Abstract
The production of interleukin (IL)-26 was initially attributed to T cells, and in particular to Th17 cells. However, more recent findings indicate IL-26 production in natural killer (NK) cells, macrophages and fibroblast-like cells as well. It is known that IL-26 binds to the IL-20R1/IL-10R2 receptor complex on certain target cells, where it causes specific intracellular signaling and the secretion of IL-1β, IL-8 and TNF-α. In line with this type of proinflammatory role, IL-26 also increases chemotaxis of human neutrophils. Interestingly, high levels of IL-26 are present even in normal human airways, and endotoxin exposure further enhances these levels; this indicates involvement in antibacterial host defense. Studies on acute inflammatory disorders are few but there are studies showing the involvement of IL-26 in rheumatoid arthritis and inflammatory bowel disease. In conclusion, IL-26 is emerging as a potentially important player in host defense and may also be a pathogenic factor in the chronic inflammatory disorders of humans.
Key Words: Asthma, Cancer, Crohn's disease, Interleukin-17, Rheumatoid arthritis, Th17 cells, Ulcerative colitis
Introduction
The realization that single cytokines may constitute important targets for novel therapies has driven academic and industrial researchers to identify and characterize novel cytokines in mammals, including humans. As a result, there are now several examples of promising therapies that target cytokines, including antibodies against granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-5, IL-4/IL-13 and IL-17 [1, 2, 3]. This field is thus rapidly evolving and is likely to prove important for future therapy.
The cytokine IL-26 was discovered more than 10 years ago in herpesvirus saimiri-transformed CD8+ T cells that displayed an increased gene expression of il26[4]. Given this essential observation, it is surprising that so few integrative studies on the role of IL-26 in vivo have been conducted up to now. However, there are now 3 fairly recent in vivo studies on IL-26 that are driving this interesting area of knowledge forward. In this review, we scrutinize the most intriguing findings so far, with a focus on the immunology of IL-26 in physiological and pathological conditions, and point out important gaps in current knowledge.
The Molecule and Its Gene
IL-26 is a 171-amino acid protein that belongs to the IL-10 family of cytokines, a family that includes IL-10, IL-19, IL-20, IL-22 and IL-24 [5]. The IL-26 protein is encoded by the IL26 gene located on chromosome 12q15 between genes for interferon (IFN)-γ and IL-22 [6, 7], and is conserved in several vertebrate species but not in mice and rats [8]. The cytokine IL-26 was originally named AK155, and the molecule comprises a signal sequence, 6 helices and 4 conserved cysteine residues [4]. The IL-26 protein is secreted as a highly basic protein that probably operates both as a monomer and as a homodimer [4]. Even though IL-26 shares approximately 25% of its amino acid homology with IL-10, and utilizes one of the IL-10 receptor subunits as a cell surface receptor [7, 9], a growing body of evidence indicates that the functional effects of IL-26 differ substantially from those of IL-10.
Receptor and Intracellular Signaling
Receptor Complex
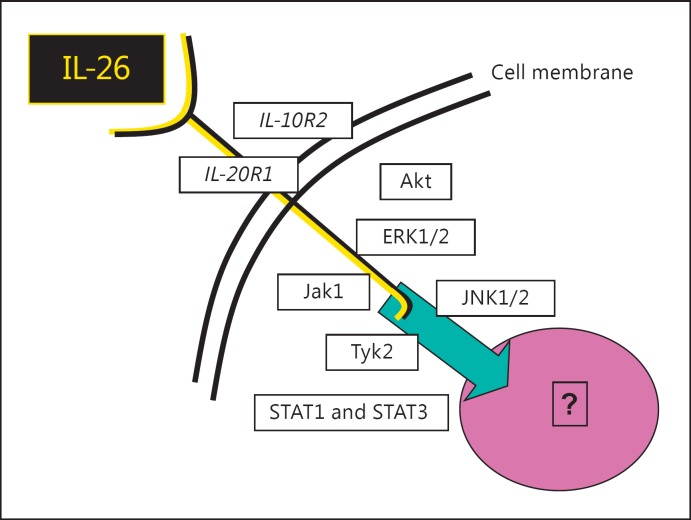
After the identification of the IL-26 gene and its encoded protein, the functional IL-26 receptor heterodimer consisting of the IL-20R1/IL-10R2 subunits was identified by two independent research groups [7, 9]. In this receptor heterodimer complex, the subunit IL-20R1 was shown to harbor the specific ligand-binding site for IL-26 whereas the IL-10R2 subunit acts as an essential second chain that completes the assembly, thereby providing an active and functional receptor complex, as summarized in figure 1[7]. As demonstrated in epithelial cells, neutralizing antibodies towards either of the 2-receptor subunits blocks IL-26 signaling, suggesting that both are needed for the function of the receptor complex [9]. However, the receptor biology for IL-26 is in need of further scrutiny, not least in hematopoietic immune cells; this is because anti-CD40-stimulated B cells respond to IL-26 with decreased secretion of immunoglobulin (Ig)A and IgG, despite the lack of IL-20R1, an observation that makes it feasible that additional receptors exist [10].
