Abstract
Hemolytic uremic syndrome (HUS), a vascular disease characterized by hemolytic anemia, thrombocytopenia, and acute renal failure, is caused by enterohemorrhagic Shiga toxin (Stx)-producing bacteria, which mainly affect children. Besides Stx, the inflammatory response mediated by neutrophils (PMN) is essential to HUS evolution. PMN can release neutrophil extracellular traps (NET) composed of DNA, histones, and other proteins. Since NET are involved in infectious and inflammatory diseases, the aim of this work was to investigate the contribution of NET to HUS. Plasma from HUS patients contained increased levels of circulating free-DNA and nucleosomes in comparison to plasma from healthy children. Neutrophils from HUS patients exhibited a greater capacity to undergo spontaneous NETosis. NET activated human glomerular endothelial cells, stimulating secretion of the proinflammatory cytokines IL-6 and IL-8. Stx induced PMN activation as judged by its ability to trigger reactive oxygen species production, increase CD11b and CD66b expression, and induce NETosis in PMN from healthy donors. During HUS, NET can contribute to the inflammatory response and thrombosis in the microvasculature and thus to renal failure. Intervention strategies to inhibit inflammatory mechanisms mediated by PMN, such as NETosis, could have a potential therapeutic impact towards amelioration of the severity of HUS.
Key Words: Neutrophils, Extracellular traps, Shiga toxin, Hemolytic uremic syndrome, Leukocyte elastase, Endothelial cells
Introduction
Infection of children with Shiga toxin (Stx)-producing Escherichia coli (STEC) is the leading cause of typical hemolytic uremic syndrome (HUS) [1], which is characterized by thrombocytopenia, hemolytic anemia, and acute renal failure. Stx, the main pathogenic factor of STEC that leads to endothelial damage, is formed by a single A subunit, which possesses both N-glycosidase activity to the 28S rRNA that promotes the inhibition of protein synthesis in eukaryotic cells, and 5 B subunits, which bind to globotriaosylceramide (Gb3Cer) on the surface of host cells [2]. Once Stx is released by bacteria in the gut, the toxin enters the bloodstream and targets the renal endothelium. Besides the cytotoxic effects of Stx, the inflammatory response mediated by neutrophils (PMN) also contributes to HUS evolution [3]. HUS patients show a high peripheral PMN count, which correlates with a poor prognosis [4]. Moreover, these cells have shown signs of previous activation, indicated by a reduced expression of different membrane molecules and intracellular components, as well as impaired cytokine-induced responses [5]. Also, in mice, depletion of peripheral PMN increases survival after Stx intoxication, indicating that these cells play an essential role in the disease [3]. Additional studies have shown that PMN can function as toxin carriers from the gut, the site of Stx production by the confined noninvasive STEC, to the kidney. Other studies have confirmed that Stx directly binds to PMN and increases the expression of activation markers [6, 7, 8].
Neutrophils have several effector functions such as phagocytosis and exocytosis of granules containing proteases and other enzymes, and they can also produce neutrophil extracellular traps (NET) [9]. NET are a meshwork of DNA fibers comprising histones and granule proteins such as elastase, myeloperoxidase (MPO), pentraxin, and lactoferrin, each with strong antimicrobial and/or immunomodulating properties [9]. Microbes are immobilized within NET and encounter a locally high and lethal concentration of effector proteins. Even though NET participate in the control of the dissemination of bacteria, high amounts of them seem to be associated with pathophysiological conditions, implicating NET as contributors to collateral damage within inflamed tissues [10, 11, 12]. When these structures are formed inside the microvasculature, they act as a stimulus for thrombus formation [13]. NET induce platelet adhesion, activation, and aggregation that finally promote fibrin deposition, indicating that NET are a link between inflammation and thrombosis [14]. In patients with sepsis, there is an increase in the level of circulating free DNA (cf-DNA) in peripheral blood [15], which has been considered a marker for NETosis.
In this study, we report the presence of NET in plasma from HUS patients and an increased capacity of HUS PMN to undergo spontaneous NETosis ex vivo. We also determine that NET stimulate secretion of the proinflammatory cytokines IL-6 and IL-8 by human glomerular endothelial cells (HGEC). Moreover, Stx type 2 (Stx2) is able to trigger NETosis in healthy control PMN. Our findings suggest that NET locally produced at the renal microvasculature level could contribute to the inflammatory response that underlies the evolution of this disease.
Materials and Methods
Patients and Samples
This study was approved by the hospital's ethical committee (Comité de Bioética del Hospital Municipal del Niño de San Justo) and the institutional ethical committee and was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. HUS patients in the acute period (n = 22) and healthy children (HC; control group, n = 23), admitted for routine surgical procedures and matched for age and sex, were enrolled after informed consent had been obtained from their parents. The diagnostic criteria for HUS were: microangiopathic hemolytic anemia, thrombocytopenia (platelet count <150 × 109/l), and acute renal failure (serum creatinine level >0.100 mM). Samples were collected before dialysis. All patients developed HUS after a prodrome of gastroenteritis with bloody diarrhea. Half of the children showed evidence of STEC O157 by stool culture and/or presence of Stx antibodies in serum. Blood samples (2 ml) from HUS patients, HC, and healthy adult donors (after informed consent) were collected into EDTA tubes for PMN purification and plasma recollection.
Proteins
Recombinant purified Stx2 was purchased from Tufts University (Boston, Mass., USA). It contained less than 5 pg of LPS (per μg of Stx), quantified by Limulus amebocyte lysate assay. When needed, Stx2 was inactivated (Stx2i) via heating for 3 h at 100°C. Stx2ΔAB was produced as previously described [16].
Isolation of Human PMN
PMN were isolated from peripheral blood drawn from healthy adult donors, HC, or HUS patients by Ficoll-Hypaque gradient centrifugation and dextran sedimentation as previously described [17]. Cell suspensions contained >97% PMN and the levels of monocyte contamination were always <0.5% as evaluated by flow cytometry. PMN were suspended in RPMI 1640 medium (EMEVE Microvet SRL Laboratories, Buenos Aires, Argentina) with 0.2% bovine serum albumin (complete medium).
Stimulation of PMN and Quantification of the NET Release
PMN were suspended in complete medium to a final concentration of 2 × 106/ml. The cells were incubated in 24-well tissue culture plates and cultured with medium (basal) or stimulated with Stx2 (0.1 μg/ml), Stx2ΔAB (0.1 μg/ml), Stx2i (0.1 μg/ml), or phorbol myristate acetate (PMA; 50 ng/ml) as a positive control of NET induction [10] for 4 h at 37°C in 5% CO2. Then, the cells were treated with a low concentration of micrococcal nuclease (MNase; 1 U/ml, Worthington, N.J., USA) for 30 min at 37°C to detach NET that might have remained attached to the PMN debris. After the addition of EDTA (5 mM) to prevent further MNase activity, the samples were centrifuged and the supernatants were collected. The DNA concentration in the PMN supernatants and the cf-DNA in the plasma samples were determined using SYBR® Gold Nucleic Acid Gel Stain (Invitrogen, Carlsbad, Calif., USA) staining as previously reported [15]. Fluorescence intensity reflects the amount of DNA and was measured using a microplate reader (VICTOR3; USA). A calibration curve with defined calf thymus DNA (Sigma, St. Louis, Mo., USA) amounts ranging from 0 to 20 μg/ml was used in all analyses.
