Abstract
Background
Mannose-binding lectin (MBL) and ficolins are pattern recognition molecules (PRMs) that play an important role during infection through activation of the lectin complement pathway. We assessed whether plasma PRM levels were associated with mortality in patients with necrotizing soft tissue infection (NSTI).
Methods
We conducted a prospective, observational study over 25 months involving 135 NSTI patients with a maximum follow-up of 2.7 years. Blood samples were taken upon admission. Non-infected patients served as controls.
Results
PRM levels were significantly lower compared with controls. A baseline Ficolin-2 level below the median was associated with mortality at the end of follow-up (p = 0.007). No significant association was found for MBL, Ficolin-1 and Ficolin-3. A Ficolin-2 level below the median had a negative predictive value of 0.94 for 28-day mortality, and a level below the optimal cut-off was independently associated with 28-day mortality when adjusted for age, sex and chronicity [hazard ratio 6.27 (95% confidence interval 2.28-17.21), p < 0.0001], also when Simplified Acute Physiology Score II was included in the analysis [hazard ratio 3.16 (95% confidence interval 1.03-9.73), p = 0.045].
Conclusions
All PRMs were significantly lower in patients with NSTI than in controls. Only baseline Ficolin-2 was associated with short- and long-term mortality. A high baseline Ficolin-2 level indicated a 94% chance of surviving the first 28 days after admission.
Key Words: Sepsis, Mortality, Bacterial infection, Immunity
Introduction
Necrotizing soft tissue infection (NSTI) is a bacterial infection causing progressive necrosis of subcutaneous tissue, fascia or muscle, partially due to release of bacterial toxins. Limited data exist on the disease burden, but the incidence is estimated to be 40 cases per 1,000,000 person-years in the United States, with 4.8 deaths per 1,000,000 person-years [1, 2], and similar data have been reported from New Zealand and the United Kingdom [3, 4].
Patients with diabetes, intravenous drug abuse or hematological malignancies are at particular risk of contracting NSTI, but 25% of the cases occur in patients without comorbidity [5]. Among several immunological processes during infection, the complement system of innate immunity plays a pivotal role. Patients with NSTI might have a different immunological response, especially considering the accumulating evidence of complement activation as an important contributor to tissue necrosis after ischemia [6].
The complement system is part of the innate immune defense and is activated through three pathways: the classical, the alternative and the lectin pathway [7]. The latter is activated by multimeric pattern recognition molecules (PRMs) comprising collagen and globular recognition domains and includes mannose-binding lectin (MBL), Ficolin-1, Ficolin-2 and Ficolin-3. MBL deficiency is associated with bacterial infections [8, 9], but there are limited data regarding ficolins and infections. Nevertheless, decreased survival was found in septic patients with polymorphisms in the promoter region of the FCN1 gene [10], and survival tended to be lower in patients with community-acquired pneumonia and low Ficolin-2 level [11].
Improvement in the clinical care of NSTI requires identification of new biomarkers that are associated with early stages of the disease and outcome. A better understanding of the mechanisms related to innate immune activation following NSTI may also lead to the development of new therapeutic agents, individualized treatment and improved patient survival. To our knowledge, no studies have investigated the role of the lectin complement pathway, including MBL and ficolin levels, in patients with NSTI.
The aim of the present study was to investigate the association between plasma PRM levels of the lectin complement pathway and mortality in patients with NSTI. We hypothesized that PRM levels were associated with 28-day and long-term mortality with a maximum follow-up of 2.7 years. Lastly, we aimed to investigate the degree of complement pathway activation in patients with NSTI and in non-infected controls.
Subjects and Methods
Setting and Patients
In this prospective, observational study we included patients presenting with NSTI over 25 months at Copenhagen University Hospital (Rigshospitalet, Copenhagen, Denmark). The study was conducted as previously described in detail [12]; some data from the present cohort have been published elsewhere [13]. The study is registered at ClinicalTrials.gov (NCT02180906; https://clinicaltrials.gov/ct2/show/NCT02180906?term=NCT02180906&rank=1).
Inclusion criteria were a diagnosis of NSTI based on surgical findings of necrosis engaging any layer of the soft tissue compartments, age ≥18 years, and admission to the intensive care unit (ICU) or surgery for NSTI at our hospital. The exclusion criterion was lack of NSTI confirmation during surgery.
Inclusion criteria for control patients were elective orthopedic surgery at our hospital and age ≥18 years. Exclusion criteria were ongoing infection or inflammatory conditions. A total of 65 control patients, matched for age and sex, were included.
Data Collection
From electronic patient records we obtained data on age, chronic disease, ICU scores, site of infection, microbial etiology and biochemistry. Vital statistics were extracted from the hospital database linked to the Danish Civil Registration System, where all persons with residency in Denmark are registered with a unique civil registration number. Follow-up was until November 2015.
We drew blood from an arterial line into EDTA tubes at four discrete time points: on admission (baseline) and on the following three days. For control patients, we drew blood by venous puncture at three discrete time points: on the day before surgery (baseline), on the first postoperative day (2-6 h postoperatively) and on the second postoperative day. The blood sample was immediately put on ice, centrifuged within 40 min (3,500 rpm, 2,400 g, 10 min) and stored at −80°C until processing.
