Abstract
The combination of cisplatin and gemcitabine is the standard first-line treatment of metastatic biliary tract cancer (BTC) patients. The benefit of second-line chemotherapy in these patients is controversial. This study aims to evaluate the activity of FOLFIRI (fluorouracil and irinotecan) after failure to the first-line platinum and gemcitabine-based chemotherapy in metastatic BTC patients. We present a single-institution, retrospective cohort study. Patients with locally advanced or metastatic BTC who progressed after at least one line of chemotherapy, consecutively treated at our Institution between 2007 and 2017 were included. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were overall survival (OS), clinical benefit rate (CBR) and safety profile of FOLFIRI. Twelve patients were included in the analysis, with a median follow up of 5 months (95% CI 2.77-7.20). The median number of cycles received was 3 (range 1 to 9). Four grade 3 toxicities were recorded; no grade 4 toxicities and no treatment-related deaths occurred. The median PFS was 1.7 months (95% CI; 0.66-2.67), and median OS was 5 months (95% CI; 2.77-7.20). Two patients presented stable disease, providing a CBR of 17%. We concluded that FOLFIRI presented a favorable toxicity profile and a modest activity in metastatic BTC patients who had progressed to platinum and gemcitabine and may be considered in patients who are able to tolerate additional lines of chemotherapy. Immunotherapy and targeted therapies selected according to the tumoral genomic profile are promising alternatives to improve the outcomes of second-line treatment in BTC.
Keywords: Biliary Tract Neoplasms, Fluorouracil, Neoplasm Metastasis
INTRODUCTION
Biliary tract cancer (BTC) is a rare tumor, with an estimated incidence of 1 to 2 new cases per 100,000 people annually in the United States.1 In Belgium, around 350 new cases are diagnosed every year, and BTC is responsible for 0.5% of the cancer deaths in males and 0.8% in females.2 Surgery provides the only possibility of curative treatment for BTC patients, although it is feasible in only 20% of the cases because 80% of the patients present with locally advanced unresectable disease or metastases at diagnosis.3-5 The prognosis of patients with metastatic BTC is dismal, with 5-year survival rates of less than 1%.6
Chemotherapy is the mainstay treatment of metastatic BTC, and its administration aims to promote symptom relief, improve the quality of life and prolong the overall survival of the patients.7 The combination of cisplatin and gemcitabine has been established as the standard first-line treatment of metastatic BTC.8 However, when disease progression occurs, there is no high-quality data to support the administration of second-line chemotherapy to BTC patients.4,9,10 Indeed, studies evaluating second-line treatments are scarce, and the benefit of chemotherapy over best supportive care in this scenario has never been demonstrated.9,10 Therefore, new strategies are needed to improve these outcomes.
Metastatic pancreatic cancer shares some histological and molecular characteristics with BTC.11 The FOLFIRINOX regimen (fluorouracil, irinotecan and oxaliplatin) has robust activity in the first-line treatment of metastatic pancreatic cancer patients, and its superiority over gemcitabine alone has been demonstrated in a phase III study.12 The results of FOLFIRINOX in pancreatic cancer generated interest in its potential activity also in BTC. Indeed, in a retrospective study with 12 metastatic BTC patients in different lines of treatment, FOLFIRINOX yielded a 41.7% clinical benefit rate (CBR) and a median progression-free survival (PFS) of 9 weeks.13 Ongoing studies are evaluating FOLFIRINOX in metastatic BTC patients [NCT03291899; NCT03778593; NCT02591030; NCT02456714; NCT01494363]. Notably, FOLFIRINOX is an intensive chemotherapy regimen that comprises 3 cytotoxic drugs administered at 14-day cycles. Thus, the risk of adverse events is relevant and represents a potential concern for the development of this regimen in BTC, especially for frail patients.12 As an alternative, the agents that compose FOLFIRINOX can be combined as doublets (fluorouracil and oxaliplatin [FOLFOX] or fluorouracil and irinotecan [FOLFIRI]) to minimize the risks of adverse events, however more data on the efficacy of FOLFOX and FOLFIRI in BTC is needed.14
The FOLFIRI regimen (fluorouracil and irinotecan) arises as a potentially effective and less toxic alternative of second line-treatment due to the absence of oxaliplatin, which commonly causes hematological toxicities and peripheral neuropathy, the latest being potentially a limiting condition for patients suffering from neuropathy derived from a first-line gemcitabine-cisplatin regimen.14,15 As a consequence, ongoing studies are evaluating FOLFIRI as a second-line treatment in metastatic BTC patients. [NCT03110510; NCT03464968] However, the administration of FOLFIRI after failure to a platinum and gemcitabine-based regimen has been only reported in few retrospective series; therefore more evidence on the activity of FOLFIRI in this setting is needed.10,16-18 The present study aims to assess and describe the activity of FOLFIRI after failure to a first-line platinum and gemcitabine regimen in metastatic BTC patients.
