OBJECTIVES:
Irritable bowel syndrome with diarrhea (IBS-D) is a functional gastrointestinal disorder with limited effective treatment options. We evaluated the efficacy and safety of eluxadoline in patients with IBS-D who reported inadequate symptom control with prior loperamide.
METHODS:
Three hundred forty-six adults with IBS-D (Rome III criteria) were randomly assigned to placebo or eluxadoline 100 mg twice daily for 12 weeks. Patients recorded daily IBS-D symptoms, including worst abdominal pain (WAP) and stool consistency (through Bristol Stool Scale). The primary endpoint was proportion of composite responders, defined as patients who met daily composite response criteria (≥40% WAP improvement and <5 Bristol Stool Scale score) for at least 50% of treatment days, and recorded ≥60 days of diary entries over the 12-week period.
RESULTS:
Over 12 weeks, a significantly greater proportion of eluxadoline patients achieved the primary composite responder endpoint compared to placebo (22.7% vs 10.3%, P = 0.002), and component endpoints of improvements in stool consistency (27.9% vs 16.7%, P = 0.01) and WAP (43.6% vs 31.0%, P = 0.02). Additionally, a greater proportion of eluxadoline patients met the composite responder endpoint assessed at monthly intervals compared to placebo (weeks 1–4: 14.0% vs 6.9%, P = 0.03; weeks 5–8: 26.7% vs 14.9%, P = 0.006; weeks 9–12: 30.8% vs 16.7%, P = 0.002). Rates of adverse events were comparable in both groups (37.4% vs 35.3%); no treatment-related serious adverse event, cases of sphincter of Oddi spasm, or pancreatitis were reported.
DISCUSSION:
Eluxadoline appears safe and effective for treating IBS-D symptoms in patients with an intact gallbladder reporting inadequate relief with prior loperamide use.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder, with an estimated global prevalence of 11% (1,2). IBS is characterized by regular abdominal pain associated with changes in stool frequency and/or consistency and is subtyped based on the predominant stool pattern: IBS with constipation, diarrhea (IBS-D), mixed, or unsubtyped. Of the subtypes, IBS-D is the most common, comprising nearly 45% of all IBS cases (3).
Effective treatment options for IBS-D are limited, and patients often resort to interventions based on relieving individual symptoms. Typical initial therapeutic interventions include dietary and lifestyle modifications, as well as over-the-counter antidiarrheals. Loperamide, a peripherally restricted μ-opioid receptor (μ-OR) agonist that mechanistically decreases gut motility and increases fluid reabsorption, is one of the most commonly used agents for the management of IBS-D (4,5) despite the recent American College of Gastroenterology IBS Monograph strongly recommending against it as an IBS therapy (6). Loperamide is not indicated for long-term use and does not alleviate abdominal pain or bloating (7–11). Therefore, there is considerable need for new and effective treatments with favorable safety profiles that hold the potential to provide sustained symptom relief for patients with IBS-D.
Eluxadoline is a locally acting, mixed μ-OR agonist, κ-opioid receptor agonist, and δ-opioid receptor (δ-OR) antagonist with minimal oral bioavailability approved by the US Food and Drug Administration (FDA) for the treatment of IBS-D (12). Owing to its combined pharmacological profile, eluxadoline reduces gut motility consistent with its primary role as a μ-OR agonist and, due to its δ-OR antagonism, decreases the potential for medication-induced constipation. Importantly, eluxadoline was also shown to improve abdominal pain in 2 randomized, multicenter, multinational, double-blind, placebo-controlled phase 3 studies (IBS-3001 and IBS-3002) conducted in more than 2,400 patients with IBS-D (13). These unique features distinguish eluxadoline from peripherally acting μ-OR agonists such as loperamide (14). The primary endpoint of the two phase 3 trials of eluxadoline was defined by the simultaneous improvement in the daily worst abdominal pain (WAP) score by ≥30% compared with baseline weekly average and a reduction in the Bristol Stool Scale (BSS) to <5 on at least 50% of days within a 12-week treatment period (FDA endpoint) and a 26-week treatment period (European Medicines Agency endpoint). The 2 studies demonstrated that eluxadoline 100 mg twice daily had significantly greater composite responder rates as compared with placebo.
Patient history of prior loperamide use was collected in both phase 3 studies, and post-hoc analyses on the efficacy of eluxadoline in patients previously treated with loperamide were conducted (15). In total, 36% of all 2,400 patients with IBS-D in these studies reported prior loperamide use, among whom 62% self-reported inadequate symptom control. In the subpopulation of those not achieving adequate symptom control with prior loperamide use, a significantly greater proportion were composite responders following treatment with eluxadoline as compared to placebo (15). However, this subgroup analysis depended on patient recollection with varied durations of prior loperamide use. Therefore, the present study was conducted to further evaluate the efficacy, safety, and tolerability of eluxadoline 100 mg twice daily in patients who reported inadequate IBS-D symptom control with loperamide in the preceding 12 months. It was anticipated that the findings from this study would further our understanding on the utility of eluxadoline among patients with IBS-D self-reported as having experienced inadequate symptom relief with loperamide.
METHODS
Study design
This was a double-blinded, randomized, placebo-controlled, prospective, multicenter, multinational phase 4 study in adult patients with IBS-D (trial registration NCT02959983). The study comprised a 1-week screening period in which patients were assessed for eligibility, followed by a 3-week pretreatment period during which patients completed electronic patient-reported outcome (ePRO) diaries to record daily information regarding their IBS-D symptoms and loperamide rescue medication use. Following the pretreatment period, eligible patients who met the study entry criteria related to ePRO diary compliance, stool consistency (assessed by BSS), average WAP, and use of loperamide rescue medication were randomized through a central randomization system to receive eluxadoline 100 mg or placebo twice daily for 12 weeks. During the treatment period, patients returned to the clinic for visits at weeks 4, 8, and 12 (end-of-treatment visit), and for a post-treatment follow-up study visit at week 14. The total study duration was up to 18 weeks, with 7 site visits for each patient. The study design is presented in Supplementary Figure 1 (see Supplementary Digital Content 1, http://links.lww.com/AJG/B246).
