Abstract
Rationale:
Pelvic tumor had great impact on patients’ quality of life. After tumor resection, how to accurately fill bone defect remained challenging for orthopedic surgeons. Due to lack of individual design, high incidence of prosthetic mismatching, and loosening were reported in pelvic reconstruction surgery with conventional modular prostheses. Nowadays, with rapid development of three-dimensional (3D) print technology, pelvic prostheses could be designed according to patients’ own anatomy. The objective of this study was to describe the application of 3D printed customized hemi-pelvic prosthesis for patients with pelvic tumor.
Patient concerns:
A 62-year-old female had developed severe right joint pain without obvious inducement from 5 months before she sought medical advice. Pain, swelling, and limited range of motion of right joint were founded during physical examination.
Diagnosis:
The patients were diagnosed as “right acetabulum metastatic carcinoma”
Intervention:
3D printed titanium alloy hemi-pelvic prosthesis was designed according the morphology of unaffected side hemi-pelvis and subsequently implanted in surgery to reconstruct the pelvis. 3D printed osteotomy guide and pelvic model were also manufactured and applied to improve accuracy of osteotomy and reduce operation time. X-Ray of pelvis, Harris score, musculoskeletal tumor society score (MSTS) and The MOS item short from health survey (SF-36) were recorded during the period of preoperation, 1, 3, 6, 12 months follow-up after operation.
Outcomes:
3D printed hemi-pelvic prosthesis matched precisely with pelvis and implanted successfully. There was no sign of prosthetic loosening within 12 months’ follow-up. No sign of peri-prosthetic infection from laboratory examination. Harris score, MSTS, and SF-36 were gradually increasing during follow-up period.
Lessons:
Satisfactory effect of pelvic reconstruction could be achieved by 3D printed hemi-pelvic prostheses. It also provided a promising way to the treatment of pelvic tumor in similar cases.
Keywords: 3D print, customized protheses, osteotomy guide, pelvic tumor
1. Introduction
Pelvic tumor was the third common malignant bone tumor and occupied approximately 10 to 15 percentage of malignant bone tumor.[1–3] Pelvic tumor has relatively low survival rate and high recurrence rate. In order to prolong patient's life and reduce recurrence rate, en-bloc resection of pelvic tumor with tumor-free margin was of great importance. Therefore, large size of bone defect was always remained after tumor resection. Currently mainstream methods of pelvic reconstruction mainly including structural allograft plus internal fixation, saddle prostheses, ice-cream cone prostheses, modular prostheses, and computer-aided design (CAD) prostheses reconstruction surgery.[4,5] However, owing to the individualized morphology of pelvis and different invasion range of tumor, conventional pelvic prosthesis could not satisfactorily restore the integrity of pelvic ring.[6–8] Owing to the poor matching degree of conventional prosthesis, surgeons had no choice but to conduct massive osteotomy to satisfy prosthetic implantation. So unaffected bone tissue could not be preserved precisely.[7–9] Currently, the emergence of CAD customized pelvic prostheses has significantly enhanced the compatibility between prostheses and resected pelvis.[8–10] But manufacture accuracy of CAD was relatively low and could not satisfy the reconstruction demand. Moreover, the surface of CAD prosthesis has no macro-porous structure which could promote bone in-growth. So long-term biological stability of pelvis was always poor.[1–3]
Nowadays with rapid development of three-dimensional (3D) print technology, 3D print custom-made implant was generally applied in orthopedics.[1–3,11] The most remarkable advantage of 3D print technology was custom-made design which could significantly improve matching degree of prostheses.[2] According to previous studies, after tumor resection, 3D printed customized pelvic prostheses could accurately fill the bone defect and reconstruct pelvis to it's original morphology.[2,3] Secondly, macro-porous structure could be designed on the interface of bone-prosthesis to promote the osseous fusion ability and long-term stability of prostheses by 3D print technology.[1,3,5,6] Mesh structure has complicated morphology which was hard to manufacture by means of conventional industrial technology.
3D printed technology was also applied in various aspect in both preoperative design and intraoperative application. First step, 3D printed pelvic model was applied to carry out simulated experiment to test matching degree of prosthesis. Second step, 3D printed osteotomy guide was designed to enhance accuracy of osteotomy. Finally, 3D printed pelvic prosthesis was implanted to fill the bone defect and reconstruct pelvis. Through this method, a promising method was attempted to explore treatment of pelvic tumor.
