Abstract
Rationale
The antimicrobial peptides (AMPs) LL-37 and calprotectin are important players in the innate immunity of human airways. In patients with diseases characterized by bacterial colonization, the airway concentrations of these AMPs are increased. Less is known about their presence and release patterns in healthy humans. Our aim was to determine whether LL-37 and calprotectin are released after the activation of the innate immune response in the peripheral airways.
Methods
Healthy volunteers underwent exposure to endotoxin and vehicle in contralateral segment bronchi. After 12 or 24 h, samples of bronchoalveolar lavage fluid (BALf) were collected bilaterally from exposed segments. Cell and AMP concentrations were assessed, as were the pro-form and active form of LL-37.
Results
Both LL-37 and calprotectin were detected in cell-free BALf from both endotoxin- and vehicle-exposed segments. The concentrations of precursor and active LL-37 and neutrophils were significantly higher in endotoxin-exposed segments after 12 and 24 h, and the concentrations of LL-37 and neutrophils correlated positively. The concentrations of calprotectin were not markedly affected by exposure to endotoxin.
Conclusions
Local endotoxin exposure elicits the release and activation of LL-37 but not calprotectin in healthy human peripheral airways, suggesting an inducible involvement of LL-37 in the local innate immune response.
Keywords: Endotoxin, Lipopolysaccharide, LL-37, Calprotectin, Lung, Neutrophils, Bronchoalveolar lavage fluid
Introduction
Antimicrobial peptides and proteins (AMPs) are important players in the innate immune response of the lungs, and can directly kill invading microorganisms as well as trigger the adaptive immune response [1]. Their different structures, variable net charges and sequence diversities give AMPs a wide functional diversity. Some AMPs are constitutively expressed, while others are inducible [1].
Human cathelicidin LL-37 is stored in and released from specific granulae of the polymorphonuclear neutrophil leukocytes (neutrophils) and from airway epithelial cells in the form of the precursor protein hCAP-18 [2]. Once hCAP-18 is released into the extracellular compartment, it is cleaved by a serine protease to the active LL-37 [3]. It has been detected in induced sputum and lung tissue from patients with chronic obstructive pulmonary disease [4, 5] and also in induced sputum from patients with cystic fibrosis [5]. This AMP is inducible and kills bacteria by destroying their lipid layers in a manner shared with other AMPs. Under certain conditions [6], LL-37 may also exert a chemotactic effect on neutrophils, macrophages, and T lymphocytes [7, 8] as well as an apoptosis-inducing effect on bronchial epithelial cells. Thus, LL-37 seems to have a dual role: both performing rapid microbial killing and modulating the innate immune response [9].
Calprotectin is a bacteriostatic AMP that is clinically used as a biomarker in inflammatory bowel disease. This AMP has been detected in the sputa, bronchoalveolar lavage fluid (BALf), and blood of patients with chronic obstructive pulmonary disease [10, 11, 12] as well as in induced sputum from patients with exacerbations of cystic fibrosis [10]. Calprotectin has also been detected in induced sputum after the inhalation of endotoxin [13].
While LL-37 and calprotectin have been detected in the airways of patients with chronic inflammatory lung diseases, their respective release patterns in healthy human airways is not known. Whether calprotectin and LL-37 can be induced by inflammatory stimuli of bacterial origin in the peripheral airways of healthy humans in vivo has not previously been studied. To address these questions, we here quantified calprotectin and LL-37 at the protein level in the peripheral airways of healthy human volunteers with and without prior exposure to the TLR-4 agonist endotoxin.
Materials and Methods
Study Design
This study was performed at the Clinical Section of the Department of Respiratory Medicine, Sahlgrenska University Hospital, Göteborg, Sweden, and was approved by the local Ethics Committee (Dnr 618-02, 065-04, 683-07). Healthy volunteers were recruited after local advertising and gave their informed consent. The clinical part of the study was performed from 11 November 2003 to 3 December 2008, and it constituted a part of a larger project on innate immune response in healthy human airways after endobronchial endotoxin exposure, from which other results have already been presented [14, 15, 16].
