Abstract
Bacterial infection often follows virus infection due to pulmonary interferon-γ (IFN-γ) production during virus infection, which down-regulates macrophage phagocytosis. The molecular mechanisms for this process are still poorly understood. In the present study, IFN-γ treatment significantly inhibited the ability of mouse macrophages to phagocytize nonopsonized chicken red blood cells (cRBCs), bacteria and beads in vitro, while it enhanced IgG- and complement-opsonized phagocytosis. IFN-γ treatment decreased the expression of MARCO (macrophage receptor with collagenous structure) in macrophages. Macrophages showed lower binding to and phagocytic ability of cRBCs when MARCO was blocked with antibody. In addition, IFN-γ induced high activity of mTOR (mammalian target of rapamycin) and decreased the expression of c/EBPβ (CCAAT enhancer-binding protein β) in macrophages. Rapamycin, a specific mTOR inhibitor, significantly reversed the inhibitory effect of IFN-γ on nonopsonized phagocytosis of macrophages and restored c/EBPβ and MARCO expression. Biochemical assays showed that c/EBPβ directly bound to the MARCO gene promoter. Rapamycin significantly hampered the viral-bacterial synergy and protected influenza-infected mice from subsequent bacterial infection. Thus, IFN-γ inhibited the nonopsonized phagocytosis of macrophages through the mTOR-c/EBPβ-MARCO pathway. The present study offered evidence indicating that mTOR may be one of the key target molecules for the prevention of secondary bacterial infection caused by primary virus infection.
Key Words: Cytokines, Host defense, Macrophages, Phagocytosis
Introduction
Influenza virus and Streptococcus pneumoniae are the two pathogens that cause the majority of respiratory infections in humans. Secondary bacterial pneumonia is a major cause of excess morbidity and mortality during influenza pandemics [1, 2]. Several mechanisms, including suppression of neutrophil function and increased bacterial adherence to epithelia, may be involved in this viral-bacterial synergy [3, 4]. It has been reported that interferon-γ (IFN-γ), which is largely produced by T cells and other immune cells in the lung after viral infection [5, 6, 7], significantly inhibits alveolar macrophage-mediated microbial clearance and, consequently, leads to enhanced susceptibility to secondary bacterial infection [6]. Phagocytosis is a key cellular process during homeostasis, infection and tissue damage/repair. Phagocytosis, including opsonized and nonopsonized types, is essential for macrophages to clear foreign particles and cellular debris. Fc receptors (FcRs) or complement receptors (CRs) on the surface of professional phagocytic cells bind to the particles coated with IgG or complement, and then trigger actin-mediated ingestion of the particles [8, 9]. The role of IFN-γ in regulating the phagocytic ability of macrophages is currently controversial. It is reported that IFN-γ enhances the antimicrobial activity of macrophages including the up-regulation of FcR and CR expression and the enhanced phagocytosis of opsonized pathogens [6, 10, 11, 12]. IFN-γ has been shown to enhance clearance of apoptotic cells by several macrophage populations although the mechanisms have not been fully elucidated [13]. However, other studies showed that IFN-γ inhibited macrophage phagocytosis and killing ability [14]. In the current study, after observing the direct inhibitory effect of IFN-γ on nonopsonized phagocytosis of macrophages, we studied the underlying molecular mechanisms. We found that IFN-γ suppressed nonopsonized phagocytosis of macrophages by down-regulating the expression of MARCO (macrophage receptor with collagenous structure), the major binding receptor on the surface of macrophages for nonopsonized particles [15, 16, 17], via an mTOR (mammalian target of rapamycin)-c/EBPβ (CCAAT enhancer-binding protein β) pathway.
Materials and Methods
Mice
Six- to 8-week-old C57BL/6 mice were purchased from the Beijing University Experimental Animal Center (Beijing, PR China). All mice were bred and maintained in specific pathogen-free conditions. Approval for all animal experiments was obtained by the Animal Ethics Committee of the Institute of Zoology, Beijing, PR China.
Antibodies
Anti-MARCO polyclonal antibody was purchased from Santa Cruz Biotechnology. Anti-c/EBPβ, anti-c/EBPα, anti-p-Akt308 and anti-p-S6 polyclonal antibodies were purchased from Cell Signaling Technology. Anti-β-actin monoclonal antibody (mAb) was purchased from Sigma.
Virus
Influenza A virus A/PR8/34 was propagated in the allantoic cavities of 10-day-old embryonic chicken eggs in specific pathogen-free conditions at 37°C for 2 days. Then, the virus in the allantoic fluid was inoculated into the MDCK (Madin-Darby canine kidney) cell line (ATCC CCL-34) for plaque assay [18]. Cells were cultured in DMEM supplemented with 10% fetal bovine serum.
Isolation and Culture of Peritoneal Macrophages
Peritoneal macrophages (PEMs) were isolated as described in previous reports [19, 20], with minor modifications. In brief, PEMs were harvested from C57BL/6 mice. The mice were sacrificed, and about 5 ml of sterile PBS were injected into the abdomen. After a gentle massage for 3 min, the peritoneal fluid was collected. This process was repeated three times. After centrifugation, PEMs were plated on culture dishes and incubated for macrophage isolation. The purity of F4/80+ macrophages was more than 90%, as reported previously [21, 22].
Macrophage Adhesion Ability Assay
It has been reported that incubation at 4°C or addition of cytochalasin D, an inhibitor of actin assembly and polymerization, decreased the microparticle uptake of macrophages [23, 24, 25]. PEMs were cultured with or without IFN-γ (1 ng/ml) for 12 h, and then they were treated with cytochalasin D (5 µg/ml) for 30 min followed by co-incubation with 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled chicken red blood cells (cRBCs) for 2 h. Additionally, some PEMs were co-incubated with cRBCs at 4°C in a freezer overnight in the presence or absence of IFN-γ. Adhesive cells were assessed by flow cytometry (FCM) or under a microscope.
IFN-γ Treatment
PEMs were treated with 1 ng/ml of recombinant mouse IFN-γ (PeproTech) for 12 h in vitro [26]. Phagocytosis of macrophages was assayed in vitro as described below.
