Abstract
Maintenance of a stable gut microbial community relies on a delicate balance between immune defense and immune tolerance. We have used Drosophila to study how the microbial gut flora is affected by changes in host genetic factors and immunity. Flies with a constitutively active gut immune system, due to a mutation in the POU transcriptional regulator Pdm1/nubbin (nub) gene, had higher loads of bacteria and a more diverse taxonomic composition than controls. In addition, the microbial composition shifted considerably during the short lifespan of the nub1 mutants. This shift was characterized by a loss of relatively few OTUs (operational taxonomic units) and a remarkable increase in a large number of Acetobacter spp. and Leuconostoc spp. Treating nub1 mutant flies with antibiotics prolonged their lifetime survival by more than 100%. Immune gene expression was also persistently high in the presence of antibiotics, indicating that the early death was not a direct consequence of an overactive immune defense but rather an indirect consequence of the microbial load and composition. Thus, changes in host genotype and an inability to regulate the normal growth and composition of the gut microbiota leads to a shift in the microbial community, dysbiosis and early death.
Key Words: Microbiota, Innate immunity, Antimicrobial peptides, Gut homeostasis, Microbial community, Microbial diversity, Epithelial immunity, Drosophila, POU, Oct factor
Introduction
The role of the commensal gut microbiota for a healthy life is attracting increasing interest and research focus. The role of commensal microbes in a normal gut metabolism and the absorption of nutrients, and as suppliers of essential nutrients, has long been recognized. Currently, it is becoming evident that microbes are also active players in shaping an organism's immune system, development, physiology and nervous system with direct effects on general health, aging and life expectancy. The genetic regulation of these host processes is unusually well characterized in Drosophila melanogaster and, in combination with the fly's superb genetic tools, relatively simple intestinal architecture and ease of cultivating under different conditions, it serves as an excellent model for studies of host-gut microbe interaction and the relation between innate immunity and pathogenic infections [recently reviewed in [1]]. In addition, several recent discoveries have revealed the usefulness of flies for a general understanding of the role of microbes in gut epithelium regeneration and intestinal stem cell activity [2, 3], as well as its consequences for inflammation, cancer development and aging [4, 5, 6, 7].
The Drosophila gut microbiota was first found to represent a relatively low level of diversity in comparison with the human intestinal system. Approximately 15-30 species, primarily Acetobacter spp. and Lactobacillus spp., have routinely been identified in studies both from wild populations and laboratory strains (reviewed in Broderick and Lemaitre [8]). Considering the huge diversity of microbes present in the food in wild habitats, the comparatively low diversity of fly gut microbiota indicates that the commensal flora is shaped to some extent by the host's gut physiology and immune system. Recent studies of the gut microbiota of different species and from different habitats, using culture-independent DNA sequencing approaches, have pointed to the existence of a much larger microbial diversity in the overall Drosophila population [9, 10, 11]. In general, a typical ‘core microbiota’ could not be distinguished, except that Gluconobacter was identified in most wild-caught flies in one of the studies [10]. These studies also indicated a substantial impact of food sources for the composition of the microbiota of flies in the wild, most likely reflecting the difference in microbial communities living on various types of fruits and flowers. However, a few reports have also indicated that the host genotype controls and changes the microbial community, at least in a laboratory setting. Mutations in genes that are negative regulators of innate immunity, such as Caudal (Cad), PIRK/PIMS and several PGRPs lead to an overactive immune system [12, 13, 14, 15, 16] and this seems to have an effect on the bacterial community in those mutant flies. A direct correlation was demonstrated between de-repression of antimicrobial peptide gene expression in Cad mutants and the dominant growth of one specific bacterium, Gluconobacter spp. strain EW707, leading to gut epithelium breakdown and host mortality [12].
The innate immune system of Drosophila is characterized by immediate immune reactions that effectively eliminate microbial infections [17, 18, 19, 20, 21]. This relies on the recognition of microbial cell wall components, primarily peptidoglycan or its derivatives in the case of bacteria [22, 23]. In addition, cell signaling via the Toll, IMD, JNK and JAK/STAT pathways promotes substantial changes in the gene regulatory profile of immunoresponsive organs [17, 24]. In particular, the fat body, an adipose tissue with key metabolic functions, constitutes a major immunoresponsive organ that expresses and secretes antimicrobial peptides into the hemolymph in response to infection [17, 18]. The combination of such efficient effector molecules with other humoral immune reactions, such as coagulation and melanization, and with cellular immune reactions, such as phagocytosis, quickly eradicates most infections [24]. The immune system in the intestine is far more complex, as it needs to balance between tolerating the commensal gut flora, block the overgrowth of commensals and activate proper responses against pathogenic organisms. To ensure this immune homeostasis, it has been found that immune pathways in the fly are negatively regulated at two levels. Firstly, the presence of low concentrations of immune elicitors in the gut does not trigger a strong response, due to the presence of transcriptional repressors such as Caudal and Nubbin (Nub), thus increasing tolerance to the commensal microbiota [12, 25]. Secondly, the acute phase of immune gene induction in the case of an infection is followed by negative feedback regulation that tunes down the signaling and expression of downstream effectors [reviewed in [21], [26], [27]]. It has been found that mutations in any of several negative regulators cause abnormally high expression levels of immune effector genes, which do not cease normally once the infection is eradicated [15, 28, 29]. In several studies, this was shown to correlate with a decreased lifetime survival, indicating that the aberrant immune reactions are detrimental to the host [27].
We recently demonstrated that the Drosophila POU domain transcription factor Nub-PD, which is a homolog to human Oct1/POU2F1 and Oct2/POU2F2, acts as a repressor of many immune and stress response genes both in the gut and in other tissues of healthy flies under normal culture conditions [25]. This derepression required the transcriptional NF-κB/Rel activator Relish, and repression was necessary for keeping a large number of immune genes silent in the presence of the commensal flora. Here we take this further and show that flies lacking Nub-PD (nub1) are very short-lived and express high levels of antimicrobial peptides throughout their lifetime. The survival of nub1 mutants could be substantially prolonged by growing the flies in the presence of antibiotics, indicating a prominent role of the bacterial community as a cause of the high mortality rate. Deep DNA sequencing demonstrated that nub1 flies serve as hosts for a large diversity of microbes in spite of their overactive immune system. We show that nub1 eclose as adults with a similar bacterial load and diversity as wild-type Oregon R (OrR) flies, and that the bacterial load increases manyfold together with a remarkable shift in the bacterial composition of nub1 flies compared to OrR. This shift is characterized by the fast growth of a large number of Acetobacter spp. and Leuconostoc spp. Thus, this demonstrates how the host genotype can drive a shift in the gut microbial community, which cannot be compensated for by an active immune response, thereby leading to severe microbial dysbiosis and premature death.