METHODS
Objectives
The primary objective of this study is to assess the activity of FOLFIRI in metastatic BTC patients who experienced disease progression after first-line platinum and gemcitabine-based chemotherapy. The primary endpoint is PFS, defined as the time between the first day of the first FOLFIRI cycle and the occurrence of disease progression or death due to any cause, whichever occurred first.
The secondary endpoints are overall survival (OS) - defined as the time between the first day of the first FOLFIRI cycle and death due to any cause; CBR - defined as the rate of patients presenting a complete response, a partial response or stable disease (per Response Evaluation Criteria in Solid Tumors [RECIST] criteria version 1.1) at the first imaging assessment performed to evaluate FOLFIRI response; and safety profile – defined as the frequency of grade ≥3 toxicities presented between the first day of the first cycle and 30 days after the last administration of FOLFIRI. Toxicities were classified and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, which was the latest version available at the time of FOLFIRI administration.
Patient’s Selection
This is a single-institution, retrospective cohort study performed at the Medical Oncology Department of Institut Jules Bordet, Brussels, Belgium. Patients with a histologically confirmed diagnosis of BTC of any histological type; with metastatic disease confirmed by imaging exams or locally advanced disease considered not suitable for surgery; who presented disease-progression as defined per RECIST after at least one line of chemotherapy including platinum and gemcitabine; and consecutively treated at our Institution between 2007 and 2017 were included. A detailed review of electronic records from each patient was performed to extract data for the study. This protocol has been submitted to the local ethics committee of Institut Jules Bordet and received approval before the study initiation.
To assess the CBR, we considered the results of the first radiological exam (computerized tomography [CT], magnetic resonance imaging [MRI] or fluorine-18 fluoro-2-deoxy-D-glucose positron emission tomography/computerized tomography [FDG–PET/CT]) performed after the initiation of FOLFIRI, which was requested according to the treating’s physician discretion. Routinely, imaging exams are performed every 2 to 3 chemotherapy cycles at our Institution to assess treatment response, which is evaluated by RECIST version 1.1. Chemotherapy is administered until the occurrence of disease progression, limiting toxicities or consent withdrawn by the patient, whichever occurs first. The standard FOLFIRI regimen administered at our Institution consists in fluorouracil 400mg/m2 bolus on D1 then 2400 mg/m2 on a 48h protracted infusion D1-2, leucovorin 200 mg/m2 on D1 and irinotecan 180 mg/m2 on D1 every 14 days, with each administration being considered 1 cycle.
Statistical Analysis
PFS and OS were calculated with the Kaplan-Meier method, and median values were reported with their respective 95% confidence intervals (CI). Patients’ characteristics were reported with their respective median and/or range values when applicable. All the statistical analyses were performed using the R software version 4.4.1.
RESULTS
From all the patients with metastatic BTC who were consecutively treated between 2007 and 2017 at our Institution, twelve patients who received at least one cycle of FOLFIRI after progression to first-line chemotherapy were identified and considered eligible. The median age was 60 years, 75% had an Eastern Cooperative Oncology Group Performance Status (ECOG) ≤1, 100% presented extrahepatic metastases and 92% had only 1 previous line of chemotherapy administered for the treatment of metastatic disease. All patients had received platinum and gemcitabine-based chemotherapy as the first-line treatment of metastatic disease. Patient’s characteristics are illustrated in Table 1.