Enzyme-Linked Immunosorbent Assays
PRMs of the Lectin Complement Pathway. Plasma levels of Ficolin-1, Ficolin-2, Ficolin-3 and MBL were quantified in specific sandwich enzyme-linked immunosorbent assays (ELISAs) with monoclonal antibodies for each marker according to previously described methods [10, 14, 15, 16]. In brief, ELISA plates were coated with specific in-house-produced monoclonal catching antibodies (anti-Ficolin-1 FCN166, anti-Ficolin-2 FCN216, anti-Ficolin-3 FCN334 or anti-MBL HYB131-1) and incubated with EDTA plasma samples diluted in phosphate-buffered saline with 0.05% Tween (Ficolin-1 1:100, Ficolin-2 1:300, Ficolin-3 1:500, MBL 1:300). PRM levels were detected with specific detection antibodies against Ficolin-1 (HP9039; Hycult Biotech, Uden, The Netherlands) and in-house-produced biotinylated antibodies against Ficolin-2 (anti-Ficolin-2 FCN219), Ficolin-3 (anti-Ficolin-3 FCN334) and MBL (anti-MBL HYB131-1). Subsequently, a polyclonal donkey anti-rabbit antibody or streptavidin-conjugated horseradish peroxidase was applied and plates were developed with o-phenylenediamine dihydrochloride. Samples were tested in duplicate against a standard pool with known concentration. Assays were optimized for automated analysis in a polystyrene 384-well format using the Biomek FX platform (Beckman Coulter, Fullerton, Calif., USA) for pipetting and a subsequent DTX880 Multimode Detector system for analyses at 490 nm.
Residual Complement Activation Capacity. Residual complement deposition via the classical, lectin and alternative pathways was quantified in ELISAs essentially as previously described, but with some modifications [17, 18]. Classical pathway activity was assessed on ELISA plates coated with human serum albumin (HSA) (Sigma, A9731)/anti-HSA (DAKO, 0001) immune complexes, and ficolin-mediated lectin pathway activity was assessed on acetylated bovine serum albumin (BSA) prepared as previously described [18]. EDTA plasma samples for classical or lectin pathway activation were diluted 1:400 or 1:100, respectively, in barbital buffer (5 mM barbital natrium, 145 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, pH 7.4) with 0.05% Tween-20 (Barb-T) to eliminate contributions from alternative pathway activation. Alternative pathway activity was assessed on ELISA plates coated with lipopolysaccharide from Salmonella typhosa (Sigma, L6386). For alternative pathway activation, EDTA samples were diluted 1:10 in Barb-T added 10 mM EDTA and 10 mM MgCl to avoid contributions from the classical and the lectin pathways. Downstream deposition of C3 was detected with biotinylated rabbit-anti-C3c (Dade Behring, Marburg, Germany) and horseradish peroxidase-conjugated streptavidin was subsequently applied. Samples were tested in duplicate against a standard pool. Residual activation capacity was calculated as percentage of the complement activation capacity in the baseline samples of the controls.
Statistical Analyses
Continuous data are presented as medians (interquartile range, IQR) and compared by the Mann-Whitney U test. Categorical data are presented as absolute numbers (%) and compared by χ2 test or Fisher's exact test. Correlations were assessed using Spearman's rank correlation test. The log-rank test was used to assess differences in long-term mortality (end of follow-up) according to median plasma PRM levels and also by optimal cut-off (maximum sum of sensitivity and specificity). We compared 28-day mortality by univariate and multivariate Cox analysis and reported the results with hazard ratio (HR) and 95% confidence interval (CI). Adjustment for sex, age, Simplified Acute Physiology Score II (SAPS II) and chronic disease (diabetes, liver cirrhosis, chronic kidney disease, cardiovascular disease, chronic obstructive pulmonary disease, peripheral vascular disease, immune deficiency, malignancy, rheumatoid disease) was performed. Receiver operating characteristic (ROC) curves were analyzed for 28-day mortality and an optimal cut-off was identified as the value corresponding to the maximum sum of sensitivity and specificity.
A p value <0.05 was considered statistically significant. Analyses were performed using Statistical Package for the Social Sciences 22.0 software (SPSS Inc., Chicago, Ill., USA) and GraphPad Prism 6.0 software (GraphPad Inc., La Jolla, Calif., USA). Sample size calculation was determined by planned recruitment of at least 82 patients based on previous studies using the same analysis [19] as described in another article [13].
Ethics
The study was approved by the regional ethics committee (H-2-2014-071) and the Danish Data Protection Agency (J. No. 30-1282). Written informed consent was obtained from all patients or their legal substitute.
Results
We enrolled 162 patients of whom 27 fulfilled the exclusion criterion. Thus, we included 135 patients with NSTI who had a 28-day mortality of 16% [n = 22 (95% CI 11–24%)] and a long-term mortality of 34% [n = 46 (95% CI 27–42%)] with a maximum follow-up of 2.7 years (median 20 months, range 3-33 months). Non-survivors had lower baseline Ficolin-2 and Ficolin-3 levels and higher SAPS II and Sepsis-Related Organ Failure Assessment (SOFA) scores (table 1).
Table 1.
