Abstract
CD4+CD25+ regulatory T cells (Tregs) are involved in the regulation of physiological and pathological hepatic immune responses, but the roles are not well explored in natural killer (NK) cell-mediated liver diseases. In this study, using the NK cell-mediated oversensitive liver injury model of hepatitis B virus transgenic (HBs-Tg) mice triggered by a low dose of concanavalin A, it was observed that an increased number of CD4+CD25+Foxp3+ Tregs were accumulated in the liver, along with the recovery of liver injury. Adoptive transfer of hepatic Tregs from HBs-Tg mice but not wild B6 mice could significantly attenuate the oversensitive liver injury via inhibiting liver accumulation and decreasing NK cell group 2D-mediated activation of NK cells in the recipient HBs-Tg mice. Furthermore, upregulated expression of membrane-bound TGF-β (mTGF-β) and OX40 on hepatic Tregs were demonstrated to account for inhibiting the NK cell-mediated hepatic injury in HBs-Tg mice through cell-cell contact, confirmed by antibody blockade and cell Transwell experiments in vivo and in vitro. Our findings for the first time indicated that CD4+CD25+ Tregs directly suppressed NK cell-mediated hepatocytotoxicity through mTGF-β and OX40/OX40L interaction in a cell-cell contact manner in HBV-associated liver disease.
Key Words: Acute liver injury, Concanavalin A, Hepatitis B virus, Natural killer cell, Treg cell
Introduction
Chronic hepatitis B virus (HBV) carriers are susceptible to hepatocyte injury, which is a serious risk for development of liver cirrhosis and hepatocellular carcinoma. We firstly demonstrated that the susceptibility to hepatocyte injury was absolutely dependent on the presence of hepatic natural killer (NK) cells by using an HBV transgenic (HBs-Tg) mouse model triggered by immunologic stimulation concanavalin A (Con A). Higher NK cell group 2D (NKG2D) activation of NK cells via recognition of the upregulated Rae-1 or Mult-1 on HBsAg-expressed hepatocytes accounted for the stronger direct cytolysis-mediated liver injury after triggering with a low dose of Con A [1]. Given that the oversensitive liver injury mediated by NK cells could autorecover in 3∼4 days, it was speculated that there exist negative regulator cells to inhibit the pathological role of NK cells in the liver.
Regulatory T cells (Tregs), as a group of heterogenous T cells, are actively engaged in the negative control of a variety of immune responses. As for the liver, strong evidence has suggested that CD4+CD25+ Tregs are involved in the regulation of physiological and pathological hepatic immune responses. Our previous published paper has demonstrated that CD4+CD25+ Tregs limited the T cell-mediated fulminant hepatitis induced by Con A via a TGF-β-dependent mechanism in wild-type mice [2]. Also, the Tregs induced by Con A stimulation could induce tolerance toward Con A rechallenge through negatively regulating NKT cell function via expressing IL-10 [3]. A recent study demonstrated that Tregs effectively suppressed dendritic cell-dependent activation of NK cells and decreased the production of proinflammatory cytokines following rhesus rotavirus infection in an experimental biliary atresia, but do not directly alter NK cell-mediated killing activity [4]. Additionally, circulating and liver-resident CD4+CD25+ Tregs actively influenced the antiviral immune responses and disease progression in patients with HBV infection. In chronic severe hepatitis B patients, the frequencies of CD4+CD25+ Tregs in both peripheral blood mononuclear cells (MNCs) and liver-infiltrating lymphocytes were significantly increased. In chronic hepatitis B patients, the circulating CD4+CD25+ Treg frequency was associated with HBV DNA, while in acute hepatitis B patients, the circulating CD4+CD25+ Treg frequency was initially low and reversibly increased with decline of ALT and HBsAg seroconversion [5]. Persistent HBV infection correlated with increased levels of Tregs, and inhibition of viral replication by drug treatment could downregulate Tregs in chronic hepatitis B [6, 7, 8]. Tregs increased in the liver rapidly after initiation of HBV replication downregulated both the antiviral activity of effector T cells and the recruitment of innate immune cells to the infected liver [9]. However, the exact mechanisms of Tregs in regulating the effector cells in the HBV-associated liver diseases are not well known, which would be influenced by the immune microenvironment in the HBV-infected liver.
Among the several molecules regulating Treg suppressive function, OX40 have recently received particular attention [10, 11]. OX40 is expressed on the majority of CD4+CD25+ Tregs, even in the absence of activation [12]. However, OX40 seems not to be required for Treg genesis, because a similar pool of Tregs with suppressive function in the periphery was observed in OX40-deficient mice as in WT mice [13]. Conversely, a key role for OX40 of CD4+CD25+ Tregs has been demonstrated in the homeostasis of intestinal Tregs and in suppression of T cell-mediated colitis, including their accumulation, suppressive function and survival signal to Tregs after activation [12]. CD4+CD25+ Tregs also suppressed mast cell degranulation and allergic responses through OX40/OX40L-dependent mechanisms [14]. Triggering OX40 blocked Treg inhibitory activity [15], whereas OX40 engagement expanded Tregs as potent suppressor cells [11, 16], so the exact impact of OX40 on Foxp3+ Tregs remains controversial and deserves further clarification. In this study, the NK cell-mediated oversensitive liver injury model of HBs-Tg mice triggered by a low dose of Con A was used [1]. Hepatic CD4+CD25+ Tregs were demonstrated to attenuate the NK cell-mediated hepatocyte injury by inhibiting the accumulation and NKG2D-mediated activation of NK cells in the liver through membrane-bound TGF-β (mTGF-β) and OX40/OX40L interaction. To our knowledge, it is the first time that CD4+CD25+ Tregs have been indicated to directly suppress NK cell hepatocytotoxicity in a cell-cell contact manner in the liver, which is critical to revealing the mechanisms of immune injury and regulation for HBV-associated liver diseases.
Materials and Methods
Animals
The 8- to 10-week-old male HBV transgenic mice C57BL/6J-TgN (AlblHBV) 44Bri (named HBs-Tg) used in our study are heterozygous mice harboring the gene encoding S, PreS1 and PreS2 domains of HBV, and were purchased from the Department of Laboratory Animal Science of Peking University, who obtained the mice from Jackson Laboratory (Bar Harbor, Me., USA). The littermate C57BL/6J mice (nontransgenic) were obtained as the controls (named B6). HBs-Tg and their littermates (B6) were housed together until required and maintained under specific pathogen-free and controlled conditions (22°C, 55% humidity, 12-hour day/night cycle) and received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals.