Participants
Eligible patients were 18–80 years of age, with a diagnosis of IBS-D per Rome III criteria (defined as loose [mushy] or watery stools ≥25% and hard or lumpy stools ≤25% of bowel movements), an average WAP score of >3.0 (on a scale from 0 [no pain] to 10 [worst imaginable pain]) in the preceding 24 hours, an average BSS score of ≥5.5 (on a scale of 1 [hard lumpy stool] to 7 [entirely liquid stool]), and a BSS score of ≥5 in ≥5 days during the week before randomization. Prospective patients who met the above diagnostic criteria were prescreened based on loperamide use in the preceding 12 months and self-reported overall inadequate IBS symptom control with loperamide for study inclusion. A follow-up questionnaire was administered, which queried the patterns of medication usage (i.e., frequency, duration, reason for stopping, and degree of satisfaction with individual symptom relief including diarrhea, abdominal pain, and improvement in bowel movement) among patients who indicated that they managed their IBS-D using loperamide, antidiarrheals other than loperamide, antidepressants, and anticholinergics/antispasmodics. Patients were not allowed to use loperamide within 14 days before randomization. Patients were also required to complete the ePRO diary on at least 5 of the 7 days during the week before randomization and at least 10 of the 14 days during the 2-week pretreatment period before randomization. Key exclusion criteria were patients with IBS with constipation, IBS with mixed, or IBS with unsubtyped, history of inflammatory or immune-mediated GI disorders, diverticulitis, pancreatitis, known or suspected biliary duct obstruction, or sphincter of Oddi disease. Patients without a gallbladder were excluded in line with the updated US label (12). Patients with current or expected use of any narcotic or opioid-containing agents (e.g., antidiarrheal medications [except loperamide rescue medication after randomization] or opioid analgesics), or those with a history of alcohol abuse, alcohol addiction, or consumption of >3 alcoholic beverages per day were also excluded. Full eligibility criteria are included in the study protocol (see Supplementary Digital Content 2, http://links.lww.com/AJG/B265). The study protocol was approved and finalized before the first patient was screened, and written informed consent was obtained from all participants before initiation of any study-related activities.
Study outcomes and assessments
During the double-blind 12-week treatment period, patients recorded daily IBS-D symptoms including stool consistency (assessed through BSS), WAP, abdominal discomfort, abdominal bloating, bowel movement frequency, number of episodes of urgency in a day, number of episodes of fecal incontinence, and loperamide rescue medication use, through the ePRO diary.
The primary efficacy endpoint was the proportion of composite responders, defined as patients who met the daily composite response criteria (daily pain response: WAP score improvement by ≥40% in the preceding 24 hours and daily stool consistency response: BSS score <5 [or absence of bowel movement accompanied by ≥40% WAP improvement compared to baseline]) for at least 50% of treatment days and had ≥60 days of diary entries over the 12-week treatment period. Patients with <60 days of diary entries were considered nonresponders for the primary efficacy endpoint.
The 3 main secondary efficacy endpoints were defined as follows: (i) proportion of stool consistency responders (i.e., patients who met the daily stool consistency for ≥50% of days with diary entries over a certain time period, defined for the full 12-week treatment period [≥60 days of diary entries for the full 12 weeks] and each 4-week interval [>20 days of diary entries for each 4-week interval]); (ii) proportion of pain responders (i.e., patients who met the daily pain response criteria for ≥50% of days with diary entries over a certain time period, defined for the full 12-week treatment period [≥60 days of diary entries for the full 12 weeks] and each 4-week interval [>20 days of diary entries for each 4-week interval]); (iii) proportion of monthly composite responders (i.e., patients who met the daily composite response criteria for >50% of days with diary entry for ≥20 days during each 4-week interval [weeks 1–4, 5–8, and 9–12]). Patients with <60 days of diary entry for the 12-week treatment period or <20 days of diary entries for the 4-week intervals were considered nonresponders for the secondary efficacy endpoints.
Composite responder data were further analyzed by weekly responders for each week up to week 12 with responders defined in 2 ways. For definition #1, weekly composite responders were defined as daily composite responders on ≥4 days for a week (daily composite responder: ≥40% WAP improvement and <5 BSS score [or absence of bowel movement accompanied by ≥40% WAP improvement compared to baseline]). For definition #2, weekly composite responders were defined as patients with weekly average WAP improvement of ≥40% from average WAP of the baseline week and with ≥50% reduction in the days of BSS score 6/7 for a study week compared with days of BSS score 6/7 during the baseline week. An analysis was also performed using ≥6-week responders, wherein patients were required to meet the 2 weekly composite responder definitions defined above for at least 6 weeks over the 12-week treatment period. Safety and tolerability assessed the incidence of treatment-emergent adverse events (TEAEs). Additional endpoints are described in the study protocol and statistical analysis plan included in the Supplementary Materials (see Study Protocol, Supplementary Digital Content 2, http://links.lww.com/AJG/B265 and Statistical Analysis, Supplementary Digital Content 3, http://links.lww.com/AJG/B266).
Statistical analyses
The trial was designed with 90% power to detect the difference of the primary efficacy endpoint response for eluxadoline vs placebo using a 2-sided χ2 test at a significance level of 0.05. Similar to the primary endpoint, the secondary efficacy endpoints were analyzed using the number and percentage of the corresponding responders with P-values from χ2 test. No adjustment for the multiplicity of endpoints was performed.
Diary compliance was summarized by compliance categories (<60 vs >60 days) during the full 12-week treatment period (∼84 days) or <20 vs >20 days for the 4-week intervals, each corresponding to at least 70% compliance. For partial diary entries, compliance was affirmative if either WAP score and BSS score or WAP score and bowel movement frequency of zero were completed. For patients who met the criteria of the minimum of 4 days of diary entry, missing diary entries were imputed using the last observation carried forward method, the percent reduction calculation used the imputed data. Safety data were summarized using descriptive statistics. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA, v20.0).
