Abstract
Glucocorticoid-induced leucine zipper (GILZ) is a potent anti-inflammatory protein, the expression of which is mainly induced by glucocorticoids (GCs) in haematopoietic cells. GILZ regulates signal transduction pathways of inflammation and plays a role in cell survival. The objective of this study was to evaluate the expression and mechanisms of action of GILZ in the apoptosis of human neutrophils. GILZ expression was induced by GCs in human neutrophils, enhanced upon phosphatidylinositol 3-kinase inhibition and resulted in apoptosis amplification. We then stably transfected PLB-985 cells with the human gilz gene and differentiated both control and GILZ-overexpressing clones in neutrophil-like cells. GILZ overexpression in PLB-985 cells led to an exacerbated apoptosis, associated with caspase-3, caspase-9 and caspase-8 activations, and a loss of mitochondrial potential, suggesting that GILZ-induced apoptosis used the mitochondrial pathway. The expression of BH3 interacting domain death agonist, Bcl-2 interacting mediator of cell death, annexin-A1 and Bcl-2-associated X was not affected in PLB-985-GILZ clones, but phosphorylation and subsequent proteasomal degradation of myeloid cell leukemia-1 (Mcl-1) were observed. Noteworthy, Mcl-1 phosphorylation was related to a significant and sustained activation of c-Jun N-terminal kinase (JNK) in PLB-985-GILZ clones. These results reveal GILZ to be a new actor in apoptosis regulation in neutrophil-like cells involving JNK and Mcl-1.
Key Words: Glucocorticoid-induced leucine zipper, Apoptosis, Neutrophils, Myeloid cell leukemia-1, c-Jun N-terminal kinase, Proteasome
Introduction
Neutrophils, the most abundant leukocytes in the circulation, are terminally differentiated cells devoid of any proliferative capacities [1]. Despite their very short life span, they meticulously control the balance between their death and survival [2, 3]. In the absence of any danger signal (infection, cell necrosis, expression of pro-inflammatory cytokines, etc.), they spontaneously die by apoptosis, comparable to growth factor withdrawal [4]. Indeed, in physiological conditions, amounts of anti-apoptotic proteins, like myeloid cell leukemia-1 (Mcl-1) and proliferating cell nuclear antigen protein (PCNA), decrease in the neutrophils, triggering apoptosis [3]. However, at the inflammation site, neutrophils become activated and the level of intracellular or extracellular reactive oxygen species generated upon NADPH oxidase activation could participate in the control of the decision between necrosis, apoptosis or cell survival [4]. Mcl-1 is unique among Bcl-2 family members in that it is essential for the survival of several cell lineages, particularly neutrophils, as has been demonstrated in vitro but also in vivo [5]. Indeed, Mcl-1 conditional knockout (KO) mice show a severely impaired survival of mature neutrophils in the periphery [6]. Mcl-1 expression is regulated at transcriptional, post-transcriptional and post-translational levels, affecting its stability and functions [5]. Notably, phosphorylation by the coordinated activity of c-Jun N-terminal kinase (JNK) and glycogen synthase kinase 3 (GSK3) mediates the proteasomal degradation of Mcl-1 and the rapid triggering of cell death [7].
Glucocorticoid-induced leucine zipper (GILZ), an anti-inflammatory and immunomodulatory protein belonging to the transforming growth factor-β stimulating clone 22 domain (TSC-22D) family, has been shown to be rapidly induced upon treatment with glucocorticoids (GCs), particularly in haematopoietic cells [8]. Initially, the anti-inflammatory and immunomodulatory effects of GILZ were extensively described in vitro and were mostly related to the inhibition of activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κB) transcription factors [8]. More recently, inhibition of colitis development was observed in vivo using TAT-GILZ fusion protein delivery in dinitrobenzene sulfonic acid-treated IL-10 KO mice, demonstrating in vivo that GILZ could present therapeutic potential [8]. In addition, GILZ plays a pivotal role in controlling cell survival and apoptosis. Indeed, GILZ inhibits activation-induced cell death (AICD) in 3D0 hybridoma T cells [8]. Furthermore, our group demonstrated that in T lymphocytes, GILZ prevents IL-2 deprivation-mediated apoptosis through forkhead box O3 (FOXO3) inhibition and consequent Bcl-2 interacting mediator of cell death (Bim) down-regulation [9]. On the contrary, GILZ induces apoptosis in some cell types such as thymocytes, involving a down-regulation of Bcl-xL expression and caspase-8 and caspase-3 activations [10]. More recently, GILZ was shown to promote apoptosis in chronic myeloid leukemia cells expressing BCR-ABL oncoprotein. In this model, GILZ specifically binds to mTORC2, leading to the inhibition of AKT phosphorylation, FOXO3 transcriptional activation and Bim expression [11]. Altogether, these results highlight that GILZ regulations and functions are particularly dependent on the cell types. Our aim was first to document GILZ expression in neutrophils, and second to evaluate its role in apoptosis.
In this study, we observed the induction of GILZ expression in human blood neutrophils, which could promote apoptosis of these cells. To specifically address GILZ functions in this setting, we used the human promyelocytic leukemia PLB-985 cell line stably transfected with the human gilz gene and differentiated into neutrophil-like cells. We found that GILZ overexpression led to an exacerbated apoptosis, involving the mitochondrial pathway and associated with a sustained activation of JNK and the down-regulation of Mcl-1.
Materials and Methods
Chemicals and Reagents
All-trans retinoic acid (ATRA), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), 3,3′-dihyloxacarbocyanine iodide (DiOC6), the JNK inhibitor SP600125 and the GSK3 inhibitor SB216763 were obtained from Sigma-Aldrich (Lyon, France). Antibodies for flow cytometry (CD11b and mouse IgG1), the BD kit Cytofix/Cytoperm™ and annexin V/7AAD were obtained from BD Biosciences (San José, Calif., USA). Fluorescein-conjugated zymosan A was obtained from Invitrogen (Cergy-Pontoise, France). N,N-dimethylformamide (DMF) was obtained from Carbo Erba (Rodano, Italy). The pan-caspase inhibitor Q-VD-OPh was from Biovision (Mountain View, Calif., USA). HBSS was obtained from Gibco Life Technologies (Saint Aubin, France) and the proteasome inhibitor MG-262 from Merck-Millipore (Nottingham, UK). Dexamethasone (DEX) was purchased from Sigma-Aldrich (St Louis, Mo., USA). LY294002 was purchased from Calbiochem.
Neutrophil Isolation
Neutrophils were isolated from healthy donors' peripheral blood provided by the Etablissement Français du Sang (Rungis, France). Whole-blood centrifugation (20 min at 690 g and 20°C) on lymphocyte separation medium (Eurobio, Les Ulis, France) was used to separate PBMC (supernatant) from neutrophils and erythrocytes (pellet). Neutrophils were then isolated by pellet sedimentation on 5% dextran T500 (Pharmacia, Uppsala, Sweden) in 0.9% saline at a ratio of 4:1. Contaminating erythrocytes were removed by hypotonic lysis, and neutrophils (consistently >95% pure) were resuspended in RPMI-1640 medium, containing 0.1 mg/ml streptomycin, 100 U/ml penicillin, 1% sodium pyruvate (Fisher Scientific, Illkirch, France) and 10% foetal calf serum (PAA, Les Mureaux, France).
PLB-985 Culture and Differentiation
The human myeloid leukemia cell line PLB-985 was maintained in RPMI-1640 medium, containing 0.1 mg/ml streptomycin, 100 U/ml penicillin, 1% sodium pyruvate (Fisher Scientific, Illkirch, France) and 10% fetal calf serum (PAA). Cells were maintained at a density of between 0.1 and 1 × 106/ml. For granulocytic differentiation, exponentially growing cells at a starting density of 3 × 105/ml were cultured in RPMI-1640 supplemented with 0.5% DMF (Carbo Erba), 1 µM ATRA, 1% sodium pyruvate and 5% foetal calf serum. The medium was changed once on day 3 during the 5 days differentiation period and cells were adjusted to 1 × 106/ml [12]. On day 5, granulocytic differentiation was assessed by the measure of CD11b cell surface expression and hydrogen peroxide (H2O2) production as described below. Cell viability was assessed before experiments by trypan blue exclusion and was routinely >95%.
