Abstract
Background
Few studies have evaluated the association between preexisting vitamin D deficiency and incident tuberculosis (TB). We assessed the impact of baseline vitamins D levels on TB disease risk.
Methods and findings
We assessed the association between baseline vitamin D and incident TB in a prospective cohort of 6,751 HIV-negative household contacts of TB patients enrolled between September 1, 2009, and August 29, 2012, in Lima, Peru. We screened for TB disease at 2, 6, and 12 months after enrollment. We defined cases as household contacts who developed TB disease at least 15 days after enrollment of the index patient. For each case, we randomly selected four controls from among contacts who did not develop TB disease, matching on gender and year of age. We also conducted a one-stage individual-participant data (IPD) meta-analysis searching PubMed and Embase to identify prospective studies of vitamin D and TB disease until June 8, 2019. We included studies that assessed vitamin D before TB diagnosis. In the primary analysis, we defined vitamin D deficiency as 25–(OH)D < 50 nmol/L, insufficiency as 50–75 nmol/L, and sufficiency as >75nmol/L. We estimated the association between baseline vitamin D status and incident TB using conditional logistic regression in the Lima cohort and generalized linear mixed models in the meta-analysis. We further defined severe vitamin D deficiency as 25–(OH)D < 25 nmol/L and performed stratified analyses by HIV status in the IPD meta-analysis. In the Lima cohort, we analyzed 180 cases and 709 matched controls. The adjusted odds ratio (aOR) for TB risk among participants with baseline vitamin D deficiency compared to sufficient vitamin D was 1.63 (95% CI 0.75–3.52; p = 0.22). We included seven published studies in the meta-analysis and analyzed 3,544 participants. In the pooled analysis, the aOR was 1.48 (95% CI 1.04–2.10; p = 0.03). The aOR for severe vitamin D deficiency was 2.05 (95% CI 0.87–4.87; p trend for decreasing 25–(OH)D levels from sufficient vitamin D to severe deficiency = 0.02). Among 1,576 HIV-positive patients, vitamin D deficiency conferred a 2-fold (aOR 2.18, 95% CI 1.22–3.90; p = 0.01) increased risk of TB, and the aOR for severe vitamin D deficiency compared to sufficient vitamin D was 4.28 (95% CI 0.85–21.45; p = 0.08). Our Lima cohort study is limited by the short duration of follow-up, and the IPD meta-analysis is limited by the number of possible confounding covariates available across all studies.
Conclusion
Our findings suggest vitamin D predicts TB disease risk in a dose-dependent manner and that the risk of TB disease is highest among HIV-positive individuals with severe vitamin D deficiency. Randomized control trials are needed to evaluate the possible role of vitamin D supplementation on reducing TB disease risk.
Megan B. Murray and colleagues combine data from a case-control study in Lima, Peru, with previously published prospective studies to investigate whether low vitamin D is associated with risk of developing tuberculosis disease.
Author summary
Why was this study done?
Although multiple lines of evidence suggest that vitamin D may play a role in host susceptibility to tuberculosis (TB) disease, the impact of low vitamin D on the risk of developing TB disease has not yet been firmly established.
What did the researchers do and find?
We conducted a study in Lima, Peru, in which we measured serum 25–(OH)D levels in individuals at high risk for TB disease and followed them for development of TB over 1 year.
We pooled individual-level data from seven previously published prospective studies conducted worldwide and from our Lima cohort.
We found that individuals with low levels of vitamin D were at higher risk of future progression to TB disease.
What do these findings mean?
These findings suggest that vitamin D deficiency is a risk factor for developing TB disease.
Randomized control trials are needed to determine whether vitamin D supplementation reduces risk of TB disease.
Introduction
The global burden of tuberculosis (TB) remains high, with approximately one-fourth to one-third of the world’s population infected with Mycobacterium tuberculosis (MTB), and the World Health Organization (WHO) estimates 10 million people developed TB disease in 2017 [1]. Concurrently, vitamin D deficiency (VDD) is a widespread problem globally, with a high degree of geographic variability; reported adult prevalence of VDD ranges from 10% in North America to >80% in parts of Asia [2,3]. Vitamin D is an important regulator of the immune system [4], and in vitro studies have elucidated multiple mechanisms by which vitamin D influences the pathogenesis of TB infection or disease [5–8]. At the level of the macrophage, vitamin D is involved in cathelicidin- and interferon gamma (IFN-γ)-mediated activity against mycobacteria, [5,6], induction of oxidative species [7], and the promotion of phagolysosome fusion, which leads to degradation of mycobacteria [8]. Discoveries about the various ways vitamin D modulates specific host immune responses to TB infection have focused attention on the possibility that low vitamin D levels may contribute to TB disease progression.
Numerous observational studies have also documented lower serum vitamin D levels among TB patients compared to healthy controls, and prior meta-analyses investigating the association between vitamin D and TB have concluded that low vitamin D increases TB disease risk [9–12]. However, most studies were cross-sectional studies and assessed vitamin D status after the diagnosis of active TB disease, rather than the impact of preexisting vitamin D levels on the risk of progression to TB disease. Given TB disease can induce profound metabolic abnormalities, it is unclear whether VDD increases TB disease risk or whether underlying TB infection or disease leads to decreased serum 25–(OH)D levels. Furthermore, prior studies evaluating the association between vitamin D and TB disease have used different cutoffs to categorize vitamin D levels or define VDD [9–12]. Hence, it is challenging to determine whether there is a vitamin D threshold below which individuals are at the greatest risk of TB disease.
Here, we address the association of vitamin D status on the risk of TB progression in two ways. We first report results of a case-control analysis nested in a prospective cohort study of household contacts (HHCs) of TB patients that we conducted in Lima, Peru. We next pool these data with those from other published prospective studies of vitamin D status and TB risk to conduct an individual-participant data (IPD) meta-analysis synthesizing available evidence on the association between vitamin D status and incident TB disease.
Methods
Lima cohort study
Ethics statement
The Lima cohort study was approved by the Institutional Review Board of Harvard School of Public Health and the Research Ethics Committee of the National Institute of Health of Peru. All study participants or guardians provided written informed consent.
Study setting and population
We enrolled a prospective longitudinal cohort of HHCs of index TB patients in Lima, Peru, between September 1, 2009, and August 29, 2012, for a parent study that was designed to identify various host risk factors for TB infection and disease after exposure. We performed secondary analyses nested in this longitudinal cohort evaluating nutritional determinants of TB disease; we did not follow a prespecified protocol for these secondary analyses of nutritional risk factors. We provide a STROBE checklist of items specific to case-control analyses (S1 Text). Details of the nested study design and methods are described elsewhere [13,14]. At the time of diagnosis of an index patient, HHCs were assessed for symptoms of TB (fever, night sweats, weight loss, cough, malaise) and underwent a brief physical exam (lung auscultation, assessment for lymphadenopathy and signs of recent weight loss) conducted by a clinician to rule out TB disease. We referred those with any signs or symptoms of TB for diagnostic evaluation with chest radiograph as well as sputum smear microscopy and mycobacterial culture according to Peru’s national guidelines [15]. Among HHCs without a prior history of TB infection or disease, we assessed baseline TB infection status with the tuberculin skin test (TST). We used a structured questionnaire to obtain clinical, sociodemographic, and environmental information from HHCs. We offered all HHCs HIV testing. We also invited HHCs to provide a baseline blood sample; 60% of HHCs aged 10 years and older complied. According to national guidelines [15], local healthcare staff offered isoniazid preventive therapy (IPT) to all children under 5 years of age, to TST-positive children between ages 5 and 19 years, and to adults with specified comorbidities (HIV, malignancies, immune deficiencies).
We visited households and reevaluated all HHCs for pulmonary and extrapulmonary TB disease at 2, 6, and 12 months after enrollment. We classified HHCs as having incident secondary TB disease if they were diagnosed at least 15 days after index case enrollment and co-prevalent TB disease if they were diagnosed earlier.
At the completion of follow-up, we identified “cases” from among the HHC cohort; these were HIV-negative HHCs with blood samples who developed incident secondary TB disease within 1 year of follow-up. For each case, we randomly selected four controls from among HHCs who were not diagnosed with TB disease during the study period, matching on gender and age by year.
Laboratory methods
We stored all blood samples at −80°C from enrollment until end of follow-up. All samples were handled identically, and laboratory personnel were not aware of the case or control status of specimens. Levels of total 25–(OH)D were measured with a commercial competitive enzyme immunoassay kit (Immunodiagnostic Systems, Fountain Hills, AZ, United States), which is sensitive to 5.0 nmol/L. The interassay coefficient of variation for 25–(OH)D ranged from 4.6% to 8.7%. We also measured retinol levels using the high-performance liquid chromatography (HPLC) method described by El-Sohemy and colleagues [16]. Since testing of all blood samples occurred after the end of study follow-up, participants were not diagnosed with VDD during study period and were not offered supplementation.
Statistical analysis
We defined VDD as serum 25–(OH)D < 50 nmol/L, vitamin D insufficiency (VDI) as 50–75 nmol/L, and sufficiency as >75 nmol/L [17]. We defined vitamin A deficiency (VAD) as serum retinol < 200 μg/L [18]. We classified adults ≥ 20 years old as underweight (body mass index [BMI] < 18.5 kg/m2), normal weight (BMI 18.5–<25 kg/m2), and overweight (BMI ≥ 25 kg/m2). For children and adolescent HHCs < 20 years, we used WHO age- and gender-specific BMI z-scores tables to classify those with BMI z-score < −2 as underweight and those with z-score > 2 as overweight [19]. We conducted a principal components analysis that included housing type, number of rooms, water supply, sanitation facilities, lighting, composition of exterior walls and floor, and roof materials weighted by household size to compute a socioeconomic status (SES) score, which was then categorized into tertiles [20]. We classified HHCs as ever TB infected at baseline if they reported history of TB disease or positive TST or had a TST result ≥ 10 mm at enrollment.