Baseline characteristics of patients with NSTI stratified according to 28-day mortality from admission
| Survivors (n = 113) | Non-survivors (n = 22) | p value | |
|---|---|---|---|
| Age, years | 61 (51 – 68) | 66 (55 – 70) | 0.22 |
| Male sex | 69 (61%) | 15 (68%) | 0.53 |
| Chronic diseasea | 70 (62%) | 16 (73%) | 0.34 |
| Simplified Acute Physiology Score IIb | 43 (33 – 50) | 61 (49 – 77) | <0.0001 |
| Sepsis-Related Organ Failure Assessment score (day 1)c | 7 (4 – 9) | 11 (7 – 13) | 0.00 |
| Laboratory risk indicator for necrotizing fasciitis scored | 8 (6 – 9) | 7 (4 – 10) | 0.59 |
| Lectin markers | |||
| MBL, ng/ml | 664.6 (244.5 – 1,463.0) | 460.9 (158.4 – 1,292.8) | 0.19 |
| Ficolin-1, ng/ml | 322.3 (207.9 – 523.0) | 306.3 (147.4 – 649.4) | 0.77 |
| Ficolin-2, µg/ml | 3.6 (1.7 – 6.2) | 1.3 (0.8 – 2.1) | <0.0001 |
| Ficolin-3, ug/ml | 15.8 (10.6 – 23.0) | 12.1 (6.0 – 15.6) | 0.01 |
| Primary site of infection | |||
| Head/neck | 19 (17%) | 2 (9%) | 0.53 |
| Chest | 3 (3%) | 2 (9%) | 0.19 |
| Abdomen | 8 (7%) | 3 (14%) | 0.39 |
| Extremities | 50 (44%) | 12 (54%) | 0.38 |
| Genital/perineum | 33 (29%) | 3 (14%) | 0.13 |
| Microorganism | |||
| Group A streptococcus | 34 (30%) | 3 (14%) | 0.11 |
| Group B streptococcus | 2 (2%) | 0 (0%) | 0.53 |
| Group C streptococcus | 2 (2%) | 2 (9%) | 0.12 |
| Group G streptococcus | 3 (3%) | 0 (0%) | 0.44 |
| Staphylococcus aureus | 12 (11%) | 2 (9%) | 0.74 |
| Other | 28 (29%) | 12 (54%) | 0.08 |
| Negative findings | 32 (23%) | 3 (14%) | 0.08 |
| Biochemistry | |||
| Hemoglobin, mmol/l | 5.8 (5.0 – 6.6) | 4.7 (4.1 – 6.1) | 0.002 |
| Leukocyte count, x109/l | 16.7 (11.1 – 23.5) | 18.8 (8.3 – 31.0) | 0.53 |
| C-reactive protein, mg/l | 219 (150 – 299) | 162 (79 – 303) | 0.10 |
| Creatinine, μmol/l | 104 (72 – 169) | 228 (131- 261) | 0.001 |
| Lactate, mmol/l | 2.1 (1.2 – 3.5) | 7.3 (1.6 – 16.5) | 0.01 |
| Outcomes | |||
| Septic shock upon admission | 82 (73%) | 14 (64%) | 0.40 |
| ICU admissione | 104 (92%) | 19 (86%) | 0.41 |
| Mechanical ventilation within 7 days | 100 (89%) | 22 (100%) | 0.13 |
| Renal replacement therapy within 7 days | 22 (20%) | 12 (55%) | 0.001 |
| Amputation of extremity within 7 days | 13 (12%) | 8 (36%) | 0.01 |
Values denote median (IQR) or number (%). Differences according to 28-day mortality were analyzed with the Mann-Whitney U test or χ2 test/Fisher's exact test.
One or more of the following: diabetes, liver cirrhosis, chronic kidney disease, cardiovascular disease, chronic obstructive pulmonary disease, peripheral vascular disease, immune deficiency, malignancy, rheumatoid disease.
Patients with missing value: n = 5 (n = 4 survivors, n = 1 non-survivors).
Patients with missing value: n = 6 (n = 5 survivors, n = 1 non-survivors).
Patients with missing value: n = 15 (n = 14 survivors, n = 1 non-survivors).
Three patients died before admission to the ICU.
PRMs of the Lectin Complement Pathway
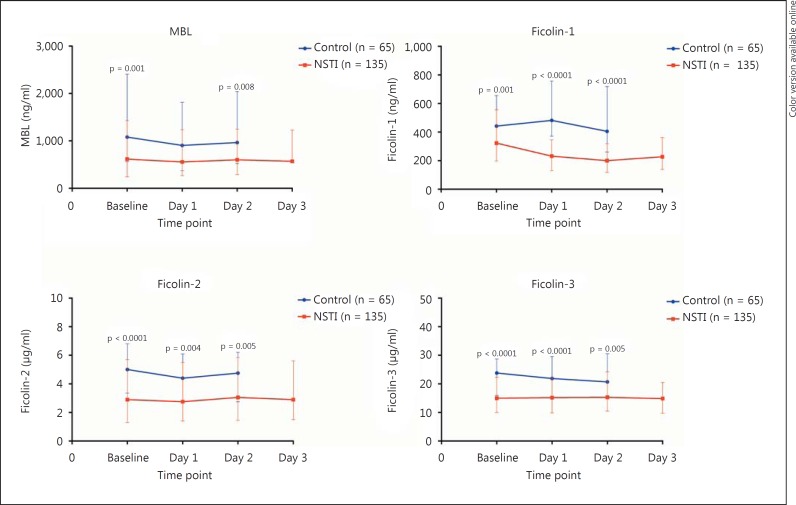
Patients with NSTI had significantly lower baseline PRM levels compared with non-infected controls, and the levels remained low for the following 3 days (fig. 1). The Ficolin-2 level correlated negatively with SAPS II (ρ = −0.26, p = 0.002) and MBL with SOFA score at baseline (ρ = −0.18, p = 0.046). We found no correlation between clinical scores and the plasma levels of Ficolin-1 and Ficolin-3 (table 2).
Fig. 1.
Levels of PRMs upon admission (baseline) and on the following days in patients with NSTI and in non-infected control patients. Values denote medians with IQR. Differences were analyzed with the Mann-Whitney U test. Microtiter plates were coated with monoclonal antibodies against Ficolin-1, Ficolin-2, Ficolin-3 and MBL. EDTA plasma samples were diluted in phosphate-buffered saline with 0.05% Tween (Ficolin-1 1:100, Ficolin-2 1:300, Ficolin-3 1:500, MBL 1:300).
Table 2.