Table 1. Patient’s characteristics.
| Variable | Number (%) | Variable | Number (%) |
|---|---|---|---|
| Age (years) | Staging | ||
| Median | 60 | Metastatic | 12 (100%) |
| Range | (37 - 82) | Locally advanced | 0 |
| Sex | Sites of metastases | ||
| Male | 6 (50%) | Hepatic only | 0 |
| Female | 6 (50%) | Extrahepatic | 12 (100%) |
| ECOG | Previous biliary drainage | 2 (17%) | |
| 0 | 4 (33%) | Previous surgery to resect primary tumor | 2 (17%) |
| 1 | 5 (42%) | Previous radiotherapy in the primary tumor | 0 |
| 2 | 1 (08%) | Previous radiotherapy in metastases | 7 (58%) |
| 3 | 2 (17%) | Previous treatment with SIR-spheres | 1 (08%) |
| Smoking history | Previous chemotherapy lines | ||
| Current smoker | 4 (33%) | One | 11 (92%) |
| Former smoker | 1 (08%) | Two | 1 (08%) |
| Never smoker | 7 (58%) | FOLFIRI cycles | |
| Histology | Median | 3 | |
| Adenocarcinoma | 12 (100%) | Range | (1 - 9) |
| Others | 0 | FOLFIRI dose reduction | |
| Primary tumor | Yes | 3 (25%) | |
| Gallbladder | 1 (08%) | No | 9 (75%) |
| Intrahepatic | 9 (75%) | FOLFIRI cycle delays | |
| Extrahepatic | 2 (17%) | Yes | 2 (17%) |
| No | 10 (83%) | ||
| Reason for FOLFIRI discontinuation | |||
| Progressive disease | 11 (92%) | ||
| Adverse events | 1 (08%) |
ECOG, Eastern Cooperative Oncology Group performance status scale; SIR-spheres, microspheres impregnated with 90Ytrium, a beta radiating isotope.
Safety Profile
The median number of FOLFIRI cycles received by the patients was 3 (range 1 to 9). In 3 patients (25%), a dose reduction was necessary at any point due to toxicities. Cycle delays occurred in 2 patients (17%), and 1 patient (8%) had to discontinue FOLFIRI due to adverse events. (Table 1).
Four grade-3 toxicities were recorded: diarrhea (1), emesis (1), and fatigue (2); no grade-4 toxicities and no treatment-related deaths occurred. (Table 2)
Table 2. Adverse events grade ≥3 presented with FOLFIRI.
| Adverse event | Number of patients (%) | Number of patients (%) |
|---|---|---|
| Grade 3 | Grade 4 | |
| Anemia | 0 | 0 |
| Thrombocytopenia | 0 | 0 |
| Neutropenia | 0 | 0 |
| Febrile neutropenia | 0 | 0 |
| Diarrhea | 1 (08) | 0 |
| Emesis | 1 (08) | 0 |
| Fatigue | 2 (17) | 0 |
| Transaminases elevation | 0 | 0 |
| Bilirubin elevation | 0 | 0 |
| Creatinine elevation | 0 | 0 |
| Rash | 0 | 0 |
| Hand-foot syndrome | 0 | 0 |
| Mucositis | 0 | 0 |
Efficacy
No loss of follow-up occurred, and all patients were followed until the occurrence of an event (death or progression) at our Institution. The median follow-up since the first day of FOLFIRI administration was 5 months (95% CI 2.77-7.20).
