Abstract
Background
Given the small number of studies on the topic, we aimed to identify the impact of prepregnancy maternal body mass index (BMI) on adverse pregnancy outcomes (POs) in a low-risk, multiethnic population, and to calculate related population attributable fractions (PAFs).
Methods
This retrospective cohort study included 1134 nulliparous women of 50 nationalities (classified into Arab and non-Arab ethnicity) in Qatar who had their first antenatal visit at a Primary Healthcare Corporation (PHCC) facility in June 2016–March 2017 and their PO at a Hamad Medical Corporation facility before 10 November 2017. We used multiple imputation to handle missing values and multivariate logistic regression to calculate adjusted ORs (aORs) for adverse POs in overweight and women with obesity.
Results
Overweight Arab women and women with obesity were at high risk for gestational diabetes mellitus (GDM) (aOR=2.38, 95% CI 1.51 to 3.84) and caesarean section (aOR=1.57, 95% CI 1.00 to 2.48). Non-Arab women with obesity were at high risk for pre-eclampsia (aOR=3.83, 95% CI 1.00 to 15.00). PAFs showed that 41.63% of pre-eclampsia, 17.36% of pregnancy-induced hypertension, 17.17% of large for gestational age, 15.89% of preterm deliveries, 14.75% of GDM and 13.99% of caesarean sections could be avoided if all mothers had normal prepregnancy BMI. There were no major differences in PAFs by ethnicity.
Conclusion
Adverse POs were attributable to maternal obesity. This suggests that, in contrast to existing PHCC protocol, overweight and women with obesity in Qatar should be targeted earlier in their pregnancy; preferably prior to getting pregnant. We observed ethnic differences in the risk of adverse POs.
Keywords: maternal BMI, pregnancy outcomes, nulliparous, maternal health
Strengths and limitations of this study.
The study identifies overweight and nulliparous mothers with obesity who are at risk for maternal and neonatal complications using specific body mass index (BMI) cut-offs applicable for a multiethnic diverse population.
The study compares the risks across Arab and non-Arab maternal ethnic groups.
The study uses multiple imputation techniques to handle missing BMI data.
The study used nationality as a surrogate for ethnicity, which was valid in this sample given the dichotomous nature of the grouping used.
The study did not consider potential confounders such as socioeconomic status since such data were not available in the dataset.
Background
More than half of women of childbearing age in the high-income countries are either overweight or obese,1 and the percentage of women who are obese at their first antenatal visit nearly doubled between 1990 and 2004.2 Maternal obesity has been associated with infertility and can cause spontaneous pregnancy loss in early gestation.3 Gestational diabetes,4 pre-eclampsia,5 gestational hypertension, depression, instrumental vaginal delivery, caesarean section delivery6 and surgical site infection have been associated with maternal obesity.7 Maternal obesity can also impact neonatal outcomes, such as preterm birth, large-for-gestational-age babies, fetal defects, congenital anomalies and perinatal death. Length of hospital stay was also reported as an adverse outcome of maternal obesity.7
Most published studies on the effect of obesity on pregnancy outcomes have focused on primarily homogenous regional populations that are not ethnically diverse,8 but some researchers have shown that ethnic differences can have an impact on the association between obesity and adverse pregnancy outcomes.9 This impact is particularly relevant for countries like Qatar, which has a diverse, multiethnic, transient population. The 2012 WHO STEPwise approach to Surveillance survey for Qatar showed that 68.3% of adult females were overweight and 43.2% were obese,10 highlighting the significance of the maternal obesity problem in the country. The public healthcare system in Qatar is broken down into primary and secondary/tertiary care systems, with primary care administered by the Primary Healthcare Corporation (PHCC), and secondary/tertiary care administered by the Hamad Medical Corporation (HMC). Both systems classify pregnancies as high risk when the mother is obese class III or higher (body mass index (BMI)≥40 kg/m2), has a pre-existing medical condition and/or obstetric complications, as per the guidelines of the Ministry of Health. Women with high-risk pregnancies are directly referred to dedicated antenatal clinics of the HMC for specialised management. However, women with a BMI <40 kg/m2, but who are still overweight or obese, are cared for through the PHCC; they are referred to the HMC only if they develop complications or for delivery.
Population attributable fractions (PAFs) are used to identify the burden of risk factors for a disease or condition in a given population.11 Few studies have looked at PAFs of maternal obesity.12 Obesity is highly prevalent in Qatar, hence it is critical to study PAFs associated with obesity in different ethnic groups to quantify the burden of disease that can be attributed to obesity, and thereby help develop targeted management strategies. We aimed to identify the impact of prepregnancy maternal BMI on adverse pregnancy outcomes in a low-risk, multiethnic population, and to calculate related PAFs.
Methods
The PHCC Database is linked to the Birth Register of Qatar; it includes information on all PHCC visits, and thus includes prepregnancy and maternal characteristics (eg, age, nationality, pre-existing conditions), and pregnancy and neonatal outcomes. For this retrospective cohort study, we used the PHCC Database to identify all nulliparous women with singleton pregnancies who had their first antenatal visit at a PHCC facility between 1 June 2016 and 1 March 2017 and their pregnancy outcome at a HMC facility before 10 November 2017 (n=1245). The follow-up care for the mother and child initially happens at HMC and may then refer back to PHCC, however, this follow-up care was outside the scope of our study. We wanted to target women with low-risk pregnancies as defined by the Ministry of Health of Qatar. Therefore, we excluded women with high-risk pregnancies, that is, those with a pregnancy outcome prior to 24 weeks of gestation (n=8), those who were obese class II and higher (BMI≥35 kg/m2, n=80) or who were under the age of 18 at their first antenatal visit (n=23). Women who gave birth to babies with indeterminate sex (n=2), or who experienced stillbirth (n=3), fetal death (n=4) or neonatal death (n=1) were also excluded. Among the exclusions were 10 women who met more than one exclusion criterion; therefore, the final study sample consisted of 1134 women.