At the first visit, the medical history of the volunteers was recorded and physical examination, spirometry, and electrocardiogram were performed. A written informed consent was obtained from each participant. Current or past smoking, a history of allergy or atopy, and any regular medication (with the exception of oral contraceptives) constituted the exclusion criteria. All included participants had a normal ventilatory lung function defined as forced expiratory volume during 1 s (FEV1) >80% of predicted value, a normal electrocardiogram, and a normal physical status. Bronchoscopies were performed at the second and third visits, separated by an interval of 12 or 24 h. During the second visit, i.e., at the first bronchoscopy, endotoxin and saline exposure took place, and during the third visit, BAL was performed.
Bronchoscopy, Endotoxin Exposure, and BAL
All bronchoscopies were performed transorally by 1 experienced bronchoscopist, who is a Board-Certified Clinical Specialist in Respiratory Medicine (M.E.S.), with the volunteer in supine position. A slightly modified version of a previously published endotoxin exposure protocol was used [17]. Ketobemidonhydrochloride (2.5–7.5 mg depending on clinical conditions) was given as a premedication, followed by nebulized local anesthesia sprayed into the oropharynx (xylocaine 10 mg/dose, 3 × 2 doses). Further local anesthesia was given as needed through the bronchoscope (xylocaine 20 mg/mL, up to 14 mL). Endobronchial photographs were taken bilaterally to enable BALf sampling at the second bronchoscopy from exactly the same bronchial segments that had been exposed to endotoxin or vehicle.
During the first bronchoscopy, a balloon-tipped catheter was inserted through the bronchoscope, placed in a bronchial segment (either the lingula or right middle lobe) and inflated with air to seal off the chosen segment proximally before challenge. Vehicle, i.e., 10 mL of 0.9% phosphate-buffered saline (PBS), was instilled in the bronchial segment, followed by 10 mL of air. The bronchoscope was subsequently retracted and transferred to the corresponding segment in the contralateral lung, where the sealing-off procedure was followed by instillation of the TLR-4 agonist endotoxin (4 ng/kg reference-standard lipopolysaccharide [LPS] from Escherichia coli 0113:H10, USP, Rockville, MD, USA) diluted with PBS up to 10 mL of fluid, followed by 10 mL of air. The bronchoscope was then retracted and, with the volunteer in place, the head end of the operating table was elevated to 30° for 1 h to minimize coughing and the spread of the instilled fluid from the challenged segments.
During the second bronchoscopy, which was performed either 12 or 24 h after the first, vehicle- and endotoxin-exposed bronchial segments were reidentified, the bronchoscope was wedged in each of these segments, and bilateral BAL was performed (3 × 50 mL of PBS at 37°C), starting with the vehicle-exposed segment. Fluid was aspirated after each 50-mL aliquot, and the entire harvested yield was pooled in a siliconized glass container. The BALf samples were immediately transported on ice to the research laboratory.
Handling of BALf Samples
The volume recovered was measured and the BALf sample was filtered for the removal of mucus and cell debris. The filtered sample was centrifuged (at 300 g for 10 min at 4°C) and the cell-free BALf (i.e., the centrifugation supernatant) was separated and frozen at −80°C until further analysis. The cell pellet from the centrifugation was resuspended in PBS, and the cell viability was determined using trypan blue exclusion. Total cell count was determined utilizing a hemocytometer (Bürker counting chamber). The differential counts for BALf cells were performed utilizing stained cytospin preparations after counting 300 cells twice, using a conventional light microscope (Axioplan 2, Carl Zeiss®, Jena GmbH, Eching, Germany).
Quantification of Extracellular LL-37 and Calprotectin Protein
Total concentrations of LL-37 and calprotectin in the BALf were determined using a commercial enzyme-linked immunosorbent assay (ELISA; LL-37 with HK321 and calprotectin with HK325; Hycult Biotech, PB Uden, The Netherlands). The complete heterodimers of calprotectin (with both subunits), the precursor protein hCAP-18, and LL-37 itself were detected by the respective utilized ELISAs. Samples below the standard curve were given half the lowest standard curve value in both analyses.