Phagocytosis of cRBCs and Escherichia coli AB1157
A fresh single-cell suspension of cRBCs was obtained. A suspension of E. coli containing a bacterial concentration corresponding to 108 colony-forming units (CFU) was used. After two washes with PBS, 1 × 107 cells/ml cRBCs or 108 CFU E. coli were labeled with 5.0 μM CFSE (Molecular Probes, Eugene, Oreg., USA) for 15 min at 37°C. These cells were then washed thoroughly and resuspended at a concentration of 1 × 107 cells/ml. Cell viability was usually more than 95% by trypan blue exclusion. F4/80+ PEMs (5 × 105) were coincubated with 5 × 106 CFSE-labeled cRBCs or 5 × 107 CFU CFSE-labeled E. coli in 48-well plates for 2 h. The cells were firstly stained with anti-mouse FcγR mAb (2.4G2) to block any nonspecific staining and then stained with PE-conjugated anti-F4/80 mAb (BM8) [27]. After three washes with FCM buffer (PBS, pH 7.2, containing 0.1% NaN3 and 0.5% bovine serum albumin), phagocytosis (%) of F4/80+ cells was determined by FCM. At least 10,000 cells were assayed using a FASCalibur flow cytometer (Becton Dickinson), and data were analyzed with FCS expression v3.
Survival of S. pneumoniae after Coculture with Macrophages
PEMs (2 × 106 cells/well) were cultured with 1 × 107 CFU S. pneumoniae in 48-well plates for 2 h. S. pneumoniae that were not phagocytosed by macrophages were washed gently with 0.5 ml PBS for 3 times. After centrifugation, S. pneumoniae were quantified by CFU assay.
Phagocytosis of Nonopsonized and Opsonized Beads
Assays for the phagocytosis of nonopsonized and opsonized beads were performed as described previously [28]. Briefly, FITC-labeled beads (2.0 μm; Sigma, St. Louis, Mo., USA) were incubated with mouse IgG (5 mg/ml) or complement (normal rabbit serum, diluted 1:10 in PBS) for 1 h at 37°C. The beads were then rinsed three times with PBS and reconstituted with culture medium. The coated beads were phagocytosed by adherent PEMs at a ratio of 10:1. The uncoated beads were used as nonopsonized phagocytosis assays. After phagocytosis for 30 min, nonadherent beads were removed with cold PBS, and cells were stained with anti-mouse FcγR mAb (2.4G2) to block nonspecific staining and then stained with PE-conjugated anti-F4/80 mAb. Cells were assayed by FCM.
Rapamycin Treatment in vivo
Rapamycin was prepared to a concentration of 2.5 mg/ml in DMSO and stored at −80°C. In some assays, PEMs were treated with rapamycin at a concentration of 100 nM for 12 h in vitro. For the in vivo assays, mice were treated with rapamycin diluted in sterile PBS at a dose of 750 μg/kg body weight or the control buffer for 2 days via intraperitoneal injection [29]. Meanwhile, we treated mice with 2 μg of recombinant mouse IFN-γ in 100 μl of PBS or control buffer via intraperitoneal injection for 2 days. PEMs were harvested and their phagocytotic ability was detected as mentioned above.
Real-Time PCR Assay
Total RNA was isolated from PEMs treated with or without IFN-γ using TRIzol (Invitrogen, Carlsbad, Calif., USA). Total RNA (1 µg) was converted into cDNA by reverse transcription with AMV (Takara). Gene expression was quantified with real-time quantitative PCR (iCycler, Bio-Rad) using SYBR Green dye and the expression level of the target gene was normalized with HPRT [30]. The following MARCO RT-PCR primers were used: MARCO (forward, 5′-CCACCTGATCCTGCTCACGGC-3′; reverse, 5′-GCCCACTGCAGCGAGAAGAAGG-3′), as described [31]. HPRT mRNA served as an internal control (forward, 5′-AGTACAGCCCCAAAATGGTTAAG-3′; reverse, 5′-CTTAGGCTTTGTATTTGGCTTTTC-3′).
Chromatin Immunoprecipitation
For chromatin immunoprecipitation (ChIP), a ChIP assay kit from Beyotime was used, and chromatin was prepared for immunoprecipitation according to the manufacturer's instructions [20]. Sonicated chromatin was immunoprecipitated with either 5 µg of anti-c/EBPβ antibodies (Cell Signaling Technology) or normal rabbit IgG antibody as negative control. Immunoprecipitated DNA was subsequently analyzed by PCR using primers specific for the MARCO promoter region. Input chromatin was analyzed for β-actin as a positive control. PCR products were analyzed by 2.5% agarose/ethidium bromide gel electrophoresis. The following primers were used for the ChIP assay: MARCO (forward, 5′-TGCCACAGGTTCAACAGGGA-3′; reverse, 5′-TGGGTTCAAATCTCCAGACC-3′) and β-actin (forward, 5′-ACCTGTTACTTTGGGAGTGGCAAGC-3′; reverse, 5′-GTCGTCCCAGTTGGTAACAATGCC-3′).
Mouse Model of Infection
We performed virus challenge of anesthetized mice by intranasal inoculation with 100 plaque-forming units (PFU) of A/PR8/34 influenza virus in 50 μl PBS. We inoculated anesthetized mice intranasally with 1 × 105 CFU S. pneumoniae in 50 μl of Ringer's solution (NaCl 123 mM, CaCl2 1.5 mM and KCl 5 mM, pH 7.3) to induce bacterial pneumonia by 7 days after primary viral infection. Mice without virus infection were used as controls. In addition, some mice received rapamycin at a dose of 750 μg/kg body weight for 2 days via intraperitoneal injection 1 day before S. pneumoniae infection [29]. Mouse survival and body weight were followed daily. In some experiments, the bacterial load in the lung was detected by 4 h after S. pneumoniae infection.
Enzyme-Linked Immunosorbent Assay for Anti-S. pneumoniae Antibodies in the Sera
Briefly, 1 × 109 CFU S. pneumoniae were sonicated in enzyme-linked immunosorbent assay (ELISA) coating buffer (50 nM, pH 9.6, bicarbonate buffer). The bacterial lysate was coated onto ELISA plates. The ELISA plates were incubated with BSA at 4°C overnight to block nonspecific binding. The mouse serum was diluted in PBS 1:1,000 and 1:5,000, respectively. After incubation at 37°C for 1 h, the plates were washed and detected with horseradish peroxidase conjugated anti-mouse IgG antibody [32]. The positive serum was derived from the mice infected with pneumococci for 4 weeks. The solution of substrate 3,3′,5,5′-tetramethylbenzidine (BioLegend) was added for 10 min, and absorbance was measured at 450 nm by an ELISA reader.
