Abstract
The Elongator complex promotes formation of 5-methoxycarbonylmethyl (mcm5) and 5-carbamoylmethyl (ncm5) side-chains on uridines at the wobble position of cytosolic eukaryotic tRNAs. In all eukaryotic organisms tested to date, the inactivation of Elongator not only leads to the lack of mcm5/ncm5 groups in tRNAs, but also a wide variety of additional phenotypes. Although the phenotypes are most likely caused by a translational defect induced by reduced functionality of the hypomodified tRNAs, the mechanism(s) underlying individual phenotypes are poorly understood. In this study, we show that the genetic background modulates the phenotypes induced by the lack of mcm5/ncm5 groups in Saccharomyces cerevisiae. We show that the stress-induced growth defects of Elongator mutants are stronger in the W303 than in the closely related S288C genetic background and that the phenotypic differences are caused by the known polymorphism at the locus for the mRNA binding protein Ssd1. Moreover, the mutant ssd1 allele found in W303 cells is required for the reported histone H3 acetylation and telomeric gene silencing defects of Elongator mutants. The difference at the SSD1 locus also partially explains why the simultaneous lack of mcm5 and 2-thio groups at wobble uridines is lethal in the W303 but not in the S288C background. Collectively, our results demonstrate that the SSD1 locus modulates phenotypes induced by the lack of Elongator-dependent tRNA modifications.
Author summary
Modified nucleosides in the anticodon region of tRNAs are important for the efficiency and fidelity of translation. The Elongator complex promotes formation of several related modified uridine residues at the wobble position of eukaryotic tRNAs. In yeast, plants, worms, mice and humans, mutations in genes for Elongator subunits lead to a wide variety of different phenotypes. Here, we show that the genetic background modulates the phenotypic consequences of the inactivation of budding yeast Elongator. This background effect is largely a consequence of a polymorphism at the SSD1 locus, encoding a RNA binding protein that influences translation, stability and/or localization of mRNAs. We show that several phenotypes reported for yeast Elongator mutants are either significantly stronger or only detectable in strains harboring a mutant ssd1 allele. Thus, SSD1 is a suppressor of the phenotypes induced by the hypomodification of tRNAs.
Introduction
A general feature of tRNA molecules is that a subset of their nucleosides harbors post-transcriptional modifications. Modified nucleosides are frequently found in the anticodon region of tRNAs, especially at position 34 (the wobble nucleoside) and 37. Modifications at these positions typically influence the decoding properties of tRNAs by improving or restricting anticodon-codon interactions [1, 2]. Uridines present at the wobble position in eukaryotic cytoplasmic tRNAs often harbor a 5-methoxycarbonylmethyl (mcm5) or 5-carbamoylmethyl (ncm5) side-chain and sometimes also a 2-thio (s2) or 2ʹ-O-methyl group [3, 4]. The first step in the synthesis of the mcm5 and ncm5 side-chains requires the Elongator complex, which is composed of six Elp proteins (Elp1-Elp6) [5–9]. Elongator is thought to catalyze the addition of a carboxymethyl (cm) group to the 5-position of the uridine which is then converted to mcm5 by the Trm9/Trm112 complex or to ncm5 by a yet unidentified mechanism [5, 8–12].
In the budding yeast Saccharomyces cerevisiae, the inactivation of any of the six ELP genes (ELP1-ELP6) not only leads to the lack of the mcm5/ncm5 groups but also a slower growth rate and numerous additional phenotypes [5, 13]. These phenotypes include increased sensitivity to elevated temperatures and various chemical stresses as well as defects in transcription, exocytosis, telomeric gene silencing, and protein homeostasis [14–18]. Even though Elongator mutants lack mcm5/ncm5 groups in 11 tRNA species [5, 19], the pleiotropic phenotypes are suppressed by increased expression of various combinations of the hypomodified forms of the three tRNA species that normally harbor a mcm5s2U34 residue, , and [18, 20, 21]. These findings suggest the pleiotropic phenotypes of Elongator mutants are caused by a reduced functionality of the hypomodified , and in translation [20, 21]. The importance of the modified wobble residue in these tRNAs was supported by the finding that strains lacking the s2 group show the same but slightly weaker phenotypes that are also suppressed by increased expression of the three tRNAs [20, 21]. Moreover, ribosome profiling experiments have shown that the inactivation of Elongator causes an accumulation of ribosomes with AAA, CAA or GAA codons in the ribosomal A-site [18, 22, 23]. However, the pausing at the codons appears to be relatively small [18, 22] and the mechanism(s) underlying the pleiotropic phenotypes of Elongator mutants are poorly understood.
In yeast, the cell wall stress that arises during normal growth or through environmental challenges is sensed and responded to by the cell wall integrity (CWI) pathway [24, 25]. The CWI pathway is induced by several different types of stresses, including growth at elevated temperatures, hypo-osmotic shock, and exposure to various cell wall stressing agents [25]. A family of cell surface sensors (Wsc1-Wsc3, Mid2 and Mtl1) detects the cell wall stress and recruits the guanine nucleotide exchange factors Rom1 and Rom2 which activate the small GTPase Rho1. Rho1-GTP binds and activates several effectors, including the kinase Pkc1. Pkc1 activates a downstream MAP kinase cascade comprised of the MAPKKK Bck1, the two redundant MAPKK Mkk1 and Mkk2, and the MAPK Mpk1 (Slt2). The phosphorylated Mpk1 then activates factors that promote transcription of genes important for cell wall biosynthesis and remodeling.
In addition to the CWI pathway, several other factors and pathways are known to influence the cell wall remodeling that occurs upon stress, e.g. the mRNA-binding protein Ssd1. Ssd1 has been reported to bind and influence the translation, stability and/or localization of a subset of cellular mRNAs of which many encode proteins important for cell wall biosynthesis and remodeling [26–31]. The wild-type SSD1 gene was originally identified as a suppressor of the lethality induced by a deletion of the SIT4 gene, which encodes a phosphatase involved in a wide range of cellular processes [32]. The study also led to the finding that some wild-type S. cerevisiae laboratory strains harbor a mutation at the SSD1 locus that is synthetic lethal with the sit4Δ allele [32]. The SSD1 locus has since been genetically implicated in many cellular processes, including cell wall integrity, various signal transduction pathways, cell morphogenesis, cellular aging, virulence, and transcription by RNA polymerase I, II and III [33–38]. Although the mechanisms by which Ssd1 influences these processes are poorly understood, they possibly involve both direct and indirect effects of Ssd1´s influence on messenger ribonucleoprotein (mRNP) complexes [28, 29, 39]. With respect to the transcripts that encode cell wall remodeling factors, Ssd1 seems to act as a translational repressor and this function is controlled by the protein kinase Cbk1, which is a component in the RAM (Regulation of Ace2 and cellular morphogenesis) network [28]. In addition to relieving the translational repression, the phosphorylation of Ssd1 appears to promote polarized localization of some Ssd1-associated mRNAs [28, 31].
In this study, we show that increased activation of the CWI signaling pathway counteracts the temperature sensitive (Ts) growth defect of elp3Δ mutants in the W303 but not in the S288C genetic background. Further, the stress-induced growth phenotypes caused by the tRNA modification defect are generally stronger in W303- than in S288C-derived strains. We show that the phenotypic differences are due to the allelic variation at the SSD1 locus, i.e. the phenotypes are aggravated by the nonsense ssd1-d2 allele found in the W303 background. We also show that the phenotypes linking the tRNA modification defect to histone acetylation and telomeric gene silencing are caused by a synergistic interaction with the ssd1-d2 allele. The difference at the SSD1 locus also provides a partial explanation to the finding [18, 40, 41] that cells lacking both the mcm5 and s2 group are viable in the S288C but not in the W303 background.
