Abstract
Activation of complement leads to generation of the 3 anaphylatoxins C3a, C4a, and C5a. Although all 3 peptides are structurally similar, only C3a and C5a share a similar functional profile that includes the classic inflammatory activities and, more recently, developmental homing and regenerative properties among others. In contrast, the functional profile of C4a is questionable in most cases owing to contamination of C4a preparations with physiologically relevant levels of C3a and/or C5a. Combined with the absence of an identified C4a receptor and the inability of C4a to signal through the C3a and C5a receptors, it is clear that C4a should not be included in the family of complement anaphylatoxins.
Key Words: Anaphylatoxin, Complement, G-protein-coupled receptors
The complement system is composed of over 40 soluble proteins and membrane receptors and is well known for its role in initiating and modulating innate and adaptive immune responses to a wide variety of pathogens. In the last 10–20 years it has become clear that complement also contributes to many facets of biology well beyond pathogen elimination. For example, it is now established that several complement components are critical in tissue turnover, the development of bone and cartilage, liver regeneration, and homing of hematopoietic stem cells and neural progenitor cells [reviewed in [1, 2, 3, 4]]. Furthermore, recent studies have demonstrated that 2 central components of the complement system, i.e. C3 and C5, are important in limb and eye regeneration after injury [5], while C1, C3, and the complement receptor type 3 contribute to synaptic pruning in development and disease [6]. Complement even exerts psychopharmacological control on eating and drinking behavior based on studies in rodents [7, 8, 9, 10, 11, 12]. C3a and C5a, 2 polypeptides derived from proteolytic cleavage of C3 and C5, respectively, mediate a significant number of the biological activities listed above. Collectively, C3a and C5a, along with C4a, a third polypeptide derived from cleavage of C4, are known as anaphylatoxins because of their ability to induce a variety of inflammatory responses which can be as severe as type I hypersensitivity allergic responses [3, 13]. Although all 3 proteins are labeled anaphylatoxins, there is remarkably little evidence to support a role for C4a in this capacity. In this review, the case for removing C4a from the list of complement anaphylatoxins is made based on its limited range of biological activities and the absence of a specific C4a receptor to mediate these functions.
The Anaphylatoxins
Structure
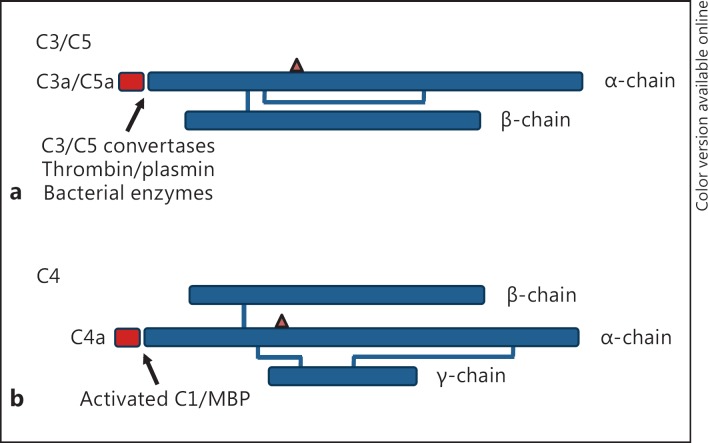
The complement anaphylatoxins are derived from cleavage of the α-chain of C3, C4, or C5 on activation of complement through the classic, lectin, alternative, or extrinsic protease pathways or by one of several bacterial enzymes [reviewed in [3]] (fig. 1). The 3 proteins have a molecular weight of approximately 9 kD and range in size from 74 to 77 amino acids. C5a is posttranslationally modified with a single complex carbohydrate chain attached through an N-linked glycosidic bond to asparagine residue 64 [14], while C3a and C4a have no carbohydrate side chains and lack the classic Asn-X-Ser/Thr glycosylation site motif. Despite similar biological functions, particularly for C3a and C5a (discussed below), there is limited sequence homology between the human anaphylatoxins. All 3 proteins have 6 invariant cysteine residues that give rise to a 4-helix core generated by 3 disulfide linkages [15, 16, 17], a limited number of conserved residue substitutions, and a carboxy-terminal arginine residue [14, 18, 19]. The sequence homology of the anaphylatoxins is high between species (as much as 70%) [3, 20, 21, 22], but it is considerably lower between the human proteins. For example, human C3a shares only 29 and 34% identity with C4a and C5a, respectively, a level of homology comparable to that between the entire coding sequence of C3, C4, and C5 [23]. The proteins share higher-order structural features, including backbone architecture, 4 antiparallel helices, and an overall compact globular shape, while the amino- and carboxy-terminal regions have no ordered conformation [24, 25, 26, 27, 28].
