by delayed gastric emptying (DGE). Loss of Nrf2 (Nuclear factor (erythroid-derived 2)-like 2) led to reduced nNOSa mediated gastric motility and DGE. The molecular signaling of cinnamaldehyde (CNM) mediated Nrf2 activation and its mechanistic role on DGE were further investigated in obese/T2D female mice. Adult female homozygous Nfe212−/− (C57BL/6J) and their wild-type (WT) littermates (Nfe2l2+/+) mice were fed with high fat diet (HFD; Obese/T2D model), or normal diet (ND) with or without CNM (50mg/kg b.w; i.p). Supplementation of CNM attenuated (p < 0.05) DGE in WT female but not in Nrf2 KO Obese/T2D mice. CNM (1) normalized serum estradiol-17β levels, (2) induced gastric Nrf2 and phase II antioxidant enzymes through extracellular signal-regulated kinase, (ERK)/c-Jun N-terminal kinase (JNK)/p38 mitogen-activated protein kinase (MAPK), (3) reduced glucose synthase kinase 3 beta (GSK3β) and aryl hydrocarbon receptor (AhR) and this was associated with (4) increased estrogen receptor expression, BH4 (Cofactor of nNOS) biosynthesis enzyme GCH-1 and nNOSα dimerization in WT Obese/T2 diabetic female mice. In addition, CNM restored impaired nitrergic relaxation in hyperglycemic conditions. These findings emphasize the importance of Nrf2 in maintaining nNOSα mediated GE and may have a translational relevance to treat obese/diabetic gastroparesis in women.
Keywords: Diabetic gastroparesis, Gastric emptying, Nrf2, nNOS, Estrogen receptors, GSK-3β
1. Introduction
Gastroparesis (GP) is characterized by gastric dysmotility with delayed or accelerated gastric emptying (GE). Gastroparesis is more commonly found in patients with type-1 diabetes (insulin dependent, T1D) and obese type-2 diabetes (non-insulin dependent, Obese/T2D) [1,2]. Almost 80% of diabetic patients suffering from gastroparesis are women [3]. Although gastroparesis is a significant health problem, the molecular mechanisms responsible for gastroparesis are not well understood [4].
Increase in oxidative stress is one of the leading causes for the development of diabetes mellitus [5]. Nrf2 (NF-E2-related factor 2) is a redox-sensitive, basic leucine zipper transcriptional factor upregulates antioxidant gene expression by binding to the promoter region of the antioxidant response element (ARE) [6]. Diminished nitric oxide (NO) and enhanced oxidative stress due to lack of tetrahydrobiopterin (BH4, a cofactor for nNOS) play a central role in several pathophysiologic pathways [7]. Deletion of nNOS resulted in delaying gastric emptying in the mice [8,9]. We have reported that loss of Nrf2 (Nfe2/2−/− female mice) resulted in decreased levels of BH4, inhibited nNOS mediated gastric nitrergic relaxation and reduced nitrite levels which led to delayed gastric emptying [10]. These reports support the notion that loss of Nrf2 expression in diabetes impaired gastric antioxidant gene expression, nNOS function which deregulates NO synthesis, thereby contributing to the development of gastroparesis [3,10]. Earlier, we have provided evidence that depletion of estradiol-17β (E2) impaired gastric Nrf2 expression, nitrergic relaxation and delayed gastric emptying suggesting that Nrf2/nNOS mediated gastric motility is depended on sex hormones [11]. In addition, we have reported that gastric Nrf2/BH4/nNOS expression and nNOS function is altered in the onset of diabetes and other rodent models [12–15]. However, the mechanistic role of Nrf2 and hormones in nNOS mediated gastric emptying remain inconclusive.
In the past decade, it has been shown that dietary compounds targeting Nrf2 activation and BH4 can be used therapeutically in reducing the risk of obesity and type 2 diabetes [3,12,13]. Cinnamonum zeyla-nicum (cinnamon) is known to be an Nrf2 activator via AKT/JNK pathway and is a popular traditional medicine to treat diabetes [16,17]. Previous studies have shown that supplementation of the cinnamalde-hyde (CNM) bioactive compound present in cinnamon, improved renal function and delayed gastric emptying in the onset of diabetes in male rodents [18,19]. However, the molecular signaling of Nrf2 activation and its mechanistic role on regulating gastric emptying has not been well delineated in female Obese/T2D mice.
Hence, our studies are aimed to investigate the mechanistic role of cinnamaldehyde, as an Nrf2 activator in gastric nitrergic relaxation and solid gastric emptying in high fat diet-induced (HFD) obesity and type 2 diabetic gastroparesis using adult female homozygous Nfe2l2−/− (B6.129X1-Nfe2l2 tm1Ywk/J, KO) and their wild-type (WT) littermates (Nfe2l2+/+) mice. Our results demonstrate that CNM restored GE in WT-HFD but not in Nfe2l2−/− female mice.
2. Materials and methods
2.1. Animals
The Institutional Animal Care and Use Committee (IACUC) at Meharry Medical College (MMC) approved all animal experiments in this study. Adult (8–9 week-old) female homozygous Nfe2l2−/− mice (B6.129X1-Nfe2l2 tm1Ywk/J, KO) and their wild-type (WT) littermates were purchased from Jackson Laboratories (The Jackson Laboratory, Bar Harbor, ME). All animals were allowed access to food and water ad libitum. The diet consisted of either a normal diet (ND, 6.2% energy as fat, Teklad Global 2018, Teklad Diets, Madison WI) or a high fat diet (HFD, 70% energy as fat, 19% protein, and 11% carbohydrate, HFD; 5SSV, TestDiet, St. Louis, MO).
2.2. Experimental design
Mice were randomly assigned to one of six treatment groups (n = 6–8 per group): (i) wild type-normal diet (WT-ND); (ii) wild type-high fat diet (WT-HFD); (iii) wild type-high fat diet + cinnamaldehyde (WT-HFD + CNM, 50 mg/kg); (iv) knock out-normal diet (KO-ND); (v) knock out-high fat diet (KO-HFD); (vi) knock out-high fat diet + cinnamaldehyde (KO-HFD + CNM). Previous studies have shown that 50 mg/kg b.w CNM is effective to restore renal function in the diabetic mouse [19]. Therefore, we have selected the similar CNM dose regimen for the current study. Cinnamaldehyde was prepared in mineral oil (carrier solution) and administered intraperitoneally (i.p.), three times a week for twelve weeks. At week 12, mice from all the groups were sacrificed by CO2 asphyxiation. Stomach tissues were snap frozen and stored at −80 °C.
2.3. Fasting blood glucose, glucose tolerance test and insulin tolerance test
Fasting blood glucose levels were monitored every alternate week for the duration of the study period using standard protocol [20]. During the 10th week of the study, intraperitoneal glucose tolerance test (IPGTT) and insulin tolerance test (ITT) was carried out at 11th was performed as reported previously [20]. Data were expressed as the absolute values of blood glucose concentrations.
2.4. Assessment of estrus cycle
The progression of two consecutive estrus cycles (Proestrus, estrus, metaestrus and diestrus) were assessed by vaginal smears after female mice were fed on ND or HFD for 12 weeks [21]. Vaginal smears were collected with a smooth edged glass Pasteur pipette filled with 10 μl of normal saline (0.9% NaCl). The vaginal smear containing cells were placed on an untreated glass microscopic slide and viewed at 20× and 40× magnification to record the stage of estrus cycle.
2.5. Obesity markers, 17β-estradiol (E2) and serum nitrite assay
Animals were sacrificed by CO2 asphyxiation and blood was drawn immediately by cardiac puncture. Serum was separated from the blood by centrifugation (2000×g at 4 °C, 15 min). Aliquots of serum was stored in − 80 °C until used for biochemical analysis. Serum cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-10, leptin, Insulin-like growth factor 1 (IGF-1), IL-6, IL-1a, IL-1b, and Monocyte chemoat-tractant protein-1 (MCP-1)] were measured by multiplex Mouse Cytokine 8-plex Assay (Signosis, Santa Clara, CA). The absorbance at 450 nm was recorded using a micro-plate reader (BioTek, Winooski, VT). Nitrite levels in serum were analyzed as total nitrite (metabolic byproduct of NO) according to the manufacturer’s protocol (Bio vision, Milpitas, CA). Serum 17 beta-estradiol (E2) levels were assayed using an ELISA kit (Enzo Life Sciences, Farmingdale, NY) as per the manufacturer’s instructions. The absorbance at 405 nm was recorded using a micro-plate reader (BioTek, Winooski, VT).
