Abstract
Although fecal calprotectin (Fcal) and the fecal immunochemical test (FIT) have been associated with endoscopic activity in ulcerative colitis (UC), the clinical implications of each marker depending on the mucosal status are not well known.
A total of 174 results obtained from 128 patients with UC who simultaneously underwent colonoscopy and fecal tests were retrospectively evaluated from March 2015 to February 2018. The correlation and predictability of fecal markers as a surrogate marker of endoscopic activity, and the sensitivity, specificity, and predictive value of fecal tests for mucosal healing were statistically evaluated.
Both fecal tests showed a statistically significant correlation with Mayo Endoscopic Subscore (MES) (Fcal: r = 0.678, P < .001 and FIT: r = 0.635, P < .001) and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) (Fcal: r = 0.711, P < .001 and FIT: r = 0.657, P < .001). Fcal was statistically superior to FIT in predictive accuracy for endoscopic activity (area under the curve [AUC]: 0.863 vs 0.765 with MES, P < .001 and AUC; 0.847 vs 0.757 with UCEIS, P < .001). FIT was superior to Fcal in sensitivity for mucosal healing (98.0% vs 78.4% with MES, 94.9% vs 74.6% with UCEIS).
Fcal and FIT were well correlated with endoscopic activity in UC and can be surrogate markers of mucosal inflammation. Depending on mucosal status, Fcal was more accurate in predicting the endoscopic activity in active inflammation, whereas FIT was more sensitive in predicting the achievement of mucosal healing.
Keywords: fecal calprotectin, fecal immunochemical test, ulcerative colitis
1. Introduction
Ulcerative colitis (UC), a type of inflammatory bowel disease, is a chronic inflammatory disorder that repeatedly worsens and improves in the large intestine. Recently, mucosal healing has been highlighted as the most important target in the treatment of UC, because it is associated with a reduced risk of relapse and improved clinical outcomes, which reduces the risk of surgery,[1,2] steroid dependency,[2] and hospitalization.[1,2] Therefore, it is essential to modify the medication after periodic inspection of the mucosal status for achievement of mucosal healing. However, endoscopic assessment, an essential method for evaluating mucosal status, is not only an invasive and inconvenient test, but it can also aggravate symptoms in patients with UC.[3] Thus, a reliable, noninvasive surrogate marker to replace the endoscopic assessment is needed.
Among surrogate markers for mucosal inflammation, both fecal calprotectin (Fcal) and the fecal immunochemical test (FIT) have been shown to be well correlated with endoscopic activity[4–7] and have predictability for recurrence in patients with UC.[8–11] However, both tests are used to assess the mucosal status in different ways; that is, Fcal is a surrogate marker for detecting a cytosolic granulocyte protein associated with neutrophil migration to the intestinal tract, and FIT is a surrogate marker for detecting stool hemoglobin derived from occult blood loss in mucosal ulceration.[12] Thus, the results of both tests for mucosal inflammation or healing may have different clinical implications.
In the present study, we aimed to evaluate the correlation among endoscopic activity and fecal tests, and to determine the clinical implications of each test on the mucosal status for proper application of both fecal tests in patients with UC.
2. Methods
2.1. Patients
From March 2015 to February 2018, the medical records of patients with UC at the Pusan National University Yangsan Hospital (PNUYH) in the Republic of Korea were retrospectively reviewed. During the study period, 195 results of 149 patients with UC who underwent colonoscopy, Fcal, and FIT were evaluated. Among them, 6 patients whose diagnosis was not clear and 15 patients whose stool tests were not submitted on the day of endoscopy day were excluded. Finally, 174 results, contributed by 128 patients, were included (Fig. 1). This study was approved by the Institutional Review Board (IRB) of PNUYH (IRB number: 05–2018–070). Informed consent was obtained from each patient. There are no conflicts of interest or sponsors for this study.
Figure 1.

Flow chart of the enrolled patients. Fcal = fecal calprotectin, FIT = fecal immunochemical test, UC = ulcerative colitis.
2.2. Fecal sampling and analysis
All fecal samples were collected in two separate stool containers at 1 or 2 days before bowel preparation and submitted on the day of colonoscopy.
Samples submitted for calprotectin analysis were stored at −70°C until they were transported to the laboratory (Green Cross Laboratories, GC Labs, Korea). Calprotectin was measured using the EliATM Calprotectin enzyme-linked immunosorbent assay kit (Thermo Fisher Scientific, Bremen, Germany). The quantitative range was between 11.5 and 2000 μg/g for calprotectin, after an appropriate 1:100 dilution of the fecal samples.