Results
The Ts phenotype of W303-derived elp3Δ cells is partially suppressed by increased expression of factors in the CWI signaling pathway
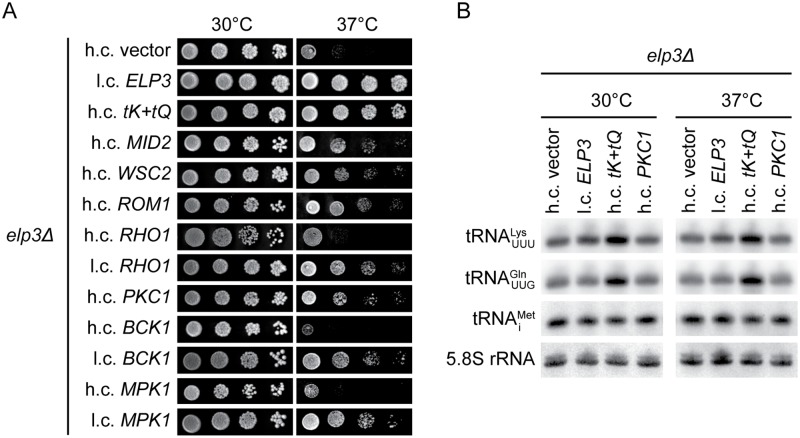
The observation that Elongator mutants are sensitive to cell wall stressing agents, e.g. calcofluor white and caffeine, implies a defect in cell wall integrity [14]. This notion is further supported by the finding that the Ts growth defect of Elongator-deficient cells is partially suppressed by osmotic support (1 M sorbitol) [42]. As the caffeine sensitivity and Ts growth defect are suppressed by increased levels of the hypomodified and [20], the phenotypes are likely caused by reduced functionality of these tRNAs in translation. To further define the wall integrity defect in Elongator mutants, we investigated, in the W303 genetic background, if the Ts phenotype of elp3Δ cells is suppressed by increased expression of factors in the CWI signaling pathway. The analyses revealed that the introduction of a high-copy MID2, WSC2, ROM1, or PKC1 plasmid into the elp3Δ strain partially suppressed the growth defect at 37°C (Fig 1A). No suppression of the phenotype was observed when the cells carried a high-copy RHO1, BCK1 or MPK1 plasmid (Fig 1A). As the overexpression of neither the upstream GTPase (Rho1) nor the downstream kinases (Bck1 and Mpk1) suppressed the Ts phenotype, we considered the possibility that the levels of these factors may be too high when expressed from a high-copy plasmid. Accordingly, low-copy RHO1, BCK1 and MPK1 plasmids suppress the Ts phenotype of elp3Δ cells to a level similar to that observed for the high-copy MID2, WSC2, ROM1, and PKC1 plasmids (Fig 1A). The level of suppression is, however, smaller than that observed for increased tK(UUU) and tQ(UUG) dosage, encoding and , respectively (Fig 1A).
Fig 1. Increased expression of factors in the CWI signaling pathway counteracts the Ts phenotype of W303-derived elp3Δ cells.
(A) Growth of the elp3Δ (UMY3269) strain carrying the indicated high-copy (h.c.) or low-copy (l.c.) LEU2 plasmids. The high copy plasmid carrying the tK(UUU) and tQ(UUG) genes [74] is abbreviated as h.c. tK+tQ. Cells were grown over-night at 30°C in liquid synthetic complete medium lacking leucine (SC-leu), serially diluted, spotted on SC-leu plates, and incubated at 30°C or 37°C for 3 days. (B) Northern analysis of total RNA isolated from elp3Δ (UMY3269) cells carrying the indicated plasmids. The cells were grown in SC-leu medium at 30°C or 37°C. The blot was probed for , , , and 5.8S rRNA using radiolabeled oligonucleotides. 5.8S rRNA serves as the loading control.
Since the Ts phenotype of elp3Δ cells is counteracted by elevated and levels [20], it was possible that the activation of the CWI pathway leads to an increase in their relative abundance. To investigate this possibility, we used northern blotting to analyze the effect of increased PKC1 dosage on the levels of and in elp3Δ cells grown at either 30°C or 37°C. The blots were also probed for and 5.8S rRNA, which served as the loading control. These RNAs were selected as does not contain an Elongator-dependent tRNA modification and 5.8S rRNA is transcribed by a different RNA polymerase (RNA polymerase I). In contrast to the ≈2-fold increase in and levels induced by increased tK(UUU) and tQ(UUG) dosage (Fig 1B and S1 Table), the abundance of the tRNAs was largely unaffected by increased PKC1 dosage at 30°C (Fig 1B and S1 Table). At 37°C, the increased PKC1 dosage correlated with a slight increase in the levels of (Fig 1B and S1 Table). However, a similar effect was observed in the elp3Δ strain complemented with the ELP3 gene, making it difficult to assess if the increase is the cause or the consequence of the improved growth at 37°C. Nevertheless, our results suggest that the Ts phenotype of elp3Δ cells is, at least in the W303 genetic background, partially suppressed by increased activation of the CWI signaling pathway.
The allelic variant at the SSD1 locus modulates the growth phenotypes of elp3Δ cells
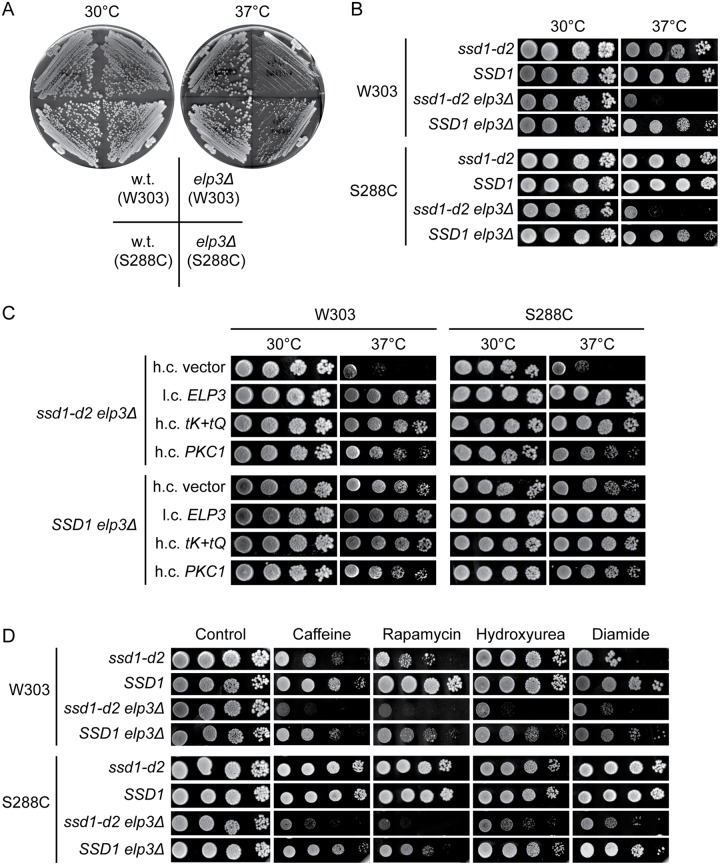
Phenotypes caused by a mutation in an individual gene can be modulated by the genetic background of the cell. In fact, the Ts phenotype induced by an elp3Δ allele is more pronounced in strains derived from W303 than in those from S288C (Fig 2A). As the inactivation of Elongator causes a lack of wobble mcm5/ncm5 groups in both strain backgrounds [5, 43], the Ts phenotype is likely modulated by genetic variation between W303 and S288C. The difference in phenotype (Fig 2A) prompted us to investigate if increased MID2, WSC2, ROM1, RHO1, PKC1, BCK1 or MPK1 dosage counteracts the Ts phenotype of elp3Δ cells in the S288C background. The analyses showed that none of the plasmids counteracted the phenotype (S1A Fig). Moreover, the growth defect of elp3Δ cells at 37°C is counteracted by osmotic support (1 M sorbitol) in the W303, but not in the S288C background (S1B Fig). Importantly, the Ts phenotype of elp3Δ cells is suppressed by increased tK(UUU) and tQ(UUG) dosage in both genetic backgrounds (Fig 1A and S1A Fig), showing that the underlying cause is the hypomodified and . We conclude that the genetic background influences the phenotypes linking the tRNA modification defect to cell wall integrity.
Fig 2. The growth phenotypes elp3Δ cells are modulated by the allele at the SSD1 locus.
(A) Growth of elp3Δ mutants in the W303 and S288C genetic backgrounds. The wild-type (W303-1A and BY4741) and elp3Δ strains (UMY3269 and MJY1036) were streaked on SC plates and incubated at 30°C or 37°C for 3 days. (B) Effects of the ssd1-d2/SSD1 alleles on the growth of elp3Δ strains in the W303 and S288C genetic backgrounds. The ssd1-d2 (W303-1A and UMY4432), SSD1 (UMY3385 and BY4741), ssd1-d2 elp3Δ (UMY3269 and UMY4439) and SSD1 elp3Δ (UMY4456 and MJY1036) strains were grown over-night at 30°C in liquid SC medium, serially diluted, spotted on SC plates, and incubated at 30°C or 37°C for 3 days. (C) Effects of increased PKC1 dosage on the growth of elp3Δ ssd1-d2 and elp3Δ SSD1 strains. The relevant strains (UMY3269, UMY4456, UMY4439, and MJY1036) carrying the indicated plasmids were grown over-night at 30°C in liquid SC-leu medium, serially diluted, spotted on SC-leu plates, and incubated for 3 days at 30°C or 37°C. (D) Influence of the ssd1-d2 allele on growth phenotypes induced by various stress-inducing agents. The strains from B were grown over-night at 30°C in liquid SC medium, serially diluted, and spotted on SC plates and SC plates supplemented with caffeine, rapamycin, hydroxyurea or diamide. The plates were incubated for 3 days at 30°C.