Materials and Methods
Fly Stocks and Culture
The following fly strains were used: OrR was used as the wild-type control and nub1b1pr1 used as the nub1 mutant in all the experiments. This nub1 strain has been kept as a homozygous stock for many generations, which may have affected the composition of the microbial gut flora that is transferred between generations. The flies were maintained on instant potato medium (consisting of, per liter, 12.6 g dry yeast, 500 ml syrup, 40 g instant mashed potato powder, 10 g agar, 8.5 ml Nipagin® and 0.25 g ascorbic acid) in mixed female/male populations at 25°C with a 12 h light/12 h dark cycle.
Survival Assay and Antibiotic Treatment
The survival rate of OrR and nub1 flies was measured in four parallel, independent sets of 25 newly eclosed flies of both sexes, in the presence (germ free; GF) and absence (conventionally reared; CR) of an antibiotic cocktail. The flies were transferred to fresh food in new vials every 3-4 days to prevent them from sticking to the food and to facilitate the recording of survival. The antibiotic cocktail was added during preparation of the culture medium to a final concentration of 100 μg/ml of carbenicillin, 100 μg/ml of neomycin, 50 μg/ml of vancomycin and 100 μg/ml of metronidazole [12].
RNA Isolation, Quantitative PCR and RT-PCR Analysis
Intestines were dissected from three pools of 20 flies of both sexes at 1, 12, 21 and 26 days of age. The intestines (pooled from both sexes) and the rest of the fly bodies (carcass, females and males separately) were analyzed separately. RNA extraction, DNase treatment, reverse transcription and PCR were carried out as previously described [30]. When possible, primers and/or probes covered intron/exon boundaries to ensure the specific amplification of cDNA.
For the analysis of 16S bacterial DNA, 34 ng of total DNA from the guts (see below for the isolation procedure) was used per technical replicate reaction. The following quantitative PCR conditions were used: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. The universal 16S primers and probe used for detection were: F: 5′-TCCTACGGGAGGCAGCAGT-3′, R: 5′-GGACTACCAGGGTATCTAATCCTGTT-3′, probe: (6-FAM)-5′-CGTATTACCGCGGCTGCTGGCAC-3′-(TAMRA) [31]. All samples were analyzed in triplicate and the measured mRNA or DNA concentrations were normalized relative to the control RpL32 values. The normalized data were used to quantify the relative concentration of a given mRNA according to comparative cycle threshold (2−ΔΔCT) analysis [32, 33]. Statistical significance was calculated using the paired t test and p values <0.05 were considered significant.
DNA Isolation, PCR, Template Preparation and MiSeq Sequencing
The intestines were dissected from 10 surface-sterilized flies under aseptic conditions. Total DNA was isolated from manually homogenized tissues with the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The DNA concentration was measured with a Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, Calif., USA). Preparation of 16S amplicon libraries with two consecutive PCRs was performed according to the principles described in Hugerth et al. [34], using PCR primer pairs that target the 16S V3-V4 region, 341-F (ACACTCTTTCCCTAC ACGACGCTCTTCCGATCT-NNNNN-AGGATTAGATACCCTGGTA, where NNNNN are five random nucleotides that aid in clustering during sequencing) and 805-R (AGACG TGTGCTCTTCCGATCTCRRCACGAGCTGACGAC). The PCR reactions were set up as 50-μl reactions, using 2 ng of DNA template and 2× Phusion Master Mix (New England Biolabs, Ipswich, Mass., USA). The PCR conditions used were 98°C for 30 s, 20 cycles of 98°C for 10 s, 58°C for 30 s and 72°C for 6 s, followed by 72°C for 2 min. The PCR products were purified using the Qiaquick PCR purification kit (Qiagen) or AMPure XP beads (Beckman Coulter). The second outer PCR reaction was performed in 50-μl reaction volumes containing 23 μl of inner PCR template, specific Multiplex primers (barcoded) and 2× Phusion Master Mix. The PCR conditions used were 98°C for 30 s, 10 cycles of 98°C for 10 s, 62°C for 30 s and 72°C for 7 s, followed by 72°C for 2 min, and then purification as above. DNA concentrations were measured using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, Mass., USA). All samples were pooled and precipitated to a final volume of 15 μl with a concentration of 10 nM/sample. The sequencing was carried out at the Science for Life Laboratory (SciLifeLab), Stockholm, on a MiSeq (Illumina Inc., San Diego, Calif., USA). For more details, including the Multiplex barcoded primers, see https://github.com/EnvGen/LabProtocols/blob/master/Amplicon_dual_index_prep.rst.
MiSeq Data Analysis, OTU Clustering and Diversity Analysis
The 5′ and 3′ reads from each sequenced amplicon were merged, quality controlled and clustered into OTUs (operational taxonomic units) using the UPARSE protocol of USEARCH [35]. Universal OTUs were formed from unique sequences from all samples, excluding sequences only occurring once, with a radius of 1.5% difference from the seed sequence. The taxonomic affinity of OTUs was established by aligning seed sequences to the SILVA database (SSURef 111) [36], using the SINA aligner [37]. Selected OTUs were blasted [38] against NCBI's 16S ribosomal RNA sequences for Bacteria and Archaea to increase the taxonomic resolution of key OTUs beyond the genus level identification that the SILVA database provides. Statistical analysis of the OTU matrix was performed with Explicet [39] and EdgeR [40, 41]. The Shannon diversity of the OTUs was calculated using Explicet (CITE) for each independent sample set. Each estimate is the average of 100 calculations based on subsampling to the size of the smallest sample.
Contamination Analysis
Possible contaminants were identified in the samples by correlation analysis (data not shown) and subtracted from all analyses of OTU abundances. Furthermore, blank samples were prepared by applying all laboratory steps from DNA isolation to PCR and sequence library preparation to purified water. PCR products from the blank samples were sequenced separately and found to be within the sequence diversity of the suspected contaminant OTUs (data not shown).
Data Availability
Sequences of all 509 OTUs, the number of reads for each and the taxonomic classification, including accession numbers of selected species, are available in the online supplementary tables 2 and 3 (see www.karger.com/doi/10.1159/000446368 for all online suppl. material). The raw sequence data were deposited in the European Nucleotide Archive (http://www.ebi.ac.uk/ena/data/view/PRJEB13129).
Results
The Lifetime Survival of nub Mutant Flies Is Doubled upon Treatment with Antibiotics
We previously demonstrated that nub1 mutant flies express high levels of antimicrobial peptide genes, as well as other genes involved in immune and stress responses [25]. To investigate if this aberrant immune gene expression affects lifespan, we followed the survival time of nub1 compared with OrR flies. To also analyze if the presence of the commensal flora would influence the survival length, we carried out the experiment both under CR conditions and in GF conditions (online suppl. fig. 1a).