Plasmid Constructs and Transfection
pcDNA3-Myc and pcDNA3-Myc-GILZ constructs were described previously [9]. PLB-985 cells were transfected with pcDNA3-Myc or pcDNA3-Myc-GILZ using an AMAXA system, program U015 in dendritic cells buffer (Amaxa, La Ferte Mace, France). Four million cells were transfected with 4 µg of plasmid. A selection of stably transfected cells was initiated 48 h after electroporation using 1.2 mg/ml G418 (Fisher Scientific). Cells were then cloned by limiting dilution.
Phenotypic Analysis of Differentiated PLB-985 Cells
3 × 105 differentiated PLB-985 cells were washed once in PBS and incubated in PBS containing 0.05% BSA for 30 min at 4°C with anti-CD11b-PE (phycoerythrin) and appropriate antibody isotypes, i.e. mouse IgG1 (both from BD Biosciences). Cells were then washed twice in PBS 0.05% BSA, once in PBS and fixed in PBS + 1% formaldehyde. For each sample, 10,000 cells were acquired from a gated homogenous cell population using a FACSCalibur flow cytometer (BD Biosciences) and results were analyzed using the CellQuest software. Results were expressed as the mean fluorescence intensity (MFI) of cells labeled with antigen-specific antibody divided by the MFI of isotype labelled cells.
Hydrogen Peroxide Production
As previously described [9], H2O2 production of differentiated PLB-985 cells (106/ml in HBSS) was measured by flow cytometry after preincubation with 1.25 µM DCFH-DA and stimulation for 15 min at 37°C with 50 ng/ml PMA (both from Sigma-Aldrich). A stimulation index (termed ‘fold induction’) was calculated as the MFI ratio of PMA-treated cells versus unstimulated cells.
Phagocytosis Assay
Fluorescein-conjugated zymosan A (Invitrogen) particles were opsonised by incubation for 1 h at 37°C in pooled and decomplemented human sera diluted by half with PBS. Particles were then washed 3 times in PBS. Differentiated PLB-985 cells (5 × 106/ml) were then incubated for 30 min at 37°C with the particles. The ratio of 10 particles per cell was found optimal. Synchronization of the interaction between cells and particles was achieved by rapidly sedimenting the cells together with zymosan particles. Phagocytosis was stopped by adding cold PBS and trypan blue (1.2 mg/ml final concentration) to quench the fluorescence of the non-ingested particles that remain associated with the cells. Cells were also incubated with zymosan particles at 4°C as a negative control. The percentage of phagocytic cells was determined using a FACSCalibur flow cytometer.
Apoptosis Measurements
Measurement of the Mitochondrial Trans-Membrane Potential
The mitochondrial transmembrane potential (ΔΨm) was measured by a flow cytometric method using the lipophilic cationic fluorescent dye DiOC6. Differentiated PLB-985 cells (1 × 106/ml in culture medium) were loaded with 100 nM DiOC6 for 1 h at 37°C and then washed 3 times in PBS before fluorescence measurement by flow cytometry. The loss of mitochondrial transmembrane potential was expressed as the percentage of cells with a low DiOC6 fluorescence intensity. Fluorescence was measured by flow cytometry using a FACSCAlibur device. Results were expressed as the percentage of low-fluorescence cells.
Caspase-3 Activity Assay
Differentiated PLB-985 cells were fixed and permeabilized using the BD kit Cytofix/Cytoperm™ according to the manufacturer's protocol (BD Biosciences). Cells were incubated with 2 µl of active caspase-3 antibody (BD Biosciences) for 30 min at room temperature. After 2 washings in PermWash buffer, fluorescence was measured by flow cytometry. Results were expressed as the percentage of positive cells.
Caspase-8 Activity Assay
At day 5 and 7 of differentiation, PLB-985 cells (1 × 106/ml in culture medium) were incubated with 1 µl of FITC-IETD-FMK at 37°C (CaspGLOW fluorescein active caspase-8 kit, eBioscience, Paris, France). They were then washed 3 times in washing buffer before the measurement of fluorescence by flow cytometry. Results were expressed as the percentage of positive cells.
Annexin-V Externalization and DNA Hypodiploidy
Apoptosis was determined using flow cytometry by quantification of DNA hypodiploidy (sub-G1 peak) as previously described [9], and by quantification of annexin-V-positive cells. Harvested cells were incubated with annexin-V-PE and 7-aminoactinomycin D (7-AAD) according to the manufacturer's protocol (BD Biosciences). Data acquisition was performed using the CellQuest software (BD Biosciences).
Immunoblotting
Fresh-blood neutrophils (between 2 and 5 million cells) were lysed in 40 µl of cold Laemmi buffer (5% Tris pH 6.8 1.25 M, 10% glycerol, 10% SDS, 1 mM PMSF, 1 mM Na3VO4, 25 mM β-glycerophosphate, 10 µg/ml aprotinin, 10 µg/ml leupeptin and 10 µg/ml pepstatin), boiled and sonicated. PLB-985 cells (106 cells/ml) were harvested and washed in cold PBS before lysis in 40 µl of lysis buffer (20 mM Tris pH 7.4, 137 mM NaCl, 2 mM EDTA pH 7.4, 2 mM sodium pyrophosphate, 1% Triton, 10% glycerol, 1 mM PMSF, 1 mM Na3VO4, 25 mM β-glycerophosphate, 10 µg/ml aprotinin, 10 µg/ml leupeptin and 10 µg/ml pepstatin) and centrifuged at 17,600 g for 20 min at 4°C, before the supernatants were collected. Equal amounts of denatured proteins (50 µg) were loaded onto 15% SDS-PAGE gel and then transferred onto PVDF membranes which were incubated with antibodies raised against Mcl-1 (Santa Cruz Biotechnology, Heidelberg, Germany, sc-819), cleaved caspase-3 (Cell Signaling Technology, Danvers, Mass., USA, ASP175), caspase-9 (Cell Signaling Technology, No. 9502), Bim (Calbiochem, 22–40), BH3 interacting domain death agonist (Bid; Cell Signaling Technology, No. 2002), annexin-A1 (BD Biosciences, clone 29/annexin I), Bcl-2-associated X (Bax; Santa Cruz Biotechnology, clone N20), GILZ [9], MKP-1 (Santa Cruz Biotechnology, clone M18), phospho-JNK (Cell Signaling Technology, CST9251) and phospho Mcl-1 (Cell Signaling Technology, No. 4579). Membranes were then stripped, and reprobed with an antibody against β-tubulin (Sigma-Aldrich, clone TUB 2.1) or JNK (Cell Signaling Technology, CST9252), as a loading control. Immunoreactive bands were detected by chemiluminescence using a ChemiDoc XRS+ system (Bio-Rad, Marnes la Coquette, France). Bands were quantified using ImageLab software. Results were expressed as the ratio of the protein of interest to β-tubulin or JNK.
Real-Time PCR Analysis
PLB-985 cells were frozen at different times along differentiation in RNA Plus™ (MP Biomedicals). Total RNA was isolated according to the manufacturer's protocol. Reverse transcription and real-time PCR were conducted as previously described [13] using the SYBR Green technology on a Bio-Rad CFX96 system. Briefly, we used SsoAdvanced EvaGreen supermix (Bio-Rad) and primers for mcl-1 [5′-TAAGGACAAAACGGGACTGG-3′ and 5′-ACCAGCTCCTACTCCAGCAA-3′], gapdh [5′-CAGCCTCAAGATCATCAG CA-3′ and 5′-TGTGGTCATGAGTCCTTCCA-3′] and beta-2-microglobulin (b2m) [5′-ACCCCCACTGAAAA AGATGA-3′ and 5′-ATCTTCAAACCTCCATGATG-3′). The quantification was performed with the Bio-Rad CFX Manager software, and data were analyzed using the ΔΔCt method. Ratio was calculated as the geometrical mean of (1 + E)-ΔΔCt values, where E is the efficiency and ΔΔCt is the target gene expression of treated cells compared with normal levels in untreated cells and corrected using the expression of the reference genes gapdh and b2m. Results were expressed as variation compared to wild-type (WT) cells, i.e. the ratio of (1 + E)−ΔΔCt of clone cells/(1 + E)−ΔΔCt of WT cells.
