Abstract
Endoplasmic reticulum (ER) stress is associated with chronic pulmonary inflammatory diseases. We hypothesized that the combined activation of both Toll-like receptor (TLR) signaling and ER stress might increase inflammatory reactions in otherwise tolerant airway epithelial cells. Indeed, ER stress resulted in an increased response of BEAS-2B and human primary bronchial epithelial cells to pathogen-associated molecular pattern stimulation with respect to IL6 and IL8 production. ER stress elevated p38 and ERK MAP kinase activation, and pharmacological inhibition of these kinases could inhibit the boosting effect. Knockdown of unfolded protein response signaling indicated that mainly PERK and ATF6 were responsible for the synergistic activity. Specifically, PERK and ATF6 mediated increased MAPK activation, which is needed for effective cytokine secretion. We conclude that within airway epithelial cells the combined activation of TLR signaling and ER stress-mediated MAPK activation results in synergistic proinflammatory activity. We speculate that ER stress, present in various chronic pulmonary diseases, boosts TLR signaling and therefore proinflammatory cytokine production, thus acting as a costimulatory danger signal.
Key Words: Endoplasmic reticulum, Cystic fibrosis, Inflammation, Epithelial cells
Introduction
Airway epithelial cells represent the first line of defense against potentially harmful microorganisms in inspired air. They constitute a tight physical barrier which is well equipped to restrict invading pathogens. Over recent years, it has been discovered that airway epithelial cells are also involved in shaping an organ-specific immune microenvironment [1, 2]. Bronchial epithelial cells express various pattern recognition receptors of the innate immune system, among others Toll-like receptors (TLR), by which they are able to recognize pathogen-associated molecular patterns (PAMPs) [3, 4, 5, 6]. Subsequent signaling results in the transcription of inflammatory mediators such as IL6 or IL8, thereby actively modulating local immunity in the lung [7]. However, direct stimulation of bronchial epithelial cells through PAMP/TLR only results in a moderate synthesis of proinflammatory cytokines [5, 8, 9]. This has been interpreted as a mechanism to avoid overshooting reactions against harmless airborne stimuli. Nevertheless, an important contribution of TLR signaling in bronchial epithelial cells in defeating invading pathogens has been described [10, 11, 12]. In addition, several chronic inflammatory pulmonary diseases have been associated with increased epithelial proinflammatory cytokine production [13]. Therefore, conditions must exist by which bronchial epithelial cells are able to sense and to react towards PAMPs. At mucosal linings with frequent contact to microbes, such a condition might be a damage of the barrier which would only be induced by virulent microbes or otherwise pathological conditions.
It has been previously demonstrated that endoplasmic reticulum (ER) stress has the potential of proinflammatory signaling [14, 15, 16, 17]. Moreover, ER stress has been implicated in several pulmonary pathologies, including viral and bacterial infections, cystic fibrosis (CF), asthma, or chronic obstructive pulmonary disease (COPD) [18, 19]. ER stress activates the unfolded protein response (UPR) in order to restore cell homeostasis. The UPR is an essential adaptive signaling program which alters a cell's transcriptional and translational machinery to match the ER folding capacity with mRNA translation. It is conducted via three ER transmembrane receptors: PERK (double-stranded RNA-activated protein kinase-like ER kinase), IRE1 (inositol requiring enzyme 1), involved in XBP1 mRNA splicing, and ATF6 (activating transcription factor 6) [20]. Overall, on the one hand the UPR induces attenuation of protein synthesis via PERK-mediated eIF2α phosphorylation, and on the other hand XBP1- and ATF6-dependent chaperone transcription to relieve ER stress. However, in the case of prolonged ER stress, the UPR promotes apoptosis via PERK-ATF4-mediated expression of the transcription factor CHOP [21]. Current knowledge on the UPR affecting TLR-induced inflammation is still limited. Given that the UPR signaling cascades seem to intersect with immune functions, we hypothesized that the combined activation of TLR and the UPR signaling might push epithelial cells towards a strong inflammatory reaction pattern. We therefore sought to investigate whether previously ER-stressed bronchial epithelial cells display a more proinflammatory phenotype.
Materials and Methods
Materials
RPMI 1640 was purchased from Biochrom (Berlin, Germany). FCS was from Life Technologies (Carlsbad, Calif., USA). Penicillin and streptomycin were from PAA Laboratories (Pasching, Austria). PBS was obtained from PAN-Biotech (Aidenbach, Germany). EBSS was from Sigma-Aldrich (Saint Louis, Mo., USA). BEBM (bronchial epithelial basal medium) and supplementary growth factors were all from Lonza (Walkersville, Md., USA). DNase I, and Protease XIV, as well as thapsigargin and tunicamycin, were from Sigma-Aldrich. GSK2606414 PERK inhibitor was purchased from Tocris Bioscience (Bristol, UK). SB203580 p38 MAPK (mitogen-activated protein kinases) inhibitor was from Merck Millipore (Darmstadt, Germany). U0126 ERK pathway inhibitor and SP600125 JNK inhibitor were from Sigma-Aldrich. Lipopolysaccharide (LPS) from Salmonella minnesota was kindly provided by U. Seydel (Division of Biophysics, Research Center Borstel, Borstel, Germany). Polyinosinic-polycytidylic acid (pIC) and purified flagellin from Bacillus subtilis (FLA) were purchased from InvivoGen (Toulouse, France). Primary antibodies detecting PERK, CHOP, β-actin, IRE1α, XBP1s, pS536-p65, p-p38, p-ERK, p-JNK, p38, ERK, JNK, and H3 were all from Cell Signaling Biotechnology (Frankfurt, Germany). Primary antibody detecting ATF6 and CHOP (F-168) antibody used for immunohistochemistry were from Santa Cruz Biotechnology (Heidelberg, Germany).
Immunohistochemistry Staining
Lung tissue samples (airway tissue of CF patients and donors) were provided by the tissue bank of the TI Biobanking of the German Centre for Infection Research (DZIF) in accordance with the regulation of the tissue bank and approval by the ethics committee of Heidelberg University. Immunohistochemical staining of formalin-fixed, paraffin-embedded tissue samples for CHOP was performed using the Dako autostainer with 1:50 dilution. Beforehand, airway tissue sections (4 µm) were pretreated in citrate buffer at pH 6.0. All sections were counterstained with hematoxylin.
Cell Culture
Human bronchial epithelial BEAS-2B cells were cultured in RPMI medium, supplemented with 10% FCS, 1% penicillin/streptomycin at 37°C in a humidified incubator at 5% CO2. Human primary bronchial epithelial cell cultures (hpBrEpC) were bought from Lonza (Visp, Switzerland) or isolated from biopsies from individuals treated by lobectomy because of non-small-cell lung carcinoma at the Thoraxklinik, Heidelberg. Ethics approval was obtained from the regional Ethics Committee at the University of Heidelberg, and all study participants provided a written informed consent. Two methods of bronchial epithelial cell isolation were used [22, 23]. Primary cell cultures were maintained at 37°C in a humidified incubator at 5% CO2. Culture medium (BEBM + supplements) was changed every 2-3 days. Cells were passaged when reaching 70–90% confluence (see online suppl. material for a more detailed protocol; see www.karger.com/doi/10.1159/000447668 for all online suppl. material).