Samples submitted to FIT analysis were immediately processed using the HM-JACK system (Kyowa Medex, Shizuoka, Japan), a fully automated quantitative FIT system. The HM-JACK system can accurately measure the fecal hemoglobin concentration within a range of 7 to 1200 ng/mL.
2.3. Assessment of endoscopic activity
Endoscopic activity was evaluated using the Mayo Endoscopic Subscore (MES) and Ulcerative Colitis Endoscopic Index of Severity (UCEIS).[13,14] All endoscopic examinations were performed by experienced endoscopists. Endoscopic mucosal healing was defined as MES of 0 and UCEIS of 0–1 in this study.
2.4. Statistical analysis
Spearman's rank correlation was used to determine the correlation among fecal tests and instruments to assess the endoscopic severity of UC. The comparison between the area under the receiver operating characteristic (ROC) curve was analyzed using DeLong's test. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), with associated 95% confidence intervals, of fecal tests for mucosal healing were evaluated. A P-value < .05 was considered statistically significant. Statistical calculations were performed with SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patients’ characteristics
One hundred seventy-four results contributed by 128 patients (87 men and 41 women; median age, 47.2 years) were included in this study. Baseline characteristics of the enrolled patients are shown in Table 1. Mean disease duration was 48.9 months (range, 0–240 months). In most cases (98.8%), treatment was a suppository or oral 5-aminosalicylate, and in 85 cases (48.9%), the following additional medications were administered as treatment: systemic steroids in 31 (17.8%), azathioprine in 32 (18.4%), and antitumor necrosis factor-alpha therapy in 22 (2.6%) cases. During the study period, 39 patients were hospitalized for severe UC. Three patients underwent surgery for severe UC refractory to medical therapy. One patient underwent surgery for advanced colon cancer. Three patients were hospitalized for reasons other than UC itself, and one of them died of acute myocardial infarction.
Table 1.
Baseline characteristics of 128 patients and 174 cases.

3.2. Correlation between endoscopic activity and the fecal tests
Colonoscopy was performed in all patients. Sixty-six patients (38.0%) had extensive colitis, 74 (42.5%) had left-sided colitis, and 34 (19.5%) had proctitis. Endoscopic activity assessed by MES was as follows: mucosal healing (0) in 51 cases (29.3%), mild activity (1) in 54 cases (31.0%), moderate activity (2) in 41 cases (23.6%), and severe activity (3) in 28 cases (16.1%). Endoscopic activity assessed by UCEIS was as follows: mucosal healing (0–1) in 59 cases (33.9%), mild activity (2–4) in 55 cases (31.6%), moderate activity (5–6) in 47 cases (27%), and severe activity (7–8) in 13 cases (7.5%).
Fcal was measured with an average value of 699.1 μg/g (range, negative to 2000 μg/g), and FIT was measured with an average value of 132.0 ng/mL (range, negative to 1200 ng/mL) in the enrolled patients. Both Fcal and FIT results were significantly correlated with MES and UCEIS. Fcal was positively correlated with endoscopic activity; Spearman's rho correlation coefficients were 0.678 with MES (P < .001) and 0.711 with UCEIS (P < .001). FIT was also positively correlated with endoscopic activity; Spearman's rho correlation coefficients were 0.635 with MES (P < .001) and 0.657 with UCEIS (P < .001). The correlation between endoscopic activity and the fecal tests is shown in Figure 2.
Figure 2.

Correlation between fecal tests and endoscopic activity. (A) Fecal calprotectin and Mayo endoscopic subscore, (B) fecal immunochemical test and Mayo endoscopic subscore, (C) fecal calprotectin and Ulcerative colitis endoscopic index of severity, and (D) fecal immunochemical test and Ulcerative colitis endoscopic index of severity.
3.3. Comparison of predictability of endoscopic activity between Fcal and FIT
As a negative cut-off value in this study, FIT was based on 100 ng/mL, which is used for colorectal cancer screening in most studies, and Fcal was set at 170 μg/g based on the ROC curve for mucosal inflammation (Fig. 3). The predictive accuracy of Fcal for MES was statistically better than that of FIT (AUC, 0.863 vs 0.765, z = 3.429, P < .001). The predictive accuracy of Fcal for UCEIS was also statistically better than that of FIT (AUC, 0.847 vs 0.757, z = 2.988, P < .001). The comparison between two fecal tests is shown in Figure 4.