Observing the lifetime survival of four independent sets of fly cultures of CR-nub1, GF-nub1, CR-OrR and GF-OrR flies showed that the survival curves differed significantly both between genotype and treatment (p < 0.0005; fig. 1). Importantly, nub1 flies exhibited a considerably reduced lifespan (T50 = 21.7 days) compared to OrR (T50 = 68.5) under CR conditions (fig. 1). Transferring newly eclosed flies to food supplemented with a potent cocktail of antibiotics [12] increased the total lifespan of both nub1 and OrR. However, the effect was significantly more pronounced for nub1, whose mean lifetime survival (T50; fig. 1b) increased from 21.7 to 43.8 days (102% lifespan extension), while in OrR it increased from 68.5 to 85.5 days (25% lifespan extension) in the presence of antibiotics, revealing a substantial difference in the relative lifetime extensions between nub1 and OrR (p = 0.0018). In addition, there was considerable variation in the survival curves between the four sets of CR-nub1 flies, while OrR flies displayed consistent survival curves for all independent sets (online suppl. table 1), indicating that separated cultures of nub1 flies provide different survival conditions, and that this effect was more pronounced in CR conditions, which allows microbial growth. This suggests that the number and/or composition of microbes differ between the individual sets of nub1 cultures, and that this has a direct effect on the lifespan of nub1 flies.
Fig. 1.
The lifespan of nub1 flies is considerably shorter than that of OrR flies, and significantly extended in the presence of antibiotics. a The mean survival of OrR and nub1 flies from four independent experiments (replicates of 25 flies kept as isolated cultures) maintained either under CR conditions or as GF cultures in the presence of antibiotics, as depicted with a Kaplan-Meier survival plot. The log-rank test showed strong significant differences between all four survival curves [χ2 (3) = 435.33, p < 0.0005]. The log-rank test of the replicate cultures for each genotype and treatment shows no significant difference for OrR-CR (p < 0.90) and OrR-GF (p < 0.44), while for nub1 cultures there was a difference, especially for nub1-CR (p < 0.0091), but less significant for nub1-GF (p < 0.0374; online suppl. table 1). b The mean survival time (T50) for each replicate culture of the four experimental variants is indicated with a dot. One-way ANOVA followed by Tukey's pairwise comparison tests at level 0.005 showed significant differences in the mean survival between all pairs.
In summary, the survival curves of nub1 and OrR in the absence and presence of antibiotics indicate that nub1 flies may host opportunistic infections. Nonetheless, the abolishment of microbes did not restore the lifespan of nub1 to wild-type proportions, demonstrating that the loss of Nub-PD protein has other negative effects on the general fitness of nub1 flies. This was expected as we previously have shown that the absence of Nub-PD protein affects the expression of numerous genes involved in stress, metabolism, development and differentiation, either directly or indirectly [25]. However, the strong effect on nub1 survival by treating them with antibiotics was surprising in the light of our earlier results, which had indicated that nub1 flies do not carry any cultivatable bacteria in their intestines [25]. Consequently, it raised several interesting questions on the primary cause(s) of the short lifespan of nub1 flies - is it caused by the overactive immune system, by the growth of pathogenic bacteria and intestinal dysbiosis, or by a lack of normal gut epithelium renewal (intestinal homeostasis)? To answer some of these questions we repeated the survival study and analyzed: (1) the expression of two key antimicrobial peptide genes as read-out of immune gene activity; (2) the bacterial load by 16S rDNA quantitative PCR measurements, and (3) the composition of the gut microbiota by deep DNA sequencing (see online suppl. fig. 1b for an overview of the whole experimental setup). Flies were collected and analyzed at time points reflecting the 75, 50 and 25% survival of nub1 (CR), i.e. 75% (T75 = 12 days), 50% (T50 = 21 days) and 25% (T25 = 26 days) survival.
Immune Gene Expression Is Constantly High throughout the Lifespan of nub1 Mutant Flies, Also in the Presence of Antibiotics
We chose to follow the expression of two well-known effectors of the Drosophila immune system, the genes for the antimicrobial peptides CecropinA1 (CecA1) and Diptericin (Dipt), during the lifespan of nub1 flies. Both these genes were previously found to be upregulated in 5- to 10-day-old nub1 flies in a Relish-dependent manner [25]. As expected, a Dipt-mCherry reporter was highly expressed in the midgut of nub1 flies (fig. 2d, e) compared to control OrR flies (fig. 2b, c), confirming the transcriptional derepression of an immune effector gene in the midgut epithelium.
Fig. 2.
Antimicrobial peptide genes are overexpressed throughout the lifespan of nub1 flies. a Schematic drawing of the adult Drosophila digestive system. The different regions of the intestine are indicated: anterior midgut (ant mg), posterior midgut (post mg), copper cell region (cc) and hindgut (hg). b-e Expression of Dipt-mCherry in dissected adult anterior midgut (ant mg) and posterior midgut (post mg) in control flies (b, c) and in nub1 mutants (d, e). Scale bar = 100 µm. f, g Quantification of CecA1 and Dipt mRNA levels in extracts of dissected intestines by reverse transcriptase quantitative PCR. CR and antibiotics-treated/GF flies were dissected on days 12, 21 and 26 posteclosure. All expression levels are related to CR OrR on day 12, which was set to 1. The data are mean values from at least three independent experiments and error bars indicate the standard deviation. Statistical significance was calculated using the paired t test, * p < 0.05, *** p < 0.001.
The expression of CecA1 and Dipt was then analyzed in extracts of dissected intestines and in the rest of the fly body (carcass) by reverse transcriptase quantitative PCR. The expression of CecA1 and Dipt was significantly higher in the intestines dissected from nub1 compared to OrR, both in CR and GF conditions (fig. 2f, g; online suppl. fig. 2b) Also in the carcass samples, CecA1 and Dipt expression was higher in nub1 than in OrR (online suppl. fig. 2a, c). There was an obvious increase in AMP gene expression with time, especially of Dipt in nub1 gut samples at 21 and 26 days (fig. 2g), and this was more pronounced under CR conditions. However, as Dipt expression also increases in the gut of OrR at days 21 and 26, the relative fold change in age-matched pairwise comparisons indicates that the increase is primarily age related and not linked to genotype (online suppl. fig. 2b, c). Thus, nub1 flies express immune genes excessively throughout their lifetime also when grown under GF conditions, confirming that Nub-PD is a strong negative regulator of immune gene expression also in the absence of microbes.