In Lima, Peru, the monthly average temperature ranges between 24 and 26°C from January to April and starts to decrease in May, reaching an annual low temperature (18°C) in August; and temperatures begin to rise again in October [21]. Based on this yearly pattern, we classified a year into three seasons: winter (June through September), spring (October through December), and summer (January through May).
We used univariate and multivariate conditional logistic regression models (adjusting for matching factors) to evaluate the association between baseline vitamin D status and risk of TB disease [22]. Multivariate models included baseline covariates identified a priori as potential confounders. We used complete case analysis in the regression models. Because we had previously observed that VAD increases risk of TB disease in this cohort [13], we also adjusted for retinol levels and evaluated the interaction between VAD and VDD on incident TB risk.
In sensitivity analyses, we repeated our regressions, restricting the analysis to people who developed incident TB that was diagnosed at least 60 days after enrollment of index patient and their matched controls. We also performed a sensitivity analysis in which we included only patients with microbiologically confirmed TB and their matched controls.
Based on peer review feedback, we further examined the association between VDD and risk of incident TB diagnosed less than 60 days after index case enrollment.
Data were analyzed using SAS v9.4 (SAS Institute, Cary, NC, USA, 2013)
Systematic review and IPD meta-analysis
Upon completing our nested case-control study analysis, we conducted a systematic review of prior prospective studies investigating the association between vitamin D status and incident TB disease. After identifying fewer than 10 studies, most of which were conducted in small cohorts and used different thresholds for categorizing vitamin D, we undertook an IPD meta-analysis to harmonize the definition of VDD and increase the power to detect differences in risk of TB by vitamin D status.
Search strategy and data sources
We conducted the systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) guidelines (S2 Text, S3 Text) [23,24]. All studies included in the IPD meta-analysis received relevant institutional or country-specific ethics approval, and participants provided written or oral informed consent.
We initially searched the PubMed database via the NCBI Entrez system (https://www.ncbi.nlm.nih.gov/pubmed) and Embase (https://www.embase.com) for all available studies up to December 31, 2017, on the association between vitamin D and incident TB disease. Box 1 provides details of our search strategy.
Box 1. Search strategy for studies on the association between vitamin D and incident TB disease
PubMed
Mesh Terms:
-
1
“Vitamin D” OR “Vitamin D deficiency” AND
-
2
Tuberculosis
OR
Text Terms:
-
3
“Vitamin D” OR “Vitamin D deficiency” OR “hypovitaminosis D” OR “25-hydroxyvitamin D” OR “1,25-dihydroxyvitamin D” OR “Vitamin D2” OR “Vitamin D3” OR “Ergocalciferol” OR “Cholecalciferol” AND
-
4
Tuberculosis
Embase
Text Terms:
-
1
“Vitamin D” OR “Vitamin D deficiency” OR “hypovitaminosis D” OR “25-hydroxyvitamin D” OR “1,25-dihydroxyvitamin D” OR “Vitamin D2” OR “Vitamin D3” OR “Ergocalciferol” OR “Cholecalciferol” AND
-
2
Tuberculosis
We updated the search during peer review process to identify additional eligible studies published between January 1, 2018, and June 8, 2019.
Study eligibility and inclusion criteria
We placed no restrictions on language. We included all prospective longitudinal studies of human participants at risk for TB if the study measured vitamin D levels in baseline blood samples obtained prior to a diagnosis of TB disease. Studies were included if they confirmed TB diagnosis by microbiological criteria or specified their clinical criteria for ascertaining TB disease and if they collected data on age and gender. We considered any clinical criteria for TB disease based on physician assessment, imaging studies, or national guidelines. We did not place restrictions on laboratory method used to assess vitamin D levels and did not require studies to control for possible confounders. We also considered reports from conference abstracts. We excluded the following: case reports, animal or in vitro studies, case-control or cross-sectional studies that measured vitamin D levels among individuals already diagnosed with TB disease, studies that did not report vitamin D levels, studies of other diseases or non-TB-related outcomes, studies of vitamin D and TB treatment outcomes or TB infection or TB immune reconstitution inflammatory syndrome (IRIS), reviews, meta-analyses, letters, editorials, and protocols.
Data collection and data items
For each eligible study, two reviewers (OA and SC) independently performed full text reviews and extracted the following information: first author’s last name, year of publication, study design and study aim, country of study, calendar years of study, length of follow-up, number of incident TB cases, total number of subjects analyzed, criteria for diagnosing TB disease, laboratory method of vitamin D assay, method of categorizing vitamin D, assessment of HIV status, and covariates in multivariate analyses. Discrepancies were resolved by consensus.
We contacted all authors of the eligible studies identified from the systematic review and requested IPD. Lead investigators from all the studies agreed to provide data for analysis. Data were de-identified prior to transfer via email. We requested all available data on possible confounders of the association between vitamin D and TB disease, including age, gender, HIV status, weight, height, BMI, IPT, baseline TST result, TB disease history, and comorbid diseases. We also requested baseline vitamin D levels, incident TB disease status during study follow-up, time from enrollment to TB diagnosis, and index case smear status, if applicable. Data were reviewed for consistency with published data, and authors were contacted for clarifications or missing information as needed.
We assessed study quality and risk of bias using the Newcastle-Ottawa Scale (NOS) for assessing quality of nonrandomized studies in meta-analyses; the NOS evaluates observational studies on participant selection, comparability of study groups, and ascertainment of exposure or outcome [25]. Based on the 9-point scoring scale, we classified study quality as good (≥7 points), fair (5–6 points), and poor (<5 points).
Statistical analysis
We conducted a one-step IPD meta-analysis combining data from the eligible studies and from the Peru-based study reported above. We used the unified criteria described above to categorize BMI and to define VDD as serum 25–(OH)D less than 50 nmol/L and insufficiency as 50–75 nmol/L. We further defined severe VDD as 25–(OH)D less than 25 nmol/L. To account for geographic variability, we considered datasets from each single-country study and from each country within a multicountry study as independent data sources. We used generalized linear mixed univariate and multivariate models to evaluate the association between baseline vitamin D status and risk of incident TB, including an indicator for each independent dataset as a random effect to account for within-study correlation. Multivariate models were adjusted for age, gender, BMI, and HIV status. Given sparse data on other variables, we did not adjust for any other potential confounders. We also separately evaluated the association between severe VDD, compared to sufficient levels, and TB disease risk. To determine whether the effect of vitamin D on incident TB differed by HIV status, we conducted a stratified analysis. In a sensitivity analysis, we restricted the main analysis to incident TB cases diagnosed at least 60 days after enrollment. We calculated the Rone-step2 statistic to assess heterogeneity [26].
The IPD meta-analysis was conducted using the R package "lme4” [27]. Datasets for the Lima cohort study and IPD meta-analysis provided as supporting information (S1 Data, S2 Data).
Results
Lima cohort study
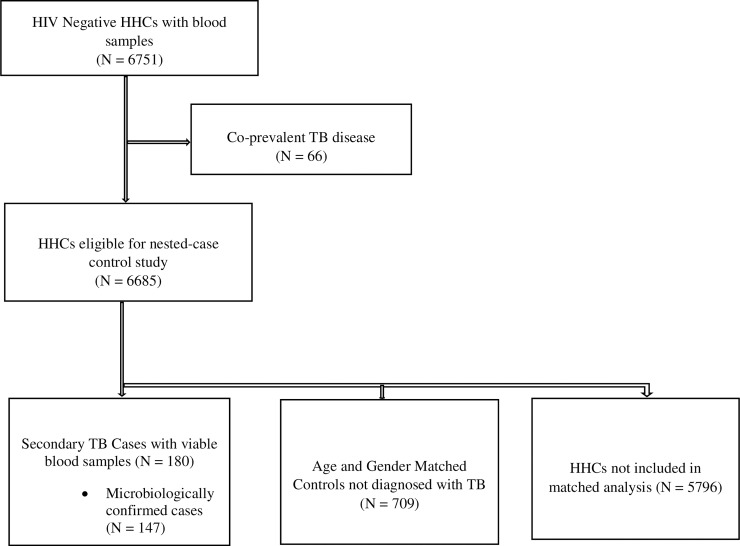
Among 6,751 HIV-negative HHCs with baseline blood samples, 258 developed TB disease, 66 within 15 days of enrollment and 192 thereafter. Among these 192 secondary TB cases, 152 (79.1%) were microbiologically confirmed, and viable blood samples were available for analysis for 180 (93.8%) at the end of follow-up (Fig 1). Monthly incident of TB cases is shown in S1 Fig. Among the 180 TB cases analyzed, 147 (81.7%%) were microbiologically confirmed. Table 1 lists baseline characteristics of the incident cases and their matched controls. The median levels of 25–(OH)D at baseline were similar among cases (53.9 nmol/L; interquartile range [IQR] 42.7–64.0 nmol/L) and controls (54.7 nmol/L; IQR 44.5–67.1 nmol/L; p = 0.32) (Table 2). Median serum 25–(OH)D levels during spring/summer (57.2 nmol/L; IQR 46.1–69.0 nmol/L) were higher than during winter (49.6 nmol/L; IQR 40.5–59.9 nmol/L; p < 0.001) (S2 Fig).
Fig 1. Flow diagram for selection of cases and controls in Lima cohort.
HHC, household contact; TB, tuberculosis.