Fig. 1.
Schematic of the polypeptide structure of human C3, C4, and C5. a C3 and C5 are composed of an α- and a β-chain that have inter- and intrachain disulfide bridges. C3a and C5a are derived by cleavage of the amino-terminal end of the α-chain by C3/C5 convertases, activated coagulation proteases including thrombin, plasmin, and factors IX through XI, and by bacterial enzymes. b C4 is composed of 3 polypeptide chains (α, β, and γ) which also have inter- and intrachain disulfide bridges. C4a is derived by cleavage of the amino-terminal end of the α-chain by activated C1 on activation of the classical, or by activated mannose binding protein on activation of the lectin pathway. The triangle indicates the relative position of the thioester bond found in the α-chain of C3 and C4.
Function
Despite the structural similarity between the complement anaphylatoxins, the functional profile of these proteins is dramatically different with respect to C4a. As outlined in table 1, C3a and C5a share an amazing array of diverse functional properties that includes modulation of the innate and adaptive immune response, cell homing, and tissue regeneration. In contrast, C4a mediates almost none of these functions and, in those cases where a functional overlap is reported, closer inspection of the data suggests a significant weakness in the studies. The fundamental problem in most cases is the purity of the C4a preparations. For example, the initial studies identifying C4a as an anaphylatoxin were performed using human C4a in a guinea pig ileum contraction assay. C4a could induce ileum smooth muscle contraction but at concentrations ∼100-fold higher than those used for C3a [34]. Furthermore, C4a induced erythema and wheal formation upon injection into the skin of human volunteers, but again concentrations several orders of magnitude higher were required to elicit the same response via injection of C3a and C5a. The C4a preparations used in these studies were contaminated with physiologically relevant levels of C3a (0.002%) and C5a (0.006%), suggesting that the biological activity attributed to C4a was in fact due almost entirely to C3a/C5a [34]. Given that C3a and C5a are functional in the nanogram range, this level of contamination (due most likely to the biochemical preparatory methods employed) would be sufficient to induce the reported biological responses. A subsequent study examining the ability of the anaphylatoxins to cross-desensitize guinea pig platelets revealed that C4a had only 3% of the activity of C3a on a molar basis [38]. Given that the C4 used in the study was prepared in exactly the same way as in the study of Gorski et al. [34], it is clear that C3a and C5a contamination complicates the interpretation of the reported results. More recently it was reported that C4a induced Ca++ mobilization in guinea pig macrophages and that these cells were still sensitive to C3a-mediated Ca++ mobilization, suggesting that C4a has its own receptor [56]. The authors did not, however, assess for trace amounts of C3a and C5a in the C4a used in the study, which had been prepared using a method similar to that of Gorski et al. [34], raising concerns about the purity of C4a and the validity of the data. In a study using recombinant C4a and C5a (thus overcoming the contamination issue), the ability of C4a to inhibit C5a-mediated neointima injury was assessed [55]. Coinfusion of C4a and C5a resulted in reduced neointima formation relative to infusion of C5a alone. However, interpretation of the data is complicated by the fact that dose-response experiments were not performed and the amount of both anaphylatoxins administered to mice was exceedingly high (0.1 mM) relative to physiological levels. Finally, in a study proposing a new function for C4a, the ability to inhibit blood monocyte chemotaxis, possible C3a/C5a contamination, and the ill-defined components of zymosan-activated serum significantly weakened the interpretation of the results [32].
Table 1.
