Abstract
NK cells play a central role in innate immunity, acting directly through cell-mediated cytotoxicity and by secreting cytokines. TNFα activation of TNFR2 enhances NK cell cytotoxicity, but its effects on the other essential function of NK cells - cytokine production, for which IFNγ is paramount - are poorly defined. We identify the expression of both TNFα receptors on human peripheral blood NK cells (TNFR2 > TNFR1) and show that TNFα significantly augments IFNγ production from IL-2-/IL-12-treated NK cells in vitro, an effect mimicked by a TNFR2 agonistic antibody. TNFα also enhanced murine NK cell IFNγ production via TNFR2 in vitro. In a mouse model characterized by the hepatic recruitment and activation of NK cells, TNFR2 also regulated NK cell IFNγ production in vivo. Specifically, in this model, after activation of an innate immune response, hepatic numbers of TNFR2-expressing and IFNγ-producing NK cells were both significantly increased; however, the frequency of IFNγ-producing hepatic NK cells was significantly reduced in TNFR2-deficient mice. We delineate an important role for TNFα, acting through TNFR2, in augmenting cytokine-induced NK cell IFNγ production in vivo and in vitro, an effect with significant potential implications for the regulation of innate and adaptive immune responses.
Key Words: Cytokines, Inflammation, NKT cells, Innate immunity, Liver disease, Hepatitis
Introduction
The immune system is classically divided into innate and adaptive arms. NK cells are an important component of the innate immune system, and as such NK cells are critically involved in host immune responses to infectious agents (e.g. viruses and fungi) and to malignantly transformed cells [1, 2, 3]. In response to tissue injury, NK cells are rapidly recruited from the blood into affected tissues, where they are subsequently activated and directly and/or indirectly impact immune responses [3]. Activation of NK cells during pathological responses can occur as a result of direct cell-cell interactions or through indirect mechanisms including the release of cytokines from other innate immune cells within an inflamed tissue which subsequently activate NK cells [3, 4, 5, 6]. The cytokines most commonly implicated in this process of NK cell activation are IL-2, IL-12, IL-15, and IL-18 [5, 6, 7]. Subsequent immune effector roles of NK cells within tissues can be mediated through both cytotoxic and cytokine-producing capacities [5, 8]. IFNγ is a major cytokine produced by activated NK cells, and release of IFNγ within tissues has profound immunomodulatory effects [9]. Specifically, IFNγ release can lead to downstream activation of other innate and adaptive immune cells, enhanced recruitment of immune cells into affected tissues, a shift in cytokine responses to a more Th1-biased response, and direct antiviral effects [9, 10]. However, the roles of other cytokines, including TNFα, in enhancing NK cell production of IFNγ are not well understood.
TNFα is a pleiotropic cytokine that can be produced by numerous immune cells, including T cells, macrophages, NK cells, and NKT cells [11]. Enhanced TNFα production is an important regulator of innate immunity in a variety of diseases, in both patients and animal models [11, 12]. To exert its biological effects, TNFα interacts with two cell surface receptors, TNFR1 and TNFR2 [12, 13]. TNFR1 is ubiquitously expressed on all cells, whereas expression of TNFR2 is more restricted, being found mainly on certain T cell subtypes (i.e. CD4 and CD8), endothelial cells, and cells within the brain [11, 12, 13]. In general, the immunoregulatory effects of TNFα have been mainly attributed to interactions of TNFα with TNFR1, which in turn involves intracellular signaling pathways containing a death domain [11]. However, although less well characterized, TNFR2 (which is not linked to a death domain) has been increasingly implicated in the effects of TNFα in immune regulation [14]. It has been appreciated for many years that TNFα can augment the effects of IL-2 to induce NK cell differentiation and activation, and enhance NK cell-driven cytotoxicity towards target cells in vitro [15, 16]. This effect of TNFα on NK cell-mediated cytotoxicity has been attributed, at least in part, to TNFα stimulation of TNFR2 expressed on both human and murine NK cells [17, 18]. However, an effect of TNFα upon NK cell production of IFNγ has not been directly examined, but has clear potential immunological implications. In a recent study, the potential involvement of TNFα in enhancing NK cell IFNγ release was noted in an in vitro NK cell coculture system with macrophages; however, the TNFR subtype involved in this effect was not characterized [19].
In this series of experiments, we demonstrate a significant expression of TNFR2 on the surface of NK cells and delineate a central role for TNFα interacting with TNFR2 in NK cell activation and IFNγ production in both human and murine NK cells. These observations highlight the importance of TNFα-TNFR2 interactions in NK cell production of IFNγ. Moreover, our current findings may provide new insight into the clinical consequences and commonly reported adverse outcomes associated with TNFα-neutralizing/-inhibiting therapeutic strategies in patients with infectious and autoimmune diseases [20], in which both IFNγ and NK cells are known to play important immunomodulatory roles [2, 7, 10, 21].
Materials and Methods
Antibodies and Reagents for Human NK Cell Studies
The following reagents, antibodies, and appropriate isotype controls were obtained from the indicated sources. RPMI 1640 medium, HEPES, fetal bovine serum (FBS), 2-mercaptoethanol, nonessential amino acids, sodium pyruvate, penicillin-streptomycin, phosphate-buffered saline (PBS) were all obtained from Invitrogen, Life Technologies, Carlsbad, Calif., USA. FITC anti-human CD56 (clone B159) and APC Annexin V were obtained from BD Biosciences, Mississauga, Ont., Canada. Brefeldin A solution, monensin solution, cell stimulation cocktail, IC Fixation Buffer, permeabilization buffer, fixable viability dyes, human Fc receptor-binding inhibitor, and anti-human IFNγ eFluor 450 (clone 4S.B3) were all obtained from eBioscience Inc., San Diego, Calif., USA. Anti-human TNFRI/TNFRSF1A-APC (clone 16803), anti-human TNFRII/TNFRSF1B-APC (clone 22235), and anti-human TNFRII/TNFRSF1B polyclonal were all obtained from R&D Systems Inc. Minneapolis, Minn., USA. Specific TNFR2 agonist antibody (clone TY010) was kindly provided by Dr. Denise Faustman (Immunology Laboratories, Massachusetts General Hospital East, Charlestown, Mass., USA) [22].
Human Peripheral Blood NK Cell Isolation
For isolation of primary human NK cells, peripheral blood was obtained by venipuncture from healthy volunteers (in compliance with the University of Calgary Conjoint Health Research Ethics Board of the University of Calgary, Protocol No. 23363) and anticoagulated with heparin (10 U/ml blood). Peripheral blood mononuclear cells (PBMCs) were purified as previously described [23]. Briefly, blood was centrifuged on a Ficoll-Hypaque (GE Healthcare, Mississauga, Ont., Canada) density gradient and washed 3 times in Hanks' balanced salt solution (Invitrogen, Carlsbad, Calif., USA). NK cells were magnetically separated through LS columns using a MACS NK cell isolation kit (Miltenyi Biotec, Auburn, Calif., USA) as per the manufacturer's instructions. NK cells collected in the negative fraction were labeled with anti-human CD56 to assess the purity (which was routinely measured and consistently found to be between 95 and 97%) and to define CD56hi and CD56lo subsets.