Table 1. Baseline characteristics of participants in Lima cohort study.
| Characteristic | Cases, n (%) | Na (Cases) | Controls, n (%) |
Na (Controls) | Nonmatched Household Contacts, n (%) | Na (Household Contacts) |
|---|---|---|---|---|---|---|
| Age categories in years | 180 | 709 | 5,796 | |||
| 0–14 | 15 (8.3) | 60 (8.5) | 600 (10.4) | |||
| 15–24 | 74 (41.1) | 298 (42.0) | 1,380 (23.8) | |||
| ≥25 | 91 (50.6) | 351 (49.5) | 3,816 (65.8) | |||
| Male | 94 (52.2) | 366 (51.6) | 2,413 (41.6) | |||
| BMI categoriesb | 179 | 706 | 5,745 | |||
| Underweight | 8 (4.5) | 6 (0.9) | 64 (1.1) | |||
| Overweight | 45 (25.1) | 299 (42.4) | 2,881 (50.2) | |||
| Normal | 126 (70.4) | 401 (56.8) | 2,800 (48.7) | |||
| SESc | 171 | 698 | 5,607 | |||
| Lowest tertile | 77 (45.0) | 228 (32.7) | 1,847 (32.9) | |||
| Middle tertile | 66 (38.6) | 326 (46.7) | 2,565 (45.8) | |||
| Highest tertile | 28 (16.4) | 144 (20.6) | 1,195 (21.3) | |||
| Heavy alcohol used | 14 (8.1) | 174 | 64 (9.3) | 692 | 444 (7.9) | 5,632 |
| Current smoking | 13 (7.4) | 176 | 78 (11.2) | 699 | 488 (8.5) | 5,711 |
| Self-reported diabetes | 6 (3.4) | 179 | 11 (1.6) | 701 | 135 (2.4) | 5,739 |
| Comorbid diseasee | 37 (20.6) | 180 | 175 (24.7) | 709 | 1,380 (23.8) | 5,795 |
| Isoniazid preventive therapy | 7 (3.9) | 180 | 108 (15.2) | 709 | 713 (12.3) | 5,790 |
| BCG scar | 159 (88.3) | 180 | 628 (88.6) | 709 | 5,133 (88.6) | 5,795 |
| History of TB | 34 (18.9) | 55 (7.8) | 708 | 545 (9.4) | 5,782 | |
| Baseline TST positive (≥10 mm) | 85 (71.4) | 119 | 255 (39.4) | 647 | 2,498 (46.6) | 5,362 |
| Ever TB infected at baseline | 145 (82.4) | 176 | 281 (41.1) | 683 | 2,768 (49.0) | 5,649 |
| Mean days to TB diagnosis (± SD) | 118.5 (±114.5) | 180 | NA |
NA |
||
| Median days to TB diagnosis (IQR) | 66.0 (20.5–198.0) | 180 | NA | NA |
||
| Season of blood sample collection | 180 | 709 |
5,796 |
|||
| Spring/summer | 136 (75.6) | 483 (68.1) | 3,821 (65.9) | |||
| Winter | 44 (24.4) | 226 (31.9) | 1975 (34.1) | |||
| Index Patient Characteristics | ||||||
| Smear positive | 156 (86.7) | 180 | 486 (68.7) | 707 | 4,150 (71.6) | 5,793 |
| Cavitary disease | 54 (30.2) | 179 | 175 (25.1) | 697 | 1,424 (24.9) | 5,722 |
a Total number of subjects with data for corresponding variable.
b We classified adults ≥ 20 years old as underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–<25 kg/m2), and overweight (BMI ≥ 25 kg/m2). For children and adolescents < 20 years old, we used WHO age- and gender-specific BMI z-scores tables to classify those with BMI z-score < –2 as underweight and those with z-score > 2 as overweight.
c We conducted a principal components analysis that included housing type, number of rooms, water supply, sanitation facilities, lighting, composition of exterior walls and floor, and roof materials weighted by household size to compute an SES score, which was then categorized into tertiles [20].
d Self-reported consumption of ≥40 g or ≥3 alcoholic drinks daily.
e Heart disease, high blood pressure, asthma, kidney disease, use of steroids or chemotherapy or immunosuppressant, any other self-reported chronic illness.
Abbreviations: BCG, Bacillus Calmette–Guérin; BMI, body mass index; IQR, interquartile range; SES, socioeconomic status; TB, tuberculosis; TST, tuberculin skin test; WHO, World Health Organization
Table 2. Baseline levels of vitamin D among cases and controls in Lima cohort study.
| Vitamin D levels | Cases (N = 180) Median (IQR) or n (%) |
Control (N = 709) Median (IQR) or n (%) |
p-Valuea |
|---|---|---|---|
| 25–OH Vitamin D (nmol/L) | 53.9 (42.7–64.0) | 54.7 (44.5–67.1) | 0.32 |
| Vitamin D deficient (<50 nmol/L) | 76 (42.2) | 259 (36.5) | 0.13 |
| Vitamin D insufficient (50–75 nmol/L) | 84 (46.7) | 348 (49.1) | 0.45 |
| Vitamin D sufficient (>75 nmol/L) | 20 (11.1) | 102 (14.4) | 1.00 |
a Univariate p values adjusted for matching factors (age and sex).
Abbreviation: IQR, interquartile range
In the univariate analysis, the differences in the risk of incident TB disease among HHCs with baseline VDD and baseline VDI compared to those with sufficient levels were not statistically significant (odds ratio [OR] VDD 1.54; 95% CI 0.88–2.71; p = 0.13 and OR VDI 1.23; 95% CI 0.72–2.08; p = 0.45) (Table 3). After we adjusted for BMI categories, SES, heavy alcohol consumption, tobacco use, IPT, TB infection status, comorbid disease, self-reported DM, index patient smear status, and season of sample collection, compared to those with sufficient vitamin D levels, the ORs for HHCs with baseline VDD and VDI were 1.63 (95% CI 0.75–3.52; p = 0.22) and 1.12 (95% CI 0.57–2.23; p = 0.75), respectively (Table 3). When we further adjusted for VAD, the ORs for HHCs with VDD and VDI were 1.59 (95% CI 0.71–3.55; p = 0.26) and 1.12 (95% CI 0.55–2.27; p = 0.75), respectively. A test for interaction between VAD and VDD was not statistically significant (p for interaction = 0.07) (S1 Table).
Table 3. Association between vitamin D status and risk of TB disease among household contacts of TB patients in Lima cohort study.
| Vitamin D status | Cases/Controls | Univariate OR (95% CI) N = 889 | p-Value | Multivariate ORa (95% CI) N = 822 | p-Value |
|---|---|---|---|---|---|
| Vitamin D deficient (<50 nmol/L) | 76/259 | 1.54 (0.88–2.71) | 0.13 | 1.63 (0.75–3.52) | 0.22 |
| Vitamin D insufficient (50–75 nmol/L) | 84/348 | 1.23 (0.72–2.08) | 0.45 | 1.12 (0.57–2.23) | 0.74 |
| Vitamin D sufficient (>75 nmol/L) | 20/102 | 1.00 | 1.00 |
a Adjusted for matching factors (age and sex), BMI categories, socioeconomic status, heavy alcohol consumption, tobacco use, isoniazid preventive therapy, ever TB infected, comorbid disease, self-reported DM, index patient smear status, and season of sample collection.
Abbreviations: BMI, body mass index; DM, type 2 diabetes mellitus; OR, odds ratio; TB, tuberculosis
Our conclusions did not differ from results of the main analysis when we restricted our analyses to cases (and matched controls) diagnosed at least 60 days after index patient enrollment or to microbiologically confirmed TB cases (Table 4). We did not find a statistically significant association between VDD and risk of TB diagnosed less than 60 days after index case enrollment (OR 0.98; 95% CI 0.20–4.72; p = 0.98).
Table 4. Vitamin D status and risk of TB disease stratified by date of TB diagnosis and microbiologically confirmed TB in Lima cohort study.
| Timing of TB diagnosis or TB type | Cases/Controls | Multivariate ORa (95% CI) | p-Value |
|---|---|---|---|
| TB diagnosed < 60 days | |||
| Vitamin D deficient (<50 nmol/L) | 50/172 | 0.98 (0.20–4.72) | 0.98 |
| Vitamin D insufficient (50–75 nmol/L) | 34/137 | 1.12 (0.28–4.45) | 0.88 |
| Vitamin D sufficient (>75 nmol/L) | 5/37 | 1.00 | |
| TB diagnosed ≥ 60 days | |||
| Vitamin D deficient (<50 nmol/L) | 26/87 | 1.89 (0.70–5.11) | 0.21 |
| Vitamin D insufficient (50–75 nmol/L) | 50/211 | 1.21 (0.52–2.80) | 0.65 |
| Vitamin D sufficient (>75 nmol/L) | 15/65 | 1.00 | |
| Microbiologically confirmed TB disease | |||
| Vitamin D deficient (<50 nmol/L) | 60/209 | 1.91 (0.78–4.70) | 0.16 |
| Vitamin D insufficient (50–75 nmol/L) | 72/284 | 1.36 (0.61–3.07) | 0.45 |
| Vitamin D sufficient (>75 nmol/L) | 15/84 | 1.00 |
a Adjusted for matching factors (age and sex), BMI categories, socioeconomic status, heavy alcohol consumption, tobacco use, isoniazid preventive therapy, ever TB infected, comorbid disease, self-reported DM, index patient smear status, and season of sample collection.