Statistical Analysis
Experiments were performed at least 3 times and results were presented as mean ± standard error of the mean (SEM). The Mann-Whitney U test was used to compare pairs of means (generally ‘Myc’ group versus ‘GILZ’ group). Results were considered significant when p < 0.05. Statistical analyses were performed with GraphPad Prism software (San Diego, Calif., USA).
Results
GILZ Is Expressed in Human Blood Neutrophils
GCs are among the most widely used treatments for inflammatory diseases and are also the best characterized inducers of GILZ in hematopoietic cells such as thymocytes, peripheral T cells [8, 14] or dendritic cells [15]. In freshly isolated human neutrophils treated for 12 h with increasing concentrations (10-9, 10-8, 10-7M) of DEX, gilz expression was induced in a dose-dependent manner at the mRNA (fig. 1a) and protein levels (fig. 1b, d). Moreover, the proteasome inhibitor MG262 caused a marked accumulation of GILZ in the absence of DEX, and more substantially in the presence of DEX (fig. 1b), suggesting that GILZ could be subject to proteasomal degradation.
Fig. 1.
Expression of GILZ in human neutrophils. a Relative expression of gilz in peripheral blood neutrophils in the presence of increasing concentrations of DEX. Cells were isolated and treated with DEX (10-9, 10-8, 10-7M). gilz mRNA expression was measured using qRT-PCR. The results represent the means ± SEM of 10 independent experiments. b Expression of GILZ in peripheral blood neutrophils in the presence of GCs and/or of the proteasome inhibitor MG262. Cells were isolated and treated for 12 h with DEX (10-7M) and/or MG-262 (1 µM). Representative Western blot using anti-GILZ polyclonal antibody. Ct = Control. c Expression of gilz in peripheral blood neutrophils in the presence of DEX and/or the PI3K inhibitor LY294002. Cells were isolated and treated with DEX (10-9, 10-8, 10-7M) and/or the LY294002 inhibitor (10 µM). gilz mRNA expression was measured using qRT-PCR. The results represent the means ± SEM of 4 independent experiments. d Expression of GILZ in peripheral blood neutrophils in the presence of DEX and/or the PI3K inhibitor LY294002. Cells were isolated and treated with DEX (10-9, 10-8, 10-7M) and/or the LY294002 inhibitor (10 µM). Western blot with anti-GILZ polyclonal antibody. e Apoptosis of peripheral blood neutrophils in the presence of increasing concentrations of DEX (10-9, 10-8M) and/or LY294002 (10 µM). Cells were isolated and treated with DEX (10-9, 10-8, 10-7M) and/or the LY294002 inhibitor (10 µM). Apoptosis was quantified using the sub-G1 technique. Representative of 3 experiments.
GCs are known to paradoxically inhibit the apoptosis of human neutrophils, at least in part, through the upregulation of the antiapoptotic protein Mcl-1 [16]. Inhibition of the phosphatidylinositol 3-kinase (PI3K) signalling pathway was shown to reduce neutrophil survival and Mcl-1 expression in the presence of DEX [16]. Neutrophils were then treated with DEX and LY294002, a potent, cell-permeable inhibitor of PI3K, and both GILZ expression and apoptosis were evaluated. Results showed that inhibition of the PI3K pathway enhanced gilz induction with suboptimal concentrations of 10-9 and 10-8M of DEX (fig. 1c). Moreover, LY294002 suppressed GC-induced Mcl-1 while efficiently promoting GILZ expression at the suboptimal concentrations of 10-9 and 10-8M of DEX (fig. 1d). Interestingly, LY294002 also partly abrogated DEX-induced neutrophil survival as assessed by sub-G1 analysis (fig. 1e), suggesting that upon GC stimulation, apoptosis of neutrophils could depend on the balance between GILZ and Mcl-1 expressions. In the absence of Mcl-1, GILZ could promote apoptosis. To test this hypothesis without GC stimulation, which is known to regulate the expression of numerous target genes, and because neutrophils cannot be subject to genetic manipulation, we decided to stably transfect a GILZ expression vector in the neutrophil-like PLB-985 cells and to investigate apoptosis in this model once differentiation had taken place.
GILZ Expression Does Not Affect PLB-985 Cell Differentiation
Human neutrophils are short-lived, terminally differentiated cells which cannot be genetically modified. Once differentiated, PLB-985 cells share many functional features with peripheral-blood mature neutrophils and are therefore widely used to study the molecular mechanisms of neutrophil biology [12, 17]. Interestingly, in these cells, GILZ was not detected in undifferentiated cells and strongly induced by a 2- to 3-hour treatment with GCs (10-7M DEX) after 5 days of differentiation (fig. 2a) as previously demonstrated in human neutrophils (fig. 1d). To specifically investigate the role of GILZ expression in these cells, we generated GILZ overexpressing clones stably transfected with the human gilz gene (PLB-GILZ clones) and control clones (PLB-Myc) transfected with the empty vector (fig. 2b). GILZ clones were selected based on GILZ expression and control clones were selected by comparison with WT cells (PLB-985-WT). GILZ expression was found to be cytoplasmic in PLB-GILZ clones (data not shown), as previously described in the laboratory in HL-60 cells [18].
Fig. 2.
Characterization of PLB-GILZ clones. a Expression of GILZ in the presence of GCs. Cells were differentiated for 5 days in the presence of ATRA and DMF and treated for 1, 2 and 3 h with 10-7M DEX. Representative Western blot with polyclonal anti-GILZ antibody. b Expression of GILZ in stably transfected PLB-985 clones. Representative Western blot using polyclonal anti-GILZ antibody. Control clones (PLB-Myc) were termed M3, M4, M9 and M10. PLB-GILZ clones were termed G2, G7, G11, G19, G24 and G48. c Kinetics of CD11b expression in PLB-Myc clones (full lines) and PLB-GILZ clones (broken lines). CD11b membrane expression was assessed by flow cytometry. MFI was expressed as CD11b MFI corrected by the MFI of the control isotype. The results represent the means ± SEM of 4 independent experiments. d Percentage of cells expressing CD11b in the course of the differentiation. PLB-Myc clones (full lines) and PLB-GILZ clones (broken lines). e Respiratory burst capacities of PLB-Myc and PLB-GILZ clones on day 5 of differentiation. Cells were incubated with 1.25 µM DCFH-DA and stimulated or not with 50 ng/ml PMA. A stimulation index (fold increase) was calculated as the MFI ratio of PMA-treated cells versus unstimulated cells. The results represent the means ± SEM of 4 independent experiments. f Phagocytosis capacities of PLB-Myc and PLB GILZ clones. Cells on day 5 of differentiation were incubated with fluorescein-conjugated zymosan A particles (10 particles/cell). After 30 min, the percentage of cells integrating zymosan was determined by flow cytometry. The results represent the means ± SEM of 4 independent experiments.
PLB-985 cells can be differentiated in vitro in neutrophil-like cells using ATRA and DMF [12, 17]. After 5 days of differentiation, no difference in CD11b expression was observed in PLB-GILZ compared with PLB-Myc clones and PLB-985-WT cells as expressed by the MFI (fig. 2c) or percentage of CD11b-positive cells (fig. 2d). Basal CD11b cell surface expression in WT or PLB clones on day 5 of differentiation was similar to that obtained with neutrophils freshly isolated from human blood (data not shown), reflecting the terminal granulocytic maturation of these cells. In the absence of stimulation, PLB-Myc and PLB-GILZ clones had comparable intracellular basal levels of reactive oxygen species, as assessed by DCFH-DA oxidation (online suppl. data 1; for all online suppl. material, see www.karger.com/doi/10.1159/000439052). Using a gate restricted to viable cells (in order not to analyze apoptotic cells), H2O2 production in response to PMA was similar in the PLB-GILZ and PLB-Myc control clones (fig. 2e). Finally, the phagocytosis of fluorescein-conjugated zymosan particles was similar in PLB-Myc and PLB-GILZ differentiated clones (fig. 2f). Altogether, these results indicate that differentiated PLB-985 cells provide a neutrophil-like model suitable for evaluating GILZ functions in neutrophils. Day 5 of differentiation was chosen for further functional experiments, since we observed the highest basal CD11b expression (fig. 2d).