Human NK Cell Flow Cytometry Analysis and Gating Strategy
Freshly isolated NK cells were subjected to direct immunofluorescence analyses using multicolor flow cytometry staining. Data from the samples were acquired either using a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif., USA) or an Attune Acoustic Focusing flow cytometer (Applied Biosystems, Burlington, Ont., Canada). The data were analyzed using FlowJo software (Tree Star, Ashland, Oreg., USA) or Attune Cytometric Software v2.1 (Applied Biosystems). Gating proceeded as follows: gating of live cells, excluding duplet cells, followed by gating on forward scatter and side scatter areas to identify regions appropriate to define lymphocytes. CD56+ cells were identified in the lymphocyte gate. Cells expressing TNFR1 and TNFR2 were identified in the CD56+ gate. For IFNγ staining, cells were first stained for extracellular receptors, fixed, permeabilized, and then stained for IFNγ. Fluorescence-minus-one controls were used for the accurate designation of cells with fluorescence above background levels [24]. Appropriate isotype controls were used to determine the specificity of all antibodies used.
In vitro Human NK Cell Studies
To assess NK cell production of IFNγ, purified NK cells were allowed to rest overnight and were resuspended in 200 μl of fresh RPMI 1640 complete medium supplemented with 10% (v/v) FBS, 2.0 mML-glutamine, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin at a density of 1 × 106 cells/ml (250,000 cells/200 μl) in flat-bottomed 96-well multiwell plates (Sarstedt Inc., Newton, Mass., USA). To stimulate submaximal IFNγ production from NK cells, cells were treated with IL-2 and IL-12 (each 20 ng/ml) for 24 h (concentrations were determined in pilot experiments and are consistent with those in previous reports) [25, 26]. For FACS (fluorescence-activated cell sorting) analyses, medium was supplemented with protein transport inhibitors (brefeldin A and monensin at 3.0 µg/ml and 2 mM, respectively, for the last 5 h of incubation). To test for an effect of TNFα or TNFR2 agonist antibody, TNFα or TNFR2 agonist antibody was added to NK cell-containing wells (concentrations of 100 ng and 2.5 µg/ml, respectively) [22] just prior to adding the IL-2 and IL-12. Addition of TNFα alone to the incubation medium did not increase NK cell Annexin V expression [stained with APC Annexin V (BD Pharmingen, San Jose, Calif., USA) and Fixable Viability Dye eFluor® 780 (eBioscience)] compared to NK cells incubated in buffer alone (online suppl. fig. 5; for all online suppl. material, see www.karger.com/doi/10.1159/000448077). For measurement of NK cell secretion of IFNγ into culture media, cell culture supernatants were harvested 24 h after stimulation. Samples were centrifuged (at 3,000 g) prior to aliquoting to remove debris. The cell culture supernatants were then stored at −20°C until assayed. IFNγ was measured in cell culture supernatant samples using a Luminex® assay [Luminex 100 system (Millipore, USA) assay performed by Eve Technologies Corporation, Calgary, Alta., Canada]. The results are expressed as pg/ml supernatant. To assess effects of cytokines (IL-2, IL-12, and TNFα) on NK cell TNFR2 expression, purified human NK cells were treated with IL-2 + IL-12 (each 20 ng/ml), TNFα alone (100 ng/ml), or a combination of all three cytokines for 24 h. At the end of the experiment, cells were harvested and cytokine-induced changes in NK cell TNFR2 expression evaluated by flow cytometry. TNFR2 expression was expressed both in terms of changes in frequencies of TNFR2-positive cells and mean fluorescence intensity (MFI).
Mouse Studies
For all experiments, male wild-type C57BL/6 mice and TNFR2-deficient mice (on a C57BL/6 background) were used (B6.129S2-Tnfrsf1btm1Mwm/J, 8-10 weeks of age; The Jackson Laboratory, Bar Harbor, Maine, USA). CD1d-deficient mice (C57BL/6 genetic background) were obtained as previously described [27]. All procedures in this study were approved by the Animal Care Committee of the University of Calgary and were performed in accordance with the guidelines established by the Canadian Council on Animal Care. To induce hepatic NK cell recruitment and activation, the mice were treated with a single intravenous (i.v.) injection of α-galactosylceramide (αGalCer; Alexis Biochemicals, San Diego, Calif., USA; 2 μg in 100 μl vehicle: 0.04% Tween 20 in sterile PBS) [28, 29]. Controls received 100 μl of vehicle. αGalCer treatment rapidly activates hepatic NKT cells, which in turn secrete large amounts of numerous cytokines, including TNFα [30]. TNFα produced by activated hepatic NKT cells in this manner is a critical driver of the subsequent robust recruitment of NK cells into the liver, and NK cells recruited in this fashion are activated and produce IFNγ [28, 31, 32].
Murine Hepatic Lymphocyte Isolation and Flow Cytometry Analyses
Eight, 16, and 48 h after αGalCer or vehicle administration, hepatic lymphocytes were isolated using methods as previously described [27, 29]. Briefly, livers were perfused with ice-cold normal saline and were then treated with digestion buffer [0.05% collagenase 2 (Cedarlane Laboratories, Burlington, Ont., Canada) and 0.02% DNase I (Roche Diagnostics, Laval, Que., Canada) in Hanks' balanced salt solution with Ca2+ and Mg2+] for cell isolation. Hepatic lymphocytes were isolated by discontinuous Percoll® gradient (GE Healthcare Bio-Sciences, Baie-d'Urfé, Que., Canada) as previously described [27, 29]. Cell viability was assessed by Trypan Blue dye exclusion. Single-cell suspensions (0.5-1.0 × 106 cells/sample) were prepared in binding buffer (1% FBS in PBS) for flow cytometry staining using CellQuest software (Becton Dickinson). For cell surface staining, 0.5-1 × 106 cells were incubated with various antibodies for 30 min at 4°C. Anti-mouse antibodies included: PerCP anti-mouse CD3e (145-2C11; BD Pharmingen), FITC or PE anti-mouse NK1.1 (PK136; BD Pharmingen), PE anti-mouse CD120b receptor (TNFR2, TR75-8; BD Pharmingen), and FITC anti-mouse TNFα (MP6-XT22; BD Pharmingen). Hepatic NKT cells were identified as being CD3+ PBS-57 loaded CD1d tetramer positive (provided by the NIH Tetramer Facility, Emory University Vaccine Center, Atlanta, Ga., USA). Hepatic NK cells were defined as being NK1.1+CD3- cells. Intracellular expression of IFNγ was assessed on permeabilized cells as previously described using a Cytofix/Cytoperm Plus Kit (BD Pharmingen) with PE anti-mouse IFNγ (XMG 1.2; BD Pharmingen).
In vitro Stimulation of Murine Splenic NK Cells
Single-cell suspensions were prepared from the spleens of naive wild-type and naive TNFR2-deficient mice using RPMI 1640 medium (supplemented with 10% fetal calf serum, nonessential amino acids, L-glutamine, β-mercaptoethanol, and penicillin-streptomycin; all reagents from Invitrogen, Canada). Briefly, spleens from naive wild-type and TNFR2-deficient mice were gently squeezed between sterile frosted slides, passed through a 70-μm cell strainer (BD Pharmingen), and then placed in ammonium chloride lysis buffer to remove red blood cells. Next, freshly isolated splenocytes were enriched for NK cells using a negative selection NK cell isolation kit (130-090-864; Miltenyi Biotec). Enriched NK cells (1 × 106 cells/well) in supplemented RPMI 1640 medium were treated in vitro with the following murine recombinant cytokines: unstimulated, murine IL-2 (10 ng/ml; eBioscience) alone; murine IL-12 (5 ng/ml; Peprotech, London, UK) alone; murine TNFα (10 ng/ml; Peprotech) alone, or either the combination of rmIL-2 + rmIL-12 or rmIL-2 + rmIL-12 + rmTNFα for 16 h at 37°C and 5% CO2. Following in vitro stimulation, supernatants were collected for quantification of IFNγ levels by Luminex® assay (Eve Technologies Corporation). Additionally, NK cells were removed and stained extracellularly with FITC anti-mouse NK1.1 mAb (BD Pharmingen) and then intracellularly for PE anti-mouse IFNγ (BD Pharmingen) as described above.