2.6. Solid gastric emptying studies
Solid gastric emptying studies were performed as described previously [10]. Briefly, after fasting overnight (providing water), known amounts of food were fed to the animals for 3 h. At the end of 3 h, the remaining food was weighed in order to establish the amount of food intake. Animals were fasted for 2 h without food and water and then sacrificed. Gastric tissue were collected and the weight of the whole stomach was recorded. The rate of gastric emptying was calculated according to the following equation: gastric emptying (% in 2 h) = (1 − gastric content/food intake) × 100.
2.7. Organ bath studies
As reported earlier, electric field stimulation (EFS)-induced non-adrenergic non-cholinergic relaxation (NANC) was studied in WT (n = 4/group) circular gastric antrum neuromuscular strips [3,9–11]. To investigate the in-vitro effect of CNM (100 μM; 30min) under normoglycemic or hyperglycemic conditions (50 mM, 30 min), muscle strips were pre-incubated and NANC dependent nitrergic relaxation (nNOS function) was determined at 2Hz [3,9–11]. In a separate set of experiments, to investigate the role of Nrf2 signaling pathways, muscle strips were preincubated with SP600125 (10 μM), an inhibitor of ERK/JNK for 30 min in the presence or absence of CNM. The NO dependence of nitrergic relaxation was confirmed with NG-nitro-L-arginine-methyl ester (L-NAME, 100 μM, 30 min). Comparisons between groups were performed by measuring the area under the curve (AUC/mg of tissue) of the EFS-induced relaxation (AUCR) for 1 min and the baseline for 1 min (AUCB), according to the formula “(AUCR-AUCB)/weight of tissue (mg) = AUC/mg of tissue”.
2.8. nNOSα dimerization in gastric neuromuscular tissue
nNOSα monomer and dimer expressions were quantified by Western blotting via low temperature (LT)-polyacrylamide gel electrophoresis (PAGE) in gastric antrum homogenates as described previously (3, 8, 9). A polyclonal antibody (N-terminal) specific to nNOSα (1:500, Thermo Fisher Scientific, Waltham, MA) and anti-rabbit IgG conjugated with horseradish peroxidase (1:10000, Sigma Chemical, St. Louis, MO) were used as the primary and secondary antibodies, respectively.
2.9. Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Total tissue RNA was isolated from the mice gastric neuromuscular tissues by a single-step guanidine thiocyanate method using Trizol (Invitrogen, CA). The quality of RNA was determined by NanoDrop™ (Thermo Fisher Scientific). The iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) was used to synthesize cDNA. RT-qPCR amplification was performed using the Syber-Green (Bio-Rad, Hercules, CA) method. Expression levels were determined using the 2-∆∆Ct method. Relative amounts of mRNA were normalized to p-actin. Primers used for quantitative analysis are listed in Table 1. All studies were performed in the MMC Molecular Core Laboratory.
Table 1.
Primers used for quantitative real-time PCR.
| Gene | Forward | Reverse |
|---|---|---|
| SOD 1 | 5’-CGGTTGAGATAGACAGG-3’ | 5’-TTAAGTGGTCTTGCACTCG-3’ |
| CAT | 5’-GCGGATTCCTGAGAGAGTGGTAC-3’ | 5’-GCCTGACTCTCCAGCGACTGTGGAG-3’ |
| nNOS α | 5’—CCCAACGTCATTTCTGTCCGT-3’ | 5’-TCTACCAGGGGCCGATCATT-3’ |
| GCH-1 | 5’-GAGCATCACCTTGTTCCATTTG −3’ | 5’-GCCAAGTTTACTGAGACCAAGGA −3’ |
| ER α | 5’—CCCGCCTTCTACAGGTCTAAT-3’ | 5’-CTTTCTCGTTACTGCTGGACAG-3’ |
| ER β | 5’-CTGTGATGAACTACAGTGTTCCC | 5’-CACATTTGGGCTTGCAGTCTG-3’ |
| β-actin | 5’-TGGAATCCTGTGGCATCCATGAAAC-3’ | 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’ |
2.10. Western blot analysis
Proteins from gastric antrum neuromuscular tissue lysates were separated by SDS-PAGE. The membrane was immunoblotted with polyclonal Nrf2, Kelch-like ECH-associated protein 1 (Keap-1), super-oxide dismutase 1 (SOD 1), catalase (CAT) and GTP cyclohydrolase 1 (GCH-1) from Santa Cruz (CA), p-ERK, p-JNK, p38/MAPK (Cell Signaling, Danvers, MA), nNOS α (N— terminal, Abcam, Cambridge, MA), primary antibody and anti-rabbit IgG conjugated with horseradish peroxidase as secondary antibody (1: 10,000, Sigma Chemicals, St. Louis, MO). All primary antibodies were probed as per the manufacturer’s recommendation (1:500 or 1:1000) dilutions respectively. Binding of antibodies to the blots was detected using an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Piscataway, NJ) following the manufacturer’s instructions. Stripped blots were re-probed with β-actin-specific polyclonal antibodies (Sigma Chemical) to enable normalization of signals between samples. Band intensities were analyzed using ImageQuant LAS 500 (GE Health Sciences, Pittsburgh, PA).
2.11. Statistical analysis
Data were presented as the mean ± standard error (SE). Statistical comparisons between groups were determined by the Student’s t-test or Tukey’s test after one-way Analysis of Variance (ANOVA), using the GraphPad Prism Version 5.0 (GraphPad Software, San Diego, CA). A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Effect of cinnamaldehyde-on body weight and blood glucose levels
High fat diet (HFD) group in both Nrf2 KO (Nfe2l2−/−) and wild type mice showed a significant (p < 0.05) higher body weight when compared to the normal diet (ND) fed group (Table 2). Supplementation of CNM significantly lowered total body weight (27.1 ± 0.9 g, p < 0.01) when compared to HFD group in both WT and Nrf2 KO mice. In addition, CNM significantly attenuated elevated blood glucose levels in both WT and Nrf2 KO mice those fed with HFD (Table 2).
Table 2.
Body weight, blood glucose and insulin levels in WT and Nrf2 KO female mice at 12 weeks.
| Body Weight (g) | Blood Glucose (mg/dL) | |
|---|---|---|
| WT-ND | 24.2 ± 1.12 | 119 ± 5.06 |
| WT-HFD | 37.19 ± 0.99* | 274 ± 15.57* |
| WT-HFD + CNM | 27.11 ± 0.89# | 152 ± 18.32# |
| KO-ND | 27.98 ± 1.01 | 136 ± 13.42 |
| KO-HFD | 45.02 ± 3.15* | 291 ± 14.78* |
| KO-HFD + CNM | 29.52 ± 0.64# | 160 ± 13.95# |
Results are expressed as mean ± SEM.
p < 0.05 compared to normal diet,
p < 0.05 compared to High fat diet respectively.
3.2. Cinnamaldehyde reverses the effects of HFD on estrus cycle in a Nrf2 dependent manner
As depicted in Fig. 1, HFD fed mice showed abnormalities in cycling phases; progression and cycle length (7–8 as compared to 4–5 in ND fed mice, p < 0.05). These abnormalities include; elongation of phases (e.g. diestrus for 4 consecutive days), skipping of phases (e.g. metestrus), or a combination of both indicating disruption in the reproductive cycle (Fig. 1). CNM significantly restored cycle predominance to the estrus/meta estrus phases (Fig. 1A – C, 1G) as well as decreased cycle length (6 days, p < 0.05) in WT mice, but not in Nrf2 KO mice (8 days) (Fig. 1D – F, 1H). While glucose tolerance was apparent in HFD group, mice with CNM supplementation significantly improved glucose tolerance (Fig. 1Iand J). In concurrence with this observation, ITT data indicates that CNM maintained insulin sensitivity at the normal level similar to that of mice fed on a normal diet (Fig. 1K & L).