2. Case presentation
2.1. Ethical statement
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Before execution, this study was authorized by the Ethics Committee of the second hospital of Jilin University. “Informed consent” was obtained from the patient and her family members before operation. Patient's data were kept anonymous to ensure patient's privacy.
2.2. Clinical data
A 62-year-old female was diagnosed as “right acetabulum metastatic carcinoma (Enneking IIB stage), right renal clear-cell carcinoma.” Osseous destruction of right ilium and acetabulum was founded in pelvic computed tomography (CT) image (Fig. 1A, B, and D). Maximum shade of intercondylar soft tissue was 51 × 42 mm in magnetic resonance imaging image (Fig. 1C). Right hip joint “4 experiment” (+) was founded in physical examination. Range of motion of affected hip: forward flexion 10°, backward extension 0°, adduction 10°, abduction 15°.
Figure 1.
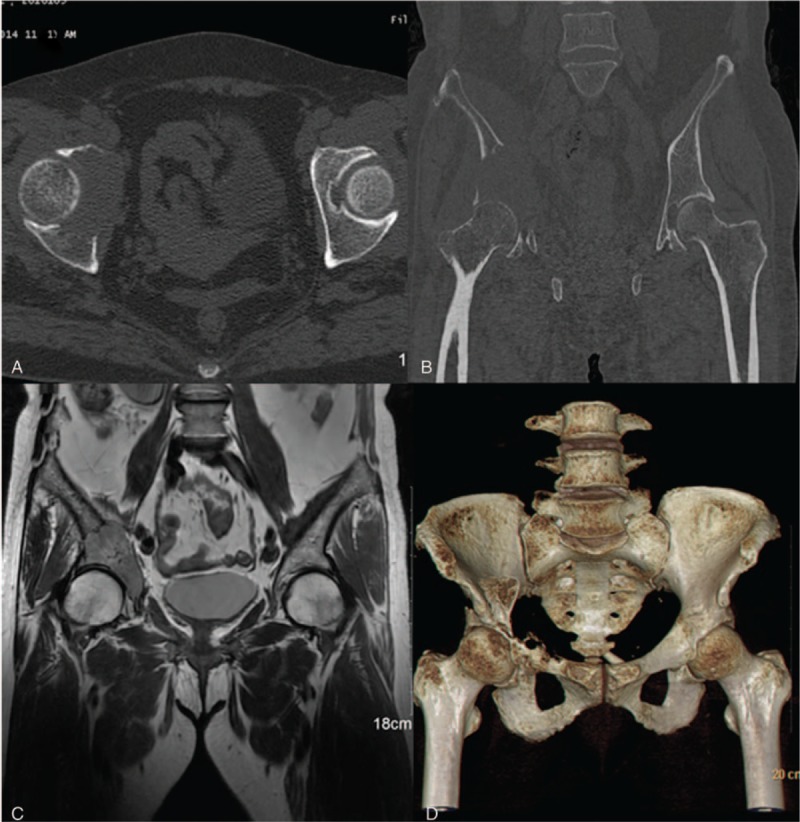
Preoperative image of a 62-yr-old female patient who was diagnosed as metastatic acetabulum carcinoma: (A): Axial view CT image. (B): Coronal view CT image. (C): Coronal view MRI image. (D) Three-dimensional reconstruction CT image. CT = computed tomography, MRI = magnetic resonance imaging.
2.3. Preoperative design
2.3.1. Data acquisition and image processing
Pelvic CT scan was conducted by 256 row spiral CT machine (Philips Corporation. Japan). Parameters of CT scan was as follow: Kilovolt peak: 120 kV, tube current: 232 mA, rotation time: 0.75 seconds, layer thickness: 0,9 mm, increment: 0.45 mm. After acquisition of digital imaging and communications in medicine format CT data, it was exported into Mimics 17.0 Software (Materialise Corporation, Belgium).
2.3.2. Manufacture of 3D printed stereolithography apparatus resin pelvic model
CT data were exported into Mimics 17.0 Software to conduct 3D reconstruction. Stereolithography (STL) format pelvic model was acquired and imported into the rapid prototyping Magics 18.0 Software (Materialise Corporation) to conduct model pretreatment and support generation. Finally, the data was imported into resin 3D print machine (Shining ISLA 450, China) to manufacture STL apparatus resin model of pelvis. Pelvic model was applied as auxiliary instrument to evaluate bone defect preoperative. In addition, it was also used to test matching degree of prosthesis before operation.