Although W303 is closely related to S288C, comparisons of the genomes identified polymorphisms in ≈800 genes that lead to variations in the amino acid sequence [44, 45]. To identify the cause of the phenotypic differences between the elp3Δ strains, we examined polymorphisms that have been shown to be physiologically relevant. The polymorphism at the SSD1 locus was a good candidate as SSD1 has been genetically implicated in many cellular processes, including the maintenance of cellular integrity [32, 33, 46–50]. Ssd1 is a RNA binding protein that associates with a subset of mRNAs of which many encode proteins important for cell wall biosynthesis and remodeling [26–28]. The SSD1 allele in the S288C background encodes the full-length Ssd1 protein (1250 amino acids) whereas the allele in W303, designated ssd1-d2, contains a nonsense mutation that introduces a premature stop codon at the 698th codon of the open reading frame [32, 35]. To investigate if the allele at the SSD1 locus contributes to the phenotypic differences between the elp3Δ mutants, we analyzed congenic ssd1-d2, SSD1, ssd1-d2 elp3Δ, and SSD1 elp3Δ strains in both genetic backgrounds. By analyzing the growth of the strains, we found that the ssd1-d2 allele augments the Ts phenotype of elp3Δ cells in both backgrounds (Fig 2B and S2 Table). The ssd1-d2 allele also appears to cause a slightly reduced growth rate of strains with a wild-type ELP3 gene at the elevated temperature (S2 Table). Even though the elp3Δ mutants grow slower than the ELP3 strains at 30°C, the effect of the ssd1-d2 allele is less pronounced at that temperature (S2 Table). In both genetic backgrounds, the increased activation of the CWI signaling pathway, through increased PKC1 dosage, suppresses the Ts phenotype of the ssd1-d2 elp3Δ, but not the SSD1 elp3Δ strains (Fig 2C). Although the elp3Δ strains show increased sensitivity to caffeine, irrespective of the allele at the SSD1 locus, the ssd1-d2 elp3Δ cells are in both backgrounds more caffeine-sensitive than the SSD1 elp3Δ cells (Fig 2D). This observation is consistent with the previous finding that the ssd1-d2 allele enhances the growth inhibitory effects of caffeine [32].
The inactivation of Elongator not only leads to increased sensitivity to caffeine, but also to other stress-inducing agents [14, 17, 18]. To investigate if the ssd1-d2 allele influences these phenotypes, we analyzed the growth of the ssd1-d2, SSD1, ssd1-d2 elp3Δ, and SSD1 elp3Δ strains on medium containing rapamycin, hydroxyurea, or diamide. The analyses revealed that the ssd1-d2 allele, irrespective of background, also increases the sensitivity of elp3Δ cells to these compounds (Fig 2D). To ensure that the effect of the ssd1-d2 allele is not restricted to one set of congenic strains, we repeated the growth assays, in both genetic backgrounds, using a second set of ssd1-d2, SSD1, ssd1-d2 elp3Δ, and SSD1 elp3Δ isolates. The stress-induced growth defects of elp3Δ cells were also in these strains augmented by the ssd1-d2 allele (S2 Fig). As translational readthrough of the premature stop codon in the ssd1-d2 mRNA could generate low levels of full-length functional Ssd1 protein, we also investigated the effect of an ssd1Δ allele on the growth phenotypes of elp3Δ mutants. In both genetic backgrounds, the effect of the ssd1Δ allele is comparable to the ssd1-d2 mutation (S3 Fig). Collectively, these results show that the allele at the SSD1 locus influences stress-induced growth defects of elp3Δ cells.
Ssd1 does not appear to influence the abundance, modification, or function of tRNAs
As the phenotypes of Elongator mutants are largely caused by the reduced functionality of the hypomodified and [20], it seemed possible that the ssd1-d2 allele may influence tRNA abundance or function. To investigate if the allele at the SSD1 locus influence tRNA abundance, we used northern blotting to determine the levels of , , and in the ssd1-d2, SSD1, ssd1-d2 elp3Δ, and SSD1 elp3Δ strains. The analyses revealed that the relative levels of the tRNAs are, in both genetic backgrounds, largely unaffected by the allelic variant at the SSD1 locus (S4 Fig and S3 Table). Moreover, HPLC analyses of the nucleoside composition of total tRNA isolated from the strains showed that the levels of ncm5U, mcm5U, and mcm5s2U are comparable in the ssd1-d2 and SSD1 strains and not detectable in the ssd1-d2 elp3Δ and SSD1 elp3Δ mutants (S4 Table). The HPLC analyses also revealed that the allele at the SSD1 locus has no apparent effect on the abundance of other modified nucleosides present in tRNAs (S5 Fig and S4 Table).
To investigate if the allele at the SSD1 locus influences tRNA function, we utilized a +1 frameshifting reporter system [51, 52]. By using this system, it was previously shown that elp3Δ cells show elevated levels of +1 frameshifting on a CUU AAA C frameshifting site [52]. Further, this increase is caused by slow entry of the hypomodified into the ribosomal A-site and the consequent slippage of the P-site tRNA [52]. Although analyses of the ssd1-d2, SSD1, ssd1-d2 elp3Δ, and SSD1 elp3Δ strains confirmed that the elp3Δ mutants show elevated levels of +1 frameshifting on the CUU AAA C sequence, we detected no influence of the allele at the SSD1 locus (Table 1). Thus, Ssd1 does not appear to influence the functionality of the hypomodified .
Table 1. The allele at the SSD1 locus does not influence +1 frameshifting.
| Strain | Percent frameshiftinga |
|---|---|
| ssd1-d2 (W303-1A) | 0.32 ± 0.09 |
| ssd1-d2 elp3Δ (UMY3269) | 2.28 ± 0.33 |
| SSD1 (UMY3385) | 0.20 ± 0.09 |
| SSD1 elp3Δ (UMY4456) | 2.61 ± 0.09 |
a The value for the β-galactosidase activity in cells carrying the frameshift construct (CUU AAA C, pABY2139) was divided by the value for the in-frame control (pABY2144). The values represent the mean from four independent experiments. The standard deviation is indicated.
The ssd1-d2 allele is required for the histone acetylation and telomeric gene silencing defects of elp3Δ mutants
In the W303 background, elp3Δ mutants show reduced acetylation of histone H3 [15]. Although the phenotype was originally thought to reflect a function of Elongator in RNA polymerase II transcription [15], the reduced acetylation of lysine-14 (K14) in histone H3 was subsequently shown to be an indirect consequence of the tRNA modification defect [20]. To investigate if the ssd1-d2 allele contributes to the phenotype, we analyzed, in the W303 background, the histone H3 K14 acetylation levels in the ssd1-d2, ssd1-d2 elp3Δ, SSD1, and SSD1 elp3Δ strains. As previously shown [15, 20], the level of K14 acetylation is lower in the ssd1-d2 elp3Δ mutant than in the ssd1-d2 strain (Fig 3A). However, the SSD1 and SSD1 elp3Δ strains show comparable levels of K14 acetylated histone H3, indicating that it is the combination of the elp3Δ and ssd1-d2 alleles that induces the histone H3 acetylation defect.
Fig 3. The ssd1-d2 allele is required for the histone acetylation and telomeric gene silencing defects of elp3Δ cells.