Figure 3.

The receiver-operator characteristics curve of fecal calprotectin for predicting mucosal inflammation, MES 1-3. AUC = area under curve, MES = Mayo endoscopic subscore.
Figure 4.
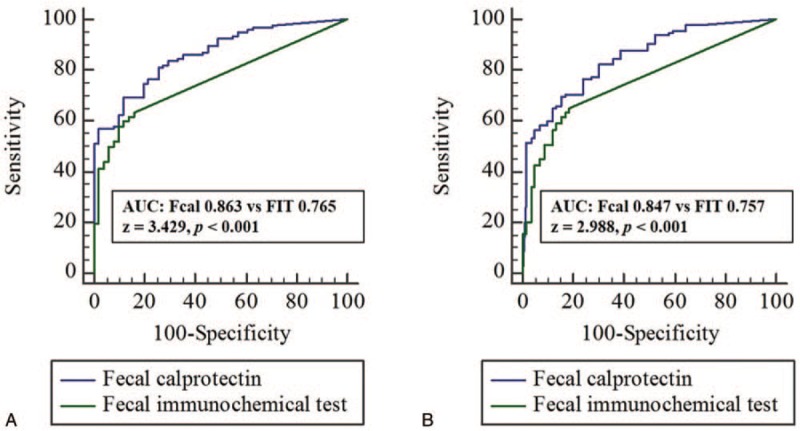
Comparison between fecal calprotectin and fecal immunochemical test for mucosal inflammation. AUC = area under curve, Fcal = fecal calprotectin, FIT = fecal immunochemical test, MES = Mayo endoscopic subscore, ROC = receiver-operator characteristics, UCEIS = ulcerative colitis endoscopic index of severity.
3.4. Sensitivity, specificity, and predictive value of the fecal tests for mucosal healing
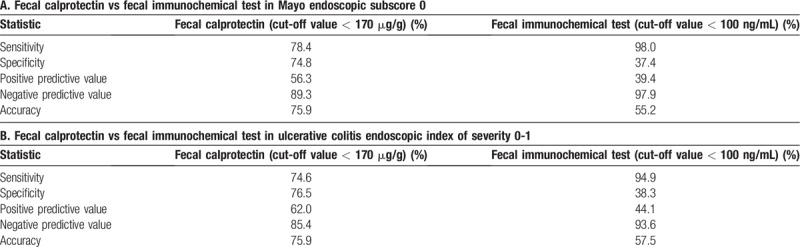
The sensitivity, specificity, PPV, NPV, and accuracy of the two fecal tests for mucosal healing are summarized in Table 2. When the values indicative of mucosal healing were applied, Fcal showed a sensitivity of 78.4%, specificity of 74.8%, PPV of 56.3%, NPV of 89.3%, and accuracy of 75.9%; and FIT showed a sensitivity of 98.0%, specificity of 37.4%, PPV of 39.4%, NPV of 97.9%, and accuracy of 55.2% in identifying patients with MES of 0 (Table 2A). Similar results were obtained for UCEIS. Fcal showed a sensitivity of 74.6%, specificity of 76.5%, PPV of 62.0%, NPV of 85.4%, and accuracy of 75.9%; and FIT showed a sensitivity of 94.9%, specificity of 38.3%, PPV of 44.1%, NPV of 93.6%, and accuracy of 57.5% in identifying patients with UCEIS of 0–1 (Table 2B).
Table 2.
The sensitivity, specificity, and predictive values of the two fecal tests for mucosal healing.
4. Discussion
In this study, we confirmed a positive correlation between endoscopic activity and the fecal tests, and different predictability between the fecal tests depending on the mucosal status in patients with UC. Although several studies suggested that Fcal and FIT were well correlated with endoscopic activity and mucosal healing[6,15–19] and that a negative FIT has a high sensitivity to mucosal healing in patients with UC,[6,17–19] there has been no conclusion as to which of the two tests is superior. This study showed that Fcal was statistically more relevant to endoscopic activity with MES and UCEIS, and FIT was superior to Fcal in sensitivity for mucosal healing with MES and UCEIS.