Abbreviations: BMI, body mass index; DM, type 2 diabetes mellitus; OR, odds ratio; TB, tuberculosis
Systematic review and IPD meta-analysis
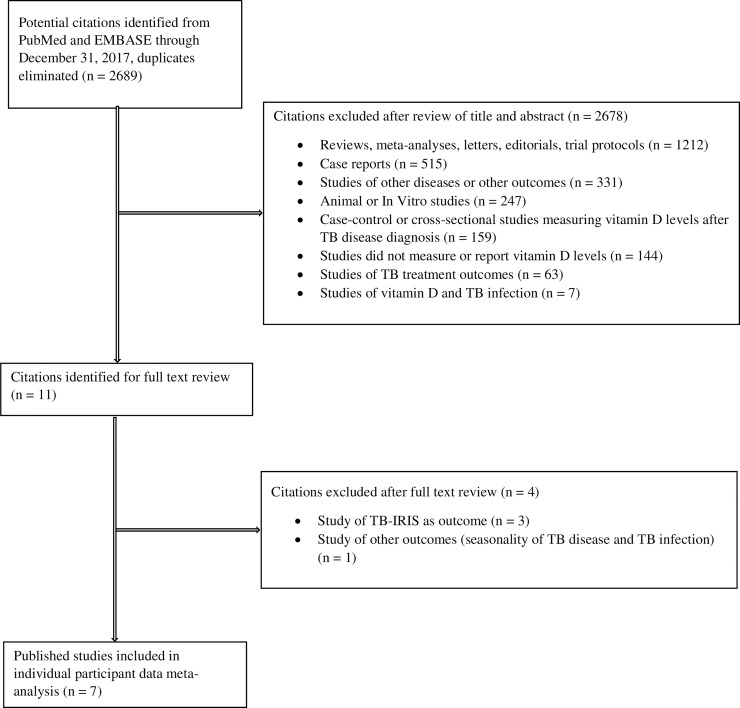
We identified 2,689 citations from the initial PubMed and Embase searches through December 31, 2017. After screening titles and abstracts, we excluded 2,678 articles because they were reviews, meta-analyses, letters, editorials or protocols (n = 1,212), case reports (n = 515), studies of other diseases or other outcomes (n = 331), animal or in vitro studies (n = 247), case-control or cross-sectional studies that assessed vitamin D status after TB disease diagnosis (n = 159), studies that did not measure vitamin D (n = 144), studies of TB treatment outcomes (n = 63), and studies of TB infection (n = 7) (Fig 2). We reviewed full texts of the remaining 11 articles [28–38] and further excluded three studies that assessed outcomes of TB-IRIS [35–37] and one study of TB infection with seasonality of TB [38]. Table 5 provides information about the seven eligible published studies [28–34] identified from the systematic review through December 31, 2017. All seven studies included in the IPD meta-analysis attained at least seven points on the NOS scale and were categorized as “good quality” studies (S2 Table).
Fig 2. Flow diagram for selection of studies for the IPD meta-analysis.
IPD, individual-participant data; IRIS, immune reconstitution inflammatory syndrome; TB, tuberculosis.
Table 5. Summary of studies included in the IPD meta-analysis.
| Study [Reference] | Country (Latitude) | Study Design and Study Population | Total Number of Participants | Number of TB Cases (%) | Median Age, Years (IQR) | Female, N (%) | HIV-Positive Cases, N (%) | Method of Measuring Vitamin D | Median Baseline 25-OH Vitamin D, nmol/L (IQR) | Length of Follow-up, Yearsa | TB Disease Definition | Adjusted Effect Estimate (95% CI) Reported From Original Study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arnedo-Pena et al., 2015 [28] | Spain (40.4637° N, 3.7492° W) | Prospective cohort study of household and community contacts of TB cases | 523 | 3 (0.6) | 37.0 (28.0–46.0) | 255 (48.8) | NAb | ECLIAs and CLIAs | 60.0 (42.5–79.3) | Mean 1.6 (± 0.9) | Smear or culture positive | aHR for continuous vitamin D and microbiologically confirmed TB: 0.88 (0.80–0.97) |
| Gupta et al., 2016 [29] | South Africa (30.5595° S, 22.9375° E) | Prospective case-cohort study of HIV-positive and HIV-exposed infants (no previous known TB exposure) | 366 | 100 (27.3) | 0.7 (0.5–0.7) | 196 (53.6) | 193 (52.7) | Immunoassay | 90.8 (75.8–109.0) | 3.7 | 2004 South African NTP criteria for definite, probable, or possible TBc | aHR for vitamin D < 80 nmol/L and any TB: 1.76 (1.01–3.05) |
| Mave et al., 2015 [30] | India (20.5937° N, 78.9629° E) | Nested case-control study of HIV-positive breastfeeding mothers (TB exposure status not specified) | 120 | 33 (27.5) | 23.0 (21.0–25.0) | 120 (100.0) | 120 (100.0) | Radioimmunoassay | 39.4 (24.4–47.5) | 1.0 | Culture confirmed OR Probable TB: (1) smear positive; and (2) histological and clinical features suggestive of TB and response to anti-TB treatment |
aOR for vitamin D < 50 nmol/L and any TB: 1.57 (0.49–4.98) |
| Owolabi et al., 2016 [31]d | The Gambia (13.4432° N, 15.3101° W) | Prospective cohort study of household contacts of TB cases | 139 | 12 (8.6) | 24.0 (20.0–37.0) | 72 (51.8) | 0 (0.0) | ELISA | 47.4 (37.4–56.4) | 2.0 | smear/culture positive | Adjusted linear regression estimate for continuous vitamin D and microbiologically confirmed TB: 3.65 (0.59–6.71) |
| Sudfeld et al., 2013 [32] | Tanzania (6.3690° S, 34.8888° E) | Prospective cohort study of HIV- positive patients initiating ART (TB exposure status not specified) | 1,092 | 50 (4.6) | 37.0 (32.0–42.8) | 752 (68.9) | 1,092 (100.0) | High-performance liquid chromatography | 73.5 (60.3–86.8) | Median 1.7 (IQR 0.7–2.8) | smear positive or chest radiograph | aHR for vitamin D < 50 nmol and microbiologically confirmed TB: 2.89 (1.31–7.41) |
| Talat et al., 2010 [33] | Pakistan (30.3753° N, 69.3451° E) | Prospective cohort study of household contacts of TB cases | 109 | 8 (7.3) | 20.0 (15.0–35.0) | 59 (54.1) | NA | ELISA | 23.5 (13.8–43.5) | 4.0 | smear positive or chest radiograph | aHR for 1-log decrement in continuous vitamin D and any TB: 5.1 (1.2–21.3) |
| Tenforde et al., 2017 [34] | Brazil (14.2350° S, 51.9253° W), Haiti (18.9712° N, 72.2852° W), India (20.5937° N, 78.9629° E), Malawi (13.2543° S, 34.3015° E), Peru (9.1900° S, 75.0152° W), South Africa (30.5595° S, 22.9375° E), Thailand (15.8700° N, 100.9925° E), US (37.0902° N, 95.7129° W), Zimbabwe (19.0154° S, 29.1549° E) |
Prospective case-cohort study of HIV-positive patients initiating ART (TB exposure status specified by history of TB disease) | 306 | 70 (22.9) | 35.0 (29.0–41.0) | 141 (46.1) | 306 (100.0) | Immunoassay | 80.0 (57.5–97.5) | 1.8 | ACTG criteria for confirmed, probable, or clinical TBe | aHR for vitamin D < 50 nmol and any TB: 3.66 (1.16–11.51) |
| Lima cohort study | Peru (9.1900° S, 75.0152° W) | Nested case-control study of HIV-negative household contacts of TB cases | 889 | 180 (20.2) | 24.0 (18.0–37.0) | 429 (48.3) | 0 (0.0) | Immunoassay | 54.5 (44.1–66.8) | 1.0 | Peru’s NTP criteria for TB diagnosis [15] | aOR for vitamin D < 50 nmol and any TB: 1.70 (0.84–3.46) aOR for vitamin D < 50 nmol and microbiologically confirmed TB: 1.78 (0.79–4.03) |
a Data as reported from original study either in total length or median/mean length.
b Three incident TB cases were HIV-negative; otherwise, HIV status is not available for remaining study participants.
c The South African National Tuberculosis Control Program: Practical Guidelines 2004. Available from: http://www.kznhealth.gov.za/chrp/documents/Guidelines/Guidelines%20National/Tuberculosis/SA%20TB%20Guidelines%202004.pdf
d Original study reported results from case-control analysis but baseline vitamin D levels for entire cohort of household contacts provided for individual participant data meta-analysis.
e Campbell TB, Smeaton LM, Kumarasamy N, Flanigan T, Klingman KL, Firnhaber C, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9(8): e1001290.
Abbreviations: ACTG, AIDS Clinical Trial Group; aHR, adjusted hazard ratio; aOR, adjusted OR; ART, antiretroviral therapy; CLIA, chemiluminescence immunoassay; ECLIA, electrochemiluminescence immunoassay; IPD, individual-patient data; IQR, interquartile range; NTP; OR, odds ratio; TB, tuberculosis
In the updated search during peer review, we identified one additional eligible study published between January 1, 2018, and June 8, 2019, that had not been included in the IPD meta-analysis [39]. Details are provided as supporting information (S3 Fig, S3 Table).