Patient and public involvement
This study involved secondary analysis of data collected by PHCC during its routine interactions with the patients. No additional patient or public contact was undertaken in this study.
Maternal ethnicity
Included women were of 50 unique nationalities, and maternal ethnicity was categorised as Arab or non-Arab based on nationality. Women were designated as Arab if they were citizens of one of the 22 countries included in the list of League of Arab States (LAS).13 Our Arab study women came from 18 of these countries (Algeria, Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Sudan, Syria, Tunis, United Arab Emirates and Yemen). The LAS have a common language (Arabic) and share a number of cultural, social and dietary habits which may put them at different lifestyle and obesity-related risks as compared with non-Arabs. Women of other nationalities were designated as non-Arabs.
Prepregnancy body mass index
Prepregnancy BMI was calculated based on information recorded at the most recent prepregnancy visit available in the PHCC Database. If this primary care visit was prior to 12 weeks from the date of the first antenatal visit, the BMI was treated as missing. Prepregnancy BMI was categorised as normal weight (BMI<25 kg/m2), overweight (BMI 25–29.99 kg/m2) and obese (BMI≥30 kg/m2) based on standard WHO guidelines.14 However, different BMI cut-offs were applied to Asians (n=405, overweight: BMI 23–27.5 kg/m2; obese: BMI≥27.5 kg/m2), as recommended by the WHO expert consultation.15 Sensitivity analyses, the results of which are not presented in this manuscript, showed that our application of different BMI cut-offs for Asians was valid, as using standard cut-offs underestimated the risk in that population.
Adverse pregnancy outcomes, adverse neonatal outcomes and risk factors
Investigated adverse pregnancy outcomes included GDM, pregnancy-induced hypertension (PIH), pre-eclampsia, preterm delivery, assisted vaginal delivery and caesarean section. Investigated adverse neonatal outcomes included macrosomia, large for gestational age, small for gestational age, neonatal intensive care unit referrals and Apgar score <7 at 1 min. Risk factors considered included maternal age, maternal ethnicity and pre-existing conditions such as diabetes mellitus type 1 or 2, hypertension and thyroid conditions. Retrospective review of patient medical records including doctors’ notes were used to identify pre-existing conditions and most adverse outcomes such as GDM. Diagnosis of macrosomia, large for gestational age and small for gestational age was based on the weight percentiles calculator available from the WHO website.16 17
Statistical analysis
Maternal characteristics and risk factors were tabulated using the observed data, and differences in the demographics across BMI categories were compared using Pearson’s χ2 test. Thereafter, as prepregnancy BMI was not available for 101 (8.9%) of the study subjects, multiple imputation was performed to assign these values, with the underlying assumption that BMI values were missing at random.18 19 Univariate and multivariate associations between maternal BMI category and adverse pregnancy and neonatal outcomes were assessed by logistic regression, using the imputed dataset.
Known and potential risk factors such as maternal age at first antenatal visit, pre-existing diabetes mellitus type 1 or 2, pre-existing hypertension and pre-existing thyroid conditions were included as covariates in the final model. Maternal ethnicity was assessed as potential risk factor while computing adjusted ORs (aORs) for associations between BMI category and adverse pregnancy and neonatal outcomes. Subgroup analysis was conducted by computing crude and aORs for adverse pregnancy and neonatal pregnancy outcomes separately for Arab and non-Arab mothers.
The aORs were used to compute PAFs of overweight and obesity for Arab and non-Arab mothers. PAFs were used to estimate the proportion of adverse pregnancy and neonatal outcomes that could be prevented if either one of two scenarios were true20: 1) if overweight and women with obesity were all of normal weight before pregnancy; 2) if overweight and obese mothers had a one-category drop in BMI before pregnancy (ie, obese to overweight, and overweight to normal weight). Scenario 0 was denoted the reference scenario, that is, the current population as represented by the data. These scenarios were also analysed by ethnicity.
PAFs and corresponding 95% CIs were computed using a user-written procedure (punaf) in the Stata software.20 All analyses in this research report were performed using the Stata 1521 software package and Microsoft Excel. The significance level for this study was set at 5%, so p value ≤0.05 was considered to be statistically significant.
Results
In our study sample, 86.33% of women were aged 18–30 years and 59.17% were of Arab ethnicity. The study sample included women from 50 countries, representing an extremely diverse data set. The most common risk factor among women with obesity was pre-existing thyroid conditions (5.22%). Term deliveries (gestational age 37–41 weeks) accounted for 89.77% of births in the study sample (table 1a).
GDM was the most common adverse pregnancy outcome and was observed in 35.89% of the study sample, followed by caesarean section (24.96%). Pre-eclampsia was observed in 3.44% of the study sample. The prevalence of GDM, caesarean section and pre-eclampsia increased with increasing BMI category (table 1b).
Table 1A.