Fig. 1.
Overview of the IL-26 receptor complex, with the receptor subunits (IL-20R1 and IL-10R1) plus the involved intracellular signaling molecules. Akt = Protein kinase B; ERK1/2 = extracellular signal-related kinase; Jak = receptor-associated Janus tyrosine kinase; JNK1/2 = c-Jun N-terminal kinase; Tyk = receptor-associated Janus tyrosine kinase.
Intracellular Signaling
Most likely, once the IL-26 receptor complex is fully assembled, it signals via the receptor-associated Janus tyrosine kinases Jak1 and Tyk2, involving altered activation of both signal transducers and activators of transcription (STAT)1 and, in particular, STAT-3, as indicated in figure 1 [4, 7, 9]. Moreover, IL-26 activates the extracellular signal-related kinase (ERK)-1/2, stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK)-1/2, mitogen-activated protein (MAP) kinases, and also protein kinase B, commonly known as Akt [11]. However, the intracellular signaling cascade farther downstream of the IL-26 receptor complex is in need of further characterization.
Receptor Expression
While IL-10R2 is ubiquitously expressed [12], the expression of IL-20R1 is absent in unstimulated human peripheral blood cells [13] and cancer cell lines from the human liver, cervix and pancreas. In contrast to this, IL-20R1 is expressed in colon carcinoma and keratinocyte cell lines [9]. This was logically interpreted as a proof for nonhematopoietic cell types to constitute the main targets for IL-26. Whilst the effect on nonhematopoietic cells has been verified, a very recent study shows that IL-26 per se causes the upregulation of the IL-20R1 gene in primary immune cells from human lungs - and that the gene expression for IL-20R1, IL-10R2, STAT1 and STAT3, respectively, is increased by IL-26 in both the immune and epithelial cells from human lungs [14]. Interestingly, this suggests that IL-26 can alter the responsiveness to itself in immune cells and certain structural cells (i.e. epithelial cells), thereby providing evidence for a positive feedback loop. Whether or not the expression of IL-26 receptor subunits and its downstream signaling molecules is altered during the time course of inflammation has still to be addressed.
In terms of cells residing in tissue, the gene expression of IL-20R1 has been demonstrated in human fetal skin, but this type of expression is absent in the fetal heart, kidney, small intestine [7] and liver [15]. Moreover, under physiological conditions, IL-20R1 appears to be expressed in the adult brain, especially in the cerebellum, in the medulla and spinal cord as well as in the heart, lung, stomach, testis, placenta, salivary glands, pancreas, ovary, uterus and prostate [7, 15]. In contrast, IL-20R1 has not been observed in the human spleen, liver, kidney and colon. However, whether the presence of IL-20R1 is altered in tissues in patients with acute or chronic inflammatory disorders still needs to be investigated.
Cellular Sources
T Cells
An early report claimed gene expression for IL-26 in T cells, based upon RT-PCR analyses in CD4+, CD8+ and γ∂ herpesvirus-transformed or mitogenic-stimulated T cell lines [4]. According to the same report, there is gene expression for IL-26 even in naive T cells, but to a lesser extent than in activated T cells. Upon considering the gene regulation, it is interesting to note that in 1 study, costimulation of T cells with anti-CD3 and anti-CD28 showed no significant effect on the gene expression for IL-26 [13]. However, Th1-polarized cells can display upregulation of the gene expression for IL-26 as well whereas T cells polarized towards a regulatory phenotype display a corresponding downregulation of IL-26 [13].