Murine Peripheral Blood Isolation and Flow Cytometry Analyses
For evaluation of CD27 and TNFR2 expression on murine NK cells, blood from naive mice was collected into BD Vacutainers containing EDTA and PBMCs isolated by discontinuous Percoll® gradient (GE Healthcare Bio-Sciences) as previously described [27, 29]. The PBMCs were then stained with anti-mouse CD3 Alexa Fluor® 488 (17A2; BioLegend, San Diego, Calif., USA), anti-mouse PerCP-Cyanine5.5 NK1.1 (PK136; eBioscience), anti-mouse APC/Cy7 CD27 antibody (LG.3A10; BioLegend), anti-mouse PE TNFR2 antibody (TR75-89; BioLegend). NK cells were first identified as CD3-NK1.1+ cells, and were then further divided into CD27lo and CD27hi subsets. Differential expression of TNFR2 on the CD27lo and CD27hi subsets was then determined.
Statistics
All data are shown as means ± SEM. Statistical significance was assessed using an unpaired Student t test for comparisons between two groups, or with an ANOVA followed by the Student-Newman-Keuls post hoc test for comparisons between more than two groups, using GraphPad InStat 3 software (GraphPad Software Inc., La Jolla, Calif., USA). Differences between means were considered significant when p < 0.05.
Results
Differential Expression of TNFR Subtypes on NK Cells
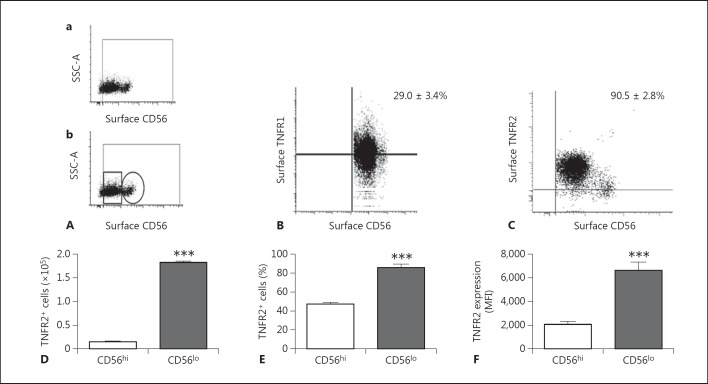
Conflicting reports have previously been published with regard to the relative differential expression of TNFR1 and TNFR2 on human NK cells, with the reported NK cell expression of TNFR1 ranging from <3 to 16% and the expression of TNFR2 ranging from 9 to 68% of NK cells [17, 33]. We found that TNFR2 expression was relatively enriched relative to TNFR1 within the NK cell population as a whole [expression as percent of total NK cells: TNFR1 29.0 ± 3.4% (n = 5 donors) and TNFR2 90.5 ± 2.8% (n = 6 donors)] (fig. 1). The NK cell population has typically been divided into two main groups based on CD56 expression. CD56hi NK cells have classically been positioned as major cytokine producers, and CD56lo NK cells as being mainly cytotoxic, although this separation by functional properties has recently been questioned [34, 35]. In our current study, we have extended the phenotypic characterization of NK cell subpopulations for expression of TNF receptors. Flow cytometry analysis revealed a differential expression of TNFR2 within the CD56hi and CD56lo NK cell populations. Specifically, NK cell surface TNFR2 expression was greater in the CD56lo subpopulation of NK cells (both as percent positive cells and as MFI) (fig. 1E, F; online suppl. fig. 4). Similarly, the proportion of CD56hi NK cells expressing TNFR1 was significantly smaller than the CD56lo NK cell population (online suppl. fig. 7). A differential expression of TNFR2 within these two NK cell populations is interesting and has not been previously reported.
Fig. 1.
Flow cytometry analysis of TNFR1 and TNFR2 expression on human peripheral blood CD56+ NK cells. Purified peripheral blood NK cells from healthy donors were stained with CD56, TNFR1, or TNFR2 and analyzed by flow cytometry. A Representative flow cytometry dot plot showing the NK cell gating strategy (a) and the gating strategy used to determine CD56lo (small rectangular gate) and CD56hi (small circular gate) NK cells (b). SSC- A = Side scatter area. B Representative flow cytometry dot plot of CD56+ NK cells vs. TNFR1 expression (mean 29.0 ± 3.4%, n = 5). C Representative flow cytometry dot plot of CD56+ NK cells vs. TNFR2 expression (mean 90.5 ± 2.8%, n = 6). D Total number of TNFR2+ NK cells in CD56lo vs. CD56hi NK cell subsets. *** p < 0.0001 (n = 3/group). E Frequency of CD56lo vs. CD56hi NK cells expressing TNFR2. *** p < 0.0001 (n = 3/group). F TNFR2 expression as MFI on CD56lo vs. CD56hi NK cells. *** p < 0.0001 (n = 3/group).
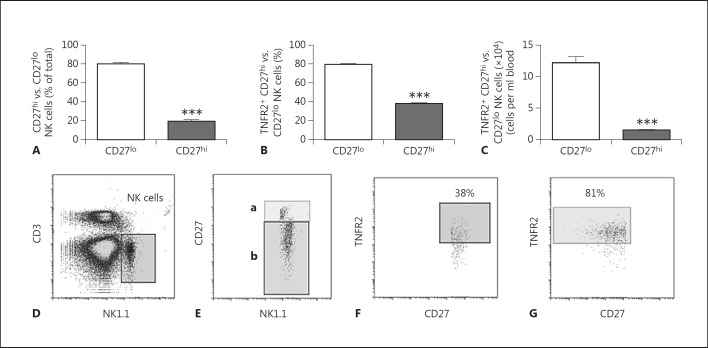
Murine NK cells do not express CD56; however, CD27 expression on murine NK cells has been suggested to be equivalent to CD56 expression on human NK cells [36, 37]. Therefore, we determined differential TNFR2 expression on CD27hi and CD27lo murine peripheral blood NK cells by flow cytometry (fig. 2), and demonstrate findings similar to those obtained using CD56 in human NK cells (fig. 1).
Fig. 2.
Flow cytometry analysis of TNFR2 expression on murine peripheral blood CD27+ NK cells. Peripheral blood from naïve mice was analyzed by flow cytometry to determine NK cell CD27 and TNFR2 expression. A Frequency of CD27lo- and CD27hi-expressing NK cells within the total NK cell population. *** p ≤ 0.0001 vs. CD27lo group (n = 5 mice). B, C Frequency (B) and total number (C) of TNFR2+ cells in CD27hi and CD27lo subsets. *** p ≤ 0.0001 vs. CD27lo (n = 5 mice). D, E Representative flow cytometry dot plots showing the NK cell gating strategy (D) and the gating strategy used to determine CD27lo (b) vs. CD27hi (a) NK cells (E). F Representative flow cytometry dot plot of TNFR2 expression on CD27hi NK cells. G Representative flow cytometry dot plot of TNFR2 expression on CD27lo NK cells.