Spearman rank correlation between clinical scores or complement activity and concentration of biomarkers at baseline (upon admission) in patients with NSTI
| MBL |
Ficolin-1 |
Ficolin-2 |
Ficolin-3 |
|||||
|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | p | p | p | |
| SAPS II | –0.10 | 0.252 | –0.07 | 0.454 | –0.26 | 0.002 | –0.02 | 0.847 |
| SOFA | –0.18 | 0.046 | –0.10 | 0.273 | –0.17 | 0.059 | 0.02 | 0.821 |
| LRINEC | –0.08 | 0.395 | 0.12 | 0.181 | 0.09 | 0.329 | 0.08 | 0.376 |
| CPa | 0.09 | 0.06 | 0.21 | 0.02 | 0.31 | <0.0001 | 0.45 | <0.0001 |
| LPa | 0.12 | 0.18 | 0.24 | 0.007 | 0.47 | <0.0001 | 0.59 | <0.0001 |
| APa | 0.33 | <0.0001 | 0.37 | <0.0001 | 0.46 | <0.0001 | 0.40 | <0.0001 |
SAPS II = Simplified Acute Physiology Score II; SOFA = Sepsis-Related Organ Failure Assessment score; LRINEC = Laboratory Risk Indicator for Necrotizing Fasciitis score; CP = classical pathway; LP = ficolin-mediated lectin pathway; AP = alternative pathway.
Baseline complement pathway residual activation capacity.
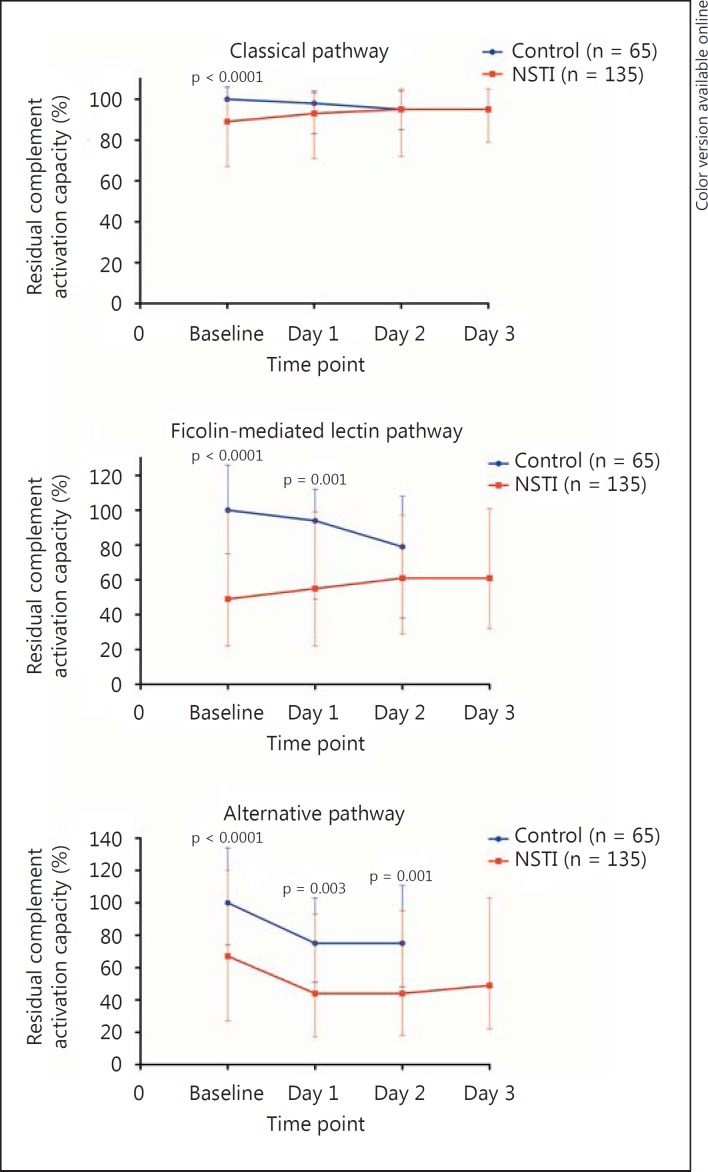
Additionally, patients with NSTI had significantly lower baseline activation potential of the three complement pathways, but to a varying degree: an 11% difference in the classical pathway, a 51% difference in the ficolin-mediated lectin pathway, and an 33% difference in the alternative pathway (fig. 2). The ficolin-mediated lectin pathway activation capacity was significantly associated with SAPS II (ρ = −0.24, p = 0.005), but not the classical (ρ = −0.16, p = 0.063) or alternative pathway activation capacity (ρ = −0.06, p = 0.498). In addition, Ficolin-2 and Ficolin-3 showed the strongest correlation to the residual activation capacity of the ficolin-mediated lectin pathway (table 2).
Fig. 2.
Residual activation capacity of the complement system in patients with NSTI and in non-infected control patients. Values denote medians with IQR. Differences were analyzed with the Mann-Whitney U test. Assessment of classical pathway activity was performed on anti-human albumin/albumin immune complexes (plasma samples diluted 1:400), ficolin-mediated lectin pathway activity on acetylated BSA (plasma samples diluted 1:1,000) and alternative pathway activity on lipopolysaccharide (plasma samples diluted 1:10).
Mortality Analyses of Patients with NSTI
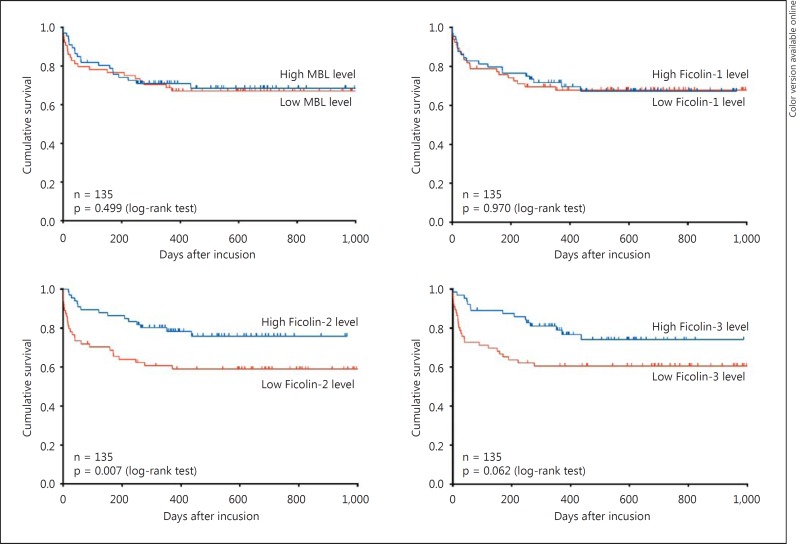
A baseline Ficolin-2 level below the median was significantly associated with long-term mortality (p = 0.007) (fig. 3). A similar association was found between 28-day mortality and low Ficolin-2 and Ficolin-3 levels [HR 5.01 (1.70-14.83), p = 0.004, and HR 3.56 (1.31-9.65), p = 0.013, respectively] (table 3). The associations were not significant when we adjusted for SAPS II.