Fig. 1. Effect of high fat diet (HFD) and cinnamaldehyde (CNM) on estrus cycle in wild type (WT) and Nrf2 KO mice. Glucose tolerance and insulin sensitivity in HFD fed mice. Insulin tolerance test (ITT) in wild type (WT) and Nrf2 KO mice.
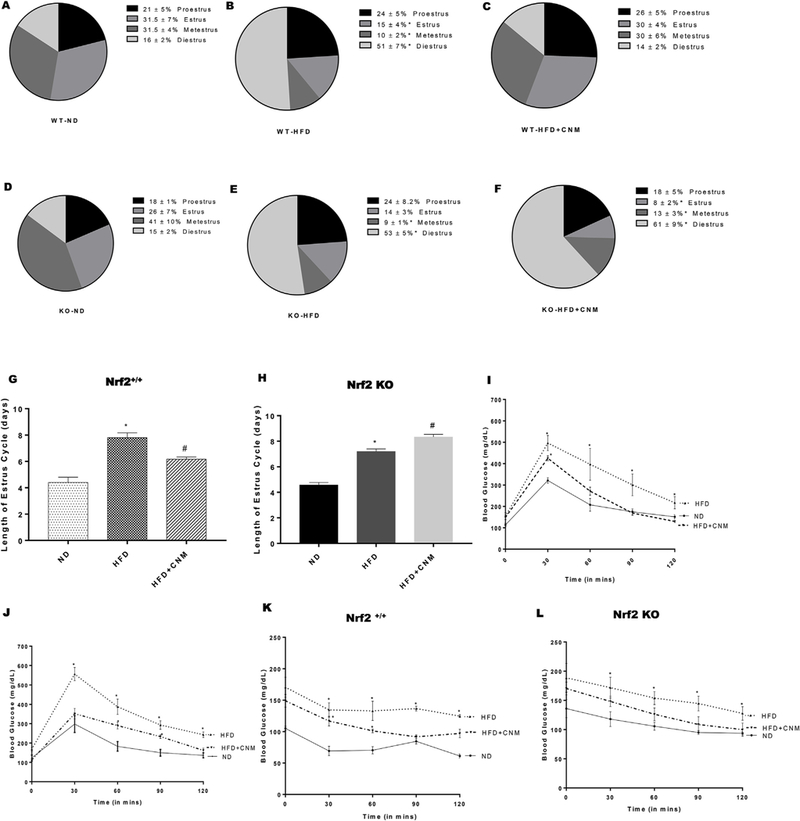
(A–F) Representation of cycling days (G-H). (I, J) Intraperitoneal glucose tolerance test (IPGTT); profile of blood glucose concentration versus time; (K, L) profile of blood glucose concentration (percentage of initial value) as a function of time upon intraperitoneal injection of insulin. Data were analyzed using one way ANOVA by using graph pad prism software. Data are means ± SEM (n = 6). *P < 0.05 compared with normal diet (ND) mice; #P < 0.05 compared to HFD mice.
3.3. Cinnamaldehyde restored serum obesity markers, 17β-estradiol (E2) and nitrite levels
Serum leptin concentrations exhibited very strong correlation with body weight (Fig. 2A). As shown in Fig. 2B–G, obesity marker levels were significantly (p < 0.05) higher in HFD mice compared to ND mice. CNM significantly (p < 0.05) normalized obesity markers, in WT HFD-fed mice but not in Nrf2 KO mice. As shown in Fig. 2Hand I, serum E2 levels were elevated in HFD-fed groups (WT and KO) in comparison to ND groups whereas serum nitrite levels were significantly (p < 0.05) reduced in HFD-fed groups (WT and KO) (Fig. 2K & L). Supplementation of CNM normalized both E2 and nitrite levels in WT-HFD but not in KO-HFD group.
Fig. 2. Effect of cinnamaldehyde (CNM) on serum obesity markers, 17β-estradiol and nitrite levels.
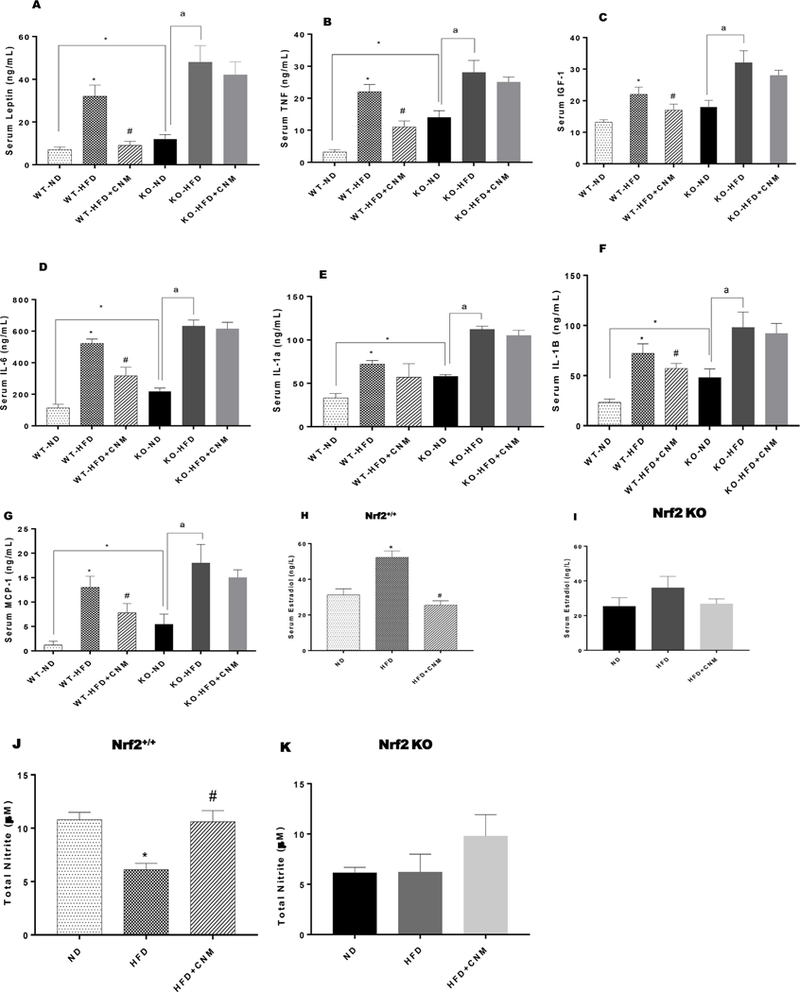
(A-G) serum obesity markers; (H-I) serum estradiol levels and (J-K) serum total nitrite (NO) levels. Data were analyzed using one way ANOVA by using graph pad prism software. Data are means ± SEM (n=6). *P < 0.05 compared with ND mice; #P < 0.05 compared to HFD mice.
3.4. Nrf2 is required for cinnamaldehyde-mediated reversal of HFD-induced delay of gastric emptying in wild type mice
As shown in Fig. 3, HFD fed mice displayed a significant decrease (38%) in solid GE compared to ND fed mice (77.4 ± 3.4% vs 29.3 ± 4.9%). CNM normalized delayed gastric emptying significantly (75.7 ± 10.0% vs 29.3 ± 4.9%, p < 0.01) compared to WT-HFD but not in Nrf2 KO-HFD fed mice (Fig. 3Aand B).
Fig. 3. Effect of cinnamaldehyde (CNM) on solid gastric emptying in wild type (WT) and Nrf2 KO high fat diet (HFD) fed female mice.
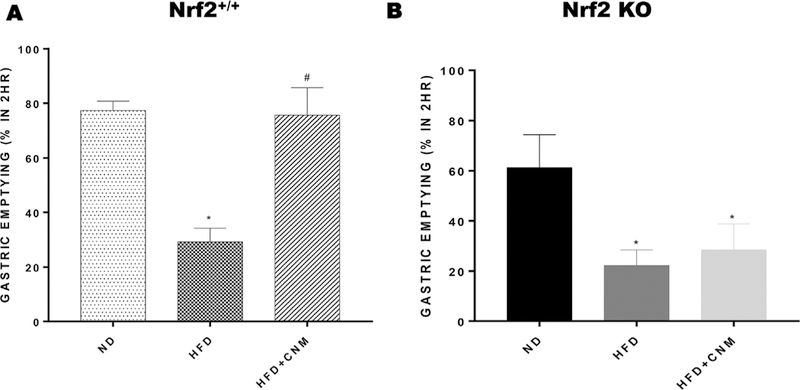
A group of WT and Nrf2 KO female mice were fed with normal diet (ND). Data were analyzed using one way ANOVA by using graph pad prism software. Data are means ± SEM (n = 6). *P < 0.05 compared with ND mice; #P < 0.05 compared to HFD mice.