Th17 Cells
In a seminal study on T-cell biology, it was demonstrated that exposure to IL-23 and IL-1β induces the development of Th17 cells, an intriguing subpopulation that is characterized by gene expression for not only IL-17A, but also for IL-17F, IL-22, IFN-γ, CCL20, the transcription factor RORγt and IL-26 [16]. This observation was supported by a second study on Th17 cells from the blood that demonstrated a correlation between the gene expressions of IL-26 and IL-17A [17]. Moreover, an interesting mechanistic link between Th17 cells and IL-26 was implicated by the study from Corvaisier et al. [18], showing that not only is IL-26 produced by infiltrating Th17 cells in the synovia of patients with rheumatoid arthritis (RA) but it also upregulates the intracellular production and secretion of IL-17A by memory CD4+ T cells. The same study implicated IL-26 in the increases of numbers of Th17 cells, but not Th1 or Th2 cells. This observation suggests that IL-26 drives a functional and positive feedback loop, by which its signaling sustainability and influence on inflammation is preserved. This finding was recently verified and expanded when a functional deviation of Th17 cells was demonstrated in vitro when IL-4Rα stimulation (the receptor subunit shared by IL-4 and IL-13) resulted in diminished IL-17F gene expression whilst the gene expression of IL-26 remained unaltered [19].
B Cells
The first and only report so far indicates a very weak gene expression of IL-26 in human herpesvirus 8-transformed B cells [4].
Natural Killer Cells
A follow-up study indicated that although the expression of the IL-26 gene mainly takes place in memory T cells (CD4+CD45RO+), it is also present to some extent in natural killer (NK) cells [13]. The finding that NK cells express low levels of IL-26 mRNA was subsequently followed up by a study showing that NKp44+ cells, representing a human NK cell subset that is localized in mucosa-associated lymphoid tissues, produce substantial amounts of IL-26 and IL-22 in response to stimulation with the archetype Th17 regulator [20].
Macrophages
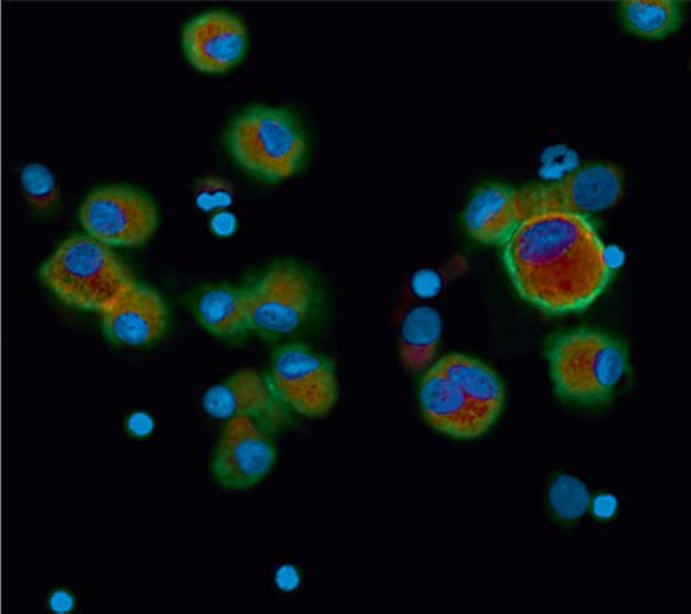
The first published study on the topic suggested that monocytes do not express the gene for IL-26 but it examined a cell line, not primary cells [4]. It was therefore of conceptual importance that a recent study on primary airway cells from healthy human volunteers demonstrated the gene expression and secretion of IL-26 in alveolar macrophages in vivo and ex vivo; the expression was further enhanced by exposure to bacterial endotoxin [14]. As shown in figure 2, the study demonstrated an abundance of IL-26 protein in airway macrophages in vivo, and this finding warrants further study of macrophages in human organs other than the lungs.
Fig. 2.
Immunocytofluorescent image of alveolar macrophages expressing IL-26 protein. The macrophage marker CD68 is indicated in red, the nuclei in blue and IL-26 protein in green. Reprinted with permission of The American Thoracic Society© [14].
Structural Cells
In addition to what is described above, the first published study on the topic suggested that fibroblasts and transformed epithelial-gland cells do not express the gene for IL-26 but, again, this study examined cell lines [4]. In comparison to the case for monocytes/macrophages presented above, a subsequent study on primary fibroblasts demonstrated gene expression for IL-26 in a synoviolin-positive subset of these structural cells, i.e. cells that were harvested from the hyperplastic lining cell layer of the synovia of patients with RA [18]. In fact, these fibroblasts appeared to constitute the major source of IL-26 in the inflamed synovia of the referred patients.