Con A-Induced Hepatitis
Con A (type IV; Sigma-Aldrich, St. Louis, Mo., USA) was dissolved in pyrogen-free phosphate-buffered saline (PBS) at a concentration of 1 mg/ml and stored at −20°C. HBs-Tg mice and the littermate B6 mice were i.v. injected with Con A at a dose of 3 μg/g body weight or 15 μg/g body weight. Control mice were injected with the same volume of pyrogen-free PBS.
Assay for Serum Transaminase Activities and Cytokine Levels
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were determined by the standard photometric method using the serum transaminase test kit (Rong Sheng, Shanghai, China). Serum TGF-β1 and IL-10 levels were analyzed using a commercially available ELISA kit (Quantikine; R&D Systems) according to the manufacturers' instructions.
Histological Examination
Liver samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections of 5 μm thickness were affixed to slides, deparaffinized, dehydrated and then stained with hematoxylin and eosin (HE) using routine methods.
MNC Preparation
Briefly, the liver was pressed through a 200-gauge stainless steel mesh and then suspended in PBS medium. After one washing, the cells were resuspended in 40% Percoll (Gibcol BRL, Grand Island, N.Y., USA) solution and the cell mixture was gently overlaid onto 70% Percoll solution and then centrifuged at 1,260 g for 30 min at room temperature. The interface cells between the Percoll solutions were aspirated and washed twice with PBS medium. Splenocytes were passed through a 200-gauge stainless steel mesh and were treated with RBC lysis solution (155 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA and 170 mM Tris, PH 7.3). Peripheral blood was collected from the orbital sinus of each mouse and suspended in HBSS containing 100 U/ml heparin. The cells were then treated with RBC lysis solution as described above. Thymocytes were passed through a 200-gauge stainless steel mesh. Single cell suspensions were diluted proportionally in 3% acetic acid according to the cell concentration, and then cell numbers were evaluated.
Flow Cytometry Analysis
Hepatic or splenic MNCs were stained with the optimal amount of the indicated fluorescence-labeled mAbs at 4°C for 30 min in darkness for the surface antigens, and then washed three times and acquired by FACScalibur (Becton Dickinson) and analyzed with WinMDI 2.8 or Flowjo software. For the intracellular assay of Foxp3, after the surface antigens were stained, cells were fixed and permeabilized using a Foxp3 staining buffer set (eBioscience, San Diego, Calif., USA). The mAbs included fluorescein isothiocyanate (FITC)-anti-NK1.1 (Ms IgG2a, κ), FITC-anti-CD25 (rat IgM, κ), FITC-anti-CD69 (ArH IgG1, λ3; PharMingen, San Diego, Calif., USA); phycoerythrin (PE)-anti-NK1.1 (Ms IgG2a, κ), PE-anti-CD69 (ArH IgG1, λ3), PE-anti-CD25 (rat IgG1, λ; PharMingen), PE-anti-OX40 (rat IgG2a, κ; eBioscience), PE-anti-OX40L (goat IgG, FAB1236P; R&D Systems), PE-anti-Foxp3 (rat IgG2a, κ; eBioscience), PE-CY5-anti-CD4 (Rat IgG2a, κ), Percp-CY5.5-anti-CD3e (AH IgG1, κ), Percp-CY5.5-anti-CD4 (rat IgG2a, κ), Percp-CY5.5-anti-NK1.1 (Ms IgG2a, κ; PharMingen); APC-anti-NKG2D (rat IgG1, κ; eBioscience); Alexa-647-anti-Foxp3 (rat IgG2a, κ; eBioscience); APC-CY7-anti-CD3e (AH IgG1, κ; PharMingen).
CD4+CD25+ Treg Isolation and Adoptive Transfer
A CD4+CD25+ Regulatory T Cell Isolation Kit (130-091-041; Miltenyi Biotec Inc., Bergisch Gladbach, Germany) was used. Under ether anesthesia, isolated hepatic Tregs (2 × 105 or 3 × 105) suspended in 100 μl of pyrogen-free PBS were injected into the lateral left lobe of the liver at a rate of 10 μl/s using a 29-gauge needle attached to a 1-ml syringe, followed by i.v. injection of Con A, as described previously [17]. The sham mice received only 100 μl of pyrogen-free PBS without Tregs.
Isolation of Mouse Hepatocytes
Mice were anesthetized with sodium pentobarbital (30 mg/kg, i.p.), and then the portal vein was cannulated. The liver was subsequently perfused with EGTA solution (5.4 mM KCl, 0.44 mM KH2PO4, 140 mM NaCl, 0.34 mM Na2HPO4, 0.5 mM EGTA and 25 mM tricine, pH 7.2) and digested with 0.075% collagenase solution [18]. The viable hepatocytes were then suspended in DMEM (Life Technologies, Gaithersburg, Md., USA) solution and were separated by 40% Percoll (Gibcol BRL) solution with centrifugation at 400 g for 10 min at 4°C.
Purification of NK Cells
The stained MNCs were automatically sorted by FACS Aria (Becton Dickinson) in PBS buffer with a total volume of 1 ml/1 × 107 cells. NK cells (CD3-NK1.1+) were then collected for the in vitro experiments. The separated cells had a purity of ≥95%.
Cytotoxicity Assay
The cytotoxicity of hepatic NK cells against hepatocytes was determined by a 4-hour AST release assay [18]. Hepatic NK cells purified from 2-hour Con A-treated HBs-Tg mice were added to the freshly isolated hepatocytes from 2-hour Con A-treated HBs-Tg mice at the indicated effector to target (E/T) cell ratio of 10:1. 1 × 104 hepatocytes were used as target cells in the assay. After 4 h, the supernatant was harvested and AST activity was measured. The specific cytotoxicity was calculated as: [(ASTexperimental- ASTspontaneous)/(ASTmaximum-ASTspontaneous)] × 100% [1]. Furthermore, hepatic Tregs (suppressor cells) were added to the culture mixture at an effector to suppressor ratio of 1:2. Mouse anti-TGF-β antibody (MAB1835, clone 1D11, mouse IgG1; R&D Systems) or anti-OX40L antibody (MAB1236, clone 182601, rat IgG2a; R&D Systems) was added to the culture mixture at a final concentration of 40 or 20 μg/ml, respectively. A dose of 40 μg/ml mouse IgG1 (MAB002, clone 11711; R&D Systems) or 20 μg/ml rat IgG2a (MAB006, clone 54447; R&D Systems) was used as the control. A Transwell experiment was used to confirm the interaction manner of Tregs and NK cells.