Cell Stimulation
The experimental stimulation set-up included a pretreatment with DMSO as a solvent control, either thapsigargin (1 µM), or tunicamycin (5 μg/ml) for 1 h to induce ER stress, followed by stimulation with either LPS (100 ng/ml), pIC (10 μg/ml), or flagellin (1 μg/ml). Where indicated, cells were treated either with GSK2606414 PERK inhibitor (300 nM) for 1 h, or with SB203580 (10 µM), U0126 (10 µM), or SB00125 (10 µM) MAPK inhibitor 15 min prior to DMSO/thapsigargin pretreatment. Supernatants were removed at the indicated periods of time and used for measurement of cytokine levels by enzyme-linked immunosorbent assay (ELISA). Cells were then lysed and total RNA was isolated for downstream analysis by quantitative RT-PCR (qRT-PCR).
RNA Interference
RNA interference was performed with siRNAs against human PERK (s18102; Ambion, Life Technologies, Carlsbad, Calif., USA), IRE1α (SI00605248; Qiagen, Hilden, Germany), XBP1 (s14913; Ambion), ATF6 (115889; Ambion), and GFP (5′-GCAAGCUGAAGUUCAU-3′, sense; Biomers, Ulm, Germany) as a negative control. BEAS-2B cells were seeded into a 24-well plate 7 h before transfection. siRNAs (10 nM) were transfected into BEAS-2B cells (grown to approx. 60% confluence) using Lipofectamine RNAiMax transfection reagent (Invitrogen, Carlsbad, Calif., USA) according to the manufacturer's protocol.
RNA Isolation and qRT-PCR
Total cellular RNA was isolated using peqGold Total RNA Kit (peqlab Biotechnology, Erlangen, Germany) according to the manufacturer's standard protocol. RNA isolation included DNase digestion using an RNase-free DNase set (Qiagen). In order to perform qRT-PCR, total RNA was first reverse transcribed into single-stranded cDNA using a High Capacity cDNA RT Kit (Applied Biosystems, Foster City, Calif., USA). For RT-PCR analysis, 2 µl of cDNA (diluted 1:4) was used as a template in a final reaction volume of 15 µl, combined with SYBR® Green PCR Master Mix Fast (Applied Biosystems) and corresponding primers (sequences available upon request). The analysis was performed on a StepOne Plus RT-PCR platform (Applied Biosystems) in a 96-well format. Each gene was measured in duplicates of each cDNA sample. The baseline and threshold values were detected automatically and the Ct values of the endogenous constitutively expressed reference gene were subtracted from the determined Ct values resulting in a ΔCt for each target gene, which was then used to calculate the relative expression, rE = 2-ΔCt. To control reaction specificity, all measurements included samples without the reverse transcriptase enzyme. Melting curves were used to prove specific amplification.
Measurement of Cytokine Secretion
Sandwich ELISA was performed using commercially available kits to determine the amount of secreted human IL6 and IL8 (BD Biosciences, Heidelberg, Germany) cytokines in the cell-free supernatants of stimulated cells. Different samples were tested in duplicate and the assays were performed according to the manufacturers' instructions. Cytokines were detected by measuring the absorbance at 490 nm with a 650-nm reference in a photometer (Sunrise Reader, Tecan, Salzburg, Austria). Cytokine concentrations were calculated according to a standard dilution of the respective recombinant cytokines using Magellan v.5.0 software (Tecan, Salzburg, Austria).
Luciferase Assay
BEAS-2B cells grown in 24-well plates were transfected with a luciferase reporter gene pNFκB-Luc (pGL4.32[luc2P/NFκB-RE/Hygro]; Promega, Madison, Wis., USA) using the peqFECT DNA (peqlab Biotechnology, Erlangen, Germany) transfection reagent. After 42 h of cultivation and stimulation as indicated in the experiment, cells were lysed and luciferase activities in the lysates were measured using a luminometer (LUMIstar OPTIMA system, BMG LABTECH).
Western Blot Analysis
BEAS-2B and human primary bronchial epithelial cells, grown in either 6- or 24-well plates, respectively, were washed with cold PBS, lysed, and incubated at 95°C for 10 min. Following separation by SDS-PAGE, proteins were transferred to a nitrocellulose membrane by a semidry blotting procedure. Unspecific binding was blocked by incubating the membranes in a 5% BSA solution in 1× TBST (TBS, 0.05% Tween 20) for at least 1 h. Incubation with specific primary antibodies (all Cell Signaling antibodies 1:1,000 and anti-ATF6 antibody from Santa Cruz 1:400, diluted in 5% BSA blocking solution) was done overnight at 4°C, followed by three 10-min washing steps in 1× TBST at room temperature. Blots were incubated with HRP-conjugated secondary antibody (1:4,000 diluted in 1× TBST, anti-rabbit-HRP or anti-mouse-HRP; Cell Signaling Biotechnology) for 45 min at room temperature and washed three times for 10 min with 1× TBST prior to chemiluminescence detection using enhanced chemiluminescence substrate (PerkinElmer, Rodgau, Germany). Densitometry was performed using ImageJ software (National Institutes of Health, Bethesda, Md., USA) [24].
Statistical Analysis
All experiments were repeated three times unless stated otherwise. Data are shown as the mean + SD. The statistical significance of comparisons between three or more unmatched groups was determined by one-way ANOVA, and if multiple comparisons were performed two-way ANOVA was used (both including Bonferroni's post hoc test). Statistics on quantitative PCR data were performed on previously log-transformed data to achieve a normal distribution. All statistical analyses were done using GraphPad Prism software (5.00 and 6.05; GraphPad, San Diego, Calif., USA). Significant differences were considered at p values <0.05 (*), 0.01 (**), 0.001 (***), or 0.0001 (****).