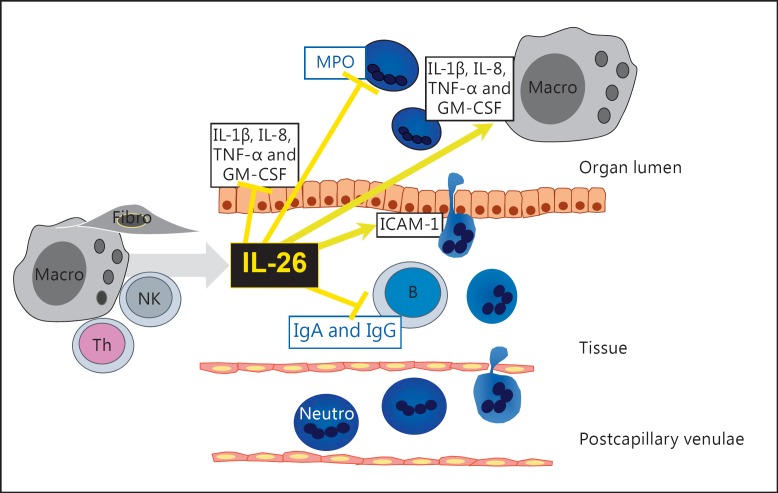
Tentatively, as summarized in figure 3, there is evidence to indicate that several types of classic, hematopoietic immune cells produce and secrete IL-26, and evidence is emerging that certain nonhematopoietic, structural cells are also capable of producing it.
Fig. 3.
Proposed cellular sources and actions of IL-26 in human organs. Fibro = Fibroblasts; Macro = macrophages; Neutro = neutrophils.
Effector Functions
Even though the current knowledge remains superficial from a wholistic point of view, there is now evidence that the effector function of IL-26 depends upon the type of cellular target and the costimulatory conditions [14, 18, 21]. Despite the structural similarity with the archetype anti-inflammatory cytokine IL-10, and the sharing of one of its heterodimer receptor subunits, functional studies are compatible with IL-26 driving or sustaining inflammation rather than suppressing it. However, it should be emphasized that hitherto, very little has been known about the functions of IL-26 in homeostasis during physiological or pathological conditions.
Host Defense
It seems reasonable to assume that mucosal surfaces are constantly being exposed to microbial stimuli and that there is a delicate balance whereby microbes induce a sufficiently effective antimicrobial immune response without causing long-lasting mucosal inflammation with a harmful effect on the tissue of the host. In this context, IL-26 may prove functionally important, but, so far, limited research efforts have been made with reference to its production after microbial challenges [14].
Host Defense against Extracellular Bacteria
Interestingly, IL-26 may be involved in host defense against components from the cell membrane of extracellular bacteria, as indicated by a recent in vivo and in vitro study on human blood monocytes exposed to endotoxin (LPS) from Escherichia coli[13]. While stimulation of these monocytes with LPS for a substantial period (up to 18 h) did not alter the gene expression of IL-26 [13], the very same stimulus upregulated the gene expression in differentiated lung macrophages, and caused the release of IL-26 protein in these innate effector cells [14]. It thus seems possible that, after extravasation, monocytes mature and differentiate into tissue macrophages that develop the capacity to produce IL-26 in response to Gram-negative bacteria. The relevance of this for conditions in vivo is indicated by the observation that local exposure to endotoxin enhances IL-26 protein concentrations in human airways, and this increase in IL-26 is strongly associated with the local accumulation of macrophages [14].
Host Defense against Intracellular Bacteria
The involvement of IL-26 in host defense against intracellular bacteria has also been indicated [22]. A recent study described the potential role of IL-26 infection with Mycobacterium tuberculosis in isolated human blood monocytes cultured in vitro [22]. By screening for altered gene expression with microarray analysis, it was discovered that the IL-26 gene was downregulated in monocytes from tuberculosis patients. However, while blood monocytes from healthy controls secreted IL-26 protein into the conditioned medium, the monocytes that were infected with M. tuberculosis secreted less IL-26. Moreover, when IL-26 was added into a whole-blood culture of M. tuberculosis, this cytokine did in fact inhibit the observed pathogen-killing capability in vitro.