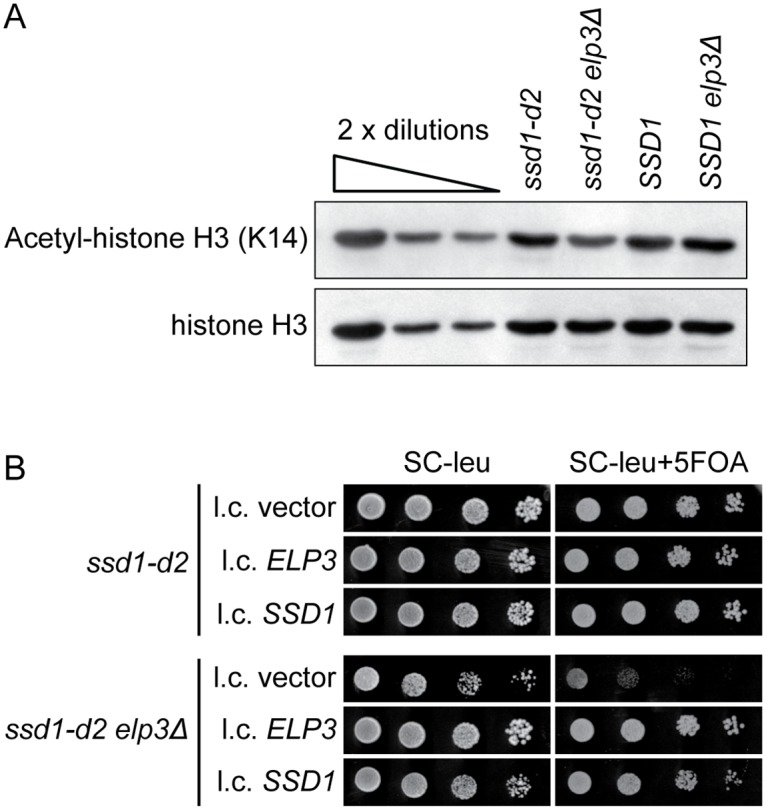
(A) Western analysis of histones isolated from the ssd1-d2 (W303-1A), ssd1-d2 elp3Δ (UMY3269), SSD1 (UMY3385) and SSD1 elp3Δ (UMY4456) strains grown in SC medium at 30°C. Polyclonal anti-acetyl-histone H3 and anti-histone H3 antibodies were used to detect the indicated proteins. The blot is a representative of three independent experiments. (B) Influence of the ssd1-d2 allele on telomeric gene silencing in elp3Δ cells. The ssd1-d2 TELVIIL::URA3 (UMY2584) and ssd1-d2 elp3Δ TELVIIL::URA3 (UMY3790) strains carrying the indicated plasmids were grown over-night at 30°C in liquid SC-leu medium, serially diluted, spotted on SC-leu and SC-leu+5-FOA plates, and incubated for 3 days at 30°C.
W303-derived Elongator mutants have also been reported to show delayed transcriptional activation of the GAL1 and GAL10 genes upon a shift from raffinose- to galactose-containing medium [20, 53]. To determine if the ssd1-d2 allele influences this phenotype, we analyzed the induction of the GAL1 mRNA in the W303-derived ssd1-d2, ssd1-d2 elp3Δ, SSD1, and SSD1 elp3Δ strains. Unexpectedly, we observed no obvious delay in the induction of GAL1 transcripts in the elp3Δ strains regardless of the nature of the allele at the SSD1 locus (S6 Fig). As the phenotype is thought to reflect a reduced ability of Elongator mutants to adapt to new growth conditions [53], it is possible that differences in media or the handling of the cultures can explain why elp3Δ cells show rapid GAL1 induction in our experiments.
Another phenotype reported for Elongator mutants in the W303 background is a defect in telomeric gene silencing [17, 21]. The telomere silencing defect of Elongator mutants was inferred from experiments where the expression of a URA3 gene integrated close to the left telomere of chromosome VII was assayed [17, 21]. The defect in telomeric gene silencing leads to increased expression of the URA3 gene, which is scored as reduced growth on plates containing 5-fluoroorotic acid (5-FOA) [17, 21]. Even though the integration of an SSD1 allele into ssd1-d2 cells does not influence the level of telomeric gene silencing [36], the inactivation of SSD1 does increase the expression of a reporter gene at the silent mating type locus HMR [54]. The latter finding implies that the ssd1-d2 allele may influence the assembly of silent chromatin and consequently contribute to the silencing defect in Elongator mutants. Accordingly, the introduction of a low-copy SSD1 plasmid into the ssd1-d2 elp3Δ TELVIIL::URA3 strain [21] complemented the 5-FOA sensitivity to a level similar to that observed with a plasmid carrying the wild-type ELP3 gene (Fig 3B). Thus, the telomeric gene silencing defect in Elongator mutants is caused by a synergistic interaction between the ssd1-d2 and elp3Δ alleles.
The ssd1-d2 allele augments phenotypes induced by the simultaneous lack of mcm5 and s2 groups
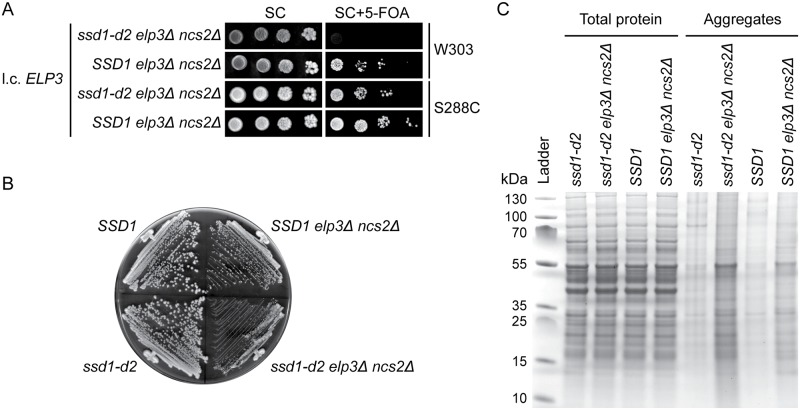
In the formation of mcm5s2U34, Elongator promotes synthesis of the mcm5 side-chain whereas the thiolation of the 2-position is catalyzed by the Ncs2/Ncs6 complex [40, 43, 55–57]. The simultaneous lack of mcm5 and s2 groups was originally reported to be lethal [40]. However, those experiments were performed in the W303 background and more recent studies have shown that strains lacking both groups are viable in the S288C background [18, 41]. To investigate if the allele at the SSD1 locus accounts for the difference in viability, we constructed ssd1-d2 elp3Δ ncs2Δ and SSD1 elp3Δ ncs2Δ strains in both backgrounds all carrying a wild-type ELP3 gene on a low-copy URA3 plasmid. Analyses of the strains revealed that the elp3Δ ncs2Δ double mutant is viable in the W303 background if it encompasses an SSD1 allele (Fig 4A; S7 Fig shows the same plates after a 2-day incubation). In the S288C background, the ssd1-d2 elp3Δ ncs2Δ strain is viable but it grows slower than the SSD1 elp3Δ ncs2Δ strain (Fig 4A and 4B). These observations not only show that allele at the SSD1 locus influences the viability of elp3Δ ncs2Δ cells, but they also indicate that the growth phenotype is modulated by additional genetic factors.
Fig 4. The growth and protein homeostasis defects of elp3Δ ncs2Δ cells are augmented by the ssd1-d2 allele.
(A) Influence of the ssd1-d2 allele on the growth of elp3Δ ncs2Δ cells. The ssd1-d2 elp3Δ ncs2Δ (UMY4454 and MJY1159) and SSD1 elp3Δ ncs2Δ (UMY4467 and MJY1058) strains carrying the l.c. URA3 plasmid pRS316-ELP3 were grown over-night at 30°C in SC medium, serially diluted, spotted on SC and SC+5-FOA plates, and incubated for 3 days at 30°C. (B) Growth of S288C-derived SSD1 elp3Δ ncs2Δ and ssd1-d2 elp3Δ ncs2Δ strains. The wild-type (BY4741 and UMY4432) and elp3Δ ncs2Δ strains (MJY1058 and UMY4449) were streaked on a SC plate and incubated at 30°C for 2 days. (C) Effects of the ssd1-d2 allele on protein aggregation in elp3Δ ncs2Δ cells. Total protein and protein aggregates was analyzed from the ssd1-d2 (UMY4432), ssd1-d2 elp3Δ ncs2Δ (UMY4449), SSD1 (BY4741) and SSD1 elp3Δ ncs2Δ (MJY1058) strains grown in SC medium at 30°C. The gel is a representative of two independent experiments.
The lack of the mcm5 and/or s2 groups has, in the S288C background, been shown to correlate with an increased accumulation of protein aggregates [18]. This effect was most pronounced in a strain lacking both groups and the phenotype was suggested to be a consequence of co-translational misfolding due to slower decoding of AAA and CAA codons by the hypomodified and [18]. Moreover, the increased load of aggregates during normal growth was proposed to account for observation that the double mutant is impaired in clearing diamide-induced protein aggregates [18]. As strains deleted for SSD1 show a defect in the disaggregation of heat-shock-induced protein aggregates [58], it seemed possible that the ssd1-d2 allele would augment the protein homeostasis defect in elp3Δ ncs2Δ cells. To investigate this possibility, we isolated aggregates [18, 59] from the ssd1-d2, SSD1, ssd1-d2 elp3Δ ncs2Δ, and SSD1 elp3Δ ncs2Δ strains. Analyses of the insoluble fractions revealed that the levels of aggregated proteins are comparable in the ssd1-d2 and SSD1 strains (Fig 4C). However, the ssd1-d2 elp3Δ ncs2Δ mutant shows increased accumulation of aggregates compared to the SSD1 elp3Δ ncs2Δ strain (Fig 4C). Thus, the allele at the SSD1 locus modulates the protein homeostasis defect induced by the simultaneous lack of the wobble mcm5 and s2 groups.