Expression of GILZ Promotes Apoptosis in PLB-985 Differentiated Cells
Circulating neutrophils enter spontaneously into apoptosis as terminally differentiated cells [2]. Differentiated PLB-985 cells also undergo apoptosis when their differentiation is completed. So we evaluated whether GILZ expression could alter their spontaneous apoptosis process. The sub-G1 analysis showed that, on day 5 of differentiation, GILZ expression led to an exacerbated apoptosis in all the clones tested (fig. 3a). A representative dot plot of annexin V/7AAD staining for the clones M10 and G48 is presented in figure 3b, showing that GILZ-overexpressing cells are mainly early apoptotic (annexin V+/7AAD-) with few cells in secondary necrosis (annexin V+/7AAD+); no primary necrotic cells were detected (annexin V-/7AAD+). Furthermore, to better characterize the kinetics of apoptosis, DNA hypodiploidy was followed during differentiation in representative clones. We observed that apoptosis started after 3 days of differentiation in PLB-GILZ G2 and G48 clones and to a much lesser extent in control clones (fig. 3c). Apoptosis was significantly increased in PLB-GILZ G2 and G48 clones at day 4 and day 5 when compared to control M4 and M10 clones, suggesting that GILZ overexpression could probably accelerate or strengthen the spontaneous apoptosis process initiated by differentiation.
Fig. 3.
Apoptosis of PLB-GILZ clones. a Apoptosis of PLB-Myc and PLB-GILZ clones on day 5 of differentiation. Cells were stained with annexin V and 7AAD, and analyzed by FACS. Annexin V+/7AAD- and annexin V+/7AAD+ cells were assumed to be apoptotic cells. The percentage of annexin V+ (AV+) cells was significantly higher in PLB-GILZ clones than in PLB-Myc clones. Mann-Whitney U test. # p < 0.01. The results represent the means ± SEM of 4 independent experiments. b Apoptosis of PLB-Myc M10 and PLB-GILZ G48 clones on day 5 of differentiation. The dual parametric dot plots combining annexin V-FITC and 7AAD fluorescence show the viable cell population in the bottom left quadrant (annexin V-/7AAD-), the early apoptotic cells in the bottom right quadrant (annexin V+/7AAD-) and the late apoptotic cells in the top right quadrant (annexin V+/7AAD+). The apoptotic cells are indicated as a percentage of gated cells. c Kinetics of apoptosis of PLB-Myc and PLB-GILZ clones. Two representative PLB-Myc clones (M4/M10, full lines) and 2 representative PLB-GILZ clones (G2/G48, broken lines) were used. Apoptosis was quantified using the sub-G1 technique. PLB-GILZ clones were significantly more apoptotic from day 3 to day 7. Mann-Whitney U test. * p < 0.05, # p < 0.01. The results represent the means ± SEM of 4 independent experiments. d Measurement of mitochondrial potential in PLB-Myc and PLB-GILZ clones. PLB-Myc clones (full lines) and PLB-GILZ clones (broken lines). Cells were loaded with 100 µM DiOC6 for 1 h, and the mitochondrial potential was followed using cytofluorometry. The difference was statistically significant using the Mann-Whitney U test on days 4-7. * p < 0.05. The results represent the means ± SEM of 4 independent experiments. e Comparison of mitochondrial potential loss in PLB-Myc and PLB-GILZ clones. Slopes were determined from the observed kinetics of each clone. PLB-GILZ clones slope values were statistically higher than those of PLB-Myc clones. Mann-Whitney U test. # p < 0.01.
Alteration of Mitochondrial Potential in PLB-GILZ Clones
Loss of mitochondrial potential is a hallmark of the classic pathway of apoptosis. Using DiOC6 staining and flow cytometry analysis, a significant increase in mitochondrial potential alteration was found in PLB-GILZ clones compared to PLB-Myc clones. This loss of mitochondrial potential was found earlier in PLB-GILZ clones (fig. 3d), as shown by the slope calculated from each clone kinetics (fig. 3e), consistent with the sub-G1 kinetics reported above.
Apoptosis of PLB-GILZ Clones Is Caspase-Dependent
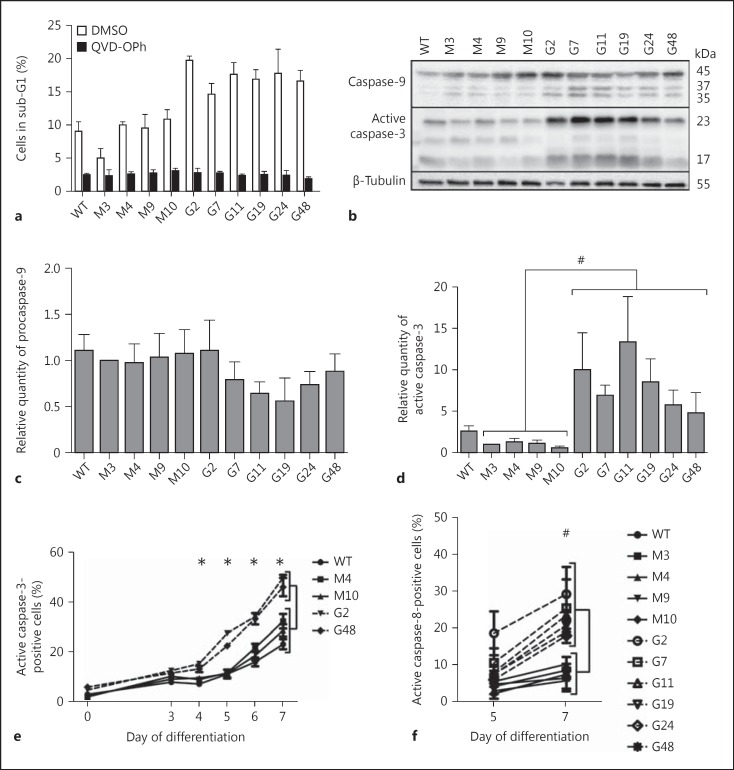
Caspases are key players in neutrophil apoptosis [4]. To clarify whether caspases were involved in GILZ- induced exacerbation of PLB-985 apoptosis, cells were treated from day 3 to day 5 of differentiation with a pan-caspase inhibitor, Q-VD-OPh, which is more potent, more specific and less cytotoxic (even at high concentrations) than z-VAD-fmk [19]. Results showed that Q-VD-OPh treatment strongly prevented apoptosis in both PLB-Myc and PLB-GILZ clones (fig. 4a), suggesting that caspases are involved in GILZ-related apoptosis.
Fig. 4.
Implication of caspases in GILZ-induced apoptosis. a Effect of a pancaspase inhibitor on PLB-985 apoptosis at day 5 of differentiation. Cells were treated with 100 µM QVD-OPh or with DMSO from day 3 to day 5 of differentiation. Cells were fixed with ethanol, stained with propidium iodide and analyzed for hypodiploidy using flow cytometry. The results represent the means ± SEM of 4 independent experiments. b Activation of caspase-9 and caspase-3 in PLB-Myc and PLB-GILZ clones on day 5 of differentiation. Representative Western blot of 4 independent experiments. Caspase-9 antibody recognized the 45-kDa pro-form and the 37- and 35-kDa active forms. Caspase-3 antibody recognized the 23-kDa active form. c Densitometric analysis of caspase-9 expression on day 5 of differentiation. Results were normalized to the densitometric values of β-tubulin. The results represent the means ± SEM of 4 independent experiments. d Densitometric analysis of active caspase-3 expression (p23 subunit) on day 5 of differentiation. Results were normalized to the densitometric values of β-tubulin. Caspase-3 was significantly more activated in PLB-GILZ clones than in PLB-Myc clones. Mann-Whitney U test. # p < 0.01. The results represent the means ± SEM of 4 independent experiments. e Kinetics of caspase-3 activation in PLB-Myc and PLB-GILZ clones. PLB-Myc clones (full lines) and PLB-GILZ clones (broken lines). Caspase-3 activation was monitored using flow cytometry with an antibody directed to the p23 active form of caspase-3. Differences were statistically significant using the Mann-Whitney U test on days 4-7. * p < 0.05. The results represent the means ± SEM of 4 independent experiments. f Activation of caspase-8 in PLB-Myc and PLB-GILZ clones. PLB-Myc clones (full lines) and PLB-GILZ clones (broken lines). Caspase-8 activation was followed with FITC-conjugated IETD-FMK and cytofluorometry. Differences were observed on day 7 of differentiation but not on day 5 (statistically significant using the Mann-Whitney U test). # p < 0.01. The results represent the means ± SEM of 4 independent experiments.