Quantification of Nucleosomes
Nucleosomes were quantified by ELISA (Cell Death Detection Kit; Roche, USA) according to the manufacturer's instructions. One unit of nucleosomes refers to the average amount of nucleosomes quantified in plasma from HC.
Evaluation of Elastase Activity
Elastase activity was determined by using the specific substrate N-methoxysuccinyl-Ala-Ala-Pro-Val (Sigma) and evaluating the absorbance at 405 nm by spectrophotometry.
Induction of NET by Monosodium Urate Crystals
PMN from healthy adult donors (2 × 106/ml) were cultured with monosodium urate (MSU) crystals (300 µg/ml) or vehicle for 1 h at 37°C, and then the cells were centrifuged to remove the crystals, resuspended in complete medium in the presence or absence of MNase (10 U/ml), and cultured for 3 additional hours. Thereafter, the cells were subjected to a 60-second ultrasonic pulse (40 Hz) to detach NET that might have remained attached to the PMN debris, and the supernatants were collected to quantify the DNA and elastase activity as readouts of the NET concentration. Other aliquots were seeded over Vero cells or HGEC as indicated below.
Immunofluorescence Staining and Confocal Microscopy Examination of NET
Freshly isolated PMN (50,000/300 µl) were seeded on poly-L-lysine-coated coverslips and stimulated with vehicle (basal), PMA (50 ng/ml), MSU (300 µg/ml), or Stx2 (0.1 µg/ml) for 4 h at 37°C in 5% CO2. Then, the cells were fixed with 4% paraformaldehyde (PFA; Sigma), permeabilized with 0.5% Triton X-100 in PBS for 1 min, and blocked with 5% calf serum for 60 min. In some experiments, freshly isolated PMN (600,000/300 µl) were cultured in a Lab-Tek chambered coverglass (Nunc, Rochester, N.Y., USA) with vehicle (basal), PMA (50 ng/ml), MSU (300 µg/ml), or Stx2 (0.1 µg/ml) for 4 h at 37°C in 5% CO2. The cells were fixed with 4% Paraformaldehyde (PFA, Sigma, Mo., USA) for 1 h and then incubated with 0.1 M glycine for 15 min. After that, the cells were permeabilized with cold acetone for 5 min, washed with PBS, and blocked with 5% calf serum for 60 min. Then, samples were incubated with a rabbit polyclonal antibody anti-elastase or rabbit IgG antibody as an isotype control (1 µg/ml; Calbiochem, USA) and a secondary DyLight 488-conjugated anti rabbit antibody (3 µg/ml; Jackson Immunoresearch Labs, West Grove, Pa., USA). After that, DNA was stained with propidium iodide (PI; 1 µg/ml; Sigma-Aldrich, St. Louis, Mo., USA). Alternatively, NET were stained with an FITC-conjugated anti-MPO antibody (Biolegend, San Diego, Calif., USA). NET were visualized by confocal laser scanning microscopy (CLSM) using a FluoView FV1000 confocal microscope equipped with a Plapon ×60/1.42 objective (Olympus, Tokyo, Japan). Images were acquired by sequentially scanning with the optimal settings for each fluorophore used.
Alternatively, to evaluate the kinetics of NETosis, PMN (600,000/300 µl) isolated from HUS patients and HC were cultured in a Lab-Tek chambered coverglass at 37°C without stimulation (basal) for 1 h or with PMA (50 ng/ml) for 1, 2, and 3 h. Then, the cells were fixed with 4% PFA overnight, stained with PI (1 μg/ml) and analyzed via CLSM.
NET Detection in Plasma by Immnunofluorescence Staining
Plasma samples (100 µl) were obtained from blood samples after centrifugation and seeded on poly-L-lysine-coated coverslips for 1 h. Then, the samples were fixed with 4% PFA overnight at 4°C. After that, the coverslips were washed with PBS and stained with anti-MPO FITC and PI (1 µg/ml). Then, the slides were analyzed via CLSM.
Detection of the Capacity of Peripheral Blood PMN to Undergo Spontaneous NETosis ex vivo
Whole-blood samples (100 µl) from HUS patients and HC were lysed (hypotonic shock) and centrifuged, and then the leukocytes were resuspended in RPMI medium (200 µl) and cultured in a Lab-Tek chambered coverglass at 37°C for 1 h. After that, the cells were fixed with 4% PFA overnight, washed with PBS, stained with anti-MPO FITC and PI (1 µg/ml), and analyzed via CLSM.
Reactive Oxygen Species Generation
PMN from healthy adult donors (1 × 106 cells/ml) were incubated with dihydrorhodamine (DHR) 123 (1 mM; Sigma Chemical Co., St. Louis, Mo., USA), a fluorescent probe for determination of reactive oxygen species (ROS), for 15 min at 37°C in 5% CO2. Then, the cells were stimulated and incubated for 15 additional minutes and analyzed by flow cytometry.
PMN Degranulation Assays
PMN from healthy adult donors were incubated with Stx2 for 180 min at 37°C, and then the cells were incubated for 30 min at 4°C with a PE-conjugated anti-CD11b monoclonal antibody, an FITC-conjugated anti-CD66b monoclonal antibody (Becton Dickinson, Franklin Lakes, N.J., USA), or isotype controls and washed and analyzed by flow cytometry. The analysis was made on 10,000 events for each sample using the CellQuest program. PMN were identified and gated according to their forward and light scattering (FSC/SSC) dot plot profiles.
In vitro Evaluation of the Capacity of NET to Modulate Stx Cytotoxicity
A Vero cell line sensitive to Stx2 was grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin (GIBCO, USA) (complete medium). To determine the effect of NET on Stx2 toxicity, one cytotoxic dose of Stx2 that kills 50% of VERO cells (670 pg of Stx2, cytotoxic dose) was incubated with MSU-induced NET (NET-MSU) or the corresponding controls for 1 h at 37°C. Then, the samples were seeded on Vero cells grown in 96-well microplates and incubated for 48 h at 37°C in 5% CO2. Afterwards, the cells were washed with PBS and stained with crystal violet dye, and after 10% acetic acid treatment the absorbance was determined at 570 nm.