3.5. Cinnamaldehyde restored gastric ERK, JNK and p38/MAP signaling pathways, Keap-1, phase II detoxifying, GSK-3β and AhR expression
Shen et al., demonstrated that Nrf2 transactivation required ERK and JNK signaling [22]. Having shown that CNM-mediated rescue of GE requires Nrf2, we next investigated if HFD affected these signaling pathways. Our data in Fig. 4A and B shows that feeding HFD to WT and Nrf2 KO female mice resulted a statistically significant decrease (p < 0.05) in both ERK and JNK phosphorylation in gastric neuro-muscular tissue. Supplementation of CNM reversed HFD induced decrease of these pathways in WT but not in KO female gastric tissue.
Fig. 4. Effect of cinnamaldehyde (CNM) on protein expressions of wild type (WT) and Nrf2 KO female mice in gastric neuromuscular tissues.
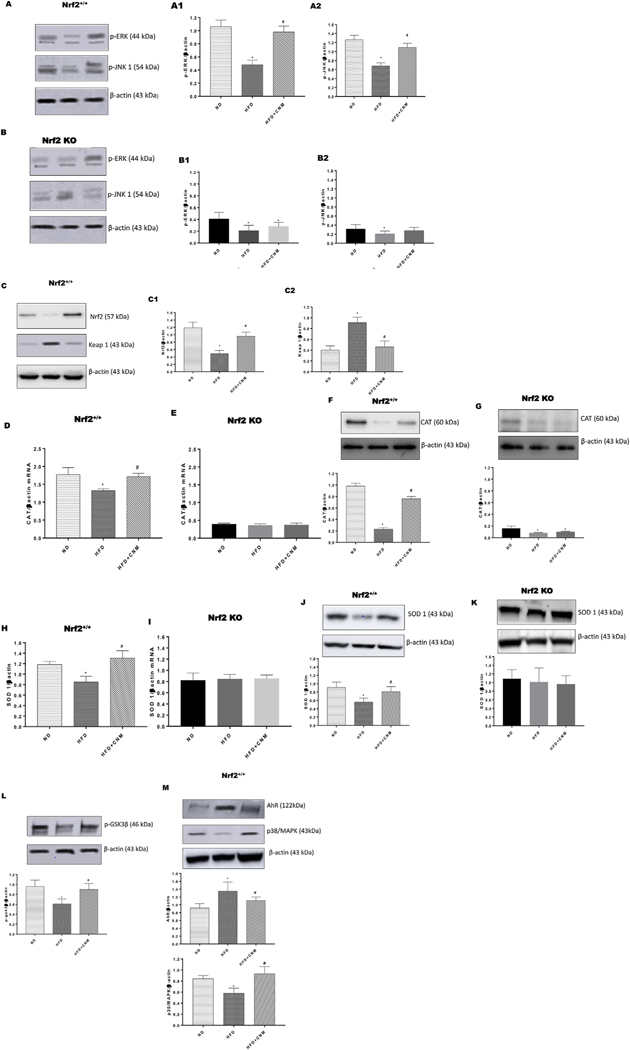
Representative immunoblot and densitometry analysis data for (A1–2) p-ERK/JNK protein expression in WT, (B1–2) p-ERK/JNK protein expression in Nrf2 KO, (C1–2) Nrf2/KEAP-1 protein expression in WT, (D) CAT mRNA expression in WT, (E) CAT mRNA expression in Nrf2 KO, (F) CAT protein expression in WT, (G) CAT protein expression in Nrf2 KO, (H) SOD 1 mRNA expression in WT, (I) SOD 1 mRNA expression in Nrf2 KO, (J) SOD-1 protein expression in WT, (K) SOD-1 protein expression in Nrf2 KO (L) p-GSK3β protein expression and (M) AhR/p38 MAPK protein expression in WT female mice gastric neuromuscular tissue. Stripped blots were re-probed with β-actin. Data were normalized with housekeeping gene or protein (β-actin). Bar graphs showed a ratio of target gene or protein with β-actin. Data were analyzed using one way ANOVA by using graph pad prism software. Values are mean ± SE (n = 4). *p < 0.05 compared with ND; #p < compared with HFD fed mice.
In addition, our results depicted in Fig. 4C demonstrate that HF diet suppresses Nrf2, induces Keap-1 expression, whereas CNM increases Nrf2 protein levels and reduces the protein expression of Keap-1, the negative regulator of Nrf2 phase II antioxidant enzyme genes. Finally, we also have noticed that CNM attenuated decreased expression of Nrf2 targeted gastric phase II antioxidant enzyme genes; CAT and SOD 1 (p < 0.05) in WT but not in Nrf2 KO mice. (Fig. 4 D–K).
Since GSK-3β is inhibited by phosphorylation at Ser9 by Ser/Thr protein kinases via AKT, it has been suggested that Nrf2 might be up-regulated through activation of AKT and inactivation of GSK-3 [23]. The results indicate that supplementation of CNM phosphorylate GSK-3β in WT Obese/T2D gastric neuromuscular tissues (Fig. 4L). In addition, activation of hydrocarbon receptor (AhR) is linked to major diseases, including obesity, and also evidenced that activated AhR inhibits the expression of E2 induced genes [24–26]. We investigated the expression of AhR in WT-HFD and Nrf2 KO mice gastric tissue. The results shows that supplementation of CNM substantially attenuated elevated expression of gastric AhR in WT-HFD mice but not in Nrf2 KO mice fed with HFD (Fig. 4M).
3.6. Effect of cinnamaldehyde on gastric estrogen receptor expression
Previous studies have shown that p38/MAPK and AhR may contribute to E2 mediated ER binding and function [26,27]. As shown in Fig. 5, both gastric ERα and ERβ mRNA & protein expressions were suppressed in HFD fed mice. In addition, CNM supplementation significantly (p < 0.5) upregulated both ER’s expression only in WT-HFD but not in Nrf2 KO mice (Fig. 5 A–H).
Fig. 5. Effect of cinnamaldehyde (CNM) on mRNA and protein expressions of estrogen receptor alpha (ER α) and estrogen receptor beta (ER β) in wild type (WT) and Nrf2 KO female mice gastric neuromuscular tissues.
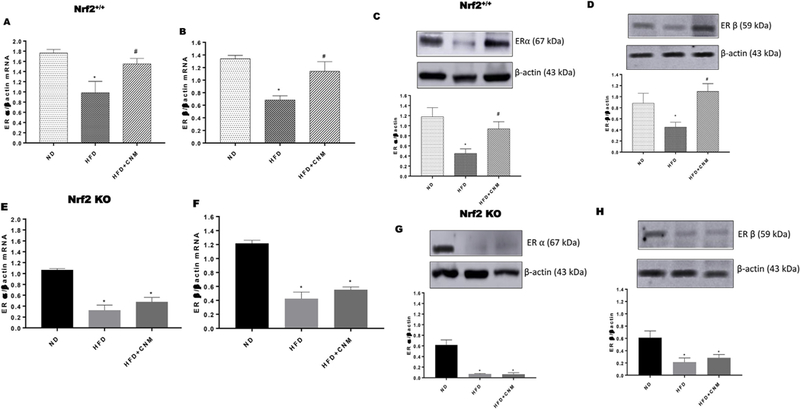
Representative immunoblot and densitometric analysis data for (A) ER α mRNA expression in WT, (B) ER α mRNA expression in WT, (C) ER α protein expression in WT, (D) ER β protein expression in WT, (E) ER α mRNA expression in Nrf2 KO, (F) ER β mRNA expression in Nrf2 KO, (G) ER α protein expression in Nrf2 KO, (H) ER β protein expression in Nrf2 KO in female mice gastric neuromuscular tissue. Stripped blots were re-probed with β-actin. Data were normalized with housekeeping gene or protein (β-actin). Bar graphs showed a ratio of target gene or protein with β-actin. Data were analyzed using one way ANOVA by using graph pad prism software. The values are mean ± SEM (n = 4). *p < 0.05 compared with control group; #p < compared with the diabetic group.