Bacterial Load Increases with Age and Is Generally Higher in nub1 Mutants Compared to OrR Flies
We previously demonstrated that dissected intestines from nub1 mutants contain no or very few cultivatable bacteria [25]. However, preliminary analysis of the gut content showed the presence of bacterial 16S rDNA, indicating that nub1 mutant flies do harbor noncultivatable bacteria in spite of the high levels of immune gene expression in the intestine and the rest of the fly. To investigate this further, we followed the bacterial load of OrR and nub1 flies at the same time points as before (online suppl. fig. 1b). Each independent set of flies were transferred to fresh vials every third day, a procedure which has been shown to allow the establishment of a normal microbial community, as adult flies and eggs carry bacteria externally, which will contaminate the food and then be ingested by larvae and flies [42]. Flies were surface-sterilized and their intestines dissected on day 12, 21 or 26 (online suppl. fig. 1b). Bacterial 16S rDNA and Drosophila RpL32 DNA were measured by qPCR (fig. 3) and, in parallel, the same samples were used for DNA sequence analysis to compare the richness and overall diversity of the bacterial communities (fig. 4). The sequence analysis was also used for the identification of changes in the presence of specific OTUs in the intestines of nub1 and OrR flies (fig. 5, 6; online suppl. table 2).
Fig. 3.
The bacterial load is low in newly eclosed flies and increases with age both in OrR and in nub1 flies, but reaches magnitudes higher in nub1 samples. Bacterial 16S rDNA relative to Drosophila RpL32 DNA in three sample sets of dissected intestines of CR OrR and nub1 flies is shown. The three sample sets were maintained separately in continuous culture, and flies were withdrawn from each set on days 12, 21 and 26 (online suppl. fig. 1b). The samples taken on day 1 (black crosses, x) were taken from another set of independent cultures. Statistical significance was calculated using the paired t test, * p < 0.05, ** p < 0.01.
Fig. 4.
Microbial diversity increases with age in both OrR and nub1 flies. a Alpha-diversity (Shannon) of OTUs for each independent sample set. Mean values are indicated with a black bar. Statistical significance was calculated using the paired t test, * p < 0.05. b Multidimensional scaling plot of all samples based on EdgeR estimates of OTU counts. d = Days.
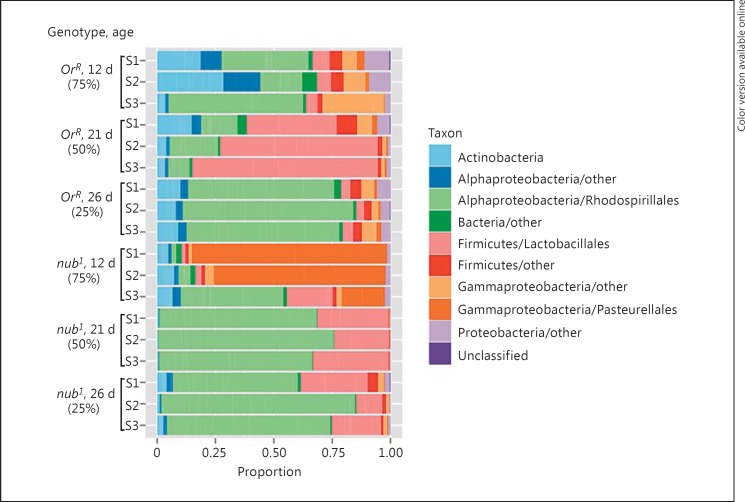
Fig. 5.
The microbial composition shifts radically with age in nub1 flies. The relative abundance of different phyla and dominant orders as assessed by 16S rDNA OTUs for each biological replicate/sample. d = Days.
Fig. 6.
A large number of OTUs are preferentially abundant in nub1 flies. A significant difference existsin the abundance of 16S rDNA sequence reads/counts of OTUs in nub1 and OrR on days (d) 26, 21 and 12, and over all time points. For each graph, positive values on the x-axis (logFC = base 2 logarithm of calculated fold change) reflects significant overrepresentation of the indicated OTU in nub1 compared to OrR guts, while negative values reflect overrepresentation in OrR guts. The y-axis (logCPM = base 2 logarithm of average counts per million) reflects the abundance of the indicated OTU. For calculated numbers, significance and taxonomy, see online supplementary table 4.
The relative bacterial 16S rDNA level (normalized against Drosophila RpL32 DNA) was first measured on day 1 posteclosure, which showed that both strains have very low 16S rDNA values (fig. 3), indicating that neither nub1 nor OrR carry any substantial numbers of bacteria in their guts at eclosure. In OrR flies, the low number of bacteria persisted until day 12, but then increased to higher loads at 21 and 26 days of age (fig. 3). This corroborates earlier studies showing that laboratory strains of Drosophila carry no or very few bacteria in their guts at the time of eclosure, followed by increasing bacterial loads during their adult life [1, 42]. Surprisingly, nub1 flies carried high levels of 16S rDNA in their guts already by day 12. Samples at later time points varied, with some samples showing a very high concentration of 16S rDNA, reflecting extreme bacterial loads in the guts of these flies, while other samples were similar to OrR (fig. 3). This indicates that bacterial growth is established faster in nub1 flies than in OrR, and that it can multiply to excessive numbers compared to what is normally found in laboratory strains.
Diversity and Taxonomic Distribution
To analyze the diversity and composition of the gut microbiota during the relatively short lifespan of nub1 flies, and to compare it with age-matched OrR flies, we extracted DNA from dissected intestines in groups of 10 from each independent set of flies (online suppl. fig. 1b). To allow the detection of rare microbes, we sequenced the DNA on an Illumina MiSeq flow cell. Primers were designed against the hypervariable regions V3 and V4 of the 16S rRNA gene in combination with sample-specific barcode sequences, enabling parallel in-depth analysis of all the samples in one channel [34, 43]. In total, this provided 27 million reads with an average length of 251 nucleotides. After merging and quality filtering, the reads were assigned to 509 OTUs at 1.5% difference from the seed sequence using the UPARSE protocol [35] (online suppl. table 2). The subtraction of contaminant OTUs (see Materials and Methods) resulted in a total of 486 OTUs used in further analyses (online suppl. table 3).
To compare the overall diversity, we analyzed the alpha diversity within each sample and then compared this for genotype and age. We found that the mean diversity of the taxonomic composition is relatively even between genotypes and age (fig. 4a), but due to large variation between the biological replicates, especially at the early time points when the bacterial load was low, most differences were not significant. In general, nub1 samples had slightly less diversity than OrR. At 26 days of age, the nub1 samples revealed significantly reduced diversity compared to OrR, correlating with a shift in microbial communities in nub1, dominated by a few bacterial families, as described below.
We then analyzed the differences in microbial composition between the two genotypes and the three age groups, and determined how much variation there is between individual samples/cultures by carrying out a multidimensional scaling analysis based on estimated OTU abundances (fig. 4b). This showed that the bacterial composition between individual samples of the same genotype and age is relatively similar as they clustered together with the exception of a few samples. The plot shows that nub1 flies separated well from the rest of the samples on days 21 and 26, suggesting that nub1 flies carry their own distinct bacterial communities at 21 and 26 days of age.