HGEC were isolated from kidneys removed from different pediatric patients undergoing nephrectomies performed at the Hospital Nacional Alejandro Posadas (Buenos Aires, Argentina). Written informed consent was obtained and this project was approved by Ethics Committee of the Universidad de Buenos Aires. The method used for HGEC isolation was adapted from that described by McGinn et al. [18] and others [19]. To evaluate the impact of NET on Stx2 cytotoxicity, HGEC were cultured in the presence or absence of NET-MSU or NET-MSU together with Stx2 (1 ng/ml) for 24 or 48 h at 37°C in 5% CO2. Afterwards, the supernatants were recovered and kept at −20°C for cytokine evaluation, and cell viability was evaluated via a neutral red staining assay.
Cytokine Evaluation
Commercial ELISA kits were used according to the manufacturer's recommendations for immunological quantification of IL-6 and IL-8 (BD Biosciences, USA).
Statistical Evaluation
Statistical analysis was performed using the Mann-Whitney test to compare 2 groups of values at the same time or the Kruskal-Wallis test with Dunn's post hoc test. Analyses were performed using GraphPad Prism software (version 5; GraphPad Software, San Diego, Calif., USA). p < 0.05 was considered statistically significant.
Results
Evidence of Increased NETosis in HUS patients
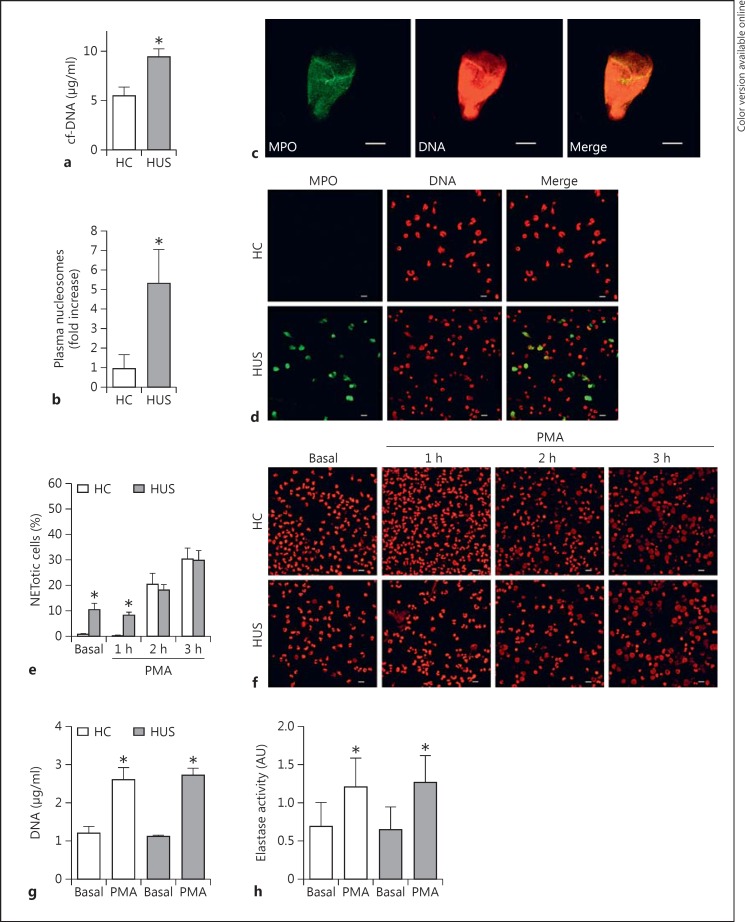
The concentration of cf-DNA was measured in plasma from HUS patients as a marker for NETosis as previously described [15]. As seen in figure 1a, plasma samples from HUS patients in the acute period showed significantly increased cf-DNA concentrations compared to plasma samples from HC. HUS samples also showed increased levels of circulating nucleosomes determined by a specific ELISA which comprises a capture antibody against an epitope shared by all histones and a detecting antibody against DNA (fig. 1b). To confirm that the quantified cf-DNA belonged to NET, we immobilized plasma samples on microscope slides and evaluated the presence of DNA and MPO by immunofluorescence and CLSM. We observed that MPO colocalized with DNA fibers (fig. 1c), supporting the concept that cf-DNA and nucleosomes detected in plasma came from PMN that underwent NETosis in vivo before blood samples were collected from the patients. Of note, DNA structures which colocalized with MPO were observed in plasma from HUS patients (n = 6) but not in HC plasma samples (n = 6), probably as a consequence of the low sensitivity of this method.
Fig. 1.
Evidence of NETosis in HUS patients. cf-DNA levels (a) and quantification of nucleosomes (b) in plasma from HUS patients (n = 16) and HC (n = 16). Results are expressed as means ± SEM. * p < 0.05 vs. HC (Mann-Whitney test). c Identification of NET in a plasma sample from an HUS patient (green: FITC-conjugated anti-MPO; red: PI). Scale bars = 10 μm. d Evaluation of the capacity of HUS PMN to undergo spontaneous NETosis. DNA was revealed with PI (red) and MPO with an FITC-conjugated specific antibody (green) in blood samples from both an HUS patient and an HC donor. Scale bars = 10 μm. Colors refer to the online version only. e Comparison of the kinetics of the NETosis process in HUS patients and HC. PMN (6 × 105 cells/0.3 ml) from HC or HUS patients were incubated in a Lab-Tek chambered coverglass in the absence (basal) or presence of PMA (50 ng/ml) for the indicated periods of time. Then, the cells were fixed and stained with PI (1 µg/ml), and NET formation was evaluated via CLSM. Viable PMN were identified by their lobulated nuclei, while the presence of decondensed DNA occupying the whole cell volume was used to identify NETotic cells. Results are depicted as the mean ± SEM of 5 samples of HC and HUS samples; * p < 0.05 vs. HC at the same time point (Mann-Whitney test). f Representative CLSM images from 1 independent experiment of 5 performed with isolated PMN from HC and HUS patients. Scale bars = 10 μm. DNA concentrations (g) and elastase activity (h) in the supernatants of culture of PMN isolated from HUS patients and HC, stimulated with PMA (50 ng/ml) for 4 h. Data are depicted as the mean ± SEM of experiments performed with 6 donors. * p < 0.05 vs. vehicle (Mann-Whitney test).
We then evaluated whether PMN from HUS patients exhibited differences in their capacity to undergo spontaneous NETosis. For this purpose, whole-blood leukocytes were cultured in Lab-Tek chambered coverglasses for 1 h without stimulation and the percentage of NETotic cells was determined by confocal microscopy. As shown in figure 1d, samples from HUS patients, in contrast to HC cells, exhibited spontaneous NETosis after 1 h of culture. Quantification of the images acquired indicated that 25 ± 3% of the leukocytes from HUS patients (n = 3) underwent NETosis, while only 0.3 ± 0.2% of the cells experienced this cell death in HC samples (n = 3) (percentages were calculated from at least 100 cells counted per donor, * p < 0.05, Mann-Whitney test). These findings indicate that PMN from HUS patients were more prone to undergoing spontaneous NETosis than PMN from HC, supporting the concept that the higher levels of cf-DNA and nucleosomes found in plasma from HUS patients could originate from in vivo occurring NETosis.