Analysis of the Pro-Form and Active Forms of LL-37 Protein
The BALf (4 mL) was cleared from debris by centrifugation (3,000 rpm for 10 min). To enrich for peptides and proteins, the supernatant was applied to reversed-phase OASIS-columns (1 mL; Waters Corp., Milford, MA, USA). The columns were washed with 0.1% trifluoroacetic acid (TFA) in water, and eluation was done with 1 mL acetonitrile (80% in 0.1% TFA). The eluates were lyophilized overnight and the pellets were dissolved in 20 µL 0.1% TFA. The protein content was measured with a NanoDrop system (Thermo Fisher Scientific, Waltham, MA, USA). For Western blot analysis, 20 µg of peptide extract was applied to each lane on gradient NuPAGE-gels (4–12%), using 2-(N-morpholino) ethanesulfonic acid (MES) buffer. The pro-form and the active form of LL-37 were detected with monoclonal anti-LL-37 [18]. An anti-human IgG-antibody (rabbit) coupled to horseradish peroxidase was used as a secondary reagent. The signal was developed by the ECL prime system (Amersham, GE Healthcare UK Ltd., Little Chalfont, UK). For densitometry, all films were scanned and analyzed in ImageJ. Data were normalized to the internal control for each gel (2 ng LL-27, positive control). Nondetectable levels were arbitrarily defined as 0.01. The Western blot images were finalized in Photoshop (Adobe Systems Inc., San José, CA, USA).
Statistical Considerations
The SPSS 19.0 (SPSS Inc., Chicago, IL, USA) software package was used for the statistical analyses, except for the expression of LL-37 in BALf, for which GraphPad Prism v6.0 (GraphPad Software Inc., La Jolla, CA, USA) was used. Differences between endotoxin- and vehicle-exposed segments of the lungs of the same volunteer were analyzed using the nonparametric Wilcoxon signed-rank test. For differences between time points in samples from different volunteers, comparisons were made by calculating the difference between the results from the endotoxin- and vehicle-exposed segment of each volunteer, at each time point, followed by a comparison of these differences between 12 and 24 h, using the Mann-Whitney U test. Data are presented as median and individual values with range. For correlations between individual data in different groups, the Spearman two-tailed rank correlation test was applied. p < 0.05 was accepted as statistically significant.
Results
Study Population
Nineteen healthy nonsmoking volunteers (20–27 years of age, 9 males and 10 female) were included and completed the study protocol. They were allocated into 2 groups; this was decided on a practical basis determined by hospital logistics, which resulted in 7 subjects for the second bronchoscopy at 12 h, and 12 subjects at 24 h.
Influx of Leukocytes
While the recovery of BALf volume from the endotoxin-exposed bronchial segments was comparable to that from the vehicle-exposed segments (100 [89–113] vs. 102 [91–115] mL), the cell viability was significantly higher in BALf samples from the endotoxin-exposed segments (96 [91–99] vs. 87 [77–93]%), in line with previous studies [19]. There were no clear gender differences in either recovery or cell viability.
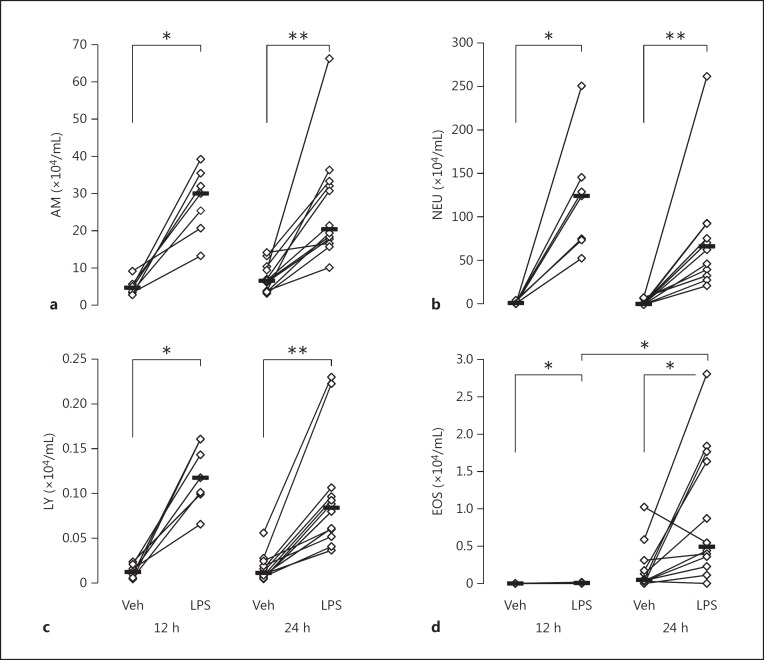
The total cell concentration was considerably higher in BALf samples from the endotoxin-exposed segments than from the vehicle-exposed ones at both 12 h (172 [75–297] vs. 9 [4–17] × 104 cells/mL; p < 0.05) and 24 h (118 [37–352] vs. 13 [5–26] × 104 cells/mL; p < 0.01), giving, on average, a 19-fold-higher concentration on the endotoxin-exposed side at 12 h. At the same time point, the concentration of neutrophils was (on average) 66-fold, that of alveolar macrophages 7-fold, and that of lymphocytes 9-fold-higher on the endotoxin-exposed side. This effect of endotoxin largely remained at 24 h after exposure (Fig. 1).