Fig. 3.
Kaplan-Meier curves of long-term mortality up to 2.7 years in patients with NSTI stratified by median level of MBL (≤627.3 ng/ml), Ficolin-1 (≤322.3 ng/ml), Ficolin-2 (≤2.9 µg/ml) and Ficolin-3 (≤15.0 µg/ml). Differences were assessed by the log-rank test.
Table 3.
Cox regression analysis of mortality up to day 28 (time of censoring) in patients with NSTI based on high versus low concentrations of the biomarkers according to median baseline values
| Unadjusted |
Adjusted for age, sex, chronic disease |
Adjusted for age, sex, chronic disease, SAPS IIa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| MBL | |||||||||
| High (>627.3 ng/ml) | 1 | 1 | 1 | ||||||
| Low (≤627.3 ng/ml) | 2.25 | 0.92 – 5.52 | 0.077 | 2.23 | 0.91 – – 5.48 | 0.080 | 2.64 | 1.01 – 6.92 | 0.048 |
| Ficolin-1 | |||||||||
| High (>322.3 ng/ml) | 1 | 1 | 1 | ||||||
| Low (≤322.3 ng/ml) | 0.98 | 0.43 – 2.27 | 0.969 | 0.85 | 0.36 – 2.01 | 0.713 | 0.67 | 0.27 – 1.70 | 0.404 |
| Ficolin-2 | |||||||||
| High (>2.9 µg/ml) | 1 | 1 | 1 | ||||||
| Low (≤2.9 µg/ml) | 5.01 | 1.70 – 14.83 | 0.004 | 4.73 | 1.59 – 14.07 | 0.005 | 2.14 | 0.65 – 7.04 | 0.209 |
| Ficolin-3 | |||||||||
| High (>15.0 µg/ml) | 1 | 1 | 1 | ||||||
| Low (≤15.0 µg/ml) | 3.56 | 1.31 – 9.65 | 0.013 | 3.42 | 1.26 – 9.29 | 0.016 | 2.67 | 0.83 – 8.48 | 0.099 |
| Optimal Ficolin-2 cut-offb | |||||||||
| High (>1.9 µg/ml) | 1 | 1 | 1 | ||||||
| Low (≤1.9 µg/ml) | 6.68 | 2.46 – 18.14 | <0.0001 | 6.27 | 2.28 – 17.21 | <0.0001 | 3.16 | 1.03 – 9.73 | 0.045 |
Five patients were not included in the analysis due to missing data of SAPS II.
Found by maximum sum of sensitivity (0.77) and specificity (0.70).
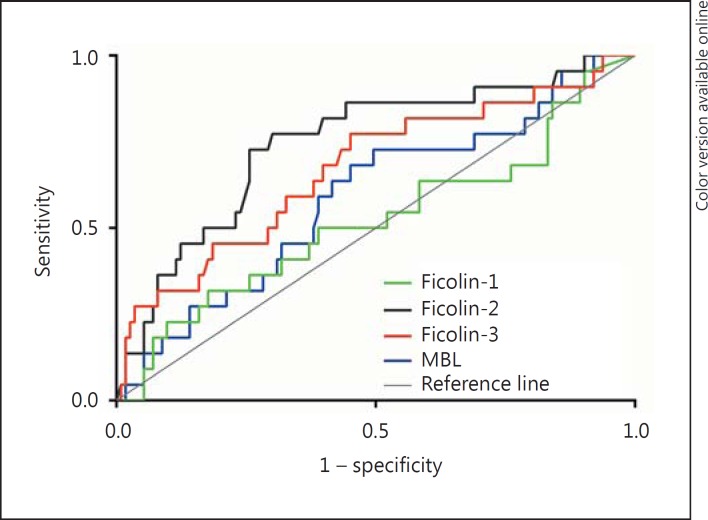
Interestingly, Ficolin-2 was shown to have the highest sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and area under the ROC curve (table 4; fig. 4). The optimal cut-off for Ficolin-2 was 1.9 µg/ml (sensitivity = 0.77, specificity = 0.70). When the optimal cut-off was used instead of the median, baseline Ficolin-2 level proved to be a significant independent predictor of 28-day mortality [HR 6.27 (2.28-17.21), p < 0.0001], also when SAPS II was included in the statistical model [HR 3.16 (1.03-9.73), p = 0.045] (table 3).
Table 4.