Antibody Neutralization and Blockade in vivo
At 24 h after Con A treatment, a dose of 200 μg of anti-TGF-β antibody (MAB1835, clone 1D11, mouse IgG1; R&D Systems) or a dose of 200 μg of anti-OX40L antibody (MAB1236, clone 182601, rat IgG2a; R&D Systems) was injected i.p. to the mouse for the neutralization and blockade in vivo. A dose of 200 μg of mouse IgG1 (MAB002, clone 11711; R&D Systems) or 200 μg of rat IgG2a (MAB006, clone 54447; R&D Systems) was used as the control.
Statistical Analysis
The results were analyzed by Student's t test or ANOVA where appropriate. All data are presented as the mean ± SEM. p < 0.05 was considered to be statistically significant: * p < 0.05, ** p < 0.01, *** p < 0.001.
Results
Liver Accumulation of CD4+CD25+ Tregs in NK Cell-Mediated Liver Injury in HBs-Tg Mice
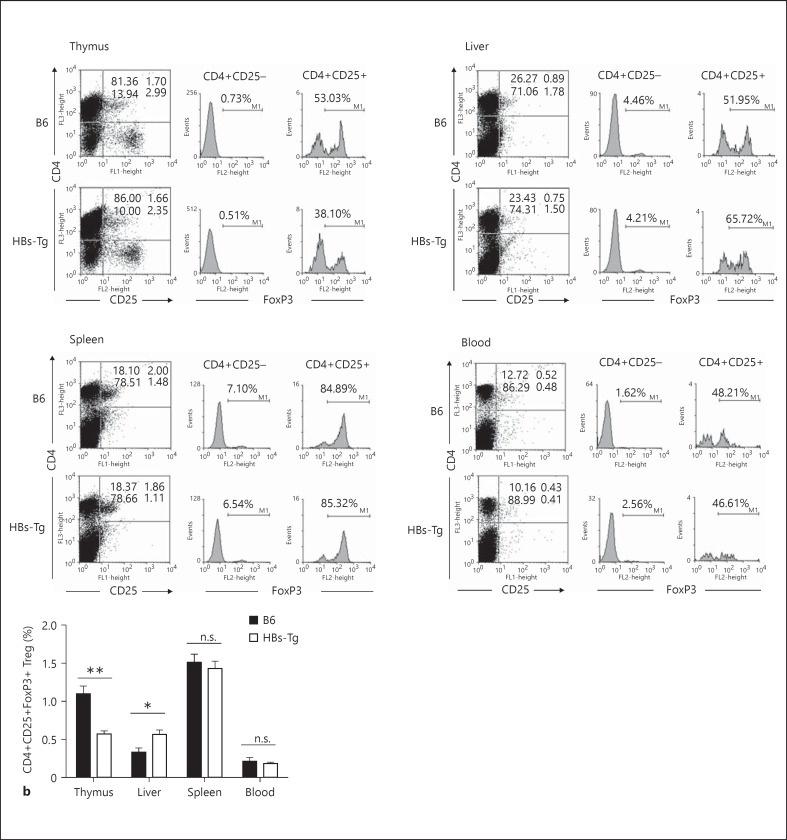
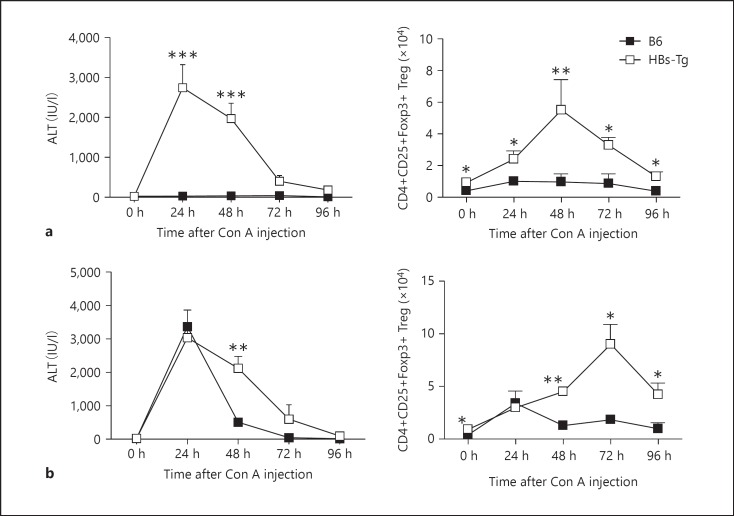
The frequencies and numbers of CD4+CD25+ Tregs in cohoused HBs-Tg mice and B6 mice were detected. As shown in figure 1, the percentage of CD4+CD25+Foxp3+ Tregs was significantly higher in the liver, but not spleen or peripheral blood, of HBs-Tg mice than of B6 mice, while the percentage of CD4+CD25+Foxp3+ Tregs was significantly lower in the thymus. The absolute number of hepatic CD4+CD25+Foxp3+ Tregs in HBs-Tg mice was greater than that in B6 mice in a steady state (fig. 2). HBs-Tg mice were oversensitive to NK cell-mediated liver injury triggered by a low dose of Con A stimulation [1]. As shown by the levels of serum ALT, a low dose of Con A (3 μg/g body weight) injection induced severe liver injury in HBs-Tg mice but not in B6 mice (fig. 2a). With the recovery of liver injury, the number of CD4+CD25+Foxp3+ Tregs was significantly increased at 48 and 72 h, and gradually declined to normal at 96 h after Con A stimulation in HBs-Tg mice (fig. 2a). A high dose of Con A (15 μg/g body weight) injection induced the peak level of serum ALT both in HBs-Tg mice and B6 mice (fig. 2b). The number of CD4+CD25+Foxp3+ Tregs was significantly increased at 24 h after Con A stimulation in both HBs-Tg mice and B6 mice (fig. 2b), and more CD4+CD25+Foxp3+ Tregs were accumulated in the liver of HBs-Tg mice at 48, 72 and 96 h after Con A stimulation along with the recovery of oversensitive liver injury (fig. 2b). These results indicated that CD4+CD25+Foxp3+ Treg accumulation was correlated to the automatic recovery of NK cell-mediated oversensitive liver injury triggered by Con A stimulation in HBs-Tg mice.