Regarding the clinical application of our results, a conclusion similar to that in a recent study can be made. Hiraoka et al suggested that FIT confirms mucosal healing and is favorable for subsequent relapse prediction, suggesting that Fcal is more effective in monitoring patients with active inflammation.[11] In this study, Fcal was more superior to FIT in predictability of endoscopy activity and had a low sensitivity for mucosal healing, so it could be used for monitoring UC patients with active inflammation. However, FIT had a high sensitivity for mucosal healing, and thus, could be used to monitor relapse in UC patients with mucosal healing. Based on these results, the strategy to use one of the two fecal tests depending on the mucosal status may be more economical rather than only using Fcal regardless of the mucosal status because FIT is inexpensive. On the basis of these results, we created a simple algorithm for managing patients with UC by using the two fecal tests (Fig. 5). After induction treatment, patients with UC should undergo endoscopy to confirm mucosal healing. If patients have mucosal healing and no symptoms, FIT should be used to regularly monitor them every 6–12 months. However, an endoscopic evaluation should be performed when FIT result is elevated to 100 ng/mL in these patients. If patients failed to show mucosal healing after induction treatment, Fcal level should be assessed after 3–6 months. According to Fcal level, an endoscopy to confirm mucosal healing should be performed in patients with Fcal level <170 μg/g and no symptoms, or re-monitoring of Fcal level should be performed after 3–6 months in patients with Fcal level ≥170 μg/g.
Figure 5.

Algorithm for managing patients with UC using fecal tests. Fcal = fecal calprotectin, FIT = fecal immunochemical test, UC = ulcerative colitis.
Recently, simple and noninvasive tests that replace endoscopy have been studied in the evaluation of UC. Among several tests, Fcal is most frequently used. Fcal is a neutrophil-derived protein released in stool in response to mucosal inflammation, and it indicates the amount of inflammatory cells.[20] Fcal has been reported to be associated with various conditions of UC. The level of Fcal is related to endoscopic severity,[15] prediction of mucosal healing,[16] and prediction of relapse,[21] and it is useful in monitoring patients’ response to treatment.[22,23] Conversely, FIT indicates the amount of blood coming from the damaged mucosa, and it is used for colorectal cancer screening.[24] There are a relatively small number of studies about FIT compared to Fcal in UC, and there are few reports related to mucosal healing.[17–19] A recent meta-analysis reported that the sensitivity and specificity of the FIT result for predicting mucosal healing in UC were 0.77 and 0.81, respectively.[25] In our study, the sensitivity and specificity of FIT for predicting mucosal healing in UC were 0.98 and 0.38, respectively. The lower specificity of FIT for predicting mucosal healing in UC in this study than in the meta-analysis may be caused by the strict definition of mucosal healing and cut-off values of the present study. Actually, the definition of mucosal healing and the cut-off value were MES of 0 and 100 ng/mL in the present study, respectively, whereas those were MES of 0–1 and 50–280 ng/mL in studies included in the meta-analysis, respectively.
Several scoring systems are used for evaluating endoscopic activity of UC, among which MES is the most representative.[13] More recently, UCEIS has been introduced[14] and is known to reflect clinical outcomes and long-term prognosis.[26,27] Ikeya et al suggested that UCEIS more accurately reflects clinical outcomes and long-term prognosis than MES.[26] In this study, both scoring systems were useful because MES and UCEIS were statistically significantly correlated (r = 0.923, P < .001). The definition of mucosal healing is not clearly defined, but recent studies have defined mucosal healing as only MES of 0.[7,28,29] UCEIS does not yet have a standard value for mucosal healing. Herein, endoscopic activity was evaluated by both MES and UCEIS, and mucosal healing was defined as MES of 0 and UCEIS of 0–1.
In the present study, FIT had a better sensitivity for mucosal healing than Fcal. However, the sensitivity may change depending on the determined cut-off value. For the FIT, usually a cut-off value of 100 ng/mL is used for screening for colorectal cancer. However, Fcal does not have a defined cut-off value. Therefore, most studies have used cut-off values of Fcal based on ROC curves. The cut-off value of Fcal for mucosal healing (MES 0) varied in each study: 180 μg/g in Hiraoka et al,[11] 187 μg/g in Lee et al,[30] and 194 μg/g in Yamaguchi et al.[7] In these studies, the sensitivity and specificity were reported as 71% to 85% and 58% to 89%, respectively.[7,11,30] In our study, the cut-off value was also determined based on the ROC curve, and the cut-off value of Fcal for MES of 0 could be set to 170 μg/g. Additionally, the sensitivity and specificity in our study were not significantly different from those in other studies.