Clearly, IL-26 may be involved in host defense against bacteria in more ways than affecting macrophages. Recent data on isolated neutrophils indicate that IL-26 inhibits the spontaneous release of myeloperoxidase (MPO) from these innate effector cells in vitro [14]. The release of MPO from azurophilic granules of neutrophils is important for the production of reactive oxygen species that kill bacteria [23], so this observation is compatible with IL-26 exerting a modulatory role in antibacterial host defense during certain conditions or time points of the immune response. However, MPO is also known to stimulate apoptosis in neutrophils [24, 25;] it is thus just as possible that the decreased release of MPO from neutrophils in response to recombinant IL-26 mirrors suppresses apoptosis, thereby emphasizing the potential of IL-26 as a neutrophil-regulating cytokine. Yet, the role of IL-26 appears dualistic with regard to neutrophils, given that IL-26 enhances chemotaxis (directed migration) of human blood neutrophils, caused by the C-X-C chemokine IL-8 or the bacterial compound N-formyl peptide in vitro, but inhibits chemokinesis (undirected migration) at the same time [14]. Taken together, these observations are compatible with IL-26 being important for favoring the meaningful migration of neutrophils to sites of infection and inflammation, while at the same time limiting less meaningful, undirected migration of neutrophils and, finally, for increasing the survival of these critical innate effector cells.
Host Defense against Viruses
Given that the discovery of gene expression for IL-26 was due to the transformation of T cells by a virus, it seems feasible that IL-26 plays a role in antiviral host defense. In line with this, epithelial cells that constitute a first physical ‘barrier’ against many viruses, do express IL-20R1 [11, 26]. A recent study examined the role of IL-26 in viral infection models of epithelial cells [27]. Here, rhabdovirus vesicular stomatitis infection of epithelial cells was strongly enhanced by stimulation with IL-26, while human cytomegalovirus infection was inhibited and herpes simplex virus type 1 infection remained unaltered by IL-26 stimulation. However, the increased infectivity of vesicular stomatitis virus was independent of the expression of the IL-26 receptor on the epithelial cells, and the investigators attributed the effect of IL-26 to interference between the negatively charged surfaces of viruses and target cells instead of the expected signaling via the IL-26 receptor complex [27]. Moreover, a large-cohort study on patients with chronic infection by hepatitis C virus indicated markedly elevated serum levels of IL-26 in these patients, especially in those with severe disease. This particular study demonstrated IL-26 in infiltrating CD3+ lymphocytes, but not in the resident hepatocytes in fibrotic and inflammatory liver lesions. Due to the functional importance of NK cells in antiviral hepatitis C virus immunity, the authors also investigated the effect of IL-26 on the NK cells of healthy donors, and obtained evidence that the NK cells were sensitive to IL-26 [28]. In fact, the IL-26-stimulated NK cells killed hepatitis C virus-infected hepatocytes in a TRAIL-dependent manner as efficiently as IFN-1-stimulated NK cells [28].
Inflammation
Specifically, it has been established that fibroblast-like synovial cells from patients with RA increase IL-26 secretion in response to IL-1β and IL-17A. Also, monocytes from the referred patients respond to IL-26 in a dose- and time-dependent manner and secrete IL-1β, IL-6 and TNF-α [18]. It is interesting to note that such responses are all prevented in the presence of IL-4, IL-10, IL-13 or TGF-β. While no upregulation of the IL-26 gene was detected in IL-26-stimulated monocytes, these cells showed increased gene expression of IL-24 and macrophage inflammatory protein 3α (CCL20) [18]. In another study, IL-26 stimulated the release of IL-8 in keratinocytes [9]. In an even more recent study, IL-26 stimulated primary immune cells from human airways to release neutrophil-mobilizing cytokines, including IL-1β, TNF-α, IL-8 and GM-CSF [14]. In the same study, IL-26 suppressed the corresponding cytokine release in human primary bronchial epithelial cells [14].
Evidently, there is an intriguing ‘duality’ of IL-26 in terms of displaying classic proinflammatory and anti- inflammatory effects (fig. 3). The gene expression and protein secretion of IL-26 is upregulated in response to archetype proinflammatory mediators, whereas stimulation by IL-26 per se causes the production of the same archetype proinflammatory mediators, plus the recruitment of innate effector cells to the site of infection. Further characterization of these features may prove critical for understanding the immunology of IL-26 under physiological and pathological conditions.