We obtained IPD from all eligible seven studies published through December 31, 2017. One study provided patient data from a multisite evaluation conducted in nine countries [34]. Six of the seven studies were prospective cohort or case-cohort studies [28, 29, 31–34], whereas one study was a nested case-control study [30]. The final combined dataset with our Lima cohort study included 3,544 participants from 13 countries: Brazil, The Gambia, Haiti, India, Malawi, Pakistan, Peru, South Africa, Spain, Tanzania, Thailand, US, and Zimbabwe. We analyzed a total of 456 TB cases. The median time to TB diagnosis from enrollment was 151.0 days (IQR 44.0–342.0 days). Table 6 lists the baseline characteristics of all patients analyzed. The majority of the participants (86.5%) were over 15 years of age. HIV status was unknown for 629 (17.7%) patients, whereas 1,711 (48.3%) were HIV positive. One study assessed serum 25–(OH)D levels using HPLC [32], whereas others used immunoassay [28–30,34] and ELISA [31,33]. The median baseline level of 25–(OH)D was 65.0 nmol/L (IQR 48.8–83.5 nmol/L). The prevalence of VDD at baseline was 26.2% and of severe VDD was 4.8%. Most of the participants with severe VDD were from studies conducted in Pakistan (35.7%) [33], Spain (25.2%) [28], and India (17.5%) [30]. Median serum 25–(OH)D levels were higher among HIV-positive participants (74.3 nmol/L; IQR 58.0–90.0 nmol/L) compared to HIV-negative individuals (56.5 nmol/L; IQR 44.6–72.5 nmol/L; p < 0.0001). The studies in the IPD meta-analysis did not collect similar covariates; therefore, we did not compare additional baseline variables by HIV status.
Table 6. Baseline demographic and clinical characteristics of participants in the IPD meta-analysis (N = 3,544).
| Characteristic | n (%) or Median (IQR) |
|---|---|
| Male | 1,520 (42.9) |
| Age categories in years | |
| 0–14 | 478 (13.5) |
| 15–24 | 688 (19.4) |
| ≥25 | 2,378 (67.1) |
| HIV | |
| Positive | 1,711 (48.3) |
| Negative | 1,204 (34.0) |
| Unknown | 629 (17.7) |
| BMI categoriesa | |
| Underweight | 433 (12.2) |
| Overweight | 873 (24.6) |
| Normal | 1,858 (52.4) |
| Unknown | 380 (10.7) |
| Isoniazid preventive therapy | |
| Yes | 366 (10.3) |
| No | 2,504 (70.7) |
| Unknown | 674 (19.0) |
| Baseline tuberculin skin test | |
| Positive (≥10 mm) | 750 (21.2) |
| Negative | 965 (27.2) |
| Unknown | 1,829 (51.6) |
| History of TB | |
| Yes | 558 (15.7) |
| No | 2,447 (69.1) |
| Unknown | 539 (15.2) |
| Comorbid diseaseb | |
| Yes | 1,224 (34.5) |
| No | 1,181 (33.3) |
| Unknown | 1,139 (32.1) |
| Index smear status among studies of household contacts of TB casesc | |
| Positive | 1,155 (69.6) |
| Negative | 364 (21.9) |
| Unknown | 141 (8.5) |
| Antiretroviral therapy use among HIV positived | |
| Yes | 1,588 (92.8) |
| No | 121 (7.1) |
| Unknown | 2 (0.1) |
| Baseline CD4 count among HIV positive (cell/μL)e | 167 (82–272) |
| 25–OH vitamin D (nmol/L) | 65.0 (48.8–83.5) |
| Vitamin D deficient (<50 nmol/L) | 930 (26.2) |
| Vitamin D insufficient (50–75 nmol/L) | 1,357 (38.3) |
| Vitamin D sufficient (>75 nmol/L) | 1,257 (35.5) |
a We classified adults ≥ 20 years old as underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–<25 kg/m2), and overweight (BMI ≥ 25 kg/m2). For children and adolescents < 20 years old, we used WHO age- and gender-specific BMI z-scores tables to classify those with BMI z-score < −2 as underweight and those with z-score > 2 as overweight.
b Presence of any other diseases or diagnoses as defined or ascertained in original study.
c N = 1,660.
d N = 1,711.
e N = 1,353.
Abbreviations: BMI, body mass index; IPD, individual-patient data; IQR, interquartile range; TB, tuberculosis; WHO, World Health Organization
In the univariate analysis, baseline VDD was associated with a 49% increased risk of progression to TB disease (OR 1.49; 95% CI 1.07–2.07; p = 0.02), and the OR for VDI compared to vitamin D sufficiency was 1.26 (95% CI 0.95–1.66; p = 0.11) [Table 7]. Both VDD and VDI remained associated with an increased risk of TB disease after we adjusted for age, gender, BMI, and HIV status (adjusted OR [aOR] for VDD: 1.48; 95% CI 1.04–2.10; p = 0.03; R2 = 0.97 and aOR for VDI: 1.33; 95% CI 1.00–1.78; p = 0.05; R2 = 0.98).
Table 7. Association between selected baseline characteristics and risk of incident TB disease in the IPD meta-analysis.
| Characteristic | Univariate ORa (95% CI) N = 3,544 | p-Value | Multivariate ORb (95% CI) N = 2,769 | p-Value |
|---|---|---|---|---|
| BMI categoriesc | ||||
| Underweight | 1.43 (1.02–2.03) | 0.04 | 1.37 (0.95–1.95) | 0.09 |
| Overweight | 0.41 (0.30–0.55) | <0.001 | 0.40 (0.30–0.56) | <0.001 |
| Normal | 1.00 | 1.00 | ||
| HIV positived | 1.44 (0.92–2.25) | 0.11 | 1.22 (0.77–1.95) | 0.40 |
| Vitamin D deficient (<50 nmol/L) | 1.49 (1.07–2.07) | 0.02 | 1.48 (1.04–2.10) | 0.03 |
| Vitamin D insufficient (50–75 nmol/L) | 1.26 (0.95–1.66) | 0.11 | 1.33 (1.00–1.78) | 0.05 |
| Vitamin D sufficient (>75 nmol/L) | 1.00 | 1.00 | ||
| p trend = 0.02 | p trend = 0.03 |
a Adjusted for age and gender because of presence of age- and gender-matched case-control study in the combined dataset.
b Adjusted for age, gender, BMI categories, and HIV status.
c N = 3,164.
d N = 2,915.
Abbreviations: BMI, body mass index; IPD, individual-patient data; OR, odds ratio; TB, tuberculosis
When we stratified by HIV status, HIV-positive individuals with VDD were twice as likely to develop TB disease compared to those with normal levels (aOR 2.18; 95% CI 1.22–3.90; p = 0.01; R2 = 0.97), whereas the aOR for TB disease among HIV-negative participants with VDD was 1.20 (95% CI 0.74–1.93; p = 0.46; R2 = 0.99) (Table 8, p for interaction = 0.17).
Table 8. Vitamin D deficiency and risk of incident TB disease stratified by HIV status in the IPD meta-analysisa.
| HIV Positive | HIV Negative | |||||
|---|---|---|---|---|---|---|
| Characteristic | N | Multivariate ORb (95% CI) | p-Value | N | Multivariate ORb (95% CI) | p-Value |
| Vitamin D deficient (<50 nmol/L) | 146 | 2.18 (1.22–3.90) | 0.01 | 419 | 1.20 (0.74–1.93) | 0.46 |
| Vitamin D insufficient (50–75 nmol/L) | 623 | 1.32 (0.90–1.94) | 0.16 | 509 | 1.18 (0.76–1.84) | 0.46 |
| Vitamin D sufficient (>75 nmol/L) | 807 | 1.00 | 265 | 1.00 | ||
| p trend = 0.01 | p trend = 0.52 | |||||
| BMI categories | ||||||
| Underweight | 392 | 1.02 (0.68–1.54) | 0.92 | 34 | 3.99 (1.79–8.87) | 0.001 |
| Overweight | 231 | 0.41 (0.21–0.78) | 0.01 | 449 | 0.41 (0.29–0.59) | <0.001 |
| Normal | 953 | 1.00 | 710 | 1.00 | ||
p Test for interaction between HIV status and serum 25–(OH)D levels = 0.17.
a Excluded datasets from Arnedo-Pena and colleagues [28] and Talat and colleagues [33] because of lack of information on HIV status.
b Adjusted for age, gender, and BMI categories.
Abbreviations: BMI, body mass index; IPD, individual-patient data; OR, odds ratio; TB, tuberculosis
In the entire IPD cohort, the aOR for incident TB among those with severe VDD was 2.05 (95% CI 0.87–4.87; p trend for stepwise decrease in serum 25–(OH)D levels from sufficient to severe deficiency = 0.02; R2 = 0.91) (Table 9). Among HIV-positive individuals, the OR for severe VDD compared to sufficient vitamin D levels was 4.28 (95% CI 0.85–21.45; p = 0.08; R2 = 0.90). In contrast, among HIV-negative individuals, the OR for severe VDD was 1.55 (95% CI 0.55–4.34; p = 0.41; R2 = 0.97) (Table 9, p for interaction 0.17).
Table 9. Severe vitamin D deficiency and risk of incident TB disease stratified by HIV status in the IPD meta-analysis.
| Vitamin D status | N | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|
| All participants (N = 2,769)a | |||
| Vitamin D < 25 nmol/L | 38 | 2.05 (0.87–4.87) | 0.10 |
| Vitamin D 25–<50 nmol/L | 527 | 1.45 (1.01–2.06) | 0.04 |
| Vitamin D insufficient (50–75 nmol/L) | 1,132 | 1.33 (1.00–1.78) | 0.05 |
| Vitamin D sufficient (>75 nmol/L) | 1,072 | Ref | |
| p trend = 0.02 | |||
| HIV positive (N = 1,576)b | |||
| Vitamin D < 25 nmol/L | 12 | 4.28 (0.85–21.45) | 0.08 |
| Vitamin D 25–<50 nmol/L | 134 | 2.06 (1.13–3.76) | 0.03 |
| Vitamin D insufficient (50–75 nmol/L) | 623 | 1.32 (0.90–1.95) | 0.16 |
| Vitamin D sufficient (>75 nmol/L) | 807 | Ref | |
| p trend = 0.01 | |||
| HIV negative (N = 1,193)b | |||
| Vitamin D < 25 nmol/L | 26 | 1.55 (0.55–4.34) | 0.41 |
| Vitamin D 25–<50 nmol/L | 393 | 1.17 (0.73–1.90) | 0.51 |
| Vitamin D insufficient (50–75 nmol/L) | 509 | 1.18 (0.76–1.84) | 0.46 |
| Vitamin D sufficient (>75 nmol/L) | 265 | Ref | |
| p trend = 0.46 |
p Test for interaction between HIV status and serum 25–(OH)D levels = 0.17.
a Model adjusted for age, gender, BMI categories, and HIV status.
b Model adjusted for age, gender, BMI, categories.