There are some limitations to this study. First, there are inaccuracies and limitations of the fecal tests. FIT can be positive in different situations unrelated with UC, such as anal bleeding. Fcal has no standard cut-off value and produces different results depending on measurement kits and diurnal variation.[31] Second, the sensitivity of FIT to mucosal healing was significantly higher but the specificity was lower than that reported in other studies, and FIT can show high false-positive results. However, FIT is an inexpensive and fast test, so we can perform it easily and frequently. Third, this study had a single-center, retrospective design with a small sample size. Nevertheless, when compared with other studies confirming the usefulness of fecal tests in patients with UC, the number of participating patients was relatively high, and only those patients who submitted a stool test on the day of colonoscopy were included in this study.
In conclusion, this study revealed that both Fcal and FIT were well correlated with endoscopic activity in patients with UC. Between the two tests, Fcal was statistically more correlated and had better predictive accuracy with endoscopic activity. On the other hand, FIT was more sensitive for predicting mucosal healing with MES and UCEIS. Therefore, we suggest that Fcal may be used to evaluate disease activity and treatment response in active UC patients with mucosal inflammation, and FIT may be used to monitor recurrence in patients with UC with mucosal healing. Further well-designed prospective, randomized, large-volume trials are required to determine proper application of the two fecal tests in patients with UC.
Author contributions
Conceptualization: Hyung Wook Kim, Su Bum Park.
Data curation: Dae Gon Ryu.
Formal analysis: Dae Gon Ryu.
Investigation: Su Bum Park, Cheol Woong Choi, Su Jin Kim, Hyeong Seok Nam.
Methodology: Dae Gon Ryu, Hyung Wook Kim.
Project administration: Dae Gon Ryu, Hyung Wook Kim, Su Jin Kim.
Software: Su Bum Park, Cheol Woong Choi, Hyeong Seok Nam.
Supervision: Dae Hwan Kang.
Validation: Dae Gon Ryu, Hyung Wook Kim, Su Bum Park.
Visualization: Dae Gon Ryu, Su Bum Park.
Writing – original draft: Dae Gon Ryu, Hyung Wook Kim.
Writing – review & editing: Dae Gon Ryu, Hyung Wook Kim.
Footnotes
Abbreviations: AUC = area under the curve, Fcal = fecal calprotectin, FIT = fecal immunochemical test, MES = Mayo endoscopic subscore, NPV = negative predictive value, PPV = positive predictive value, ROC = receiver operating characteristic, UC = ulcerative colitis, UCEIS = Ulcerative Colitis Endoscopic Index of Severity.
How to cite this article: Ryu DG, Kim HW, Park SB, Kang DH, Choi CW, Kim SJ, Nam HS. Clinical implications of fecal calprotectin and fecal immunochemical test on mucosal status in patients with ulcerative colitis. Medicine. 2019;98:36(e17080).
The authors have no conflict of interest to disclose.
References
- [1].Peyrin-Biroulet L, Ferrante M, Magro F, et al. Results from the 2nd Scientific Workshop of the ECCO. I: impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis 2011;5:477–83. [DOI] [PubMed] [Google Scholar]
- [2].Reinink AR, Lee TC, Higgins PD. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta-analysis. Inflamm Bowel Dis 2016;22:1859–69. [DOI] [PubMed] [Google Scholar]
- [3].Mukewar S, Costedio M, Wu X, et al. Severe adverse outcomes of endoscopic perforations in patients with and without IBD. Inflamm Bowel Dis 2014;20:2056–66. [DOI] [PubMed] [Google Scholar]
- [4].Kato J, Hiraoka S, Nakarai A, et al. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin? Intest Res 2016;14:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis 2013;19:332–41. [DOI] [PubMed] [Google Scholar]
- [6].Mooiweer E, Fidder HH, Siersema PD, et al. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis 2014;20:307–14. [DOI] [PubMed] [Google Scholar]
- [7].Yamaguchi S, Takeuchi Y, Arai K, et al. Fecal calprotectin is a clinically relevant biomarker of mucosal healing in patients with quiescent ulcerative colitis. J Gastroenterol Hepatol 2016;31:93–8. [DOI] [PubMed] [Google Scholar]
- [8].Lasson A, Simren M, Stotzer PO, et al. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm Bowel Dis 2013;19:576–81. [DOI] [PubMed] [Google Scholar]