Role of TNFR2 in Human NK Cell Production of IFNγ
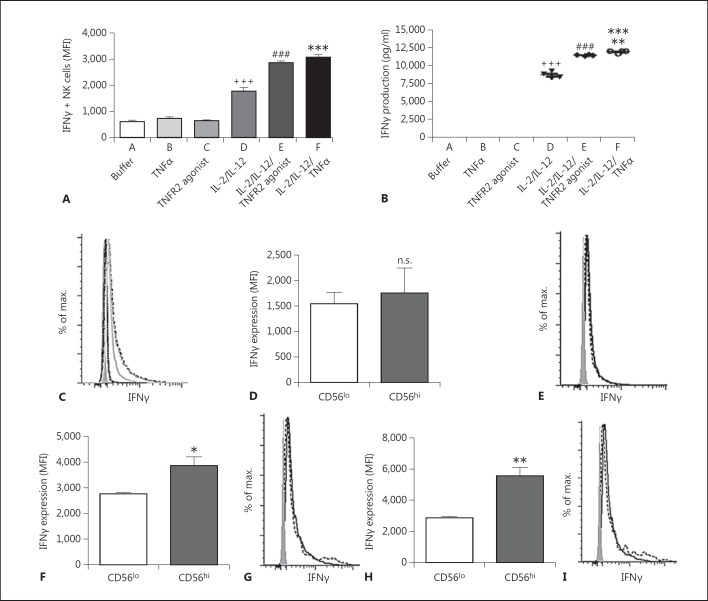
TNFR2 has been implicated in the enhancement of NK cell cytotoxicity [17]; however, the role of TNFR2 in altering the other major property of NK cells, cytokine production, remains unclear. To address this issue, we examined NK cell production of IFNγ using an in vitro assay. We found that treatment of NK cells in vitro with TNFα alone, or with a specific TNFR2 agonistic antibody alone, did not induce NK cell IFNγ production (fig. 3a, b, c). In contrast, as reported previously, administration of a combination of IL-2 + IL-12 enhanced IFNγ production in NK cells (fig. 3a, b, c) [25, 26]. Surprisingly, coadministration of TNFα, or a TNFR2 agonistic antibody, to NK cells that were also treated with IL-2 + IL-12 resulted in a synergistic augmentation of IFNγ production (fig. 3a, b, c). We have shown that TNFR2 is differentially expressed on NK cell subpopulations (see above). Therefore, we sought to delineate the capacity of CD56hi and CD56lo NK cell subpopulations to produce IFNγ after TNFα costimulation. We found that IFNγ production was similar in CD56hi and CD56lo NK cell subpopulations in response to IL-2 + IL12 treatment (as determined by MFI, a measure of IFNγ production per cell) (fig. 3D, E). However, addition of TNFα or the TNFR2 agonistic antibody to IL-2 + IL-12-treated NK cells synergistically increased overall IFNγ production, and induced more robust IFNγ production in CD56hi NK cells compared to CD56lo NK cells (fig. 3F, G, H, I).
Fig. 3.
Effect of TNFα or TNFR2 agonistic antibody on IFNγ production by human CD56+ NK cells in vitro. Human peripheral blood NK cells were cultured for 24 h in the presence of buffer alone, TNFα alone, TNFR2 agonist antibody alone, IL-2 + IL-12, IL-2 + IL-12 + TNFα, or IL-2 + IL-12 + TNFR2 agonist antibody. A NK cell IFNγ expression as MFI. *** p ≤ 0.001 group F vs. groups A, B, C, and D; ### p ≤ 0.001 group E vs. groups A, B, C, and D; +++ p ≤ 0.001 group D vs. groups A, B, C, E, and F. B IFNγ production in NK cell culture supernatant determined by ELISA. *** p ≤ 0.001 group F vs. groups A, B, C, and D; ** p ≤ 0.01 group F vs. group E; ### p ≤ 0.001 group E vs. groups A, B, C, and D; +++ p ≤ 0.001 group D vs. groups A, B, C, E, and F. D, F, H CD56hi and CD56lo NK cell IFNγ production after 24 h of incubation with IL-2 + IL-12 (D; n.s., not significant), IL-2 + IL-12 + TNFR2 agonist antibody (F; * p ≤ 0.05), or IL-2 + IL-12 + TNFα (H; ** p ≤ 0.01). Data are shown as the mean ± SEM of 4 replicates from 1 donor. Similar results were obtained from 4 additional donors. C Representative flow cytometry histograms depicting IFNγ expression for the data presented in A. Buffer (far left, black dashed line), TNFR2 agonist (left, gray dotted line), TNFα (middle, black dotted line), IL-2 + IL-12 (right, gray solid line), IL-2 + IL-12 + TNFα (far right, black long-dashed line), IL-2 + IL-12 + TNFR2 agonist (far right, gray long-dashed line). E Representative flow cytometry histograms depicting IFNγ expression for the data presented in D. The solid line represents IFNγ expression by the CD56lo subset and the dotted line represents IFNγ expression by the CD56hi subset in the presence of IL-2 + IL-12. G Representative flow cytometry histograms depicting IFNγ expression for the data presented in F. The solid line represents IFNγ expression by the CD56lo subset and the dotted line represents IFNγ expression by the CD56hi subset in the presence of IL-2 + IL-12 + TNFR2 agonist. I Representative flow cytometry histograms depicting IFNγ expression for the data presented in H. The solid line represents IFNγ expression by the CD56lo subset and the dotted line represents IFNγ expression by the CD56hi subset the in presence of IL-2 + IL-12 + TNFα. C, E, G, I The shaded histograms represent isotype staining controls.