Complement anaphylatoxin functions
| Function | C3a | C4a | C5a | Reference |
|---|---|---|---|---|
| Defined receptor | + | _ | + | 3, 4, 29–31 |
| Chemotaxis | + | ? | + | 3, 4, 13, 32, 33 |
| Smooth-muscle contraction | + | ? | 3, 4, 13, 34 | |
| Vascular permeability | + | ? | + | 34, 35 |
| Myeloid cell activation | + | – | + | 3, 4, 13, 36 |
| Platelet activation | + | – | + | 3, 4, 13, 37, 38 |
| CD4/CD8 and γδ T cell modulation | + | – | + | 39–44 |
| Induction of acute phase response (cytokine production) | + | – | + | 3, 4, 13, 45 |
| Developmental homing | + | – | + | 46–49 |
| Regeneration | + | – | + | 1, 2, 50, 51 |
| Antimicrobial | + | + | – | 27, 52–54 |
| Cross-desensitization | ? | 55, 56 |
For many of the biological activities listed in table 1, such as developmental homing or tissue regeneration, C4a has yet to be assessed relative to C3a and C5a. Thus it cannot be ruled out that C4a shares no functional overlap with the other anaphylatoxins. In fact, C4a and C3a both share antimicrobial activity toward Gram-negative and Gram-positive bacteria based on studies using purified proteins and 20-mer peptides derived from the sequence of various regions of both peptides [27, 53]. It should be noted however, that C3a/C4a antimicrobial activity is receptor independent, relying instead on the overall net charge, percentage of hydrophobic amino acids, and degree of amphipathicity of the peptides [57]. Nevertheless, it is striking that the C4a biological activity is so limited relative to that of C3a and C5a. Although it is possible that C4a contributes to C3a/C5a-mediated immune functions, until more rigorous studies are performed, the in vitro and in vivo data to date fails to support its moniker as an anaphylatoxin.
The Anaphylatoxin Receptors
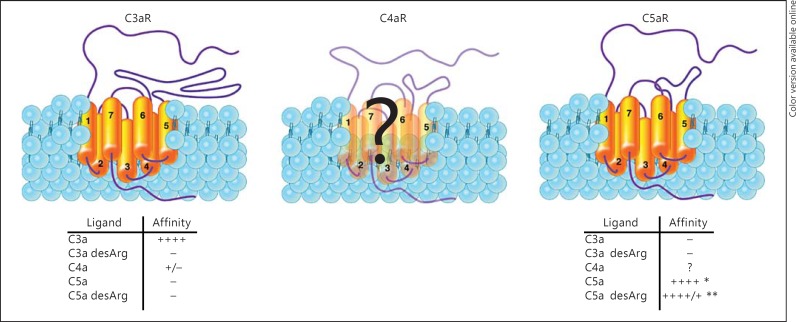
Perhaps the most compelling argument that C4a is not an anaphylatoxin is the absence of an identified C4a-specific receptor and the inability of C4a to effectively signal through any known human receptor. To date, a single C3aR and 2 C5aR (C5aR1 and C5aR2) have been characterized in humans and many other species (fig. 2) [30, 31, 58, 59, 60], all of which are members of the large G-protein-coupled receptor family (GPCR) [61, 62]. The C3a and C5a receptors are part of a subgroup of receptors that includes the N-formyl-methionyl receptors and several related orphan receptors. There is no indication that any of the orphan GPCR serves as a C4a receptor. In fact, the branching pattern and evolutionary distance argues against C4a as a ligand for these receptors [62]. Could C4a bind and signal through other GPCR family members outside the complement anaphylatoxin and FMLP subgroup, particularly within the peptide receptor subgroupings? It seems unlikely since many of these receptors bind small molecules such as nucleotides, lipids, biogenic amines, cyclic peptides, and glutamate. Of the remaining peptide receptor subgroups, most of the members bind smaller peptides with conformational features markedly different from that of C4a [62].
Fig. 2.
Schematic of the human complement anaphylatoxin receptor structure. A schematic structure of the human C3a and C5 receptors is shown. Both receptors are G protein-coupled, 7-membrane-spanning family members. Each transmembrane domain is numbered. The C3aR is unique among GPCRs due to the unusually large extracellular loop between transmembrane domains 4 and 5. The as yet unidentified C4a receptor is shown as partially transparent in the center panel. Shown below the C3aR and C5aR are the relative binding affinities for their known ligands. * The affinity of C5a for both C5aR is in the low nanomolar range. ** The affinity for C5a desArg for C5aR1 is in the low nanomolar range, while the affinity for C5aR2 is in the micromolar range.
The complement anaphylatoxin receptors bind their respective ligands with a low nanomolar affinity [30, 31, 58], with the exception of no binding between C3adesArg and the C3aR [63, 64] and a low affinity binding between C5a-desArg and C5aR1 (see fig. 2). Neither C3a nor C5a serves as an effective agonist in cross-desensitization assays, a finding supported by studies mapping distinct ligand:receptor binding sites for both peptides [65, 66, 67, 68, 69, 70]. C4a weakly binds the human C3aR when present in micromolar amounts but does not induce calcium mobilization, indicating that a functional C4aR must be distinct from the C3aR [29, 71]. Interestingly, human C4a binds the guinea pig C3aR and induces calcium mobilization in in vitro assays when used in micromolar amounts [71]. This latter finding raises evolutionary questions regarding C4a. Does C4a in lower species preferentially signal through the C3a or C5a receptors? Perhaps signaling through the C4aR was functionally redundant and the receptor was selected against over time. Studies designed to address these and other questions are feasible and may shed light on evolutionary divergence of the anaphylatoxin receptors and their ligand interactions.