Detection of IFN-γ in the Bronchoalveolar Lavage Fluid
Mice were inoculated with 100 PFU of A/PR8/34 influenza virus. On day 7 after infection, mice were treated with rapamycin diluted in sterile PBS at a dose of 750 μg/kg body weight via intraperitoneal injection. Bronchoalveolar lavage fluid (BALF) was collected by washing the lung twice with 1 ml PBS, pH 7.2. The amount of IFN-γ in BALF various days after influenza infection was assessed by ELISA.
Statistical Analysis
All data are presented as the mean ± SD. One- or two-way ANOVA was used for comparisons among multiple groups with SPSS 16.0 software according to the type of the data. Student's unpaired t test was used to compare means between two groups. A value of p < 0.05 was considered to be statistically significant.
Results
IFN-γ Inhibits Nonopsonized Phagocytosis but Increases Opsonized Phagocytosis of Macrophages
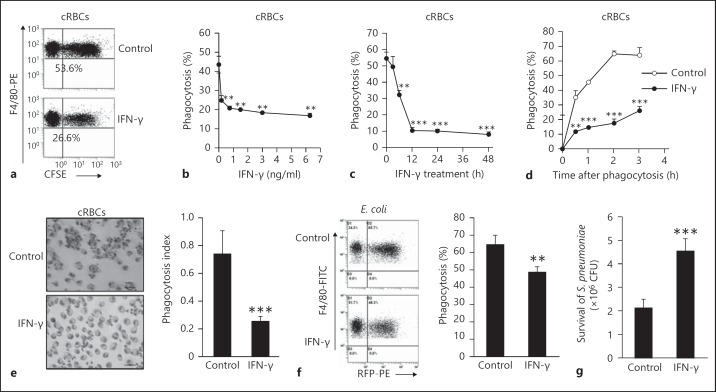
To determine the role of IFN-γ on nonopsonized phagocytosis of macrophages, PEMs isolated from C57BL/6 mice [33] were used to phagocytose nonopsonized particles (cRBCs and E. coli) in the presence or absence of IFN-γ. The phagocytosis data indicated that IFN-γ suppressed nonopsonized phagocytosis in a dose- and time-dependent manner (fig. 1a-c). The efficiency of phagocytosis of IFN-γ-treated PEMs was significantly lower than that of control PEMs even if increasing phagocytosis time (fig. 1d). In parallel, when phagocytic efficiency was determined microscopically, the percentage of phagocytosed cRBCs of IFN-γ-treated PEMs was less than half that of the control PEMs (fig. 1e). In addition, PEMs treated with IFN-γ had only two thirds of the phagocytic capacity to E. coli (fig. 1f). Furthermore, in coculture experiments of S. pneumonia with macrophages in the presence of IFN-γ or control buffer, significantly more S. pneumonia survived in the coculture medium of IFN-γ-treated macrophages compared with untreated macrophages (p < 0.001; fig. 1g). These data suggest that IFN-γ had an inhibitory effect on nonopsonized phagocytosis of macrophages.
Fig. 1.
IFN-γ significantly inhibited the nonopsonized phagocytosis of macrophages. The F4/80+ PEMs isolated from C57BL/6 mice were subjected to phagocytic nonopsonized CFSE-cRBCs (5 × 106) in the presence or absence of IFN-γ (1 ng/ml), and were then stained with PE-conjugated anti-F4/80 mAb (BM8). After three washes with FCM buffer, phagocytosis (%) of F4/80+ cells was determined by FCM. a One representative FCM displays gated F4/80+ cells. b, c IFN-γ inhibited the nonopsonized phagocytosis of macrophages in a dose- (b) and time-dependent manner (c). d IFN-γ inhibited the nonopsonized phagocytosis of macrophages even after prolonged phagocytosis as determined by FCM. e IFN-γ inhibited the nonopsonized phagocytosis of macrophages as determined under a microscope. ×40. f The freshly isolated F4/80+ PEMs were cocultured with CFSE-labeled E. coli (5 × 107) in the presence or absence of IFN-γ, and then phagocytosis (%) of F4/80+ cells was determined by FCM. g Survival of S. pneumoniae after coculture with IFN-γ-treated and control macrophages. Data are the mean ± SD (n = 3–5/group) from one of at least two independent experiments with identical results. * p < 0.05, ** p < 0.01 and *** p < 0.001, vs. the control group or the indicated group. PE = Phycoerythrin; RFP = red fluorecent protein.
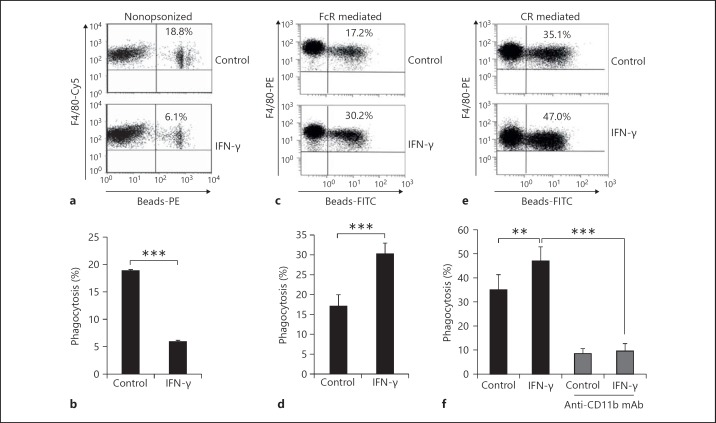
To determine whether IFN-γ impacts opsonized phagocytosis of macrophages, PEMs isolated from C57BL/6 mice were used to phagocytose FITC-glass beads opsonized with none, IgG or complements in the presence or absence of IFN-γ. As shown in figure 2, in parallel with the results showing that IFN-γ inhibited the phagocytosis of macrophages to cRBCs and E. coli, IFN-γ significantly decreased the phagocytosis of macrophages to nonopsonized FITC-glass beads (p < 0.001; fig. 2a). However, IFN-γ significantly enhanced opsonized phagocytosis of PEMs regardless of its dependence on IgG (FcR) or complements (CR, CD11b; p < 0.01; fig. 2c-f). Collectively, these results indicate that IFN-γ had distinct effects on opsonized and nonopsonized phagocytic macrophage pathways.
Fig. 2.
IFN-γ significantly inhibited nonopsonized phagocytosis of macrophages but enhanced opsonized phagocytosis of macrophages. F4/80+ PEMs isolated from C57BL/6 mice were subjected to phagocytic FITC-glass beads without opsonized treatment (a, b) or beads pre-opsonized with IgG (c, d) or complement (e, f) in the presence or absence of IFN-γ. Phagocytosis (%) of F4/80+ cells was determined by FCM. Data are the mean ± SD (n = 3–5/group) from one of at least two independent experiments with identical results. ** p < 0.01 and *** p < 0.001, vs. the indicated group. PE = Phycoerythrin.