Diagnostic accuracy of low baseline biomarker levels in predicting 28-day mortality in patients with NSTI
| MBL | Ficolin-1 | Ficolin-2 | Ficolin-3 | |
|---|---|---|---|---|
| Sensitivity | 0.68 (0.47 – 0.85) | 0.50 (0.30 – 0.70) | 0.82 (0.61 – 0.94) | 0.77 (0.56 – 0.91) |
| Specificity | 0.53 (0.49 – 0.56) | 0.50 (0.46 – 0.54) | 0.56 (0.52 – 0.58) | 0.55 (0.51 – 0.58) |
| PPV | 0.22 (0.15 – 0.27) | 0.16 (0.10 – 0.23) | 0.27 (0.20 – 0.30) | 0.25 (0.18 – 0.30) |
| NPV | 0.90 (0.83 – 0.95) | 0.84 (0.77 – 0.90) | 0.94 (0.87 – 0.98) | 0.93 (0.86 – 0.97) |
| Area under the ROC curve | 0.59 (0.46 – 0.72) | 0.52 (0.38 – 0.66) | 0.75 (0.63 – 0.86) | 0.67 (0.54 – 0.80) |
Data are presented as fractions (95% CI). The prevalence of 28-day mortality was 16%. Low baseline level is defined by the median.
Fig. 4.
ROC curve of 28-day mortality in patients with NSTI according to the baseline level of the lectin markers.
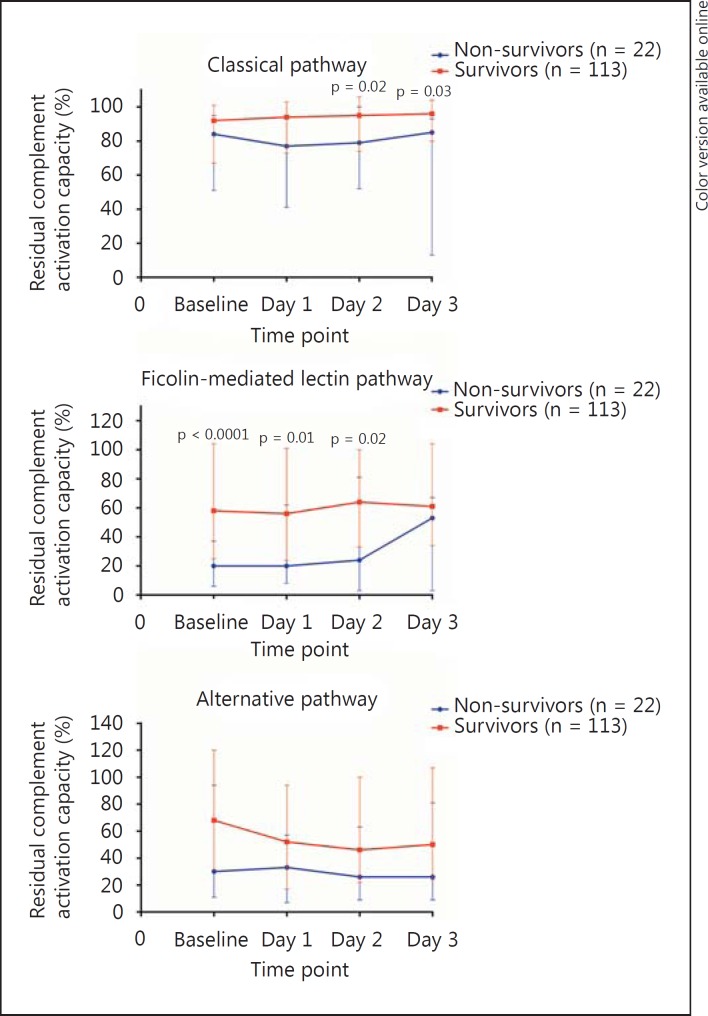
In continuation of this, univariate Cox regression analysis revealed that an activation capacity of the ficolin-mediated pathway below the median upon admission was associated with 28-day mortality [HR 7.05 (2.08-23.83), p = 0.002], but this was not the case for the classical or alternative pathways [HR 1.85 (0.78-4.41), p = 0.166, and HR 1.81 (0.76-4.32), p = 0.179, respectively]. Moreover, non-survivors had significantly lower residual activation capacity of the ficolin-mediated lectin pathway upon admission compared with survivors (fig. 5). The difference could not be found in the activation capacity of the classical pathway and the alternative pathway. However, on day 3 there was no significant difference in the ficolin-mediated pathway according to 28-day mortality, whereas a significant difference was observed in the classical pathway.
Fig. 5.
Residual activation capacity of the complement system in patients with NSTI according to 28-day mortality. Values denote medians with IQR. Differences were analyzed with the Mann-Whitney U test. Assessment of classical pathway activity was performed on anti-human albumin/albumin immune complexes (plasma samples diluted 1:400), ficolin-mediated lectin pathway activity on acetylated BSA (plasma samples diluted 1:1,000) and alternative pathway activity on lipopolysaccharide (plasma samples diluted 1:10).
Discussion
We performed a detailed analysis of the lectin pathway PRMs during the first 4 days in the ICU in 135 patients with NSTI and found a significant association between low baseline Ficolin-2 levels and the risk of both 28-day and long-term mortality. Conversely, a baseline Ficolin-2 level above the median had a high NPV of 0.94.
A main strength of this study was the size of the study population, which to date is the largest cohort of patients with NSTI who had biological material prospectively collected. Additionally, extensive follow-up was possible due to the access to the Danish Civil Registration System, which gave us the opportunity to investigate the distinctive mortality pattern and long-term outcome in this patient group. We found that more than 1 in 3 patients died during the 2.7-year follow-up, indicating that even though the patients survive the first 28 days after admission, twice as many will die within a few years. However, we do not know why. The treatment of NSTI has been centralized at a national level at our hospital, thus minimizing the risk of selection bias and increasing the internal and external validity.
The plasma concentrations of the four PRMs of the lectin complement pathway were lower in patients with NSTI than in non-infected controls at all investigated time points. We interpret this as a consumption phenomenon seen in critically ill patients, who are massively exposed to bacterial toxins, and as being related to the hyperinflammatory state in patients with sepsis. It is new knowledge that the PRMs play an important role in the hyperinflammatory response. Notably, a low baseline Ficolin-2 level, but not MBL, Ficolin-1 or Ficolin-3 level, was independently associated with both short- and long-term mortality in patients with NSTI, and we found a moderate to high sensitivity and a high NPV of baseline Ficolin-2 according to 28-day mortality. This could indicate a specific role for Ficolin-2 in the pathophysiology of the severity of NSTI.