Conclusions and Perspectives
The anaphylactic activity of complement was initially postulated over 100 years ago [72], and subsequent studies in passive cutaneous anaphylaxis directly demonstrated a role for complement but did not implicate the complement anaphylatoxins as we know them today [73, 74, 75]. In the late sixties, multiple groups independently documented the isolation and functional activity of C3a and C5a using purified complement proteins [76, 77, 78]. Since that time, C3a and C5a have been shown to contribute to a broad array of biological functions well beyond that of their classical role as anaphylatoxins inducing smooth muscle contraction, wheal and flare reactions, or platelet activation. Expression of the receptors for C3a and C5a on nearly all cells types provides broad support for these newly described biological activities [reviewed in [4]]. From an evolutionary point of view, the C3a and C5a receptors have integrated anaphylatoxin-mediated control of adaptive immune responses at the level of the bony fishes since these receptors have been conserved in teleost fish for over 300 million years [79, 80]. The control of other biological functions by C3a and C5a in organisms phylogenetically simpler than the teleost fish remains to be established. In contrast, C4a appears to be the dunsel of the complement anaphylatoxin family. It fails to meet the minimum expectations for a classic complement anaphylatoxin and has yet to be shown to contribute to adaptive immune responses or any of the more recently described developmental or regenerative activities of C3a and C5a. The absence of a C4a receptor, coupled with the inability to bind and signal effectively through the C3aR and C5aR, suggests that the primary function of C4a is to serve as the ‘pro’ portion of C4, stabilizing C4 and its thioester bond until the classical or lectin pathways are activated. Until C4a is shown to contribute to immune responses, it should not be considered an anaphylatoxin and, as suggested by Klos et al. [4] it may make more sense to eliminate the term anaphylatoxin altogether given their broad functional array.
Disclosure Statement
The author declares no competing interests.
Acknowledgements
The author thanks David Fisher for the anaphylatoxin receptor illustration and Dr. Robert Ames for critical reading of this paper.
References
- 1.Rutkowski MJ, Sughrue ME, Kane AJ, Ahn BJ, Fang S, Parsa AT. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. 2010;59:897–905. doi: 10.1007/s00011-010-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Fang S, Parsa AT. Complement and the central nervous system: emerging roles in development, protection and regeneration. Immunol Cell Biol. 2010;88:781–786. doi: 10.1038/icb.2010.48. [DOI] [PubMed] [Google Scholar]
- 3.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klos A, Wende E, Wareham KJ, Monk PN. International union of basic and clinical pharmacology. LXXXVII. Complement peptide c5a, c4a, and c3a receptors. Pharmacol Rev. 2013;65:500–543. doi: 10.1124/pr.111.005223. [DOI] [PubMed] [Google Scholar]
- 5.Kimura Y, Madhavan M, Call MK, Santiago W, Tsonis PA, Lambris JD, Del Rio-Tsonis K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- 6.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 7.Schupf N, Williams CA, Hugli TE, Cox J. Psychopharmacological activity of anaphylatoxin C3a in rat hypothalamus. J Neuroimmunol. 1983;5:305–316. doi: 10.1016/0165-5728(83)90051-6. [DOI] [PubMed] [Google Scholar]
- 8.Williams CA, Schupf N, Hugli TE. Anaphylatoxin C5a modulation of an alpha-adrenergic receptor system in the rat hypothalamus. J Neuroimmunol. 1985;9:29–40. doi: 10.1016/s0165-5728(85)80004-7. [DOI] [PubMed] [Google Scholar]
- 9.Schupf N, Williams CA, Berkman A, Cattell WS, Kerper L. Binding specificity and presynaptic action of anaphylatoxin C5a in rat brain. Brain Behav Immun. 1989;3:28–38. doi: 10.1016/0889-1591(89)90003-2. [DOI] [PubMed] [Google Scholar]