The Taxonomic Composition Shifts Dramatically in nub1 Mutants
At the phylum level, Proteobacteria and Firmicutes dominated the taxonomic composition in all samples, but it also contained Actinobacteria and Bacteroidetes. Most samples were dominated by the Rhodospirillales and Lactobacillales orders (fig. 5). Further in-depth taxonomic analysis and comparison between individual sample sets (see online suppl. table 3) revealed that the largest orders found in our samples of the Actinobacteria are Micrococcales and Corynebacteriales, of the Firmicutes is Lactobacillales, of the Alphaproteobacteria is Rhodospirillales (Acetobacteraceae), of the Betaproteobacteria are Burkholderiales and Neisseriales, and of the Gammaproteobacteria is Pasteurellales (Orbaceae).
By comparing the dominant phyla within OrR and nub1, respectively, between different time points, one can see that the proportion of Firmicutes/Lactobacillales was higher on day 21 in OrR than in all other samples. It is clear that the microbial composition shifted radically in nub1 flies between days 12 and 21, with a remarkable increase in Alphaproteobacteria/Rhodospirillales (fig. 5). In fact, the nub1 gut samples on day 12 (75% survival) exhibited the most distinct pattern: a large fraction, but varying between replicates, of Pasteurellales (Orbus spp.). Gammaproteobacteria have not frequently been identified with any high abundance in laboratory-reared Drosophila [reviewed in [8]], except for the frequent presence of endosymbiotic Wolbachia, and in one study Enterobacteriaceae Group Orbus [9], now reclassified as Pasteurellales/Orbus spp.
The most abundant species among the Firmicutes/Lactobacillales showed an interesting host distribution. Lactobacillaceae were abundant in both OrR and nub1, with Lactobacillus plantarum highly abundant in both genotypes, while L. brevis was primarily present in nub1 at days 21 and 26. L. plantarum is a commensal bacterium of the Drosophila intestine [12, 44, 45], shown to confer beneficial and growth-promoting effects on its host [16, 46, 47], while L. brevis has been correlated with some pathogenic properties [48]. Interestingly, Leuconostoc pseudomesenteroides, a well-known fermenting bacterium, previously isolated primarily from wild-caught Drosophila [11, 49], was only found in the nub1 gut samples. The Firmicutes also included abundant sequence reads in most samples of OTUs matching to different species of Gemella, Streptococcus and Staphylococcus (online suppl. table 3). The Bacteroidetes were generally not very abundant, but several OTUs belonging to Prevotella were identified in most of the samples. Within the Alphaproteobacteria, the Acetobacteraceae order contained many different OTUs corresponding to a whole range of Acetobacter species, as described in more detail below. The Alphaproteobacteria also included OTUs with abundant reads in many of the samples matching to Brevundimonas spp. Phyllobacterium and Brucella spp. [32]. OTUs corresponding to Betaproteobacteria were not very frequent in total, but a few genera came up with abundant sequence reads, among them Delftia spp., Massilia spp. and Neisseria spp. Gammaproteobacteria, other than the Pasteurellales, were present primarily in OrR, and among them several Enterobacteriales were abundant, such as Escherichia coli and Serratia marcescens, and OTUs matching Haemophilus parainfluenzae were found in most of the samples (online suppl. table 3).
Significant Differences in the Bacterial Composition between nub1 Flies and OrR
To analyze if the composition of the microbial community could explain the short lifespan of nub1 mutant flies, we performed a comparative analysis of the taxonomic distribution, by examining the differences in abundance (number of sequence reads) of all 486 OTUs between age-matched nub1 and OrR flies, using the Bioconductor tool edgeR[41]. The significant differences of individual OTUs is presented in figure 6 and online supplementary table 4, showing independent graphs at each of the three time points, 12, 21 and 26 days, reflecting 75, 50 and 25% survival of nub1, and a graph comparing the OTUs in each genotype over all time points. One of the most striking observations from this comparison is that many OTUs are overrepresented in nub1 flies, while very few OTUs are overrepresented in OrR (fig. 6). This indicates that several OTUs grow better in nub1 flies, leading to an unbalanced repertoire with some dominating families and genera.
Most surprisingly, several insect pathogens were abundant in OrR but nearly absent in nub1 flies. On day 12, OrR samples carried S. marcescens (out 113) and Erwinia carotovora (Pectobacterium carotovorum; OTU 026), and at all time points Bacillus thurigiensis (OTU 039; fig. 6). As these pathogenic bacteria were essentially absent in nub1 flies (online suppl. table 4) it suggests that these pathogens do not readily use nub1 as a host.
Young nub1 flies (12 days, 75% survival) were frequent carriers of bacteria of the Gammaproteobacteria, order Pasteurellales, genus Orbus (OTUs 008 and 506; fig. 6), while these OTUs were absent in OrR. The two closest known species were O. sasakiae, a bacterium previously isolated from the gut of wild populations of the butterfly Sasakiae charonda[48], and to O. hercynius[50]. However, the sequence identity was only 92% to these species, and we therefore refer to these OTUs as Group Orbus [9]. In addition, Acetobacter persici (OTUs 225, 313 and 416) was overrepresented in nub1 flies on day 12.
The bacterial community of nub1 flies was highly diverse on days 21 (50% survival) and 26 (25% survival), but the community was dominated by species belonging to a few bacterial families. In line with this, more than 20 OTUs were significantly more abundant in nub1 compared to OrR samples (fig. 6). Most of the OTUs, overrepresented in nub1, belong to the group of acetic acid bacteria (AAB), especially A. indonesiensis and A. estunensis, and to lactic acid bacteria, such as Lactobacillus and Leuconostoc (fig. 6; and online suppl. table 4). In addition, OTU 037, matching Corynebacterium variabile (Actinobacteria), another diary fermenting bacterium, was highly overrepresented in nub1 flies and almost absent in OrR (fig. 6; online suppl. table 4), while a large number of OTUs matching other Corynebacteria were present in both nub1 and OrR. Finally, a significant overrepresentation of Enterococcus faecalis (OTU 011) was observed, as it was consistently found at high loads in all samples and age groups of nub1 flies but almost absent from OrR samples (fig. 6; online suppl. table 4).
Discussion
Earlier studies of the composition of the commensal microbiota, based on culture-dependent methods, indicated that animals and humans carry a ‘core microbiota’ with a shared number of microbial species, which has coevolved with the host. Today, direct sequencing approaches have on the contrary demonstrated that the variation in the microbial community between human individuals is enormous, each constituting a complex ecological community [51, 52]. Likewise, most studies of the intestinal microbial community in Drosophila have been based on culture-dependent or bacterial DNA cloning studies, and it has been argued that flies typically harbor at most 15-30 bacterial species [recently reviewed in [8]].