Of particular interest was the investigation of the different complement pathways, which in these assays detected the residual activation capacity of C3. We found that the activation capacity of all pathways was significantly lower at baseline in patients with NSTI compared with control patients, but that the difference in baseline residual activation capacity was only 11% in the classical pathway assay and 33% in the alternative pathway assay, while the difference was 51% in the ficolin-mediated lectin pathway assay (fig. 2). The only difference in terms of components involved in the classical pathway assay and the ficolin-mediated lectin pathway assay are the PRMs and the associated serine proteases, while all downstream components are the same (C4, C2 and C3). This strongly indicates that more PRMs of the lectin pathway are consumed compared with C1q, which is the PRM of the classical pathway [20]. Therefore, the ficolin-mediated lectin pathway may play a particularly important role in patients with NSTI. This is supported by our findings that a ficolin-mediated activation capacity below the median upon admission was associated with 28-day mortality.
In line with this, the ficolin-mediated lectin pathway proved to be the only pathway with significantly lower activation capacity in non-survivors upon admission and the following day, whereas the classical pathway also became significant on day 2. On day 3, however, only the classical pathway activation capacity was significantly lower in non-survivors. This proves the dynamic interplay among the different complement pathways. We speculate that the ficolin-mediated lectin pathway plays a particularly important role during the first days of infection. The observed differences in the classical pathway might be explained by the different kinetics of the molecules or a larger reserve of the classical pathway molecules, thus taking longer before the consumption becomes significant. Even though the residual activation capacity of the ficolin-mediated lectin pathway increases on day 3, a following cytokine storm might explain the 28-day mortality. Future experimental studies are needed to elucidate the exact mechanisms. However, we focused on the baseline level, as this is important from a clinical point of view because it can be used to guide the initial acute phase of the treatment before information regarding microbial etiology etc. is available.
Ficolin-2 is mainly secreted from the hepatocytes [21]. It differs from the other ficolins by having four distinct binding grooves instead of one [22] and the ability to bind various capsulated bacteria, among these human pathogens such as staphylococci and streptococci, which are found in many patients with NSTI [23, 24]. Of further relevance, Ficolin-2 has been shown to initiate complement activation via the lectin pathway by binding necrotic and apoptotic cells, thus participating in the maintenance of tissue homeostasis [25, 26]. Another explanation for low baseline Ficolin-2 levels in non-surviving patients might therefore be consumption due to extensive tissue necrosis. It would be interesting to investigate the correlation between the extent of tissue damage and the Ficolin-2 level in future studies.
We believe that this new knowledge could be used at the bedside for the following reasons. Ficolin-2, but not the other investigated PRMs, could be useful in the risk stratification of patients with NSTI. A low Ficolin-2 level indicates that patients have an increased risk of dying within the first 28 days after admission, thereby suggesting a more aggressive treatment approach, while the patients with high levels may be treated more conservatively. In line with this, a high baseline Ficolin-2 level indicates that the NSTI patients have a 94% chance of surviving the first 28 days after admission (with a risk of missing 13%) (table 4). Importantly, bedside kits for Ficolin-2 analysis can be developed, which means that the Ficolin-2 level can be detected easily and quickly. In contrast, it takes 24 h before the physician can calculate a SAPS II score, and sometimes one or more of the 17 variables are missing. Therefore, Ficolin-2 may be used in the acute assessment and in cases where SAPS II cannot be calculated. However, this should be tested in future studies specifically designed to elucidate this. Additionally, Ficolin-2 might be combined with biomarkers such as PTX3, which has shown interesting results in this group of patients [13], together with clinical information and scores in order to provide good and reliable information for prognosis assessment as well as a timely, individualized treatment strategy.
Some limitations of the study should be mentioned. First, the results from the regression analyses had wide 95% CIs, which makes the interpretation of the clinical importance of low Ficolin-2 levels difficult. However, our findings clearly indicate that patients with NSTI have an increased risk of dying when having a low Ficolin-2 level upon admission. Second, estimation of genotype distribution in the patients was not possible. Thus we do not know whether the low levels were due to primary Ficolin-2 deficiency or depletion as a consequence of consumption. Nevertheless, the influence of polymorphic variance on the Ficolin-2 plasma concentration is modest and a genetically based deficiency state of Ficolin-2 in humans has not been described yet [27].
Conclusion
We found that the PRMs of the lectin complement pathway were significantly lower in patients with NSTI than in non-infected control patients upon admission. This was compatible with the 51% decreased residual activation capacity observed upon admission in the ficolin-mediated lectin pathway assay. A low Ficolin-2 level upon admission was significantly associated with short- and long-term mortality, whereas patients with high Ficolin-2 levels had a 94% chance of surviving the first 28 days after admission.
Disclosure Statement
Martin B. Madsen is sub-investigator on a randomized controlled trial that is partly funded by CSL Behring. The remaining authors report no conflict of interest related to this submission.
Acknowledgments
Special thanks go to Jesper Andresen for help with the laboratory analyses. We also thank the project team for their help with patient inclusion and collection of blood samples.
This work was supported by the European Union's Seventh Framework Program (305340), the Rigshospitalet Research Foundation (E-22514-02 to M.B.H.), the Aase and Ejnar Danielsens Foundation (10-001274), the Hans and Nora Buchards Foundation (7334), the Director Jacob Madsen and Olga Madsens Foundation (5323), the Novo Nordisk Research Foundation (bmkk to P.G.), the Danish Heart Association Research Foundation (15-R99-A5943-22922 to P.G.), and the Tryg Foundation (109662 to L.S.R.).