Progression-Free Survival (PFS)
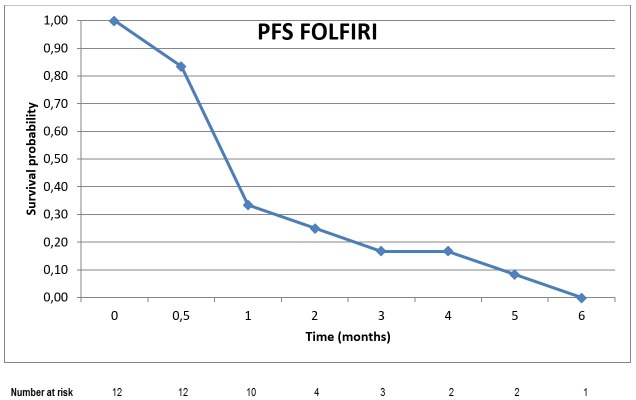
Eleven patients (92%) discontinued FOLFIRI due to disease progression. All patients included in the analysis presented disease progression confirmed by imaging exams. The median PFS achieved with FOLFIRI was 1.7 months (95% CI; 0.66-2.67). (Figure 1)
Figure 1. Kaplan Meier curve of progression-free survival with FOLFIRI.
Overall Survival (OS)
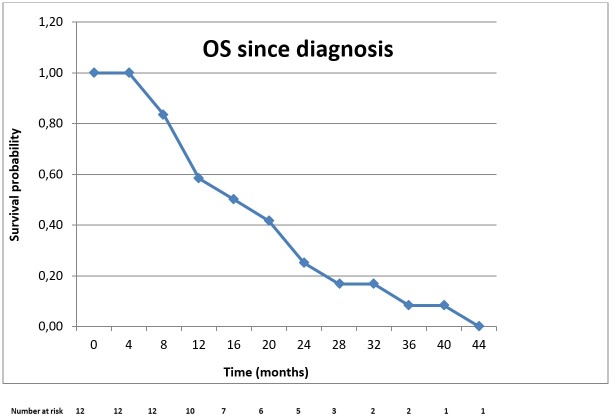
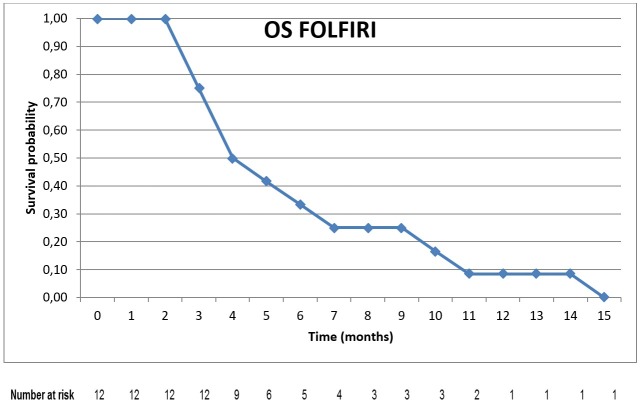
All patients included in the analysis had died before the date of data collection for this study. The median OS from the date of diagnosis of BTC until death was 16 months (95% CI; 9.97-22.06) (Figure 2). The median OS from the first day of FOLFIRI administration until death (median OS with FOLFIRI) was 5 months (95% CI; 2.77-7.20) (Figure 3).
Figure 2. Kaplan Meier curve of overall survival since the moment of diagnosis of biliary tract cancer.
Figure 3. Kaplan Meier curve of overall survival with FOLFIRI.
Clinical Benefit Rate
At the moment of the first evaluation of treatment response, 10 patients (83%) presented radiological evidence of progressive disease, whereas 2 patients presented stable disease and continued FOLFIRI, providing a CBR of 17%.
DISCUSSION
The population of our study reflects the characteristics of the patients with metastatic BTC reported in other series in terms of age, tumor primary site, and histology, and the OS of 16 months observed is in-line with data reported in the medical literature.19 The administration of FOLFIRI was feasible and presented a manageable toxicity profile, with clinically relevant adverse events being observed in the minority of the patients. The short median PFS and OS and the modest CBR observed in our study suggest that FOLFIRI has limited activity as second-line chemotherapy in metastatic BTC patients.