Abbreviations: BMI, body mass index; IPD, individual-patient data; OR, odds ratio; TB, tuberculosis
When we separately considered incident cases diagnosed at least 60 days after enrollment, VDI remained associated with increased risk of TB disease (aOR 1.40; 95% CI 1.02–1.92; p = 0.04; R2 = 0.97), whereas VDD was no longer associated with incident TB (aOR 1.02; 95% CI 0.67–1.57; p = 0.92; R2 = 0.95). Only 51.1% of incident TB cases in the IPD meta-analysis cohort had data on microbiologic confirmation, and we therefore did not conduct a sensitivity analysis.
In the one publication we identified that appeared after the target dates for the IPD meta-analysis, Maceda and colleagues [39] reported on a nested case-control study of 72 male prisoners in Brazil. Mean 25–(OH)D levels did not differ significantly among cases (92.5 ± 37.0 nmol/L) and controls (93.8 ± 27.5 nmol/L), and there was no association between serum 25–(OH)D < 75 nmol/L and risk of incident TB disease during 1 year of follow-up (aOR 0.59; 95% CI 0.13–2.62) (S3 Table).
Discussion
Our IPD meta-analysis provides consistent support for a modest dose-dependent effect of vitamin D on future progression of TB disease across multiple studies conducted in diverse contexts. In the IPD, the association of low serum 25–(OH)D levels with increased TB disease risk was most pronounced among HIV-positive individuals with severe VDD.
Although some previous studies have documented lower vitamin D levels among patients with active TB compared to healthy controls [9,10,12,40], others have not confirmed the association between VDD and increased TB disease risk [11]. Furthermore, even among studies that found lower vitamin D levels in TB patients compared to healthy controls, the causal direction of this association is difficult to infer, since TB disease can lead to reduced dietary intake and micronutrient deficiencies, which resolve with successful treatment [10]. Although a number of intervention studies have found little evidence of an impact of vitamin D supplementation on TB treatment outcomes [10,41–46], a recent study demonstrated improved outcomes in a subgroup of multidrug-resistant patients who received supplementation [47]. In contrast to studies of micronutrients and TB treatment outcomes [10,41–46], relatively few studies have prospectively investigated the role of preexisting vitamin D status in the development of TB disease.
Our findings in these human studies support the role of vitamin D in TB infection and disease that has been inferred from more fundamental research that has enumerated multiple mechanisms by which VDD modulates host immune response to MTB. In macrophages, vitamin D is implicated in the activation of cathelicidin-mediated killing of ingested mycobacteria [5,48] induction of IFN-γ-mediated activity in macrophages [6], induction of reactive oxygen and nitrogen species [7], stimulation of phagolysosome fusion in infected macrophages [8], and inhibition of matrix metalloproteinases involved in the pathogenesis of cavitary pulmonary TB [49]. Recent evidence has also demonstrated vitamin D is involved in reduced dendritic cell–mediated priming of the adaptive immune response [50].
In addition to its impact on immunity, vitamin D status has also been linked to human metabolic phenotypes that may be involved in the pathogenesis of TB. In vitro studies have demonstrated various ways by which vitamin D promotes insulin sensitivity [51], and animal models have shown VDD impairs insulin secretion in pancreatic beta cells [51,52]. Numerous observational studies have also found an inverse association between vitamin D levels and incident type 2 diabetes mellitus (DM) [51]. Given DM is a well-described risk factor for TB disease [53,54], VDD may also contribute to increased TB risk through its role in modifying risk of diabetes.
Two other lines of evidence point to a possible association between vitamin D and TB. First, multiple studies have reported an association between specific polymorphisms of vitamin D receptor (VDR) and vitamin D binding protein (VDBP) and increased TB risk [10,55–57]. Although it is not clear that the functional effect of these polymorphisms recapitulates the impact of low vitamin D levels, several studies show that the impact of VDR variants is stronger in the presence of VDD [29,55,58]. Secondly, TB incidence varies with season and peaks in spring and summer months when vitamin D levels are highest. Some observers have postulated that low levels of sunshine, and hence vitamin D, in winter contribute to an increase in TB infection followed by a rise in TB disease incidence after a 6-month lag [38,59–61].
We also note previous studies have reported that low vitamin A is a strong predictor of incident TB disease [34, 62]. Vitamins A and D mediate changes at the cellular level by binding to nuclear hormone receptors, retinoic acid receptor, and VDR, respectively; and both receptors bind to retinoid X receptor (RXR) [63]. Some in vitro evidence further suggests vitamins A and D have synergistic activity in restricting MTB entry and reducing survival within macrophages [64]. Although we did not find evidence of a statistically significant interaction between vitamins A and D deficiencies on risk of TB, our study may not have been powered to detect this interaction. In a previous analysis of the Lima cohort, we found VAD conferred a 10-fold increase in TB disease risk [13], and here, we show that adjustment for vitamin A modestly attenuates the impact of vitamin D. Similarly, Tenforde and colleagues also reported that adjusting for retinol levels attenuated the effect of vitamin D on TB disease risk [34]. This raises the possibility that vitamin D levels correlate with other micronutrients implicated in the pathogenesis of TB, and these micronutrients may be potent mediators of increased TB risk.
Although we did not detect a statistically significant interaction between vitamin D and HIV status in the IPD, our findings raise the possibility that the effect of low serum 25–(OH)D on TB risk may be more pronounced among HIV-positive patients. Studies have shown that among HIV-infected individuals, VDD is associated with deleterious immune activation [65], lower CD4 counts [65,66], higher viral loads [65], and accelerated HIV disease progression [65,67]. Thus, VDD may exacerbate existing immune dysregulation in HIV infection to further increase TB risk, or low vitamin D levels may reflect severity of HIV-related immunosuppression. We also note that vitamin D status fluctuates with season, with declines in serum 25–(OH)D in the winter when UVB exposure is lower [68,69], and in vitro evidence suggests there is winter-associated increase in HIV replication [68]. Vitamin D also restricts mycobacterial growth in the presence of HIV infection [70]. A clinical trial is currently underway to evaluate the efficacy of vitamin D supplementation in preventing incident TB among adults with HIV in Tanzania [71]; the results may help clarify role of vitamin D in HIV-associated TB disease. We also plan to measure inflammatory markers in the Peru cohort to explore association between VDD and immune dysregulation.
We considered possible explanations for why we did not detect a significant association between VDD and TB risk in the Peru cohort, despite its relatively large size. First, since TB incidence is highest in summer [60,61] and HHCs were recruited when the index case was diagnosed, they are more likely to have been recruited and assessed when their vitamin D levels were highest. If levels later fell and this fall precipitated TB progression, this would not have been detected. Secondly, VDR variants are heterogeneously distributed in different populations and may modify the effect of vitamin D on TB risk. We did not measure VDR variants in Peru and are therefore unable to assess the prevalence of different VDR genotypes in this cohort. The Peru study is also limited by the relatively short (1-year) period of follow-up and the fact that it was only powered to detect a 3-fold or greater difference in TB incidence among people with VDD.
Our IPD meta-analysis also has some important limitations. Firstly, many possible confounding covariates were not measured across all studies. Therefore, we were unable to account for other important confounders such as baseline infection status, other micronutrient levels, and comorbidities, including DM, that might be associated with both VDD and TB risk. Secondly, although we only examined prospective studies of incident TB disease, the included studies were all observational, and we cannot exclude the possibility that participants had early, undiagnosed TB at baseline that lowered vitamin D levels. Although we addressed this by conducting a sensitivity analysis excluding incident TB cases diagnosed less than 60 days after enrollment, the smaller number of incident cases diagnosed after 60 days reduced the power to detect a statistically significant association. Thirdly, we also cannot exclude the possibility of publication bias if studies with nonsignificant findings on the link between vitamin D and incident TB have remained unpublished. We did not construct a funnel plot to assess publication bias, because we analyzed seven studies, and guidelines suggest tests for funnel plot asymmetry are not sufficiently powered to distinguish real asymmetry from chance with fewer than 10 studies [72]. These studies further used different methods to categorize vitamin D levels and therefore provided effect estimates that are not directly comparable on a funnel plot. During our systematic review, we attempted to address this by considering data reported from meeting abstracts, and none met our inclusion criteria. Of note, in the publication identified after the initial meta-analysis target dates, although Maceda and colleagues found no association between serum 25–(OH)D < 75 nmol/L and increased TB risk, our effect estimate for VDD in the IPD (OR 1.48) falls within the confidence intervals of this small cohort study [39].
We also note that the different 25–(OH)D assays employed in the meta-analysis studies vary in their sensitivity and precision. However, it is unlikely such variability introduced a bias in one direction, since within any given study, 25–(OH)D levels were analyzed using the same assay in individuals that progressed to TB and nonprogressors. The effect of any imprecision would be more likely to increase the noise:signal ratio, which would bias results of the analysis toward the null.