Results
ER Stress Amplifies TLR-Induced Cytokine Production in Airway Epithelial Cells
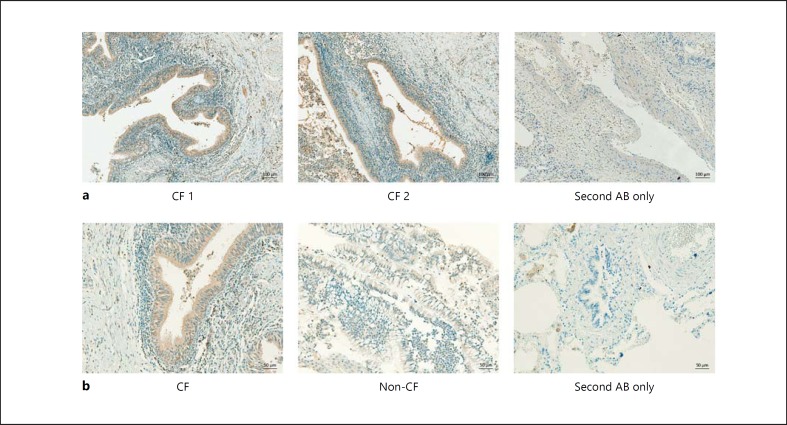
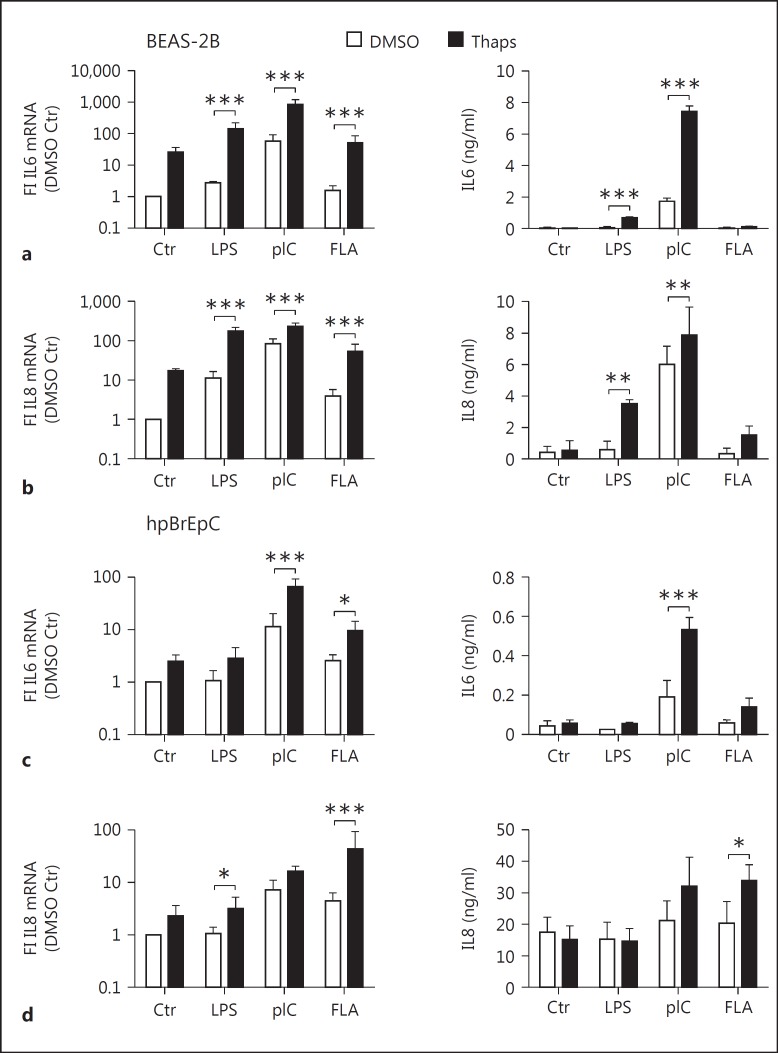
ER stress has been shown to occur in various pulmonary diseases. In order to verify previous observations, we analyzed the expression of the ER stress marker CHOP in biopsies taken from cystic fibrotic lungs using immunohistochemistry. Indeed, we observed strong expression of CHOP, especially in the epithelial cells (fig. 1a). Moreover, staining for CHOP was stronger in samples from CF compared to non-CF lung (fig. 1b), confirming the previous association of CF with ER stress [18, 19]. Since the airway epithelium of CF lungs has been shown to display increased proinflammatory cytokine production upon PAMP stimulation [13], and given that both ER stress and TLR signaling have been linked to proinflammatory cytokine production, we wondered if epithelial ER stress might modulate PRR signaling. We observed that IL6 and IL8 mRNA and protein levels were only moderately induced after a 5-hour stimulation of TLR4, TLR3, or TLR5 with LPS, pIC, or flagellin, respectively (fig. 2a, b), indicating that TLR stimulation alone is not a sufficiently strong trigger of airway epithelial cells. In order to analyze if ER stress is able to modulate TLR-induced cytokine production, we preincubated BEAS-2B cells for 1 h with 1 µM thapsigargin before adding LPS, pIC, or flagellin (fig. 2a, b). The addition of thapsigargin in combination with LPS, pIC, or flagellin significantly increased IL6 and IL8 mRNA production as well as protein secretion. On the other hand, thapsigargin alone showed only a minor induction of IL6 and IL8 mRNA in comparison to combined thapsigargin and TLR stimulation. Given that the relative expression of the cytokines itself were low, this induction did not result in detectable protein levels (fig. 2a, b). To confirm our findings, we next treated human primary bronchial epithelial cells (hpBrEpC) according to the same protocol. We observed that prestimulation with thapsigargin significantly boosted pIC- and FLA-induced IL6 mRNA and pIC-induced IL6 protein secretion (fig. 2c). Similar to results on IL6 mRNA and protein levels, LPS- and FLA-induced IL8 mRNA and FLA-induced IL8 protein levels were significantly increased by pretreatment with thapsigargin (fig. 2d). Human primary bronchial epithelial cells were generally less responsive to LPS. As some of our material derives from smokers' airways, this hyporesponsiveness to LPS with respect to IL6 and IL8 protein production could be due to cigarette smoke-affected and thus suppressed TLR4 signaling, which has been reported before [25, 26]. Therefore, in subsequent experiments, hpBrEpC were stimulated with pIC which was highly stimulatory and gave rise to more homogenous results. Induction of the UPR by thapsigargin was verified by the upregulation of CHOP mRNA in all settings (data not shown).
Fig. 1.
CHOP expression is detected in the epithelium of CF airways. a Immunohistochemistry of paraffin-embedded human CF airway tissue sections (4 µm) stained for CHOP (brown), including a nonspecific, secondary antibody control. Original magnification ×10. Scale bars = 100 µm. The images show representative stainings for n = 5 CF lungs. b Immunohistochemistry of paraffin-embedded human CF and control (non-CF) airway tissue sections (4 µm) stained for CHOP (brown), including a nonspecific, secondary antibody control. Original magnification ×20. Scale bars = 50 µm. All sections were counterstained with hematoxylin.
Fig. 2.
ER stress increases reactivity towards TLR agonists in BEAS-2B and human primary bronchial epithelial cells. a, b Human bronchial epithelial BEAS-2B cells were pretreated with DMSO or 1 µM of thapsigargin (Thaps) for 1 h and subsequently stimulated with LPS (100 ng/ml), pIC (10 µg/ml), or FLA (1 µg/ml) for 5 h (n = 3-6). c, d hpBrEpC cells were treated in the same manner as BEAS-2B cells (n = 3-4). Fold induction (FI) of the relative mRNA expression of IL6 and IL8 was determined by qRT-PCR. Secreted IL6 and IL8 levels were measured by ELISA. Data are shown as the mean + SD.
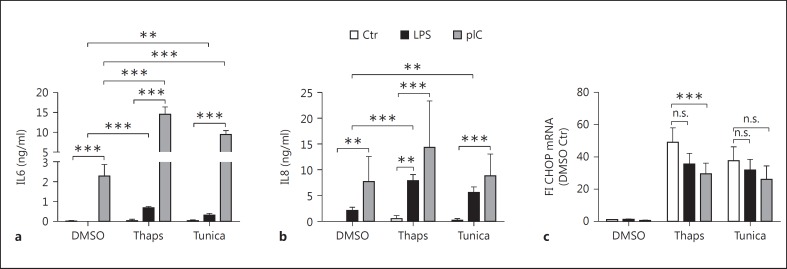
To exclude an effect specific to thapsigargin, we used tunicamycin, which is another ER stress inducer. Even though both chemicals induce ER stress, they act through two different and unrelated mechanisms. ER stress induced by tunicamycin was able to boost LPS- and pIC-mediated cytokine production to a similar extent as observed with thapsigargin (fig. 3a, b). CHOP mRNA levels were in the same range in both thapsigargin- and tunicamycin-treated cells (fig. 3c). Moreover, LPS or pIC alone did not induce CHOP expression (fig. 3c). These findings indicate that ER stress induced by different means is able to boost TLR-induced cytokine production within airway epithelial cells.