Evaluation of efficacy and patient demographics was based on the intent-to-treat population, which included all randomized patients. For the intent-to-treat population, patients were analyzed according to their randomization assignment, regardless of the actual treatment received. Evaluation of safety endpoints was conducted using the safety population, which included all enrolled patients who received at least one dose of study drug. For the safety population, patients were grouped and analyzed according to the treatment they actually received. Additional details related to statistical analysis are described in the study protocol and statistical analysis plan included in the Supplementary Materials (see Study Protocol, Supplementary Digital Content 2, http://links.lww.com/AJG/B265 and Statistical Analysis, Supplementary Digital Content 3, http://links.lww.com/AJG/B266).
Data availability
Data reported in this manuscript are available within the article and its supplementary materials. Additional data from the RELIEF study may be requested at http://www.allerganclinicaltrials.com/PatientDataRequest.htm.
RESULTS
Patient disposition
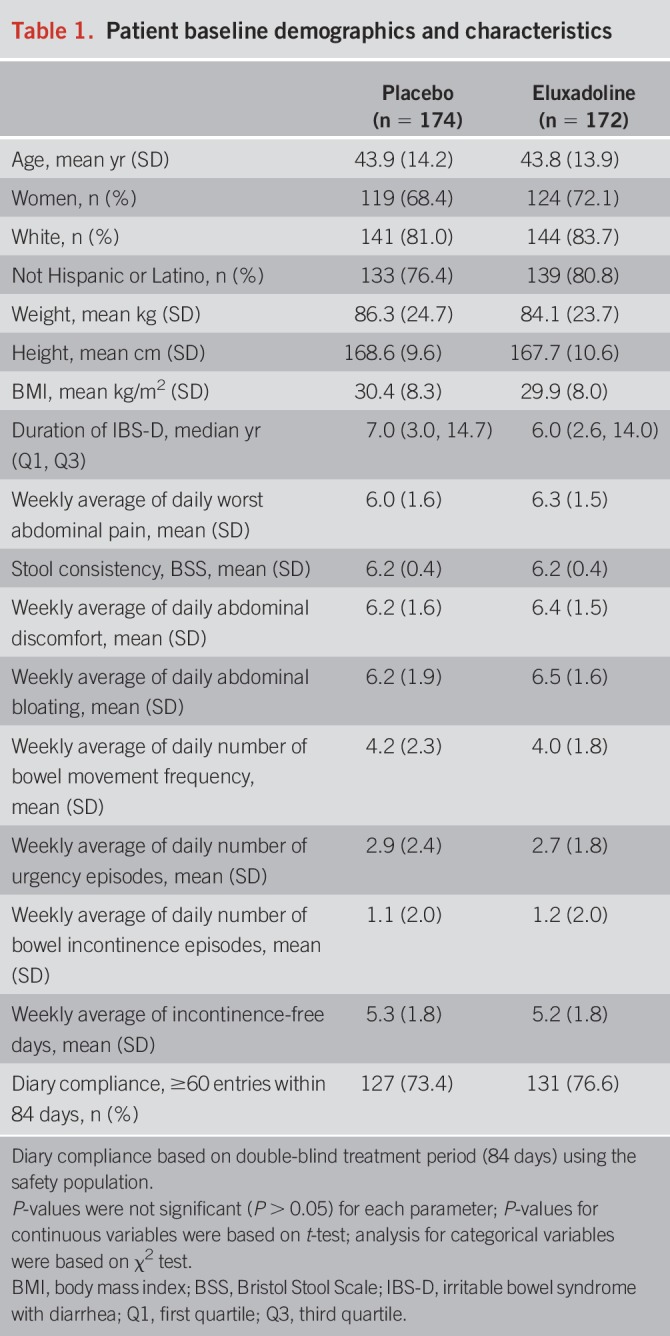
The study was conducted from November 2016 to January 2018 in 82 study sites across the United States and Canada. Of the 660 screened for eligibility, 346 patients were randomized to either placebo (n = 174) or eluxadoline (n = 172), and 295 (85.3%) completed the trial (see Figure 2, Supplementary Digital Content 1, http://links.lww.com/AJG/B246). Baseline characteristics among the patients were similar between the placebo and eluxadoline groups. The mean age was 44 years, 70% were women, and the median time since IBS-D diagnosis was 6 years. Overall, baseline IBS-D symptoms, including WAP, stool consistency, and patterns of bowel movements, were also similar between the 2 groups (Table 1).
Table 1.