3.7. Cinnamaldehyde normalized gastric nNOSα mRNA & protein expression and dimerization in wild type but not Nrf2 KO mice
As depicted in Fig. 6, nNOSα expression was significantly (p < 0.05) reduced in HFD fed mice, whereas CNM supplementation enhanced the expression of nNOS α expression in WT mice but not in Nrf2 KO mice. To measure whether the decrease in the nNOSα was due to altered nNOS dimer (an indirect measurement for enzyme activity) levels, we performed the dimerization study by LT-PAGE gel. As shown in Fig. 6E, a significant (p < 0.01) decrease in the dimer/monomer ratio was noticed in HFD fed mice in both WT and Nrf2 KO mice. Whereas, supplementation of CNM significantly restored dimerization on WT + HFD but not in Nrf2 KO obese/T2D mice. (Fig. 6E and F).
Fig. 6. Effect of cinnamaldehyde (CNM) on mRNA and protein expression of nNOS α.
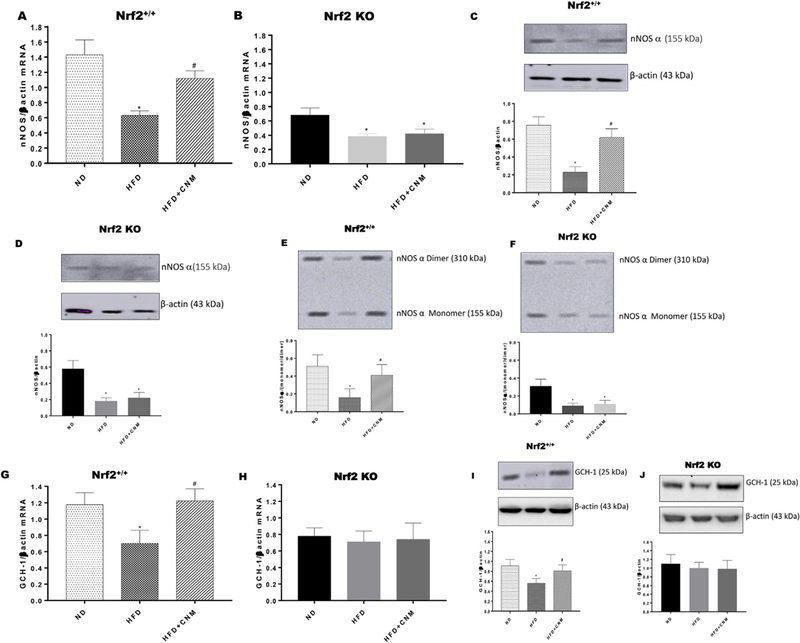
(A-D), nNOSα dimerization (E, F) and GCH-1 (G-J) in WT and Nrf2 KO female mice gastric neuromuscular tissues. Representative immunoblot and densitometric analysis data for (A) nNOSα mRNA expression in WT; (B) nNOSα mRNA expression in Nrf2 KO, (C) nNOSα protein expression in WT; (D) nNOSα protein expression in Nrf2 KO, (E) nNOSα dimerization in WT and (F) nNOSα dimerization in Nrf2 KO; (G) GCH-1 mRNA expression in WT; (H) GCH-1 mRNA expression in Nrf2 KO; (I) GCH-1 protein expression in WT and (J) GCH-1 protein expression in Nrf2 KO in female mice gastric neuromuscular tissue. Stripped blots were re-probed with β-actin. Data were normalized with housekeeping gene or protein (β-actin). Bar graphs showed a ratio of target gene or protein with β-actin. Data were analyzed using one way ANOVA by using graph pad prism software. The values are mean ± SEM (n = 4). *p < 0.05 compared with control group; #p < compared with the diabetic group.
3.8. Cinnamaldehyde attenuated diabetes induced decrease in gastric GTP cyclohydrolase I (GCH-1) expression
GCH-1 play a role in BH4 biosynthesis via de novo pathway [28]. As depicted in Fig. 6 G – J, HFD significantly (p < 0.05) reduced GCH-expression in WT but not in Nrf2 KO. In addition, supplementation of CNM restored GCH-1 expression.
3.9. Cinnamaldehyde restored diabetes-induced impairment of gastric nitrergic relaxation in in vitro
As shown in Fig. 7A, a statistically significant (p < 0.05) decrease in nitrergic relaxation was observed in neuromuscular tissues exposed to hyperglycemic conditions when compared with normal glucose group. Further, pre-incubation with CNM (100 μM) resulted in attenuation of hyperglycemia impaired gastric nitrergic relaxation (Fig. 7A). As shown in Fig. 7B, inhibition of JNK/ERK signaling pathway with SP600125 significantly impaired nitrergic relaxation of gastric neuromuscular tissue. Furthermore, pre-incubation with CNM restored gastric nitrergic relaxation suggesting that Nrf2 is playing a critical role in nitrergic neuron function (Fig. 7B).
Fig. 7. Nitrergic relaxation of in-vitro following electric field stimulation (EFS (2Hz) in circular gastric antrum strips.
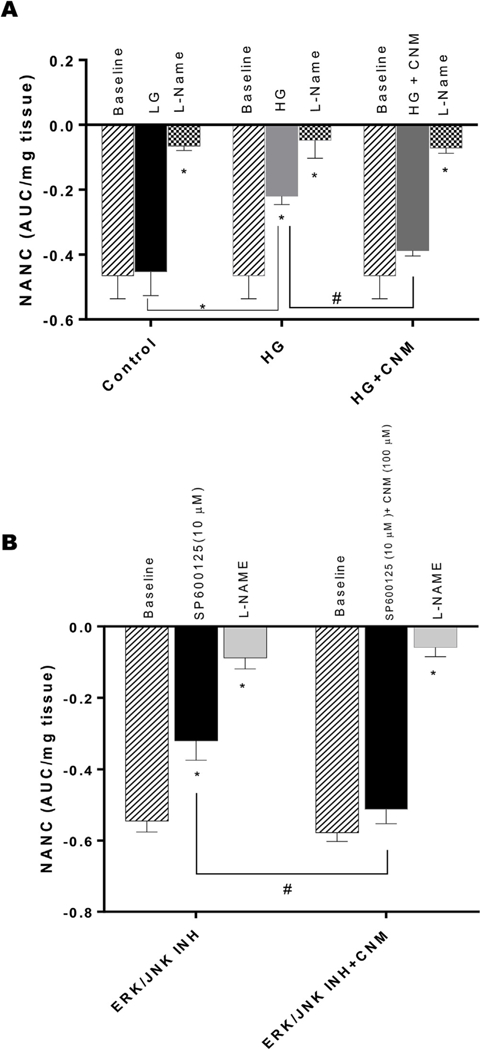
The nitric oxide (NO) dependence of the NANC relaxations was confirmed by preincubation (30 min) with the NO inhibitor nitro-L-arginine methyl ester (L-NAME; 100 μM). (A) the absence (time control), or in the presence of either high glucose (50 mM) and preincubation of CNM (100 μM) and (B) effect of SP600125 for 30 min and then challenged with 100 μM cinnamaldehyde. Data were analyzed using one way ANOVA by using graph pad prism software. The values are mean ± SE (n = 4), *p < 0.05 compared with the response in the absence of L-NAME.