Fig. 3.
ER stress-mediated increase in proinflammatory activity is induced through different stressors. BEAS-2B cells were pretreated with DMSO, thapsigargin (Thaps; 1 µM), or tunicamycin (Tunica; 5 µg/ml) for 1 h and subsequently stimulated with LPS (100 ng/ml) or pIC (10 µg/ml) for 5 h. Secreted IL6 (a) and IL8 (b) levels were measured by ELISA. Fold induction of relative CHOP (c) mRNA expression was determined by qRT-PCR (n = 3). Data are shown as the mean + SD.
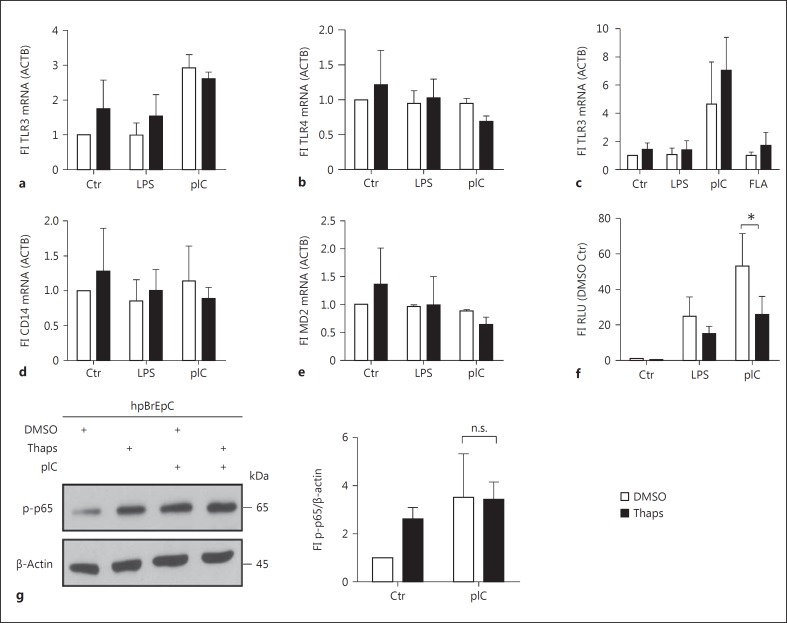
TLR Expression and NFκB Signaling Is Not Modified by ER Stress
It has been shown previously that expression of TLRs can be modulated by ER stress in HeLa cells, thereby increasing the expression of cytokines [27]. Thus, we analyzed the expression of TLR3 and TLR4 by qRT-PCR in BEAS-2B cells (fig. 4a, b), and TLR3 expression in hpBrEpC (fig. 4c). Surprisingly, we did not observe any significant changes within the different stimulation protocols. In addition, it has been postulated that low MD2 or CD14 expression leads to a relative insensitivity of airway epithelial cells towards TLR4 agonists [5, 9]. Similar to our findings regarding TLR4 expression, we did not observe any significant alterations of CD14 or MD2 mRNA expression (fig. 4d, e). Downstream signaling of TLR4 and TLR3 leads to activation of NFκB, which is a central transcription factor for IL6 and IL8. We analyzed NFκB activity using a luciferase reporter construct driven by five consecutive NFκB response elements (fig. 4f). As expected, LPS and pIC stimulation led to a 20- or 60-fold induction of luciferase activity compared to DMSO or thapsigargin alone. However, the combined stimulation with thapsigargin and LPS or pIC did not result in increased NFκB activation (fig. 4f). To support our findings, we additionally analyzed p65 phosphorylation (S536) in hpBrEpC by Western blotting (fig. 4g). As expected, there was no significant difference in phosphorylation of p65 after pIC alone and combined thapsigargin and pIC treatment. Interestingly, thapsigargin alone led to an increase of p65 phosphorylation, which is in concert with the literature and the observed mRNA induction (fig. 2). These results indicate that an ER stress-mediated increase of TLR responses is neither due to regulation of the LPS or pIC receptor nor due to a direct modulation of NFκB signaling.
Fig. 4.
UPR activation does not alter the TLR3/NFκB or TLR4/NFκB axis directly. a, b, d, e BEAS-2B cells were pretreated with DMSO or thapsigargin (Thaps) for 1 h and subsequently stimulated with LPS (100 ng/ml) or pIC (10 µg/ml) for 5 h. Fold induction of the relative expression of TLR3 (a), TLR4 (b), CD14 (d), and MD2 (e) mRNA was determined by qRT-PCR (n = 3). c, f hpBrEpC were treated in the same manner as BEAS-2B. Fold induction of relative expression of TLR3 (c) mRNA was determined by qRT-PCR (n = 3). Following transfection with a luciferase reporter plasmid for NFκB activity, BEAS-2B cells were pretreated with DMSO or Thaps for 1 h and subsequently stimulated with LPS or pIC for 5 h (f). Luciferase activity was normalized to β-galactosidase activity (n = 3-4). hpBrEpC were pretreated with DMSO or Thaps for 1 h and then stimulated with pIC for 30 min. Protein levels of phosphorylated p65 (p-p65) and β-actin as the loading control were analyzed by Western blotting (representative of n = 3). Quantification of the blots, represented as a ratio of phosphorylated p65 and the loading control, is depicted next to the blots (g). Data are shown as the mean + SD.
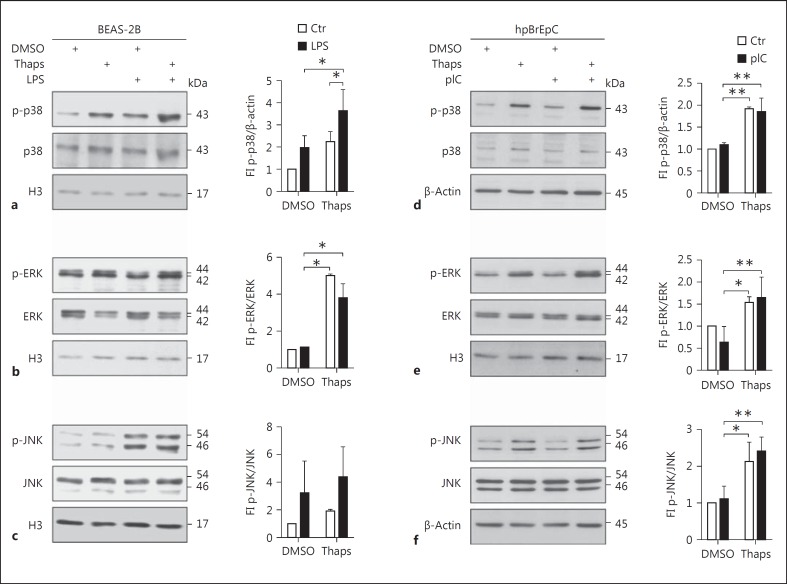
ER Stress Increases Activation of p38 and ERK MAPK Activity, Thereby Boosting Proinflammatory Cytokine Secretion
Since we did not observe any significant changes in NFκB activity (fig. 4f, g), we analyzed MAPK activation by Western blotting (fig. 5). Surprisingly, LPS in BEAS-2B or pIC in human primary bronchial epithelial cells was only moderately able to increase the phosphorylation of p38 and ERK in airway epithelial cells (fig. 5a, b, d, e) but led to a strong phosphorylation of JNK in BEAS-2B cells (fig. 5c). ER stress induction alone resulted in a slight activation of p38, but more prominently of ERK. Increased and strong phosphorylation of p38 and ERK upon LPS stimulation could only be observed in the additional presence of thapsigargin in BEAS-2B (fig. 5a, b). In line with this observation, in human primary bronchial epithelial cells, thapsigargin alone was able to significantly increase phosphorylation of p38, ERK, and JNK (fig. 5d, e, f). These results indicate that ER stress is able to add MAPK activation to TLR-mediated NFκB activity in airway epithelial cells.