The combination of cisplatin and gemcitabine is the standard-of-care first-line treatment of metastatic BTC patients, based on the results of the phase III study ABC-02, which demonstrated an overall survival benefit with cisplatin-gemcitabine compared to gemcitabine alone (median OS 11.7 vs. 8.1 months respectively; hazard ratio [HR] 0.64; 95% CI, 0.52-0.80; p<0.001).4,8 Unfortunately, disease progression invariably occurs after first-line chemotherapy, and no second-line treatment has been established as a standard for metastatic BTC patients so far.9,10 Notably, disease progression is generally associated with fatigue, jaundice, weight loss, pain and a decrease in performance status.19 As a consequence of this clinical deterioration that frequently occurs at progression, the administration of second-line treatments is feasible only in a minority of the patients, with best supportive care being recommended in most of the cases.9,10,20,21
The evidence to support the recommendation of second-line chemotherapy in BTC patients is scarce, consisting mainly in retrospective series and phase II studies.9,10 Second-line chemotherapy can be considered in patients who present a good performance status and a low predicted risk of toxicities, although its efficacy is modest.9,10,21 The regimens most frequently administered are those based on fluoropyrimidines and taxanes, which yield response rates of around 10% and median PFS rates of approximately 3 months.9,10 Polychemotherapy regimens are associated with an increased risk of adverse events and do not seem to increase response rates or PFS in comparison to single-agent chemotherapy.10 Targeted-therapies such as bevacizumab (monoclonal antibody that targets the vascular endothelial growth factor [VEGF]), erlotinib (tyrosine kinase inhibitor that blocks the epithelial growth factor receptor [EGFR]), cetuximab and panitumumab (monoclonal antibodies targeting the EGFR) presented also a modest activity as second-line treatments in BTC patients.22-29
The use of FOLFIRI after failure to first-line chemotherapy has been evaluated in retrospective series, with response rates of around 11% and a median PFS of approximately 3 months (Table 3).16-18,30,31 In the largest of these series, 98 patients who received FOLFIRI after progression on first-line platinum and gemcitabine chemotherapy were evaluated. Sixty-five out of the 98 patients had response assessments documented in their medical records, with 11% presenting a partial response and 35% presenting stable disease. The median PFS and OS were 2.4 and 6.6 months respectively.31 In a prospective single-arm phase II study that evaluated FOLFIRI as the first-line treatment of 30 patients with unresectable or metastatic BTC, the response rate was 10%, whereas 10% of the patients presented stable disease; the median PFS was 2.8 months for patients with an intrahepatic primary tumor and 5.2 months for those with extrahepatic tumors.30 The results from our study are thus similar to those reported in the literature, supporting the concept that the activity of FOLFIRI in metastatic BTC is at best modest, although a subset of patients may derive benefit from this treatment.9,10
Table 3. Studies reporting outcomes of metastatic biliary tract cancer patients treated with FOLFIRI.
| 1st author year | Regimen | N | Design | Line | ORR | mPFS | mOS |
|---|---|---|---|---|---|---|---|
|
Feisthammel30 2007 |
FOLFIRI | 30 | Single-arm PHASE II | 1st | 10% | 2.8 m (intrahepatic) 5.2 m (extrahepatic) |
5.4 m (intrahepatic) 9 m (extrahepatic) |
|
Brieau16 2015 |
FOLFIRI | 64 b | Retrospective | 2nd | 11.5% | 2.6 m | 6.1 m |
|
Moretto17 2013 |
FOLFIRI | 24 b 30 p |
Retrospective | 38% 2nd | 42.9% (CBR) | 3.5 m | 6.2 m |
|
Sebbagh18 2015 |
FOLFIRI | 52 | Retrospective | 2nd | N/A | 3.2 m | 8.4 m |
|
Mizrahi31 2019 |
FOLFIRI | 98 | Retrospective | 2nd and beyond | 11% | 2.4 m | 6.6 m |
|
Caparica 2019 |
FOLFIRI | 12 | Retrospective | 2nd and beyond | 17% (CBR) |
1.7 m | 5 m |
b = biliary; CBR = clinical benefit rate; m = months; mPFS = median progression-free survival; mOS = median overall survival; N = number of patients; ORR = overall response rate; p = pancreatic; N/A = not available.