In the present work, we compared the taxonomic composition of gut microbiota using Illumina sequencing of 16S rDNA between laboratory-reared wild-type (OrR) and nub1 mutant flies, during the course of the short lifespan of nub1 flies. In both genotypes, the microbial density and diversity increased considerably during this period, and we identified a total of 486 bacterial OTUs. Each biological replicate typically carried between 150 and 200 OTUs, which is considerably more than in previous studies. We ascribe the larger microbial community identified in the present work to differences in sequencing methods and fly culture conditions compared to previous studies. Direct sequencing is definitely an advantage, as culturing and cloning strategies are known to reduce the number of identified taxa considerably. In addition, the large number of sequence reads provided by Illumina MiSeq sequencing ensures that also rare OTUs will be identified. Furthermore, in many studies on laboratory-reared Drosophila, flies have been kept on food complemented with bacteriostatic compounds which inhibit microbial growth. In the present study, flies were transferred to boiled fly food in the absence of any such compounds, and the stocks had been cultivated like this routinely for many generations. It is possible that this has enabled the establishment of a larger microbial community, which is transferred vertically between generations. From the results presented in this study, we can conclude that the richness and diversity of the microbial composition in the Drosophila gut have been greatly underestimated. A few recent studies support this conclusion, also indicating that Drosophila might harbor a much richer microbiota than previously anticipated, which varies greatly within and between populations and species [10, 11, 53]. These studies also showed that the microbial composition of both laboratory-reared and wild-caught flies was greatly affected by environmental factors, such as local habitat and food source/composition. Taken together, it suggests that the microbial richness in Drosophila may differ by less than one magnitude of that of the human gut, which typically harbors around 1,000 ‘species-level’ phylotypes in each individual [52].
Previous studies have shown that flies eclose without a gut microbiota, which is then established de novo by the consumption of exogenous bacteria [reviewed in [8]], much in resemblance to how human babies are born sterile and how the microbial community is acquired during early childhood [54]. In Drosophila, Blum et al.[42] showed that frequent transfer (every day) of newly eclosed flies to sterile food prevented the establishment of a microbial gut community, while transfer every third day readily allowed the creation of a gut microbiota. In the present study, we adopted a scheme of transferring the flies to new vials every third day and, as expected, the bacterial load within each replicate of OrR flies were low on day 1 and increased with age (fig. 3). Interestingly, the microbial composition varied substantially between replicate samples in young flies (fig. 4b). This suggests that the early microbial colonization is stochastic, leading to high diversity between separate cultures (beta diversity). In older OrR flies (day 26), the alpha-diversity was high within all samples (fig. 4a) but there was less variation between the samples (beta-diversity; fig. 4b), indicating the existence of selective forces that shape the communities to become more similar, although they are not in direct contact with each other. This shaping of the communities, or ‘homogenization’, is likely a result of different host factors, such as immunity, metabolism and physiology, in combination with competition between the microbes, thereby allowing a stable community to be established. However, it may also reflect that certain bacteria have evolved to better colonize guts than other. Most interestingly, such community shaping resembles to a high degree the initial establishment and shaping of microbial communities in humans, in which the microbiota in young children (up to 2 years of age) is highly variable and unstable, while adults who live in similar environments harbor relatively uniform and resilient microbial communities [52, 55]. Thus, the evolutionary forces and ecological constraints maintaining environmental and compositional homeostasis of the microbial gut community seem to act similarly in organisms as divergent as flies and humans.
Contrary to the development of a stable, healthy gut microbiota in OrR flies, the nub1 mutant flies revealed a dramatic shift in their communities at later time points. The microbial load in nub1 increased to extreme levels in some samples (fig. 3) and the microbial composition within nub1 samples continued to diversify (fig. 4a) but, most strikingly, the composition shifted radically in all nub1 samples compared to OrR samples (fig. 4b). This indicates that neither host constraints nor microbial competition were acting normally. This was most extreme on day 21 (50% survival) and 5 days later (day 26) only 25% of the flies were alive and most of these were moribund within a few days, demonstrating the evolvement of a host-threatening state, either due to general bacterial overgrowth or the presence of opportunistic species, or both.
The microbial composition at the phylum level in our samples was dominated by Proteobacteria and Firmicutes (fig. 5a), but also contained Actinobacteria and Bacteroidetes. Interestingly, this profile is similar to the microbial composition of the human gut, which is dominated by Firmicutes and Bacteroidetes, but also contains Proteobacteria, Actinobacteria, Fusobacteria, Verrumicrobia and Cyanobacteria [43, 52, 55]. As mentioned above, the microbiota of laboratory-reared Drosophila has previously been considered to be very sparse. However, wild-caught insects have been reported to display a much greater microbial diversity, especially among the Proteobacteria. Gammaproteobacteria was recently reported as the major bacterial class in the guts of free-living house flies (Musca domestica)[56], and has been isolated frequently from D. melanogaster [44, 57, 58]. Another large group of OTUs in our samples belong to Corynebacteria (Actinobacteria), with 100-500 sequence reads in all samples (online suppl. table 3), while one OTU (037) matching C. variabile was only identified in nub1 flies (fig. 6; online suppl. table 4). This reflects how the lactic acid bacteria C. variabile is common in dairy products, while other well-known Corynebacteria spp. are human pathogens.
We searched for over- and underrepresented OTUs that could explain the fast decline and death of the nub1 flies, either due to the overrepresentation of potential pathogens or due to loss/underrepresentation of beneficial microbes that may support longevity. Direct comparison of the number of sequence reads between OrR and nub1 revealed that very few OTUs were underrepresented in nub1. Most interestingly, some of the underrepresented OTUs in nub1 are well-known insect pathogens, such as S. marcescens, E. carotovora and B. thuringiensis. This strongly indicates that growth of these Drosophila pathogens is effectively blocked in nub1 flies, correlating with the high constitutive expression of AMPs and other immune effectors in this host [25], and suggests that nub1 flies may in fact be protected against these pathogens. However, while several pathogens succumb in this host, other OTUs seemed to have a selective advantage and some of those grew to very high loads.
It may be important to note that the nub1 strain used in this study had been cultivated as a homozygous stock for many generations. This may have created a selective advantage for microbes that confer resistance to AMPs and other immune effectors, as homozygous nub1 flies have an overactive immune system. This could at least in part explain why the bacterial load in nub1 reaches much higher than in OrR, as AMP-resistant bacteria would not be effectively controlled by the host immune system. It will be interesting in future experiments to analyze newly established homozygous nub1 strains in parallel with the strain used here, as well as analyzing how many generations it will take for a homozygous nub1 strain to regain a shifted composition of gut microbiota after treatment with antibiotics. Finally, the growth conditions for bacteria in nub1 mutants may be changed in a complex manner compared to the OrR host. We previously reported that numerous genes encoding proteins involved in metabolic functions are dysregulated in nub1 mutants [25]. Thus, it would be interesting to follow the metabolic profile of nub1 flies compared to OrR, and especially to measure if the concentrations of sugars and other nutrients are changed to confer a more growth-promoting environment.