Fig. 5.
UPR induction results in enhanced p38 and ERK activity upon LPS stimulation. BEAS-2B cells were pretreated with DMSO or thapsigargin (Thaps) for 1 h and then stimulated with LPS for 15 min. Protein levels of activated, phosphorylated (p)-p38 (a), p-ERK (b), and p-JNK (c), the unphosphorylated proteins, and histone 3 (H3) as the loading control were analyzed by Western blotting (representative of n = 3-4). hpBrEpC were pretreated with DMSO or Thaps for 1 h and then stimulated with pIC for 30 min. Protein levels of activated p-p38 (d), p-ERK (e), and p-JNK (f), the unphosphorylated proteins, and histone 3 or β-actin as the loading control were analyzed by Western blotting (representative of n = 3-4). Quantification of the blots, represented as a ratio of p-p38 and the loading control, or phosphorylated and unphosphorylated ERK and JNK, is depicted next to the blots. Data are shown as the mean + SD.
As MAPK signaling participates in proinflammatory gene induction, we next wanted to analyze the relevance of ER stress-induced MAPK activation. Therefore, we preincubated BEAS-2B cells with inhibitors of p38, ERK, or JNK, and analyzed IL6 and IL8 expression (data not shown) and secretion (fig. 6a, b). Inhibition of JNK using SP600125 did not affect IL6 protein (fig. 6a) or mRNA levels (data not shown) but did marginally decrease IL8 protein (fig. 6b) and mRNA (data not shown). In contrast, inhibition of p38 and ERK using SB203580 and U0126, respectively, resulted in decreased IL6 and IL8 protein levels (fig. 6a, b). Similarly, inhibition of JNK in human primary bronchial epithelial cells did not significantly affect IL6 (fig. 6c) or IL8 (fig. 6d) protein, unlike p38 and ERK inhibition. Interestingly, pIC-induced IL8, but not IL6, protein levels were also significantly reduced by inhibition of p38 and ERK. These results support the findings that ER stress-mediated p38 and ERK activation is required for high expression and secretion of IL6 and IL8 in airway epithelial cells.
Fig. 6.
p38 and ERK mediate increased reactivity of bronchial epithelial cells to LPS and pIC upon ER stress induction. BEAS-2B (a, b) and hpBrEpC cells (c, d) were pretreated with the inhibitors of p38 (SB203580, 10 µM), ERK (U0126, 10 µM), and JNK (SP600125, 10 µM) for 15 min and then with DMSO or thapsigargin (Thaps) for 1 h. Subsequently, cells were stimulated with LPS or pIC for 5 h. Secreted IL6 (a, c) and IL8 (b, d) levels were determined by ELISA (n = 4-6). Data are shown as the mean + SD.
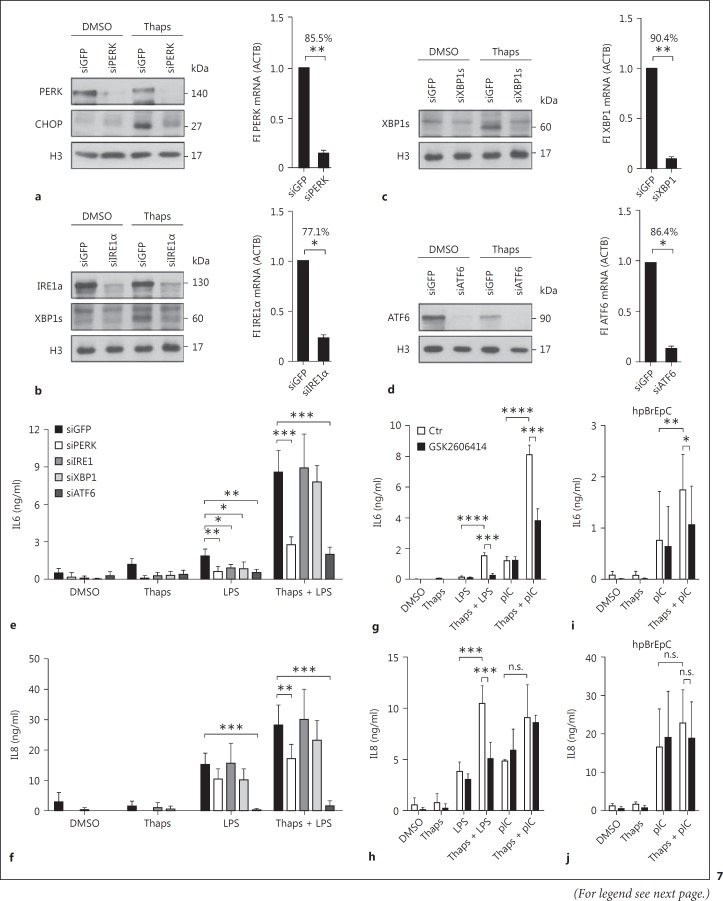
PERK and ATF6 Mediate ER Stress-Induced Hyperreactivity
Since the UPR is mediated via various receptors, we analyzed which of the three main receptors - PERK, IRE1α, and ATF6 - mediates the synergy between ER stress and TLR signaling. Therefore, we transfected BEAS-2B cells with siRNA against GFP (control), PERK, IRE1α, XBP1, and ATF6, and measured mRNA and protein levels of the respective genes (fig. 7). Knockdown efficacies for siPERK, siIRE1α, siXBP1, or siATF6 compared to siGFP were 85.5, 77.1, 90.4, and 86.4%, respectively. Downregulation could also be verified on the protein level by Western blotting for PERK, CHOP, IRE1α, XBP1s, and ATF6 (fig. 7a, b, c, d). We observed that the knockdown of PERK and ATF6 were most potent in inhibiting IL6 and IL8 protein secretion after combined thapsigargin and LPS stimulation (fig. 7e, f). However, ATF6 knockdown also affected the proliferation of BEAS-2B cells, suggesting we might overestimate its effect on IL6 and IL8 protein. Silencing IRE1α did not have a significant effect on IL6 and IL8 protein secretion in ER-stressed LPS-stimulated cells. However, knockdown of IRE1α and XBP1 under non-ER stress conditions was able to reduce IL6 protein secretion (fig. 7e, f) in line with a previous observation made in macrophages [28]. The involvement of IRE1α and XBP1 under basal but not in ER-stressed conditions might be an indication of two unrelated mechanisms operating. In order to confirm our knockdown data, pretreatment with PERK inhibitor GSK2606414 showed similar results as the transfection with siRNA: the inhibitor reduced thapsigargin-induced hyperresponsiveness to LPS and pIC in BEAS-2B (fig. 7g, h) and in human primary bronchial epithelial cells (fig. 7i, j).