The main challenges in the treatment of metastatic BTC are the small proportion of patients that are eligible to receive a second line treatment when disease-progression occurs, the absence of effective treatments after the first-line and the lack of biomarkers to predict which patients benefit from treatment.9,10,19 Promising strategies to overcome these limitations are under evaluation. As an example, immunotherapy is based on the rationale that the activation of the immune system can generate an anti-tumor immune response.32 This concept has proven to be effective in several malignancies and is currently being also investigated in metastatic BTC patients.33 In the preliminary reports of two phase II studies that enrolled metastatic BTC patients who had received several lines of chemotherapy, the anti-programmed death-1 receptor antibodies pembrolizumab and nivolumab presented response rates of 5.8% and 17%, respectively. Interestingly, the median duration of response was not reached in both studies, suggesting that patients who respond to immunotherapy may present a long-term benefit with this treatment.34,35
Notably, BTC have a heterogeneous genomic profile, and different abnormalities were found in these tumors, such as mutations/amplifications in the genes of the human epidermal growth factor (HER) family, fibroblast growth factor (FGFR) mutations/rearrangements, isocitrate dehydrogenase (IDH) mutations and hyperexpression or mutations in the genes involved in the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways.36 Targetable alterations, meaning those genomic abnormalities for which a potential therapy exists, were found in 68% of the cases in one study with 34 metastatic BTC patients.37 Interestingly, the genomic profile of BTC varies according to the site of origin of the primary tumor: while IDH and FGFR alterations are more frequent in intrahepatic tumors, abnormalities in the genes of the HER family are more common in extrahepatic and gallbladder tumors.36 The incorporation of tumoral genomic profile as a stratification factor in studies evaluating targeted therapies arises as a promising strategy to optimize the treatment of BTC.37 As an example, the combination of a BRAF inhibitor (dabrafenib) with a mitogen-activated extracellular kinase (MEK) inhibitor (trametinib) yielded a median PFS of 9.2 months in a phase II study that enrolled 33 patients with BRAF mutated metastatic BTC. Partial responses were observed in 14 patients (42%), while stable disease occurred in 15 patients (45%).38 Although direct comparisons between different studies are precluded, these results are more robust than the ones observed in our study and in the literature with second-line chemotherapy in general.9,10
The potential limitations of the present study need to be considered when interpreting our findings. The retrospective character of our series increases the risks of bias and missing data, limiting the assessment of toxicities and treatment response since all the information was retrieved from the data reported in patient’s medical records. Since most patients who experience progressive disease after first-line chemotherapy are too debilitated to receive a second-line treatment, the population included in our study may be composed of selected patients with good prognostic features or with indolent tumors who were eligible to receive additional chemotherapy. The small sample size reduces the robustness of our analyses, and a logistic regression model that was originally pre-planned to seek for potential prognostic or predictive factors could not be performed. All patients were treated at a single tertiary cancer center in Belgium, which possesses high-quality facilities and vast expertise in the management of BTC, a fact that may limit the extrapolation of these results to low-resource scenarios. However, due to the paucity of studies on the topic, our findings are important to provide additional data on the activity of FOLFIRI after progression to first-line chemotherapy in metastatic BTC patients.
In conclusion, FOLFIRI presented a manageable toxicity profile but a modest activity in metastatic BTC patients who had progressed to platinum and gemcitabine chemotherapy and may be considered an option for patients who can tolerate additional lines of chemotherapy. The enrolment of metastatic BTC patients in clinical trials must be encouraged, due to the paucity of effective treatments in this scenario. Immunotherapy and targeted therapies selected according to the tumoral genomic profile arise as promising alternatives to improve the efficacy of second-line treatment in BTC patients.
Footnotes
How to Cite: Caparica R, Lengelé A, Bekolo W, Hendlisz A. FOLFIRI as second-line treatment of metastatic biliary tract cancer patients. Autops Case Rep [Internet]. 2019;9(2):e2019087. https://doi.org/10.4322/acr.2019.087
This protocol has been submitted to the local ethics committee of Institut Jules Bordet and received approval before the study initiation.