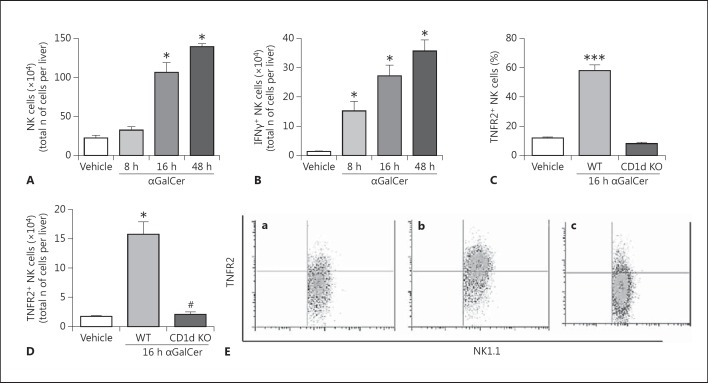
NK Cell Recruitment and Activation in the Liver of Mice after αGalCer Administration
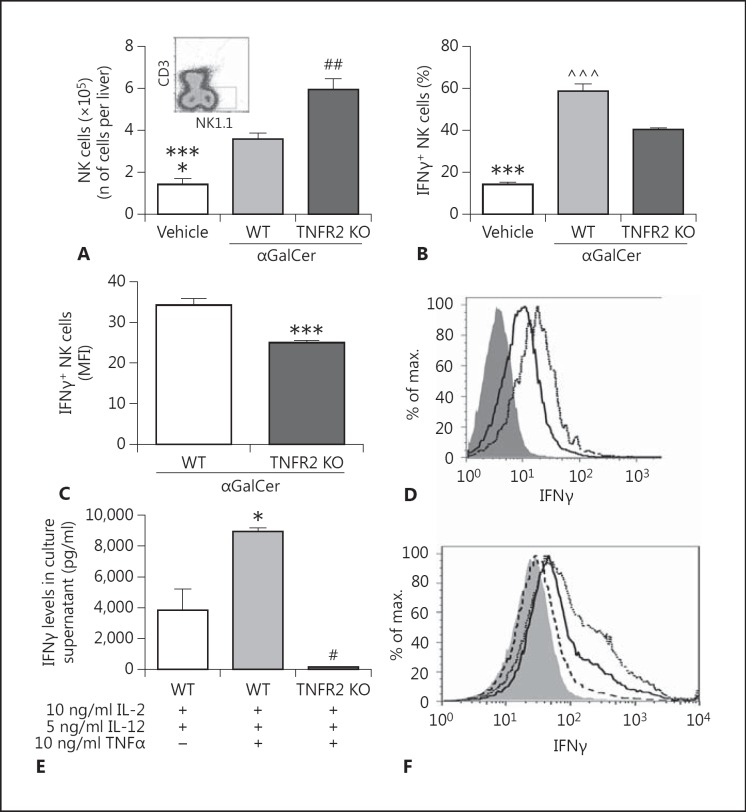
The glycolipid αGalCer specifically activates hepatic NKT cells via their invariant T cell receptor, leading to the rapid and robust recruitment and activation of NK cells within the liver [27, 28, 32]. Therefore, this model was used to determine the in vivo role of TNFR2 in tissue-recruited NK cell activation and IFNγ production. Consistent with previous reports, administration of αGalCer to mice resulted in a significant increase in the overall numbers of hepatic NK1.1+CD3- (NK) cells compared to vehicle-treated controls [27, 32]. Moreover, elevated hepatic NK cell numbers persisted for at least 48 h after treatment (fig. 4A; online suppl. fig. 1A). Administration of αGalCer also led to enhanced numbers of IFNγ-producing NK cells within the liver compared to vehicle-treated controls (fig. 4B; online suppl. fig. 1B). αGalCer treatment of mice rapidly (within 2 h) activates hepatic NKT cells which subsequently produce TNFα, and these activated NKT cells constitute the major TNFα-producing cell type in the liver after αGalCer treatment [31]. We have confirmed that αGalCer does indeed rapidly activate hepatic NKT cells to produce TNFα, as reflected in a significant increase in the percentage of hepatic NKT cells producing TNFα 2 h after αGalCer treatment compared to vehicle-treated mice (64.9 ± 2.6 vs. 4.1 ± 0.7%; n = 5 mice/group; p < 0.0001) (online suppl. fig. 6). We next determined whether NK cells isolated from livers of αGalCer-treated mice expressed TNFR2. Indeed, numbers of hepatic TNFR2+ NK cells were increased in αGalCer-treated compared to vehicle-treated mice (fig. 4C, D, E). Moreover, hepatic recruitment of TNFR2+ NK cells after αGalCer administration was dependent upon the presence of NKT cells, as NK cells were not recruited to the liver in CD1d knockout (KO) mice (which are NKT cell deficient) [27] treated with αGalCer (fig. 4C, D, E).
Fig. 4.
TNFR2-expressing NK cells are recruited to the liver in mice after αGalCer treatment. Recruitment of NK cells (identified as NK1.1+CD3- cells) to the liver after αGalCer administration was evaluated by flow cytometry. Mice were treated with either αGalCer (2 µg i.v./mouse) or vehicle; 8, 16, and 48 h later, hepatic lymphocytes were isolated. A Time course of NK cells recruited to the liver after αGalCer treatment. There was an increase in the number of NK1.1+CD3- cells recruited to the liver in αGalCer-treated mice in comparison to vehicle-treated mice. * p ≤ 0.01 (n = 6/group) vs. vehicle-treated group. B Time course of IFNγ-producing NK cells recruited to the liver after αGalCer treatment. There was an increase in the number of IFNγ+NK1.1+CD3- cells recruited to the liver in αGalCer-treated mice in comparison to vehicle-treated mice. * p ≤ 0.01 vs. vehicle-treated group (n = 6/group). C, D There were increases in the frequencies (C) and numbers (D) of TNFR2+ NK cells recruited to the liver 16 h after αGalCer treatment in comparison to vehicle-treated mice. The frequencies and total numbers of TNFR2+ NK cells recruited to the liver after αGalCer treatment did not change in CD1d KO mice compared to vehicle-treated wild-type mice. C*** p < 0.001 vs. vehicle-treated group (n = 6/group). D * p ≤ 0.01 vs. vehicle-treated group (n = 6/group). NK cell deficiency (i.e. CD1d KO mice) prevented the hepatic recruitment of TNFR2+ NK cells to the liver after αGalCer treatment. # p ≤ 0.01 vs. wild-type αGalCer-treated mice. E Representative flow cytometry dot plots for the data presented in C. a Vehicle-treated group. b Wild-type mice 16 h after αGalCer treatment. c CD1d KO mice 16 h after αGalCer treatment.
TNFR2 Regulates Murine NK Cell IFNγ Production in vivo and in vitro
To assess the role of TNFR2 in IFNγ production by liver-recruited NK cells in vivo, we treated TNFR2-deficient and wild-type mice with αGalCer. Surprisingly, αGalCer-treated TNFR2 KO mice demonstrated significantly greater total NK cell recruitment to the liver than did similarly treated wild-type mice (fig. 5A). Although the overall total number of hepatic IFNγ+ NK cells was not altered in TNFR2 KO versus wild-type mice treated with αGalCer (online suppl. fig. 2), the percentage of IFNγ+ NK cells within the liver 16 h after αGalCer administration was significantly reduced in TNFR2-deficient mice compared to similarly treated wild-type controls (fig. 5B), and the fluorescent intensity of IFNγ labeling in NK cells was also lower (fig. 5C, D). To further establish the role of TNFα signaling via TNFR2 in NK cell IFNγ production, TNFR2 KO or wild-type splenic NK cells were purified by MACS and cultured with rmIL-2, rmIL-12, and rmTNFα for 16 h. Levels of IFNγ in culture supernatants were then quantitated by Luminex® assay. We found a significant decrease in IFNγ levels in TNFR2 KO splenic NK cells treated with rmIL-2 + rmIL-12 + rmTNFα compared to similarly treated wild-type splenic NK cells (fig. 5E). A similar pattern of results was obtained when intracellular IFNγ expression in NK cells was determined by flow cytometry (fig. 5F). Importantly, NK cells isolated from TNFR2 KO mice are not defective in their overall ability to produce IFNγ, since splenic NK cells isolated from wild-type and TNFR2 KO mice stimulated with PMA (phorbol 12-myristate 13-acetate) and ionomycin in vitro demonstrate similar IFNγ expression (online suppl. fig. 3).
Fig. 5.