Discussion
The phenotypic penetrance of a mutation is often impacted by the genetic background, a phenomenon frequently observed in monogenic diseases [60, 61]. In this study, we investigate the effect of genetic background on the phenotypes of S. cerevisiae mutants defective in the formation of modified wobble uridines in tRNAs. We show that the phenotypes of Elongator mutants are augmented by the ssd1-d2 allele found in some wild-type laboratory strains. Moreover, the histone H3 acetylation and telomeric gene silencing defects reported for Elongator mutants are only observed in cells harboring the ssd1-d2 allele. Thus, the ssd1-d2 allele sensitizes yeast cells to the effects induced by the lack of mcm5/ncm5 groups in U34-containing tRNAs.
Although the pleiotropic phenotypes of Elongator mutants are largely caused by the reduced functionality of the hypomodified , and , the basis for individual phenotypes is poorly understood. Several not necessarily mutually exclusive models have been proposed to explain how the lack of the mcm5/ncm5 groups can lead to a particular phenotype. One model postulates that phenotypes can be induced by inefficient translation of mRNAs enriched for AAA, CAA and/or GAA codons and the consequent effects on the abundance of the encoded factors [21, 62–64]. In this model, the inefficient decoding of the mRNAs leads to reduced protein output without affecting transcript abundance. The mechanism by which the slower decoding of the codons leads to reduced protein levels is unclear, but it may involve the inhibition of translation initiation by the queuing of ribosomes. Alternative models suggest that the phenotypes can be caused by defects in protein homeostasis and/or by indirect effects on transcription [18, 22]. The inactivation of SSD1 not only influences translation and stability of the transcripts normally bound by Ssd1, but it also leads to altered abundance of many transcripts that do not appear to be Ssd1-associated [28, 39]. Thus, the effects of the ssd1-d2 allele on the phenotypes of elp3Δ cells could be due to either direct or indirect effects on gene expression. Moreover, the ribosome profiling experiments of Elongator mutants have been performed in the S288C background [18, 22, 23] and it remains possible that the lack of Ssd1 influences decoding of the AAA, CAA, and GAA codons. However, we observed no apparent effect of the ssd1-d2 allele on the +1 frameshifting induced by the lack of Elongator (Table 1), indicating that Ssd1 does not influence the A-site selection rate.
While the difference at the SSD1 locus partially explains the nonviability of elp3Δ ncs2Δ cells in the W303 background, the W303-derived SSD1 elp3Δ ncs2Δ strain grows slower than the corresponding strain in the S288C background (S7 Fig). Moreover, the ssd1-d2 elp3Δ ncs2Δ strain is viable, although with a growth defect, in the S288C background. These findings indicate that the growth phenotypes of elp3Δ ncs2Δ cells strains are modulated by additional genetic factors. Consistent with the finding that ssd1Δ cells show a defect in Hsp104-mediated protein disaggregation [58], the ssd1-d2 elp3Δ ncs2Δ strain shows increased accumulation of protein aggregates compared to the SSD1 elp3Δ ncs2Δ strain in the S288C background (Fig 4C). It is, however, unclear if this increase is the cause or the consequence of the reduced growth of the ssd1-d2 elp3Δ ncs2Δ strain.
Materials and methods
Yeast strains, plasmids, media and genetic procedures
Strains and plasmids used in this study are listed in S5 and S6 Tables. Yeast media were prepared as described [65, 66]. The medium was where appropriate supplemented with 2.5 ng/ml rapamycin (R0395, Sigma-Aldrich), 7 mM caffeine (C0750, Sigma-Aldrich), 100 mM hydroxyurea (H8627, Sigma-Aldrich), 0.25 mg/ml diamide (D3648, Sigma-Aldrich), or 1 mg/ml 5-fluoroorotic acid (R0812, Thermo Fisher).
To generate ssd1-d2 derivatives of BY4741 and BY4742 (S288C background) [67], we first replaced the sequence between position 2907 and 3315 of the SSD1 ORF with a URA3 gene PCR-amplified from pRS316 [68]. The oligonucleotides used for strain constructions are described in S7 Table. The generated strains were transformed with an ssd1-d2 DNA fragment PCR-amplified from W303-1A [69]. Following selection on 5-fluoroorotic acid (5-FOA)-containing plates and subsequent single cell streaks, individual clones were screened for the integration of ssd1-d2 allele by PCR and DNA sequencing. The generated strains (UMY4432 and UMY4433) were allowed to mate producing the homozygous ssd1-d2/ssd1-d2 strain (UMY4434).
Strains deleted for SSD1, ELP3, or NCS2 were constructed by transforming the appropriate diploid (UMY3387, UMY2836 or UMY4434) with an ssd1::KanMX4, elp3::KanMX4, or ncs2::KanMX4 DNA fragment with appropriate homologies. The DNA fragments were PCR-amplified from ssd1::KanMX4 (Open Biosystems deletion collection), elp3::KanMX4 (UMY3269), or ncs2::KanMX4 (UMY3442) strains. Following PCR confirmation of the deletion, the generated heterozygous diploids were allowed to sporulate and the W303 ssd1Δ (UMY4558), S288C ssd1Δ (UMY4559), W303 elp3Δ SSD1 (UMY4456 and UMY4457), W303 ncs2Δ SSD1 (MJY1019), S288C elp3Δ ssd1-d2 (UMY4438 and UMY4439), S288C ncs2Δ ssd1-d2 (UMY4442), S288C elp3Δ SSD1 (MJY1036), and S288C ncs2Δ SSD1 (MJY1021 strains were obtained from tetrads. The ssd1Δ elp3Δ mutants (MJY1227 and UMY4574) were obtained from crosses between the relevant ssd1Δ and elp3Δ strains. The elp3Δ ncs2Δ SSD1 mutants (MJY1058 and UMY4467) were obtained from crosses between the relevant strains. The diploids used to generate the elp3Δ ncs2Δ ssd1-d2 strains (MJY1159 and UMY4454) were transformed with pRS316-ELP3 [40] before sporulation. MJY1159 was able to lose the plasmid generating strain UMY4449.
To construct plasmids carrying individual genes for factors in the CWI signaling pathway, we PCR-amplified the gene of interest using oligonucleotides that introduce appropriate restriction sites (S7 Table). The DNA fragment was then cloned into the corresponding sites of pRS425 [70] or pRS315 [68].
RNA methods
The abundance of individual tRNA species was determined in total RNA isolated from exponentially growing cultures at an optical density at 600 nm (OD600) of ≈0.5 [66]. Samples containing 10 μg of total RNA were separated on 8M urea-containing 8% polyacrylamide gels followed by electroblotting to Zeta-probe membranes (Bio-Rad). The blots were sequentially probed for , , , and 5.8S rRNA using 32P-labeled oligonucleotides (S7 Table). Signals were detected and analyzed by phosphorimaging using a Typhoon FLA 9500 biomolecular imager and Quantity One software.
To analyze the induction of GAL1 transcripts, cells were grown at 30°C in 50 ml synthetic complete (SC) medium containing 2% raffinose (SC/Raf) to OD600≈0.45. The culture was harvested by centrifugation at 1,500 x g for 5 min at room temperature, and the cell pellet resuspended in 15 ml pre-warmed (30°C) SC/Raf medium. Following reincubation in the shaking water bath for 10 min, transcription of GAL1 was induced by the addition of 1.5 ml pre-warmed 20% galactose. Aliquots were harvested [66] at various time points after the addition of galactose and the cell pellets frozen on dry ice. The procedures for determining mRNA levels have been described [66].
To analyze the nucleoside composition of total tRNA, the tRNA was isolated from exponentially growing cultures at OD600≈0.8 [21]. The tRNA was digested to nucleosides using nuclease P1 (Sigma-Aldrich, N8630) and bacterial alkaline phosphatase (Sigma-Aldrich, P4252) and the hydrolysate analyzed by HPLC [71, 72]. The compositions of the elution buffers were as described [72] with the difference that methanol concentration in buffer A was changed to 5% (v/v).
β-galactosidase assays
Cells transformed with pABY2139 or pABY2144 were grown in synthetic complete medium lacking uracil (SC-ura) to OD600≈0.5. Cells representing 10 OD units were harvested and the β-galactosidase activity determined in protein extracts as described previously [65]. The +1 frameshifting levels were determined by dividing the β-galactosidase activity in extracts of cells containing the frameshift construct (pABY2139) with that of cells containing the in-frame control (pABY2144).