Fig. 1.
Higher content of CD4+CD25+ Tregs in the liver of HBs-Tg mice. MNCs prepared from thymus, spleen, liver and peripheral blood of HBs-Tg and B6 mice were analyzed by flow cytometry. a The percentages represent the net percentage of cells in the appropriate quadrant. Furthermore, CD4+CD25- and CD4+CD25+ cells were gated to analyze their Foxp3 expression. These are from a single experiment representative of 4 mice in each group. b Data are presented as the mean ± SEM. * p < 0.05, ** p < 0.01 compared with the control group.
Fig. 2.
Accumulation of CD4+CD25+ Tregs in NK cell-mediated liver injury of HBs-Tg mice. A Low dose of Con A (3 μg/g body weight; a) or high dose of Con A (15 μg/g body weight; b) was injected i.v. into HBs-Tg and B6 mice. At 24, 48, 72 and 96 h after injection, serum ALT activity was determined and MNCs were prepared from the liver and then analyzed by flow cytometry. The number of CD4+CD25+Foxp3+ Tregs was calculated according to the percentage of cells multiplied by total viable MNCs. Data are shown as the mean ± SEM in each group. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control group.
CD4+CD25+ Tregs Inhibited the NK Cell-Mediated Oversensitive Liver Injury in HBs-Tg Mice
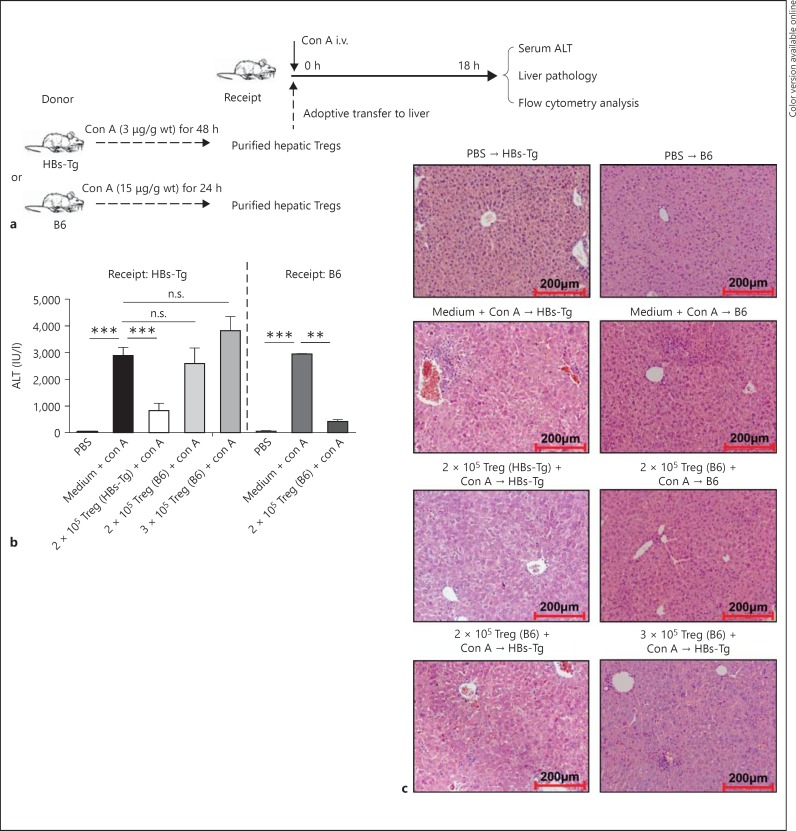
To investigate the role of CD4+CD25+ Tregs in the NK cell-mediated oversensitive liver injury in HBs-Tg mice, adoptive hepatic CD4+CD25+ Treg transfer was performed (fig. 3a). Hepatic CD4+CD25+ Tregs were purified from HBs-Tg mice treated with Con A (3 μg/g body weight, 48 h) or from B6 mice treated with Con A (15 μg/g body weight, 24 h). The proportions of Foxp3+ cells among CD4+CD25+ cells are 64.61 ± 3.41% in HBs-Tg mice and 41.95 ± 1.09% in B6 mice. As shown by the serum ALT levels, adoptive transfer of hepatic CD4+CD25+ Tregs (2 × 105) from HBs-Tg mice, but not hepatic CD4+CD25+ Tregs (2 × 105) from B6 mice (2,589 ± 586 U/l), significantly inhibited the liver injury in Con A-triggered recipients in HBs-Tg mice (825 ± 272 U/l) compared with the sham group (2,886 ± 307 U/l). To exclude the possibility that Tregs from B6 mice are too few to suppress Con A-induced liver injury in HBs-Tg mice, we increased the number of hepatic CD4+CD25+ Tregs from B6 mice to 3 × 105 to make the contained Foxp3+ cells equal to that from HBs-Tg mice. Nevertheless, these higher numbers of B6 Tregs (3 × 105) still could not suppress the liver injury in HBs-Tg mice (3,814 ± 539 U/l), as shown in figure 3b. Furthermore, it was demonstrated that hepatic CD4+CD25+ Tregs (2 × 105) from B6 mice could inhibit the liver injury in Con A-triggered recipients of B6 mice (420 ± 73 U/l) compared with the sham control (2,942 ± 14 U/l; fig. 3b). These results indicated that hepatic CD4+CD25+ Tregs from B6 mice were functionally suppressive in B6 mice but not in HBs-Tg mice. Liver histopathological changes further demonstrated the inhibitory role of hepatic CD4+CD25+ Tregs from HBs-Tg mice in limiting the NK cell-mediated oversensitive liver injury in HBs-Tg mice (fig. 3c).
Fig. 3.
CD4+CD25+ Tregs inhibited NK cell-mediated liver injury in HBs-Tg mice. a The procedures of adoptive CD4+CD25+ Treg transfer into the liver of HBs-Tg mice. Hepatic CD4+CD25+ Tregs were purified from HBs-Tg mice treated with Con A [3 μg/g body weight (wt)] for 48 h or from B6 mice treated with Con A (15 μg/g body weight) for 24 h. Tregs (2 × 105 or 3 × 105) were adoptively transferred into the liver of recipient HBs-Tg or B6 mice, followed by Con A injection (3 μg/g body weight for HBs-Tg and 15 μg/g body weight for B6). Sham group mice were transferred with 100 μl of medium followed by Con A injection. b At 18 h after Treg transfer, serum ALT levels were measured in the recipient mice. c At 18 h after Treg transfer, liver injury of the recipient mice was observed by liver pathology (HE stain. ×200). Data are shown as the mean ± SEM in each group. ** p < 0.01, *** p < 0.001 compared with the control group.