2.3.3. Design and manufacture of 3D printed osteotomy guide
The basic shape of osteotomy guide was designed according to morphology of affected hemi-pelvis combined with projected osteotomy scope. Osteotomy lines were located at least 30 mm away from the boundaries of tumor invasion region to ensure thorough tumor resection and tumor-free margin. Four osteotomy lines were designed around the acetabulum, including anterior ilium, posterior ilium, pubis, and ischium osteotomy lines (Fig. 2). Conformable surface was projected on 3D printed osteotomy guide which had consistent shape with affected hemi-pelvis. Surgeon could locate osteotomy guide at preoperative-designed location and fix it with Kirschner wires to conduct accurate osteotomy. After shape design, osteotomy guide was manufactured by EOS P 110 3D print machine.
Figure 2.
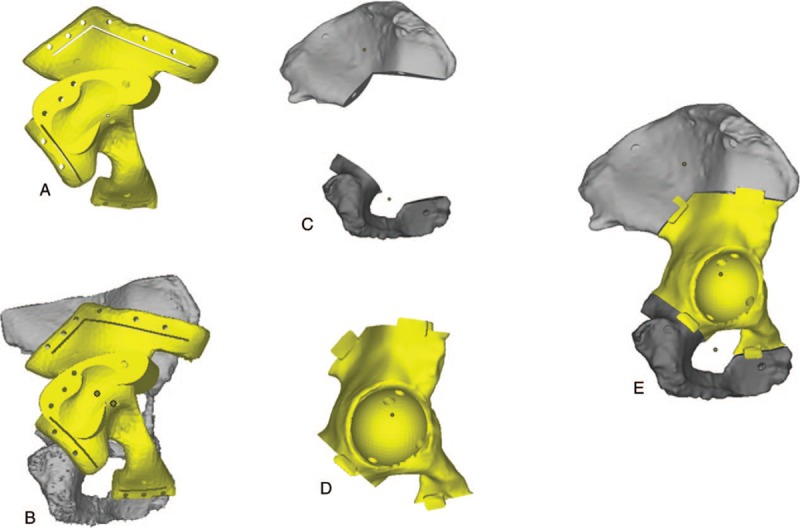
Flow chart of design procedure and matching experiment of 3D printed osteotomy guide and prosthesis: (A): Osteotomy guide. (B): Simulative matching of osteotomy guide and affected side hemi-pelvis. (C): Residual hemi-pelvis after tumor resection. (D): Basic morphology of 3D printed pelvic prosthesis. (E): Simulative matching of residual hemi-pelvis and pelvic prosthesis. 3D = three-dimensional.
2.3.4. Design and manufacture procedure of individual 3D printed prosthesis
3D printed prosthesis was designed according to mirror image of unaffected side hemi-pelvis combined with projected osteotomy scope. 3D printed macro-porous structure was designed on the interface of bone-prosthesis to promote bone in-growth and prosthetic stability. Pore diameter and porosity of macro-porous structure were set to 400 μm and of 60%, respectively. Ti6Al4V was chosen as material of prosthesis. Finally, titanium alloy prosthesis was manufactured by Arcam A1 3D print machine (Arcam Corporation, Beijing) by means of electron beam melting (EBM) 3D print technique (Fig. 3A and B).
Figure 3.