- 10.Ohinata K, Inui A, Asakawa A, Wada K, Wada E, Yoshikawa M. Albutensin A and complement C3a decrease food intake in mice. Peptides. 2002;23:127–133. doi: 10.1016/s0196-9781(01)00588-5. [DOI] [PubMed] [Google Scholar]
- 11.Ohinata K, Takagi K, Biyajima K, Kaneko K, Miyamoto C, Asakawa A, Eguchi N, Urade Y, Inui A, Yoshikawa M. Complement C5a stimulates food intake via a prostaglandin D(2)- and neuropeptide Y-dependent mechanism in mice. Prostaglandins Other Lipid Mediat. 2009;90:81–84. doi: 10.1016/j.prostaglandins.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Ohinata K, Yoshikawa M. Food intake regulation by central complement system. Adv Exp Med Biol. 2008;632:35–46. [PubMed] [Google Scholar]
- 13.Ember JA, Jagels MA, Hugli TE. Characterization of complement anaphylatoxina and their biological responses. In: Volanakis JE, Frank MM, The Human Complement System in Health and Disease, editors. New York: Dekker; 1998. pp. pp 241–284. [Google Scholar]
- 14.Fernandez HN, Hugli TE. Primary structural analysis of the polypeptide portion of human c5a anaphylatoxin: polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978;253:6955–6964. [PubMed] [Google Scholar]
- 15.Nettesheim DG, Edalji RP, Mollison KW, Greer J, Zuiderweg ER. Secondary structure of complement component C3a anaphylatoxin in solution as determined by NMR spectroscopy: differences between crystal and solution conformations. Proc Natl Acad Sci U S A. 1988;85:5036–5040. doi: 10.1073/pnas.85.14.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Boyar W, Toth MJ, Wennogle L, Gonnella NC. Structural definition of the C5a C terminus by two-dimensional nuclear magnetic resonance spectroscopy. Proteins. 1997;28:261–267. doi: 10.1002/(sici)1097-0134(199706)28:2<261::aid-prot13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Zuiderweg ER, Nettesheim DG, Mollison KW, Carter GW. Tertiary structure of human complement component C5a in solution from nuclear magnetic resonance data. Biochemistry. 1989;28:172–185. doi: 10.1021/bi00427a025. [DOI] [PubMed] [Google Scholar]
- 18.Hugli TE. Human anaphylatoxin (C3a) from the third component of complement: primary structure. J Biol Chem. 1975;250:8293–8301. [PubMed] [Google Scholar]
- 19.Moon KE, Gorski JP, Hugli TE. Complete primary structure of human C4a anaphylatoxin. J Biol Chem. 1981;256:8685–8692. [PubMed] [Google Scholar]
- 20.Hugli TE, Vallota EH, Muller-Eberhard HJ. Purification and partial characterization of human and porcine C3a anaphylatoxin. J Biol Chem. 1975;250:1472–1478. [PubMed] [Google Scholar]
- 21.Smith MA, Gerrie LM, Dunbar B, Fothergill JE. Primary structure of bovine complement activation fragment C4a, the third anaphylatoxin: purification and complete amino acid sequence. Biochem. 1982;207:253–260. doi: 10.1042/bj2070253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui L, Carney DF, Hugli TE. Primary structure and functional characterization of rat C5a: an anaphylatoxin with unusually high potency. Protein Sci. 1994;3:1169–1177. doi: 10.1002/pro.5560030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetsel RA, Lemons RS, Le Beau MM, Barnum SR, Noack D, Tack BF. Molecular analysis of human complement component C5: localization of the structural gene to chromosome 9. Biochemistry. 1988;27:1474–1482. doi: 10.1021/bi00405a012. [DOI] [PubMed] [Google Scholar]
- 24.Huber R, Scholze H, Paques EP, Deisenhofer J. Crystal structure analysis and molecular model of human C3a anaphylatoxin. Hoppe Seylers Z Physiol Chem. 1980;361:1389–1399. doi: 10.1515/bchm2.1980.361.2.1389. [DOI] [PubMed] [Google Scholar]
- 25.Paques EP, Scholze H, Huber R. Purification and crystallization of human anaphylatoxin, C3a. Hoppe Seylers Z Physiol Chem. 1980;361:977–980. [PubMed] [Google Scholar]
- 26.Greer J. Comparative structural anatomy of the complement anaphylatoxin proteins C3a, C4a and C5a. Enzyme. 1986;36:150–163. doi: 10.1159/000469285. [DOI] [PubMed] [Google Scholar]
- 27.Pasupuleti M, Walse B, Nordahl EA, Morgelin M, Malmsten M, Schmidtchen A. Preservation of antimicrobial properties of complement peptide C3a, from invertebrates to humans. J Biol Chem. 2007;282:2520–2528. doi: 10.1074/jbc.M607848200. [DOI] [PubMed] [Google Scholar]