Despite the aforementioned limitations, we present findings from one of the largest cohorts to date evaluating the role of vitamin D on incident TB disease with comprehensive adjustment for possible confounders. The concurrent IPD meta-analysis further increased the sample size, providing the statistical power to detect more modest associations and enabling the evaluation of this relationship across different locations and by HIV status.
In conclusion, in our meta-analysis of prospective studies, we found low serum 25–(OH)D levels were associated with increased risk of future progression to TB disease in a dose-dependent manner. Randomized control trials are needed to determine whether vitamin D supplementation among individuals at high risk can mitigate the risk of developing TB disease.
Supporting information
(XLSX)
(CSV)
(DOC)
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
(DOC)
IPD, individual-patient data.
(DOCX)
TB, tuberculosis.
(TIF)
(TIF)
Diagram describes process for identifying eligible studies published between January 1, 2018, and June 8, 2019, that were not included in the IPD meta-analysis. IPD, individual-patient data.
(TIF)
TB, tuberculosis.
(DOCX)
NOS, Newcastle-Ottawa Scale.
(DOCX)
(DOCX)
Acknowledgments
We would like to thank study participants from all sites.
Abbreviations
- ACTG
AIDS Clinical Trial Group
- aHR
adjusted hazard ratio
- aOR
adjusted odds ratio
- BCG
Bacillus Calmette–Guérin
- BMI
body mass index
- CLIA
chemiluminescence immunoassay
- DM
type 2 diabetes mellitus
- ECLIA
electrochemiluminescence immunoassay
- HCC
household contact
- HPLC
high-performance liquid chromatography
- IFN-γ
interferon gamma
- IPD
individual-participant data
- IPT
isoniazid preventive therapy
- IQR
interquartile range
- IRIS
immune reconstitution inflammatory syndrome
- MTB
Mycobacterium tuberculosis
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RXR
retinoid X receptor
- SES
socioeconomic status
- TB
tuberculosis
- TST
tuberculin skin test
- VAD
vitamin A deficiency
- VDBP
vitamin D binding protein
- VDD
vitamin D deficiency
- VDI
vitamin D insufficiency
- VDR
vitamin D receptor
- WHO
World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
MBM acknowledges funding from the National Institute of Health (NIH) and National Institute of Allergy and Infectious Diseases (NIAID) (U19AI076217, U01AI057786, TBRU U19AI111224; https://report.nih.gov/). OA acknowledges funding from the National Institute on Drug Abuse (NIDA) (T32DA013911); https://www.drugabuse.gov/international/research-funding-landing) and National Institute of Mental Health (NIMH) (R25MH083620; https://report.nih.gov/). AG, GM, and SAS acknowledge funding for the NWCS113 International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) study provided by National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). Support of the sites was provided by NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network (NICHD contract number N01-DK-9-001/HHSN267200800001C), NIAID (UM1AI069465 to AG), and NINDS (5R01NS077874 to SAS; https://report.nih.gov/). MWT and AG acknowledge the NWCS319 project was supported by Award Number U01AI068636 to the AIDS Clinical Trials Group from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH) and National Institute of Dental and Craniofacial Research (NIDCR). The work was also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (U01AI069497, R01AI080417, UM1AI069465 to AG). The parent trial A5175 was also supported in part by Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline. MWT also acknowledges funding from NIH (F32AI140511; https://report.nih.gov/). AG, VM, and NG acknowledge research reported in this publication was supported by Gilead Foundation (https://www.gilead.com/purpose/giving/gilead-foundation), Ujala Foundation (Newtown Square, PA, USA), and Wyncote Foundation (Philadelphia, PA, USA; https://www.gilead.com/purpose/giving/gilead-foundation). VM, NG, RB, AK, and AG acknowledge funding for the SWEN trial was supported by the NIAID (R01 AI45462), the NIH - Fogarty International Center Program of International Training Grants in Epidemiology Related to AIDS (D43-TW0000), the NIAID Byramjee Jeejeebhoy Medical College HIV Clinical Trials Unit (U01 AI069497), and the NIAID’s Baltimore-Washington-India Clinical Trials Unit (UM1 AI069465; https://report.nih.gov/). CRS acknowledges funding from the National Institute of Child Health and Human Development (R01 HD32257; https://report.nih.gov/). NTI acknowledges funding from the National Commission on Biotechnology (PCST/NCB-AC3/2003), the Higher Education Commission (HEC#20/796/ R&D/06; http://www.hec.gov.pk/english/pages/home.aspx), the International Research Support Initiative Program of the Higher Education Commission Government of Pakistan (http://www.hec.gov.pk/english/scholarshipsgrants/IRSIP/Pages/default.aspx), and the Bill and Melinda Gates Foundation (https://www.gatesfoundation.org/). SC acknowledges funding from the NIH Fogarty International Center (K01TW010829; https://report.nih.gov/). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (WHO). Global Tuberculosis Report 2017. Available from: http://www.who.int/tb/publications/global_report/en/
- 2.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144: 138–145. 10.1016/j.jsbmb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111(1): 23–45. 10.1017/S0007114513001840 [DOI] [PubMed] [Google Scholar]
- 4.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5: 151 10.3389/fphys.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4): 2060–2063. 10.4049/jimmunol.179.4.2060 [DOI] [PubMed] [Google Scholar]
- 6.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for IFN-gamma mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104): 104ra102 10.1126/scitranslmed.3003045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276: 35482–35493. 10.1074/jbc.M102876200 [DOI] [PubMed] [Google Scholar]
- 8.Hmama Z, Sendide K, Talal A, Garcia R, Dobos K, Reiner NE. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1alpha,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J Cell Sci. 2004;117: 2131–2140. 10.1242/jcs.01072 [DOI] [PubMed] [Google Scholar]
- 9.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37: 113–119. 10.1093/ije/dym247 [DOI] [PubMed] [Google Scholar]
- 10.Sutaria N, Liu CT, Chen TV. Vitamin D status, receptor gene polymorphisms, and supplementation on tuberculosis: A systematic review of case-control studies and randomized controlled trials. J Clin Transl Endocrinol. 2014;1(4):151–160. 10.1016/j.jcte.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng J, Wu G, Yang W, Gu X, Liang W, Yao Y, et al. A serum vitamin D level < 25 nmol/l pose high tuberculosis risk: a meta-analysis. PLoS ONE. 2015;10(5): e0126014 10.1371/journal.pone.0126014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SJ, Wang XH, Liu ZD, Cao WL, Han Y, Ma AG, et al. Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther. 2016;11: 91–102. 10.2147/DDDT.S79870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aibana O, Franke MF, Huang CC, Galea JT, Calderon R, Zhang Z, et al. Impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin Infect Dis. 2017;65(6): 900–909. 10.1093/cid/cix476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aibana O, Franke MF, Huang CC, Galea JT, Calderon R, Zhang Z, et al. Vitamin E status is inversely associated with risk of incident tuberculosis disease among household contacts. J Nutr. 2018;148(1): 56–62. 10.1093/jn/nxx006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peru Ministerio de Salud. Norma Técnica de Salud para el Control de la Tuberculosis. Dirección General de Salud de las Personas. Estrategia Sanitaria Nacional de Prevención y Control de la Tuberculosis. 2006. Available from: ftp://ftp2.minsa.gob.pe/descargas/dgsp/ESN-tuberculosis/normaspublicaciones/NTSTBC.pdf
- 16.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr. 2002;76: 172–179. 10.1093/ajcn/76.1.172 [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US); 2011. [PubMed]
- 18.Rice AL, West KP Jr, Black RE. Vitamin A deficiency. Global and regional burden of disease attributable to selected major risk factors. Vol 1 World Health Organization; 2004. Available from: http://www.who.int/healthinfo/global_burden_disease/cra/en/ [Google Scholar]
- 19.World Health Organization (WHO). Child growth standards. 2011. Available from: http://www.who.int/childgrowth/software/en/
- 20.Odone A, Calderon R, Becerra MC, Zhang Z, Contreras CC, Yataco R, et al. Acquired and Transmitted Multidrug Resistant Tuberculosis: The Role of Social Determinants. PLoS ONE. 2016;11(1): e0146642 10.1371/journal.pone.0146642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Meteorological Organization. World Weather Information Service. Available from: http://worldweather.wmo.int/en/city.html?cityId=108
- 22.Pearce N. Analysis of matched case-control studies, BMJ. 2016;352: i969 10.1136/bmj.i969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7): e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340: c221 10.1136/bmj.c221 [DOI] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 26.Chen B, Benedetti A. Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. Syst Rev. 2017;6(1): 243 10.1186/s13643-017-0630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1): 1–48. [Google Scholar]
- 28.Arnedo–Pena A, Juan-Cerdán JV, Romeu-García A, García-Ferrer D, Holguín-Gómez R, Iborra-Millet J, et al. Vitamin D status and incidence of tuberculosis among contacts of pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2015;19(1): 65–69. 10.5588/ijtld.14.0348 [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Montepiedra G, Gupte A, Zeldow B, Jubulis J, Detrick B, et al. Low vitamin-D levels combined with PKP3-SIGIRR-TMEM16J host variants is associated with tuberculosis and death in HIV-infected and -exposed infants. PLoS ONE. 2016;11(2): e0148649 10.1371/journal.pone.0148649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mave V, Chandanwale A, Bhosale R, Shere D, Gupte N, Suryavanshi N, et al. Vitamin D deficiency and risk of postpartum tuberculosis among HIV-infected breastfeeding mothers in India. Int J Tuberc Lung Dis. 2015;19(3): 302–304. 10.5588/ijtld.14.0658 [DOI] [PubMed] [Google Scholar]