IFN-γ Decreased the Adhesive Ability to Target Cells of Macrophages
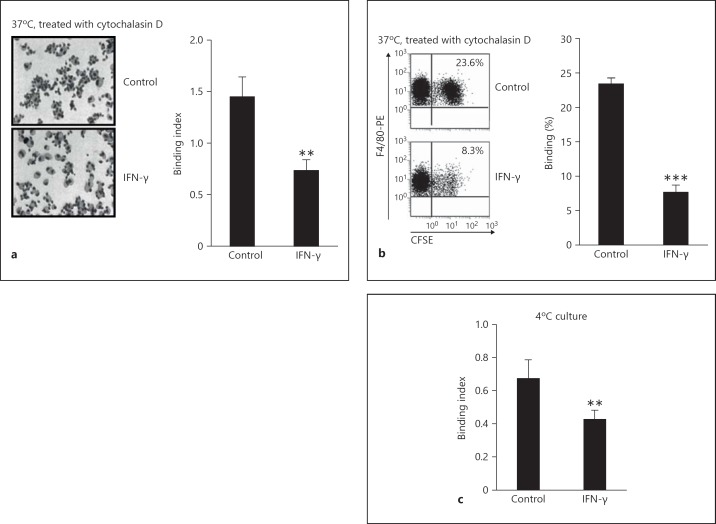
We examined whether IFN-γ suppressed the adhesive ability of PEMs to cRBCs using two methods described in the Materials and Methods. PEMs treated with cytochalasin D plus IFN-γ only had about one third of the adhesive ability of control PEMs (p < 0.01; fig. 3a, b). Consistent with the above results, the adhesive ability of PEMs treated with IFN-γ was reduced to ∼70% in control PEMs incubated at 4°C (p < 0.01; fig. 3c). Together, these data suggest that IFN-γ inhibited the adhesive ability to nonopsonized target cells of macrophages.
Fig. 3.
IFN-γ significantly suppressed the adhesive ability of macrophages to nonopsonized cRBCs. The freshly isolated PEMs were cultured with or without IFN-γ (1 ng/ml) for 12 h and were treated with cytochalasin D (5 µg/ml) for 30 min followed by co-incubation with CFSE-labeled cRBCs for 2 h. a The binding ability was observed under a microscope. ×40. b Adhesive ability of F4/80+ macrophages to nonopsonized cRBCs was determined by FCM. c PEMs were co-incubated with cRBCs at 4°C in a freezer overnight in the presence or absence of IFN-γ (1 ng/ml). The adhesive ability of F4/80+ macrophages to nonopsonized cRBCs was determined by FCM. Data are the mean ± SD (n = 3–5/group) from one of two independent experiments with identical results. ** p < 0.01 and *** p < 0.001, vs. the control group.
IFN-γ Decreased Nonopsonized Phagocytosis of Macrophages through Down-Regulation of MARCO Expression
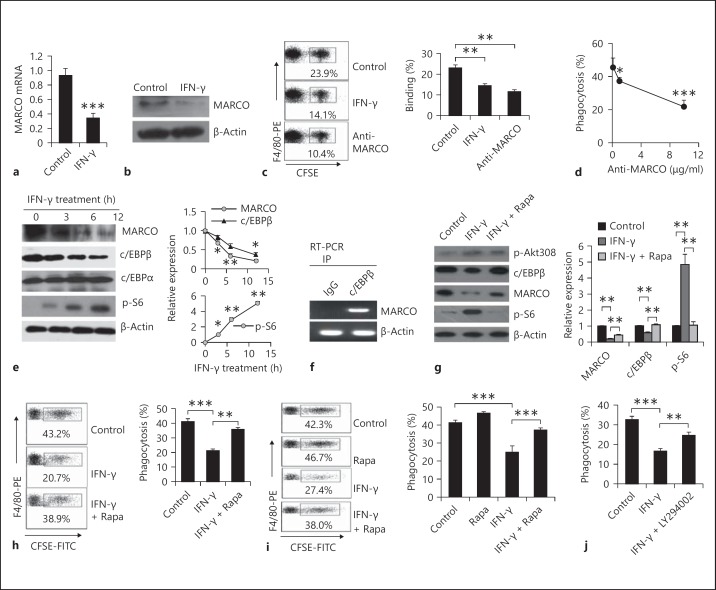
Several lines of evidence indicate that MARCO is the major binding receptor on the surface of macrophages for nonopsonized particles [15, 16, 17]. MARCO belongs to the scavenger receptor family characterized by their broad ligand specificity and thought to be pattern recognition receptors involved in innate immune recognition [34, 35, 36, 37]. We, therefore, investigated whether IFN-γ decreased the expression of MARCO on macrophages. Total mRNA was isolated from PEMs treated with or without IFN-γ and was subjected to real-time PCR using MARCO-specific primers. The mRNA level of MARCO was decreased in IFN-γ-treated PEMs compared to that of control PEMs (fig. 4a). To further analyze the inhibitory function of IFN-γ on PEMs, we collected PEMs treated with or without IFN-γ and lysed with RIPA buffer containing protease inhibitors. The lysates were subjected to Western blot with the anti-MARCO antibody. Consistently, the MARCO protein level of IFN-γ-treated PEMs was reduced to about 30% of control PEMs (fig. 4b). These data indicate that the reduced efficacy of macrophages to phagocytize nonopsonized particles is likely linked to down-regulation of MARCO protein levels.
Fig. 4.