Inflammatory Disorders
Acute Inflammatory Disorders
To the best of our knowledge, no studies have focused on the role of IL-26 in acute inflammatory disorders, such as acute ischemia or the systemic inflammatory response syndrome. However, since IL-26 can be released in high quantities in response to classic proinflammatory stimuli and is involved in mediating neutrophil mobilization in human lungs [14], it seems likely that this intriguing cytokine is involved in acute inflammatory disorders.
Chronic Inflammatory Disorders
RA constitutes a classic example of a chronic inflammatory condition, and elevated concentrations of IL-26 have been detected in the synovial fluid and serum of patients with this disease [18]. Moreover, 2 single-nucleotide polymorphisms in or near the IL-26 gene are associated with an increased risk to develop RA, even though only in a gender-specific manner [29].
Another classic example of chronic inflammatory disorders is Crohn's disease; patients suffering from this disorder display increased gene expression of IL-26 in inflamed colonic mucosa and this expression correlates significantly with upregulated gene expression for IL-22 and IL-8 [11]. In addition, the number of IL-26-expressing cells per se is increased in inflamed tissue, a finding that can, at least in part, be attributed to the infiltration of Th17 cells. This local increased expression of IL-26 is also reflected in the serum of Crohn's patients compared to healthy controls [11]. Another potentially important observation is that the gene expression of IL-26 is increased in the serum and lesional skin tissue of psoriasis patients [30] as well as in the sera of patients suffering from multiple sclerosis [31]. Additional observations that support the role of IL-26 in chronic inflammatory disorders is the fact that IL-26-producing Th17 cells constitute up to 30% of infiltrating T lymphocytes that have been directly isolated from inflamed lesions of patients with psoriasis vulgaris and RA or in the bronchial biopsies of patients with severe asthma [32]. Finally, as recently demonstrated, a third identified single nucleotide polymorphism in the IL-26 gene strongly correlates with the development of the chronic inflammatory disorder ulcerative colitis [33].
Tentatively, observational studies on patients with mucosal-associated infections as well as chronic inflammatory disorders in the intestines, skin and joints all suggest involvement and upregulation of IL-26 in the pathology. Even though the specific pathophysiological role of IL-26 needs to be defined better, there are indications that variations in the IL-26 gene are associated with the development of chronic inflammatory disorders involving mucosal surfaces. Future studies may reveal whether epigenetic alterations of the IL-26 gene or its controlling mechanisms are involved in these disorders.
Cancer
Intriguingly, the stimulation of colon carcinoma cells with recombinant IL-26 causes the upregulation of intercellular adhesion molecule 1 (ICAM-1) protein and the release of IL-10 and IL-8 protein [9]. The functional meaning of these results is difficult to interpret, given that the expression of ICAM-1 is important for the extravasation of leukocytes (particularly neutrophils) into the tissue. Whereas IL-8 recruits neutrophils into the tissue, IL-10 instead counteracts this type of leukocyte mobilization. Thus, the net outcome of these seemingly counteracting events has still to be verified in functional studies. In another study, IL-26 caused decreased proliferation in a colorectal-cancer cell line [11]. In human gastric-cancer cells, on the other hand, IL-26 induces STAT3-dependent upregulation of Bcl-2, Bcl-xl and c-myc malignant carcinoma cells, thereby promoting both cell survival and proliferation [21]. Thus, IL-26 exerts potentially important effects on cancer cells but the current data appear conflicting and it remains for the conceptual importance of IL-26 in cancer to be clarified.
Conclusions
From a conceptual point of view, current understanding of the immunology and pathology of IL-26 remains sparse. Evidently, classic hematopoietic immune cells as well as nonhematopoietic structural cells can constitute sources of IL-26 protein release and both extra- and intracellular bacteria may trigger this release. It seems feasible that IL-26 can drive inflammation by enhancing the influx of neutrophils and stimulating the release of proinflammatory cytokines and chemokines from mature macrophages, monocytes, fibroblasts and epithelial cells. In addition, IL-26 may mobilize Th17 cells in infected or inflamed tissues. Moreover, IL-26 is involved in chronic inflammatory disorders like inflammatory bowel disease and RA, but its role in more acute inflammatory disorders has still to be established. As indicated by the specific molecular and cellular events related to IL-26 in some chronic inflammatory disorders, IL-26 may be involved in autoimmune disease as well. Clearly, the involvement of IL-26 in tumor biology is in need of further study. Thus, even when the limitations of current knowledge are taken into account, IL-26 emerges as an important player in host defense and inflammation, with the potential to constitute a therapeutic target in infections and chronic inflammatory disorders.