Histone preparation and immunoblot analyses
Histones were isolated from cells grown in SC medium at 30°C to OD600≈0.8. Cells representing 100 OD600 units were harvested, washed once with water, resuspended in 30 ml of buffer A (0.1 mM Tris-HCl at pH 9.4, 10 mM DTT), and incubated on a rotator at 30°C for 15 min. Cells were collected, washed with 30 ml buffer B (1 M Sorbitol, 20 mM HEPES at pH7.4) and resuspended in 25 ml of buffer B containing 600 U yeast lytic enzyme. After 1 hour incubation on a rotator at 30°C, the sample was mixed with 25 ml of ice-cold buffer C (1 M Sorbitol, 20 mM PIPES at pH 6.8, 1 mM MgCl2) followed by centrifugation at 1,500 x g for 5 min. The pellet was resuspended in 40 ml nuclei isolation buffer [73] and the suspension incubated with gentle mixing at 4°C for 30 min. Cell debris were removed by centrifugation at 1,500 x g for 5 min. The supernatant was homogenized with 5 strokes in a Dounce homogenizer followed by centrifugation at 20,000 x g for 10 min. Histones in the pelleted nuclei were extracted by re-suspension in 5 ml of cold 0.2 M H2SO4 and overnight incubation on a rotator at 4°C. After centrifugation at 10,000 x g for 10 min, proteins in the supernatant were precipitated by adding 0.5 volumes of 100% trichloroacetic and 30 min of incubation on ice. Following centrifugation, the pellet was washed twice with acetone and then dissolved in 200 μl 10 mM Tris-HCl at pH 8.0. Fractions (10 μl) were resolved by 15% SDS-PAGE and transferred to Immobilon-P (Millipore) membranes. The blots were incubated with rabbit anti-acetyl-histone H3 (Lys14) antibodies (1:1,000 dilution, Millipore, 07–353) and then with horseradish peroxidase-linked donkey anti-rabbit IgG (NA934, GE Healthcare). Blots were stripped and reprobed with rabbit anti-histone H3 antibodes (1:5,000 dilution, Millipore, 07–690). Proteins were detected using ECL Western blotting detection reagents (GE Healthcare, RPN2209) and Amersham Hyperfilm ECL (GE Healthcare, 28906836).
Analysis of protein aggregates
Protein aggregates were analyzed in exponentially growing cultures in SC medium at 30°C. Cells representing 50 OD600 units were harvested at OD600≈0.5 and protein aggregates were isolated [18, 59] from samples containing 5 mg of total protein. 1/10 of the aggregates and 5μg of total protein were resolved on a 4–12% NuPAGE Bis-Tris gel (Thermo Fisher, NP0321BOX) followed by staining with the Colloidal Blue Staining Kit (Thermo Fisher, LC6025).
Supporting information
(A) Growth of the elp3Δ (MJY1036) strain carrying the indicated high-copy (h.c.) or low-copy (l.c.) LEU2 plasmids. Cells were grown over-night at 30°C in liquid SC-leu medium, serially diluted, spotted on SC-leu plates, and incubated at 30°C or 37°C for 3 days. (B) The wild-type (W303-1A and BY4741) and elp3Δ (UMY3269 and MJY1036) strains were grown over-night at 30°C in liquid SC medium, serially diluted, and spotted on SC plates and SC plates supplemented with 1M sorbitol. The plates were incubated for 3 days at 30°C or 37°C.
(PDF)
The ssd1-d2 (W303-1B and UMY4433), SSD1 (UMY3386 and BY4742), ssd1-d2 elp3Δ (UMY2843 and UMY4438) and SSD1 elp3Δ (UMY4457 and MJY1037) strains were grown over-night at 30°C in liquid SC medium, serially diluted, and spotted on SC plates and SC plates supplemented with caffeine, rapamycin, hydroxyurea, or diamide. The plates were incubated at 30°C or 37°C for 3 days.
(PDF)
The ssd1-d2 (W303-1A and UMY4432), ssd1Δ (UMY4558 and UMY4559), SSD1 (UMY3385 and BY4741), ssd1-d2 elp3Δ (UMY3269 and UMY4439), ssd1Δ elp3Δ (UMY4574 and MJY1227), and SSD1 elp3Δ (UMY4456 and MJY1036) strains were grown over-night at 30°C in liquid SC medium, serially diluted, and spotted on SC plates and SC plates supplemented with caffeine, rapamycin, hydroxyurea, or diamide. The plates were incubated for 3 days at 30°C or 37°C.
(PDF)
Northern analysis of total RNA isolated from the ssd1-d2 (W303-1A and UMY4432), ssd1-d2 elp3Δ (UMY3269 and UMY4439), SSD1 (UMY3385 and BY4741), and SSD1 elp3Δ (UMY4456 and MJY1036) strains grown in SC medium at 30°C. The blot was probed for , , , and 5.8S rRNA using radiolabeled oligonucleotides.
(PDF)
The peaks representing ncm5U, mcm5U, mcm5s2U, pseudouridine (Ψ), cytidine (C), uridine (U), guanosine (G), adenosine (A), 1-methyladenosine (m1A), 5-methylcytidine (m5C), 2'-O-methylcytidine (Cm), 1-methylguanosine (m1G), N2-methylguanosine (m2G), N4-acetylcytidine (ac4C), and N2, N2-dimethylguanosine () are indicated. The asterisk indicates a peak that is a contamination from the bacterial alkaline phosphatase.
(PDF)
Northern analysis of total RNA isolated from the ssd1-d2 (W303-1A), ssd1-d2 elp3Δ (UMY3269), SSD1 (UMY3385) and SSD1 elp3Δ (UMY4456) strains. Cells were grown in SC medium containing 2% raffinose followed by induction of GAL1 transcription by the addition of 0.1 volumes 20% galactose. Time points after the addition of galactose are indicated above the lanes. The blot was probed for GAL1 transcripts using a randomly labelled DNA fragment. 18S rRNA was detected using a oligonucleotide probe. The blot is a representative of two independent experiments.
(PDF)
The figure shows a shorter incubation (2 days) of the plates in Fig 4A.