The mTOR-c/EBPβ-MACRO pathway was involved in IFN-γ-reduced nonopsonized phagocytosis of macrophages. The freshly isolated mouse PEMs were treated with IFN-γ for 12 h. MARCO mRNA (a) and protein (b) expression were determined by real-time PCR and Western blots. The adhesive (c) and phagocytic (d) ability of PEMs was examined by FCM after pretreatment with the anti-MARCO antibody. e PEMs were stimulated with IFN-γ for 12 h, and then lysed for Western blot to analyze the expression of MARCO, c/EBPβ, c/EBPα and p-S6. The ratios of the intensity of each dot to the β-actin dot were summarized. f PEMs were subjected to ChIP using anti-c/EBPβ antibody or normal mouse IgG. c/EBP-binding regions of the MARCO gene were amplified by specific primers as described in the Materials and Methods. g PEMs were treated with IFN-γ alone or IFN-γ plus rapamycin (100 nM/ml) and then lysed. The cell lysate was analyzed by Western blot with the indicated antibodies. The ratio of the intensity of each dot to the β-actin dot was summarized. h PEMs were primed with IFN-γ alone or IFN-γ plus rapamycin (100 nM) for 12 h. These PEMs were then cocultured with CSFE-labeled cRBCs for 2 h at 37°C. The phagocytosis percentage of the F4/80+ cells was determined by FCM. i C57BL/6 mice were intraperitoneally injected with IFN-γ alone (2 μg/mouse) or IFN-γ plus rapamycin (750 μg/kg body weight) for 2 days. PEMs were isolated and subjected to phagocytosis assays in vitro. j PEMs were primed with IFN-γ alone or IFN-γ plus LY294002 (20 μM). These cells were then cocultured with CSFE-labeled cRBCs as described in the Materials and Methods. Phagocytosis of F4/80+ cells was determined by FCM. Data are the mean ± SD (n = 3–5/group) from one of at least two independent experiments with identical results. Rapa = Rapamycin. * p < 0.05, ** p < 0.01 and *** p < 0.001, vs. the control group or the indicated group. PE = Phycoerythrin; IP = immunoprecipitation.
To confirm whether the decreased MARCO level was responsible for the down-regulation of nonopsonized phagocytosis induced by IFN-γ, we detected the adhesive and phagocytic abilities of PEMs blocked with the anti-MARCO antibody. The adhesive capacity of PEMs blocked with the anti-MARCO antibody was about 50% that of control PEMs (fig. 4c). Moreover, treatment with the anti-MARCO antibody impaired phagocytosis of macrophages to cRBCs in a dose-dependent manner (fig. 4d). Taken together, IFN-γ inhibited nonopsonized phagocytosis of macrophages through down-regulation of MARCO expression.
IFN-γ Decreased Nonopsonized Phagocytosis of Macrophages by the mTOR-c/EBPβ Pathway
In a previous report, MARCO expression was deficient in c/EBPβ-knockout mice [31]. To determine whether c/EBPβ was involved in the IFN-γ-induced reduction in nonopsonized phagocytosis of macrophages, we examined c/EBPβ expression of IFN-γ-treated PEMs. Meanwhile, c/EBPα, another c/EBP family member, was detected as a control. As shown in figure 4, c/EBPβ expression was gradually reduced along with the prolongation of IFN-γ treatment and synchronized with MARCO expression (fig. 4e). We performed ChiP assays to investigate whether MARCO expression was directly regulated by the upstream promoter element c/EBPβ. ChIP assay results revealed that c/EBPβ could directly bind to the c/EBP-binding site of the MARCO gene promoter range (fig. 4f).
It was demonstrated that rapamycin could increase the protein level of c/EBPβ in a bacterial keratitis model [38]. We first confirmed that mTORC1 was activated by IFN-γ treatment (fig. 4e) using Western blot, which is consistent with the previously reported results [39]. Therefore, we speculated that inhibition of mTOR by rapamycin may rescue the IFN-γ-inhibitory effect on c/EBPβ expression in PEMs. To investigate this hypothesis, PEMs were treated with IFN-γ plus rapamycin for 12 h, and the lysate extracted from differently treated PEMs was subjected to Western blot with the indicated antibodies. We found that rapamycin could, at least partially, recover the IFN-γ-inhibitory effect on c/EBPβ and MARCO expression (fig. 4g). We next determined whether rapamycin could recover the suppressive effect of IFN-γ on nonopsonized phagocytosis of macrophages. As shown in figure 4h, rapamycin treatment significantly recovered the suppressive influence induced by IFN-γ on nonopsonized phagocytosis of macrophages in vitro. To further verify the role of mTOR on nonopsonized phagocytosis of macrophages in vivo, we treated mice with IFN-γ in the presence or absence of rapamycin, as described in the Materials and Methods. As expected, IFN-γ treatment significantly inhibited nonopsonized phagocytosis of macrophages, whereas rapamycin also reversed this effect of IFN-γ (fig. 4i). In addition, we have included LY294002, an inhibitor of phosphatidylinositol 3-kinase (PI3K), to verify involvement of the PI3K-Akt pathway. The data showed that LY294002 (20 μM) significantly reversed the inhibitory effect of IFN-γ on the nonopsonized phagocytosis function of macrophages (p < 0.01; fig. 4j). These data collectively demonstrate that IFN-γ participates in down-regulating nonopsonized phagocytosis through the PI3K-Akt-mTOR-c/EBPβ-MARCO pathway.
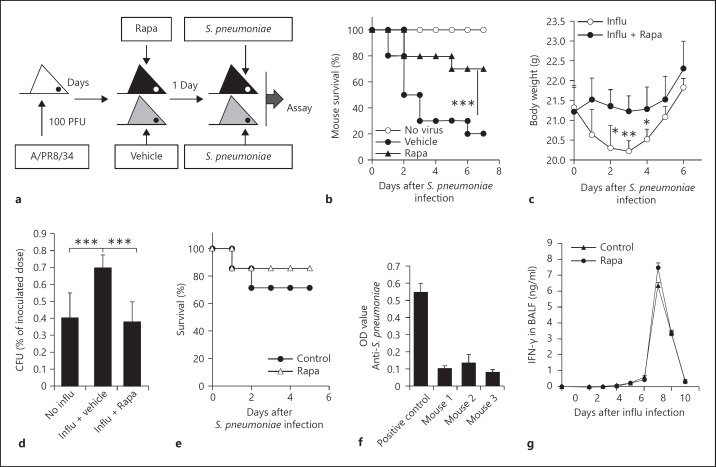
Rapamycin Protected Mice from Bacterial Attack during Recovery from Influenza Infection
It was previously reported that pulmonary IFN-γ produced during T cell responses to influenza infection in mice inhibited initial bacterial clearance from the lung by alveolar macrophages [6]. To better understand the biological significance of our finding, we examined whether rapamycin could protect mice from S. pneumoniae infection during recovery from influenza infection. We performed viral challenge by intranasal inoculation with 100 PFU of A/PR8/34 influenza virus and treatment of mice with rapamycin at a dose of 750 μg/kg body weight for 2 days beginning 1 day before S. pneumoniae infection, as described in figure 5a. Notably, treatment with rapamycin resulted in a much higher number of mice surviving S. pneumoniae infection than did treatment with control buffer (fig. 5b). Moreover, rapamycin-treated mice lost less body weight and cleared bacteria faster than control mice (p < 0.001; fig. 5c, d). In addition, rapamycin treatment failed to cause significant effects on the survival in S. pneumoniae-infected mice if the hosts did not receive a pre-infection of influenza (fig. 5e). On the other hand, we also studied the importance of opsonized phagocytosis mediated by natural antibody directed against S. pneumoniae in this model. These mice had very low levels of anti-S. pneumoniae antibody in the sera compared with the S. pneumoniae-infected positive control mice by ELISA assays (p < 0.001; fig. 5f). In order to determine whether short-term treatment with rapamycin affects local IFN-γ levels in the lung of influenza-infected mice, we assessed IFN-γ levels in BALF after infection with influenza. IFN-γ levels in BALF of rapamycin-treated mice were similar to those of rapamycin-untreated mice after influenza infection (p > 0.05; fig. 5g). Thus, we speculated that nonopsonized phagocytosis may be the major approach for macrophages to phagocytize S. pneumoniae in these mice, although we could not at all exclude opsonized phagocytosis in this model. Thus, treatment with rapamycin during recovery from influenza infection enhanced protection against S. pneumoniae.