Patient baseline demographics and characteristics
IBS-D treatment before randomization
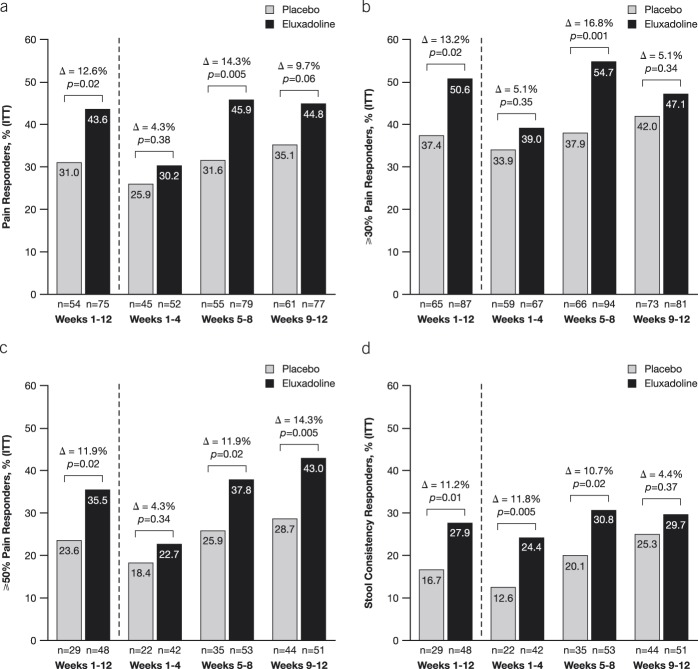
Previous treatment for IBS-D was balanced between the 2 groups. Use of antidiarrheal medications other than loperamide was reported by 27 (15.5%) patients in the placebo group and 23 (13.4%) in the eluxadoline group (see Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/B246). In addition, patients also reported managing their IBS-D using antidepressants, anticholinergics/antispasmodics, and in fact, >60% of patients tried at least one additional method, including lifestyle changes. For most patients (approximately 85%), loperamide was taken on an as-needed basis, while approximately 11% reported daily loperamide use (Table 2). Duration of loperamide use varied among the patients, with 80 (46.5%) in the placebo group and 67 (39.4%) in the eluxadoline group having taken loperamide for longer than 1 year. At randomization, 40 (23.3%) patients in placebo as compared to 55 (32.4%) patients in the eluxadoline group reported continued loperamide use, whereas >40% of patients in each group reported that they had stopped taking loperamide due to lack of improvement in their abdominal and bowel symptoms. Although all patients self-reported overall inadequate symptom relief with prior loperamide use in line with protocol requirement, ∼55% and ∼42% of patients in each treatment group indicated varying degrees of satisfaction (ranging from “a little satisfied” to “very satisfied”) with individual symptom relief of diarrheal and abdominal pain with loperamide, respectively. Conversely, ∼45% and ∼58% were “not at all satisfied” with the degree of individual diarrheal and abdominal pain relief, respectively, from prior loperamide use. Similarly, ∼55% of patients in each group reported varied satisfaction with decreased urgency and improvement in bowel movement with prior loperamide use, whereas ∼45% reported lack of satisfaction. Except for the rates of continued loperamide use, patterns of loperamide usage in the 12 months preceding randomization were balanced between the 2 groups (Table 2).
Table 2.
Patterns of loperamide usage in the 12 months preceding randomization
Primary endpoint
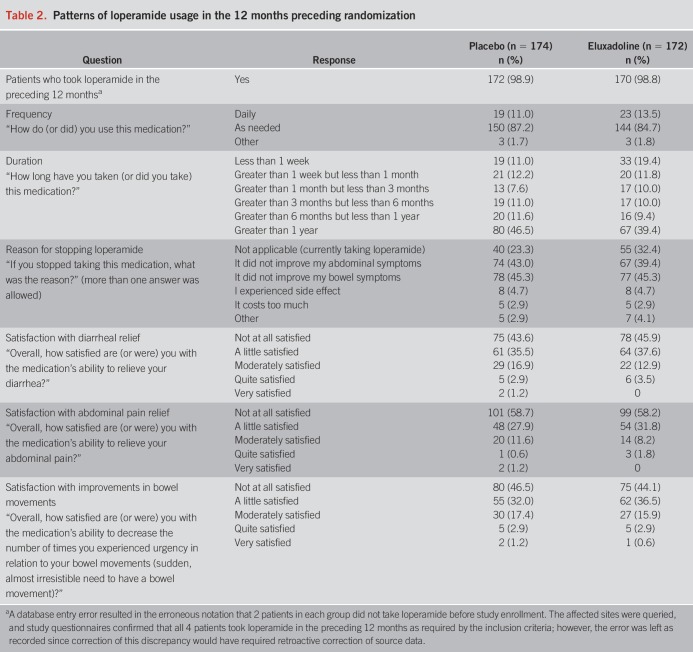
A statistically significantly greater proportion of eluxadoline-treated patients achieved the primary composite responder endpoint compared with patients treated with placebo (eluxadoline: 22.7% [39/172], placebo: 10.3% [18/174]; P = 0.002) (Figure 1a). Analysis of the monthly composite endpoint responder population showed that a greater proportion of patients treated with eluxadoline met the composite responder endpoint during each 4-week interval compared to placebo-treated patients (weeks 1–4: 14.0% vs 6.9%, P = 0.03; weeks 5–8: 26.7% vs 14.9%, P = 0.006; weeks 9–12: 30.8% vs 16.7%, P = 0.002) (Figure 1a). Separation of response in the daily composite responders was observed within the initial 14 days of treatment that favored eluxadoline and persisted throughout the study (Figure 1c).
Figure 1.
Analyses of composite responders. (a) Composite endpoint of daily responders (i.e., patients who meet daily composite response criteria on ≥50% of days, defined as ≥40% improvement in WAP compared with baseline and BSS score <5 [or the absence of a bowel movement if accompanied by ≥40% improvement in WAP]). Monthly composite responders are patients who met the daily composite response criteria on ≥50% of days and had a minimum of 20 days of diary entries for each 4-week interval. (b) A post-hoc analysis of composite responders defined at ≥30% improvement in WAP compared with baseline pain and daily stool consistency response. For both analyses, any patient with fewer than 20 days of diary entries for the 4-week interval was considered as a nonresponder. (c) Daily composite responders (≥40% WAP improvement and BSS score <5) over time. BSS, Bristol Stool Scale; WAP, worst abdominal pain. P-values are based on χ2 test.
A post-hoc analysis of daily composite endpoint responders was performed based on the primary composite endpoint criteria previously used in the phase 3 studies and consistent with FDA guidance for the design and completion of IBS-D trials (daily ≥30% improvement in WAP and daily stool consistency response [BSS score <5 or the absence of a bowel movement if accompanied by ≥30% improvement in WAP]) (16). A significantly greater proportion of patients treated with eluxadoline met this alternate composite responder endpoint during each of the 4-week intervals and the overall 12-week period as compared to patients treated with placebo (weeks 1–12: 26.2% vs 13.8%, P = 0.004; weeks 1–4: 19.2% vs 8.6%, P = 0.005; weeks 5–8: 29.7% vs 18.4%, P = 0.013; weeks 9–12: 31.4% vs 20.1%, P = 0.016) (Figure 1b).