Selected characteristics of the study sample by prepregnancy maternal body mass index (BMI) in Qatar (n=1134)
| Prepregnancy BMI category | ||||||
| Normal weight (n=404) |
Overweight (n=399) | Obese (n=230) |
Missing (n=101) |
Total (n=1134) |
P value | |
| Maternal age (years) | <0.001 | |||||
| 18–24 | 212 (52.48%) | 141 (35.34%) | 73 (31.74%) | 48 (47.52%) | 474 (41.8%) | |
| 25–30 | 157 (38.86%) | 195 (48.87%) | 114 (49.57%) | 39 (38.61%) | 505 (44.53%) | |
| 31–44 | 35 (8.66%) | 63 (15.79%) | 43 (18.7%) | 14 (13.86%) | 155 (13.67%) | |
| Maternal ethnicity | 0.005 | |||||
| Arab | 254 (62.87%) | 240 (60.15%) | 114 (49.57%) | 63 (62.38%) | 671 (59.17%) | |
| Non-Arab | 150 (37.13%) | 159 (39.85%) | 116 (50.43%) | 38 (37.62%) | 463 (40.83%) | |
| Pre-existing hypertension | ||||||
| Yes | 8 (1.98%) | 10 (2.51%) | 4 (1.74%) | 2 (1.98%) | 24 (2.12%) | 0.94 |
| No | 396 (98.02%) | 389 (97.49%) | 226 (98.26%) | 99 (98.02%) | 1110 (97.88%) | |
| Pre-existing diabetes mellitus type 1 or 2 | ||||||
| Yes | 5 (1.24%) | 4 (1%) | 4 (1.74%) | 0 (0%) | 13 (1.15%) | 0.67 |
| No | 399 (98.76%) | 395 (99%) | 226 (98.26%) | 101 (100%) | 1121 (98.85%) | |
| Pre-existing thyroid condition | ||||||
| Yes | 18 (4.46%) | 10 (2.51%) | 12 (5.22%) | 2 (1.98%) | 42 (3.7%) | 0.22 |
| No | 386 (95.54%) | 389 (97.49%) | 218 (94.78%) | 99 (98.02%) | 1092 (96.3%) | |
| Gestational age at delivery (weeks) | ||||||
| <37 | 32 (7.92%) | 40 (10.03%) | 28 (12.17%) | 10 (9.9%) | 110 (9.7%) | 0.60 |
| 37–41+6 days | 370 (91.58%) | 357 (89.47%) | 200 (86.96%) | 91 (90.1%) | 1018 (89.77%) | |
| ≥42 | 2 (0.5%) | 2 (0.5%) | 2 (0.87%) | 0 (0%) | 6 (0.53%) | |
Values shown as number (%).
χ2 test was used to determine differences across the BMI categories.
Fisher’s exact test was used when cell count was <5.
Bold values are statisticaly significant.
Table 1B.
Distribution of adverse pregnancy and neonatal outcomes by prepregnancy maternal body mass index (BMI) in Qatar (N=1134)
| Prepregnancy BMI category | ||||||
| Normal weight (n=404) |
Overweight (n=399) |
Obese (n=230) |
Missing (n=101) |
Total (n=1134) |
P value | |
| Pregnancy outcomes | ||||||
| Gestational diabetes mellitus | 118 (29.21%) | 140 (35.09%) | 108 (46.96%) | 41 (40.59%) | 407 (35.89%) | <0.001 |
| Pregnancy-induced hypertension | 14 (3.47%) | 14 (3.51%) | 17 (7.39%) | 1 (0.99%) | 46 (4.06%) | 0.038 |
| Pre-eclampsia | 8 (1.98%) | 14 (3.51%) | 15 (6.52%) | 2 (1.98%) | 39 (3.44%) | 0.013 |
| Preterm delivery | 32 (7.92%) | 40 (10.03%) | 28 (12.17%) | 8 (7.92%) | 108 (9.52%) | 0.21 |
| Assisted vaginal delivery | 66 (16.34%) | 62 (15.54%) | 40 (17.39%) | 11 (10.89%) | 179 (15.78%) | 0.831 |
| Caesarean section | 80 (19.8%) | 107 (26.82%) | 69 (30%) | 27 (26.73%) | 283 (24.96%) | 0.008 |
| Neonatal outcomes | ||||||
| Macrosomia | 11 (2.72%) | 8 (2.01%) | 13 (5.65%) | 1 (0.99%) | 33 (2.91%) | 0.034 |
| Large for gestational age | 25 (6.19%) | 31 (7.77%) | 23 (10%) | 7 (6.93%) | 86 (7.58%) | 0.22 |
| Small for gestational age | 65 (16.09%) | 55 (13.78%) | 32 (13.91%) | 22 (21.78%) | 174 (15.34%) | 0.606 |
| NICU referral | 36 (8.91%) | 46 (11.53%) | 22 (9.57%) | 10 (9.9%) | 114 (10.05%) | 0.449 |
| Apgar score <7 at 1 min | 19 (4.7%) | 17 (4.26%) | 14 (6.09%) | 4 (3.96%) | 54 (4.76%) | 0.582 |
Values shown as number (%).
χ2 test was used to determine differences across the BMI categories.
Bold values are statisticaly significant.
NICU, neonatal intensive care unit.