Considering the spontaneous NETosis observed in leukocytes from HUS patients, PMN isolated from both clinical groups were cultured in basal conditions or with PMA (the gold-standard nonphysiologic NET inducer) for different periods of time to evaluate whether they exhibited differences in the kinetics of stimulated NETosis. Then, the cells were stained with IP to allow quantification of PMN, showing changes compatible with NETosis (chromatin decondensation, disintegration of the nuclear envelope, and occupation of the whole volume of the cell by the nuclear material) [10, 20]. In agreement with the spontaneous NETosis observed in experiments performed with whole-blood leukocytes, HUS samples showed an increased percentage of NETotic PMN at early time points (1 h) in both spontaneous (basal) and PMA-stimulated conditions (fig. 1e, f). However, no differences were observed in the percentage of NETotic cells between HUS and HC PMN in either basal or stimulated conditions at later time points (fig. 1e, f). The evidence provided by microscopy was further confirmed when the concentration of DNA (fig. 1g) and the elastase activity (fig. 1h) were evaluated at the last time point (4 h) as surrogate markers for NET formation. Samples from both clinical groups showed similar levels of DNA and elastase activity in culture supernatants under basal and stimulated conditions.
Stx2 Induces NET Release
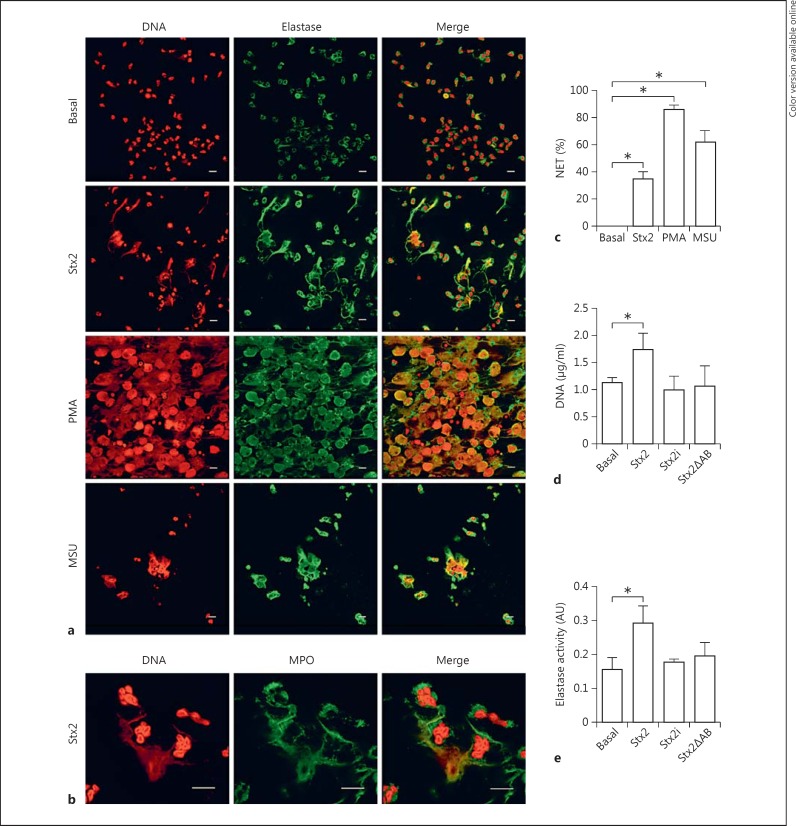
Since cf-DNA was detected in plasma from HUS patients and Stx2 is the main pathogenic factor of HUS, we evaluated whether Stx2 was able to induce PMN from healthy adult donors to undergo NETosis by fluorescence staining and CLSM. We determined that PMN, upon treatment with Stx2 for 4 h, extruded DNA which colocalized with elastase (fig. 2a) and MPO (fig. 2b). Additional stimuli such as PMA and MSU crystals were also assessed in parallel. PMA is the gold-standard stimulus for NET induction. On the other hand, it has been reported that children with HUS have elevated levels of serum uric acid [21], which is released from injured cells and might crystallize and form MSU crystals. Since it has been described that MSU crystals trigger NETosis [22], they could be potential NET inducers during HUS. Thus, we confirmed that PMN released NET in response to both PMA and MSU stimulation (fig. 2a, c).
Fig. 2.
Capacity of Stx2 to induce NETosis in PMN from HC. a PMN (600,000/300 μl) from HC were incubated with medium (basal), PMA, MSU, or Stx2 for 4 h. Then, NET formation was revealed by staining DNA with PI, and elastase (a) or MPO (b) with specific antibodies. The images, representatives of 1 experiment of 8 performed with PMN isolated from healthy donors, were acquired via CLSM. Scale bars = 10 μm. c Quantification of the percentage of NETotic cells analyzed by CLSM. Viable cells and NETotic cells were counted in 5 photographs (50 cells) from each treatment. Then, the NET percentage was calculated as the number of NET over the number of total cells. Data are presented as the mean percentage ± SEM of cells evidencing characteristics of NETotic cells (decondensed chromatin and disintegrated nuclei) in which DNA colocalized with elastase, over the total number of cells. At least 50 cells per condition were counted in each experiment (n = 5). * p < 0.05 (Kruskal-Wallis test followed by Dunn's post hoc test). Extracellular DNA concentration (d) and elastase activity (e) in supernatants of the culture of PMN (4 × 105 cells/ml) isolated from healthy donors incubated with medium (basal), Stx2, Stx2i, or Stx2ΔAB (0.1 μg/ml) for 4 h. Data are expressed as the mean ± SEM of 5 independent experiments. * p < 0.05 (Kruskal-Wallis test followed by Dunn's post hoc test).
To determine whether the capacity of Stx2 to induce NETosis depends on its enzymatic function, the effect of a mutant Stx2 devoid of its catalytic activity (Stx2ΔAB) was analyzed. We also evaluated the effect of Stx2i to confirm that the native structure of Stx2 is required to trigger NETosis. PMN were treated with these Stx2 preparations for 4 h, and then the DNA concentration and the elastase activity in the culture supernatants were quantified as readouts of the NET release. As expected, Stx2 induced a significant increase in the extracellular DNA concentration and elastase activity compared to the values determined in supernatants of unstimulated PMN. In contrast, when PMN were stimulated with Stx2i or Stx2ΔAB, an increase in DNA or elastase activity was not detected (fig. 2d, e). These results not only suggest that the specific activity of Stx2 was required to stimulate NETosis but also rule out that traces of LPS that might have remained in the Stx2 preparation were responsible for the effects ascribed to Stx2.