Fig. 1.
a–d. Cell concentrations in BALf at 12 h (n = 7) and 24 h (n = 12) after endobronchial exposure to either vehicle (Veh) or endotoxin (LPS) in healthy human airways. a Alveolar macrophages (AM). b Neutrophils (NEU). c Lymphocytes (LY). d Eosinophils (EOS). Median and individual values are shown. Significant differences between lungs (paired analysis) at each time point and between time points (unpaired analysis) are indicated. * p < 0.05; ** p < 0.01.
Quantification of Extracellular LL-37 and Calprotectin Protein
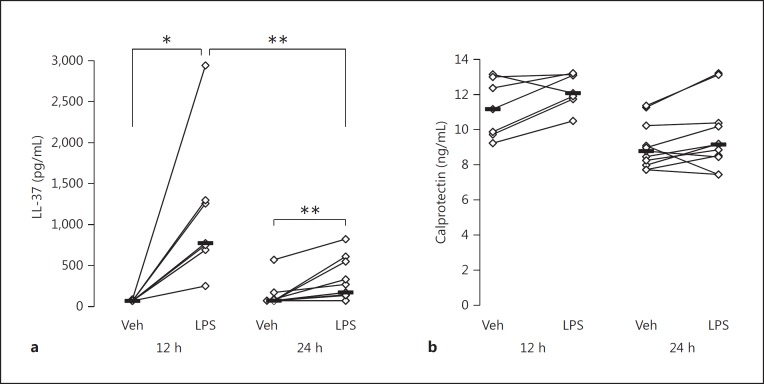
Calprotectin protein was detected in all samples from both vehicle- and endotoxin-exposed segments at both time points. As for LL-37, detectable concentrations were found in 4/19 samples from the vehicle-exposed segments and in 16/19 samples from the endotoxin-exposed segments at both time points.
As judged from the ELISA measurements, the median concentration of LL-37 protein was higher in the BALf from the endotoxin-exposed bronchial segments than in the vehicle-exposed ones at both time points (Fig. 2). This difference was numerically larger at 12 h (median 776 [252–2,946] vs. 70 [70–89] pg/mL) than at 24 h (median 170 [70–822] vs. 70 [70–569] pg/mL). The concentrations of calprotectin did not differ significantly between endotoxin- and vehicle-exposed segments at either time point, or between time points.
Fig. 2.
Concentration of LL-37 (a) in BALf at 12 h (n = 7) or 24 h (n = 12) after endobronchial exposure to either vehicle (Veh) or endotoxin (LPS) in contralateral lung segments, and concentration of calprotectin (b) at 12 h (n = 7) or 24 h (n = 11) after the same endobronchial exposure. Median and individual values are shown. Significant differences between lungs (paired analysis) at each time point and between time points (unpaired analysis) for LL-37 are indicated. * p < 0.05; ** p < 0.01.