- [9].De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis 2013;19:2111–7. [DOI] [PubMed] [Google Scholar]
- [10].Jauregui-Amezaga A, Lopez-Ceron M, Aceituno M, et al. Accuracy of advanced endoscopy and fecal calprotectin for prediction of relapse in ulcerative colitis: a prospective study. Inflamm Bowel Dis 2014;20:1187–93. [DOI] [PubMed] [Google Scholar]
- [11].Hiraoka S, Inokuchi T, Nakarai A, et al. Fecal immunochemical test and fecal calprotectin results show different profiles in disease monitoring for ulcerative colitis. Gut Liver 2018;12:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ma C, Lumb R, Walker EV, et al. Noninvasive fecal immunochemical testing and fecal calprotectin predict mucosal healing in inflammatory bowel disease: a prospective cohort study. Inflamm Bowel Dis 2017;23:1643–9. [DOI] [PubMed] [Google Scholar]
- [13].Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- [14].Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012;61:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- [16].Theede K, Holck S, Ibsen P, et al. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:1929–36. e1921. [DOI] [PubMed] [Google Scholar]
- [17].Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol 2013;108:83–9. [DOI] [PubMed] [Google Scholar]
- [18].Takashima S, Kato J, Hiraoka S, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am J Gastroenterol 2015;110:873–80. [DOI] [PubMed] [Google Scholar]
- [19].Ryu DG, Kim HW, Park SB, et al. Assessment of disease activity by fecal immunochemical test in ulcerative colitis. World J Gastroenterol 2016;22:10617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol 1999;34:50–4. [DOI] [PubMed] [Google Scholar]
- [21].Garcia-Sanchez V, Iglesias-Flores E, Gonzalez R, et al. Does fecal calprotectin predict relapse in patients with Crohn's disease and ulcerative colitis? J Crohns Colitis 2010;4:144–52. [DOI] [PubMed] [Google Scholar]
- [22].Molander P, af Bjorkesten CG, Mustonen H, et al. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFalpha blocking agents. Inflamm Bowel Dis 2012;18:2011–7. [DOI] [PubMed] [Google Scholar]
- [23].Theede K, Kiszka-Kanowitz M, Nielsen AM, et al. The correlation between fecal calprotectin, simple clinical colitis activity index and biochemical markers in ulcerative colitis during high-dose steroid treatment. Scand J Gastroenterol 2014;49:418–23. [DOI] [PubMed] [Google Scholar]
- [24].Chen LS, Yen AM, Chiu SY, et al. Baseline faecal occult blood concentration as a predictor of incident colorectal neoplasia: longitudinal follow-up of a Taiwanese population-based colorectal cancer screening cohort. Lancet Oncol 2011;12:551–8. [DOI] [PubMed] [Google Scholar]
- [25].Dai C, Jiang M, Sun MJ, et al. Fecal immunochemical test for predicting mucosal healing in ulcerative colitis patients: A systematic review and meta-analysis. J Gastroenterol Hepatol 2018;33:990–7. [DOI] [PubMed] [Google Scholar]
- [26].Ikeya K, Hanai H, Sugimoto K, et al. The ulcerative colitis endoscopic index of severity more accurately reflects clinical outcomes and long-term prognosis than the Mayo endoscopic score. J Crohns Colitis 2016;10:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Arai M, Naganuma M, Sugimoto S, et al. The ulcerative colitis endoscopic index of severity is useful to predict medium- to long-term prognosis in ulcerative colitis patients with clinical remission. J Crohns Colitis 2016;10:1303–9. [DOI] [PubMed] [Google Scholar]
- [28].Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflamm Bowel Dis 2016;22:623–30. [DOI] [PubMed] [Google Scholar]
- [29].Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J Crohns Colitis 2016;10:13–9. [DOI] [PubMed] [Google Scholar]
- [30].Lee SH, Kim MJ, Chang K, et al. Fecal calprotectin predicts complete mucosal healing and better correlates with the ulcerative colitis endoscopic index of severity than with the Mayo endoscopic subscore in patients with ulcerative colitis. BMC Gastroenterol 2017;17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Patel A, Panchal H, Dubinsky MC. Fecal calprotectin levels predict histological healing in ulcerative colitis. Inflamm Bowel Dis 2017;23:1600–4. [DOI] [PubMed] [Google Scholar]