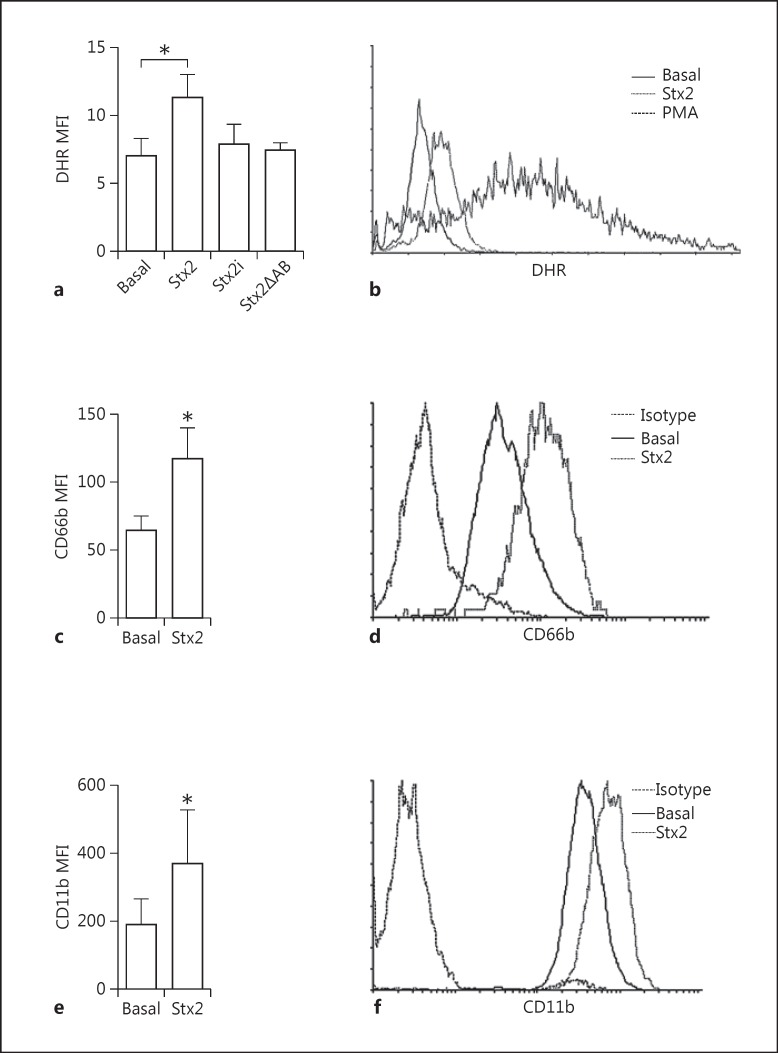
Stx2 Induces ROS Generation in PMN
It has been reported that the NETosis process requires NADPH oxidase activation. To gain insight into the mechanism via which Stx2 is able to induce NETosis, we evaluated the capacity of this toxin to induce ROS production. The response triggered by PMA, a strong NADPH oxidase inducer, was determined as a positive control. We observed that Stx2 induced ROS production, evaluated as the increase in DHR 123 mean fluorescence intensity by flow cytometry (fig. 3a, b). In contrast, when PMN were incubated with Stx2i or Stx2ΔAB, no change was observed compared to basal fluorescence (fig. 3a, b), in agreement with the incapacity of Stx2ΔAB and Stx2i to stimulate NETosis.
Fig. 3.
Stx2 directly induces the activation of PMN. ROS production was measured in PMN from healthy donors. a Results are expressed as the mean fluorescence intensity (MFI) of DHR after culture with medium (basal) or 0.1 µg/ml Stx2, Stx2i, or Stx2ΔAB for 15 min. Each bar represents the mean SEM of 5 experiments. * p < 0.05 (Kruskal-Wallis test followed by Dunn's post hoc test). b Representative histograms of DHR fluorescence of PMN stimulated or not with Stx2 or PMA as the positive control. c-f Stx2-dependent degranulation of PMN. Neutrophils were incubated with medium (basal) or Stx2 for 3 h. Then, the cells were washed and immunostained with specific antibodies anti-CD66b (c, d) and anti-CD11b (e, f) and the expression of these markers was determined by flow cytometry. Each bar represents the mean ± SEM of 5 experiments. * p < 0.05 vs. basal (Mann-Whitney test). Representative histograms of CD66b (d) and CD11b (f) expression in basal, Stx2, and isotype control conditions.
Stx2 Induces Upregulation of Activation Markers in PMN
To gain additional data in support of the concept that Stx2 is able to directly activate PMN, the cell surface expression of CD11b and CD66b was analyzed after incubation with Stx2. We observed a significant increase in the expression of both degranulation markers with respect to basal levels when PMN were cultured with Stx2 (fig. 3c, d, e, f). Taking together the ROS production and the degranulation of PMN, we conclude that Stx2 is capable of activating PMN.
NET Do Not Modulate the Cytotoxic Effects Exerted by Stx2
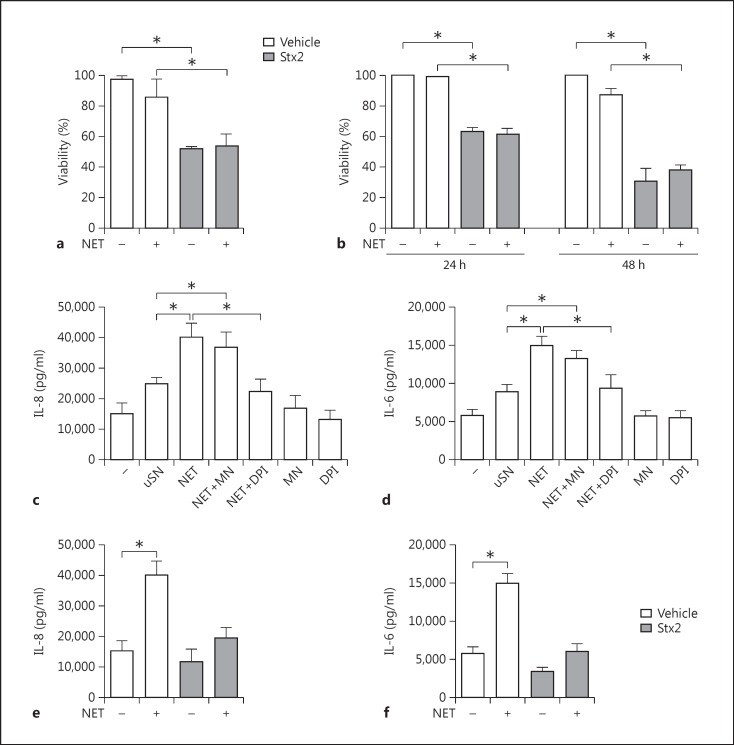
We performed experiments to gain insight on whether NET might contribute to the pathogenesis of HUS. For this purpose, we first investigated the impact of NET on both cell viability and the cytotoxic effect of Stx2 on Vero cells, a cell line sensitive to the toxic effects of this toxin [23]. In preliminary assays, we confirmed that Stx2, even at a concentration 100-fold lower than that used to trigger NETosis (0.7 ng/ml), induced a significant decrease in Vero cell viability, while MSU (300 µg/ml) did not (viability ± SEM: control, 100%; Stx2, 52.0 ± 3.9%, and MSU, 94.0 ± 3.2%). Thus, the following experiments were performed with NET induced by MSU to avoid the contribution of traces of Stx2 that could remain in NET preparations leading to artifact results. NET-containing supernatants were collected from MSU-stimulated PMN, seeded over Vero cell monolayers with or without Stx2, and cultured for 48 h. The cytotoxic effect of Stx2 in the absence of NET was evaluated in parallel. As expected, Stx2 reduced the cell viability compared to untreated cells (basal) (fig. 4a). In contrast, NET neither significantly affected cell viability per se nor modulated the cytotoxicity induced by Stx2 (fig. 4a).