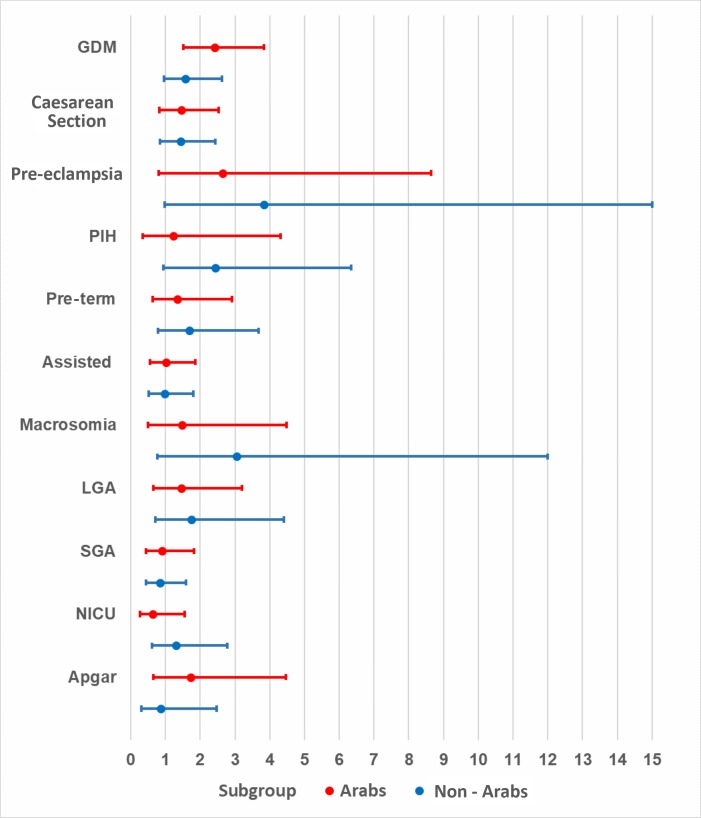
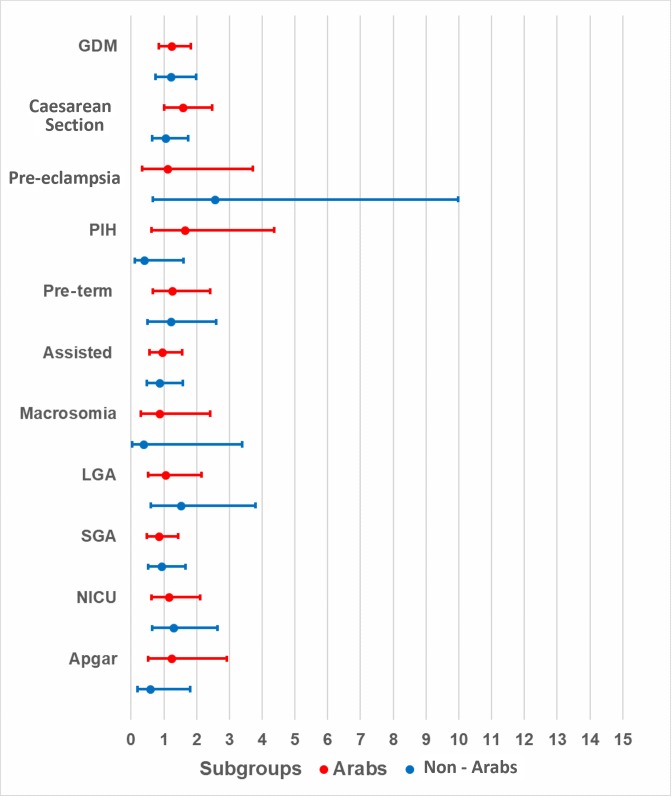
Obese Arab mothers had statistically significant, higher odds of developing GDM (aOR=2.41, 95% CI 1.51 to 3.84, p<0.01) when compared with normal-weight Arab mothers. Although the odds were higher for obese non-Arab mothers when compared with their normal-weight counterparts (aOR=1.58, 95% CI 0.96 to 2.62, p=0.07), these odds were not as high as those observed for Arab mothers, and the association was not statistically significant. Non-Arab women with obeseity had significantly higher odds (aOR=3.83, 95% CI 0.98 to 15.00, p=0.05) of developing pre-eclampsia when compared with normal-weight non-Arab women. Finally, the odds of caesarean section were significant among overweight Arab mothers, as compared with their normal-weight counterparts (aOR=1.57, 95% CI 1.00 to 2.48, p=0.05), but this association was not significant among obese Arab mothers or among obese non-Arab mothers when compared with their normal-weight counterparts (table 2). Although higher odds were observed in obese (figure 1) and overweight (figure 2) mothers in both ethnic groups, p values were not statistically significant for most adverse pregnancy or neonatal outcomes when compared with normal-weight counterparts.
Table 2.
ORs and 95% CIs of the association between adverse pregnancy and neonatal pregnancy outcomes and body mass index (BMI) category by ethnicity after multiple imputation (n=1134)
| Arabs | Non-Arabs | |||||
| Prevalence | Adjusted OR (95% CI) |
P value | Prevalence | Adjusted OR (95% CI) |
P value | |
| Pregnancy outcomes | ||||||
| Gestational diabetes mellitus | ||||||
| Overweight | 92 (34.98%) | 1.24 (0.84 to 1.82) | 0.28 | 64 (36.99%) | 1.23 (0.76 to 2.00) | 0.40 |
| Obese | 66 (51.97%) | 2.38 (1.51 to 3.84) | <0.01 | 54 (43.2%) | 1.60 (0.97 to 2.65) | 0.07 |
| Pregnancy-induced hypertension | ||||||
| Overweight | 11 (4.18%) | 1.66 (0.63 to 4.38) | 0.31 | 3 (1.73%) | 0.41 (0.11 to 1.61) | 0.20 |
| Obese | 4 (3.15%) | 1.21 (0.34 to 4.31) | 0.76 | 13 (10.4%) | 2.41 (0.93 to 6.327) | 0.07 |
| Pre-eclampsia | ||||||
| Overweight | 6 (2.28%) | 1.11 (0.33 to 3.71) | 0.86 | 9 (5.2%) | 2.56 (0.66 to 9.97) | 0.18 |
| Obese | 7 (5.51%) | 2.64 (0.81 to 8.65) | 0.11 | 8 (6.4%) | 3.83 (0.98 to 15.00) | 0.05 |
| Preterm delivery | ||||||
| Overweight | 26 (9.89%) | 1.26 (0.66 to 2.41) | 0.48 | 18 (10.4%) | 1.22 (0.57 to 2.61) | 0.61 |
| Obese | 14 (11.02%) | 1.35 (0.63 to 2.91) | 0.45 | 17 (13.6%) | 1.69 (0.78 to 3.68) | 0.18 |
| Assisted vaginal delivery | ||||||
| Overweight | 38 (14.45%) | 0.94 (0.57 to 1.55) | 0.82 | 28 (16.18%) | 0.87 (0.48 to 1.58) | 0.65 |
| Obese | 20 (15.75%) | 1.01 (0.55 to 1.86) | 0.97 | 22 (17.6%) | 0.97 (0.52 to 1.81) | 0.93 |
| Caesarean section | ||||||
| Overweight | 66 (25.1%) | 1.57 (1.00 to 2.48) | 0.05 | 51 (29.48%) | 1.05 (0.64 to 1.74) | 0.83 |
| Obese | 32 (25.2%) | 1.46 (0.83 to 2.53) | 0.20 | 45 (36%) | 1.43 (0.84 to 2.44) | 0.19 |
| Neonatal outcomes | ||||||
| Macrosomia | ||||||
| Overweight | 7 (2.66%) | 0.86 (0.30 to 2.42) | 0.77 | 1 (0.58%) | 0.37 (0.04 to 3.39) | 0.38 |
| Obese | 6 (4.72%) | 1.48 (0.49 to 4.48) | 0.49 | 7 (5.6%) | 3.05 (0.77 to 12.06) | 0.11 |