PLB-Myc and PLB-GILZ clones were differentiated for 5 days and caspase-9, caspase-3 and caspase-8 activations were followed using Western blot and flow cytometry. We found the highest activation of caspase-9 at day 5 of differentiation in PLB-GILZ clones compared to control clones, although this did not reach statistical significance (fig. 4b, c). A significant increase of procaspase-3 cleavage was observed by Western blot in PLB-GILZ clones compared to PLB-Myc clones (fig. 4b, d), with a marked detection of the active p23 subunit. These results were confirmed by flow cytometry using an antibody against p23 (fig. 4e). Interestingly, GILZ expression induced an earlier caspase-3 activation during differentiation, consistent with the hypothesis of an acceleration of the apoptosis process in these cells. Differences were evident from day 4 by flow cytometry (fig. 4e) and from day 5 by Western blot (online suppl. data 2), reflecting the different sensitivities of these 2 techniques. Finally, results obtained by flow cytometry showed a late but noteworthy activation of caspase-8 in PLB-GILZ clones that was statistically significant compared to PLB-Myc clones on day 7 of differentiation (fig. 4f).
Expression of Apoptotic-Regulating Proteins in PLB-985 Differentiated Cells
Mitochondrial integrity and cell apoptosis are controlled by proteins from the Bcl-2 family [3, 4]. On day 5 of differentiation, Bid expression was not impacted by GILZ expression, and no truncation could be detected (fig. 5a, b). Bim long and Bim small were not detected in the 2 clones (data not shown). The expression of Bim extra-long (Bim EL) was decreased in PLB-GILZ clones (fig. 5a, c). The expression of annexin A1 [3], a protein known to be pro-apoptotic in neutrophils, was not impacted by GILZ in PLB-985 differentiated cells (fig. 5a, d).
Fig. 5.
Expression of apoptosis-regulating proteins in PLB-Myc and PLB-GILZ clones. a Bid, Bim EL and annexin A1 expressions on day 5 of differentiation. Representative Western blot of 4 independent experiments. b Densitometric analysis of Bid expression on day 5 of differentiation in PLB-Myc and PLB-GILZ clones. Results were normalized to the densitometric values of β-tubulin. Bid expression was not modulated by GILZ over-expression. Mann-Whitney U test. The results represent the means ± SEM of 4 independent experiments. c Densitometric analysis of Bim EL expression on day 5 of differentiation in PLB-Myc and PLB-GILZ clones. Results were normalized to the densitometric values of β-tubulin. Bim EL was less expressed in PLB-GILZ clones than in PLB-Myc clones. Mann-Whitney U test. * p < 0.05. The results represent the means ± SEM of 4 independent experiments. d Densitometric analysis of annexin A1 expression on day 5 of differentiation in PLB-Myc and PLB-GILZ clones. Results were normalized to the densitometric values of β-tubulin. Annexin A1 expression was not modulated by GILZ over-expression. Mann-Whitney U test. The results represent the means ± SEM of 4 independent experiments. e Expression of Bax during the differentiation of PLB-Myc and PLB-GILZ clones. Western blot with clones M4/M10 and G2/G48 is representative of 3 independent experiments. f Densitometric analysis of Bax expression on days 0, 3 and 5 of differentiation in PLB-Myc and PLB-GILZ clones. Results were normalized to the respective densitometric values of β-tubulin. Bax expression was not modulated by GILZ over-expression during the differentiation. Mann-Whitney U test. The results represent the means ± SEM of 4 independent experiments.
The loss of mitochondrial potential may be consecutive to pore formation in mitochondrial membrane and is often associated with Bax activation, especially in neutrophils [20]. Bax expression increased in both types of clones during differentiation, probably in relation to the spontaneous apoptosis process (fig. 5e). At days 0, 3 and 5 of differentiation, no statistical difference in Bax expression was observed (fig. 5f), confirmed by Western blot performed for all the clones at day 5 of differentiation (data not shown). Bax activation could be documented by an increase of expression, but also by a change in protein conformation which was evaluated using an antibody directed against ‘active’ Bax, the 6A7 clone [21]. No differences between PLB-Myc and PLB-GILZ clones were observed (data not shown).
Expression of Mcl-1 Is Down-Regulated in PLB-GILZ Clones
Mcl-1 is a pro-survival member of the Bcl-2 family critical in neutrophil apoptosis control [5]. Almost all molecules promoting neutrophil survival lead to Mcl-1 expression or stabilization [5, 22]. Interestingly, our results showed that apoptosis of PLB-GILZ clones was associated with a down-regulation of Mcl-1 observed at the protein level on day 5 of differentiation (fig. 6a, b), but not at the transcriptional level (fig. 6c). The decrease of Mcl-1 expression occurred between days 4 and 5 (data not shown).
Fig. 6.
Mcl-1 down-regulation in PLB-GILZ clones. a Mcl-1 expression on day 5 of differentiation. Representative Western blot of Mcl-1 out of 4 independent experiments. b Densitometric analysis of Mcl-1 expression on day 5 of differentiation in PLB-Myc and PLB-GILZ clones. Results were normalized to the densitometric values of β-tubulin. Mcl-1 expression was significantly down-regulated in PLB-GILZ clones compared to PLB-Myc clones. Mann-Whitney U test. * p < 0.05. The results represent the means ± SEM of 4 independent experiments. c mcl-1 mRNA expression on day 5 of differentiation. qRT-PCR was performed using specific primers for mcl-1, gapdh and b2m. Relative quantity of mcl-1 mRNA was expressed as the mcl-1 mRNA expression level divided by the mean of gapdh and b2m mRNA levels (Materials and Methods). The results represent the means ± SEM of 3 independent experiments.
Sustained Activation of JNK in PLB-GILZ Clones and Mcl-1 Phosphorylation
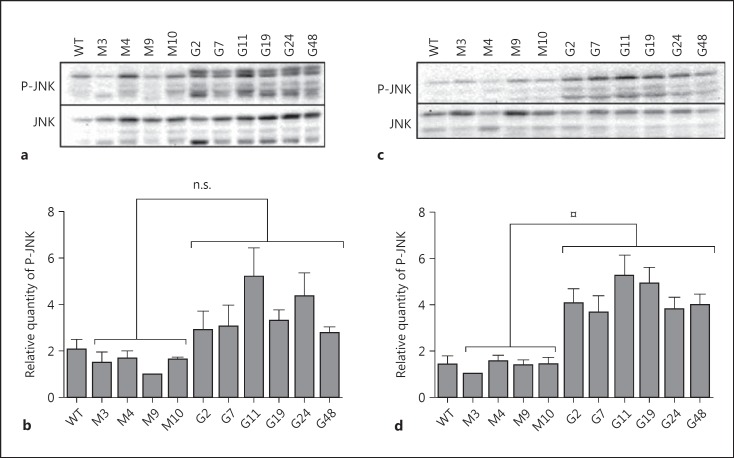
Mcl-1 protein turnover is mostly controlled by phosphorylation [7]. In particular, the phosphorylation of Mcl-1 by JNK on the Ser159 residue may favour GSK3 phosphorylation on Ser155 and Ser159 residues in the PEST, i.e. rich in proline (P), glutamic acid (E), serine (S) and threonine (T), domain, followed by polyubiquitination and targeting of Mcl-1 to the proteasome [7]. On day 3, a trend towards an increase in JNK phosphorylation in PLB-GILZ clones was found (fig. 7a, b). However, a significantly increased and sustained activation of JNK in PLB-GILZ clones was observed on day 5 of differentiation concomitant with apoptosis (fig. 7c, d). Moreover, GILZ effect on JNK phosphorylation was not likely to be the consequence of altered mitogen-activated protein kinase phosphatase-1 (MKP-1) expression (online suppl. data 3).
Fig. 7.
