Abstract
Background:
Although several previous studies demonstrated the feasibility and efficacy of indocyanine green (ICG) for thyroid cancer surgery, ICG was administered through venous injection and focused on parathyroid gland protection. We thus aimed to study the feasibility of imaging using ICG combined with carbon nanoparticles (CNs) in the identification of sentinel lymph nodes (SLNs) in patients diagnosed with papillary thyroid microcarcinoma (PTMC).
Methods:
Two approaches were applied to detect lymph nodes in PTMC surgery. Patients were randomized into 2 groups. ICG and CNs were injected into the thyroid in Group A. In Group B, only CNs was injected. Black-stained or fluorescent nodes observed using near-infrared fluorescence imaging systems were defined as SLNs. SLN and central lymph node (CLN) dissection was completed in both groups. The pathological and postoperative outcomes were compared between 2 groups.
Results:
There were 40 patients in Group A and 60 in Group B. A total of 138 SLNs were identified; 72 and 66 SLNs were detected and dissected in Groups A and B, respectively. The number of SLNs identified (per patient) in Group A was higher than that in Group B (P = .027). The number of harvested CLNs was 161 and 192 in Groups A and B, respectively, out of which 45 and 48 lymph nodes with metastasis were confirmed by permanent pathology. The CLN metastatic rate in Group A was higher than that in Group B (P = .048).
Conclusion:
Imaging using ICG combined CNs is feasible and safe for SLN identification in PTMC patients. Compared with using only CNs, more SLNs can be removed and more metastatic lymph nodes can be confirmed when using the combined method. Although the combined method appears to accurately stage tumors, further research is needed.
Keywords: carbon nanoparticles, indocyanine green, papillary thyroid microcarcinoma, sentinel lymph node
1. Introduction
The incidence of thyroid cancer has doubled between 1975 and 2009, and it is now the fifth most common malignant tumor among women in the United States.[1,2] The majority (>90%) of these new diagnoses are papillary thyroid cancer, and surgery is usually needed in patients with differentiated thyroid cancer.[3] In the postoperative pathological examination, the micrometastasis rate of the central lymph nodes (CLNs) is about 27% to 90% in papillary thyroid microcarcinoma (PTMC).[4–6] Central lymph node dissection (CLND) can improve the prognosis of PTMC and reduce tumor recurrence as well as provide accurate information for the evaluation of tumor staging. Sentinel lymph nodes (SLNs) are the lymph nodes involved when metastasis occurs; they are also the first station in the primary tumor drainage of PTMC. As such, SLN involvement is an accurate predictor of central compartment metastasis.[7] Accurate evaluation of the status of SLNs is important for treatment and follow-up after PTMC surgery. Sentinel lymph node biopsy is the standard procedure to retrieve the lymph nodes and it has become part of the routine treatment protocol for some malignant tumors, such as melanoma and breast cancer.[8,9]
Previously, only a few studies have focused on SLN detection in PTMC. In these studies, different mapping methods were used, including blue dye, radioisotope, and carbon nanoparticles (CNs). Detection rates varied drastically depending on whether the 3 agents were used separately or in combination.[10–12]
CNs as a new tracer for lymph nodes are widely used in studies conducted in China.[13] With an average diameter larger than blood capillaries but smaller than lymphatic vessels, CNs can only enter lymphatic vessels and be taken in by macrophages accumulating in lymph nodes when it is injected directly to the peritumoral area. The lymph nodes are black stained as a result, making it easier for identification and resection,[13] which is advantageous in thyroid cancer surgeries. However, if not managed properly, CNs can stain tissues surrounding lymph nodes making it harder to find the lymph nodes. Furthermore, although nanocarbon has the potential to increase the detection rate of lymph nodes, some lymph nodes are affected by factors impairing the lymphatic flow, which may prevent them from becoming black-stained. Similar problems have been observed in other imaging methods involving dyes. Therefore, a new method for detecting thyroid SLNs with higher specificity and sensitivity is needed.