4. Discussion
Gastroparesis is more prominent in both obese and diabetic patients. Gastric emptying depends on many factors such as autonomic and enteric nerve damage (excitatory and inhibitory nerves), interstitial cells of Cajal (ICC) malfunction, drastic fluctuations in blood glucose, medications related to incretin and aggravated by a mental factor via autonomic mechanisms [29]. Earlier studies from our laboratory have shown that supplementation of BH4/sepiapterin restores nNOS mediated gastric emptying by alleviating Nrf2-Phase II enzymes in diabetic rodents [3,13,30,31]. Deletion of Nrf2 gene reduced nitrergic relaxation and delayed gastric emptying [10]. In addition, previous studies identify that Nrf2 pathway is a novel target for management of obesogenesis as well as glucose homeostasis in male mice fed with HFD [20,32]. However, studies are limited to investigate the molecular signaling of Nrf2 activation and its mechanistic role on regulating gastric emptying in female obese/T2D mice. In the current study, by using C57BL/6J WT and Nrf2 KO high-fat diet-fed mouse models, we have demonstrated that activation of Nrf2 attenuated delayed gastric emptying by normalizing (1) body weights, (2) fasting glucose and IPGTT’s/ITT, (3) serum E2, NO, obese markers (4) gastric ERK/JNK/Keap-1 (5) GSK3β, AhR, p38/MAPK, ERα and ERβ, (6) BH4 (Cofactor of nNOS) biosynthesis enzyme GCH-1 (de novo), (7) nNOSα protein & dimerization and (5) Nrf2 and phase II antioxidant enzymes (Fig. 8). Collectively, our data suggest that activation of Nrf2 by CNM is not only important in bringing glucose homeostasis and normalizing circulatory markers but also regulating normal gastric emptying by restoring altered gastric nNOS function [20].
Fig. 8. Schematic illustration depicting the effects of cinnamaldehyde (CNM) on mechanistic signaling of Nrf2 in nNOS mediated gastric motility and gastric emptying in obesity/T2D female mice.
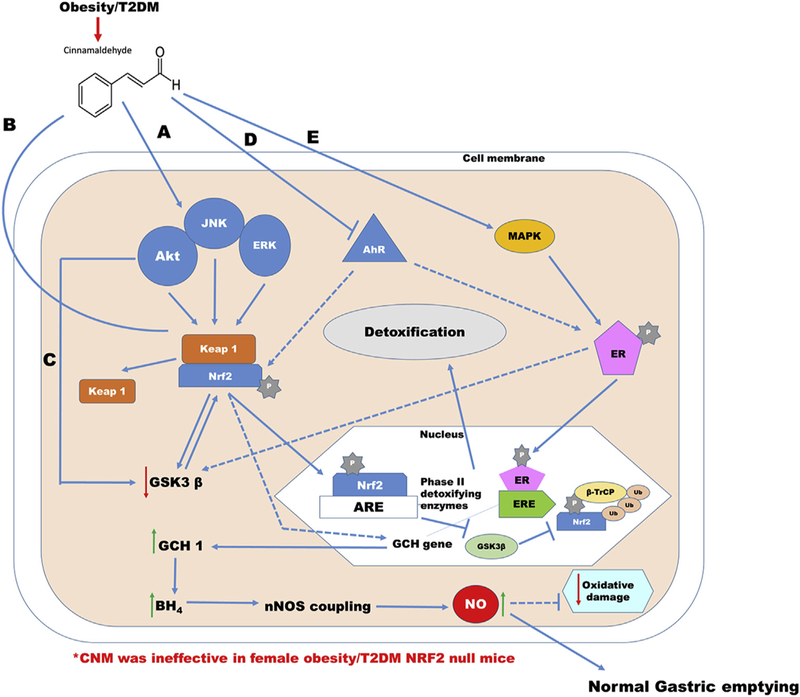
Supplementation of CNM restores delayed gastric emptying via (1) ERK/JNK/Keap-1 (2) GSK3β, MAPK, AhR, ERα and ERβ, (3) BH4 (Cofactor of nNOS) biosynthesis enzyme GCH-1 (de novo), (4) nNOSα protein & dimerization and (5) Nrf2 and phase II antioxidant enzymes in WT Obese/T2D but not in Nrf2 KO female mice. The solid connections indicate the complexes are tethered through protein-protein interactions to a transcription factor complex that connects the gene promoters to execute transcription of target genes. The dotted connections indicate possible interactions between the proteins. The reversible arrows indicate possible cross talk between the proteins. Arrow indicates activation, whereas bar indicates inhibition. ↑ or ↓marks in the parenthesis indicate increase or decrease levels, respectively
In addition to cinnamaldehyde, several other activators such as sulforaphane and curcumin activates Nrf2 and trigger various cell-signaling mechanisms in type 2 diabetes [16]. Cinnamaldehyde and its metabolites are well distributed in all the organs such as heart, liver, spleen, lung, kidney, and brain [33]. In this study, we have selected cinnamaldehyde because it exerts its beneficial effects on multiple cell signaling pathways via Nrf2 [33]. Several studies have demonstrated the vital role of various Nrf2 activators in controlling diabetes and its secondary complications [16]. However, none of the above studies have investigated the mechanistic role of Nrf2 activators with context to diabetic gastroparesis. Recent studies have shown significant alterations in circulatory inflammatory cytokines in gastroparesis patients [34]. Inflammation is commonly seen in all obesity and diabetic humans and in animal models that can be primarily initiated by nutrient overload [35]. Our current data demonstrated that supplementation of CNM, neutralized pro-inflammatory cytokines as well as leptin suggesting an effective strategy for improving glucose tolerance and insulin sensitivity in HFD induced obesity. Many studies have suggested that Nrf2-deficient mice are more prone to cell death, inflammation and genotoxic effects induced by oxidants as well as electrophiles [6,30,36,37]. The results from WT and Nrf2 KO mice in this study further indicate that activation of Nrf2 by CNM plays a protective role against inflammation and oxidative stress and normalizes altered gastric emptying.
Sex steroid hormones mediate their biological actions through their nuclear and cytoplasmic/membrane receptors [38]. Tamir et al. [39], reported that oxidative stress, a well-known consequence of diabetes, differentially regulates the expression of ERα and ERβ in various cell types. Our results demonstrate that estrogen receptors have been reduced in HFD fed mice stomach tissue while serum estrogen levels are elevated. Supplementation of CNM improved gastric estrogen receptors, normalized estrus cycle, serum estrogen and nitrite and accelerated gastric emptying in WT but not in Nrf2 KO fed with HFD. Jasik and Lustig [40], reported that large amount of circulatory estrogen are being released from adipose tissue due to increased conversion of testosterone in mice fed with HFD. Long-term obesity decreases liver functions by estrogen 2-hydroxylation, which also increases circulatory estrogen levels [41]. Our data suggest that Nrf2 may have a direct or indirect effect on ovarian as well as adipose endocrine system and maintain normal gastric emptying in HFD induced obese/T2D female mice. In addition, these studies may have a direct clinical relevance to address the beneficial role of ovarian estrogens and/or their gastric receptors in diabetic gastroparesis women. Studies are underway to investigate the potential role of estrogens and/or their gastric receptors in NO mediated gastric function.
In addition to demonstrating the therapeutic potential of CNM in suppressing diabetic GP, we have investigated the mechanism by which Nrf2 activation was able to attenuate the delayed GE. Nrf2 signaling pathways regulate several biochemical processes such as gene expression, localization of proteins and its expression, cell cycle, and stress responses [6]. Thus, from a regulatory viewpoint, it is important to delineate the effect of signaling pathways involved in the regulation of CNM mediated Nrf2 activation and phase II enzyme expression. Consequently, the extent of gastric p-ERK/p-JNK and p38/MAPK kinases was further investigated following CNM treatment in both WT and Nrf2 KO with or without fed with HFD in female mice. The results in this study reveal that CNM increased both ERK/JNK, p38/MAP kinase signaling pathways and phase II protein expression through Nrf2 activation in WT but not in Nrf2 KO fed with HFD in female mice. Our studies for the first time highlight the importance of Nrf2 activation on regulating gastric emptying and underlying mechanisms in HFD female rodents.