Fig. 5.
Rapamycin (Rapa) treatment enhanced host protection against S. pneumoniae infection following influenza (influ) infection. a Experimental design. Mice were infected with 100 PFU of A/PR8/34 influenza virus. On day 6 after infection, mice were injected with rapamycin at a dose of 750 μg/kg body weight for 2 days beginning 1 day prior to S. pneumoniae infection on day 7 after virus infection. b, c Survival (b) and body weight (c) of C57BL/6 mice given intraperitoneal injection of rapamycin or control buffer were analyzed. d The bacterial loads in mice were detected as described in the Materials and Methods. Survived S. pneumoniae (%) are shown (mean CFU ± SD, 4 mice/group). * p < 0.05, ** p < 0.01 and *** p < 0.001, vs. the control group or the indicated group. e Naive C57BL/6 mice were infected with 1 × 105 CFU S. pneumoniae. In addition, some mice were injected with rapamycin at a dose of 750 μg/kg body weight or control buffer for 2 days beginning 1 day before S. pneumoniae infection. The survival of mice was analyzed (7 mice/group). f The natural anti-S. pneumoniae antibodies in the sera of 6- to 8-week-old C57BL/6 mice were detected by ELISA. The sera of S. pneumoniae-infected C57BL/6 mice were used as a positive control. g Rapamycin treatment did not significantly alter the levels of IFN-γ in the BALF of influenza-infected mice. Data are the mean ± SD (n = 3–5 mice/group). * p < 0.05, ** p < 0.01 and *** p < 0.001, vs. the control group.
Discussion
Macrophages play critical roles in the initial defense against pathogens and macrophage immunity significantly influences subsequent acquired immune responses [40, 41]. Phagocytosis is one of the key processes for host defense mediated by macrophages. So far, most of our understanding of the signaling pathways leading to phagocytosis in macrophages comes from studies of opsonized phagocytosis, especially FcR-mediated phagocytosis. There are few reports about the mechanism of regulation of nonopsonized phagocytosis. Here, we have elucidated the mechanism by which IFN-γ regulated nonopsonized phagocytosis of macrophages through the mTORC1-c/EBPβ-MARCO pathway.
The present studies demonstrated that IFN-γ had dual functions on regulating phagocytosis of macrophages. IFN-γ priming has been shown to enhance FcR- and CR-mediated phagocytosis of macrophages, but to reduce nonopsonized phagocytosis. We found that the expression of MARCO, the major nonopsonized phagocytosis receptor, was significantly decreased in macrophages, not only at the mRNA but also at the protein level, by IFN-γ treatment. In addition, macrophages blocked with anti-MARCO antibody showed lower adhesive and phagocytic ability to cRBCs. These data provide evidence that IFN-γ participated in the down-regulation of nonopsonized phagocytosis through inhibition of MARCO expression on macrophages.
Rapamycin, a specific inhibitor of mTOR, remarkably reversed the IFN-γ-suppressive effect on nonopsonized phagocytosis of macrophages in vivo and in vitro, indicating that IFN-γ might inhibit nonopsonized phagocytosis of macrophages through mTOR-dependent pathways. mTOR, a serine-threonine kinase, plays a critical role in cell growth and proliferation, ribosome biogenesis, cytoskeletal organization, antibody switching and germinal centers [38, 42, 43, 44]. mTOR complexes are activated by many factors, such as growth factors, hormones and cytokines for example [45]. It is reported that mTORC1 can be activated by IFN-γ via its downstream PI3K-AKT pathway [39]. Therefore, we speculated that a PI3K inhibitor might recover the IFN-γ-suppressive effect on nonopsonized phagocytosis. We used LY294002, an inhibitor of PI3K, to verify the deduction. The data showed that LY294002 significantly recovered the inhibitory effect of IFN-γ on the nonopsonized phagocytosis function of macrophages. These data demonstrated that IFN-γ participates in the down-regulation of nonopsonized phagocytosis via the PI3K-Akt-mTOR pathway.
It is reported that c/EBPβ expression is dramatically increased during macrophage differentiation [46, 47] and macrophages from c/EBPβ-deficient mice have a defective ability to kill bacteria and clear tumor cells [48, 49, 50]. It is suggested that the production of nitric oxide (NO) is necessary but not sufficient for bacterial and tumor killing by activated macrophages [49]. These data indicate that there exists an NO-independent mechanism of bacterial and tumor killing that is mediated by c/EBPβ in activated macrophages. We herein identified another mechanism by c/EBPβ to regulate macrophage function, i.e. c/EBPβ positively controls nonopsonized phagocytosis of macrophages. In the present study, we showed that IFN-γ inhibited c/EBPβ expression in macrophages in a time-dependent manner. Importantly, our data showed that c/EBPβ could directly bind to the MARCO gene promoter, indicating the direct regulatory role of c/EBPβ on MARCO expression. Further studies indicated that mTOR activation induced by IFN-γ could markedly inhibit c/EBPβ expression in macrophages. Thus, the activation of PI3K-AKT-mTOR decreased c/EBPβ expression, which subsequently resulted in decreased expression of MARCO, and finally caused the deficient nonopsonized phagocytosis in IFN-γ-stimulated macrophages.