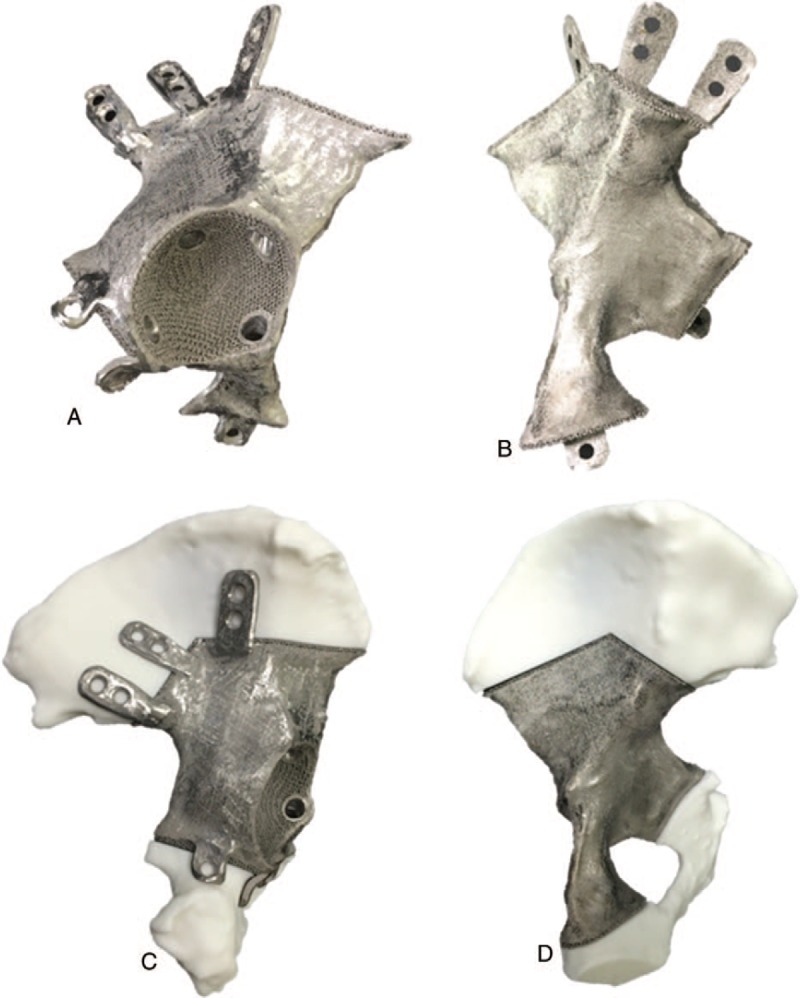
Preoperative matching test of 3D printed Ti6Al4V hemi-pelvic prosthesis: Anterior view (A) and posterior view (B) of 3D printed Ti6Al4V hemi-pelvic prosthesis. Preoperative in vitro experiment was carried out to test matching degree between 3D printed Ti6Al4V hemi-pelvic prosthesis and residual hemi-pelvis (C, D). 3D = three-dimensional.
2.3.5. Preoperative in vitro simulative experiment
3D printed osteotomy guide and model of affected side hemi-pelvis were applied in this study to conduct in vitro simulative osteotomy experiment. After that, simulative matching experiment between residual hemi-pelvic model and 3D printed pelvic prosthesis was carried out to ensure the compatibility attribute of prosthesis before operation. 3D printed prosthesis matched perfectly with pelvic model during in vitro simulation (Figure 3C and D).
2.4. Surgical procedure
After general anesthesia, patient was placed as left-lateral recumbent position. Right hip K-L incision with 30 cm length was adopted to expose focus. Skin, subcutaneous tissue, and gluteus maximus were incised successively. Internal rotation and adduction of right lower limb were positioned to amply expose femoral neck. After cutting off external rotation muscle group, joint capsule was incised with “T shape” incision. Osteotomy of femoral neck was conducted by swing saw from the intersection region between greater trochanter and femoral neck to 10 mm above small trochanter. Then femoral head was removed. After total exposure of the tumor, bone destruction of ilium, pubis, and partial ischium was found. 3D printed osteotomy guide was located and fixed at the corresponding location of the hemi-pelvis to carry out osteotomy (Fig. 4A). Under assistance of osteotomy guide, osteotomy was conducted with minimal distance 30 mm away from the boundary of tumor. After hemostasis of operative field, 3D printed Ti6Al4V pelvic prosthesis was fixed firmly at ilium and pubis by flank screws (Fig. 4B). Bone cement type acetabular cup with size of 46/28 mm was implanted (Fig. 4C). Femoral medullary cavity was reamed along major axis of femur. Biological femoral prosthesis with size #10 was inserted into femoral medullary cavity (Fig. 4D). Femoral head with 28/-4 mm size was also assembled. After restoration of hip joint, satisfactory hip joint range of motion was insured by surgeons. Then muscle, subcutaneous tissue, skin were sawed step by step.
Figure 4.