| Large for gestational age | ||||||
| Overweight | 19 (7.22%) | 1.06 (0.53 to 2.14) | 0.87 | 15 (8.67%) | 1.52 (0.61 to 3.79) | 0.37 |
| Obese | 13 (10.24%) | 1.46 (0.66 to 3.20) | 0.35 | 12 (9.6%) | 1.75 (0.70 to 4.41) | 0.23 |
| Small for gestational age | ||||||
| Overweight | 34 (12.93%) | 0.84 (0.49 to 1.43) | 0.52 | 30 (17.34%) | 0.93 (0.52 to 1.67) | 0.82 |
| Obese | 17 (13.39%) | 0.90 (0.44 to 1.82) | 0.77 | 19 (15.2%) | 0.84 (0.44 to 1.60) | 0.59 |
| NICU referral | ||||||
| Overweight | 26 (9.89%) | 1.15 (0.63 to 2.11) | 0.65 | 23 (13.29%) | 1.30 (0.64 to 2.64) | 0.46 |
| Obese | 8 (6.3%) | 0.64 (0.26 to 1.55) | 0.32 | 17 (13.6%) | 1.30 (0.61 to 2.77) | 0.49 |
| Apgar score <7 at 1 min | ||||||
| Overweight | 13 (4.94%) | 1.24 (0.53 to 2.92) | 0.63 | 6 (3.47%) | 0.59 (0.19 to 1.80) | 0.47 |
| Obese | 8 (6.3%) | 1.72 (0.66 to 4.46) | 0.27 | 6 (4.8%) | 0.86 (0.30 to 2.48) | 0.50 |
For each ethnic group, normal weight was considered the reference category (OR=1) for calculating ORs. All outcomes were adjusted for maternal age at first antenatal visit. In addition, pre-eclampsia for pre-existing diabetes, and pre-existing hypertension, preterm delivery for pre-existing comorbid conditions, assisted vaginal delivery for pre-existing comorbid conditions, caesarean section for pre-existing comorbid conditions, macrosomia for pre-existing diabetes, large for gestational age for pre-existing diabetes and small for gestational age was adjusted for pre-existing diabetes and pre-existing hypertension.
NICU, neonatal intensive care unit.
Figure 1.
Adjusted ORs and 95% CIs for obese nulliparous mothers by ethnicity. GDM, gestational diabetes mellitus; PIH, pregnancy-induced hypertension; NICU, neonatal intensive care unit; LGA, large for gestational age; SGA, small for gestational age.
Figure 2.
Adjusted ORs and 95% CIs for overweight nulliparous mothers by ethnicity. C-section, caesarean section; GDM, gestational diabetes mellitus; PIH, pregnancy-induced hypertension; NICU, neonatal intensive care unit; LGA, large for gestational age; SGA, small for gestational age.
Population attributable fractions
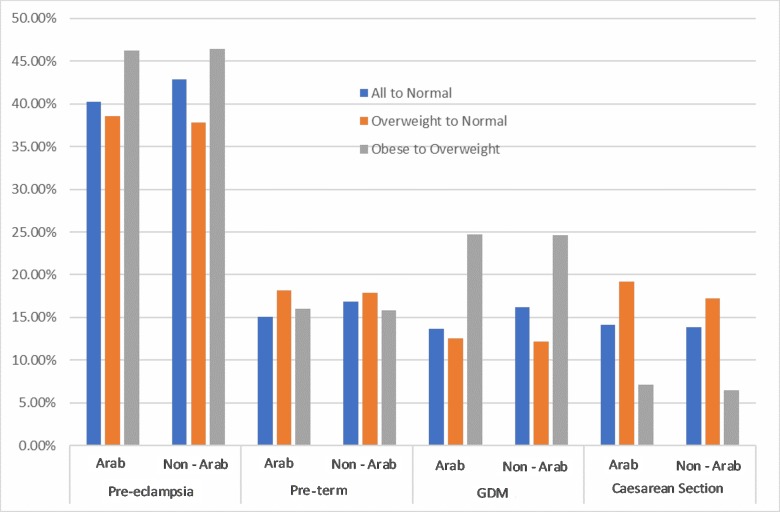
For pre-eclampsia, a one-category reduction in BMI among Arab mothers corresponded to a PAF of 46.28% for women with obesity and 38.61% for overweight women (figure 3). For PIH, a one-category drop in BMI for women with obesity corresponded to a PAF of 50.83%, meaning that 50.83% of PIH cases could be avoided if obese mothers reduced their BMI category to overweight (figure 3). Corresponding PAFs for neonatal outcomes were 65.23% for macrosomia and 30.42% for Apgar score <7 at 1 min. Some of the CIs and PAFs were negative (eg, small for gestational age and assisted vaginal delivery), indicating a protective effect of obesity on these outcomes, which means that removing obesity could increase these risks (tables 3 and 4).
Figure 3.
Population attributable fractions for Arab and non-Arab mothers for pregnancy outcomes for scenarios 1 (all to normal weight) and scenario 2 (one-category decrease in body mass index). C-section, caesarean section; GDM, gestational diabetes mellitus.
Table 3.