Nrf2 is regulated principally by two different mechanisms. First mechanism is by Keap-1 an ubiquitin E3 ligase substrate adapter for a Cullin3/Rbx1-dependent E3 ubiquitin ligase complex; proteasomal degradation of Nrf2 happens by binding of Keap-1 to Nrf2 [42]. The second mechanism is by GSK-3β, which phosphorylates Nrf2 creating a recognition site for β-Transducin Repeat Containing E3 Ubiquitin Protein Ligase (β-TrCP) which leads to Cullin-1/Rbx1-mediated Nrf2 ubi-quitination and its subsequent degradation in the cytosol [43]. Thus GSK-3β is also known to degrade Nrf2 in the nucleus via the β-TrCP-GSK3β axis [44]. Glycogen synthase kinase (GSK) 3β has emerged to be an integration point and acts as a pivotal role in controlling Nrf2 activity. Glycogen synthase kinase-3 is a serine/threonine kinase with important roles in the regulation of glycogen synthesis, protein synthesis, gene transcription, and cell differentiation in various cell types. Impaired ability of insulin to activate glucose disposal and glycogen synthase are linked to GSK-3 overexpression in skeletal muscle of obese rodent models and diabetic humans [45]. Nrf2 might be upregulated through activation of AKT and permanent inactivation of GSK-3 by phosphorylation at Ser9 by Ser/Thr protein kinases [46,47]. In this study, we have demonstrated that treatment with CNM disrupts the Keap-1/Nrf2 interaction and through the GSK-3β/Nrf2 signaling pathway, provides a double mechanism of activation of Nrf2.
Aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor that mediates toxicological responses and has been reported to influence the development of obesity and metabolic disorders [48]. A recent study suggests that modern western diet activates AhR signaling [48]. Previous studies suggest that a cross talk between Nrf2 and AhR exists while Nrf2 controls AhR signaling [49,50]. In addition to interactions with Nrf2 signaling, AhR also modulates its function on estrogen receptor (ERα and ERβ) and androgen receptor (AR). AhR activation by assembling a ubiquitin ligase complex, CUL4BAHR was shown to promote the ubiquitination and proteasomal degradation of ERs and AR [51,52]. Our data highlight the fact that supplementation of CNM reduced gastric AhR by which enhances ER’s expression and protects against diet-induced obesity and its metabolic complications in WT + HFD. In addition, our data shows that CNM activated estrogen receptor dependent p38/MAPK signaling pathway. Previous studies have shown that activation of p38/MAPK known to promote ER receptor efficiency to translocate into the nucleus, which can then bind directly to estrogen response element (EREs). Several lines of evidence suggest that estrogens maintain gastric emptying in both rodents and humans [9]. Shah et al., studies demonstrated that nNOS mediated gastric relaxation is regulated by estrogens [53]. Collectively, the above data suggest activation of Nrf2 by CNM regulate gastric ER and maintain nNOS mediated gastric emptying in female T2D mice.
Neuronal nitric oxide synthase (nNOS) activity represents a critical signaling node for regulating gastric motor function. nNOS catalyzes the formation of nitric oxide (NO), which initiates smooth muscle relaxation. nNOS activity in turn is regulated by tetrahydrobiopterin (BH4) a cofactor for nNOS dimerization and enzyme activity [13]. Loss of Nrf2 plays a significant role on the BH4 levels in turn on nNOS function [10]. Our current studies demonstrate that supplementation of CNM attenuated reduced gastric nNOSa and nNOSα dimerization in WT HFD but not in Nrf2 KO HFD mice. In addition, supplementation of CNM restored the protein expression of GCH-1 (de novo enzyme for BH4 biosynthesis) but not DHFR (salvage) in WT fed with HFD. Finally, our studies demonstrate that CNM restored hyperglycemia induced impairment of gastric nitrergic relaxation via AKT/ERK/JNK mechanism. These results suggest that obesity-induced hyperglycemia contribute to decreased availability of BH4 and nNOSα uncoupling which results in impaired nitrergic relaxation and delayed gastric emptying. In addition, Xue et al. [54], suggested that GCH-1 activated by Nrf2, increases BH4 and inhibits NOS uncoupling, which reduces the generation of ROS. Collectively, the above data suggest that activation of Nrf2 by CNM enhances the expression of BH4 enzyme GCH-1, nNOS dimerization and normalizes delayed gastric emptying in obese/T2D female mice.
In conclusion, results obtained in this study showed that CNM effectively prevented HFD-induced obesity and T2D. The possible mechanism involved in controlling diabetic gastroparesis is through modulation of key regulating detoxifying enzymes via Nrf2 mediated gastric ER and nNOS function. This suggests that CNM possess potential therapeutic value for treating and/or management of diabetes and its secondary complications such as gastroparesis. This bioactive compound could be a suitable candidate for use in clinical studies as a pharmaconutritive agent for alleviation of diabetic gastroparesis.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under award number-SC1GM121282.
Guarantors: P.G. and C.S. will take the responsibility in data integrity and access to the data.
Footnotes
Author disclosure statement
The authors declare no conflicts of interests.
References
- [1].Parkman HP, Fass R, Foxx-Orenstein AE, Treatment of patients with diabetic gastroparesis, Gastroenterol. Hepatol. 6 (2010) 1–16 http://www.ncbi.nlm.nih.gov/pubmed/20733935. [PMC free article] [PubMed] [Google Scholar]
- [2].Ma J, Rayner CK, Jones KL, Horowitz M, Diabetic gastroparesis: diagnosis and management, Drugs 69 (2009) 971–986, 10.2165/00003495-200969080-00003. [DOI] [PubMed] [Google Scholar]
- [3].Gangula PR, Challagundla KB, Ravella K, Mukhopadhyay S, Chinnathambi V, Mittal MK, Sekhar KR, Sampath C, Sepiapterin alleviates impaired gastric nNOS function in spontaneous diabetic female rodents through NRF2 mRNA turnover and miRNA biogenesis pathway, Am. J. Physiol. Liver Physiol. 315 (2018) G980–G990, 10.1152/ajpgi.00152.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kashyap P, Farrugia G, Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years, Gut 59 (2010) 1716–1726, 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tangvarasittichai S, Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus, World J. Diabetes 6 (2015) 456–480, 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ma Q, Role of nrf2 in oxidative stress and toxicity, Annu. Rev. Pharmacol. Toxicol. 53 (2013) 401–426, 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Forstermann U, Sessa WC, Nitric oxide synthases: regulation and function, Eur. Heart J. 33 (2012) 829–837, 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mashimo H, Kjellin A, Goyal RK, Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice, Gastroenterology 119 (2000) 766–773 http://www.ncbi.nlm.nih.gov/pubmed/10982771. [DOI] [PubMed] [Google Scholar]
- [9].Gangula PRR, Sekhar KR, Mukhopadhyay S, Gender bias in gastroparesis: is nitric oxide the answer? Dig. Dis. Sci. 56 (2011) 2520–2527, 10.1007/s10620-011-1735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mukhopadhyay S, Sekhar KR, Hale AB, Channon KM, Farrugia G, Freeman ML, Gangula PR, Loss of NRF2 impairs gastric nitrergic stimulation and function, Free Radic. Biol. Med. 51 (2011) 619–625, 10.1016/j.freeradbiomed.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ravella K, Al-Hendy A, Sharan C, Hale AB, Channon KM, Srinivasan S, Gangula PR, Chronic estrogen deficiency causes gastroparesis by altering neuronal nitric oxide synthase function, Dig. Dis. Sci. 58 (2013) 1507–1515, 10.1007/s10620-013-2610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gangula PRR, Mukhopadhyay S, Pasricha PJ, Ravella K, Sepiapterin reverses the changes in gastric nNOS dimerization and function in diabetic gastroparesis, Neuro Gastroenterol. Motil. 22 (1325–31) (2010) e351–e352, 10.1111/j.1365-2982.2010.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gangula PRR, Mukhopadhyay S, Ravella K, Cai S, Channon KM, Garfield RE, Pasricha PJ, Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats, Am. J. Physiol. Liver Physiol. 298 (2010) G692–G699, 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gangula PR, Chinnathambi V, Hale AB, Mukhopadhyay S, Channon KM,Ravella K, Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice, Neuro Gastroenterol. Motil. 