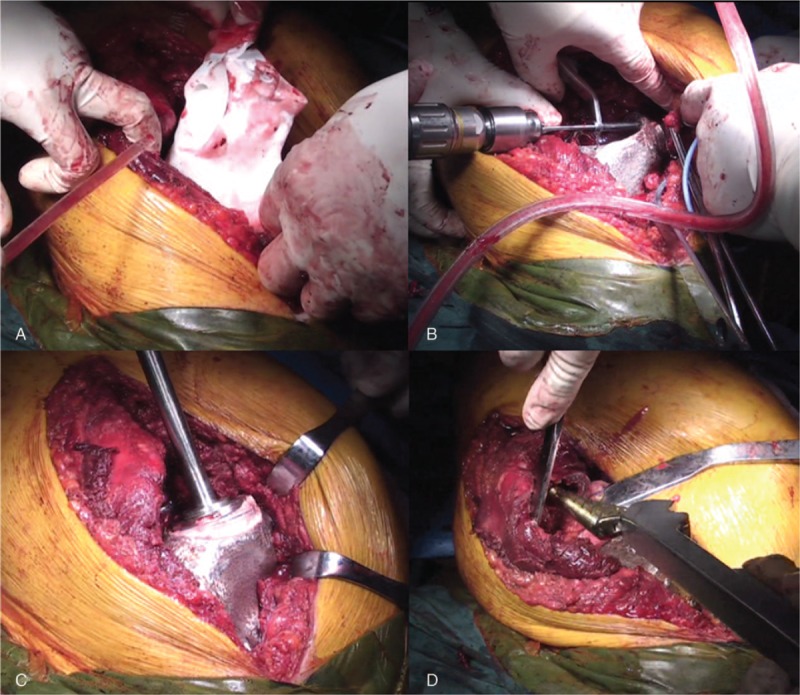
Surgical procedure: (A): 3D printed prosthesis model was applied as guidance of osteotomy in operation. (B): 3D printed Ti6Al4V pelvic prosthesis was fixed at ilium, pubis, and ischium by flank screws. (C): Cement type acetabular cup was implanted. (D): Biological femoral stem was inserted into femoral medullary cavity. 3D = three-dimensional.
3. Outcomes and follow-up
3.1. Surgical result
3D printed prosthesis was implanted to fill the bone defect successfully. Anatomical reconstruction of pelvis was achieved. Satisfactory motion range of hip joint was restored. Intraoperative amount of blood was 900 ml. Operation time was 104 minutes.
3.2. Follow-up result
Musculoskeletal tumor society score (MSTS), SF-36, and Harris score have improved respectively from 7, 52.7, and 19 points preoperative to 16, 108.2, and 52 points at 12 months’ follow-up as described in Table 1. Functional training of lower limbs under assistance of crutch was allowed 2 days after operation. Full weight bear exercise was allowed 2 weeks after operation. X-ray and CT indicated satisfactory prosthetic location during postoperative 1 to 12 months follow up (Fig. 5). No radiolucent line which indicated prosthetic loosening and migration was detected until the latest follow-up. There was also no sign of periprosthetic fractures in X-ray image. There was also no sign of periprosthetic infection. Erythrocyte sedimentation rate and C-reactive protein were normal during follow-up. Patient could walk over 1 km under assistance at 6 months postoperative. Daily activities could be achieved by herself at latest follow-up.
Table 1.
Postoperative function score.

Figure 5.
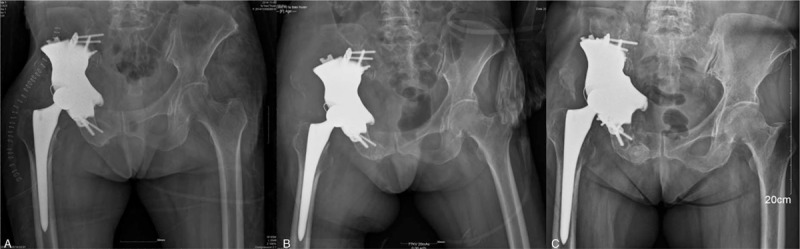
Postoperative follow-up pelvic X-Ray image. (A): Intraoperative X-ray image. (B): Postoperative 3th mo follow-up X-ray image; (C): Postoperative 12th mo follow-up X-ray image. Satisfactory location of 3D printed prosthesis was investigated in pelvic X-ray image. 3D = three-dimensional.