Key secondary efficacy outcomes
A prespecified analysis of composite responder data was performed for weekly responders for each week up to week 12 with responders defined in 2 ways (see METHODS section for the definitions). During the study, a greater proportion of patients treated with eluxadoline met the criteria for weekly composite responders by both definitions as compared to the placebo group at weeks 4, 5, 6, 7, 9, 11, and 12 (see Figure 3, Supplementary Digital Content 1, http://links.lww.com/AJG/B246). Furthermore, as compared to the placebo group, treatment with eluxadoline resulted in a significantly higher proportion of ≥6-week composite responders for the analyzed weeks 1–6 (see Figure 4, Supplementary Digital Content 1, http://links.lww.com/AJG/B246) and weeks 1–12 (Figure 2) periods during the 12-week treatment period for both responder definitions.
Figure 2.
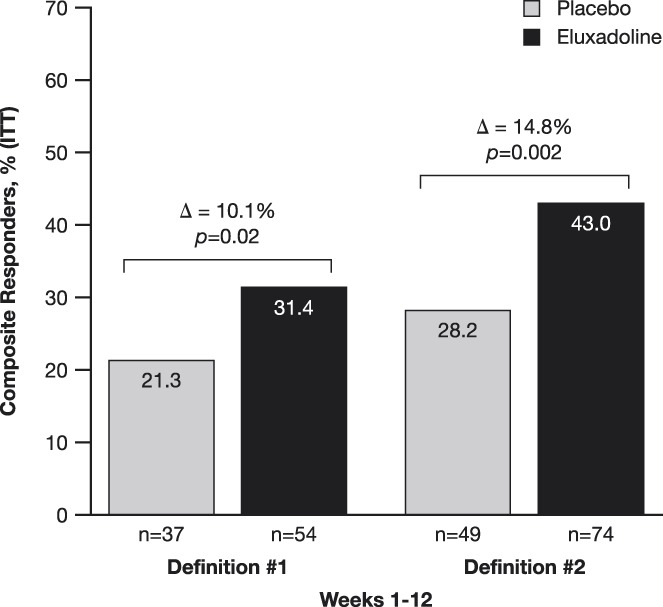
Weekly composite responder during the 12-week treatment period, and for at least 6 weeks out of 12 weeks from weeks 1–12 by 2 definitions. Definition #1 = weekly composite responder is defined as daily composite responder on ≥4 days for a week. Daily composite responder = WAP improvement by ≥40% compared with baseline and BSS score <5 (or the absence of bowel movement if accompanied by ≥40% improvement in WAP) compared to baseline. Definition #2 = weekly composite responder is defined as patients with weekly average WAP improvement of ≥40% from average WAP of the baseline week and with ≥50% reduction in the days of BSS score 6/7 for a week comparing with days of BSS score 6/7 during the baseline week. BSS, Bristol Stool Scale; WAP, worst abdominal pain. P-values are based on χ2 test.
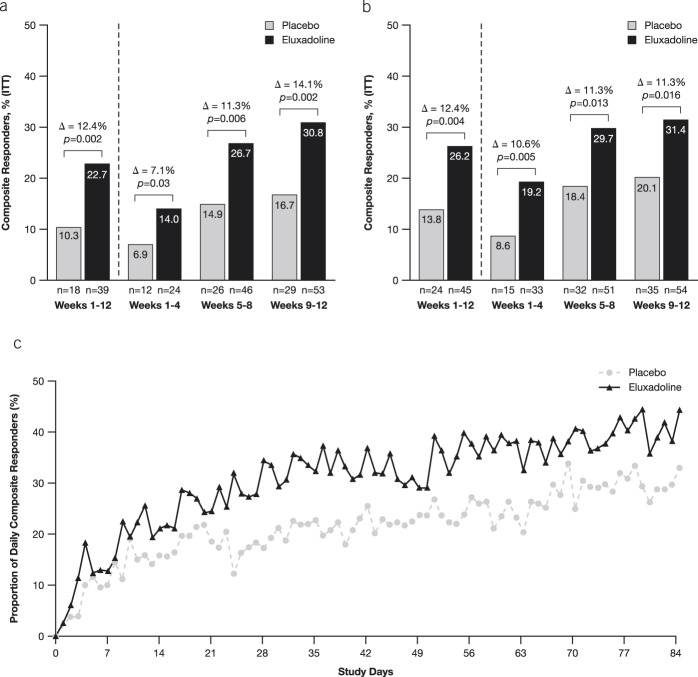
A significantly greater proportion of patients treated with eluxadoline met the abdominal pain response endpoint over the 12-week period as compared with placebo (43.6% [75/172] vs 31.0% [54/174]; P = 0.02) (Figure 3a). During each 4-week interval, treatment with eluxadoline resulted in improvements in WAP response over placebo (weeks 1–4: 30.2% vs 25.9%, P = 0.38; weeks 5–8: 45.9% vs 31.6%, P = 0.005; weeks 9–12: 44.8% vs 35.1%, P = 0.06). Abdominal pain responder data were further analyzed for patients who met daily 30% and 50% pain response criteria for ≥50% of days with diary entries over either the full 12-week treatment period or the three 4-week intervals. Treatment with eluxadoline resulted in a greater proportion of pain responders with both 30% and 50% improvement in WAP as compared with placebo during the overall 12-week treatment period and over the 4-week intervals (Figures 3b,c). In addition, a significantly greater proportion of patients treated with eluxadoline over the 12-week period were stool consistency responders as compared with placebo (27.9% [48/172] vs 16.7% [29/174]; P = 0.01) (Figure 3d). During each of the 4-week intervals, greater improvements in stool consistency response were observed with eluxadoline as compared to placebo. Collectively, these data demonstrated greater symptom improvement in key secondary endpoints throughout the 12-week treatment period with eluxadoline as compared to placebo. A summary of key efficacy endpoints is provided in Supplementary Table 2 (see Supplementary Digital Content 1, http://links.lww.com/AJG/B246).