TNFR2 deficiency attenuates murine NK cell activation to produce IFNγ. A Hepatic IFNγ+NK1.1+CD3- cells were evaluated by flow cytometry 16 h after vehicle or αGalCer treatment. Inset Representative FACS profile demonstrating the hepatic NK cell gate. Bars represent means ± SEM of the data from 5 mice/group and indicate an increase in hepatic numbers of NK cells in αGalCer-treated TNFR2 KO vs. αGalCer-treated wild-type mice. ## p ≤ 0.01 vs. wild-type αGalCer-treated mice; *** p ≤ 0.001 vs. αGalCer-treated TNFR2 KO mice; * p ≤ 0.05 vs. αGalCer-treated wild-type mice. B Frequency of IFNγ+ NK cells in vehicle-treated mice and in wild-type and TNFR2 KO mice 16 h after αGalCer treatment. *** p ≤ 0.001 vs. both αGalCer-treated groups; ^^^ p ≤ 0.001 vs. αGalCer-treated TNFR2 KO mice (n = 5 mice/group). C NK cell production of IFNγ as measured by MFI in wild-type and TNFR2 KO mice treated 16 h previously with αGalCer. *** p ≤ 0.001 vs. wild-type mice (n = 5 mice/group). D Representative flow cytometry histograms depicting the lower IFNγ expression in hepatic NK cells isolated from αGalCer-treated TNFR2 KO mice (solid line; 41%) vs. αGalCer-treated wild-type mice (dotted line; 55%). The shaded histogram represents the isotype. E TNFR2 KO or wild-type splenic NK cells were purified and cultured with rmIL-2 + rmIL-12 ± rmTNFα, and 16 h later IFNγ levels were quantitated in culture supernatant. * p ≤ 0.05 comparing wild-type splenic NK cells cultured with rmIL-2 + rmIL-12 + rmTNFα with wild-type splenic NK cells cultured with rmIL-2 + rmIL-12 (n = 4/group); # p ≤ 0.01 comparing TNFR2 KO splenic NK cells cultured with rmIL-2 + rmIL-12 + rmTNFα with similarly cultured wild-type splenic NK cells (n = 4/group). Baseline IFNγ release in vitro from TNFR2 KO and wild-type NK cells was similar (20.6 and 18.5 pg/ml, respectively; n = 2/group). Stimulation of TNFR2 KO NK cells in vitro with IL-2 + IL-12 or IL-2 + IL-12 + TNFα resulted in a similar low-level release of IFNγ (IL-2 + IL-12 = 141.0 ± 9.8 pg/ml; IL-2 + IL-12 + TNFα = 145.3 ± 30.5 pg/ml; n = 3/group; not significant). F Representative flow cytometry histograms (n = 3 replicates/group) showing the lower IFNγ expression in splenic NK cells isolated from TNFR2 KO mice (dashed line; 11%) 16 h after in vitro stimulation with rmIL-2 + rmIL-12 + rmTNFα vs. similarly activated wild-type NK cells (dotted line; 42%). IFNγ production from splenic NK cells isolated from wild-type mice and stimulated in vitro for 16 h with rmIL-2 and rmIL-12 is shown as the solid line (31%). The shaded histogram represents the isotype. Experiments were repeated at least twice, with similar results.
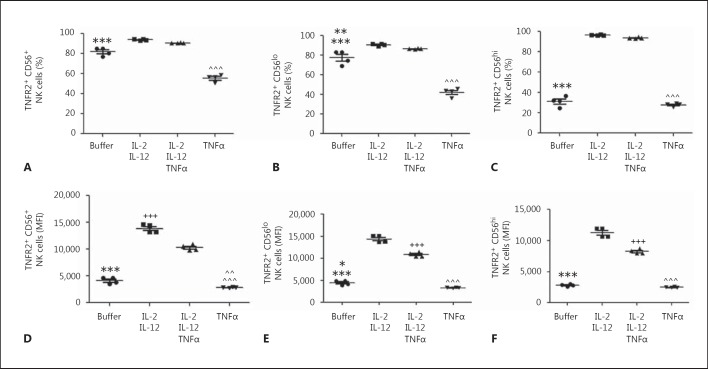
Effect of IL-2 + IL-12 and TNFα on Human NK Cell Expression of TNFR2
The effect of cytokine-mediated activation of NK cells upon the expression of TNFR2 is unknown, as is the effect of TNFα stimulation. Therefore, we assessed the impact of stimulation of NK cells with IL-2 + IL-12 in vitro, in the presence or absence of TNFα, on total NK cell - and CD56hi and CD56lo NK cell - expression of TNFR2. We now demonstrate that activation of NK cells with IL-2 + IL-12 increased the percentage of NK cells expressing TNFR2 (fig. 6A). In contrast, incubation of NK cells with TNFα alone decreased the percentage of NK cells expressing TNFR2 (fig. 6A). Moreover, this pattern of IL-2 + IL-12 and TNFα-stimulated alterations in the percentages of TNFR2-expressing total NK cells was paralleled in the CD56lo NK cell subset (fig. 6B). However, CD56hi NK cells demonstrated a more pronounced enhancement in the frequency of TNFR2-expressing cells after stimulation with IL-2 + IL-12 versus the CD56lo subset (fig. 6C). In contrast to our findings in CD56lo NK cells, TNFα alone did not alter the TNFR2-expressing NK cell frequency amongst the CD56hi NK cell subset (fig. 6C). Moreover, addition of TNFα to IL-2 + IL-12-stimulated NK cells did not significantly change the percentage of NK cells expressing TNFR2 (fig. 6a, b, c). However, when NK cell TNFR2 expression was examined by MFI, addition of TNFα significantly attenuated the upregulation of TNFR2 expression induced by IL-2 + IL-12 in the total NK cell population (fig. 6D) and in both the CD56lo and CD56hi NK cell subsets (fig. 6E, F).
Fig. 6.
Cytokine activation of human NK cells in vitro differentially regulates TNFR2 expression (the role of TNFα). Purified human peripheral blood NK cells were cultured for 24 h in the presence of buffer alone, TNFα alone, IL-2 + IL-12, or IL-2 + IL-12 + TNFα. A Changes in the frequency of TNFR2-expressing NK cells induced by activation with different cytokine mixtures. *** p ≤ 0.001 buffer group vs. all other groups; ^^^ p ≤ 0.001 TNFα group vs. all other groups (n = 4/group). B Changes in the frequency of CD56lo NK cells expressing TNFR2. *** p ≤ 0.001 buffer vs. TNFα group; ** p < 0.01 buffer group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups; ^^^ p ≤ 0.001 TNFα group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups (n = 4/group). C Changes in the frequency of CD56hi NK cells expressing TNFR2. *** p ≤ 0.001 buffer group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups; ^^^ p ≤ 0.001 TNFα group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups. D-F TNFR2 expression by NK cells as quantified in terms of MFI. D TNFR2 expression on all NK cells. *** p ≤ 0.001 buffer group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups; ^^^ p ≤ 0.001 TNFα group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups; ^^ p ≤ 0.01 TNFα vs. buffer group; +++ p ≤ 0.001 IL-2 + IL-12 vs. IL-2 + IL-12 + TNFα group (n = 4/group). E TNFR2 expression on CD56lo NK cells. *** p ≤ 0.001 buffer group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups; * p ≤ 0.05 buffer vs. TNFα group; +++ p ≤ 0.001 IL-2 + IL-12 vs. IL-2 + IL-12 + TNFα group; ^^^ p ≤ 0.001 TNFα group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups. F TNFR2 expression on CD56hi NK cells. *** p ≤ 0.001 buffer group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups; +++ p ≤ 0.001 IL-2 + IL-12 vs. IL-2 + IL-12 + TNFα group; ^^^ p ≤ 0.001 TNFα group vs. IL-2 + IL-12 and IL-2 + IL-12 + TNFα groups. Similar results were obtained from 3 different donors.