4. Discussion
Pelvis was the third frequent site for metastatic bone tumor, which occupied approximately 10 to 15 percentage of primary metastatic bone tumor.[10–13] Pelvic tumor had relatively low survival rate and high recurrence rate.[9,14–16] Owing to lack of individual prosthetic design, conventional saddle prostheses were reported 12% incidences of prosthetic loosening.[10] Due to poor matching degree of conventional pelvic protheses, massive pelvis osteotomy always needed to be conducted in order to satisfy implantation of protheses. Massive osteotomy leads to loss of attached point of the adjacent soft tissue. This factor might interpret why poor postoperative lower limbs’ motion function was achieved in pelvic reconstruction surgery with modular prostheses.[5–9] In recent years, with rapid development of 3D print technology, this technology was gradually applied in orthopedic oncology which mainly including design and manufacture of protheses, osteotomy guide, and model.[18–20]
Restoration of original anatomy and biomechanics stability of pelvis was the foremost task of pelvic reconstruction surgery.[1–3,13–15] 3D print technology has incomparable superiority in aspect of pelvic reconstruction for it's individual prosthetic design, high accuracy of manufacturing, shorter periodicity, and commercial availability.[2,17–21] For instance, 3D printed iliac prosthesis was manufactured to fill bone defect of a patient with ilium tumor in Chen et al's study. Novel navigation system was also applied to ensure optimal location of 3D printed prostheses. Patient-specific 3D printed prostheses were highly recommended in their study.[2] The advantages of 3D print technology in pelvic reconstruction were also founded in Fan et al's study. In their study, various 3D printed prostheses were designed according to patients’ own morphology of bone tissue to carry out limb salvage surgery of clavicle, pelvis, and scapular.[3] In pelvic reconstruction surgery, operation time of was 130 minutes and amount of intraoperative bleeding was merely 800 ml. High degree MSTS of 27 points (90%) was achieved at 18th months’ follow-up. It was concluded in their study that custom-made 3D printed pelvic protheses simplified surgical procedure and improved motion function of lower limbs.[3] In Wei et al's study, “U shape” 3D printed sacral prosthesis was particularly designed and manufactured to reconstruct the pelvis of a patient with primary sacral tumor.[1] Optimal reconstruction effect of lumbosacral and pelvic ring was achieved by 3D printed sacral prosthesis. Bone defect was filled perfectly with 3D printed prostheses, therefore, the integrity of pelvis was restored intraoperatively. Meanwhile, it was founded by Wei et al that macro-porous structure on the interface of bone-prosthesis could induce bone in-growth ability hence long-term prosthetic stability. The 3D printed pelvic prosthesis applied in our study was projected on the basis of mirror entity of unaffected side hemi-pelvis. Through rigorous preoperative plan, restoration of original morphology of pelvis and high-grade matching degree of prostheses were guaranteed to the utmost. 3D printed prostheses were widely recommended as humanization design, low cost and high efficiency, and accuracy of manufacture.[20–22]
In addition to anatomical reconstruction of pelvis, long-term stability of protheses was essential to ensure the functions of lower limbs.[1–3,22] In order to reduce the incidence of prothetic lossening, macro-porous structure should be projected on the surface of bone-prothesis to promote osteointegration of prothesis.[22,23] But the complex structure of macro-porous limited it's application in this aspect. The limitation highlighted the advantage of EBM-3D print technology for it's excellent manufacturing engineering.[18,19] It was recommended in previous studies that macro-porous structure with diameter in range of 300 μm to 700 μm and porosity ranged between 51% to 73% was most effective to induce bone in-growth ability.[22,23] 3D printed scaffolds with diameter of 800 um was also designed on the surface of 3D printed iliac prosthesis in Wei et al's study.[1] In Fan et al's study, 3D printed scaffolds with various morphology were applied to augment bone in-growth capacity of clavicular, scapular, and pelvic prostheses.[3] Drawing lessons from previous studies, EBM-3D printed macro-porous structure was also designed on the surface of pelvic prothesis in our study. Diameter and porosity of macro-porous structure was set to 400 μm and 60%, respectively. Satisfactory location of 3D printed prosthesis was founded in X-ray during 12 months’ follow-up. No signs of prosthetic loosening and displacement were detected, which good osteointegration ability of prothesis.