- 28.Fredslund F, Laursen NS, Roversi P, Jenner L, Oliveira CL, Pedersen JS, Nunn MA, Lea SM, Discipio R, Sottrup-Jensen L, Andersen GR. Structure of and influence of a tick complement inhibitor on human complement component 5. Nat Immunol. 2008;9:753–760. doi: 10.1038/ni.1625. [DOI] [PubMed] [Google Scholar]
- 29.Ames RS, Tornetta MA, Foley JJ, Hugli TE, Sarau HM. Evidence that the receptor for C4a is distinct from the C3a receptor. Immunopharmacology. 1997;38:87–92. doi: 10.1016/s0162-3109(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 30.Ames RS, Li Y, Sarau HM, Nuthulaganti P, Foley JJ, Ellis C, Zeng Z, Su K, Jurewicz AJ, Hertzberg RP, Bergsma DJ, Kumar C. Molecular cloning and characterization of the human anaphylatoxin C3a receptor. J Biol Chem. 1996;271:20231–20234. doi: 10.1074/jbc.271.34.20231. [DOI] [PubMed] [Google Scholar]
- 31.Crass T, Raffetseder U, Martin U, Grove M, Klos A, Kohl J, Bautsch W. Expression cloning of the human C3a anaphylatoxin receptor (C3aR) from differentiated U-937 cells. Eur J Immunol. 1996;26:1944–1950. doi: 10.1002/eji.1830260840. [DOI] [PubMed] [Google Scholar]
- 32.Tsuruta T, Yamamoto T, Matsubara S, Nagasawa S, Tanase S, Tanaka J, Takagi K, Kambara T. Novel function of C4a anaphylatoxin: release from monocytes of protein which inhibits monocyte chemotaxis. Am J Pathol. 1993;142:1848–1857. [PMC free article] [PubMed] [Google Scholar]
- 33.Kato Y, Nakao M, Shimizu M, Wariishi H, Yano T. Purification and functional assessment of c3a, c4a and c5a of the common carp (Cyprinus carpio) complement. Dev Comp Immunol. 2004;28:901–910. doi: 10.1016/j.dci.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Gorski JP, Hugli TE, Muller-Eberhard HJ. C4a: The third anaphylatoxin of the human complement system. Proc Natl Acad Sci U S A. 1979;76:5299–5302. doi: 10.1073/pnas.76.10.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hugli TE, Kawahara MS, Unson CG, Molinar-Rode R, Erickson BW. The active site of human C4a anaphylatoxin. Mol Immunol. 1983;20:637–645. doi: 10.1016/0161-5890(83)90008-1. [DOI] [PubMed] [Google Scholar]
- 36.Fukuoka Y, Xia HZ, Sanchez-Munoz LB, Dellinger AL, Escribano L, Schwartz LB. Generation of anaphylatoxins by human beta-tryptase from C3, C4, and C5. J Immunol. 2008;180:6307–6316. doi: 10.4049/jimmunol.180.9.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meuer S, Ecker U, Hadding U, Bitter-Suermann D. Platelet-serotonin release by C3a and C5a: Two independent pathways of activation. J Immunol. 1981;126:1506–1509. [PubMed] [Google Scholar]
- 38.Meuer S, Hugli TE, Andreatta RH, Hadding U, Bitter-Suermann D. Comparative study on biological activities of various anaphylatoxins (C4a, C3a, C5a): investigations on their ability to induce platelet secretion. Inflammation. 1981;5:263–273. doi: 10.1007/BF00911092. [DOI] [PubMed] [Google Scholar]
- 39.Raedler H, Vieyra MB, Leisman S, Lakhani P, Kwan W, Yang M, Johnson K, Faas SJ, Tamburini P, Heeger PS. Anti-complement component C5 mAB synergizes with CTLA4Ig to inhibit alloreactive T cells and prolong cardiac allograft survival in mice. Am J Transplant. 2011;11:1397–1406. doi: 10.1111/j.1600-6143.2011.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieyra M, Leisman S, Raedler H, Kwan WH, Yang M, Strainic MG, Medof ME, Heeger PS. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. Am J Pathol. 2011;179:766–774. doi: 10.1016/j.ajpath.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, Heeger PS, Tuohy VK, Medof ME. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han G, Geng S, Li Y, Chen G, Wang R, Li X, Ma Y, Shen B, Li Y. GammadeltaT-cell function in sepsis is modulated by C5a receptor signalling. Immunology. 2011;133:340–349. doi: 10.1111/j.1365-2567.2011.03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol. 2008;180:6044–6053. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 46.Benard M, Raoult E, Vaudry D, Leprince J, Falluel-Morel A, Gonzalez BJ, Galas L, Vaudry H, Fontaine M. Role of complement anaphylatoxin receptors (C3aR, C5aR) in the development of the rat cerebellum. Mol Immunol. 2008;45:3767–3774. doi: 10.1016/j.molimm.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 47.Shinjyo N, Stahlberg A, Dragunow M, Pekny M, Pekna M. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells. 2009;27:2824–2832. doi: 10.1002/stem.225. [DOI] [PubMed] [Google Scholar]