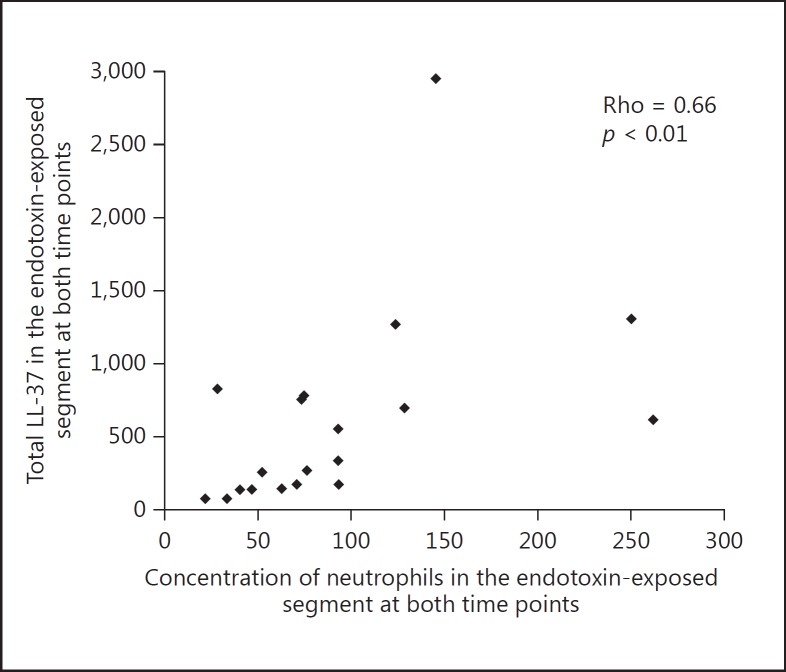
There was a positive correlation (Rho = 0.66; p = 0.002) between the total concentrations of LL-37 protein and neutrophils in the endotoxin-exposed lung segments (Fig. 3), but not between LL-37 and alveolar macrophages or lymphocytes.
Fig. 3.
Correlation between concentrations of neutrophils and total LL-37 in BALf after endobronchial exposure to endotoxin (both time points are included).
Analysis of the Pro-Form and Active Form of LL-37 Protein
The precursor of LL-37 was detected in all except 1 sample from both vehicle- and endotoxin-exposed segments at both time points. The active form of LL-37 was detected in 2/18 samples from the vehicle-exposed segments and in 12/18 samples from the endotoxin-exposed segments at both time points.
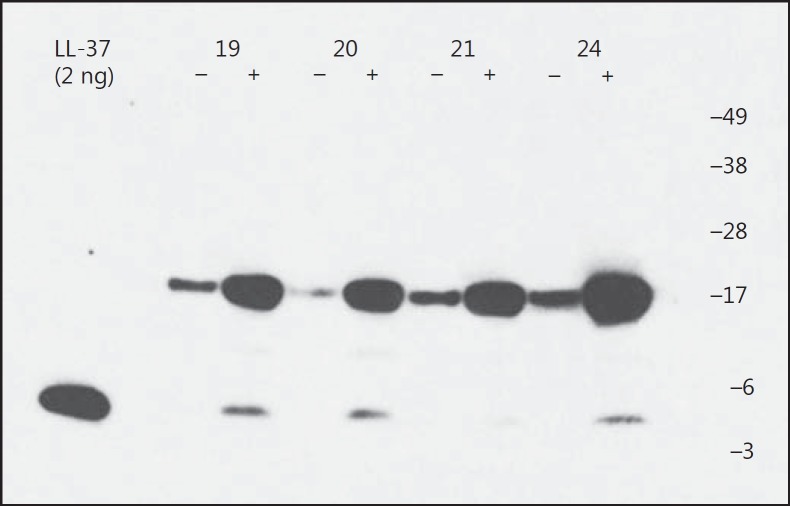
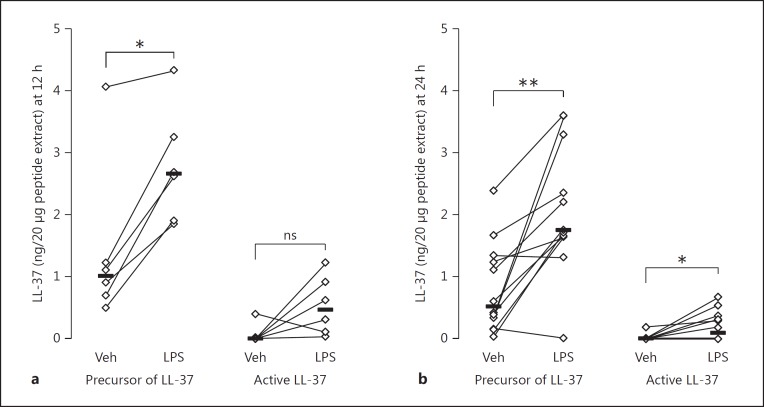
As judged from the Western blot analysis, the median concentration of the precursor of LL-37 was higher in the BALf from the endotoxin-exposed bronchial segments than in the vehicle-exposed ones, at both time points, while the concentration of the active form of LL-37 was significantly higher in BALf from the endotoxin-exposed segments at 24 h only (Fig. 4, 5). There were no clear differences between time points in either the pro-form or the active form of LL-37.