JNK phosphorylation in PLB-GILZ clones. P-JNK = Phospho-JNK. a Phosphorylation of JNK on day 3 of differentiation. Representative Western blot out of 4 independent experiments. b Densitometric analysis of P-JNK expression on day 3 of differentiation in PLB-Myc and PLB-GILZ clones. Results were normalized to the densitometric values of JNK. JNK was not significantly (n.s.) more phosphorylated in PLB-GILZ clones than in PLB-Myc clones. Mann-Whitney U test. p > 0.05, n.s. The results represent the means ± SEM of 4 independent experiments. c Phosphorylation of JNK on day 5 of differentiation. Representative Western blot out of 4 independent experiments. d Densitometric analysis of P-JNK expression on day 5 of differentiation in PLB-Myc and PLB-GILZ clones. Results were normalized to the densitometric values of JNK. JNK was significantly more phosphorylated in PLB-GILZ clones than in PLB-Myc clones. Mann-Whitney U test. ¤ p < 0.001. The results represent the means ± SEM of 4 independent experiments.
We then evaluated whether sustained activation of JNK could trigger the phosphorylation of Mcl-1. To address this question, PLB-Myc and PLB-GILZ clones were treated for 24 h from day 4 to day 5 of differentiation with the proteasome inhibitor MG-262. We then used an antibody specifically directed against Mcl-1 amino acid residues phosphorylated by JNK and GSK3. A phosphorylated protein of 115 kDa was detected on Western blot in PLB-GILZ clones on day 5 of differentiation and was also recognized by the anti-Mcl-1 antibody (fig. 8a, b). This protein was hardly detected in PLB-Myc control clones, suggesting that, under proteasomal inhibition, this phosphorylated form of Mcl-1 accumulated specifically in PLB-GILZ clones. The high molecular mass of the protein detected suggested that it was probably poly-ubiquitinated but not degraded, due to proteasome inhibition. Furthermore, a concomitant treatment with the pharmacological inhibitors of JNK (SP600125) or GSK3 (SB216763) strongly decreased the detection of phospho-Mcl-1 (fig. 8c), confirming the implication of JNK and GSK3 in the phosphorylation of the 115-kDa protein detected.
Fig. 8.
Mcl-1 phosphorylation in PLB-GILZ clones. a Phospho-Mcl-1 expression on day 5 of differentiation under proteasome inhibition. Cells were treated with MG-262 (1 µM) from day 4 to day 5 of differentiation. A protein migrating at 115 kDa was recognized by anti-phospho-Mcl-1 and anti-Mcl-1 antibodies. Representative Western blot out of 4 independent experiments. b Densitometric analysis of phospho-Mcl-1 expression on day 5 of differentiation in PLB-Myc and PLB-GILZ clones under proteasome inhibition. Results were normalized to the densitometric values of β-tubulin. Phospho-Mcl-1 was significantly less expressed in PLB-GILZ clones than in PLB-Myc clones. Mann-Whitney U test. ¤ p < 0.001. The results represent the means ± SEM of 4 independent experiments. c Phospho-Mcl-1 protein expression in the presence of JNK or GSK3 pharmacological inhibitors. Cells were treated with 1 µM of MG-262 from day 4 to day 5 of differentiation and 20 µM of JNK inhibitor SP600125 or 40 µM of SB216763 GSK3 inhibitor. The protein at 115 kDa was recognized by phospho-Mcl-1 antibody. Representative Western blot out of 3 independent experiments for 2 representative clones. d Apoptosis of PLB-Myc and PLB-GILZ clones in the presence of the JNK pharmacological inhibitor SP600125. Cells were treated with 10 µM of SP600125 from day 3 to day 4 of differentiation. Apoptosis was quantified using the sub-G1 technique on day 5. Results are shown as a fold increase in apoptosis of 2 representative GILZ clones (G2 and G48) and Myc clones (M4 and M10) in the presence of DMSO or SP600125. The bars represent the means ± SEM of 5 independent experiments. In the presence of SP600125, PLB-GILZ clone apoptosis was found to be significantly less induced. Mann-Whitney U test. ** p < 0.01.
Altogether, these results suggest that, in PLB-GILZ clones, increased Mcl-1 phosphorylation and degradation could be related to the sustained activation of JNK, resulting in an amplification of the apoptosis process initiated in these cells when differentiation is completed. To explore this hypothesis, 2 representative PLB-Myc (M4 and M10) and PLB-GILZ (G2 and G48) clones were treated for 24 h between day 3 and day 4 of differentiation with the pharmacologic inhibitor of JNK (SP600125) at 10 µM. Apoptosis was evaluated using sub-G1 analysis on day 5 of differentiation. Results showed that SP600125 efficiently and significantly reduced the induction of apoptosis of PLB-GILZ clones (fig. 8d), confirming the role of JNK in the apoptosis due to GILZ overexpression.
Discussion
GILZ is a protein that is rapidly expressed upon treatment with GCs and is believed to relay part of the anti-inflammatory and immunomodulatory effects of GCs [23]. GILZ is also involved in cell survival, by exerting pro- or anti-apoptotic effects, depending on the cell type and the stimuli used [9, 11, 24]. In this work, we specifically addressed the role of GILZ expression on neutrophil functions, with a special emphasis on apoptosis. We demonstrated that GILZ was induced by GCs in human neutrophils and could be subject to proteasomal degradation, allowing its levels to change quickly in response to some specific stimuli. The combination of DEX and an inhibitor of the PI3K signalling pathway up-regulated GILZ levels, as previously described in multiple myeloma cells [25] and lung epithelial cells [26] or, more recently, in normal human lung fibroblasts [27]. The gilz promoter, which was characterized in the laboratory [9], comprises 6 GC-responsive elements and 3 FOXO responsive elements which functionally cooperate for optimal activation of the promoter [14]. Here, interruption of the PI3K/Akt pathway using pharmacological inhibitors could result in the activation of FOXO proteins.
Concomitant DEX and LY294002 treatment was linked with the enhanced apoptosis of neutrophils, associated with GILZ up-regulation and the inhibition of Mcl-1 induction. Altogether, these results suggest that upon GC stimulation, apoptosis of neutrophils could be controlled by the relative expressions of GILZ and Mcl-1. In the absence of Mcl-1, GILZ could shift the balance in favour of apoptosis. It was recently suggested that, even if GCs are known to delay neutrophil apoptosis in vitro, thereby paradoxically limiting the control of neutrophilic inflammation, some conditions at inflamed sites (severe hypoxia or inflammatory mediators) could create a microenvironment where a neutrophil pro-survival effect is abrogated [28]. We cannot exclude that GILZ expression could be favoured and locally promoted in vivo in such specific microenvironments.
To specifically address the role of GILZ in neutrophils, we stably expressed the human gilz gene in the PLB-985 cell line that differentiates into fully mature and functional neutrophil-like cells [12, 29]. After granulocytic differentiation, PLB-985 cells were shown to express neutrophil-like surface markers, become responsive to physiological neutrophil stimuli such as fMLP or TNF-α [17, 30, 31] and display functional characteristics such as calcium mobilization, granule exocytosis as well as phagocytosis [32]. GILZ expression in PLB-985 cells did not alter PLB-985 cell differentiation kinetics after ATRA and DMF addition, CD11b basal expression, phagocytosis or PMA-induced oxidative burst. Upon differentiation, PLB-985 cells undergo apoptotic cell death and can therefore be used as a model of apoptosis of resting neutrophils [29]. This apoptotic process was observed from the third day of differentiation. Noteworthy, when GILZ was overexpressed in PLB-985 cells, a significant increase of apoptosis was found in PLB-GILZ clones compared to PLB-Myc clones, strongly suggesting that GILZ could provoke apoptosis or take part in the spontaneous apoptotic process in these cells. It is generally admitted that neutrophils can engage the intrinsic or the extrinsic death pathways resulting in the activation of effector caspases [4]. Particularly, in neutrophils undergoing spontaneous apoptosis, caspase-3 is known to be activated in a time-dependent manner and to play a central role in the execution of the apoptotic process. Apoptosis of PLB-Myc and PLB-GILZ cells was caspase-dependent, significantly abrogated by the pan-caspase inhibitor QVD-OPh and characterized by an earlier activation of the same caspases (caspase-9 and caspase-3). In PLB-GILZ cells, caspase-8 activation occurred 2 days after the detectable activation of caspase-3, suggesting that its activation was not direct. Active caspase-3 could induce late activation of caspase-8 as an amplification loop, as found in other cell types [33]. Neutrophils display few mitochondria, which hardly participate in ATP synthesis. Nevertheless, the mitochondrial death pathway is functional in these cells, probably due to elevated cytoplasmic Apaf-1 levels [2]. Our results showed an alteration of the mitochondrial potential in PLB-GILZ clones, and the kinetics of mitochondrial potential loss and caspase-3 activation were quite similar. Altogether, these results suggest that apoptosis of PLB-GILZ clones involved an exacerbated activation of the mitochondrial pathway.