The procedure of tumor resection and pelvic reconstruction surgery was complicated in which long operation time and massive amount of bleeding widely existed. According to previous studies, 10 patients with acetabulum tumors were conducted allograft surgeries in which operation time and intraoperative bleeding were 5.2 hours and 2700 ml.[15] In Sun et al's study, average operation time and amount of bleeding of 16 cases primary pelvic tumors resection surgeries were 3.5 hours and 2600 ml. Long operation time and massive bleeding increased the probability of infection.[1,5,11] In modular hemi-pelvic endoprosthesis, high incidence of postoperative infection (14%) was reported by Wang et al.[5] In our study, in order to simplify surgical procedure and reduce operation time, 3D printed osteotomy guide and pelvis model were manufactured to assisted implantation of 3D printed prothesis. Owing to rigorous pro-operative design and fast osteotomy, operation time and intraoperative bleeding have been reduced to merely 104 minutes and 900 ml which were far less than the data reported in relative studies. Among the above factors, application of 3D printed osteotomy guide was considered as a key point to simplify surgical procedure by surgeon of this operation.
Patient-specific osteotomy guide could significantly improve osteotomy accuracy. Currently, it has been widely applied in resection of bone tumor.[9–12] Osteotomy accuracy of 3D printed guide and free-hand osteotomy were compared by Jentzsch et al.[13] Maximum error of 3D printed guide was 4 mm, which was significantly lower than 28 mm of free-hand osteotomy. Owing to complex anatomy of pelvis, accurate tumor resection could not be insured by free-hand pelvic osteotomy.[11–14] A simulative osteotomy study with 24 pelvic models was carried out by Cartiaux et al.[14] Under assistance of osteotomy guide, high-precision osteotomy accuracy of 1 mm was achieved. Accurate osteotomy could also avoid the incidence of intraoperative lesion of adjacent blood vessel and nerve.[11,13] In our study, 4 osteotomy lines were planned surrounding acetabulum. The osteotomy guide matched perfectly with pelvis in operation. The application of 3D printed osteotomy guide lay foundation for the successful implantation of 3D printed prosthesis.
Though satisfactory surgical effect and follow-up result were achieved, there were also several limitations in this study. First, only 1 case pelvic reconstruction surgery with 3D printed prosthesis was reported in this study. More cases would be brought into further study. Second, there was no quantitative result to assess osteotomy accuracy of 3D printed guide. Finally, biomechanical tests were not conducted to forecast stability of prosthesis preoperatively. Further study was expected to remedy above limitations.
Accurate tumor resection and pelvic reconstruction were successfully achieved with application of 3D printed hemi-pelvic prosthesis. Follow-up result within 12 months was satisfactory. A promising method for treatment of pelvic tumor was provided in this study. More convincing conclusions require further biomechanical tests and more clinical cases to verify.
Author contributions
Conceptualization: Qing Han, Jincheng Wang.
Data curation: Kesong Zhang, Yong Zhang, Chenyu Wang, Kerong Yang, Bingpeng Chen.
Funding acquisition: Qing Han, Bingpeng Chen.
Investigation: Qing Han, Kesong Zhang.
Methodology: Kesong Zhang, Yun Zou, Bingpeng Chen.
Resources: Jincheng Wang.
Software: Chenyu Wang, Kerong Yang.
Supervision: Qing Han.
Writing – original draft: Qing Han, Kesong Zhang, Jincheng Wang.
Writing – review and editing: Qing Han, Jincheng Wang.
Footnotes
Abbreviations: 3D = three-dimensional, CAD = computer-aided design, CT = computed tomography, EBM = electron beam melting, MSTS = musculoskeletal tumor society score, SF-36 = the MOS item short from health survey, STL = stereolithography.
How to cite this article: Han Q, Zhang K, Zhang Y, Wang C, Yang K, Zou Y, Chen B, Wang J. Individual resection and reconstruction of pelvic tumor with three-dimensional printed customized hemi-pelvic prosthesis. Medicine 2019;98:36(e16658).
This work was supported by the National Natural Science Foundation of China (grant number 81802174); Department of Science and Technology of Jilin Province, P.R.C (grant numbers 20170204004GX and 20180520115JH); Jilin Province Development and Reform Commission, P.R.C (grant number 2018C010).
The authors have no conflicts of interest to disclose.