Fig. 4.
LL-37 expression in BALf at both 12 and 24 h after exposure. A peptide/protein-extract was made from BALf from 18 healthy volunteers after endobronchial exposure to either vehicle or endotoxin in contralateral lung segments, marked with a “-” and “+”, respectively. A representative Western blot analysis, using a monoclonal antibody against LL-37 (1 out of 5 gels), is shown. As a positive control, 2 ng of LL-37 was used (left lane). Numbers designate identification of the volunteers, 2 from each time point (Nos. 19 and 20 at 24 h, Nos. 21 and 24 at 12 h).
Fig. 5.
LL-37 expression in BALf at 12 h (a, n = 6) or 24 h (b, n = 12) after endobronchial exposure to either vehicle (Veh) or endotoxin (LPS) in contralateral lung segments. Densitometric semiquantitative analysis of the scanned films from Western blot analysis (in Fig. 4), using ImageJ software. Nondetectable levels were arbitrarily defined as “0.01” in the graph. Significant differences between lungs (paired analysis) at each time point are indicated. * p < 0.05; ** p < 0.01; ns, nonsignificant (p = 0.094).
Discussion
We found that LL-37 was detectable in endotoxin-exposed bronchial segments already 12 h after exposure but that it was seldom detectable in the unexposed segments. This response started to recede already 24 h after exposure. In contrast, we found no corresponding endotoxin-induced effect on calprotectin at either time point. Our study therefore suggests that there is a rapid and inducible release of LL-37 but not calprotectin in response to a bacterial stimulus in the peripheral airways of healthy humans.
This study provides new evidence for the inducible involvement of LL-37 but not calprotectin in the innate immune response to the TLR4-agonist endotoxin in the peripheral airways of healthy humans. First, we verified that a single intrabronchial exposure to endotoxin in this compartment leads to a prompt innate immune response, represented by a massive accumulation of leukocytes, especially neutrophils, within 12 h after the exposure and in parallel to the local increase of LL-37 protein. Second, and more importantly, we found that the innate immune response to the TLR4-agonist endotoxin derived from E. coli is associated with either measurable or higher concentrations of LL-37 protein, indicating release from its cellular source(s), with the concentrations at 12 and 24 h after exposure paralleling those of key cellular players in the innate immune response, i.e., neutrophils. Third, we found evidence of activation of the secreted precursor molecule of LL-37, with enhanced concentrations of active LL-37 in the endotoxin-exposed bronchial segment at 24 h, and the same tendency, though not significant, at 12 h. Fourth, we found a clear positive correlation between the concentrations of LL-37 and neutrophils in BALf samples from the endotoxin-exposed segments at both time points, in support of previous reports on neutrophils as an important source of LL-37 [3]. Taken together, our evidence clearly indicates the inducible involvement of LL-37 in the innate immune response of the peripheral human airways.
To become active, LL-37 must be cleaved by a serine protease from its inactive precursor hCAP-18 molecule, and this normally takes place in the extracellular compartment [3]. Interestingly, the median concentration of active LL-37 was higher 24 h after exposure than at 12 h. In a previous study, we showed that the net serine protease activity after endotoxin exposure is higher 24 h after exposure than at 48 h [14]. Here, we demonstrated markedly higher levels of the active form of LL-37 24 h after endotoxin exposure in the same human model. Thus, the sum of these findings suggest that Gram-negative bacterial stimulation causes the release of LL-37 and its potential activation factor serine protease from neutrophils, although the functional interaction between the 2 remains to be verified in molecular studies.
Notably, the AMP LL-37 has been shown to act together with human-β-defensin-2 (hBD-2) [20], an AMP whose presence we have previously demonstrated in healthy airways 12 and 24 h after endotoxin exposure, in the very same human model that we utilized in this study [15]. We find it interesting that these 2 AMPs have previously been shown to exert synergistic effects in the killing of Staphylococcus aureus, a Gram-positive bacterium of relevance for human pneumonia [21, 22]. Each of the mentioned proteins possesses the individual capacity to kill Gram-negative bacteria [23, 24] and their potential synergy is a motivation for conducting further studies on human airways.