Fig. 7.
PERK and ATF6 mediate ER stress-induced hyperreactivity. BEAS-2B cells were transfected with the indicated siRNAs. After 42 h, cells were pretreated with DMSO or thapsigargin (Thaps) for 1 h and subsequently stimulated with LPS for 5 h. Knockdown efficacies of the siRNAs against PERK (a), IRE1α (b), XBP1 (c), and ATF6 (d) were determined by analyzing the respective proteins or their downstream targets by Western blotting. Relative mRNA expression of the respective genes was determined by qRT-PCR and these are shown next to the blots. Secreted IL6 (e) and IL8 (f) protein levels were determined by ELISA (n = 3). BEAS-2B cells were pretreated with the inhibitor of PERK (GSK2606414, 300 nM) for 1 h and then with DMSO or Thaps for another 1 h. Subsequently, cells were stimulated with LPS or pIC for 5 h. Secreted IL6 (g) and IL8 (h) levels were determined by ELISA (n = 3). hpBrEpC cells were pretreated with the inhibitor of PERK (GSK2606414, 300 nM) for 1 h and then with DMSO or Thaps for another 1 h. Subsequently, cells were stimulated with pIC for 21 h. Secreted IL6 (i) and IL8 (j) levels were determined by ELISA (n = 11). Data are shown as the mean + SD.
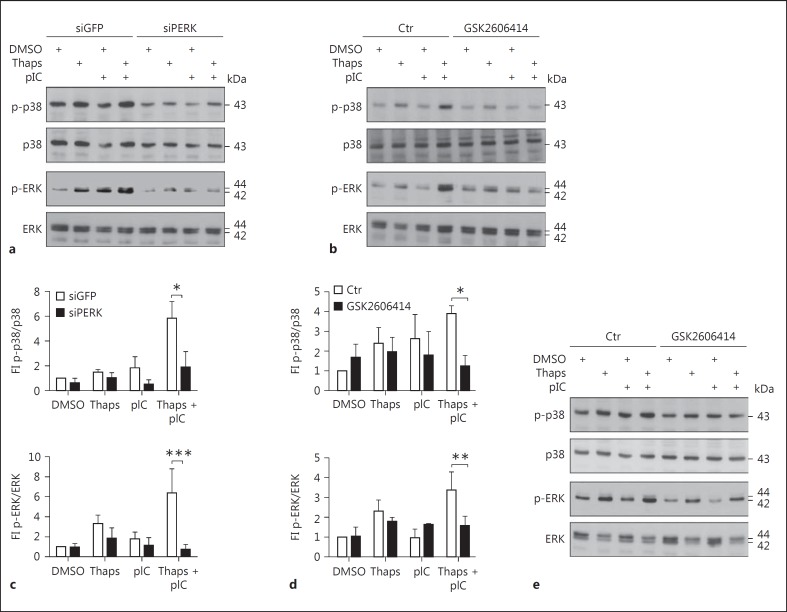
PERK and ATF6 Modulate p38 MAPK Phosphorylation
We showed that PERK and ATF6 pathways of the UPR were mainly involved in the regulation of IL6 and IL8 upon ER stress following LPS stimulation (fig. 7). Furthermore, p38 and ERK MAPK had a strong impact on IL6 and IL8 induction in airway epithelial cells (fig. 6). Therefore, we next investigated the relation of PERK and ATF6 branches on MAPK activation by Western blotting. In the control cells that were treated with an unrelated siGFP control, we confirmed the previously observed induction of p38 and ERK activation upon combined thapsigargin and TLR stimulation (fig. 8; online suppl. fig. 1). Silencing PERK by siRNA led to a significant decrease in p38 phosphorylation within all experimental conditions. Most importantly, the strong ER stress-mediated induction of p38 phosphorylation upon combined thapsigargin/pIC treatment was completely abolished with siPERK (fig. 8a, c). Moreover, we analyzed ERK phosphorylation within the same experimental setup. Similar to the p38 phosphorylation pattern, phospho-ERK levels were also significantly reduced by silencing PERK (fig. 8a, c). The same could be shown using PERK inhibitor GSK2606414 for both p-p38 and p-ERK (fig. 8c, d), indicating that MAPK activation is downstream of PERK. Furthermore, knockdown of ATF6 resulted in a general decrease of p38 and a minor but significant reduction of ERK activation after stimulation with thapsigargin and pIC (online suppl. fig. 1). In line with these results, treatment of human primary bronchial epithelial cells with the PERK inhibitor GSK2606414 resulted in decreased p38 phosphorylation to a similar extent as in BEAS-2B. Phosphorylated ERK levels were reduced but the ER stress-mediated induction was still noticeable (fig. 8e). However, due to donor variability, we were not able to quantify these blots. Taken together, these results indicate that the hyperreactivity of bronchial epithelial cells towards PAMPs is regulated by PERK- and ATF6-mediated MAPK activation, resulting in increased IL6 and IL8 secretion (fig. 9).
Fig. 8.
PERK modulates p38 and ERK activation. a BEAS-2B cells were transfected with siRNA against PERK. After 42 h, cells were pretreated with DMSO or thapsigargin (Thaps) for 1 h and subsequently stimulated with pIC for 5 min. b BEAS-2B cells were pretreated with the inhibitor of PERK (GSK2606414, 300 nM) for 1 h and then with DMSO or Thaps for another 1 h. Subsequently, cells were stimulated with pIC for 5 min. e hpBrEpC cells were pretreated with the inhibitor of PERK (GSK2606414, 300 nM) for 1 h and then with DMSO or Thaps for another 1 h. Subsequently, cells were stimulated with pIC for 30 min. Protein levels of activated, phosphorylated (p)-p38, p-ERK, and the respective unphosphorylated proteins were analyzed by Western blotting (representative of n = 3-4). The quantification of a and b is represented as a normalized ratio of phosphorylated p38 or phosphorylated ERK and their respective unphosphorylated forms (fold induction, FI) and depicted in c and d, respectively. Data are shown as the mean + SD.
Fig. 9.
ER stress, via PERK- and ATF6-mediated p38 and ERK activation, is able to boost TLR- and NFκB-mediated inflammation in airway epithelial cells: under homeostatic conditions, direct stimulation of bronchial epithelial cells through PAMP/TLR (LPS and pIC in BEAS-2B cells, and pIC in hpBrEpC cells activating TLR4 and TLR3, respectively) is followed by only marginal MAPK activation on the one hand, but prominent NFκB activation on the other hand. This results in only a moderate synthesis of proinflammatory cytokines (IL6, IL8). However, in the ER stress-affected airway epithelial cells, UPR boosts TLR/NFκB-mediated inflammation via PERK- and ATF6-mediated increased p38 and ERK MAPK activation. This results in highly enhanced proinflammatory cytokine production (IL6, IL8).