Population attributable fractions (%) and 95% CIs of adverse pregnancy and neonatal outcomes by prepregnancy body mass index among Arab mothers
| All to normal weight (scenario 1) |
Obese to overweight (scenario 2) |
Overweight to normal (scenario 2) |
|
| Pregnancy outcomes | |||
| Gestational diabetes | 13.64 (2.81 to 23.27) | 24.72 (8.88 to 37.8) | 12.52 (−7.28 to 28.66) |
| Pregnancy-induced hypertension | 14.62 (−28.68 to 43.35) | 50.83 (1.46 to 75.46) | −3.46 (−116.78 to 50.63) |
| Pre-eclampsia | 40.26 (−8.34 to 67.06) | 46.28 (−8.98 to 73.52) | 38.61 (−45.72 to 74.14) |
| Preterm delivery | 15.04 (−11.09 to 35.03) | 16.02 (−33.05 to 46.99) | 18.14 (−28.65 to 47.91) |
| Assisted vaginal delivery | −3.11 (−22.13 to 12.95) | 8.23 (−32.19 to 36.29) | −8.5 (−50.1 to 21.57) |
| Caesarean section | 14.09 (−0.9 to 26.86) | 7.14 (−21.13 to 28.81) | 19.24 (−5.27 to 38.03) |
| Neonatal outcomes | |||
| Macrosomia | 6.78 (−47.09 to 40.91) | 65.23 (17.74 to 85.31) | −44.54 (−257.57 to 41.58) |
| Large for gestational age | 16.03 (−13.93 to 38.11) | 22.26 (−29.92 to 53.48) | 17.23 (−38.3 to 50.46) |
| Small for gestational age | −7.96 (-27.51 to 8.59) | −1.19 (-53.4 to 33.24) | −14.47 (−61.48 to 18.85) |
| NICU referral | 7.91 (−18.08 to 28.18) | −25.28 (-104.33 to 23.18) | 18.37 (−24.71 to 46.57) |
| Apgar score <7 at 1 min | 1.67 (−37.59 to 29.73) | 30.42 (−38.63 to 65.08) | −10.89 (−111.84 to 41.95) |
Bold values are statisticaly significant.
NICU, neonatal intensive care unit.
Table 4.
Population attributable fractions (%) and 95% CIs of adverse pregnancy and neonatal outcomes by prepregnancy body mass index among non-Arab mothers
| All to normal weight | Obese to overweight | Overweight to normal | |
| Pregnancy outcomes | |||
| Gestational diabetes | 16.2 (4.79 to 26.23) | 24.58 (8.6 to 37.77) | 12.18 (−7.12 to 28) |
| Pregnancy-induced hypertension | 19.98 (−26.05 to 49.2) | 50.24 (1.25 to 74.93) | −3.41 (−114.55 to 50.16) |
| Pre-eclampsia | 42.92 (−5.47 to 69.11) | 46.42 (−9.27 to 73.73) | 37.79 (−44.56 to 73.23) |
| Preterm delivery | 16.89 (−10.67 to 37.59) | 15.8 (−32.54 to 46.51) | 17.86 (−28.14 to 47.34) |
| Assisted delivery | −2.81 (−23.22 to 14.22) | 8.14 (−31.83 to 36) | −8.27 (−48.48 to 21.06) |
| Caesarean section | 13.89 (−0.47 to 26.2) | 6.48 (−18.95 to 26.47) | 17.19 (−4.73 to 34.53) |
| Neonatal outcomes | |||
| Macrosomia | 13.73 (−46.3 to 49.13) | 65.86 (17.93 to 85.8) | −45.01 (−261.54 to 41.84) |
| Large for gestational age | 18.86 (−13.59 to 42.04) | 22.44 (−30.35 to 53.85) | 17.29 (−38.52 to 50.62) |
| Small for gestational age | −8.73 (−30.31 to 9.27) | −1.13 (−50.1 to 31.86) | −13.69 (−57.58 to 17.98) |
| NICU referral | 7.09 (−20.53 to 28.37) | −24.49 (−100.23 to 22.59) | 17.79 (−23.81 to 45.41) |
| Apgar score <7 | 4.25 (−40 to 34.51) | 30.53 (−38.95 to 65.27) | −10.93 (−112.38 to 42.06) |
Bold values are statisticaly significant.
NICU, neonatal intensive care unit.
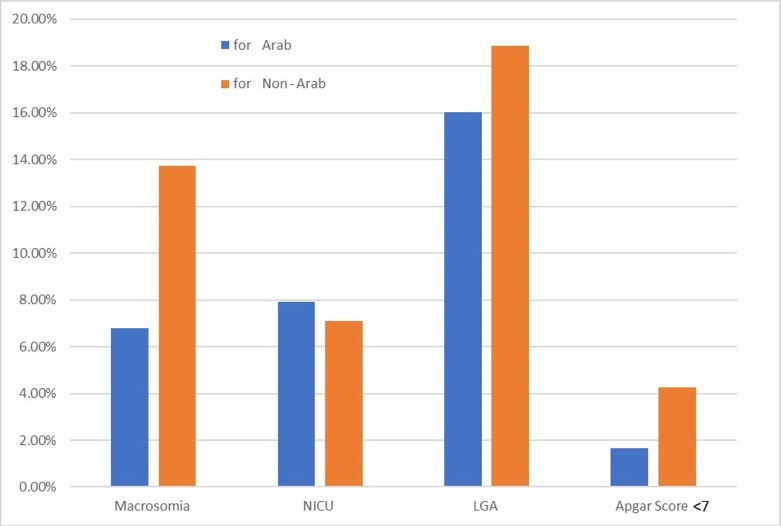
For GDM, a one-category reduction in BMI corresponded to a PAF of 24.72% and 24.58% for obese Arab and non-Arab mothers, respectively (figure 3). The PAF for macrosomia if all women were of normal weight before pregnancy was 13.73% for non-Arab women and 6.78% for Arab mothers (figure 4). The PAFs for neonatal outcomes after a one-category reduction in BMI are not shown in the figures 3 and 4, as they are mostly negative due to the small number of cases.
Figure 4.
