Abstract
We report on a clinical case with haemorrhagic small bowel metastases in a malignant melanoma patient with anaemia, diagnosed using small bowel video capsule endoscopy (VCE). A 67-year-old male patient with a previous diagnosis of malignant melanoma presented with anaemia and vertigo on admission. The standard diagnostic protocol for gastrointestinal (GI) bleeding investigation including a gastroscopy, colonoscopy and small bowel capsule endoscopy, as well as abdominal sonography and a restaging protocol including chest–abdomen–pelvis CT (CAP-CT), echocardiography and ECG was applied. Gastroscopy and colonoscopy were not conclusive in determining the bleeding source. VCE provided evidence for numerous haemorrhagic small bowel metastases. The CAP-CT was unremarkable for small bowel findings. Due to a diffuse metastatic disease diagnosed in heart, brain, liver, spleen and bone metastasis, the patient was treated in a conservative/palliative manner. VCE can provide precious information about GI bleeding of unknown origin when classical diagnostic methods are non-conclusive.
Keywords: gi bleeding, endoscopy, small intestine, small intestine cancer, dermatology
Background
Small bowel video capsule endoscopy (VCE) is a minimally invasive method for the intraluminal visualisation of the entire small bowel. Gastrointestinal (GI) bleeding of unknown origin is one of the major clinical indications for the performance of a VCE. Melanoma is a common malignant skin tumour with a global prevalence of 132 000 per year.1 It has a metastatic affinity to the GI tract above other skin cancers2 and is a rare cause of GI bleeding. Metastatic malignant melanoma is associated with a very poor prognosis, with about 5% survival after 5 years and a mortality rate of more than 95%3.
Checkpoint inhibitors can provide clinically important benefits in patients with metastatic melanoma. In this clinical setting, surgery is rarely curative, and radiotherapy can only provide palliative effects. The distinction between primary and metastatic intestinal melanoma can be difficult. The symptomatology of intestinal localisations of melanoma is not specific and includes nausea, vomiting, abdominal pain, anaemia, GI bleeding and weight loss. One of the most common indicators of a possible melanoma of the GI tract is iron-deficiency anaemia due to occult bleeding.
Case presentation
A 67-year-old male patient with a known melanoma of the right upper arm presented to our hospital with vertigo when standing up from the sitting or lying position or during stress. He was diagnosed with malignant melanoma stage IIIA and underwent surgical excision in 2015 with subsequent interferon-alpha immunotherapy. In a follow-up restaging chest–abdomen–pelvis CT in 2017, the patient showed multimetastatic disease in the liver, spleen and bones. After two cycles of immunotherapy with ipilimumab and nivolumab, the liver and spleen depositions were partially regressive, whereas the skeletal metastatic disease remained stable. Five months after the last chest–abdomen–pelvis CT, the patient presented with severe anaemia (haemoglobin 58 g/L) and vertigo on admission. All other routine lab parameters were normal.
Investigations
After transfusion of four whole-blood units, we applied the standard diagnostic protocol for GI bleeding investigation including gastroscopy, colonoscopy, small bowel capsule endoscopy, as well as abdominal sonography and an extended restaging protocol including chest–abdomen–pelvis CT, an echocardiography and an ECG.
Gastroscopy revealed a normal oesophagus, stomach and duodenum with no significant findings (figure 1A). Colonoscopy diagnosed a non-haemorrhagic melanoma metastasis in cecum (figure 1B). Neither gastroscopy nor colonoscopy was conclusive for an active bleeding source. Suspecting a source of bleeding in the small bowel, we decided to perform a small bowel VCE, which showed many active bleeding metastases in the distal terminal ileum (figure 2A). It also showed approximately 30 areas of black nodules, and in some areas, black pointed polypoid mucosa of the jejunal loops, indicative of non-bleeding metastatic melanoma foci (figure 2B). Since the small bowel bleeding could not be managed by the capsule endoscopy, we referred the patient to surgical consultation. Due to the stable haemodynamic condition, no surgical intervention took place.
Figure 1.