23 (2011), 10.1111/j.1365-2982.2011.01695.x 773-e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ravella K, Yang H, Gangula PRR, Impairment of gastric nitrergic and NRF2 system in apolipoprotein E knockout mice, Dig. Dis. Sci. 57 (2012) 1504–1509, 10.1007/s10620-012-2070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].David JA, Rifkin WJ, Rabbani PS, Ceradini DJ, The nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus, J. Diabetes Res. 2017 (2017) 1–15, 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mollazadeh H, Hosseinzadeh H, Cinnamon effects on metabolic syndrome: a review based on its mechanisms., Iran, J. Basic Med. Sci. 19 (2016) 1258–1270, 10.22038/ijbms.2016.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Camacho S, Michlig S, de Senarclens-Bezençon C, Meylan J, Meystre J, Pezzoli M, Markram H, le Coutre J, Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered Ghrelin secretion and functional impact on food intake and gastric emptying, Sci. Rep. 5 (2015) 7919, 10.1038/srep07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD, Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy, Diabetes 60 (2011) 3055–3066, 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang Y-KJ, Wu KC, Liu J, Klaassen CD, Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet, Toxicol. Appl. Pharmacol. 264 (2012) 305–314, 10.1016/j.taap.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chakraborty TR, Donthireddy L, Adhikary D, Chakraborty S, Long-term high fat diet has a profound effect on body weight, hormone levels, and estrous cycle in mice, Med. Sci. Monit. 22 (2016) 1601–1608, 10.12659/MSM.897628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, Keum Y-S, Han J, Gallo MA, Kong ANT, Regulation of Nrf2 transactivation domain activity, J. Biol. Chem. 279 (2004) 23052–23060, 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- [23].Cuadrado A, Kügler S, Lastres-Becker I, Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy, Redox Biol 14 (2018) 522–534, https://doi.org/10.1016Zj.redox.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ohtake F, Fujii-Kuriyama Y, Kato S, AhR acts as an E3 ubiquitin ligase to modulate steroid receptor functions, Biochem. Pharmacol. 77 (2009) 474–484, 10.1016/j.bcp.2008.08.034. [DOI] [PubMed] [Google Scholar]
- [25].Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP, Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10, J. Biol. Chem. 285 (2010) 5993–6002, 10.1074/jbc.M109.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Safe S, Wormke M, Inhibitory aryl hydrocarbon Receptor — Estrogen receptor α cross-talk and mechanisms of action, Chem. Res. Toxicol. 16 (2003) 807–816, 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- [27].Thomas RS, Sarwar N, Phoenix F, Coombes RC, Ali S, Phosphorylation at serines 104 and 106 by Erk ½ MAPK is important for estrogen receptor-α activity, J. Mol. Endocrinol. 40 (2008) 173–184, 10.1677/JME-07-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tayeh MA, Marletta MA, Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor, J. Biol. Chem. 264 (1989) 19654–19658 http://www.ncbi.nlm.nih.gov/pubmed/2584186. [PubMed] [Google Scholar]
- [29].Krishnasamy S, Abell TL, Diabetic gastroparesis: principles and current trends in management, Diabetes Ther 9 (2018) 1–42, 10.1007/s13300-018-0454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sireesh D, Dhamodharan U, Ezhilarasi K, Vijay V, Ramkumar KM, Association of NF-E2 related factor 2 (Nrf2) and inflammatory cytokines in recent onset type 2 diabetes mellitus, Sci. Rep. 8 (2018) 5126, 10.1038/s41598-018-22913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gangula PRR, Maner WL, Micci M-A, Garfield RE, Pasricha PJ, Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum, Am. J. Physiol. Gastrointest. Liver Physiol. (2007), 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-Imidazolide, Eur. J. Pharmacol. 620 (2009) 138–144, 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhu R, Liu H, Liu C, Wang L, Ma R, Chen B, Li L, Niu J, Fu M, Zhang D, Gao S, Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety, Pharmacol. Res. 122 (2017) 78–89, https://doi.org/10.10167j.phrs.2017.05.019. [DOI] [PubMed] [Google Scholar]
- [34].Sarosiek I, Bashashati M, Biswas S, Shankar N, Gomez Y, Bright T, McCallum RW, Sarosiek J, Terreros D, Significant alterations within the plasma cytokine profile in gastroparesis: a case-control study, Gastroenterology 152 (2017) S520, 10.1016/S0016-5085(17)31914-5. [DOI] [Google Scholar]
- [35].Monteiro R, Azevedo I, Chronic inflammation in obesity and the metabolic syndrome, Mediat. Inflamm. 2010 (2010) 1–10, 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vomund S, Schäfer A, Parnham M, Brüne B, von Knethen A, Nrf2, the master regulator of anti-oxidative responses, Int. J. Mol. Sci. 18 (2017) 2772, 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boyanapalli SSS, Paredes-Gonzalez X, Fuentes F, Zhang C, Guo Y, Pung D, Saw CLL, Kong A-NT, Nrf2 knockout attenuates the anti-inflammatory effects of phenethyl isothiocyanate and curcumin, Chem. Res. Toxicol. 27 (2014) 2036–2043, 10.1021/tx500234h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boonyaratanakornkit V, Edwards D, Receptor mechanisms mediating non-Genomic actions of sex steroids, Semin. Reprod. Med. 25 (2007) 139–153, 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- [39].Tamir S, Izrael S, Vaya J, The effect of oxidative stress on ERα and ERß expression, J. Steroid Biochem. Mol. Biol. 81 (2002) 327–332, 10.1016/S0960-0760(02)00115-2. [DOI] [PubMed] [Google Scholar]
- [40].Jasik CB, Lustig RH, Adolescent obesity and puberty: the “perfect storm,”, Ann. N. Y. Acad. Sci. 1135 (2008) 265–279, 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- [41].Khalil RA, Estrogen, vascular estrogen receptor and hormone therapy in post-menopausal vascular disease, Biochem. Pharmacol. 86 (2013) 1627–1642, 10.1016/j.bcp.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S, Antioxidant responses and cellular adjustments to oxidative stress, Redox Biol 6 (2015) 183–197, 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A, SCF/-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner, Mol. Cell Biol. 31 (2011) 1121–1133, 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wardyn JD, Ponsford AH, Sanderson CM, Dissecting molecular cross-talk between Nrf2 and NF-kB response pathways, Biochem. Soc. Trans. 43 (2015) 621–626, 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ciaraldi TP, Oh DK, Christiansen L, Nikoulina SE, Kong APS, Baxi S, Mudaliar S, Henry RR, Tissue-specific expression and regulation of GSK-3 in human skeletal muscle and adipose tissue, Am. J. Physiol. Metab. 291 (2006) E891–E898, 10.1152/ajpendo.00176.2006. [DOI] [PubMed] [Google Scholar]
- [46].Rojo AI, Rada P, Egea J, Rosa AO, López MG, Cuadrado A, Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal celldeath, Mol. Cell. Neurosci. 39 (2008) 125–132, 10.1016/j.mcn.2008.06.007. [DOI] [PubMed] [Google Scholar]
- [47].Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A, Glycogen synthase kinase-3β inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2, J. Biol. Chem. 281 (2006) 14841–14851, 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- [48].Kerley-Hamilton JS, Trask HW, Ridley CJA, DuFour E, Ringelberg CS, Nurinova N, Wong D, Moodie KL, Shipman SL, Moore JH, Korc M, Shworak NW, Tomlinson CR, Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a western diet, Environ. Health Perspect. 120 (2012) 1252–1259, 10.1289/ehp.1205003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW, NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis, Mol. Cell Biol. 27 (2007) 7188–7197, 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Furue M, Fuyuno Y, Mitoma C, Uchi H, Tsuji G, Therapeutic agents with AHR inhibiting and NRF2 activating activity for managing chloracne, Antioxidants 7 (2018) 90, 10.3390/antiox7070090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S, Dioxin receptor is a ligand-dependent E3 ubiquitin ligase, Nature 446 (2007) 562–566, 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- [52].Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW, When NRF2 talks, who’s listening? Antioxidants Redox Signal. 13 (2010) 1649–1663, 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G, E 2 and not P 4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy, Am. J. Physiol. Integr. Comp. Physiol. 280 (2001) R1546–R1554, 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- [54].Xue J, Yu C, Sheng W, Zhu W, Luo J, Zhang Q, Yang H, Cao H, Wang W, Zhou J, Wu J, Cao P, Chen M, Ding W-Q, Cao J, Zhang S, The nrf2/GCH1/BH4 Axis Ameliorates radiation-induced skin injury by modulating the ROS cascade, J. Invest. Dermatol. 137 (2017) 2059–2068, 10.1016/j.jid.2017.05.019. [DOI] [PubMed] [Google Scholar]