Secondary bacterial pneumonia results in excess morbidity and mortality during influenza pandemics [2]. Several mechanisms, including the suppression of neutrophil function, the increased bacterial adherence to epithelia and the decreased macrophage phagocytosis by IFN-γ, may be involved in the enhanced susceptibility to secondary bacterial infection in virus-infected hosts [3, 4, 6]. However, the molecular mechanisms responsible for the decreased phagocytosis by IFN-γ were not addressed. Our data showed that IFN-γ enhanced mTOR activity in macrophages, followed by hampering the expression of c/EBPβ, which is a key promoter element of MARCO. To further prove the biological significance of mTOR in IFN-γ-mediated inhibition of macrophage phagocytosis function in vivo, the viral-bacterial synergy mouse model described previously [6] has been used in the present study. Our data demonstrated that rapamycin could protect mice from a secondary bacterial attack during recovery from influenza infection, suggesting that mTOR may serve as a key molecule target for therapy of secondary bacterial infection caused by primary influenza infection. However, it is worthy to point out that the present study could not exclude the involvement of opsonized phagocytosis mediated by natural antibody directed against S. pneumoniae in this model. It is true that these mice housed under specific pathogen-free conditions had very low levels of anti-S. pneumoniae antibody in the sera and we speculated that nonopsonized phagocytosis may be the major approach for macrophages to phagocytize S. pneumoniae in these mice. The involvement of opsonized phagocytosis in this model needs to be addressed in the future.
Hinojosa et al. [51] reported that long-term rapamycin treatment (4 months) protected aged C57BL/6 mice (9–22 months) against pneumonia through reduced lung cellular senescence. However, in our study, the short-term rapamycin treatment (only two injections) did not show a protective effect on pneumonia infection in naïve young mice (6–8 weeks) without virus infection. The inconsistency is likely due to the differences in the period of rapamycin treatment and age of the hosts.
In summary, our data demonstrated that IFN-γ plays distinct roles in opsonized and nonopsonized phagocytosis. The activation of PI3K-AKT-mTOR by IFN-γ decreased c/EBPβ expression, subsequently resulted in reduced MARCO expression and finally caused a defect in nonopsonized phagocytosis in macrophages. Short-term rapamycin treatment significantly interrupted the viral-bacterial synergy and enhanced the protection against secondary S. pneumoniae in infection. Thus, the data presented here will help us to better understand the molecular mechanisms of nonopsonized phagocytosis. More importantly, rapamycin may be used to prevent potential bacterial infection caused by primary influenza infection.
Disclosure Statement
The authors declare no competing financial interests.
Acknowledgments
The authors wish to thank Dr. Lina Sun and Mr. Douglas Corley for their kind review and editing of the paper, Mrs. Jing Wang and Dr. Xiaoqiu Liu for their expert technical assistance, Mrs. Yanli Hao for her excellent laboratory management, and Mr. Baisheng Ren for his outstanding animal husbandry. This work was supported by grants from the National Basic Research Program of China (2010CB945301 and 2011CB710903 to Y.Z.), the National Natural Science Foundation for General and Key Programs (C81130055 and C81072396 to Y.Z.), the CAS/SAFEA International Partnership Program for Creative Research Teams (to Y.Z.), the ‘215’ high-level health technology project (2011 to J.L.) and the National Science and Technology Major Project ‘Prevention and Treatment of AIDS and Virus Hepatitis’ (2014ZX10002002-001-002 to J.L.).
References
- 1.Stiver HG. The threat and prospects for control of an influenza pandemic. Expert Rev Vaccines. 2004;3:35–42. doi: 10.1586/14760584.3.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Brundage JF. Cases and deaths during influenza pandemics in the United States. Am J Prev Med. 2006;31:252–256. doi: 10.1016/j.amepre.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Giebink GS, Mills EL, Quie PG. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J Infect Dis. 1981;143:836–845. doi: 10.1093/infdis/143.6.836. [DOI] [PubMed] [Google Scholar]
- 4.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J Exp Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2007;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 7.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, Yoshikai Y. Type I interferon plays opposing roles in cytotoxicity and interferon-gamma production by natural killer and CD8 T cells after influenza A virus infection in mice. J Innate Immun. 2014;6:456–466. doi: 10.1159/000356824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodridge HS, Underhill DM, Touret N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic. 2012;13:1062–1071. doi: 10.1111/j.1600-0854.2012.01382.x. [DOI] [PubMed] [Google Scholar]
- 9.Galvan MD, Greenlee-Wacker MC, Bohlson SS. C1q and phagocytosis: the perfect complement to a good meal. J Leukoc Biol. 2012;92:489–497. doi: 10.1189/jlb.0212099. [DOI] [PubMed] [Google Scholar]
- 10.Marodi L, Schreiber S, Anderson DC, MacDermott RP, Korchak HM, Johnston RB., Jr Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Invest. 1993;91:2596–2601. doi: 10.1172/JCI116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiff DE, Rae J, Martin TR, Davis BH, Curnutte JT. Increased phagocyte Fc gammaRI expression and improved Fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects. Blood. 1997;90:3187–3194. [PubMed] [Google Scholar]
- 12.Goto Y, Ogawa K, Hattori A, Tsujimoto M. Secretion of endoplasmic reticulum aminopeptidase 1 is involved in the activation of macrophages induced by lipopolysaccharide and interferon-gamma. J Biol Chem. 2011;286:21906–21914. doi: 10.1074/jbc.M111.239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan A, Magnus T, Gold R. Phagocytosis of apoptotic inflammatory cells by microglia and modulation by different cytokines: mechanism for removal of apoptotic cells in the inflamed nervous system. Glia. 2001;33:87–95. doi: 10.1002/1098-1136(20010101)33:1<87::aid-glia1008>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Speert DP, Thorson L. Suppression by human recombinant gamma interferon of in vitro macrophage nonopsonic and opsonic phagocytosis and killing. Infect Immun. 1991;59:1893–1898. doi: 10.1128/iai.59.6.1893-1898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arredouani MS, Palecanda A, Koziel H, Huang YC, Imrich A, Sulahian TH, Ning YY, Yang Z, Pikkarainen T, Sankala M, Vargas SO, Takeya M, Tryggvason K, Kobzik L. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol. 2005;175:6058–6064. doi: 10.4049/jimmunol.175.9.6058. [DOI] [PubMed] [Google Scholar]