Under non-stimulated conditions, human neutrophils constitutively express relatively stable levels of pro-apoptotic proteins such as Bax, Bak, Bim or Bid [4, 34]. Thus, neutrophil apoptosis and survival might be controlled essentially by the inducible expression or degradation of the anti-apoptotic protein Mcl-1, whereas the levels of the proapoptotic proteins Bax, Bad, Bik, and Bak remain constant [35]. Our results showed similar modulation of Bax and Bid expressions in PLB-GILZ and PLB-Myc cells. In contrast, we observed a down-regulation of Bim in PLB-GILZ cells, confirming our previous results in T cells [9, 18]. The unconformity between Bim expression levels and apoptosis found here has been already described, as inhibition of apoptosis has been reported in neutrophils despite increased Bim levels [36]. Altogether, these results suggest that in our model, the balance between death and survival signals was still in favour of apoptosis despite Bim down-regulation, probably due to alteration in the expression of anti-apoptotic proteins.
Mcl-1 is a labile and inducible anti-apoptotic protein essential in the control of neutrophil life span [5]. Our results showed that GILZ probably modulated Mcl-1 expression through a post-translational modification since mcl-1 mRNA levels were not affected. Stability of Mcl-1 is controlled by phosphorylations of serine and threonine residues present in the N-terminal-domain PEST sequence, which is associated with the targeting of proteins to the proteasome for rapid turnover [7]. In murine fibroblasts, stress-induced apoptosis is associated with Mcl-1 degradation requiring the coordinated activity of JNK and GSK3 [37]. Thus, we hypothesized that GILZ expression could lead to Mcl-1 phosphorylation through JNK activation.
We observed a sustained increase of JNK phosphorylation in PLB-GILZ cells, as soon as 3 days of differentiation and statistically significant by day 5, concomitant with Mcl-1 degradation. Since PLB-Myc and PLB-GILZ clones had similar intracellular levels of reactive oxygen species after 5 days of differentiation, we excluded excessive oxidative stress due to GILZ expression. JNK activation is regulated by the MKP-1 phosphatase, known to be regulated by GILZ in a human endothelial cell line [38, 39], but its expression was similar in the course of differentiation of PLB-Myc and PLB-GILZ clones.
We are proposing an original mechanism of neutrophil apoptosis where GILZ expression leads to JNK activation and phosphorylation of Mcl-1, its subsequent degradation by the proteasome and, consequently, apoptosis. This hypothesis was confirmed by the specific detection of JNK- and GSK3-phosphorylated Mcl-1 under proteasome inhibition in PLB-GILZ cells and by reduced apoptosis of these cells in the presence of the JNK inhibitor SP600125. JNK activation is known to contribute to apoptotic signalling [40], as in normally cycling cells at the G2/M phase or in presence of microtubule-damaging drugs such as paclitaxel, through the phosphorylation of Bcl-2, resulting in its inactivation [41]. Furthermore, it has been described that a sustained activation of JNK is associated with apoptosis, particularly in cells where the apoptotic program has been initiated, whereas the acute and transient activation of JNK may be involved in the proliferation or survival of cells [40]. Indeed, prolonged JNK activation was shown to play a pro-apoptotic role in the UV-induced apoptosis of fibroblasts, through the mitochondrial death pathway [42]. Here, sustained JNK activation promoted Mcl-1 phosphorylation and degradation, and apoptosis in neutrophil-like cells.
In this work, we show for the first time that GILZ is expressed in human neutrophils and that this expression, in a recognized cellular model of neutrophil-like cells, enhances spontaneous apoptosis through JNK activation and Mcl-1 down-regulation. We propose here a new mechanism of neutrophil apoptosis regulation by GILZ, the relevance of which in clinical inflammatory settings remains to be thoroughly evaluated. Interestingly, consistent with our in vitro observations reported here, it was very recently described in an LPS-induced pleurisy model in mice that the administration of a TAT-GILZ protein promoted resolution of neutrophilic inflammation [43].
Disclosure Statement
The authors declare no conflict of interest.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
The authors particularly thank Claudine Deloménie (IPSIT) for expert assistance for real-time PCR. Marie-Alix Espinasse is a fellow of ‘La Fondation du Souffle’ and ‘Fonds de Dotation de Recherche en Santé Respiratoire’.
References
- 1.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabelloni ML, Trevani AS, Sabatte J, Geffner J. Mechanisms regulating neutrophil survival and cell death. Semin Immunopathol. 2013;35:423–437. doi: 10.1007/s00281-013-0364-x. [DOI] [PubMed] [Google Scholar]
- 3.Witko-Sarsat V, Pederzoli-Ribeil M, Hirsch E, Sozzani S, Cassatella MA. Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 2011;32:117–124. doi: 10.1016/j.it.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011;18:1457–1469. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milot E, Filep JG. Regulation of neutrophil survival/apoptosis by Mcl-1. ScientificWorldJournal. 2011;11:1948–1962. doi: 10.1100/2011/131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 8.Ayroldi E, Riccardi C. Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 2009;23:3649–3658. doi: 10.1096/fj.09-134684. [DOI] [PubMed] [Google Scholar]
- 9.Asselin-Labat ML, David M, Biola-Vidamment A, Lecoeuche D, Zennaro MC, Bertoglio J, Pallardy M. GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal-induced apoptosis. Blood. 2004;104:215–223. doi: 10.1182/blood-2003-12-4295. [DOI] [PubMed] [Google Scholar]
- 10.Delfino DV, Spinicelli S, Pozzesi N, Pierangeli S, Velardi E, Bruscoli S, Martelli MP, Pettirossi V, Falchi L, Kang TB, Riccardi C. Glucocorticoid-induced activation of caspase-8 protects the glucocorticoid-induced protein GILZ from proteasomal degradation and induces its binding to SUMO-1 in murine thymocytes. Cell Death Differ. 2011;18:183–190. doi: 10.1038/cdd.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joha S, Nugues AL, Hetuin D, Berthon C, Dezitter X, Dauphin V, Mahon FX, Roche-Lestienne C, Preudhomme C, Quesnel B, Idziorek T. GILZ inhibits the mTORC2/AKT pathway in BCR-ABL(+) cells. Oncogene. 2011;31:1419–1430. doi: 10.1038/onc.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semiramoth N, Gleizes A, Turbica I, Sandre C, Gorges R, Kansau I, Servin A, Chollet-Martin S. Escherichia coli type 1 pili trigger late IL-8 production by neutrophil-like differentiated PLB-985 cells through a Src family kinase- and MAPK-dependent mechanism. J Leukoc Biol. 2009;85:310–321. doi: 10.1189/jlb.0608350. [DOI] [PubMed] [Google Scholar]
- 13.Migdal C, Botton J, El Ali Z, Azoury ME, Guldemann J, Gimenez-Arnau E, Lepoittevin JP, Kerdine-Romer S, Pallardy M. Reactivity of chemical sensitizers toward amino acids in cellulo plays a role in the activation of the Nrf2-ARE pathway in human monocyte dendritic cells and the THP-1 cell line. Toxicol Sci. 2013;133:259–274. doi: 10.1093/toxsci/kft075. [DOI] [PubMed] [Google Scholar]
- 14.Asselin-Labat ML, Biola-Vidamment A, Kerbrat S, Lombes M, Bertoglio J, Pallardy M. FoxO3 mediates antagonistic effects of glucocorticoids and interleukin-2 on glucocorticoid-induced leucine zipper expression. Mol Endocrinol. 2005;19:1752–1764. doi: 10.1210/me.2004-0206. [DOI] [PubMed] [Google Scholar]