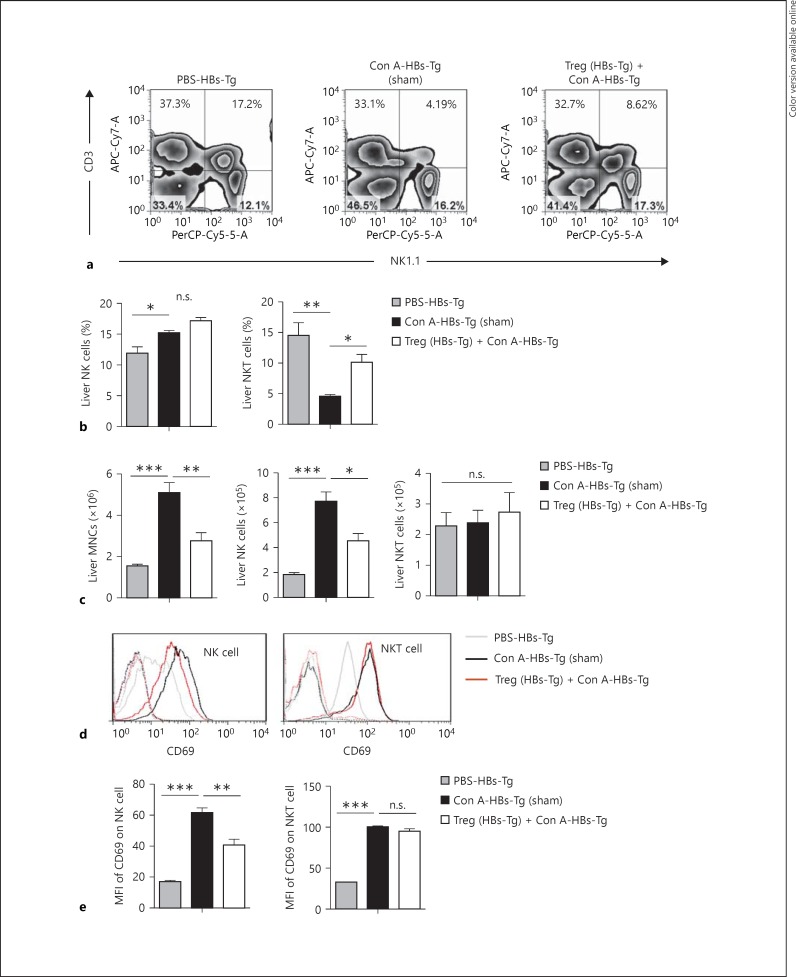
In the Con A-induced oversensitive liver injury model of HBs-Tg mice, NK cells were the effector cells with the most increased number in the liver [1]. Here, it was found that when adoptively transferred with hepatic CD4+CD25+ Tregs from HBs-Tg mice liver MNC infiltration was significantly inhibited after Con A stimulation (fig. 4c); the increase of hepatic NK cell number was markedly inhibited, but not the hepatic NKT cells (fig. 4a, b, c). As shown by the expression levels of CD69, the activation of hepatic NK cells triggered by Con A stimulation was attenuated by the adoptive transfer of hepatic CD4+CD25+ Tregs from HBs-Tg mice, but the activation of NKT cells was not significantly affected (fig. 4d, e).
Fig. 4.
NK cell activation was inhibited by CD4+CD25+ Tregs from HBs-Tg mice. Hepatic CD4+CD25+ Tregs were isolated from HBs-Tg mice treated with a low dose of Con A (3 μg/g body weight) for 48 h, and then Tregs (2 × 105) were adoptively transferred into the liver of HBs-Tg mice, followed by a low dose of Con A (3 μg/g body weight) injection. At 18 h after Treg transfer, MNCs were prepared from the liver and analyzed by flow cytometry. a The percentages represent the net percentage of cells in the appropriate quadrant. b The percentages of hepatic NK cells (CD3-NK1.1+) and NKT cells (CD3+NK1.1+) were statistically analyzed in each group. c The number of liver MNCs, NK cells and NKT cells were calculated. d The expression of CD69 on hepatic NK cells and NKT cells are shown in the histogram. e The mean fluorescence (MFI) of CD69 expressed by hepatic NK cells and NKT cells were statistically analyzed in each group. Data are presented as the mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control group.
NKG2D-Mediated Activation of NK Cells Was Regulated by CD4+CD25+ Tregs in HBs-Tg Mice
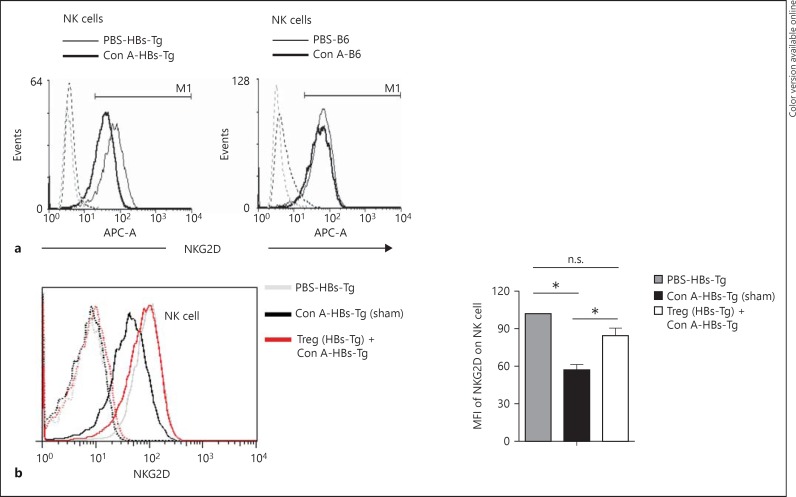
In the low dose of Con A-induced oversensitive liver injury of HBs-Tg mice, it has been well demonstrated that NK cells exerted their hepatocytotoxicity depending on NKG2D recognition of its ligands on HBsAg-expressed hepatocytes [1]. Here, it was observed that the expression of surface NKG2D on hepatic NK cells was significantly downregulated after Con A stimulation in HBs-Tg mice, but not in B6 mice (fig. 5a). It was reported that upon activation surface expression of NKG2D on NK cells was modulated to be at a lower level by internalization after interaction with its ligands [19]. Similar results were observed in our study, indicating that the internalization of NKG2D is related to the role of NK cells in the oversensitive liver injury of HBs-Tg mice. Furthermore, it was confirmed that adoptive transfer of hepatic CD4+CD25+ Tregs from HBs-Tg mice inhibited the internalization of NKG2D of hepatic NK cells, thus preventing NKG2D recognition and signaling, as shown by the higher expression of surface NKG2D on hepatic NK cells compared with the sham control, indicating the suppressive function of Tregs correlated to NKG2D-mediated NK cell activation (fig. 5b).