- 48.Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, Parsons M, Lambris JD, Mayor R. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011;21:1026–1037. doi: 10.1016/j.devcel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, Turner AR, Ratajczak MZ, Janowska-Wieczorek A. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes T, Luz-Madrigal A, Reis ES, Echeverri Ruiz NP, Grajales-Esquivel E, Tzekou A, Tsonis PA, Lambris JD, Del Rio-Tsonis K. Complement anaphylatoxin C3a is a potent inducer of embryonic chick retina regeneration. Nat Commun. 2013;4:2312. doi: 10.1038/ncomms3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malmsten M, Davoudi M, Walse B, Rydengard V, Pasupuleti M, Morgelin M, Schmidtchen A. Antimicrobial peptides derived from growth factors. Growth Factors. 2007;25:60–70. doi: 10.1080/08977190701344120. [DOI] [PubMed] [Google Scholar]
- 53.Nordahl EA, Rydengard V, Nyberg P, Nitsche DP, Morgelin M, Malmsten M, Bjorck L, Schmidtchen A. Activation of the complement system generates antibacterial peptides. Proc Natl Acad Sci U S A. 2004;101:16879–16884. doi: 10.1073/pnas.0406678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Z, Li M, Wu J, Zhang S. Interplay between invertebrate C3a with vertebrate macrophages: functional characterization of immune activities of amphioxus C3a. Fish Shellfish Immunol. 2013;35:1249–1259. doi: 10.1016/j.fsi.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Xu H, Yu W, Xie BD. Complement anaphylatoxin C4a inhibits C5a-induced neointima formation following arterial injury. Mol Med Rep. 2014;10:45–52. doi: 10.3892/mmr.2014.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murakami Y, Yamamoto T, Imamichi T, Nagasawa S. Cellular responses of guinea-pig macrophages to C4a: inhibition of C3a-induced O2- generation by C4a. Immunol Lett. 1993;36:301–304. doi: 10.1016/0165-2478(93)90104-a. [DOI] [PubMed] [Google Scholar]
- 57.Yount NY, Bayer AS, Xiong YQ, Yeaman MR. Advances in antimicrobial peptide immunobiology. Biopolymers. 2006;84:435–458. doi: 10.1002/bip.20543. [DOI] [PubMed] [Google Scholar]
- 58.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 59.Roglic A, Prossnitz ER, Cavanagh SL, Pan Z, Zou A, Ye RD. cDNA cloning of a novel G protein-coupled receptor with a large extracellular loop structure. Biochim Biophys Acta. 1996;1305:39–43. doi: 10.1016/0167-4781(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 60.Ohno M, Hirata T, Enomoto M, Araki T, Ishimaru H, Takahashi TA. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol Immunol. 2000;37:407–412. doi: 10.1016/s0161-5890(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 61.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joost P, Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002;3:RESEARCH0063. doi: 10.1186/gb-2002-3-11-research0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilken HC, Gotze O, Werfel T, Zwirner J. C3a(desArg) does not bind to and signal through the human C3a receptor. Immunol Lett. 1999;67:141–145. doi: 10.1016/s0165-2478(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 64.Johswich K, Martin M, Thalmann J, Rheinheimer C, Monk PN, Klos A. Ligand specificity of the anaphylatoxin C5L2 receptor and its regulation on myeloid and epithelial cell lines. J Biol Chem. 2006;281:39088–39095. doi: 10.1074/jbc.M609734200. [DOI] [PubMed] [Google Scholar]
- 65.Crass T, Ames RS, Sarau HM, Tornetta MA, Foley JJ, Kohl J, Klos A, Bautsch W. Chimeric receptors of the human C3a receptor and C5a receptor (CD88) J Biol Chem. 1999;274:8367–8370. doi: 10.1074/jbc.274.13.8367. [DOI] [PubMed] [Google Scholar]