- 15.Cohen N, Mouly E, Hamdi H, Maillot MC, Pallardy M, Godot V, Capel F, Balian A, Naveau S, Galanaud P, Lemoine FM, Emilie D. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood. 2006;107:2037–2044. doi: 10.1182/blood-2005-07-2760. [DOI] [PubMed] [Google Scholar]
- 16.Saffar AS, Dragon S, Ezzati P, Shan L, Gounni AS. Phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase regulate induction of Mcl-1 and survival in glucocorticoid-treated human neutrophils. J Allergy Clin Immunol. 2008;121:492–498. doi: 10.1016/j.jaci.2007.10.003. e410. [DOI] [PubMed] [Google Scholar]
- 17.Pedruzzi E, Fay M, Elbim C, Gaudry M, Gougerot-Pocidalo MA. Differentiation of PLB-985 myeloid cells into mature neutrophils, shown by degranulation of terminally differentiated compartments in response to N-formyl peptide and priming of superoxide anion production by granulocyte-macrophage colony-stimulating factor. Br J Haematol. 2002;117:719–726. doi: 10.1046/j.1365-2141.2002.03521.x. [DOI] [PubMed] [Google Scholar]
- 18.Latre de Late P, Pepin A, Assaf-Vandecasteele H, Espinasse C, Nicolas V, Asselin-Labat ML, Bertoglio J, Pallardy M, Biola-Vidamment A. Glucocorticoid-induced leucine zipper (GILZ) promotes the nuclear exclusion of FOXO3 in a Crm1-dependent manner. J Biol Chem. 2010;285:5594–5605. doi: 10.1074/jbc.M109.068346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ. 2007;14:387–391. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- 20.Croker BA, O'Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang JG, White M, Lackovic K, Cengia LH, O'Reilly LA, Bouillet P, Cory S, Strasser A, Roberts AW. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci USA. 2011;108:13135–13140. doi: 10.1073/pnas.1110358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellosillo B, Villamor N, Lopez-Guillermo A, Marce S, Bosch F, Campo E, Montserrat E, Colomer D. Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood. 2002;100:1810–1816. doi: 10.1182/blood-2001-12-0327. [DOI] [PubMed] [Google Scholar]
- 22.Wardle DJ, Burgon J, Sabroe I, Bingle CD, Whyte MK, Renshaw SA. Effective caspase inhibition blocks neutrophil apoptosis and reveals Mcl-1 as both a regulator and a target of neutrophil caspase activation. PLoS One. 2011;6:e15768. doi: 10.1371/journal.pone.0015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaulieu E, Morand EF. Role of GILZ in immune regulation, glucocorticoid actions and rheumatoid arthritis. Nat Rev. 2011;7:340–348. doi: 10.1038/nrrheum.2011.59. [DOI] [PubMed] [Google Scholar]
- 24.Delfino DV, Agostini M, Spinicelli S, Vito P, Riccardi C. Decrease of Bcl-xL and augmentation of thymocyte apoptosis in GILZ overexpressing transgenic mice. Blood. 2004;104:4134–4141. doi: 10.1182/blood-2004-03-0920. [DOI] [PubMed] [Google Scholar]
- 25.Grugan KD, Ma C, Singhal S, Krett NL, Rosen ST. Dual regulation of glucocorticoid-induced leucine zipper (GILZ) by the glucocorticoid receptor and the PI3-kinase/AKT pathways in multiple myeloma. J Steroid Biochem Mol Biol. 2008;110:244–254. doi: 10.1016/j.jsbmb.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez M, Raju SV, Viswanathan A, Painter RG, Bonvillain R, Byrne P, Nguyen DH, Bagby GJ, Kolls JK, Nelson S, Wang G. Ethanol upregulates glucocorticoid-induced leucine zipper expression and modulates cellular inflammatory responses in lung epithelial cells. J Immunol. 2010;184:5715–5722. doi: 10.4049/jimmunol.0903521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Y, Morand EF, Song W, Cheng Q, Stewart A, Yang YH. Regulation of lung fibroblast activation by annexin A1. J Cell Physiol. 2013;228:476–484. doi: 10.1002/jcp.24156. [DOI] [PubMed] [Google Scholar]
- 28.Marwick JA, Dorward DA, Lucas CD, Jones KO, Sheldrake TA, Fox S, Ward C, Murray J, Brittan M, Hirani N, Duffin R, Dransfield I, Haslett C, Rossi AG. Oxygen levels determine the ability of glucocorticoids to influence neutrophil survival in inflammatory environments. J Leukoc Biol. 2013;94:1285–1292. doi: 10.1189/jlb.0912462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witko-Sarsat V, Mocek J, Bouayad D, Tamassia N, Ribeil JA, Candalh C, Davezac N, Reuter N, Mouthon L, Hermine O, Pederzoli-Ribeil M, Cassatella MA. Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival. J Exp Med. 2010;207:2631–2645. doi: 10.1084/jem.20092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ear T, McDonald PP. Cytokine generation, promoter activation, and oxidant-independent NF-kappaB activation in a transfectable human neutrophilic cellular model. BMC Immunol. 2008;9:14. doi: 10.1186/1471-2172-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volk AP, Barber BM, Goss KL, Ruff JG, Heise CK, Hook JS, Moreland JG. Priming of neutrophils and differentiated PLB-985 cells by pathophysiological concentrations of TNF-α is partially oxygen dependent. J Innate Immun. 2011;3:298–314. doi: 10.1159/000321439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pivot-Pajot C, Chouinard FC, El Azreq MA, Harbour D, Bourgoin SG. Characterisation of degranulation and phagocytic capacity of a human neutrophilic cellular model, PLB-985 cells. Immunobiology. 2010;215:38–52. doi: 10.1016/j.imbio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira KS, Kreutz C, Macnelly S, Neubert K, Haber A, Bogyo M, Timmer J, Borner C. Caspase-3 feeds back on caspase-8, Bid and XIAP in type I Fas signaling in primary mouse hepatocytes. Apoptosis. 2012;17:503–515. doi: 10.1007/s10495-011-0691-0. [DOI] [PubMed] [Google Scholar]
- 34.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. Bcl-2 family expression in human neutrophils during delayed and accelerated apoptosis. J Leukoc Biol. 2001;70:783–792. [PubMed] [Google Scholar]
- 36.Bauer A, Kirschnek S, Hacker G. Inhibition of apoptosis can be accompanied by increased Bim levels in T lymphocytes and neutrophil granulocytes. Cell Death Differ. 2007;14:1714–1716. doi: 10.1038/sj.cdd.4402185. [DOI] [PubMed] [Google Scholar]
- 37.Morel C, Carlson SM, White FM, Davis RJ. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol Cell Biol. 2009;29:3845–3852. doi: 10.1128/MCB.00279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Q, Fan H, Ngo D, Beaulieu E, Leung P, Lo CY, Burgess R, van der Zwan YG, White SJ, Khachigian LM, Hickey MJ, Morand EF. GILZ overexpression inhibits endothelial cell adhesive function through regulation of NF-kappaB and MAPK activity. J Immunol. 2013;191:424–433. doi: 10.4049/jimmunol.1202662. [DOI] [PubMed] [Google Scholar]
- 39.Fan H, Kao W, Yang YH, Gu R, Harris J, Fingerle-Rowson G, Bucala R, Ngo D, Beaulieu E, Morand EF. Macrophage migration inhibitory factor inhibits the anti-inflammatory effects of glucocorticoids via glucocorticoid-induced leucine zipper. Arthritis Rheumatol. 2014;66:2059–2070. doi: 10.1002/art.38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome C-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 43.Vago JP, Tavares LP, Garcia CC, Lima KM, Perucci LO, Vieira EL, Nogueira CR, Soriani FM, Martins JO, Silva PM, Gomes KB, Pinho V, Bruscoli S, Riccardi C, Beaulieu E, Morand EF, Teixeira MM, Sousa LP. The role and effects of glucocorticoid-induced leucine zipper in the context of inflammation resolution. J Immunol. 2015;194:4940–4950. doi: 10.4049/jimmunol.1401722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data