Discussion
Sensing of microbial components by TLRs are important events in the production of proinflammatory cytokines. It is well established that airway epithelial cells express pattern recognition receptors including TLRs or RIG-I [3, 5, 8, 29]. Under normal, homeostatic conditions, airway epithelial cells are chronically challenged with low-dose airborne microbial substances through normal ventilation of the lung. In order to prevent chronic inflammation in the pulmonary system, airway epithelial cells are tolerant to a certain level of microbial load [5, 8, 9]. On the contrary, various chronic pulmonary diseases have been associated with increased epithelial reactions to normal PAMP levels [13]. Therefore, we hypothesized that besides the mere stimulation of TLRs, conditions might exist which change the tolerant phenotype of airway epithelial cells to a more proinflammatory phenotype.
ER stress is a condition where the protein folding capacity is in a mismatch with the ER protein load, thereby leading to an accumulation of misfolded or unfolded proteins. In order to restore ER homeostasis, the cell activates the UPR, leading to a decreased cap-dependent protein synthesis and an increased expression of chaperones. Several pulmonary diseases have been described to induce ER stress in epithelial cells [30, 31, 32]. Specifically, various chronic pulmonary diseases that are linked to chronic inflammation, including asthma, COPD, or CF, have been associated with ER stress in airway epithelium [33]. In line with these observations, we could prove the presence of ER stress in cystic fibrotic lung using immunohistochemistry. ER stress has been described to induce the expression of proinflammatory cytokines, like IL6 or IL8, in an NFκB-dependent manner. Interestingly, it has also been shown in macrophages that the LPS/TLR4 pathway engages certain UPR proteins in order to increase chaperone levels, most likely to cope with the increased cytokine synthesis rate in activated macrophages. Similar effects can be observed in activated B cells [34].
As TLR and the UPR are proinflammatory and since the UPR has been linked to inflammatory processes in the respiratory system, we hypothesized a link between ER stress and TLR signaling in airway epithelial cells. Indeed, unstressed bronchial epithelial cells displayed only a moderate increase in IL6 and IL8 protein production upon direct TLR stimulation by PAMPs, confirming the described tolerant phenotype. However, we could demonstrate by using BEAS-2B and human primary bronchial epithelial cells that ER stress, artificially induced by thapsigargin, increases TLR-mediated IL6 and IL8 expression and secretion. This effect does not seem to be specific to a single TLR since we could observe similar effects activating different TLRs (TLR4, TLR3, and TLR5). TLRs can be distinguished by their downstream signaling adaptor into MyD88- or TRIF-dependent receptors. Whereas most of the TLRs do signal via MyD88, TLR3 is strictly dependent on the TRIF adaptor. As we observed a costimulatory effect of thapsigargin on pIC, LPS, and flagellin, activating TLR3, TLR4, and TLR5, respectively, this effect seemed to be independent of the downstream signaling adaptor. Thapsigargin induces ER stress by inhibiting SERCAs, thereby depleting ER calcium stores and decreasing the calcium-dependent protein folding capacity. Calcium is a well-known secondary messenger in a variety of signaling processes. To analyze if thapsigargin-induced changes in calcium levels and not ER stress itself might be the reason for our observations, we used another ER stress inducer, acting via a calcium-unrelated mechanism. Indeed, tunicamycin, known to block N-linked protein glycosylation, was also able to boost the LPS- and pIC-induced IL6 and IL8 mRNA response, indicating that altered calcium fluxes are not the reason for the hyperreactive phenotype.
A possible explanation for the observed effects could be increased TLR expression. In line with this, it has been demonstrated that thapsigargin is able to induce TLR2 and TLR5 expression in HeLa cells [27]. However, we could not observe any effect of thapsigargin on the expression of TLR3 and TLR4 or its coreceptors CD14 and MD2 within the time period analyzed. Another explanation, increased NFκB signaling by the UPR, could also be excluded in our experiments. It has been shown that TLR signaling not only depends on NFκB activity, but MAPK activation is also needed for the high level production of cytokines [35]. MAPK are important to increase mRNA stability, but have also been proven to modulate the efficacy of mRNA translation [35]. Surprisingly, stimulation of airway epithelial cells with several PAMPs only increased the phosphorylation of JNK in BEAS-2B cells, but had only moderate effects on ERK and p38 in BEAS-2B or on all three MAPKs in human primary bronchial epithelial cells, whereas thapsigargin induced the phosphorylation of ERK and p38, and JNK additionally in human primary bronchial epithelial cells. Strong activation of all three MAPK, as can be observed in PAMP-stimulated professional innate immune cells, e.g. macrophages, could only be observed in airway epithelial cells if PAMP and thapsigargin were used together. Strong activation of the three arms of MAPK correlated with high levels of IL6 and IL8. The importance of UPR-induced MAPK phosphorylation was further supported by the fact that inhibition of p38 and ERK resulted in decreased IL6 and IL8 protein levels [36]. MAPK inhibition in BEAS-2B cells was more potent on protein than on mRNA levels (data not shown), arguing for an involvement of p38 and ERK in mRNA translation. In line with this, it has been demonstrated that p38 is involved in the translational regulation of TNF by modulating the crosstalk between HuR and TTP in murine macrophages [37]. Moreover, a recent study using airway epithelial cell lines derived from healthy or CF-diseased patients demonstrated a link between chronic p38 activation and increased IL6 and IL8 secretion upon PAMP stimulation [38]. We believe that PAMPs are needed for NFκB-mediated IL6 and IL8 mRNA transcription and the UPR is needed to support MAPK activation to induce efficient mRNA translation and protein synthesis. Thus, combined activation of both signaling pathways is needed to increase the synthesis of proinflammatory cytokines.
Analysis of the ER stress sensors revealed that PERK and ATF6 are crucially important. However, results regarding ATF6-mediated IL6 and IL8 protein synthesis might be blurred by the fact that the knockdown of ATF6 had a strong effect on the proliferation rate of BEAS-2B. This is in accordance with generally decreased p38 levels observed in siATF6-transfected cells (online suppl. fig. 1). Therefore, decreased IL6 and IL8 protein levels might be overestimated. Thus, PERK might be the central regulator of the ER stress-induced hyperreactivity towards TLR stimulation. A direct role of ER stress sensors, especially IRE1 and XBP1s, in TLR signaling pathways has been demonstrated in macrophages [28, 39]. In keeping with this, we observed a significant reduction of IL6 after the knockdown of IRE1α and XBP1 in LPS-stimulated BEAS-2B cells. On the other hand, the contribution of XBP1 to LPS-induced IL8 protein secretion was only minor and not statistically significant. A central role of PERK in this pathway is further supported by the fact that the pharmacological inhibition of PERK in BEAS-2B and human primary bronchial epithelial cells decreased cytokine levels upon TLR activation. Moreover, knockdown of PERK was sufficient to significantly decrease phosphorylation of p38 and ERK in BEAS-2B cells and hpBrEpC. Interestingly, siRNA against PERK was able to decrease IL6 and IL8 mRNA (data not shown) and protein, whereas p38 and ERK inhibition had a stronger effect on protein levels than on mRNA (data not shown). Thus, besides MAPK modulation, another PERK-dependent pathway might be involved in the ER stress-induced hyperreactivity of airway epithelial cells, modifying mainly the transcription of IL6 and IL8. A potential candidate would be NFκB, which has been reported to be activated by ER stress and PERK-mediated protein synthesis attenuation. However, we did not observe a significant increase of NFκB transcriptional activity under the ER stress conditions. Another downstream factor activated by PERK is ATF4. Indeed, a recent study using macrophages demonstrated a role of ATF4 in the regulation of metabolic stress-induced IL6 acting in synergy with TLR4 [40].