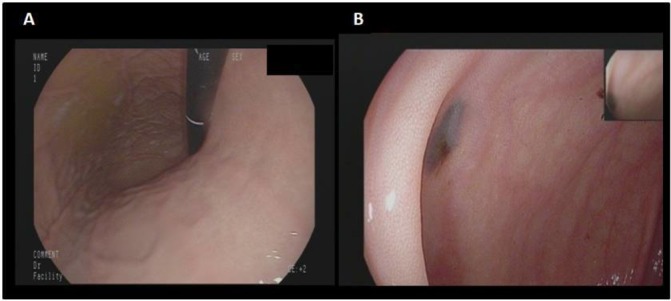
(A) Normal gastroscopy. (B) Non-haemorrhagic melanoma metastasis in cecum (colonoscopy).
Figure 2.
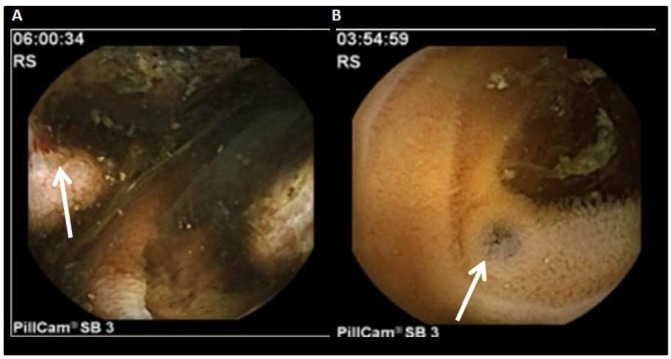
Video capsule endoscopy. (A) Ulcerated melanoma metastasis. Active bleeding (arrow). (B) Pigmentation of the mucous membrane in keeping with melanoma metastasis.
The chest–abdomen–pelvis CT restaging revealed, apart from the known liver and spleen metastatic disease (figure 3A), new cardiac metastases (figure 3B). Although the large bowel foci were evident in chest–abdomen–pelvis CT, radiological imaging failed to reveal the small bowel depositions (figure 3C,D).
Figure 3.
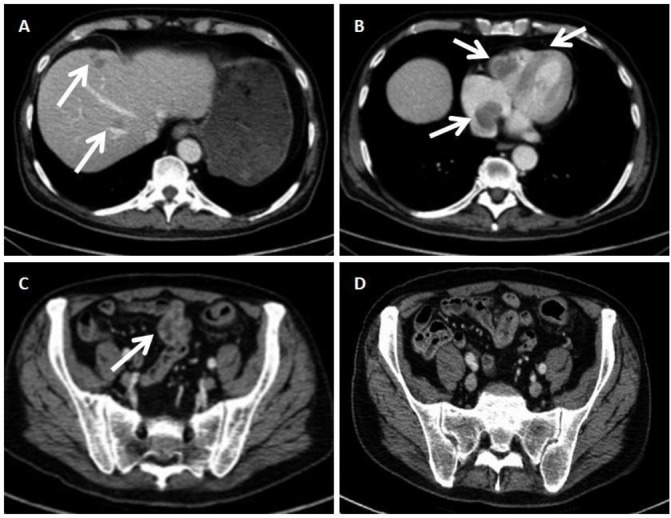
Chest–abdomen–pelvis CT. (A) Hepatic metastases. (B) Low-density, non-contrasted intracavital lesion of the right atrium and ventricle in keeping with cardiac allocations of the metastatic melanoma. (C) Dotted lesions of high density in the wall of the large bowel in keeping with the metastatic lesions shown in the colonoscopy image. (D) Proximal sigmoid colon and parts of the small bowel. In contrast to the evident lesions of the large bowel, the small bowel appears unremarkable.
Outcome and follow-up
Due to the multiple metastases and the poor prognosis, the interdisciplinary tumour board recommended palliative treatment with blood transfusions in the case of rebleeding, with the further option of endovascular treatment. We informed the patient about the poor prognosis of his illness, and we referred him to psycho-oncological consultation. The patient remained asymptomatic, with a stable haemoglobin and negative occult blood in the stools until the day of discharge. The patient stayed under close follow-up and received palliative care services until his death in March 2018.
A written informed consent for anonymised patient information to be published in this article was obtained by a patient’s legally authorised representative.