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank members of M.J.’s and A.B.’s laboratories for valuable discussions.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Magnus Bergvalls Foundation (2017-02098 to MJOJ); Åke Wibergs Foundation (M14-0207 to MJOJ); Swedish Research Council (621-2016-03949 to ASB); and Karin and Harald Silvanders Foundation/Insamlingsstiftelsen Umeå universitet (FS 2.1.6-1870-16 to ASB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Björk GR, Hagervall TG. Transfer RNA Modification: Presence, Synthesis, and Function. EcoSal Plus. 2014;6 10.1128/ecosalplus.ESP-0007-2013 [DOI] [PubMed] [Google Scholar]
- 2.Agris PF, Narendran A, Sarachan K, Vare VYP, Eruysal E. The Importance of Being Modified: The Role of RNA Modifications in Translational Fidelity. Enzymes. 2017;41: 1–50. 10.1016/bs.enz.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machnicka MA, Olchowik A, Grosjean H, Bujnicki JM. Distribution and frequencies of post-transcriptional modifications in tRNAs. RNA Biol. 2014;11: 1619–29. 10.4161/15476286.2014.992273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes Dev. 2010;24: 1832–60. 10.1101/gad.1956510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang B, Johansson MJO, Byström AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11: 424–36. 10.1261/rna.7247705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkler GS, Petrakis TG, Ethelberg S, Tokunaga M, Erdjument-Bromage H, Tempst P, et al. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J Biol Chem. 2001;276: 32743–9. 10.1074/jbc.M105303200 [DOI] [PubMed] [Google Scholar]
- 7.Dauden MI, Jaciuk M, Muller CW, Glatt S. Structural asymmetry in the eukaryotic Elongator complex. FEBS Lett. 2018;592: 502–15. 10.1002/1873-3468.12865 [DOI] [PubMed] [Google Scholar]
- 8.Kolaj-Robin O, Seraphin B. Structures and Activities of the Elongator Complex and Its Cofactors. Enzymes. 2017;41: 117–49. 10.1016/bs.enz.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Johansson MJO, Xu F, Byström AS. Elongator-a tRNA modifying complex that promotes efficient translational decoding. Biochim Biophys Acta Gene Regul Mech. 2018;1861: 401–8. 10.1016/j.bbagrm.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 10.Kalhor HR, Clarke S. Novel methyltransferase for modified uridine residues at the wobble position of tRNA. Mol Cell Biol. 2003;23: 9283–92. 10.1128/MCB.23.24.9283-9292.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazauric MH, Dirick L, Purushothaman SK, Björk GR, Lapeyre B. Trm112p is a 15-kDa zinc finger protein essential for the activity of two tRNA and one protein methyltransferases in yeast. J Biol Chem. 2010;285: 18505–15. 10.1074/jbc.M110.113100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Huang B, Anderson JT, Byström AS. Unexpected accumulation of ncm(5)U and ncm(5)S(2) (U) in a trm9 mutant suggests an additional step in the synthesis of mcm(5)U and mcm(5)S(2)U. PLoS One. 2011;6: e20783 10.1371/journal.pone.0020783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsborn T, Tukenmez H, Mahmud AK, Xu F, Xu H, Byström AS. Elongator, a conserved complex required for wobble uridine modifications in eukaryotes. RNA Biol. 2014;11: 1519–28. 10.4161/15476286.2014.992276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frohloff F, Fichtner L, Jablonowski D, Breunig KD, Schaffrath R. Saccharomyces cerevisiae Elongator mutations confer resistance to the Kluyveromyces lactis zymocin. EMBO J. 2001;20: 1993–2003. 10.1093/emboj/20.8.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkler GS, Kristjuhan A, Erdjument-Bromage H, Tempst P, Svejstrup JQ. Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc Natl Acad Sci U S A. 2002;99: 3517–22. 10.1073/pnas.022042899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahl PB, Chen CZ, Collins RN. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol Cell. 2005;17: 841–53. 10.1016/j.molcel.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Fazly AM, Zhou H, Huang S, Zhang Z, Stillman B. The elongator complex interacts with PCNA and modulates transcriptional silencing and sensitivity to DNA damage agents. PLoS Genet. 2009;5: e1000684 10.1371/journal.pgen.1000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedialkova DD, Leidel SA. Optimization of Codon Translation Rates via tRNA Modifications Maintains Proteome Integrity. Cell. 2015;161: 1606–18. 10.1016/j.cell.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson MJO, Esberg A, Huang B, Björk GR, Byström AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol. 2008;28: 3301–12. 10.1128/MCB.01542-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esberg A, Huang B, Johansson MJO, Byström AS. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell. 2006;24: 139–48. 10.1016/j.molcel.2006.07.031 [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Huang B, Eliasson M, Ryden P, Byström AS. Elongator complex influences telomeric gene silencing and DNA damage response by its role in wobble uridine tRNA modification. PLoS Genet. 2011;7: e1002258 10.1371/journal.pgen.1002258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinshteyn B, Gilbert WV. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet. 2013;9: e1003675 10.1371/journal.pgen.1003675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou HJ, Donnard E, Gustafsson HT, Garber M, Rando OJ. Transcriptome-wide Analysis of Roles for tRNA Modifications in Translational Regulation. Mol Cell. 2017;68: 978–92 e4. 10.1016/j.molcel.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69: 262–91. 10.1128/MMBR.69.2.262-291.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin DE. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189: 1145–75. 10.1534/genetics.111.128264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uesono Y, Toh-e A, Kikuchi Y. Ssd1p of Saccharomyces cerevisiae associates with RNA. J Biol Chem. 1997;272: 16103–9. 10.1074/jbc.272.26.16103 [DOI] [PubMed] [Google Scholar]
- 27.Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6: e255 10.1371/journal.pbio.0060255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen JM, Wanless AG, Seidel CW, Weiss EL. Cbk1 regulation of the RNA-binding protein Ssd1 integrates cell fate with translational control. Curr Biol. 2009;19: 2114–20. 10.1016/j.cub.2009.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohyama Y, Kasahara K, Kokubo T. Saccharomyces cerevisiae Ssd1p promotes CLN2 expression by binding to the 5'-untranslated region of CLN2 mRNA. Genes Cells. 2010;15: 1169–88. 10.1111/j.1365-2443.2010.01452.x [DOI] [PubMed] [Google Scholar]
- 30.Wanless AG, Lin Y, Weiss EL. Cell morphogenesis proteins are translationally controlled through UTRs by the Ndr/LATS target Ssd1. PLoS One. 2014;9: e85212 10.1371/journal.pone.0085212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurischko C, Kim HK, Kuravi VK, Pratzka J, Luca FC. The yeast Cbk1 kinase regulates mRNA localization via the mRNA-binding protein Ssd1. J Cell Biol. 2011;192: 583–98. 10.1083/jcb.201011061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton A, Immanuel D, Arndt KT. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol Cell Biol. 1991;11: 2133–48. 10.1128/mcb.11.4.2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaeberlein M, Guarente L. Saccharomyces cerevisiae MPT5 and SSD1 function in parallel pathways to promote cell wall integrity. Genetics. 2002;160: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson RB, Brenner AA, White TB, Engler MJ, Gaughran JP, Tatchell K. The Saccharomyces cerevisiae SRK1 gene, a suppressor of bcy1 and ins1, may be involved in protein phosphatase function. Mol Cell Biol. 1991;11: 3369–73. 10.1128/mcb.11.6.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorgensen P, Nelson B, Robinson MD, Chen Y, Andrews B, Tyers M, et al. High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics. 2002;162: 1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaeberlein M, Andalis AA, Liszt GB, Fink GR, Guarente L. Saccharomyces cerevisiae SSD1-V confers longevity by a Sir2p-independent mechanism. Genetics. 2004;166: 1661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stettler S, Chiannilkulchai N, Hermann-Le Denmat S, Lalo D, Lacroute F, Sentenac A, et al. A general suppressor of RNA polymerase I, II and III mutations in Saccharomyces cerevisiae. Mol Gen Genet. 1993;239: 169–76. 10.1007/bf00281615 [DOI] [PubMed] [Google Scholar]
- 38.Wheeler RT, Kupiec M, Magnelli P, Abeijon C, Fink GR. A Saccharomyces cerevisiae mutant with increased virulence. Proc Natl Acad Sci U S A. 2003;100: 2766–70. 10.1073/pnas.0437995100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Lu Y, Qin LX, Bar-Joseph Z, Werner-Washburne M, Breeden LL. Budding yeast SSD1-V regulates transcript levels of many longevity genes and extends chronological life span in purified quiescent cells. Mol Biol Cell. 2009;20: 3851–64. 10.1091/mbc.E09-04-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Björk GR, Huang B, Persson OP, Byström AS. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA. 2007;13: 1245–55. 10.1261/rna.558707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klassen R, Grunewald P, Thuring KL, Eichler C, Helm M, Schaffrath R. Loss of anticodon wobble uridine modifications affects tRNA(Lys) function and protein levels in Saccharomyces cerevisiae. PLoS One. 2015;10: e0119261 10.1371/journal.pone.0119261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jablonowski D, Frohloff F, Fichtner L, Stark MJ, Schaffrath R. Kluyveromyces lactis zymocin mode of action is linked to RNA polymerase II function via Elongator. Mol Microbiol. 2001;42: 1095–105. 10.1046/j.1365-2958.2001.02705.x [DOI] [PubMed] [Google Scholar]