Figure 3.
Analysis of pain and stool consistency responders. (a) Monthly pain responders (i.e., patients who met the daily pain response criteria for ≥50% of days with diary entries over a certain time period, defined for the full 12-week treatment period [≥60 days of diary entries for the full 12 weeks] and each 4-week interval [>20 days of diary entries for each 4-week interval] as ≥40% improvement in WAP compared with baseline). Analysis of responders who met the daily (b) 30% and (c) 50% improvement in WAP. (d) Analysis of monthly stool consistency responders (i.e., patients who met the daily stool consistency for ≥50% of days with diary entries over a certain time period, defined for the full 12-week treatment period [≥60 days of diary entries for the full 12 weeks] and each 4-week interval [>20 days of diary entries for each 4-week interval]) compared with baseline. WAP, worst abdominal pain. P-values are based on χ2 test.
Safety and tolerability
A total of 112 TEAEs were reported in the placebo group and 124 TEAEs in the eluxadoline group. The proportion of patients reporting at least one TEAE was comparable with eluxadoline (37.4% [64/171]) and placebo (35.3% [61/173]) (Table 3). Higher rates of treatment-related TEAEs were reported in the eluxadoline group (15.8% [27/171]) as compared to the placebo group (5.8% [10/173]). TEAEs leading to premature treatment discontinuations and study discontinuations were higher in the eluxadoline group (5.8% [10/171] and 2.9% [5/171], respectively) compared to the placebo group (1.7% [3/173] and 0.6% [1/171], respectively), whereas a higher rate of severe TEAEs was reported in the placebo group (2.9% [5/173]) as compared to the eluxadoline group (1.8% [3/171] [Table 3]). Three patients in the placebo group (1.7%) and 1 patient in the eluxadoline group (0.6%) experienced serious TEAEs (summarized in Table 3, Supplementary Digital Content 1, http://links.lww.com/AJG/B246), none of which were related to treatment.
Table 3.
Summary of treatment-emergent adverse events
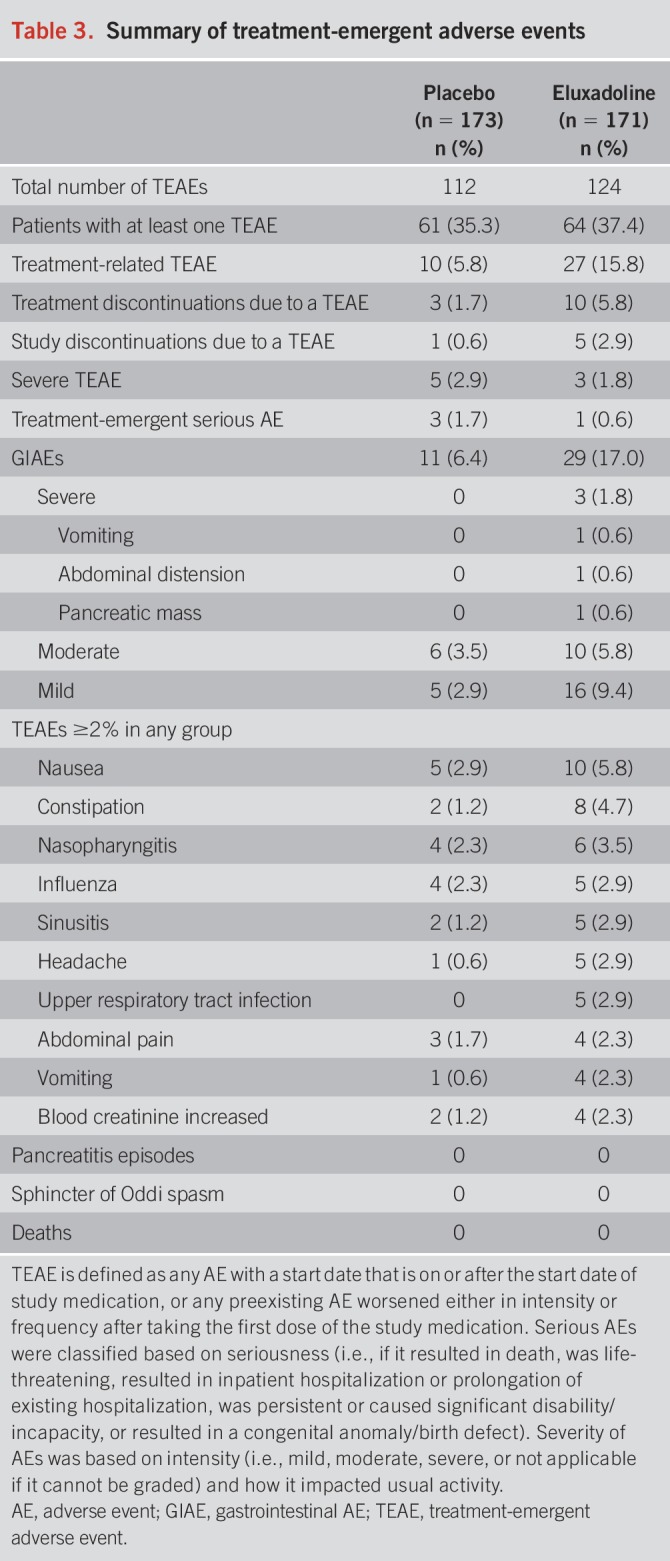
Gastrointestinal AEs (GIAEs) were the most common treatment-emergent events, with a higher rate reported in the eluxadoline group (17.0% [29/171]) as compared to the placebo group (6.4% [11/173]). Most GIAEs were mild-to-moderate in intensity, with 3 severe events reported in the eluxadoline group (Table 3). Of these, 1 event (pancreatic mass) was a serious AE, which was unrelated to treatment, from which the patient recovered and the drug was withdrawn. Of all TEAEs, nausea was the most common event at 5.8% (10/171) in the eluxadoline group as compared to the 2.9% (5/173) reported in the placebo group. A summary of TEAEs ≥2% in any group is provided by preferred term in Table 3. No episodes of pancreatitis or sphincter of Oddi spasm were reported in the study.