Fig. 5.
NKG2D function of NK cells was regulated by CD4+CD25+ Tregs in HBs-Tg mice. a A low dose of Con A (3 μg/g body weight) and a high dose of Con A (15 μg/g body weight) was injected i.v. into HBs-Tg mice and B6 mice, respectively. At 48 or 24 h after Con A injection, MNCs were prepared from the livers. The expression of surface NKG2D on NK cells (CD3-NK1.1+) was analyzed by flow cytometry. b Hepatic CD4+CD25+ Tregs were isolated from HBs-Tg mice treated with a low dose of Con A (3 μg/g body weight) for 48 h, and then Tregs (2 × 105) were adoptively transferred into the liver of HBs-Tg mice, followed by a low-dose Con A (3 μg/g body weight) injection. At 18 h after Treg transfer, MNCs were prepared from the livers and the expression of surface NKG2D on NK cells (CD3-NK1.1+) was analyzed by flow cytometry. The mean fluorescence intensity (MFI) of surface NKG2D expressed on hepatic NK cells was statistically analyzed in each group. Data are presented as the mean ± SEM. * p < 0.05 compared with the control group.
CD4+CD25+ Tregs Inhibited NK Cell Function through mTGF-β and OX40/OX40L Interaction
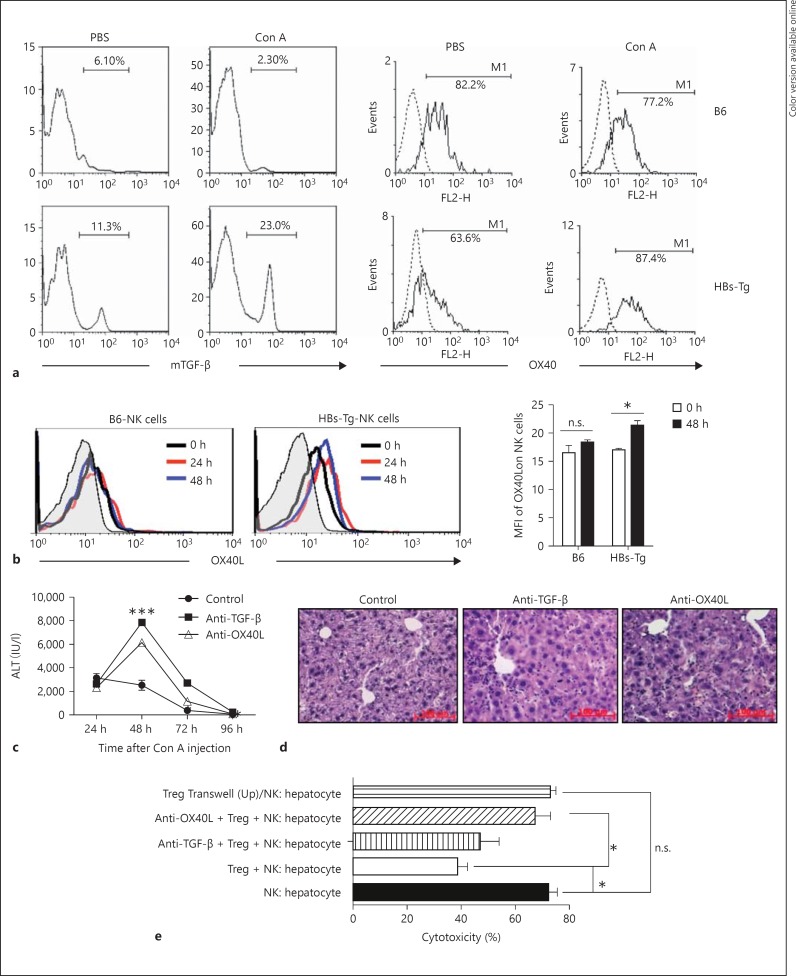
During the automatic recovery of the oversensitive liver injury in HBs-Tg mice induced by low-dose Con A stimulation, there was no significant increase in the serum level of soluble TGF-β (online suppl. fig. S1A; for all online suppl. material, see www.karger.com/doi/10.1159/000431150) and IL-10 (data not shown). The serum levels of soluble TGF-β and IL-10 were also not significantly affected by adoptive transfer of Tregs from HBs-Tg mice (online suppl. fig. S1B). These results indicated that CD4+CD25+ Tregs might exert their effects independent of soluble TGF-β and IL-10 in HBs-Tg mice, which was different from that of wild B6 mice during Con A-induced liver injury, as previously reported [2, 3]. CD4+CD25+ Tregs from HBs-Tg mice and wild B6 mice were compared, demonstrating that there was no significant difference in the expression of functional molecules including GITR, CTLA-4, CD103, CD62L and CD44 (data not shown). Noticeably, the expression of membrane-bound TGF-β (mTGF-β) on hepatic CD4+CD25+ Tregs of HBs-Tg mice was significantly higher than that of B6 mice, and even more markedly upregulated after Con A stimulation, while the expression of B6 mice was significantly downregulated (fig. 6a). Simultaneously, the expression of OX40 on hepatic CD4+CD25+ Tregs of HBs-Tg mice was markedly increased after Con A stimulation (fig. 6a). Furthermore, the expression of OX40L on hepatic NK cells of HBs-Tg mice was obvious and also significantly increased by Con A stimulation (fig. 6b). Blockade of TGF-β and OX40/OX40L interaction by pretreatment with anti-TGF-β and anti-OX40L mAb dramatically aggravated the NK cell-mediated oversensitive liver injury and postponed the recovery of liver injury in HBs-Tg mice (fig. 6c, d).