In our human airway model, calprotectin was detectable in all samples of BALf, but at levels independent of the exposure to endotoxin. Calprotectin thus appears to be constitutively expressed in the peripheral airways of healthy humans but was not inducible by endotoxin in our model. Different dynamics of synthesis and release, compared to LL-37, might explain our findings. The intragranular location of LL-37 as opposed to the cytoplasmic location of calprotectin leads to different patterns of release, in which LL-37 is more easily mobilized than calprotectin. This does not, however, exclude the possibility that the release of calprotectin can be elicited by bacterial components including endotoxin. Calprotectin has previously been detected in induced sputum after the inhalation of endotoxin, but the levels showed considerable interindividual variability and differed between time points, thereby preventing a straightforward analysis of the causative effect of endotoxin exposure [10, 13]. Moreover, the discrepancy with the results of this study may also relate to the sampling technique. The sampling of BALf and induced sputum is likely to reflect different compartments of the airways. The referred discrepancy may also relate to the dose of endotoxin, and a more widespread exposure of the airways with the inhalation procedure compared with intrabronchial instillation. In a report by Kido et al. [25], endotoxin exposure of neutrophils from human blood that were cultured in vitro resulted in a calprotectin release that peaked as rapidly as within 30 min, with calprotectin concentrations returning to baseline within 2–6 h. Such a scenario would go unnoticed in our model.
To conclude, we forward new evidence of the inducible involvement of LL-37 protein, clearly different from that of calprotectin, being associated with the innate immune response to a stimulus from Gram-negative bacteria in the peripheral airways of healthy humans. Our results are compatible with neutrophils constituting an important source of LL-37 in the investigated compartment, the peripheral airways. Although calprotectin is constitutively present, its release does not seem to be induced by a TLR-4 agonist, at least not during the time frame we examined.
Disclosure Statement
No author has any potential competing financial interest that is relevant for this study.
Funding Sources
We received financial support from the Swedish Heart-Lung Foundation (A.L., I.Q.), the Swedish Research Council (A.L., P.B.), and the King Gustav V and Queen Victoria Freemason Research Foundation (A.L.). Federal funding was obtained from Karolinska Institutet (A.L., P.B.), the Västra Götaland Region Council (A.L.) and Stockholm Region Council (A.L., P.B.).
Author Contributions
M.E.S. and M.S. contributed equally to this study. The study was conceived by M.E.S., M.S., A.L., and I.Q., clinical study sampling was performed by M.E.S., laboratory analyses were conducted by M.S., S.T., and P.B. The original draft was written by I.Q., M.E.S. and M.S. and reviewed by all coauthors, further developed and finalized by M.E.S., M.S., A.L., and I.Q., and approved by all coauthors.
Acknowledgements
Monica Lindh, biotechnician, is gratefully acknowledged for her skilful technical assistance, as is registered nurse Monica Crona for her excellent work with the clinical coordination of the study.