Fig. 4.
Effects of NET on endothelial and epithelial cells. Neutrophils from HC were cultured in the absence (uSN) or presence of MSU crystals without (NET) or with MNase (NET+MN) or DPI (NET+DPI) to digest the DNA of NET or to inhibit NET formation, respectively, following the protocol described in Materials and Methods. After 4 h, the supernatants were recovered and added to Vero cells (a) or HGEC (b-f) treated with vehicle or Stx2 as indicated, and cellular viability after 24 (a, b) or 48 h (b) was evaluated. The concentrations of IL-8 (c, e) and IL-6 (d, f) in culture supernatants from HGEC incubated for 24 h with vehicle or Stx2 were determined by ELISA. Results are expressed as the mean ± SEM of 3 independent experiments. * p < 0.05 (Kruskal-Wallis test followed by Dunn's post hoc test).
Considering that the kidney is the main affected organ during HUS, we directly evaluated whether NET could be cytotoxic for primary HGEC or could modulate the cytotoxic effect induced by Stx2. HGEC underwent ∼40 and ∼70% cell death after 24 and 48 h of exposure to Stx2 (1 ng/ml), respectively (fig. 4b), in accordance with previous studies [19]. NET did not affect HGEC viability per se or that observed upon exposure to Stx2 (fig. 4b).
NET Induce Cytokine Secretion by Primary HGEC
Simultaneously, we also analyzed whether NET were able to promote inflammation by acting on primary HGEC. Again, these assays were performed with NET induced by MSU to avoid the contribution of traces of Stx2 that could remain in NET preparations if this inducer is employed. We evaluated IL-8 and IL-6 production in the supernatants of primary HGEC incubated with NET for 24 h. Our results indicated that NET significantly increased the secretion of IL-8 (fig. 4c) and IL-6 (fig. 4d) by HGEC. Cytokine production was not inhibited when NET were pretreated with MNase to degrade their DNA content, meaning that HGEC activation by NET could not be attributed to DNA itself but is instead probably due to its associated proteins (fig. 4c, d). To confirm that NET were responsible for the stimulation of cytokine secretion by HGEC, PMN were stimulated in parallel with MSU in the presence of DPI (NET+DPI), an NADPH oxidase inhibitor that has been shown to potently inhibit NETosis. Primary HGEC incubated with the supernatants coming from PMN under this treatment (NET+DPI) released cytokine levels similar to those produced by HGEC incubated with supernatants from unstimulated PMN (fig. 4c, d). On the other hand, neither MNase nor DPI affected the cytokine production by HGEC per se. We corroborated that DPI effectively inhibited NET release and MNase induced their degradation by determining DNA concentrations (mean ± SEM) via fluorometry (unstimulated PMN, 0.13 ± 0.08 µg/ml; NET, 0.55 ± 0.13* µg/ml; NET+MN, 0.07 ± 0.05 µg/ml, and NET+DPI, 0.16 ± 0.09 µg/ml; *p < 0.05; n = 5, Kruskal-Wallis test followed by Dunn's post hoc test). Together, these results suggest that NET stimulate primary HGEC cytokine secretion. As expected, when HGEC were cultured simultaneously with NET and Stx2, the cytotoxic dose of Stx2 counteracted the cytokine induction provoked by NET (fig. 4e, f).
Discussion
Here, we demonstrated an increase in both cf-DNA and nucleosomes in plasma from HUS patients in the acute period. Interestingly, we observed cotton-like structures of cf-DNA that colocalized with MPO in plasma from HUS patients, supporting the concept that the observed cf-DNA is part of NET structures and was not due to DNA released by necrotic or apoptotic cells. We also found that PMN from HUS patients were more prone to undergoing spontaneous NETosis than HC cells, strengthening the evidence in support of the concept that the higher levels of cf-DNA and nucleosomes found in plasma from HUS patients were originated by NETosis. Under normal conditions, plasma DNA is likely to be cleaved by endogenous DNase1, but this enzyme cannot compensate the overload when high local concentrations of NET are produced [24]. In line with these results, increased levels of cf-DNA have been reported in plasma from patients suffering from different inflammatory or infectious diseases [15, 25, 26].
Neutrophilia is a typical finding in patients with HUS, and a high peripheral blood PMN count at presentation is the poor-prognosis factor most consistently reported in this disease [27, 28]. Furthermore, the degree of renal impairment has been correlated with the level of activation of circulating PMN [5, 29]. Our findings indicating that PMN from HUS patients are able to undergo spontaneous NETosis and the proinflammatory capacity of NET on HGEC are in line with these findings.
Recently, it was demonstrated that Stx2 induces an oxidative imbalance in vivo, and that ROS production by PMN may be one of the major sources of oxidative stress during Stx intoxication contributing to the amplification of microvascular injury in the kidney [30]. In this work, we also confirmed previous data indicating that Stx2 induces neutrophil ROS production [31] and demonstrated that Stx2 is also able to induce NETosis in PMN from healthy adult donors. The colocalization of DNA and elastase in structures released upon PMN treatment with Stx2 confirmed that NETosis is the mechanism of death at least in a percentage of PMN after interaction with the toxin. In agreement with these in vitro experiments, NET-associated elastase could have contributed to the high levels of elastase reported in plasma from HUS patients [32].