- 17.Palecanda A, Paulauskis J, Al-Mutairi E, Imrich A, Qin G, Suzuki H, Kodama T, Tryggvason K, Koziel H, Kobzik L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med. 1999;189:1497–1506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herve PL, Raliou M, Bourdieu C, Dubuquoy C, Petit-Camurdan A, Bertho N, Eleouet JF, Chevalier C, Riffault S. A novel subnucleocapsid nanoplatform for mucosal vaccination against influenza virus that targets the ectodomain of matrix protein 2. J Virol. 2014;88:325–338. doi: 10.1128/JVI.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, Duan K, Ma H, Niu Z, Peng J, Zhao Y. An instructive role of donor macrophages in mixed chimeras in the induction of recipient CD4(+)Foxp3(+) Treg cells. Immunol Cell Biol. 2011;89:827–835. doi: 10.1038/icb.2011.65. [DOI] [PubMed] [Google Scholar]
- 20.Hou Y, Lin H, Zhu L, Liu Z, Hu F, Shi J, Yang T, Shi X, Zhu M, Godley BF, Wang Q, Li Z, Zhao Y. Lipopolysaccharide increases the incidence of collagen-induced arthritis in mice through induction of protease HTRA-1 expression. Arthritis Rheum. 2013;65:2835–2846. doi: 10.1002/art.38124. [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Xia XP, Gong SL, Zhao Y. The macrophage heterogeneity: difference between mouse peritoneal exudate and splenic F4/80+ macrophages. J Cell Physiol. 2006;209:341–352. doi: 10.1002/jcp.20732. [DOI] [PubMed] [Google Scholar]
- 22.Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, Craig RW, Whyte MK, Dockrell DH. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest. 2005;115:359–368. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwinn WM, Qu W, Shines CJ, Bousquet RW, Taylor GJ, Waalkes MP, Morgan DL. Macrophage solubilization and cytotoxicity of indium-containing particles in vitro. Toxicol Sci. 1984;135:414–424. doi: 10.1093/toxsci/kft154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paredes-Sabja D, Sarker MR. Interactions between Clostridium perfringens spores and Raw 264.7 macrophages. Anaerobe. 2012;18:148–156. doi: 10.1016/j.anaerobe.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Wang E, Michl J, Pfeffer LM, Silverstein SC, Tamm I. Interferon suppresses pinocytosis but stimulates phagocytosis in mouse peritoneal macrophages: related changes in cytoskeletal organization. J Cell Biol. 1984;98:1328–1341. doi: 10.1083/jcb.98.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun C, Sun L, Ma H, Peng J, Zhen Y, Duan K, Liu G, Ding W, Zhao Y. The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J Cell Physiol. 2012;227:1670–1679. doi: 10.1002/jcp.22891. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Liu G, Ding W, Wu Y, Cai L, Zhao Y. Diabetes-induced alteration of F4/80+ macrophages: a study in mice with streptozotocin-induced diabetes for a long term. J Mol Med (Berl) 2008;86:391–400. doi: 10.1007/s00109-008-0304-8. [DOI] [PubMed] [Google Scholar]
- 28.Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu Y, Zhang B, Zhao L, Liu G, Ma H, Rao E, Zeng C, Zhao Y. The effect of immunosuppressive drug rapamycin on regulatory CD4+CD25+Foxp3+T cells in mice. Transpl Immunol. 2007;17:153–161. doi: 10.1016/j.trim.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu G, Hu X, Sun B, Yang T, Shi J, Zhang L, Zhao Y. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood. 2013;121:519–529. doi: 10.1182/blood-2012-05-432674. [DOI] [PubMed] [Google Scholar]
- 31.Akagi T, Thoennissen NH, George A, Crooks G, Song JH, Okamoto R, Nowak D, Gombart AF, Koeffler HP. In vivo deficiency of both C/EBPβ and C/EBPε results in highly defective myeloid differentiation and lack of cytokine response. PLoS One. 2010;5:e15419. doi: 10.1371/journal.pone.0015419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun B, Hu X, Liu G, Ma B, Xu Y, Yang T, Shi J, Yang F, Li H, Zhang L, Zhao Y. Phosphatase Wip1 negatively regulates neutrophil migration and inflammation. J Immunol. 2014;192:1184–1195. doi: 10.4049/jimmunol.1300656. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Liu G, Hou Y, Shi J, Zhu L, Jin D, Peng J, Zhao Y. Induction of M2-like macrophages in recipient NOD-scid mice by allogeneic donor CD4(+)CD25(+) regulatory T cells. Cell Mol Immunol. 2012;9:464–472. doi: 10.1038/cmi.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gough PJ, Gordon S. The role of scavenger receptors in the innate immune system. Microbes Infect. 2000;2:305–311. doi: 10.1016/s1286-4579(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 35.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 36.Pearson AM. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 37.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 38.Foldenauer ME, McClellan SA, Berger EA, Hazlett LD. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J Immunol. 2013;190:5649–5658. doi: 10.4049/jimmunol.1203094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 40.Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- 41.Sandler AD, Chihara H, Kobayashi G, Zhu X, Miller MA, Scott DL, Krieg AM. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Res. 2003;63:394–399. [PubMed] [Google Scholar]
- 42.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 43.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Zhang L, Zhang H, Xiao Y, Shao L, Li H, Yin H, Wang R, Liu G, Corley D, Yang Z, Zhao Y. Disruption of TSC1/2 signaling complex reveals a checkpoint governing thymic CD4+ CD25+ Foxp3+ regulatory T-cell development in mice. FASEB J. 2013;27:3979–3990. doi: 10.1096/fj.13-235408. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Zhang H, Li L, Xiao Y, Rao E, Miao Z, Chen H, Sun L, Li H, Liu G, Zhao Y. TSC1/2 signaling complex is essential for peripheral naive CD8+ T cell survival and homeostasis in mice. PLoS One. 2012;7:e30592. doi: 10.1371/journal.pone.0030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natsuka S, Akira S, Nishio Y, Hashimoto S, Sugita T, Isshiki H, Kishimoto T. Macrophage differentiation-specific expression of NF-IL6, a transcription factor for interleukin-6. Blood. 1992;79:460–466. [PubMed] [Google Scholar]
- 47.Scott LM, Civin CI, Rorth P, Friedman AD. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992;80:1725–1735. [PubMed] [Google Scholar]
- 48.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 50.Pizarro-Cerda J, Desjardins M, Moreno E, Akira S, Gorvel JP. Modulation of endocytosis in nuclear factor IL-6(-/-) macrophages is responsible for a high susceptibility to intracellular bacterial infection. J Immunol. 1999;162:3519–3526. [PubMed] [Google Scholar]
- 51.Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol. 2012;47:958–965. doi: 10.1016/j.exger.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]