DISCUSSION
Loperamide, a μ-OR agonist that reduces the frequency of bowel movements and improves stool consistency, is frequently used as a first-line agent for IBS-D. However, studies have revealed that loperamide is ineffective in treating the abdominal symptoms of IBS (1,7–11). Use of IBS treatments with limited efficacy may result in additional medical visits, investigations, and adoption of other therapeutic approaches, including agents with minimal evidence of benefit (17,18). Such “treatment-failure” patients are challenging to treat, as diminishing returns often are experienced with additional therapeutic interventions; moreover, these patients frequently suffer from higher psychological and nonpain comorbidities, further complicating their treatment (19). In the present study, most patients reported use of other antidiarrheals, antidepressants, anticholinergics, and lifestyle modifications before enrollment, indicating a clear need for safe and effective agents that achieve sustained global relief of IBS-D earlier and more efficaciously.
In this multicenter, randomized, controlled phase 4 study of eluxadoline vs placebo in patients with IBS-D who reported inadequate symptom control with loperamide in the 12 months preceding the study, we found that a significantly greater proportion of patients receiving eluxadoline achieved daily improvement in abdominal pain (≥40%) and stool consistency (<5 BSS score, or absence of bowel movement) for at least 50% of treatment days. These improvements were observed within the initial 4 weeks of treatment and sustained until the end of the study. Similarly, the monthly composite responder endpoints were met by a higher proportion of eluxadoline-treated patients than those who received placebo. A higher percentage of patients receiving eluxadoline also achieved the secondary endpoints of improvements in stool consistency and abdominal pain compared to placebo.
In the two phase 3 studies (IBS-3001 [52-week treatment] and IBS-3002 [26-week treatment]) evaluating the safety and efficacy of eluxadoline, the primary composite endpoint was defined as a concurrent ≥30% improvement in daily abdominal pain score compared with baseline and a BSS score <5 on at least 50% of days within a 12-week treatment period (FDA endpoint) and a 26-week treatment period (European Medicines Agency endpoint) (13). In both studies, eluxadoline demonstrated significantly greater composite responder rates compared to placebo over the respective treatment periods. Subsequently, a retrospective analysis of these phase 3 data was performed to evaluate the efficacy of eluxadoline in patients who had been previously treated with loperamide (15). Over one-third of patients (873/2,428) in the phase 3 studies reported prior loperamide use, among whom nearly 60% (538/873) self-reported inadequate loperamide symptom control; in this subpopulation, a significantly greater proportion were composite responders following treatment with 100 mg of eluxadoline compared to placebo over 12 weeks (15). Similar to the retrospective analysis of phase 3 trial data, a significantly greater proportion of loperamide-unresponsive patients in the current prospective study met the responder criteria with eluxadoline as compared to placebo over 12 weeks of treatment. Notably, in the current trial, a more stringent definition was set for daily clinical responders as a patient with IBS-D had to achieve ≥40% improvement in WAP. Despite this more stringent endpoint, the results herein are similar to those observed in the phase 3 trials.
We also performed a post-hoc analysis using the phase 3 study primary endpoint criteria (≥30% WAP improvement and a reduction to <5 in the BSS score) and found that a significantly higher proportion of eluxadoline-treated patients achieved this FDA-mandated composite responder criteria compared to placebo over 12 weeks of treatment. Moreover, a similar treatment effect on the 12-week primary endpoint was also achieved for the phase 3 subpopulation who reported lack of adequate symptom control with prior loperamide use at 12 and 26 weeks (15). Therefore, the reproducibility of the phase 3 analysis in the current study further substantiates the positive treatment effect of eluxadoline on the composite endpoint and in the context of previous loperamide failure.
IBS and its range of symptoms are often difficult to treat; however, these collective data show favorable results in the patient population who have failed loperamide, a treatment often used as standard of care. The positive outcomes of this study in a real-world setting suggest that eluxadoline may be an option for those who have previously used loperamide.
Abdominal pain, a major clinical feature of IBS, is often the most challenging to treat and is closely linked to disease severity (20–22). Analysis of a ≥40% improvement in abdominal pain threshold over 12 weeks demonstrated a significantly higher responder rate in the eluxadoline group compared to placebo. Significant differences were maintained when the data were imputed using ≥30% abdominal pain improvement criteria as was used in the phase 3 studies, as well as for the stricter ≥50% abdominal pain improvement pain response. These data are generally in line with the phase 3 studies, which showed that the ≥30% abdominal pain response component showed an improvement, although not statistically significant, for eluxadoline over placebo in the overall population. The experience of abdominal pain in IBS is complex and is influenced by a multitude of patient factors, including psychological comorbidity, previous abuse experiences, and non-GI pain comorbidities. Additionally, environmental factors including diet and stress can further modulate these symptom experiences (23). Data from the current study showed significant improvements in abdominal pain across a wide range of subjective pain endpoints. Stool consistency response was also similar to the primary composite rate over the 12-week treatment period, and collectively, our data suggest that the overall response of the composite endpoint was driven by significantly higher response in stool consistency in the early-to-mid timepoints and by significant improvements in abdominal pain in the mid-to-later timepoints of the trial. The mechanistic basis for these observed differences with the mixed μ-OR agonist eluxadoline has not been fully elucidated but are presumed related to the combined κ-opioid receptor agonist and δ-OR antagonist characteristics unique to this agent.