The capacity of Stx2 to induce NETosis was lost when either an A chain-deleted Stx2 mutant (Stx2ΔAB) or a heat-inactivated wild-type toxin was assayed, indicating that a structurally and functionally conserved Stx2 is necessary to activate this death mechanism. These findings were in agreement with the requirement of fully active Stx2 to induce the oxidative burst in PMN [31], and with previous reports indicating that the Stx-PMN interaction is mediated through the A chain of Stx [6, 33]. Finally, the upregulation of CD11b and CD66b in PMN incubated with Stx2 supports the capacity of Stx2 to directly activate these cells in culture. In agreement with this, Brigotti et al. [8] also reported that Stx1 induces a significant increase in the expression of CD66b in human PMN. The expression of a specific receptor for Stx on human PMN is a controversial issue. In this regard, both the binding [33] and the functional alterations of PMN after interaction with the toxin [34, 35] have been demonstrated in vitro. Furthermore, some authors have proposed that PMN might facilitate the systemic absorption of Stx2, which in turn would increase the risk of developing HUS [35]. In contrast, other studies have failed to detect direct binding of Stx2 to resting human PMN [36, 37, 38] or Stx2 bound to PMN in HUS patients [31, 33, 34]. However, our findings indicating that Stx2 increases the expression of activation markers, stimulates ROS production, and triggers NETosis support the notion that this toxin is able to directly activate PMN in culture.
Whether NET are beneficial or detrimental during STEC infection is difficult to predict because this probably depends on the pathophysiological context. While in the gut NET could inactivate STEC as has been reported during other intestinal infections [9], the biological activity of NET in the periphery when Stx reaches the systemic circulation and tissues is a matter of speculation. Although STEC are restricted to the luminal side of the intestine, NET might also be released from PMN in response to Stx2 and other inducers in blood vessels and tissues. Although we found that NET did not increase the endothelial or epithelial damage triggered by Stx2, they significantly induced the secretion of proinflammatory cytokines by primary HGEC. In our in vitro model, the cytotoxic impact of Stx2 overcame the cytokine-inducing effect of NET when HGEC was stimulated simultaneously with NET and Stx2. However, it should be considered that in vivo not all endothelial cells are under the protein inhibitory effect of Stx2. In fact, histological studies have shown that Stx induces focal lesions in glomeruli during HUS, and IL-6 and IL-8 released in response to NET may increase the recruitment and activation of PMN, contributing to local inflammation and organ dysfunction. Previous studies have also proposed that local NET may contribute to thrombosis by providing scaffolding for platelet adhesion and fibrin deposition [14]. In contrast to the ability of DNase1 to neutralize the procoagulant activity of cf-DNA [39], MNase was unable to counteract the capacity of NET to induce IL-6 and IL-8 secretion by HGEC. These findings suggest that, even though intact fibers may be required for thrombotic signals, DNA fragments or their associated proteins would be enough to induce cytokine secretion by endothelial cells.
Previous studies have reported the presence of NET in the glomeruli of patients with lupus nephritis [24] and thrombotic microangiopathy of different etiologies [26]. Moreover, elevation of the NET concentration in these patients is considered a significant risk factor, suggesting that NET participate in the pathogenesis of renal vascular damage [40]. Similarly, NET formation has been linked to tissue injury in small vessels in vasculitis [13] and organ dysfunction during sepsis [41]. Although in our studies NET were not cytotoxic for HGEC, they could indirectly contribute to tissue damage by recruiting and activating inflammatory cells. However, we cannot rule out that during HUS development, NET locally produced in the renal microvasculature environment also contribute to Stx2-endothelial and/or epithelial damage considering the complexity of the inflammatory signals present in the renal microenvironment that could sensitize the endothelium to Stx2/NET components.
Available evidence, together with our data indicating the presence of NET in plasma from HUS patients and the ability of Stx2 to induce NETosis, suggests that NET could participate in processes such as thrombosis, leukocyte activation, microvascular obstruction, and renal failure during the evolution of this disease.
In spite of the direct capacity of Stx2 to induce NETosis in PMN, it is not possible to rule out that in vivo other mediators are responsible for this phenomenon. In fact, patients with HUS also have elevated plasmatic levels of known effectors of NETosis, such as IL-8 and TNF-α [12] and uric acid, which could crystallize forming MSU [21].
Our present data contribute to shedding light on the role of PMN in the pathogenesis of STEC infection. These findings add to others that suggest that intervention strategies to inhibit PMN-mediated tissue damage will likely have a potential therapeutic impact towards amelioration of the severity of HUS.
Disclosure Statement
The authors declare that they have no conflict of interests.
Acknowledgements
The authors thank Federico Fuentes for his excellent technical assistance. This study was supported by research funding from the Agencia Nacional de Promoción Científica y Tecnológica of Argentina (M.V.R., M.S.P., and A.S.T.) and CONICET (M.V.R.).
References
- 1.Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;3:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donohue-Rolfe A, Acheson DW, Keusch GT. Shiga toxin: purification, structure, and function. Rev Infect Dis. 1991;13:S293–S297. doi: 10.1093/clinids/13.supplement_4.s293. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez GC, Lopez MF, Gomez SA, Ramos MV, Bentancor LV, Fernandez-Brando RJ, Landoni VI, Dran GI, Meiss R, Isturiz MA, Palermo MS. Relevance of neutrophils in the murine model of haemolytic uraemic syndrome: mechanisms involved in Shiga toxin type 2-induced neutrophilia. Clin Exp Immunol. 2006;1:76–84. doi: 10.1111/j.1365-2249.2006.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milford DV, Staten J, MacGreggor I, Dawes J, Taylor CM, Hill FG. Prognostic markers in diarrhoea-associated haemolytic-uraemic syndrome: initial neutrophil count, human neutrophil elastase and von Willebrand factor antigen. Nephrol Dial Transplant. 1991;4:232–237. doi: 10.1093/ndt/6.4.232. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez GC, Gomez SA, Rubel CJ, Bentancor LV, Barrionuevo P, Alduncin M, Grimoldi I, Exeni R, Isturiz MA, Palermo MS. Impaired neutrophils in children with the typical form of hemolytic uremic syndrome. Pediatr Nephrol. 2005;9:1306–1314. doi: 10.1007/s00467-005-1906-9. [DOI] [PubMed] [Google Scholar]
- 6.Arfilli V, Carnicelli D, Rocchi L, Ricci F, Pagliaro P, Tazzari PL, Brigotti M. Shiga toxin 1 and ricin A chain bind to human polymorphonuclear leucocytes through a common receptor. Biochem J. 2010;1:173–180. doi: 10.1042/BJ20100455. [DOI] [PubMed] [Google Scholar]