References
- 1.Garcia G, Taillé C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Anti-interleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22:251–257. doi: 10.1183/09059180.00004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Franco M, Gerardi MC, Lucchino B, Conti F. Mavrilimumab: an evidence based review of its potential in the treatment of rheumatoid arthritis. Core Evid. 2014;9:41–48. doi: 10.2147/CE.S39770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell CB, Rand H, Bigler J, Kerkof K, Timour M, Bautista E, Krueger JG, Salinger DH, Welcher AA, Martin DA. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828–3836. doi: 10.4049/jimmunol.1301737. [DOI] [PubMed] [Google Scholar]
- 4.Knappe A, Hor S, Wittmann S, Fickenscher H. Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J Virol. 2000;74:3881–3887. doi: 10.1128/jvi.74.8.3881-3887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 6.Jones EA, Flavell RA. Distal enhancer elements transcribe intergenic RNA in the IL-10 family gene cluster. J Immunol. 2005;175:7437–7446. doi: 10.4049/jimmunol.175.11.7437. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh F, Baurin VV, Lewis-Antes A, Shah NK, Smirnov SV, Anantha S, Dickensheets H, Dumoutier L, Renauld JC, Zdanov A, Donnelly RP, Kotenko SV. Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J Immunol. 2004;172:2006–2010. doi: 10.4049/jimmunol.172.4.2006. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Diaz-Rosales P, Martin SA, Secombes CJ. Cloning of a novel interleukin (IL)-20-like gene in rainbow trout Oncorhynchus mykiss gives an insight into the evolution of the IL-10 family. Dev Comp Immunol. 2010;34:158–167. doi: 10.1016/j.dci.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Hör S, Pirzer H, Dumoutier L, Bauer F, Wittmann S, Sticht H, Renauld JC, de Waal Malefyt R, Fickenscher H. The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J Biol Chem. 2004;279:33343–33351. doi: 10.1074/jbc.M405000200. [DOI] [PubMed] [Google Scholar]
- 10.Hummelshoj L, Ryder LP, Poulsen LK. The role of the interleukin-10 subfamily members in immunoglobulin production by human B cells. Scand J Immunol. 2006;64:40–47. doi: 10.1111/j.1365-3083.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 11.Dambacher J, Beigel F, Zitzmann K, De Toni EN, Göke B, Diepolder HM, Auernhammer CJ, Brand S. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58:1207–1217. doi: 10.1136/gut.2007.130112. [DOI] [PubMed] [Google Scholar]
- 12.Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int Immunopharmacol. 2004;4:577–592. doi: 10.1016/j.intimp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 14.Che KF, Tengvall S, Levänen B, Silverpil E, Smith ME, Awad M, Vikström M, Palmberg L, Qvarfordt I, Sköld M, Lindén A. Interleukin-26 in antibacterial host defense of human lungs. Effects on neutrophil mobilization. Am J Respir Crit Care Med. 2014;190:1022–1031. doi: 10.1164/rccm.201404-0689OC. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, Haugen H, Jelinek L, Kelly JD, Madden K, Maurer MF, Parrish-Novak J, Prunkard D, Sexson S, Sprecher C, Waggie K, West J, Whitmore TE, Yao L, Kuechle MK, Dale BA, Chandrasekher YA. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 16.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 17.Volpe E, Touzot M, Servant N, Marloie-Provost MA, Hupé P, Barillot E, Soumelis V. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114:3610–3614. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- 18.Corvaisier M, Delneste Y, Jeanvoine H, Preisser L, Blanchard S, Garo E, Hoppe E, Barré B, Audran M, Bouvard B, Saint-André JP, Jeannin P. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012;10:e1001395. doi: 10.1371/journal.pbio.1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolk K, Mitsui H, Witte K, Gellrich S, Gulati N, Humme D, Witte E, Gonsior M, Beyer M, Kadin ME, Volk HD, Krueger JG, Sterry W, Sabat R. Deficient cutaneous antibacterial competence in cutaneous T-cell lymphomas: role of Th2-mediated biased Th17 function. Clin Cancer Res. 2014;20:5507–5516. doi: 10.1158/1078-0432.CCR-14-0707. [DOI] [PubMed] [Google Scholar]