- 31.Owolabi O, Agbla S, Owiafe P, Donkor S, Togun T, Sillah AK, et al. Elevated serum 25-hydroxy (OH) vitamin D levels are associated with risk of TB progression in Gambian adults. Tuberculosis (Edinb). 2016;98: 86–91. 10.1016/j.tube.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudfeld C, Giovannucci EL, Isanaka S, Aboud S, Mugusi FM, Wang M, et al. Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-infected Tanzanian adults initiating antiretroviral therapy. J Infect Dis. 2013;207(3): 378–385. 10.1093/infdis/jis693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin D deficiency and tuberculosis progression. Emerg Infect Dis. 2010;16(5): 853–855. 10.3201/eid1605.091693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenforde MW, Yadav A, Dowdy DW, Gupte N, Shivakoti R, Yang WT, et al. Vitamin A and D deficiencies associated with incident tuberculosis in HIV-infected patients initiating antiretroviral therapy in multinational case-cohort study. J Acquir Immune Defic Syndr. 2017;75(3): e71–e79. 10.1097/QAI.0000000000001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price P, Haddow LJ, Affandi J, Agarwal U, Easterbrook PJ, Elliott J, et al. Short Communication: Plasma levels of vitamin D in HIV patients initiating antiretroviral therapy do not predict immune restoration disease associated with Mycobacterium tuberculosis. AIDS Res Hum Retroviruses. 2012;28(10): 1216–1219. 10.1089/AID.2011.0272 [DOI] [PubMed] [Google Scholar]
- 36.Conesa-Botella A, Meintjes G, Coussens AK, van der Plas H, Goliath R, Schutz C, et al. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012;55(7): 1004–1011. 10.1093/cid/cis577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musselwhite LW, Andrade BB, Ellenberg SS, Tierney A, Belaunzaran-Zamudio PF, Rupert A, et al. Vitamin D, d-dimer, interferon γ, and sCD14 levels are independently associated with immune reconstitution inflammatory syndrome: a prospective, international study. EBioMedicine. 2016;4: 115–123. 10.1016/j.ebiom.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingfield T, Schumacher SG, Sandhu G, Tovar MA, Zevallos K, Baldwin MR, et al. The seasonality of tuberculosis, sunlight, vitamin D, and household crowding. J Infect Dis. 2014;210(5): 774–783. 10.1093/infdis/jiu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maceda EB, Gonçalves CCM, Andrews JR, Ko AI, Yeckel CW, Croda J. Serum vitamin D levels and risk of prevalent tuberculosis, incident tuberculosis and tuberculin skin test conversion among prisoners. Sci Rep. 2018;8(1): 997 10.1038/s41598-018-19589-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keflie TS, Nölle N, Lambert C, Nohr D, Biesalski HK. Vitamin D deficiencies among tuberculosis patients in Africa: a systematic review. Nutrition. 2015;31: 1204–1212. 10.1016/j.nut.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 41.Daley P, Jagannathan V, John KR, Sarojini J, Latha A, Vieth R, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15(5): 528–534. 10.1016/S1473-3099(15)70053-8 [DOI] [PubMed] [Google Scholar]
- 42.Xia J, Shi L, Zhao L, Xu F. Impact of vitamin D supplementation on the outcome of tuberculosis treatment: a systematic review and meta-analysis of randomized controlled trials. Chin Med J (Engl). 2014;127: 3127–3134. [PubMed] [Google Scholar]
- 43.Wallis RS, Zumla A. Vitamin D as adjunctive host-directed therapy in tuberculosis: a systematic review. Open Forum Infect Dis. 2016;3(3): ofw151 10.1093/ofid/ofw151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zittermann A, Pilz S, Hoffmann H, März W. Vitamin D and airway infections: a European perspective. Eur J Med Res. 2016;21: 14 10.1186/s40001-016-0208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afzal A, Rathore R, Butt NF, Randhawa FA. Efficacy of Vitamin D supplementation in achieving an early Sputum Conversion in Smear positive Pulmonary Tuberculosis. Pak J Med Sci. 2018;34(4): 849–854. 10.12669/pjms.344.14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu HX, Xiong XF, Zhu M, Wei J, Zhuo KQ, Cheng DY. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. 2018;18(1):108 10.1186/s12890-018-0677-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jolliffe DA, Ganmaa D, Wejse C, Raqib R, Haq MA, Salahuddin N, et al. Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J. 2019;53(3) pii: 1802003 10.1183/13993003.02003-2018 [DOI] [PubMed] [Google Scholar]
- 48.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768): 1770–1773. 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 49.Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, et al. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127: 539–548. 10.1111/j.1365-2567.2008.03024.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saul L, Mair I, Ivens A, Brown P, Samuel K, Campbell JDM, et al. 1,25-Dihydroxyvitamin D3 Restrains CD4+ T Cell Priming Ability of CD11c+ Dendritic Cells by Upregulating Expression of CD31. Front Immunol. 2019;10: 600 10.3389/fimmu.2019.00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1): 205–232. 10.1016/j.ecl.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourlon PM, Billaudel B, Faure-Dussert A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J Endocrinol. 1999;160: 87–95. 10.1677/joe.0.1600087 [DOI] [PubMed] [Google Scholar]
- 53.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7): e152 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harries AD, Satyanarayana S, Kumar AMV, Nagaraja SB, Isaakidis P, Malhotra S, et al. Epidemiology and interaction of diabetes mellitus and tuberculosis and the challenges for care: a review. Public Health Action. 2013;3: S3–S9. 10.5588/pha.13.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C, Liu Q, Zhu L, Yang H, Lu W. Vitamin D receptor gene polymorphisms on the risk of tuberculosis, a meta-analysis of 29 case-control studies. PLoS ONE. 2013;8(12): e83843 10.1371/journal.pone.0083843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14(1):15–23. [PubMed] [Google Scholar]
- 57.Lee SW, Chuang TY, Huang HH, Liu CW, Kao YH, Wu LS. VDR and VDBP genes polymorphisms associated with susceptibility to tuberculosis in a Han Taiwanese population. J Microbiol Immunol Infect. 2016;49(5): 783–787. 10.1016/j.jmii.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355(9204): 618–621. 10.1016/S0140-6736(99)02301-6 [DOI] [PubMed] [Google Scholar]
- 59.Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, Bangani N, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci USA. 2011;108(47): 19013–19017. 10.1073/pnas.1111825108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parrinello CM, Crossa A, Harris TG. Seasonality of tuberculosis in New York City, 1990–2007. Int J Tuberc Lung Dis. 2012;16(1): 32–37. 10.5588/ijtld.11.0145 [DOI] [PubMed] [Google Scholar]
- 61.Fares A. Seasonality of tuberculosis. J Glob Infect Dis. 2011;3(1): 46–55. 10.4103/0974-777X.77296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Getz HR, Long ER, Henderson HJ. A study of the relation of nutrition to the development of tuberculosis; influence of ascorbic acid and vitamin A. Am Rev Tuberc. 1951;64: 381–393. [DOI] [PubMed] [Google Scholar]
- 63.Wheelwright M, Kim EW, Inkeles MS, De Leon A, Pellegrini M, Krutzik SR et al. All-trans retinoic acid-triggered antimicrobial activity against Mycobacterium tuberculosis is dependent on NPC2. J Immunol. 2014; 192(5): 2280–2290. 10.4049/jimmunol.1301686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anand P, Kaul KD, Sharma M. Synergistic action of vitamin D and retinoic acid restricts invasion of macrophages by pathogenic mycobacteria. J Microbiol Immunol Infect. 2008;41: 17–25. [PubMed] [Google Scholar]
- 65.Jiménez-Sousa MÁ, Martínez I, Medrano LM, Fernández-Rodríguez A, Resino S. Vitamin D in Human Immunodeficiency Virus Infection: Influence on Immunity and Disease. Front Immunol. 2018;9: 458 10.3389/fimmu.2018.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ezeamama AE, Guwatudde D, Wang M, Bagenda D, Kyeyune R, Sudfeld C, et al. Vitamin-D deficiency impairs CD4 + T-cell count recovery rate in HIV-positive adults on highly active antiretroviral therapy: a longitudinal study. Clin Nutr. 2016;35(5): 1110–1117. 10.1016/j.clnu.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, Hertzmark E, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE. 2010;5(1): e8770 10.1371/journal.pone.0008770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coussens AK, Naude CE, Goliath R, Chaplin G, Wilkinson RJ, Jablonski NG. High-dose vitamin D3 reduces deficiency caused by low UVB exposure and limits HIV-1 replication in urban Southern Africans. Proc Natl Acad Sci U S A. 2015;112(26): 8052–8057. 10.1073/pnas.1500909112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thieden E, Philipsen PA, Heydenreich J, Wulf HC. Vitamin D level in summer and winter related to measured UVR exposure and behavior. Photochem Photobiol. 2009;85(6): 1480–1484. 10.1111/j.1751-1097.2009.00612.x [DOI] [PubMed] [Google Scholar]
- 70.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8(5): e1002689 10.1371/journal.ppat.1002689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sudfeld CR, Mugusi F, Aboud S, Nagu TJ, Wang M, Fawzi WW. Efficacy of vitamin D supplementation in reducing incidence of pulmonary tuberculosis and mortality among HIV-infected Tanzanian adults initiating antiretroviral therapy: study protocol for a randomized controlled trial. Trials. 2017;18(1): 66 10.1186/s13063-017-1819-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane; 2011. Available from: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(CSV)
(DOC)
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
(DOC)
IPD, individual-patient data.
(DOCX)
TB, tuberculosis.
(TIF)
(TIF)
Diagram describes process for identifying eligible studies published between January 1, 2018, and June 8, 2019, that were not included in the IPD meta-analysis. IPD, individual-patient data.
(TIF)
TB, tuberculosis.
(DOCX)
NOS, Newcastle-Ottawa Scale.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.