Population attributable fractions for Arab and non-Arab mothers for neonatal outcomes for scenario 2 (one-category decrease in body mass index). NICU, neonatal intensive care unit; LGA, large for gestational age.
Discussion
Our study confirms that there is an association between prepregnancy maternal BMI and adverse pregnancy and neonatal pregnancy outcomes in multiethnic populations, using aORs and PAFs. Previous studies have shown similar strengths of association between overweight and obesity and GDM,22 PIH,22 23 preterm delivery,12 24 assisted vaginal delivery,22 25 caesarean section,22 25 macrosomia,12 large for gestational age24 and small for gestation age.24 25 However, the present study found much higher ORs for pre-eclampsia in non-Arab mothers. This could be attributed to the large portion of Asian mothers in the non-Arab group, who have been reported to be susceptible to pre-eclampsia.26
Our results confirm those of a recent study on nulliparous women in Qatar, which reported a higher incidence of GDM, caesarean section and PIH in women with obesity,27 whereas overweight women were reported to have higher caesarean section rates only.27 However, the overall incidence of these outcomes was much lower than in our study sample (GDM: 15% vs 35.89% in our study sample; caesarean section: 16% vs 24.98%). These difference cannot be explained by the data alone, since the earlier study27 was carried out in a tertiary care setting, whereas this research focused on primary care settings.
The PAFs we report are much higher than those from some published studies,12 due to the high prevalence of overweight and obesity (ie, exposure) in our study sample. Pre-eclampsia stands out, as any reduction in BMI category yielded a substantial reduction in the burden of this condition. A one-category reduction in BMI showed a much higher possibility of disease reduction among women with obesity than among overweight women, whether they were Arab or non-Arab. However, caesarean section was an exception to this trend, and showed a lesser PAF for a one-category reduction in BMI among women with obesity than overweight women. This can be explained by the higher OR for caesarean section in overweight women as compared with women with obesity in both ethnic groups. It should be noted that a causal relationship between exposures and outcomes is generally assumed in PAF calculations. However, this relationship may not necessarily exist, and PAFs should be considered accordingly.
Unlike other reports, the exposure variable in our study was not self-reported. We used standard WHO recommended BMI cut-offs, except in the Asian population, in which Asian-specific cut-offs were applied. Results of a sensitivity analysis indicated that the use of these Asian-specific cut-offs is indeed clinically important in multiethnic populations. Indeed, Asian mothers must be classified as overweight and obese using the WHO-recommended cut-offs for Asians, otherwise high-risk patients may not be properly identified during antenatal care.
Patient nationality was used as a surrogate for ethnicity, but it is common for people to change their citizenship through immigration, which could have limited the strength of the conclusions of this study. However, a detailed analysis of nationalities revealed that the number of patients claiming citizenship to countries that are most likely targets for migrants was minimal (Canadians=0, Australians=0, Americans=3, British=1, French=1). Therefore, the use of nationality as a surrogate for ethnicity is reasonable and valid for this dataset.
Other reports from Qatar showed higher rates of conditions such as diabetes mellitus and cardiovascular diseases, which were not reflected in our study. These low rates may represent an information bias which did not allow us to properly adjust for potential confounders. Data on socioeconomic status and health centre location were not available; hence it is not possible to attribute missing values to these factors or to any other variables for which data were not available.
GDM, pre-eclampsia and caesarean section were significantly associated with prepregnancy BMI among our overweight and obese mothers, who are not generally considered to have at-risk pregnancies by the healthcare system in Qatar. High prevalence of GDM in normal-weight mothers (29.21%) indicates that BMI alone cannot explain the problem, which is instead related to population norms and characteristics. High risk of pre-eclampsia in overweight and obese mothers, especially among non-Arabs, indicates that early screening and management of hypertensive disorders is needed for this group. This implies that clinical screening is indicated for all mothers regardless of their prepregnancy BMI or ethnicity, preferably at the start of pregnancy.
The rate of caesarean section was very high in our study sample (25%), especially considering that pregnancies in overweight and women with obesity are currently considered low risk by the healthcare system in Qatar. The odds of caesarean section were uncharacteristically high and similar in overweight and obese mothers regardless of ethnicity. Clinical implications cannot be drawn from these results without properly separating the caesarean sections by their indications (medically necessary vs elective), which were not available in the study sample. Our findings of an association between prepregnancy maternal BMI and caesarean section may not properly represent the true nature of the outcome risk and exposure, and should therefore be interpreted with caution.
As has been mentioned in above paragraphs, the retrospective cohort nature of this study limited our ability to validate or verify the elements of the dataset and other potential confounders through additional patient contact. The anonymity of the data by design made it impossible to undertake such a follow-up. The strength of our findings is therefore directly related to the strength of the dataset used. The number of missing values in the exposure variable, the prepregnancy BMI, meant that we were unable to ascertain a direct relationship for those records. However, the impact of such was minimised by using the multiple imputation techniques. The PAFs were computed using the punaf user written extension for Stata software and manual calculations for such cannot be undertaken due to the computational complexity of the task.
Adverse pregnancy outcomes were attributable to maternal obesity for even low-risk patients. This suggests that, in contrast to the existing PHCC protocol which focuses only on high-risk pregnancies for intervention, overweight and women with obesity in Qatar should also be targeted earlier in their pregnancy, preferably prior to getting pregnant. We observed ethnic differences in the risk of adverse pregnancy outcomes. Planning and prevention approaches at the preconception stage are needed to raise awareness and reduce the burden of these adverse outcomes on the healthcare system. There is a need for an ecological approach that addresses societal, cultural and personal influences by promoting good health at all levels. A top-down approach would work best to formulate public health policy to combat the issues raised. A combination of these public health interventions can help achieve the WHO Non-Communicable Disease Targets for the Global Action Plan by 2025.