Fig. 6.
CD4+CD25+ Tregs inhibited hepatocytotoxicity of NK cells through mTGF-β and OX40/OX40L interaction in HBs-Tg mice. a Hepatic CD4+CD25+ Tregs were analyzed for the expression of mTGF-β and OX40 in HBs-Tg mice treated with a low dose of Con A (3 μg/g body weight) for 48 h, or in B6 mice treated with a high dose of Con A (15 μg/g body weight) for 24 h. b Hepatic NK cells (CD3-NK1.1+) were analyzed for the expression of OX40L in HBs-Tg mice treated with a low dose of Con A (3 μg/g body weight) or in B6 mice treated with a high dose of Con A (15 μg/g body weight) for 24 and 48 h. Mean fluorescence intensity (MFI) data are shown as the mean ± SEM. c, d At 24 h after the low-dose Con A (3 μg/g body weight) injection, anti-TGF-β antibody or anti-OX40L antibody was injected i.p. into HBs-Tg mice, and then at each indicated time point (48, 72 and 96 h) serum ALT activity was determined. At 96 h after Con A injection, liver samples were collected for HE staining. e Four-hour AST release assay was performed to test the effect of Tregs on the cytotoxicity of hepatic NK cells against hepatocytes. Hepatic NK cells purified from 2-hour Con A-treated HBs-Tg mice were added to the freshly isolated hepatocytes (1 × 104) from 2-hour Con A-treated HBs-Tg mice at the E/T of 10/1. The Treg/NK cell ratio = 2:1. A dose of 40 μg/ml anti-TGF-β mAb or 20 μg/ml anti-OX40L mAb was added to the culture. Data are presented as the mean ± SEM from triplicates in each group. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control group.
The dependence of NKG2D-ligand interaction between NK cells and hepatocytes was confirmed by direct cytotoxicity of the purified NK cells against hepatocytes from Con A-treated HBs-Tg mice in a 4-hour AST release assay in vitro [1]. Here, CD4+CD25+ Tregs significantly inhibited NK cell hepatocytotoxicity (38.7 ± 3.7%) compared with the control (72.3 ± 3.2%). When the Tregs and NK cells were separated by the Transwell membrane, the inhibitory role of Tregs on NK cells disappeared, indicating that the cell-cell contact manner is necessary. The dependence of mTGF-β and OX40/OX40L interaction between NK cells and Tregs was also confirmed by antibody blockade in the cytotoxic assay (fig. 6e).
Discussion
In this study, using a low-dose Con A-induced oversensitive liver injury model, we provided evidence that hepatic CD4+CD25+ Tregs could attenuate the susceptibility to hepatocyte injury of HBs-Tg mice. These immunosuppressive Tregs inhibited hepatic NK cell accumulation, activation and hepatocytotoxicity via mTGF-β and OX40/OX40L interaction, which may have therapeutic implications for chronic HBV carriers. It has been well known that CD4+CD25+ Tregs play a central role in maintaining peripheral tolerance and regulating immunity in infections, transplantation, autoimmune diseases and cancer through inhibiting proliferation and effector functions of a large panel of immune cells [20, 21, 22]. It has been reported that Tregs can inhibit NK cell functions, including cytotoxic activity, cytokine production, proliferation and activating receptor expression, either via membrane-bound or soluble TGF-β or through direct killing with the granzyme/perforin pathway [22, 23, 24, 25]. To our knowledge, this is the first study revealing the role of Tregs in the direct regulation of NK cells by cell-cell contact in liver disease. Using HBV transgenic mice mimicking healthy human HBsAg carriers, this study is critical to revealing the mechanisms of immune injury and regulation during human HBV infection.
HBsAg transgenes systemically influence the host immune system [26, 27]. NKT cells, but not NK cells, were the main liver injury inducer in Con A-induced hepatitis of WT B6 mice, whose own Tregs had a suppressive function on the effector T/NKT cells [2, 3, 17, 28]. However, hepatic NK cells, but not NKT cells, were the effector that directly attacked HBsAg-positive hepatocytes in HBs-Tg mice [1]. In this work we found that only the CD4+CD25+ Tregs from Con A-treated HBs-Tg mice could inhibit the susceptibility to hepatocyte injury of HBs-Tg mice through inhibiting hepatic NK cell accumulation, activation and hepatocytotoxicity, but not the CD4+CD25+ Tregs from Con A-treated B6 mice (fig. 3, 4, 6). This represents a step forward following our finding that HBsAg transgenes changed NK cell function, and complements the immune regulation map of HBs-Tg mice.
It has been known that three inhibitory cytokines, IL-10, IL-35 and TGF-β, are key mediators of Treg cell function [29]. In Con A-induced hepatitis of wild B6 mice, CD4+CD25+ Tregs functioned via a TGF-β- or IL-10-dependent mechanism [2, 3]. However, in this study, there was no correlation between serum levels of TGF-β and IL-10 and the frequency of Tregs during the low dose of Con A-induced oversensitive liver injury (online suppl. fig. S1). By comparison, hepatic CD4+CD25+ Tregs of HBs-Tg mice expressed high levels of mTGF-β, which was further increased when simulated by Con A (fig. 6a). Simultaneously, the expression of OX40 on hepatic Tregs of HBs-Tg mice was markedly increased after Con A stimulation (fig. 6a). A high expression level of OX40 on hepatic Tregs was detected in wild B6 mice, while the expression level of OX40 on hepatic Tregs of HBs-Tg mice was significantly lower than that of wild B6 mice. A key role for OX40 of CD4+CD25+ Tregs in the homeostasis of intestinal Tregs has been demonstrated [12]. The inhibited expression of OX40 on hepatic Tregs of HBs-Tg mice might be related to their attenuated inhibitory function, resulting in the susceptibility to liver injury, which was similar to the impaired function of hepatic NK cells in HBs-Tg mice [30]. However, markedly increased expression of OX40 was induced on hepatic Tregs of HBs-Tg mice by Con A stimulation, which accounted for their inhibitory role in limiting NK cell hepatocytotoxicity. Here, we firstly demonstrated that CD4+CD25+ Tregs suppressed NK cell-mediated hepatocytotoxicity through OX40/OX40L interaction in a cell-cell contact manner (fig. 6c, d, e). It was demonstrated by Wang and colleagues [31] that increased hepatic CD4+CD25+ Tregs efficiently inhibited the antitumor ability of autologous NK cells in vitro, dependent on a direct cell-cell contact. The exact mechanism of Treg suppression was not investigated in their study but was predicted through the weakening activation signals on NK cells, which was consistent with our findings.