Financial support: None
References
- 1.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14(2):109-14. 10.1055/s-2007-1007302. [DOI] [PubMed] [Google Scholar]
- 2.Belgian Cancer Registry Cancer incidence in Belgium – Brussels 2008 [online]. Brussel: Kanker Register; 2011. [cited 2019 Apr 19]. Available from: http://www.kankerregister.org/media/docs/StK_publicatie.pdf [Google Scholar]
- 3.Bridgewater J, Galle PR, Khan SA, et al. . Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268-89. 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2016;27(Suppl 5):v28-37. 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 5.Lee SE, Kim KS, Kim WB, et al. . Practical guidelines for the surgical treatment of gallbladder cancer. J Korean Med Sci. 2014;29(10):1333-40. 10.3346/jkms.2014.29.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society Survival rates for bile duct cancer [online]. 2019. [cited 2019 Apr 5]. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
- 7.Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: an update. World J Gastrointest Oncol. 2013;5(7):171-6. 10.4251/wjgo.v5.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valle J, Wasan H, Palmer DH, et al. . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-81. 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 9.Lamarca A, Hubner RA, Ryder WD, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328-38. 10.1093/annonc/mdu162. [DOI] [PubMed] [Google Scholar]
- 10.Fornaro L, Vivaldi C, Cereda S, et al. . Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res CR. 2015;34(1):156. 10.1186/s13046-015-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmuck RB, Carvalho-Fischer CV, Neumann C, Pratschke J, Bahra M. Distal bile duct carcinomas and pancreatic ductal adenocarcinomas: postulating a common tumor entity. Cancer Med. 2016;5(1):88-99. 10.1002/cam4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy T, Desseigne F, Ychou M, et al. . FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med. 2011;364(19):1817-25. 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 13.Kus T, Aktas G, Kalender ME, Sevinc A, Camci C. Comparison of FOLFIRINOX Chemotherapy with Other Regimens in Patients with Biliary Tract Cancers: a Retrospective Study. J Gastrointest Cancer. 2017;48(2):170-5. 10.1007/s12029-016-9880-y. [DOI] [PubMed] [Google Scholar]
- 14.Mohelnikova-Duchonova B, Melichar B, Soucek P. FOLFOX/FOLFIRI pharmacogenetics: the call for a personalized approach in colorectal cancer therapy. World J Gastroenterol. 2014;20(30):10316-30. 10.3748/wjg.v20.i30.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol. 2011;18(1):18-25. 10.3747/co.v18i1.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brieau B, Dahan L, De Rycke Y, et al. . Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer. 2015;121(18):3290-7. 10.1002/cncr.29471. [DOI] [PubMed] [Google Scholar]
- 17.Moretto R, Raimondo L, De Stefano A, et al. . FOLFIRI in patients with locally advanced or metastatic pancreatic or biliary tract carcinoma: a monoinstitutional experience. Anticancer Drugs. 2013;24(9):980-5. 10.1097/CAD.0b013e328364e66b. [DOI] [PubMed] [Google Scholar]
- 18.Sebbagh S, Roux J, Dreyer C, et al. . Efficacy of a sequential treatment strategy with GEMOX-based followed by FOLFIRI-based chemotherapy in advanced biliary tract cancers. Acta Oncol. 2016;55(9-10):1168-74. 10.1080/0284186X.2016.1191670. [DOI] [PubMed] [Google Scholar]
- 19.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95-111. 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edeline J, Bonnetain F, Phelip JM, et al. . Gemox versus surveillance following surgery of localized biliary tract cancer: results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol. 2017;35(4 Suppl):225-225. 10.1200/JCO.2017.35.4_suppl.225. [DOI] [Google Scholar]