Discussion
NK cells are critical players in innate immune responses and are important for antimicrobial defense and tumor immunosurveillance [5]. To carry out these tasks, NK cells are well armed for the cytotoxic destruction of transformed cells [1, 2, 5]. However, NK cells also mediate immunoregulatory effects through the release of numerous cytokines and chemokines, with IFNγ playing a key effector role [2, 5, 9]. IFNγ is a central regulator of both innate and adaptive immune responses by promoting viral clearance and suppressing viral replication, by recruiting other immune cells into inflamed tissues, through the polarization of adaptive immunity towards a Th1 phenotype, and by promoting the maturation and activation of dendritic cells, macrophages, and T cells [38]. Importantly, both NK cells and IFNγ have also been linked to the development and/or exacerbation of autoimmunity [10, 21, 38, 39]. Production of IFNγ from NK cells can be induced by cell-cell contact, but also through cytokine stimulation [3, 4, 6]. The cytokines most closely associated with NK cell activation and IFNγ release include IL-2, IL-12, IL-18, and IL-15 [4, 9, 40]. TNFα can activate NK cells to enhance cytotoxicity [17]. However, an effect of TNFα upon NK cell production of IFNγ has not been defined, but is clearly of potential importance in better understanding the role of TNFα in the NK cell-mediated regulation of innate immune responses and autoimmunity.
We speculated that TNFα may play an important role in regulating NK cell production of IFNγ, and furthermore we postulated that TNFR2 would be important for mediating this TNFα-related effect on NK cells. In keeping with this hypothesis, TNFα activation of TNFR2 has been previously shown to enhance NK cell cytotoxicity and cytokine production during NK cell and dendritic cell interactions in mice and humans [18, 41]. In our current study, we found that the majority of human peripheral blood NK cells express TNFR2, with >90% of CD56lo and >50% of CD56hi NK cells expressing TNFR2. Similar to our findings in human peripheral blood, differential TNFR2 expression was found on murine peripheral blood NK cells subdivided into CD27hi versus CD27lo subsets, which have been reported to parallel human NK subsets divided using CD56 expression [36, 37]. Moreover, we demonstrate that TNFα can activate both human and murine cytokine-stimulated NK cells via TNFR2 to augment IFNγ production. We also demonstrate with a mouse model of hepatic innate immune activation that TNFR2 expression is important for regulating the production of IFNγ by hepatic NK cells. Moreover, it has previously been shown in this model that TNFα is a critical mediator of liver injury [31]. We now show in this model that TNFα-TNFR2 interactions regulate the severity of liver injury, as TNFR2 KO mice are less susceptible to αGalCer-induced liver injury as reflected by reduced serum alanine aminotransferase levels in αGalCer-treated TNFR2 KO versus wild-type mice (levels as IU/l: vehicle = 24.0 ± 3.2 vs. wild-type + αGalCer = 246.2 ± 34.4* vs. TNFR2 KO + αGalCer = 69.8 ± 8.5; n = 4-5/group; * p ≤ 0.001). These findings may have significant implications for the regulation of immune responses in general, and could potentially explain, at least in part, a number of observations associated with TNFα inhibition in the clinical setting.
TNFα plays a critical role in regulating the clinical expression of many immune-mediated diseases [42, 43]. As a result, inhibition of TNFα has become a widely used and effective therapy for many of these diseases, including inflammatory bowel disease, rheumatoid arthritis, and autoimmune liver disease [44, 45]. However, inhibition of TNFα in the clinical setting has been associated with the reactivation of a number of infectious diseases (e.g. hepatitis B and tuberculosis) and an increased risk for developing malignancy, indicating that TNFα is also important for the suppression of these diseases [46, 47, 48]. However, it remains unclear whether this is a direct or an indirect effect of TNFα [46]. Interestingly, TNFα inhibition has been shown to suppress IFNγ production from a mixed lymphocyte population in vitro, and to inhibit NK cell activation (as measured by cell surface CD69 expression); however, the mechanism underlying these effects was not defined [49, 50]. Recently, Serti et al. [19] demonstrated that monocytes isolated from patients with hepatitis C infection produce lower amounts of TNFα than those isolated from healthy donors. In addition, the monocytes isolated from these hepatitis C patients were less effective than monocytes isolated from healthy donors in stimulating IFNγ production from NK cells in an in vitro coculture system [19]. These findings suggest that TNFα-related antiviral effects may be mediated indirectly through the induction by TNFα of IFNγ production from NK cells, a pathway that is defective in hepatitis C-infected patients. Our current data are consistent with this suggested mechanism. In addition, we demonstrate that TNFα-induced NK cell production of IFNγ is augmented via stimulation of TNFR2. This paradigm also aligns with the observed reactivation of hepatitis B infection in patients treated with TNFα-neutralizing therapies, a disease in which IFNγ plays a key role in suppressing viral replication [46].
In response to infection or inflammation, NK cells are rapidly recruited to injured tissues, where they are in turn activated and establish immune-modulatory effector roles, including the production of IFNγ [4, 5, 6, 32, 40]. αGalCer treatment leads to the rapid activation of NKT cells within the liver and the subsequent production of numerous cytokines (including TNFα), which leads to the robust recruitment of NK cells to the liver [27, 32]. NK cells recruited in this fashion are activated and produce large amounts of IFNγ [31, 32]. Therefore, αGalCer treatment in the mouse provides a useful tool to examine the potential impact of TNFR2 deficiency in vivo upon the capacity of liver-recruited NK cells to produce IFNγ. We found that αGalCer treatment resulted in the rapid recruitment of TNFR2-expressing NK cells to the liver. Moreover, these liver-recruited NK cells produced IFNγ. Surprisingly, in the absence of TNFR2, greater numbers of NK cells overall were recruited to the liver, an observation that warrants future studies. In addition, a reduced frequency of IFNγ-producing liver-recruited NK cells was documented in TNFR2 KO versus wild-type mice treated with αGalCer. Moreover, IFNγ production per hepatic NK cell was decreased in TNFR2 KO mice versus wild-type controls treated with αGalCer, as measured by MFI. Consistent with these in vivo observations, we found by an in vitro murine NK cell culture system that TNFα acting via TNFR2 plays a key role in enhancing cytokine-mediated IFNγ production. Similarly, we also showed that cytokine-induced IFNγ production from freshly isolated human peripheral blood NK cells was augmented by TNFα as well as by a specific TNFR2 agonistic antibody. These observations clearly highlight the importance of the TNFα-TNFR2 pathway in enhancing cytokine-driven NK cell IFNγ production.
Collectively, this work establishes an important role for TNFα, acting via TNFR2, in the regulation and augmentation of NK cell production of IFNγ. In addition, we report for the first time that cytokine stimulation, as well as TNFα activation, differentially regulates TNFR2 expression on human NK cells, a finding that likely has its importance in the regulation of NK cell responses within inflamed tissues. Moreover, we confirm that this TNFα-TNFR2 pathway is important for the establishment of the presence of IFNγ-producing NK cells within the liver during an innate immune response. These novel findings have significant potential clinical implications for the role of TNFα and TNFR2 in the regulation of innate and adaptive immune responses.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
We would like to acknowledge the following individuals from the University of Calgary for their expert assistance: Mr. Tai Le for expert technical assistance, Dr. Pina Colarusso, Mrs. Carol Gwozd, and Ms. Jen Amon (Immunohistochemistry and Live Cell Imaging Core Laboratory, Snyder Institute), and Mrs. Laurie Kennedy (Flow Cytometry Core Laboratory, Faculty of Medicine).