Supplementary Material
Footnotes
Contributors: Conceptualisation: SS and UN; methodology: SS and UN; software: SS; validation: SS and UN; formal analysis: SS; investigation: SS and UN; resources: SS; data curation: SS; writing—original draft preparation: SS; writing—review and editing: SS and UN; visualisation: SS and UN; supervision: UN; project administration: SS; funding acquisition: UN.
Funding: This work has been funded by Qatar University (grant no: QUST-1-CHS-2018-14) and Qatar National Library.
Disclaimer: All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, has not received prior publication and is not under consideration for publication elsewhere.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was obtained from The Primary Health Care Corporation (Reference Number PHCC/RS/17/07/007) on 3 October 2017 and the Institutional Review Board of Qatar University (Reference QU-IRB 846-E/17) on 11 November 2017.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The database analysed for this research include information on all antenatal visits to Primary Health Care Centres, between 1 June 2016 and 1 March 2017. Data can be obtained by request from the Primary Health Corporation in Qatar.
References
- 1. Finucane MM, Stevens GA, Cowan MJ, et al. . National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. The Lancet 2011;377:557–67. 10.1016/S0140-6736(10)62037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanagalingam MG, Forouhi NG, Greer IA, et al. . Changes in Booking body mass index over a decade: retrospective analysis from a Glasgow maternity hospital. BJOG Int J Obstet Gynaecol 2005;112:1431–3. 10.1111/j.1471-0528.2005.00685.x [DOI] [PubMed] [Google Scholar]
- 3. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017;356 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torloni MR, Betrán AP, Horta BL, et al. . Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev 2009;10:194–203. 10.1111/j.1467-789X.2008.00541.x [DOI] [PubMed] [Google Scholar]
- 5. O'Brien TE, Ray JG, Chan W-S. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology 2003;14:368–74. 10.1097/01.EDE.0000059921.71494.D1 [DOI] [PubMed] [Google Scholar]
- 6. Poobalan AS, Aucott LS, Gurung T, et al. . Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women--systematic review and meta-analysis of cohort studies. Obes Rev 2009;10:28–35. 10.1111/j.1467-789X.2008.00537.x [DOI] [PubMed] [Google Scholar]
- 7. Marchi J, Berg M, Dencker A, et al. . Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16:621–38. 10.1111/obr.12288 [DOI] [PubMed] [Google Scholar]
- 8. Knight M, Kurinczuk JJ, Spark P, et al. . Inequalities in maternal health: national cohort study of ethnic variation in severe maternal morbidities. BMJ 2009;338:b542 10.1136/bmj.b542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG Int J Obstet Gynaecol 2012;119:276–82. 10.1111/j.1471-0528.2011.03156.x [DOI] [PubMed] [Google Scholar]
- 10. WHO Qatar STEPS Survey 2012 - Fact Sheet: World Health Organization, 2012. Available: http://www.who.int/chp/steps/Qatar_FactSheet_2012.pdf2017
- 11. Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993;49:865–72. 10.2307/2532206 [DOI] [PubMed] [Google Scholar]
- 12. Oteng-Ntim E, Kopeika J, Seed P, et al. . Impact of obesity on pregnancy outcome in different ethnic groups: calculating population attributable fractions. PLoS One 2013;8:e53749 10.1371/journal.pone.0053749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. LAS League of Arab states, 2018. Available: http://www.lasportal.org/en/Pages/default.aspx [Accessed 8 March 2018].
- 14. WHO Physical status: The use of and interpretation of anthropometry, Report of a WHO Expert Committee; 1995. [PubMed]
- 15. WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 16. WHO Weight Percentlies calculator. World Health Organization, 2017. https://www.who.int/reproductivehealth/topics/best_practices/weight_percentiles_calculator.xls2017 [Google Scholar]
- 17. Mikolajczyk RT, Zhang J, Betran AP, et al. . A global reference for fetal-weight and birthweight percentiles. The Lancet 2011;377:1855–61. 10.1016/S0140-6736(11)60364-4 [DOI] [PubMed] [Google Scholar]
- 18. Sterne JAC, White IR, Carlin JB, et al. . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. StataCorp LP Stata data analysis and statistical software. Special Edition Release 2017;15:733. [Google Scholar]
- 20. Newson RB. Attributable and Unattributable risks and fractions and other scenario comparisons. Stata J 2013;13:672–98. 10.1177/1536867X1301300402 [DOI] [Google Scholar]
- 21. Stata Data Analysis and Statistical Software | Stata Release 15, 2017. Available: https://www.stata.com/
- 22. El-Chaar D, Finkelstein SA, Tu X, et al. . The impact of increasing obesity class on obstetrical outcomes. J Obstet Gynaecol Can 2013;35:224–33. 10.1016/S1701-2163(15)30994-4 [DOI] [PubMed] [Google Scholar]
- 23. Metsälä J, Stach-Lempinen B, Gissler M, et al. . Risk of pregnancy complications in relation to maternal prepregnancy body mass index: population-based study from Finland 2006-102015. [DOI] [PubMed]
- 24. El Rafei R, Abbas HA, Charafeddine L, et al. . Association of Pre-Pregnancy body mass index and gestational weight gain with preterm births and fetal size: an observational study from Lebanon. Paediatr Perinat Epidemiol 2016;30:38–45. 10.1111/ppe.12249 [DOI] [PubMed] [Google Scholar]
- 25. Li C, Liu Y, Zhang W. Joint and independent associations of gestational weight gain and Pre-Pregnancy body mass index with outcomes of pregnancy in Chinese women: a retrospective cohort study. PLoS One 2015;10:e0136850 10.1371/journal.pone.0136850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Snowden JM, Mission JF, Marshall NE, et al. . The impact of maternal obesity and race/ethnicity on perinatal outcomes: independent and joint effects. Obesity 2016;24:1590–8. 10.1002/oby.21532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharara HA, Rhaman LNA, Ummunnisa F, et al. . Obese nulliparous women and the risk for maternal and fetal complications. Open J Obstet Gynecol 2014;04:239–42. 10.4236/ojog.2014.45039 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.