In 2005, Ghiringhelli et al. [32] firstly provided mechanistic insights that Tregs can suppress NK cell-mediated tumor killing by mTGF-β in vitro and in vivo, directly inhibiting NK cell effector functions and downregulated NKG2D receptor on the NK cell surface. However, in our study, coculture of Tregs from Con A-treated HBs-Tg mice did not significantly affect the expression of NKG2D on NK cells in vitro (online suppl. fig. S2). It was also reported by another group that they were unable to detect any downregulation of NKG2D on NK cells when exposed to the activated Tregs [33]. During low-dose Con A-induced oversensitive liver injury, NK cells mediated the cytotoxicity to hepatocytes via NKG2D recognition of ligands (Rae-1 and Mult-1) expressed on HBsAg-expressed hepatocytes [1], which resulted in the decreased expression of NKG2D on the cell surface (fig. 5a). In nonclassical NKT cell-mediated acute liver injury of a transgenic mouse model of primary HBV infection it was also reported that NKT cells eluted from the livers of the HBV-Env-RAG−/− mice downregulated the surface expression of NKG2D [34]. This is consistent with the fact that surface NKG2D is known to be internalized after interaction with its ligands to be a lower level [19]. In this study, the surface NKG2D on hepatic NK cells was downregulated when interacting with ligand-positive hepatocytes; however, the downregulation was significantly suppressed by adoptive transfer of CD4+CD25+ Tregs in vivo (fig. 5b), indicating that CD4+CD25+ Tregs might interfere with NKG2D/ligand signaling between NK cells and hepatocytes. How mTGF-β and OX40/OX40L interactions between Tregs and NK cells modulate the NKG2D signaling pathway between NK cells and hepatocytes deserves further investigation.
In summary, our findings firstly indicated that CD4+CD25+ Tregs directly suppressed NK cell-mediated hepatocytotoxicity through mTGF-β and OX40/0X40L interaction in a cell-cell contact manner by using HBV transgenic mice triggered by a low dose of Con A, which is critical to revealing the mechanisms of immune injury and regulation for human HBV infection. The immunosuppressive role of Tregs might have therapeutic ramifications for chronic HBV carriers.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgements
This work was supported by the Natural Science Foundation of China (81273244; 30801006). Financial support is acknowledged from the China Postdoctoral Science Foundation (20070420732), Chinese Academy of Sciences K.C. Wong Post-Doctoral Fellowships.
References
- 1.Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 2.Wei HX, Chuang YH, Li B, Wei H, Sun R, Moritoki Y, Gershwin ME, et al. CD4+CD25+ Foxp3+ regulatory T cells protect against T cell-mediated fulminant hepatitis in a TGF-β-dependent manner in mice. J Immunol. 2008;181:7221–7229. doi: 10.4049/jimmunol.181.10.7221. [DOI] [PubMed] [Google Scholar]
- 3.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 4.Miethke AG, Saxena V, Shivakumar P, Sabla GE, Simmons J, Chougnet CA. Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol. 2010;52:718–726. doi: 10.1016/j.jhep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 6.Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 7.Nan XP, Zhang Y, Yu HT, Sun RL, Peng MJ, Li Y, Su WJ, et al. Inhibition of viral replication downregulates CD4+CD25high regulatory T cells and programmed death-ligand 1 in chronic hepatitis B. Viral Immunol. 2012;25:21–28. doi: 10.1089/vim.2011.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JY, Song CH, Shi F, Zhang Z, Fu JL, Wang FS. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS One. 2010;5:e13869. doi: 10.1371/journal.pone.0013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stross L, Gunther J, Gasteiger G, Asen T, Graf S, Aichler M, Esposito I, et al. Foxp3+ regulatory T cells protect the liver from immune damage and compromise virus control during acute experimental hepatitis B virus infection in mice. Hepatology. 2012;56:873–883. doi: 10.1002/hep.25765. [DOI] [PubMed] [Google Scholar]
- 10.Piconese S, Timperi E, Pacella I, Schinzari V, Tripodo C, Rossi M, Guglielmo N, et al. Human OX40 tunes the function of regulatory T cells in tumor and nontumor areas of hepatitis C virus-infected liver tissue. Hepatology. 2014;60:1494–1507. doi: 10.1002/hep.27188. [DOI] [PubMed] [Google Scholar]
- 11.Xiao X, Gong W, Demirci G, Liu W, Spoerl S, Chu X, Bishop DK, et al. New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J Immunol. 2012;188:892–901. doi: 10.4049/jimmunol.1101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruby CE, Yates MA, Hirschhorn-Cymerman D, Chlebeck P, Wolchok JD, Houghton AN, Offner H, et al. Cutting edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K, Hayakawa Y, van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 20.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 22.Ralainirina N, Poli A, Michel T, Poos L, Andres E, Hentges F, Zimmer J. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144–153. doi: 10.1189/jlb.0606409. [DOI] [PubMed] [Google Scholar]
- 23.Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol. 2013;10:222–229. doi: 10.1038/cmi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, Blazar BR, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colonna M. NK cells: new issues and challenges. Preface. Eur J Immunol. 2008;38:2927–2929. doi: 10.1002/eji.200838883. [DOI] [PubMed] [Google Scholar]
- 26.Busca A, Kumar A. Innate immune responses in hepatitis B virus (HBV) infection. Virol J. 2014;11:22. doi: 10.1186/1743-422X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das A, Maini MK. Innate and adaptive immune responses in hepatitis B virus infection. Dig Dis. 2010;28:126–132. doi: 10.1159/000282075. [DOI] [PubMed] [Google Scholar]
- 28.Toyabe S, Seki S, Iiai T, Takeda K, Shirai K, Watanabe H, Hiraide H, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–1542. [PubMed] [Google Scholar]
- 29.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Wei H, Sun R, Tian Z. Impaired function of hepatic natural killer cells from murine chronic HBsAg carriers. Int Immunopharmacol. 2005;5:1839–1852. doi: 10.1016/j.intimp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 34.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data