Among microbes that were overrepresented in nub1 flies is a clade of Gammaproteobacteria, the Group Orbus, which was very abundant in nub1 at early time points but completely lost at later time points, indicating that it was outcompeted by faster growing bacteria. Another significant overrepresentation observed in all samples and age groups of nub1 flies was represented by E. faecalis, suggesting that nub1 is a beneficial host for this bacterium.E. faecalis has previously been isolated from the gut of many different insects [59] and found to be frequently present in some laboratory strains of D. melanogaster [44].
Interestingly, most of the OTUs overrepresented in nub1 belong to the group of AAB, such as Acetobacter (Alphaproteobacteria), and to lactic acid bacteria, such as Lactobacillus and Leuconostoc (Firmicutes). These groups of bacteria are frequently associated with insects that feed on fermenting fruits and vegetables. AAB have been suggested to represent novel insect symbionts [60], and A. pomorum was recently shown to modulate metabolic homeostasis and the developmental rate of Drosophila in a laboratory setting [61], while some AABs have been found to be opportunistic pathogens of insects [12]. It was somewhat surprising to observe such a rich diversity of Acetobacter in our laboratory-reared flies, although Cox and Gilmore [44] identified several Acetobacter spp., and subsequent studies have shown that Drosophila laboratory stocks frequently carry Acetobacter spp. [9, 10, 11, 45]. Most of these studies indicated, however, that the taxonomic diversity is usually low, i.e. few OTUs are usually identified. The high abundance and richness in Acetobacter OTUs in nub1 flies in our study indicate that this genotype is a favorable host for Acetobacter, while we did not isolate other AAB frequently found in insects, such as Gluconobacter and Commensalibacter.
The lactic acid bacteria L. brevis and L. plantarum (Firmicutes) were overrepresented in nub1 flies on days 21 and 26, respectively (fig. 6; online suppl. table 3). Both species have been isolated from Drosophila previously [12, 47, 62] and L. plantarum were found to exert beneficial properties to its fly host and to promote systemic growth [47]. Another fermenting lactic acid bacterium, L. pseudomesenteroides (Firmicutes), was one of the most abundant in nub1 flies both on day 21 and 26 (fig. 6; online suppl. table 4). This bacterium is an opportunistic human pathogen, causing nosocomial urinary tract infections. To our knowledge, it has not been isolated previously from laboratory-reared Drosophila, but from wild populations, especially from cactophilic flies in the Sonoran Desert [49]. Thus, L. pseudomesenteroides can obviously use nub1 flies as a host, but it was not abundant in the OrR samples, and it is quite possible that this is not a beneficial microbe for the fly, and that it acts as an opportunistic pathogen.
Originally, we had hypothesized that the short lifespan of nub1 flies could be a result of the overactive immune system in the absence of the Nub-PD transcriptional repressor [25]. However, the findings that the survival time of nub1 flies increased by more than 100% in the presence of antibiotics (fig. 1), concomitant with a constitutively high immune gene expression also under these GF conditions (fig. 2), indicated that early death of nub1 flies is primarily related to their microbiota and only indirectly due to the derepressed immune system. In fact, CecA1 expression was constantly high in nub1 flies, both in the absence and presence of antibiotics (fig. 2f; online suppl. fig. 2), while the expression of Diptericin followed the changes in bacterial load in the gut (compare fig. 2f and fig. 3).
It was recently reported that nub can act as a differentiation factor in the gut epithelium [63]. This raises the question of whether nub1 flies may have a disorganized or damaged gut structure that may contribute to their short lifespan. Although this is quite possible, the fact that nub1 fly guts express high levels of antimicrobial peptides strongly indicates that the guts consist of fully differentiated enterocytes that are immunocompetent. We also did not gain support for the hypothesis that nub1 flies would possess a severely damaged gut, as tested by feeding adult flies with a food dye that would leak out into the fly abdomen if the gut is damaged and cause the flies' abdomens to turn blue [64] (data not shown). Therefore, our conclusion is that the difference in lifespan between GF and CR nub1 flies is primarily due to an inability to regulate the growth and the composition of the microbiota, leading to a forceful microbial dysbiosis. This does not rule out that other processes in gut development and function are affected in nub1 flies both in GF and CR flies, as both have a shorter lifespan compared to GF OrR (fig. 1a). A radical shift in microbiota composition from Firmicutes to Proteobacteria was in fact recently reported to occur upon intestinal barrier failure during Drosophila aging, and it was shown that this shift was driving mortality [53]. Thus, it is possible that expansion of Alphaproteobacteria and Gammaproteobacteria on the expense of Firmicutes in nub1 guts mimics a progressed aging process with pleiotropic effects on health and lifespan.
In summary, we found that the microbial community of nub1 grows to very high loads until the flies succumb to death. Feeding antibiotics to nub1 flies doubled their lifespan, demonstrating the strong link between the microbial community, host genotype and early death. We hypothesize that a shift in the microbial community and selection of resistant microbes has evolved in nub1 hosts over time. This community, which is transferred vertically between generations, grows well even in the presence of high concentrations of endogenous immune effector molecules, such as antimicrobial peptides. The gut microbiota of nub1 flies may contain pathogens, but our results rather indicate that it constitutes a diversified community of primarily Acetobacter spp. and Lactobacillales, of which most may in fact be considered as commensals in Drosophila. Yet as a consequence of the resistance of these microbes, nub1 flies will not be able to fight these bacteria when they continue to multiply and reach high loads. As a result, flies will succumb to death within a short time frame due to the overgrowth of this community, most likely due to energy collapse and organ failure. Thus, we have shown that changes in host genotype, and an inability to regulate the normal growth and composition of the gut microbiota, can lead to dramatic shifts of the microbial community that subsequently causes severe dysbiosis and early death.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
We thank Fabian Staubach and Won-Jae Lee for providing samples that served as independent controls. The computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under project b2013167. Jan-Olov Persson of the Mathematical Statistics Group (SFG) at Stockholm University is gratefully acknowledged for assistance with statistical analyses. This research was supported by the Swedish Research Council, the Swedish Cancer Society and the Faculty of Science at Stockholm University.