- 7.Brigotti M, Carnicelli D, Arfilli V, Tamassia N, Borsetti F, Fabbri E, Tazzari PL, Ricci F, Pagliaro P, Spisni E, Cassatella MA. Identification of TLR4 as the receptor that recognizes Shiga toxins in human neutrophils. J Immunol. 2013;9:4748–4758. doi: 10.4049/jimmunol.1300122. [DOI] [PubMed] [Google Scholar]
- 8.Brigotti M, Tazzari PL, Ravanelli E, Carnicelli D, Barbieri S, Rocchi L, Arfilli V, Scavia G, Ricci F, Bontadini A, Alfieri RR, Petronini PG, Pecoraro C, Tozzi AE, Caprioli A. Endothelial damage induced by Shiga toxins delivered by neutrophils during transmigration. J Leukoc Biol. 2010;1:201–210. doi: 10.1189/jlb.0709475. [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;5663:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;2:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;4:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 12.Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs) - formation and implications. Acta Biochim Pol. 2013;3:277–284. [PubMed] [Google Scholar]
- 13.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;6:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;36:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margraf S, Logters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;4:352–358. doi: 10.1097/SHK.0b013e31816a6bb1. [DOI] [PubMed] [Google Scholar]
- 16.Bentancor LV, Bilen M, Brando RJ, Ramos MV, Ferreira LC, Ghiringhelli PD, Palermo MS. A DNA vaccine encoding the enterohemorragic Escherichia coli Shiga-like toxin 2 A2 and B subunits confers protective immunity to Shiga toxin challenge in the murine model. Clinical Vaccine Immunol. 2009;5:712–718. doi: 10.1128/CVI.00328-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyum A. Separation of leukocytes from blood and bone marrow (introduction) Scand J Clin Lab Invest Suppl. 1968;97:7. [PubMed] [Google Scholar]
- 18.McGinn S, Poronnik P, Gallery ED, Pollock CA. A method for the isolation of glomerular and tubulointerstitial endothelial cells and a comparison of characteristics with the human umbilical vein endothelial cell model. Nephrology (Carlton) 2004;4:229–237. doi: 10.1111/j.1440-1797.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 19.Amaral MM, Sacerdoti F, Jancic C, Repetto HA, Paton AW, Paton JC, Ibarra C. Action of shiga toxin type-2 and subtilase cytotoxin on human microvascular endothelial cells. PLoS One. 2013;7:e70431. doi: 10.1371/journal.pone.0070431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkmann V, Goosmann C, Kuhn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Frontiers in Immunology. 2013;3:413. doi: 10.3389/fimmu.2012.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balestracci A, Martin SM, Toledo I. Hyperuricemia in children with post-diarrheal hemolytic uremic syndrome. Pediatr Nephrol. 2012;8:1421–1422. doi: 10.1007/s00467-012-2181-1. [DOI] [PubMed] [Google Scholar]
- 22.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;12:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kongmuang U, Honda T, Miwatani T. Isolation of Shiga toxin-resistant Vero cells and their use for easy identification of the toxin. Infect Immun. 1988;9:2491–2494. doi: 10.1128/iai.56.9.2491-2494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;21:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng W, Paunel-Gorgulu A, Flohe S, Witte I, Schadel-Hopfner M, Windolf J, Logters TT. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm. 2012;2012:149560. doi: 10.1155/2012/149560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lammle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;6:1157–1164. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coad NA, Marshall T, Rowe B, Taylor CM. Changes in the postenteropathic form of the hemolytic uremic syndrome in children. Clin Nephrol. 1991;1:10–16. [PubMed] [Google Scholar]
- 28.Walters MD, Matthei IU, Kay R, Dillon MJ, Barratt TM. The polymorphonuclear leucocyte count in childhood haemolytic uraemic syndrome. Pediatr Nephrol. 1989;2:130–134. doi: 10.1007/BF00852893. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez GC, Gomez SA, Ramos MV, Bentancor LV, Fernandez-Brando RJ, Landoni VI, Lopez L, Ramirez F, Diaz M, Alduncin M, Grimoldi I, Exeni R, Isturiz MA, Palermo MS. The functional state of neutrophils correlates with the severity of renal dysfunction in children with hemolytic uremic syndrome. Pediatr Res. 2007;1:123–128. doi: 10.1203/01.pdr.0000250037.47169.55. [DOI] [PubMed] [Google Scholar]
- 30.Gomez SA, Abrey-Recalde MJ, Panek CA, Ferrarotti NF, Repetto MG, Mejias MP, Fernandez GC, Vanzulli S, Isturiz MA, Palermo MS. The oxidative stress induced in vivo by Shiga toxin-2 contributes to the pathogenicity of haemolytic uraemic syndrome. Clin Exp Immunol. 2013;3:463–472. doi: 10.1111/cei.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King AJ, Sundaram S, Cendoroglo M, Acheson DW, Keusch GT. Shiga toxin induces superoxide production in polymorphonuclear cells with subsequent impairment of phagocytosis and responsiveness to phorbol esters. J Infect Dis. 1999;2:503–507. doi: 10.1086/314579. [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick MM, Shah V, Filler G, Dillon MJ, Barratt TM. Neutrophil activation in the haemolytic uraemic syndrome: free and complexed elastase in plasma. Pediatr Nephrol. 1992;1:50–53. doi: 10.1007/BF00856833. [DOI] [PubMed] [Google Scholar]
- 33.Griener TP, Mulvey GL, Marcato P, Armstrong GD. Differential binding of Shiga toxin 2 to human and murine neutrophils. J Med Microbiol. 2007;11:1423–1430. doi: 10.1099/jmm.0.47282-0. [DOI] [PubMed] [Google Scholar]
- 34.Brigotti M, Carnicelli D, Ravanelli E, Barbieri S, Ricci F, Bontadini A, Tozzi AE, Scavia G, Caprioli A, Tazzari PL. Interactions between Shiga toxins and human polymorphonuclear leukocytes. J Leukoc Biol. 2008;4:1019–1027. doi: 10.1189/jlb.0308157. [DOI] [PubMed] [Google Scholar]
- 35.Brigotti M. The interactions of human neutrophils with shiga toxins and related plant toxins: danger or safety? Toxins. 2012;3:157–190. doi: 10.3390/toxins4030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geelen JM, van der Velden TJ, Te Loo DM, Boerman OC, van den Heuvel LP, Monnens LA. Lack of specific binding of Shiga-like toxin (verocytotoxin) and non-specific interaction of Shiga-like toxin 2 antibody with human polymorphonuclear leucocytes. Nephrol Dial Transplant. 2007;3:749–755. doi: 10.1093/ndt/gfl688. [DOI] [PubMed] [Google Scholar]
- 37.Holle JU, Williams JM, Harper L, Savage CO, Taylor CM. Effect of verocytotoxins (Shiga-like toxins) on human neutrophils in vitro. Pediatr Nephrol. 2005;9:1237–1244. doi: 10.1007/s00467-005-1945-2. [DOI] [PubMed] [Google Scholar]
- 38.Flagler MJ, Strasser JE, Chalk CL, Weiss AA. Comparative analysis of the abilities of Shiga toxins 1 and 2 to bind to and influence neutrophil apoptosis. Infect Immun. 2007;2:760–765. doi: 10.1128/IAI.01594-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Gunther A, Engelmann B, Preissner KT. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci USA. 2007;15:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arai Y, Yamashita K, Mizugishi K, Watanabe T, Sakamoto S, Kitano T, Kondo T, Kawabata H, Kadowaki N, Takaori-Kondo A. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;12:1683–1689. doi: 10.1016/j.bbmt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;3:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]