Taken together, we demonstrate that ER stress in combination with TLR stimulation results in a hyperreactivity of airway epithelial cells with respect to induction of proinflammatory cytokines. This costimulatory effect is mediated mainly via PERK-dependent activation of p38 and ERK, and ATF6-mediated basal expression of p38, finally resulting in the increased secretion of IL6 and IL8. These findings indicate that ER stress may signal cellular stress or damage and thus serves as a second signal for full-blown epithelial cell activation. Using two signals might help to differentiate between the mere presence of bacteria (including harmless ones) and conditions of infectious danger, which might be associated with epithelial barrier breakdown, due to virulent, invasive, cell-damaging true pathogens or otherwise harmful conditions at mucosal surfaces. Combined activation of ER stress and direct microbial sensing thus overcomes the tolerance of airway epithelial cells.
In this study, we demonstrated the role of ER stress in boosting innate immune responses and that ER stress markers are detectable by immunohistochemistry in lung biopsies of CF patients. Therefore, pharmacological inhibition of ER stress in these patients might be beneficial since it would block the overshooting of chronic inflammation regularly observed in these patients. Further research to clarify the exact molecular mechanism of how ER stress leads to activated MAPK might identify even more specific drug targets without affecting the physiological ER stress or general activation of the immune system to fight pathogens associated with CF.
Disclosure Statement
The authors declare that they have no conflicts of interest relating to the contents of this article.
Supplementary Material
Supplementary data
Acknowledgments
We thank Suzan Allam, Dr. Damir Krunic, Svenja Naumann, and Jutta Scheuerer for excellent technical support. This work was supported by the German Research Foundation (DFG; grants SFB 938/E, DA592/4 and DA592/6 to A.H.D.).
References
- 1.Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14:686–698. doi: 10.1038/nri3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weitnauer M, Mijošek V, Dalpke AH. Control of local immunity by airway epithelial cells. Mucosal Immunol. 2016;9:287–298. doi: 10.1038/mi.2015.126. [DOI] [PubMed] [Google Scholar]
- 3.Greene CM, Carroll TP, Smith SGJ, Taggart CC, Devaney J, Griffin S, et al. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 4.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 5.Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent lipopolysaccharide-induced β-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000;275:29731–29736. doi: 10.1074/jbc.M000184200. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 7.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 8.Mayer AK, Muehmer M, Mages J, Gueinzius K, Hess C, Heeg K, et al. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J Immunol. 2007;178:3134–3142. doi: 10.4049/jimmunol.178.5.3134. [DOI] [PubMed] [Google Scholar]
- 9.Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 10.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol. 2011;186:7080–7088. doi: 10.4049/jimmunol.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Maele L, Fougeron D, Janot L, Didierlaurent A, Cayet D, Tabareau J, et al. Airway structural cells regulate TLR5-mediated mucosal adjuvant activity. Mucosal Immunol. 2014;7:489–500. doi: 10.1038/mi.2013.66. [DOI] [PubMed] [Google Scholar]
- 12.McAlees JW, Whitehead GS, Harley ITW, Cappelletti M, Rewerts CL, Holdcroft AM, et al. Distinct TLR4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal Immunol. 2015;8:863–873. doi: 10.1038/mi.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218–C230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 14.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, et al. Activation of the Akt-NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasnain SZ, Lourie R, Das I, Chen AC-H, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90:260–270. doi: 10.1038/icb.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J, Rahman S, Ayaub EA, Dickhout JG, Ask K. Protein misfolding and endoplasmic reticulum stress in chronic lung disease. Chest. 2013;143:1098–1105. doi: 10.1378/chest.12-2133. [DOI] [PubMed] [Google Scholar]
- 20.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 21.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 22.Yaghi A, Zaman A, Dolovich M. Primary human bronchial epithelial cells grown from explants. J Vis Exp. doi: 10.3791/1789. DOI: 10.3791/1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 24.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greene CM. Toll-Like Receptors in Diseases of the Lung. Sharjah, Bentham Science. 2012 [Google Scholar]
- 26.Metcalfe HJ, Lea S, Hughes D, Khalaf R, Abbott-Banner K, Singh D. Effects of cigarette smoke on Toll-like receptor (TLR) activation of chronic obstructive pulmonary disease (COPD) macrophages. Clin Exp Immunol. 2014;176:461–472. doi: 10.1111/cei.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimasaki S, Koga T, Shuto T, Suico MA, Sato T, Watanabe K, et al. Endoplasmic reticulum stress increases the expression and function of toll-like receptor-2 in epithelial cells. Biochem Biophys Res Commun. 2010;402:235–240. doi: 10.1016/j.bbrc.2010.09.132. [DOI] [PubMed] [Google Scholar]
- 28.Martinon F, Chen X, Lee A-H, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismüller K-H, Godowski PJ, et al. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human β defensin-2. J Immunol. 2003;171:6820–6826. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 30.Roberson EC, Tully JE, Guala AS, Reiss JN, Godburn KE, Pociask DA, et al. Influenza induces endoplasmic reticulum stress, caspase-12-dependent apoptosis, and C-Jun N-terminal kinase-mediated transforming growth factor-Β release in lung epithelial cells. Am J Respir Cell Mol Biol. 2012;46:573–581. doi: 10.1165/rcmb.2010-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed M, Morris SH, Owczarczyk AB, Lukacs NW. Deficiency of autophagy protein Map1-LC3b mediates IL-17-dependent lung pathology during respiratory viral infection via ER stress-associated IL-1. Mucosal Immunol. 2015;8:1118–1130. doi: 10.1038/mi.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loose M, Hudel M, Zimmer K-P, Garcia E, Hammerschmidt S, Lucas R, et al. Pneumococcal hydrogen peroxide-induced stress signaling regulates inflammatory genes. J Infect Dis. 2015;211:306–316. doi: 10.1093/infdis/jiu428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osorio F, Lambrecht B, Janssens S. The UPR and lung disease. Semin Immunopathol. 2013;35:293–306. doi: 10.1007/s00281-013-0368-6. [DOI] [PubMed] [Google Scholar]
- 34.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 35.Arthur JSC, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 36.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Tiedje C, Ronkina N, Tehrani M, Dhamija S, Laass K, Holtmann H, et al. The p38/MK2-driven exchange between tristetraprolin and HuR regulates AU-rich element-dependent translation. PLoS Genet. 2012;8:e1002977. doi: 10.1371/journal.pgen.1002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blohmke CJ, Mayer ML, Tang AC, Hirschfeld AF, Fjell CD, Sze MA, et al. Atypical activation of the unfolded protein response in cystic fibrosis airway cells contributes to p38 MAPK-mediated innate immune responses. J Immunol. 2012;189:5467–5475. doi: 10.4049/jimmunol.1103661. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Joe Y, Kim HJ, Kim Y-S, Jeong SO, Pae H-O, et al. Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J Immunol. 2015;194:4498–4506. doi: 10.4049/jimmunol.1401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki Y, Suganami T, Hachiya R, Shirakawa I, Kim-Saijo M, Tanaka M, et al. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes. 2014;63:152–161. doi: 10.2337/db13-0757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data