- 21.Takahara N, Nakai Y, Isayama H, et al. . Second-line chemotherapy in patients with advanced biliary tract cancer. J Clin Oncol. 2018;36(4 Suppl):429-429. 10.1200/JCO.2018.36.4_suppl.429. 29227725 [DOI] [Google Scholar]
- 22.Philip PA, Mahoney MR, Allmer C, et al. . Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24(19):3069-74. 10.1200/JCO.2005.05.3579. [DOI] [PubMed] [Google Scholar]
- 23.Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. . Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11(1):48-54. 10.1016/S1470-2045(09)70333-X. [DOI] [PubMed] [Google Scholar]
- 24.Guion-Dusserre J-F, Lorgis V, Vincent J, Bengrine L, Ghiringhelli F. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21(7):2096-101. 10.3748/wjg.v21.i7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubner SJ, Mahoney MR, Kolesar JL, et al. . Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol. 2010;28(21):3491-7. 10.1200/JCO.2010.28.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer RV, Pokuri VK, Groman A, et al. . A multicenter phase II study of gemcitabine, capecitabine, and bevacizumab for locally advanced or metastatic biliary tract cancer. Am J Clin Oncol. 2018;41(7):649-55. 10.1097/COC.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 27.Bréchon M, Dior M, Dréanic J, et al. . Addition of an antiangiogenic thy3yerapy, bevacizumab, to gemcitabine plus oxaliplatin improves survival in advanced biliary tract cancers. Invest New Drugs. 2018;36(1):156-62. 10.1007/s10637-017-0492-6. [DOI] [PubMed] [Google Scholar]
- 28.Gruenberger B, Schueller J, Heubrandtner U, et al. . Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11(12):1142-8. 10.1016/S1470-2045(10)70247-3. [DOI] [PubMed] [Google Scholar]
- 29.Malka D, Cervera P, Foulon S, et al. . Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15(8):819-28. 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feisthammel J, Schoppmeyer K, Mössner J, Schulze M, Caca K, Wiedmann M. Irinotecan with 5-FU/FA in advanced biliary tract adenocarcinomas: a multicenter phase II trial. Am J Clin Oncol. 2007;30(3):319-24. 10.1097/01.coc.0000258124.72884.7a. [DOI] [PubMed] [Google Scholar]
- 31.Mizrahi J, Gunchick V, Mody K, et al. . FOLFIRI in advanced biliary tract cancers. J Clin Oncol. 2019;37(4 Suppl):451-451. 10.1200/JCO.2019.37.4_suppl.451. [DOI] [Google Scholar]
- 32.Weiden J, Tel J, Figdor CG. Synthetic immune niches for cancer immunotherapy. Nat Rev Immunol. 2018;18(3):212-9. 10.1038/nri.2017.89. [DOI] [PubMed] [Google Scholar]
- 33.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-5. 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai D, Kanai M, Kobayashi S, et al. . Randomized phase III study of Gemcitabine, Cisplatin plus S-1 (GCS) versus Gemcitabine, Cisplatin (GC) for Advanced Biliary Tract Cancer (KHBO1401-MITSUBA). Ann Oncol. 2018;29(Suppl 8):viii205-270. 10.1093/annonc/mdy282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim R, Kim D, Alese O, et al. . O-009 – A Phase II multi institutional study of nivolumab in patients with advanced refractory biliary tract cancers (BTC). Ann Oncol. 2018;29(1, Suppl 5):mdy149.008 . . [Google Scholar]
- 36.Verlingue L, Hollebecque A, Boige V, Ducreux M, Malka D, Ferté C. Matching genomic molecular aberrations with molecular targeted agents: are biliary tract cancers an ideal playground? Eur J Cancer. 2017;81(81):161-73. 10.1016/j.ejca.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Verlingue L, Malka D, Allorant A, et al. . Precision medicine for patients with advanced biliary tract cancers: an effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer. 2017;87(87):122-30. 10.1016/j.ejca.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Wainberg ZA, Lassen UN, Elez E, et al. . Efficacy and safety of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated biliary tract cancer (BTC): A cohort of the ROAR basket trial. J Clin Oncol. 2019;37(4 Suppl):187-187. 10.1200/JCO.2019.37.4_suppl.187. [DOI] [Google Scholar]