References
- 1.Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, Lyon JL. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293–299. doi: 10.1017/S095026880500484X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arif N, Yousfi S, Vinnard C. Deaths from necrotizing fasciitis in the United States, 2003-2013. Epidemiol Infect. 2016;144:1338–1344. doi: 10.1017/S0950268815002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das DK, Baker MG, Venugopal K. Increasing incidence of necrotizing fasciitis in New Zealand: a nationwide study over the period 1990 to 2006. J Infect. 2011;63:429–433. doi: 10.1016/j.jinf.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Hasham S, Matteucci P, Stanley PRW, Hart NB. Necrotising fasciitis. BMJ. 2005;330:830–833. doi: 10.1136/bmj.330.7495.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultan HY, Boyle AA, Sheppard N. Necrotising fasciitis. BMJ. 2012;345:e4274. doi: 10.1136/bmj.e4274. [DOI] [PubMed] [Google Scholar]
- 6.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 7.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 8.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496–1505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 9.Gao DN, Zhang Y, Ren YB, Kang J, Jiang L, Feng Z, Qu YN, Qi QH, Meng X. Relationship of serum mannose-binding lectin levels with the development of sepsis: a meta-analysis. Inflammation. 2015;38:338–347. doi: 10.1007/s10753-014-0037-5. [DOI] [PubMed] [Google Scholar]
- 10.Munthe-Fog L, Hummelshoj T, Honoré C, Moller ME, Skjoedt MO, Palsgaard I, Borregaard N, Madsen HO, Garred P. Variation in FCN1 affects biosynthesis of ficolin-1 and is associated with outcome of systemic inflammation. Genes Immun. 2012;13:515–522. doi: 10.1038/gene.2012.27. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers JD, Fleming GB, Rutherford J, Matsushita M, Kilpatrick DC, Hill AT. Serum ficolin-2 in hospitalised patients with community-acquired pneumonia. Inflammation. 2014;37:1635–1641. doi: 10.1007/s10753-014-9891-4. [DOI] [PubMed] [Google Scholar]
- 12.Hansen MB, Simonsen U, Garred P, Hyldegaard O. Biomarkers of necrotising soft tissue infections: aspects of the innate immune response and effects of hyperbaric oxygenation - the protocol of the prospective cohort BIONEC study. BMJ Open. 2015;5:e006995. doi: 10.1136/bmjopen-2014-006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen MB, Rasmussen LS, Garred P, Bidstrup D, Madsen MB, Hyldegaard O. Pentraxin-3 as a marker of disease severity and risk of death in patients with necrotizing soft tissue infections: a nationwide, prospective, observational study. Crit Care. 2016;20:40. doi: 10.1186/s13054-016-1210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munthe-Fog L, Hummelshøj T, Hansen BE, Koch C, Madsen HO, Skjødt K, Garred P. The impact of FCN2 polymorphisms and haplotypes on the Ficolin-2 serum levels. Scand J Immunol. 2007;65:383–392. doi: 10.1111/j.1365-3083.2007.01915.x. [DOI] [PubMed] [Google Scholar]
- 15.Munthe-Fog L, Hummelshøj T, Ma YJ, Hansen BE, Koch C, Madsen HO, Skjødt K, Garred P. Characterization of a polymorphism in the coding sequence of FCN3 resulting in a Ficolin-3 (Hakata antigen) deficiency state. Mol Immunol. 2008;45:2660–2666. doi: 10.1016/j.molimm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Garred P, Madsen HO, Kurtzhals JA, Lamm LU, Thiel S, Hey AS, Svejgaard A. Diallelic polymorphism may explain variations of the blood concentration of mannan-binding protein in Eskimos, but not in black Africans. Eur J Immunogenet. 1992;19:403–412. doi: 10.1111/j.1744-313x.1992.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 17.Seelen MA, Roos A, Wieslander J, Mollnes TE, Sjöholm AG, Wurzner R, Loos M, Tedesco F, Sim RB, Garred P, Alexopoulos E, Turner MW, Daha MR. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187–198. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Hein E, Honoré C, Skjoedt MO, Munthe-Fog L, Hummelshøj T, Garred P. Functional analysis of Ficolin-3 mediated complement activation. PLoS One. 2010;5:e15443. doi: 10.1371/journal.pone.0015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastrup-Birk S, Skjoedt MO, Munthe-Fog L, Strom JJ, Ma YJ, Garred P. Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS One. 2013;8:e73119. doi: 10.1371/journal.pone.0073119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I - molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita M. Ficolins: complement-activating lectins involved in innate immunity. J Innate Immun. 2010;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- 22.Garlatti V, Belloy N, Martin L, Lacroix M, Matsushita M, Endo Y, Fujita T, Fontecilla-Camps JC, Arlaud GJ, Thielens NM, Gaboriaud C. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 2007;26:623–633. doi: 10.1038/sj.emboj.7601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch NJ, Roscher S, Hartung T, Morath S, Matsushita M, Maennel DN, Kuraya M, Fujita T, Schwaeble WJ. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol. 2004;172:1198–1202. doi: 10.4049/jimmunol.172.2.1198. [DOI] [PubMed] [Google Scholar]
- 24.Krarup A, Sørensen UBS, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–1060. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuraya M, Ming Z, Liu X, Matsushita M, Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209:689–697. doi: 10.1016/j.imbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Jensen ML, Honoré C, Hummelshøj T, Hansen BE, Madsen HO, Garred P. Ficolin-2 recognizes DNA and participates in the clearance of dying host cells. Mol Immunol. 2007;44:856–865. doi: 10.1016/j.molimm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Garred P, Honoré C, Ma YJ, Rørvig S, Cowland J, Borregaard N, Hummelshøj T. The genetics of ficolins. J Innate Immun. 2010;2:3–16. doi: 10.1159/000242419. [DOI] [PubMed] [Google Scholar]