References
- 1.Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel) 2014;7:545–594. doi: 10.3390/ph7050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golec M, Reichel C, Lemieszek M, Mackiewicz B, Buczkowski J, Sitkowska J, Skorska C, Dutkiewicz J, Milanowski J, Ziesche R. Cathelicidin LL-37 in bronchoalveolar lavage and epithelial lining fluids from COPD patients and healthy individuals. J Biol Regul Homeost Agents. 2012;26:617–625. [PubMed] [Google Scholar]
- 3.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 4.Jiang YY, Xiao W, Zhu MX, Yang ZH, Pan XJ, Zhang Y, Sun CC, Xing Y. The effect of human antibacterial peptide LL-37 in the pathogenesis of chronic obstructive pulmonary disease. Respir Med. 2012;106:1680–1689. doi: 10.1016/j.rmed.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest. 2005;128:2316–2326. doi: 10.1378/chest.128.4.2316. [DOI] [PubMed] [Google Scholar]
- 6.Smeianov V, Scott K, Reid G. Activity of cecropin P1 and FA-LL-37 against urogenital microflora. Microbes Infect. 2000;2:773–777. doi: 10.1016/s1286-4579(00)90359-9. [DOI] [PubMed] [Google Scholar]
- 7.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140:103–112. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 8.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. Impact of LL-37 on anti-infective immunity. J Leukoc Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 10.Gray RD, MacGregor G, Noble D, Imrie M, Dewar M, Boyd AC, Innes JA, Porteous DJ, Greening AP. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med. 2008;178:444–452. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkel D, Rist W, Seither P, Weith A, Lenter MC. Proteomic study of human bronchoalveolar lavage fluids from smokers with chronic obstructive pulmonary disease by combining surface-enhanced laser desorption/ionization-mass spectrometry profiling with mass spectrometric protein identification. Proteomics. 2005;5:2972–2980. doi: 10.1002/pmic.200401180. [DOI] [PubMed] [Google Scholar]
- 12.Holmgaard DB, Mygind LH, Titlestad I, Madsen H, Pedersen SS, Mortensen OH, Pedersen C. Calprotectin - a marker of mortality in COPD? Results from a prospective cohort study. COPD. 2013;10:581–587. doi: 10.3109/15412555.2013.781580. [DOI] [PubMed] [Google Scholar]
- 13.Michel O, Doyen V, Leroy B, Bopp B, Dinh DH, Corazza F, Wattiez R. Expression of calgranulin A/B heterodimer after acute inhalation of endotoxin: proteomic approach and validation. BMC Pulm Med. 2013;13:65. doi: 10.1186/1471-2466-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith ME, Bozinovski S, Malmhall C, Sjostrand M, Glader P, Venge P, Hiemstra PS, Anderson GP, Linden A, Qvarfordt I. Increase in net activity of serine proteinases but not gelatinases after local endotoxin exposure in the peripheral airways of healthy subjects. PLoS One. 2013;8:e75032. doi: 10.1371/journal.pone.0075032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glader P, Smith ME, Malmhall C, Balder B, Sjostrand M, Qvarfordt I, Linden A. Interleukin-17-producing T-helper cells and related cytokines in human airways exposed to endotoxin. Eur Respir J. 2010;36:1155–1164. doi: 10.1183/09031936.00170609. [DOI] [PubMed] [Google Scholar]
- 16.Che KF, Tengvall S, Levanen B, Silverpil E, Smith ME, Awad M, Vikstrom M, Palmberg L, Qvarfordt I, Skold M, Linden A. Interleukin-26 in antibacterial host defense of human lungs. Effects on neutrophil mobilization. Am J Respir Crit Care Med. 2014;190:1022–1031. doi: 10.1164/rccm.201404-0689OC. [DOI] [PubMed] [Google Scholar]
- 17.O'Grady NP, Preas HL, Pugin J, Fiuza C, Tropea M, Reda D, Banks SM, Suffredini AF. Local inflammatory responses following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med. 2001;163:1591–1598. doi: 10.1164/ajrccm.163.7.2009111. [DOI] [PubMed] [Google Scholar]
- 18.Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, Agerberth B. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003;53:211–216. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- 19.Karimi R, Tornling G, Grunewald J, Eklund A, Skold CM. Cell recovery in bronchoalveolar lavage fluid in smokers is dependent on cumulative smoking history. PLoS One. 2012;7:e34232. doi: 10.1371/journal.pone.0034232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front Biosci. 2008;13:3760–3767. doi: 10.2741/2964. [DOI] [PubMed] [Google Scholar]
- 21.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 22.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 23.Chromek M, Arvidsson I, Karpman D. The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157:H7-mediated disease. PLoS One. 2012;7:e46476. doi: 10.1371/journal.pone.0046476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnakumari V, Packiyanathan KK, Nagaraj R. Human-beta-defensins-1–3 and analogs do not require proton motive force for antibacterial activity against Escherichia coli. FEMS Microbiol Lett. 2013;348:52–57. doi: 10.1111/1574-6968.12242. [DOI] [PubMed] [Google Scholar]
- 25.Kido J, Kido R, Kataoka M, Fagerhol MK, Nagata T. Induction of calprotectin release by Porphyromonas gingivalis lipopolysaccharide in human neutrophils. Oral Microbiol Immunol. 2004;19:182–187. doi: 10.1111/j.0902-0055.2004.00139.x. [DOI] [PubMed] [Google Scholar]