- 43.Huang B, Lu J, Byström AS. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14: 2183–94. 10.1261/rna.1184108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ralser M, Kuhl H, Ralser M, Werber M, Lehrach H, Breitenbach M, et al. The Saccharomyces cerevisiae W303-K6001 cross-platform genome sequence: insights into ancestry and physiology of a laboratory mutt. Open Biol. 2012;2: 120093 10.1098/rsob.120093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matheson K, Parsons L, Gammie A. Whole-Genome Sequence and Variant Analysis of W303, a Widely-Used Strain of Saccharomyces cerevisiae. G3 (Bethesda). 2017;7: 2219–26. 10.1534/g3.117.040022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costigan C, Gehrung S, Snyder M. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol Cell Biol. 1992;12: 1162–78. 10.1128/mcb.12.3.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee KS, Irie K, Gotoh Y, Watanabe Y, Araki H, Nishida E, et al. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol Cell Biol. 1993;13: 3067–75. 10.1128/mcb.13.5.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzoni C, Zarzov P, Rambourg A, Mann C. The Slt2 (Mpk1) map kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J Cell Biol. 1993;123: 1821–33. 10.1083/jcb.123.6.1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin H, Castellanos MC, Cenamor R, Sanchez M, Molina M, Nombela C. Molecular and functional characterization of a mutant allele of the mitogen-activated protein-kinase gene SLT2(MPK1) rescued from yeast autolytic mutants. Curr Genet. 1996;29: 516–22. [DOI] [PubMed] [Google Scholar]
- 50.Ibeas JI, Yun DJ, Damsz B, Narasimhan ML, Uesono Y, Ribas JC, et al. Resistance to the plant PR-5 protein osmotin in the model fungus Saccharomyces cerevisiae is mediated by the regulatory effects of SSD1 on cell wall composition. Plant J. 2001;25: 271–80. [DOI] [PubMed] [Google Scholar]
- 51.Belcourt MF, Farabaugh PJ. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell. 1990;62: 339–52. 10.1016/0092-8674(90)90371-k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tukenmez H, Xu H, Esberg A, Byström AS. The role of wobble uridine modifications in +1 translational frameshifting in eukaryotes. Nucleic Acids Res. 2015;43: 9489–99. 10.1093/nar/gkv832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otero G, Fellows J, Li Y, de Bizemont T, Dirac AM, Gustafsson CM, et al. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol Cell. 1999;3: 109–18. [DOI] [PubMed] [Google Scholar]
- 54.Raisner RM, Madhani HD. Genomewide screen for negative regulators of sirtuin activity in Saccharomyces cerevisiae reveals 40 loci and links to metabolism. Genetics. 2008;179: 1933–44. 10.1534/genetics.108.088443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noma A, Sakaguchi Y, Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37: 1335–52. 10.1093/nar/gkn1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leidel S, Pedrioli PG, Bucher T, Brost R, Costanzo M, Schmidt A, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458: 228–32. 10.1038/nature07643 [DOI] [PubMed] [Google Scholar]
- 57.Nakai Y, Nakai M, Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J Biol Chem. 2008;283: 27469–76. 10.1074/jbc.M804043200 [DOI] [PubMed] [Google Scholar]
- 58.Mir SS, Fiedler D, Cashikar AG. Ssd1 is required for thermotolerance and Hsp104-mediated protein disaggregation in Saccharomyces cerevisiae. Mol Cell Biol. 2009;29: 187–200. 10.1128/MCB.02271-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koplin A, Preissler S, Ilina Y, Koch M, Scior A, Erhardt M, et al. A dual function for chaperones SSB-RAC and the NAC nascent polypeptide-associated complex on ribosomes. J Cell Biol. 2010;189: 57–68. 10.1083/jcb.200910074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kammenga JE. The background puzzle: how identical mutations in the same gene lead to different disease symptoms. FEBS J. 2017;284: 3362–73. 10.1111/febs.14080 [DOI] [PubMed] [Google Scholar]
- 61.Hou J, van Leeuwen J, Andrews BJ, Boone C. Genetic Network Complexity Shapes Background-Dependent Phenotypic Expression. Trends Genet. 2018;34: 578–86. 10.1016/j.tig.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rezgui VA, Tyagi K, Ranjan N, Konevega AL, Mittelstaet J, Rodnina MV, et al. tRNA tKUUU, tQUUG, and tEUUC wobble position modifications fine-tune protein translation by promoting ribosome A-site binding. Proc Natl Acad Sci U S A. 2013;110: 12289–94. 10.1073/pnas.1300781110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bauer F, Matsuyama A, Candiracci J, Dieu M, Scheliga J, Wolf DA, et al. Translational control of cell division by Elongator. Cell Rep. 2012;1: 424–33. 10.1016/j.celrep.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez-Vazquez J, Vargas-Perez I, Sanso M, Buhne K, Carmona M, Paulo E, et al. Modification of tRNA(Lys) UUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet. 2013;9: e1003647 10.1371/journal.pgen.1003647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amberg DC, Burke DJ, Strathern JN. Methods in Yeast Genetics. Cold Spring Harbor, N Y: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- 66.Johansson MJO. Determining if an mRNA is a Substrate of Nonsense-Mediated mRNA Decay in Saccharomyces cerevisiae In: Wajapeyee N, Gupta R, editors. Eukaryotic Transcriptional and Post-Transcriptional Gene Expression Regulation. Methods Mol Biol. 1507. New York, NY: Humana Press; 2017. pp. 169–77. [DOI] [PubMed] [Google Scholar]
- 67.Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14: 115–32. [DOI] [PubMed] [Google Scholar]
- 68.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiorentini P, Huang KN, Tishkoff DX, Kolodner RD, Symington LS. Exonuclease I of Saccharomyces cerevisiae functions in mitotic recombination in vivo and in vitro. Mol Cell Biol. 1997;17: 2764–73. 10.1128/mcb.17.5.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110: 119–22. 10.1016/0378-1119(92)90454-w [DOI] [PubMed] [Google Scholar]
- 71.Gehrke CW, Kuo KC. Ribonucleoside Analysis by Reversed-Phase High Performance Liquid Chromatography In: Gehrke CW, Kuo KC, editors. Journal of Chromatography Library. Chromatography and Modification of Nucleosides Analytical Methods for Major and Modified Nucleosides: HPLC, GC, MS, NMR, UV and FT-IR. Amsterdam: Elsevier; 1990. pp. A3–A71. [Google Scholar]
- 72.Xu F, Zhou Y, Byström AS, Johansson MJO. Identification of factors that promote biogenesis of tRNACGA(Ser). RNA Biol. 2018;15: 1286–94. 10.1080/15476286.2018.1526539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edmondson DG, Smith MM, Roth SY. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 1996;10: 1247–59. 10.1101/gad.10.10.1247 [DOI] [PubMed] [Google Scholar]
- 74.Lu J, Huang B, Esberg A, Johansson MJO, Byström AS. The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA. 2005;11: 1648–54. 10.1261/rna.2172105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Growth of the elp3Δ (MJY1036) strain carrying the indicated high-copy (h.c.) or low-copy (l.c.) LEU2 plasmids. Cells were grown over-night at 30°C in liquid SC-leu medium, serially diluted, spotted on SC-leu plates, and incubated at 30°C or 37°C for 3 days. (B) The wild-type (W303-1A and BY4741) and elp3Δ (UMY3269 and MJY1036) strains were grown over-night at 30°C in liquid SC medium, serially diluted, and spotted on SC plates and SC plates supplemented with 1M sorbitol. The plates were incubated for 3 days at 30°C or 37°C.
(PDF)
The ssd1-d2 (W303-1B and UMY4433), SSD1 (UMY3386 and BY4742), ssd1-d2 elp3Δ (UMY2843 and UMY4438) and SSD1 elp3Δ (UMY4457 and MJY1037) strains were grown over-night at 30°C in liquid SC medium, serially diluted, and spotted on SC plates and SC plates supplemented with caffeine, rapamycin, hydroxyurea, or diamide. The plates were incubated at 30°C or 37°C for 3 days.
(PDF)
The ssd1-d2 (W303-1A and UMY4432), ssd1Δ (UMY4558 and UMY4559), SSD1 (UMY3385 and BY4741), ssd1-d2 elp3Δ (UMY3269 and UMY4439), ssd1Δ elp3Δ (UMY4574 and MJY1227), and SSD1 elp3Δ (UMY4456 and MJY1036) strains were grown over-night at 30°C in liquid SC medium, serially diluted, and spotted on SC plates and SC plates supplemented with caffeine, rapamycin, hydroxyurea, or diamide. The plates were incubated for 3 days at 30°C or 37°C.
(PDF)
Northern analysis of total RNA isolated from the ssd1-d2 (W303-1A and UMY4432), ssd1-d2 elp3Δ (UMY3269 and UMY4439), SSD1 (UMY3385 and BY4741), and SSD1 elp3Δ (UMY4456 and MJY1036) strains grown in SC medium at 30°C. The blot was probed for , , , and 5.8S rRNA using radiolabeled oligonucleotides.
(PDF)
The peaks representing ncm5U, mcm5U, mcm5s2U, pseudouridine (Ψ), cytidine (C), uridine (U), guanosine (G), adenosine (A), 1-methyladenosine (m1A), 5-methylcytidine (m5C), 2'-O-methylcytidine (Cm), 1-methylguanosine (m1G), N2-methylguanosine (m2G), N4-acetylcytidine (ac4C), and N2, N2-dimethylguanosine () are indicated. The asterisk indicates a peak that is a contamination from the bacterial alkaline phosphatase.
(PDF)
Northern analysis of total RNA isolated from the ssd1-d2 (W303-1A), ssd1-d2 elp3Δ (UMY3269), SSD1 (UMY3385) and SSD1 elp3Δ (UMY4456) strains. Cells were grown in SC medium containing 2% raffinose followed by induction of GAL1 transcription by the addition of 0.1 volumes 20% galactose. Time points after the addition of galactose are indicated above the lanes. The blot was probed for GAL1 transcripts using a randomly labelled DNA fragment. 18S rRNA was detected using a oligonucleotide probe. The blot is a representative of two independent experiments.
(PDF)
The figure shows a shorter incubation (2 days) of the plates in Fig 4A.
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.