References
- [1].Wei R, Guo W, Ji T, et al. One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: a technical note. Eur Spine J 2017;26:1–8. [DOI] [PubMed] [Google Scholar]
- [2].Chen X, Xu L, Wang Y, et al. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput Methods Programs Biomed 2016;125:66–78. [DOI] [PubMed] [Google Scholar]
- [3].Fan H, Fu J, Li X, et al. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: a case series and review of the literature. World J Surg Oncol 2015;13:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kitagawa Y, Ek ET, Choong PF. Pelvic reconstruction using saddle prosthesis following limb salvage operation for periacetabular tumour. J Orthop Surg (Hong Kong) 2006;14:155–62. [DOI] [PubMed] [Google Scholar]
- [5].Wang B, Xie X, Yin J, et al. Reconstruction with modular hemipelvic endoprosthesis after pelvic tumor resection: a report of 50 consecutive cases. PLoS One 2015;10:e0127263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Danişman M, Mermerkaya MU, Bekmez Ş, et al. Reconstruction of periacetabular tumours with saddle prosthesis or custom-made prosthesis, functional results and complications. Hip Int 2016;26:e14–8. [DOI] [PubMed] [Google Scholar]
- [7].Sun W, Li J, Li Q, et al. Clinical effectiveness of hemipelvic reconstruction using computer-aided custom-made prostheses after resection of malignant pelvic tumors. J Arthroplasty 2011;26:1508–13. [DOI] [PubMed] [Google Scholar]
- [8].Dai KR, Yan MN, Zhu ZA, et al. Computer-aided custom-made hemipelvic prosthesis used in extensive pelvic lesions. J Arthroplasty 2007;22:981–6. [DOI] [PubMed] [Google Scholar]
- [9].Holzapfel BM, Pilge H, Prodinger PM, et al. Customised osteotomy guides and endoprosthetic reconstruction for periacetabular tumours. Int Orthop 2014;38:1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Menendez LR, Ahlmann ER, Falkinstein Y, et al. Periacetabular reconstruction with a new endoprosthesis. Clin Orthop Relat Res 2009;467:2831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ma L, Zhou Y, Zhu Y, et al. 3D-printed guiding templates for improved osteosarcoma resection. Sci Rep 2016;6:23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ng VY, DeClaire JH, Berend KR, et al. Improved accuracy of alignment with patient-specific positioning guides compared with manual instrumentation in TKA. Clin Orthop Relat Res 2012;470:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jentzsch T, Vlachopoulos L, Fürnstahl P, et al. Tumor resection at the pelvis using three-dimensional planning and patient-specific instruments: a case series. World J Surg Oncol 2016;14:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cartiaux O, Paul L, Francq BG, et al. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery. Ann Biomed Eng 2014;42:205–13. [DOI] [PubMed] [Google Scholar]
- [15].Laffosse JM, Pourcel A, Reina N, et al. Primary tumor of the periacetabular region: resection and reconstruction using a segmental ipsilateral femur autograft. Orthop Traumatol Surg Res 2012;98:309–18. [DOI] [PubMed] [Google Scholar]
- [16].Rengier F, Mehndiratta A, von TH, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010;5:335–41. [DOI] [PubMed] [Google Scholar]
- [17].Cai L, Zhang Y, Chen C, et al. 3D printing-based minimally invasive cannulated screw treatment of unstable pelvic fracture. J Orthop Surg Res 2018;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu M, Li Y, Luo Y, et al. Uncemented three-dimensional-printed prosthetic reconstruction for massive bone defects of the proximal tibia. World J Surg Oncol 2018;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lu M, Wang J, Tang F, et al. A three-dimensional printed porous implant combined with bone grafting following curettage of a subchondral giant cell tumour of the proximal tibia: a case report. BMC Surg 2019;19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thadani VN, Riaz MJ, Singh G. The evolution of three-dimensional technology in musculoskeletal oncology. J Clin Orthop Trauma 2018;9:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Friedrich MJ, Schmolders J, Michel RD, et al. Management of severe periacetabular bone loss combined with pelvic discontinuity in revision hip arthroplasty. Int Orthop 2014;38:2455–61. [DOI] [PubMed] [Google Scholar]
- [22].Zou Y, Yang Y, Han Q, et al. Novel exploration of customized 3D printed shoulder prosthesis in revision of total shoulder arthroplasty: a case report. Medicine (Baltimore) 2018;97:e13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jana M, Jan W, Volker W, et al. Influence of different three-dimensional open porous titanium scaffold designs on human osteoblasts behavior in static and dynamic cell investigations. Materials 2015;8:5490–507. [DOI] [PMC free article] [PubMed] [Google Scholar]