Discussion
Small bowel metastases are a rare but potentially life-threatening clinical entity. They are seldom described and likely underdiagnosed (113 case reports in the last 43 years, PubMed research on March 2018). Metastatic tumours of the small intestine are more common than primary tumours. Malignant melanoma is the primary tumour in 50%–70% of cases of metastatic tumours of the small bowel.4–6
Haemorrhagic small bowel melanoma metastases are a very rare cause of GI bleeding. Small bowel involvement is found postmortem in 50%–60% of melanoma patients,7 and only 10% are diagnosed premortem. The most common location of small bowel melanoma metastases is the terminal ileum, followed by the stomach. The most common manifestation is an iron-deficiency anaemia due to the occult bleeding. It can also occur with non-specific symptoms such as obstipation and abdominal pain. Rarely is an intussusception, the primary symptom of small bowel metastases, in which case the diagnosis is made intraoperatively.8 9
The VCE is the principal method for the diagnosis of haemorrhagic small bowel metastases. Although CT imaging studies can also detect small bowel melanoma metastases, the sensitivity and specificity of VCE is definitely superior and is strongly recommended on clinical suspicion.7
There is no common consensus or standardised guidelines for the management of small bowel melanoma metastases, leading to individualised treatment in each case. Emerging evidence supports that early diagnosis and treatment could improve rates of survival.10
Hence, the identification of small bowel secondary tumours in bleeding patients suffering from malignant tumours is highly recommended.
Learning points.
Small bowel metastatic melanoma should always be suspected in any patient with a history of malignant melanoma and anaemia or even non-specific symptoms such as intestinal obstruction or weight loss.
Video capsule endoscopy (VCE) is a necessary, well-tolerated, radiation-free tool for the diagnosis of small bowel metastases.
VCE can precisely reveal not only locations of occult bleeding sites but also depositions of malignant melanoma and other tumours.
We recommend the use of VCE for melanoma patients on suspicion of a small bowel metastatic disease and, eventually, as a future standard procedure in the initial staging.
Footnotes
Contributors: AZ wrote the first draft of the manuscript. FS and NAHH consulted the small bowel video capsule endoscopy study. RL-B consulted the chest–abdomen–pelvis CT study and provided the images. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. WHO. Skin cancers. WHO Im Internet. 2019. http://www.who.int/uv/faq/skincancer/en/
- 2. Prakoso E, Selby WS. Polypoid and non-pigmented small-bowel melanoma in capsule endoscopy is common. Endoscopy 2010;42:979 10.1055/s-0030-1255879 [DOI] [PubMed] [Google Scholar]
- 3. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105–16. 10.1200/JCO.1999.17.7.2105 [DOI] [PubMed] [Google Scholar]
- 4. Amar A, Jougon J, Edouard A, et al. [Primary malignant melanoma of the small intestine]. Gastroenterol Clin Biol 1992;16:365–7. [PubMed] [Google Scholar]
- 5. Krüger S, Noack F, Blöchle C, et al. Primary malignant melanoma of the small bowel: a case report and review of the literature. Tumori 2005;91:73–6. 10.1177/030089160509100114 [DOI] [PubMed] [Google Scholar]
- 6. Yashige H, Horishi M, Suyama Y, et al. [A primary malignant melanoma of the small intestine]. Gan No Rinsho 1990;36:955–8. [PubMed] [Google Scholar]
- 7. Prakoso E, Selby WS. Capsule endoscopy in patients with malignant melanoma. Am J Gastroenterol 2007;102:1204–8. 10.1111/j.1572-0241.2007.01115.x [DOI] [PubMed] [Google Scholar]
- 8. Bilello JF, Peterson WM. Retrograde jejunojejunal intussusception secondary to metastatic melanoma. Mayo Clin Proc 2005;80:1098 10.4065/80.8.1098 [DOI] [PubMed] [Google Scholar]
- 9. Rossaak J, Watson A. Medical image. Metastatic melanoma causing small bowel obstruction. N Z Med J 2004;117:U1212. [PubMed] [Google Scholar]
- 10. Melanoma Treatment. Natl Cancer Inst Im Internet. 2019. https://www.cancer.gov/types/skin/hp/melanoma-treatment-pdq.