- 66.Chao TH, Ember JA, Wang M, Bayon Y, Hugli TE, Ye RD. Role of the second extracellular loop of human C3a receptor in agonist binding and receptor function. J Biol Chem. 1999;274:9721–9728. doi: 10.1074/jbc.274.14.9721. [DOI] [PubMed] [Google Scholar]
- 67.Sun J, Ember JA, Chao TH, Fukuoka Y, Ye RD, Hugli TE. Identification of ligand effector binding sites in transmembrane regions of the human G protein-coupled C3a receptor. Protein Sci. 1999;8:2304–2311. doi: 10.1110/ps.8.11.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikiforovich GV, Baranski TJ. Structural mechanisms of constitutive activation in the C5a receptors with mutations in the extracellular loops: molecular modeling study. Proteins. 2012;80:71–80. doi: 10.1002/prot.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nikiforovich GV, Marshall GR, Baranski TJ. Modeling molecular mechanisms of binding of the anaphylatoxin C5a to the C5a receptor. Biochemistry. 2008;47:3117–3130. doi: 10.1021/bi702321a. [DOI] [PubMed] [Google Scholar]
- 70.Nikiforovich GV, Marshall GR, Baranski TJ. Simplified modeling approach suggests structural mechanisms for constitutive activation of the C5a receptor. Proteins. 2011;79:787–802. doi: 10.1002/prot.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lienenklaus S, Ames RS, Tornetta MA, Sarau HM, Foley JJ, Crass T, Sohns B, Raffetseder U, Grove M, Holzer A, Klos A, Kohl J, Bautsch W. Human anaphylatoxin C4a is a potent agonist of the guinea pig but not the human C3a receptor. J Immunol. 1998;161:2089–2093. [PubMed] [Google Scholar]
- 72.Zinsser H. On anaphylatoxins and endotoxins of the typhoid bacillus. J Exp Med. 1913;17:117–131. doi: 10.1084/jem.17.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwab L, Moll FC, Hall T, Brean H, Kirk M, van Zandt Hawn C, Janeway CA. Experimental hypersensitivity in the rabbit: effect of inhibition of antibody formation by X-radiation and nitrogen mustards on the histologic and serologic sequences, and on the behavior of serum complement, following single large injections of foreign proteins. J Exp Med. 1950;91:505–526. doi: 10.1084/jem.91.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osler AG, Hawrisiak MM, Ovary Z, Siqueira M, Bier OG. Studies on the mechanism of hypersensitivity phenomena. 2. The participation of complement in passive cutaneous anaphylaxis of the albino rat. J Exp Med. 1957;106:811–834. doi: 10.1084/jem.106.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osler AG, Randall HG, Hill BM, Ovary Z. Studies on the mechanism of hypersensitivity phenomena. 3. The participation of complement in the formation of anaphylatoxin. J Exp Med. 1959;110:311–339. doi: 10.1084/jem.110.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jensen J. Anaphylatoxin in its relation to the complement system. Science. 1967;155:1122–1123. doi: 10.1126/science.155.3766.1122. [DOI] [PubMed] [Google Scholar]
- 77.da Silva WD, Eisele JW, Lepow IH. Complement as a mediator of inflammation. 3. Purification of the activity with anaphylatoxin properties generated by interaction of the first four components of complement and its identification as a cleavage product of C'3. J Exp Med. 1967;126:1027–1048. doi: 10.1084/jem.126.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cochrane CG, Muller-Eberhard HJ. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968;127:371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boshra H, Wang T, Hove-Madsen L, Hansen J, Li J, Matlapudi A, Secombes CJ, Tort L, Sunyer JO. Characterization of a C3a receptor in rainbow trout and Xenopus: the first identification of C3a receptors in nonmammalian species. J Immunol. 2005;175:2427–2437. doi: 10.4049/jimmunol.175.4.2427. [DOI] [PubMed] [Google Scholar]
- 80.Sunyer JO, Boshra H, Li J. Evolution of anaphylatoxins, their diversity and novel roles in innate immunity: insights from the study of fish complement. Vet Immunol Immunopathol. 2005;108:77–89. doi: 10.1016/j.vetimm.2005.07.009. [DOI] [PubMed] [Google Scholar]