References
- 1.Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 2.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 3.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 4.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Karpac J, Jasper H. Promoting longevity by maintaining metabolic and proliferative homeostasis. J Exp Biol. 2014;217:109–118. doi: 10.1242/jeb.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broderick NA, Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes. 2012;3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staubach F, Baines JF, Kunzel S, Bik EM, Petrov DA. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS One. 2013;8:e70749. doi: 10.1371/journal.pone.0070749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong AC, Chaston JM, Douglas AE. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013;7:1922–1932. doi: 10.1038/ismej.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. Innate immune homeostasis by the homeobox gene Caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 13.Kleino A, Myllymäki H, Kallio J, Vanha-aho LM, Oksanen K, Ulvila J, Hultmark D, Valanne S, Rämet M. Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol. 2008;180:5413–5422. doi: 10.4049/jimmunol.180.8.5413. [DOI] [PubMed] [Google Scholar]
- 14.Lhocine N, Ribeiro PS, Buchon N, Wepf A, Wilson R, Tenev T, Lemaitre B, Gstaiger M, Meier P, Leulier F. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe. 2008;4:147–158. doi: 10.1016/j.chom.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Paredes JC, Welchman DP, Poidevin M, Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity. 2011;35:770–779. doi: 10.1016/j.immuni.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Bosco-Drayon V, Poidevin M, Boneca IG, Narbonne-Reveau K, Royet J, Charroux B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe. 2012;12:153–165. doi: 10.1016/j.chom.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Uvell H, Engström Y. A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet. 2007;23:342–349. doi: 10.1016/j.tig.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 19.Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Davis MM, Engström Y. Immune response in the barrier epithelia: lessons from the fruit fly Drosophila melanogaster. J Innate Immun. 2012;4:273–283. doi: 10.1159/000332947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrandon D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster: from resistance to resilience. Curr Opin Immunol. 2013;25:59–70. doi: 10.1016/j.coi.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- 23.Kurata S. Peptidoglycan recognition proteins in Drosophila immunity. Dev Comp Immunol. 2014;42:36–41. doi: 10.1016/j.dci.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 25.Dantoft W, Davis MM, Lindvall JM, Tang X, Uvell H, Junell A, Beskow A, Engström Y. The Oct1 homolog Nubbin is a repressor of NF-κB-dependent immune gene expression that increases the tolerance to gut microbiota. BMC Biol. 2013;11:99. doi: 10.1186/1741-7007-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu JH, Ha EM, Lee WJ. Innate immunity and gut-microbe mutualism in Drosophila. Dev Comp Immunol. 2010;34:369–376. doi: 10.1016/j.dci.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaidman-Rémy A, Hervé M, Poidevin M, Pili-Floury S, Kim MS, Blanot D, Oh BH, Ueda R, Mengin-Lecreulx D, Lemaitre B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Kim LK, Choi UY, Cho HS, Lee JS, Lee WB, Kim J, Jeong K, Shim J, Kim-Ha J, Kim YJ. Downregulation of NF-κB target genes by the AP-1 and STAT complex during the innate immune response in Drosophila. PLoS Biol. 2007;5:e238. doi: 10.1371/journal.pbio.0050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MM, Alvarez FJ, Ryman K, Holm AA, Ljungdahl PO, Engström Y. Wild-type Drosophila melanogaster as a model host to analyze nitrogen source dependent virulence of Candida albicans. PLoS One. 2011;6:e27434. doi: 10.1371/journal.pone.0027434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 34.Hugerth LW, Wefer HA, Lundin S, Jacobsson HE, Lindberg M, Rodin S, Engstrand L, Andersson AF. DegePrime, a program for degenerate primer design for broad taxonomic-range PCR in microbial ecology studies. Appl Environ Microbiol. 2014;80:5116–5123. doi: 10.1128/AEM.01403-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 36.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pruesse E, Peplies J, Glöckner FO. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012;28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson CE, Harris JK, Wagner BD, Granger D, Browne K, Tatem B, Feazel LM, Park K, Pace NR, Frank DN. Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics. 2013;29:3100–3101. doi: 10.1093/bioinformatics/btt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson MD, McCarthy DJ, Smyth GK. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum JE, Fischer CN, Miles J, Handelsman J. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. MBio. 2013;4:e00860–13. doi: 10.1128/mBio.00860-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008;3:e2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox CR, Gilmore MS. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun. 2007;75:1565–1576. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong CN, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol. 2011;13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci USA. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Lee KA, Kim SH, Kim EK, Ha EM, You H, Kim B, Kim MJ, Kwon Y, Ryu JH, Lee WJ. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Wagner JA. Bacteria Associated with the Cactophilic Species Drosophila arizonae and Drosophila aldrichi. Tuscon, The University of Arizona. 2008 [Google Scholar]
- 50.Volkmann M, Skiebe E, Kerrinnes T, Faber F, Lepka D, Pfeifer Y, Holland G, Bannert N, Wilharm G. Orbus hercynius gen. nov., sp. nov., isolated from faeces of wild boar, is most closely related to members of the orders ‘Enterobacteriales' and Pasteurellales. Int J Syst Evolutionary Microbiol. 2010;60:2601–2605. doi: 10.1099/ijs.0.019026-0. [DOI] [PubMed] [Google Scholar]
- 51.Kuczynski J, Costello EK, Nemergut DR, Zaneveld J, Lauber CL, Knights D, Koren O, Fierer N, Kelley ST, Ley RE, Gordon JI, Knight R. Direct sequencing of the human microbiome readily reveals community differences. Genome Biol. 2010;11:210. doi: 10.1186/gb-2010-11-5-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, Rana A, Rera M, Pellegrini M, Ja WW, Walker DW. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12:1656–1667. doi: 10.1016/j.celrep.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Sommer F, Bäckhed F. The gut microbiota - masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 56.Gupta AK, Nayduch D, Verma P, Shah B, Ghate HV, Patole MS, Shouche YS. Phylogenetic characterization of bacteria in the gut of house flies (Musca domestica L.) FEMS Microbiol Ecol. 2012;79:581–593. doi: 10.1111/j.1574-6941.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 57.Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DE. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl Environ Microbiol. 2007;73:3470–3479. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juneja P, Lazzaro BP. Providencia sneebia sp. nov. and Providencia burhodogranariea sp. nov., isolated from wild Drosophila melanogaster. Int J Syst Evol Microbiol. 2009;59:1108–1111. doi: 10.1099/ijs.0.000117-0. [DOI] [PubMed] [Google Scholar]
- 59.Martin JD, Mundt JO. Enterococci in insects. Appl Microbiol. 1972;24:575–580. doi: 10.1128/am.24.4.575-580.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crotti E, Rizzi A, Chouaia B, Ricci I, Favia G, Alma A, Sacchi L, Bourtzis K, Mandrioli M, Cherif A, Bandi C, Daffonchio D. Acetic acid bacteria, newly emerging symbionts of insects. Appl Environ Microbiol. 2010;76:6963–6970. doi: 10.1128/AEM.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 62.Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–152. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Korzelius J, Naumann SK, Loza-Coll MA, Chan JS, Dutta D, Oberheim J, Glasser C, Southall TD, Brand AH, Jones DL, Edgar BA. Escargot maintains stemness and suppresses differentiation in Drosophila intestinal stem cells. EMBO J. 2014;33:2967–2982. doi: 10.15252/embj.201489072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
Sequences of all 509 OTUs, the number of reads for each and the taxonomic classification, including accession numbers of selected species, are available in the online supplementary tables 2 and 3 (see www.karger.com/doi/10.1159/000446368 for all online suppl. material). The raw sequence data were deposited in the European Nucleotide Archive (http://www.ebi.ac.uk/ena/data/view/PRJEB13129).