This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) and the Canadian Liver Foundation to M.G.S. M.G.S. also holds the Cal Wenzel Family Foundation Chair in Hepatology at the University of Calgary.
References
- 1.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 2.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 3.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Long EO. Ready for prime time: NK cell priming by dendritic cells. Immunity. 2007;26:385–387. doi: 10.1016/j.immuni.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Crouse J, Xu HC, Lang PA, Oxenius A. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol. 2015;36:49–58. doi: 10.1016/j.it.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paolini R, Bernardini G, Molfetta R, Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26:113–120. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-γ and systemic autoimmunity. Discov Med. 2013;16:123–131. [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 12.Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 13.Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Faustman DL, Davis M. TNF receptor 2 and disease: autoimmunity and regenerative medicine. Front Immunol. 2013;4:478. doi: 10.3389/fimmu.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blay JY, Bertoglio J, Fradelizi D, Chouaib S. Functional interactions of IL2 and TNF in the differentiation of LGL into LAK effectors. Int J Cancer. 1989;44:598–604. doi: 10.1002/ijc.2910440407. [DOI] [PubMed] [Google Scholar]
- 16.Favrot M, Combaret V, Blay JY, Capdeville R, Zhou DC, Clapisson G, et al. TNF alpha enhancement of NK and LAK cell functions induced by high-dose IL-2 in human peripheral blood mononuclear cells from patients pretreated with alpha IFN + IL-2. Eur Cytokine Netw. 1990;1:221–227. [PubMed] [Google Scholar]
- 17.Mason AT, McVicar DW, Smith CA, Young HA, Ware CF, Ortaldo JR. Regulation of NK cells through the 80-kDa TNFR (CD120b) J Leukoc Biol. 1995;58:249–255. doi: 10.1002/jlb.58.2.249. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Chakrabarti AK, Tan JL, Ge L, Gambotto A, Vujanovic NL. Essential role of the TNF-TNFR2 cognate interaction in mouse dendritic cell-natural killer cell crosstalk. Blood. 2007;109:3333–3341. doi: 10.1182/blood-2006-06-026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serti E, Werner JM, Chattergoon M, Cox AL, Lohmann V, Rehermann B. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 2014;147:209–220. doi: 10.1053/j.gastro.2014.03.046. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva LC, Ortigosa LC, Benard G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2:817–833. doi: 10.2217/imt.10.67. [DOI] [PubMed] [Google Scholar]
- 21.French AR, Yokoyama WM. Natural killer cells and autoimmunity. Arthritis Res Ther. 2004;6:8–14. doi: 10.1186/ar1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okubo Y, Mera T, Wang L, Faustman DL. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci Rep. 2013;3:3153. doi: 10.1038/srep03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oykhman P, Timm-McCann M, Xiang RF, Islam A, Li SS, Stack D, et al. Requirement and redundancy of the Src family kinases Fyn and Lyn in perforin-dependent killing of Cryptococcus neoformans by NK cells. Infect Immun. 2013;81:3912–3922. doi: 10.1128/IAI.00533-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 25.Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52:183–190. doi: 10.1016/j.jhep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Marr KJ, Jones GJ, Zheng C, Huston SM, Timm-McCann M, Islam A, et al. Cryptococcus neoformans directly stimulates perforin production and rearms NK cells for enhanced anticryptococcal microbicidal activity. Infect Immun. 2009;77:2436–2446. doi: 10.1128/IAI.01232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2009;49:1267–1276. doi: 10.1002/hep.22761. [DOI] [PubMed] [Google Scholar]
- 28.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 29.Wondimu Z, Santodomingo-Garzon T, Le T, Swain MG. Protective role of interleukin-17 in murine NKT cell-driven acute experimental hepatitis. Am J Pathol. 2010;177:2334–2346. doi: 10.2353/ajpath.2010.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swain MG. Natural killer T cells within the liver: conductors of the hepatic immune orchestra. Dig Dis. 2010;28:7–13. doi: 10.1159/000282059. [DOI] [PubMed] [Google Scholar]
- 31.Biburger M, Tiegs G. α-Galactosylceramide-induced liver injury in mice is mediated by TNF-α but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 32.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, et al. Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 33.Jewett A, Bonavida B. Activation of the human immature natural killer cell subset by IL-12 and its regulation by endogenous TNF-α and IFN-γ secretion. Cell Immunol. 1994;154:273–286. doi: 10.1006/cimm.1994.1077. [DOI] [PubMed] [Google Scholar]
- 34.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 35.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN-γ on activation. Proc Natl Acad Sci USA. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 38.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 39.Poggi A, Zocchi MR. NK cell autoreactivity and autoimmune diseases. Front Immunol. 2014;5:27. doi: 10.3389/fimmu.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vivier E, Ugolini S. Natural killer cells: from basic research to treatments. Front Immunol. 2011;2:18. doi: 10.3389/fimmu.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tufa DM, Chatterjee D, Low HZ, Schmidt RE, Jacobs R. TNFR2 and IL-12 coactivation enables slanDCs to support NK-cell function via membrane-bound TNF-α. Eur J Immunol. 2014;44:3717–3728. doi: 10.1002/eji.201444676. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Oppenheim JJ. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 2011;585:3611–3618. doi: 10.1016/j.febslet.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apostolaki M, Armaka M, Victoratos P, Kollias G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr Dir Autoimmun. 2010;11:1–26. doi: 10.1159/000289195. [DOI] [PubMed] [Google Scholar]
- 44.Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N, et al. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529–534. doi: 10.1016/j.jhep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Keystone EC, Ware CF. Tumor necrosis factor and anti-tumor necrosis factor therapies. J Rheumatol Suppl. 2010;85:27–39. doi: 10.3899/jrheum.091463. [DOI] [PubMed] [Google Scholar]
- 46.Vigano M, Degasperi E, Aghemo A, Lampertico P, Colombo M. Anti-TNF drugs in patients with hepatitis B or C virus infection: safety and clinical management. Expert Opin Biol Ther. 2012;12:193–207. doi: 10.1517/14712598.2012.646986. [DOI] [PubMed] [Google Scholar]
- 47.Cantini F, Lubrano E, Marchesoni A, Mathieu A, Olivieri I, Salvarani C, et al. Latent tuberculosis infection detection and active tuberculosis prevention in patients receiving anti-TNF therapy: an Italian nationwide survey. Int J Rheum Dis. 2015 doi: 10.1111/1756-185X.12708. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Dulai PS, Siegel CA. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 2014;43:525–541. doi: 10.1016/j.gtc.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Thaher F, Plankenhorn S, Klein R. Differential effects of the tumor necrosis factor alpha-blocker infliximab and etanercept on immunocompetent cells in vitro. Int Immunopharmacol. 2011;11:1724–1731. doi: 10.1016/j.intimp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Haider AS, Cohen J, Fei J, Zaba LC, Cardinale I, Toyoko K, et al. Insights into gene modulation by therapeutic TNF and IFNγ antibodies: TNF regulates IFNγ production by T cells and TNF-regulated genes linked to psoriasis transcriptome. J Invest Dermatol. 2008;128:655–666. doi: 10.1038/sj.jid.5701064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data