- 20.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You W, Tang Q, Zhang C, Wu J, Gu C, Wu Z, Li X. IL-26 promotes the proliferation and survival of human gastric cancer cells by regulating the balance of STAT1 and STAT3 activation. PLoS One. 2013;8:e63588. doi: 10.1371/journal.pone.0063588. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Guerra-Laso JM, Raposo-Garcia S, Garcia-Garcia S, Diez-Tascon C, Rivero-Lezcano OM. Microarray analysis of Mycobacterium tuberculosis-infected monocytes reveals IL-26 as a new candidate gene for tuberculosis susceptibility. Immunology. 2015;144:291–301. doi: 10.1111/imm.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanayama A, Miyamoto Y. Apoptosis triggered by phagocytosis-related oxidative stress through FLIPS down-regulation and JNK activation. J Leukoc Biol. 2007;82:1344–1352. doi: 10.1189/jlb.0407259. [DOI] [PubMed] [Google Scholar]
- 24.Milla C, Yang S, Cornfield DN, Brennan ML, Hazen SL, Panoskaltsis-Mortari A, Blazar BR, Haddad IY. Myeloperoxidase deficiency enhances inflammation after allogeneic marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L706–L714. doi: 10.1152/ajplung.00015.2004. [DOI] [PubMed] [Google Scholar]
- 25.Braum O, Pirzer H, Fickenscher H. Interleukin-26, a highly cationic T-cell cytokine targeting epithelial cells. Antiinflamm Antiallergy Agents Med Chem. 2012;11:221–229. doi: 10.2174/1871523011202030221. [DOI] [PubMed] [Google Scholar]
- 26.Braum O, Klages M, Fickenscher H. The cationic cytokine IL-26 differentially modulates virus infection in culture. PLoS One. 2013;8:e70281. doi: 10.1371/journal.pone.0070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miot C, Beaumont E, Duluc D, Le Guillou-Guillemette H, Preisser L, Garo E, Blanchard S, Hubert Fouchard I, Créminon C, Lamourette P, Fremaux I, Calès P, Lunel-Fabiani F, Boursier J, Braum O, Fickenscher H, Roingeard P, Delneste Y, Jeannin P. IL-26 is overexpressed in chronically HCV-infected patients and enhances TRAIL-mediated cytotoxicity and interferon production by human NK cells. Gut. 2014 doi: 10.1136/gutjnl-2013-306604. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Goris A, Marrosu MG, Vandenbroeck K. Novel polymorphisms in the IL-10 related AK155 gene (chromosome 12q15) Genes Immun. 2001;2:284–286. doi: 10.1038/sj.gene.6363772. [DOI] [PubMed] [Google Scholar]
- 29.Michalak-Stoma A, Pietrzak A, Szepietowski JC, Zalewska-Janowska A, Paszkowski T, Chodorowska G. Cytokine network in psoriasis revisited. Eur Cytokine Netw. 2011;22:160–168. doi: 10.1684/ecn.2011.0294. [DOI] [PubMed] [Google Scholar]
- 30.Esendagli G, Kurne AT, Sayat G, Kilic AK, Guc D, Karabudak R. Evaluation of Th17-related cytokines and receptors in multiple sclerosis patients under interferon beta-1 therapy. J Neuroimmunol. 2013;255:81–84. doi: 10.1016/j.jneuroim.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Vandenbroeck K, Cunningham S, Goris A, Alloza I, Heggarty S, Graham C, Bell A, Rooney M. Polymorphisms in the interferon-gamma/interleukin-26 gene region contribute to sex bias in susceptibility to rheumatoid arthritis. Arthritis Rheum. 2003;48:2773–2778. doi: 10.1002/art.11236. [DOI] [PubMed] [Google Scholar]
- 32.Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, Barmada MM, Klei L, Daly MJ, Abraham C, Bayless TM, Bossa F, Griffiths AM, Ippoliti AF, Lahaie RG, Latiano A, Paré P, Proctor DD, Regueiro MD, Steinhart AH, Targan SR, Schumm LP, Kistner EO, Lee AT, Gregersen PK, Rotter JI, Brant SR, Taylor KD, Roeder K, Duerr RH. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216–220. doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Xu WD, Zhu Y, Wen PF, Leng RX, Pan HF, Ye DQ. Serum levels of cytokines in systemic lupus erythematosus: association study in a Chinese population. Z Rheumatol. 2013 doi: 10.1007/s00393-013-1274-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]