The most common TEAEs in the study were GI, indicative of the local (GI) pharmacological effects of eluxadoline. GIAEs, including nausea, constipation, abdominal pain, and vomiting, were reported more frequently in the eluxadoline than the placebo group. Most GIAEs in the present study were mild or moderate in intensity. Three severe events were reported in the eluxadoline group, including vomiting, abdominal distension, and pancreatic mass, which were adjudicated as unrelated to eluxadoline. The single serious AE reported in the eluxadoline cohort was a pancreatic mass in a patient with a history of chronic alcohol consumption. This was assessed by investigators as unrelated to treatment, but treatment was withdrawn, and the pancreatic mass resolved without sequelae. It is noteworthy that no cases of pancreatitis or sphincter of Oddi spasm were reported in the present study.
In summary, results of the current study demonstrate positive treatment benefits for eluxadoline over placebo in patients with IBS-D reporting inadequate symptom relief from loperamide, based on improvements in both abdominal pain and stool consistency, the cardinal symptoms of IBS. Moreover, the safety profile of eluxadoline was comparable to placebo, with no new safety concerns identified. We conclude that this study prospectively validates previous findings revealing eluxadoline to be a safe, effective IBS-D treatment in patients reporting inadequate symptom relief with prior loperamide use.
CONFLICTS OF INTEREST
Guarantor of the article: Darren M. Brenner, MD.
Specific author contributions: D.M.B., C.R.G., and E.J. contributed to the study concept and design. D.M.B. and L.W.C.L. were principal investigators and supervised study-related activities at respective study sites. D.M.B., G.S.S., C.R.G., S.J.R.E., L.W.C.L., and B.D.C. contributed to the analysis and interpretation of data. All authors contributed to the drafting, critical revision for intellectual content, and final approval of the manuscript.
Financial support: This study was sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Germaine D. Agollah, PhD of Allergan plc and editorial assistance was also provided by Complete HealthVizion, Chicago, IL, USA. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Potential competing interests: Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. D.M.B. and G.S.S. are both consultants and speakers for Allergan plc and Ironwood Pharmaceuticals. C.R.G., E.J., and S.J.R.E. are full-time employees of Allergan plc and may own company stock. L.W.C.L. has received honoraria from Allergan, Lupin, Medtronic, AbbVie, and Takeda as a consultant and speaker, from Knight and Pendopharm as a speaker, and from Cipher as a consultant. B.D.C. has served as an advisor, consultant, or speaker for Allergan plc, Salix Pharmaceuticals, Takeda Pharmaceuticals, and Ironwood Pharmaceuticals.
Study Highlights.
WHAT IS KNOWN
✓ Eluxadoline is an FDA-approved therapy for the treatment of IBS-D.
✓ In post-hoc analyses of phase 3 clinical trials, patients failing to experience improvements in their IBS symptoms from loperamide appeared to derive symptomatic benefits from eluxadoline.
WHAT IS NEW HERE
✓ Eluxadoline improves IBS-D symptoms in individuals subjectively reporting inadequate responses to loperamide.
✓ Eluxadoline improves both pain and bowel functions in individuals reporting inadequate responses to loperamide.
✓ In a population of individuals with IBS-D with an intact gallbladder, no events of sphincter of Oddi spasm or pancreatitis occurred while taking eluxadoline.
ACKNOWLEDGEMENTS
The sponsor and authors would like to thank study participants and their families, the study investigators, research coordinators, and study staff.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B246, http://links.lww.com/AJG/B265, http://links.lww.com/AJG/B266, and http://links.lww.com/AJG/B267
REFERENCES
- 1.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015;313:949–58. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21 e4. [DOI] [PubMed] [Google Scholar]
- 4.Ruppin H. Review: loperamide—a potent antidiarrhoeal drug with actions along the alimentary tract. Aliment Pharmacol Ther 1987;1:179–90. [DOI] [PubMed] [Google Scholar]
- 5.Sayuk GS, Wolf R, Chang L. Comparison of symptoms, healthcare utilization, and treatment in diagnosed and undiagnosed individuals with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 2017;112:892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 2018;113:1–18. [DOI] [PubMed] [Google Scholar]
- 7.Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463–8. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ. Evaluation of drug treatment in irritable bowel syndrome. Br J Clin Pharmacol 2003;56:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talley NJ. Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol 2003;98:750–8. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014;147:1149–72 e2. [DOI] [PubMed] [Google Scholar]
- 11.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014;109(Suppl 1):S2–26; quiz S27. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. Viberzi. Highlights of prescribing information (https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206940s000lbl.pdf) (2015). Accessed April 26, 2018.
- 13.Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016;374:242–53. [DOI] [PubMed] [Google Scholar]
- 14.Wade PR, Palmer JM, McKenney S, et al. Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/micro opioid receptor antagonist. Br J Pharmacol 2012;167:1111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacy BE, Chey WD, Cash BD, et al. Eluxadoline efficacy in IBS-D patients who report prior loperamide use. Am J Gastroenterol 2017;112:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Guidance for Industry—Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment. (https://www.fda.gov/media/78622/download) (2012). Accessed July 15, 2019. [Google Scholar]
- 17.Pare P, Gray J, Lam S, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: Baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther 2006;28:1726–35; discussion 1710-1. [DOI] [PubMed] [Google Scholar]
- 18.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med 2008;358:1692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayuk GS, Elwing JE, Lustman PJ, et al. Predictors of premature antidepressant discontinuation in functional gastrointestinal disorders. Psychosom Med 2007;69:173–81. [DOI] [PubMed] [Google Scholar]
- 20.Lembo A, Ameen VZ, Drossman DA. Irritable bowel syndrome: Toward an understanding of severity. Clin Gastroenterol Hepatol 2005;3:717–25. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel B, Strickland A, Naliboff BD, et al. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol 2008;103:2536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegel B, Bolus R, Harris LA, et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther 2009;30:1159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Törnblom H, Drossman DA. Centrally targeted pharmacotherapy for chronic abdominal pain. Neurogastroenterol Motil 2015;27:455–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data reported in this manuscript are available within the article and its supplementary materials. Additional data from the RELIEF study may be requested at http://www.allerganclinicaltrials.com/PatientDataRequest.htm.