Near-infrared fluorescence imaging is a newly emerging modality that can enable real-time image-guided surgery. Indocyanine green (ICG) is an Food and Drug Administration approved, inexpensive, and widely available fluorescent agent. ICG has optical properties that can be detected by near-infrared fluorescence imaging systems (NIR) cameras. The use of ICG for lymphatic mapping techniques offers the ability to use NIR to visualize lymphatic anatomy and flow directly and instantly. ICG can be used safely and efficiently for lymphatic mapping in breast cancer.[14] Studies in breast cancer surgery have also shown that ICG has advantages over blue dye for SLN identification.[15]
Although several previous studies demonstrated ICG feasibility and efficacy in thyroid cancer surgery, ICG was administered through venous injection and focused on the protection of the parathyroid glands.[16,17] This novel study examined the feasibility and efficacy of ICG injected directly into the thyroid gland and combined with CNs to image SLNs.
2. Patients and methods
2.1. Case selection
From January 2017 to January 2019, the study population was patients preoperatively diagnosed with PTMC (≤10 mm) based on ultrasound-guided fine-needle aspiration cytology (FNAC) in the Department of Thyroid and Breast Surgery, Yinzhou Hospital of Ningbo University Medical College. To be included in the study, patients had to meet the following criteria: be over 14 years old at the time of the surgery, have proven PTMC on preoperative FNAC, and clinical lymph node-negative (Clinical stage N0, cN0) confirmed by ultrasound. Patients were excluded if they met any of the following criteria: presence of any other carcinomas concurrent with PTMC, history of any previous neck surgeries, severe hepatic or renal disease, or history of allergy to ICG. All patients included in this study received thyroidectomy with SLN and CLN dissection. This study was approved by the Clinical Ethics Committee of Yinzhou Hospital of Ningbo University Medical College (No. 107-W-066, January 19, 2019). Informed consent was obtained from all patients.
2.2. Study design
Patients were randomized into 2 groups: the ICG combined with CNs Group (Group A), and CNs only Group (Group B). The following clinical data were recorded for all patients:
-
(1)
Age,
-
(2)
Gender,
-
(3)
Body mass index,
-
(4)
Surgical method,
-
(5)
Tumor size,
-
(6)
Preoperative parathyroid hormone level,
-
(7)
Preoperative comorbidity.
Demographic information for each patient, pathology results, the number of SLNs, metastatic SLNs, CLNs confirmed by pathology and postoperative complications data were collected and compared between Groups A and B.
2.3. Surgical procedures
All surgeries were carried out by 2 skilled surgeons with more than a decade of clinical experiences in thyroidectomy. Open surgery for thyroidectomy was performed on all patients. A horizontal incision of 2-fingers’ width was made above the suprasternal notch. A subplatysmal dissection was performed to elevate the neck skin flaps. Strap muscles were dissected and pulled aside from the midline. The lesion side of the thyroid was exposed. The lateral and posterior parts of thyroid gland were not dissected to reduce damage to the surrounding thyroid lymphatic network. Afterwards, 0.1 ml ICG (10 ml: 25 mg, Weicai Pharmaceutical Co Ltd, China.) and 0.1 ml CN suspension (1 ml: 25 mg, Chongqing Laimei Pharmaceutical Co Ltd, China.) were injected in the middle part of the thyroid lobe in patients of Group A. The puncture point was cauterized and sealed with electric coagulation scalpel immediately as the syringe needle was withdrawn to prevent leakage. The surgical dissection started 5 minutes later. The surgical lights were turned off. The lymphatic tissue was mapped in real-time by NIR (M.D. Biomedical Technology Corporation, China.) (Fig. 1). In the central neck compartment, SLNs were defined as those that were either were black-stained by CNs or that were fluorescent from ICG (Fig. 2). After SLNs were removed, further CLND was performed. Before closing the incision, the lymph nodes in removed tissue were re-confirmed with NIR (Fig. 3). In Group B, only 0.1 ml CN suspension was injected in the middle part of the thyroid lobe. After 5 minutes, the black-stained SLNs were dissected and further CLND was performed. The thyroid and harvested CLNs were then sent to permanent pathology in both groups.
Figure 1.
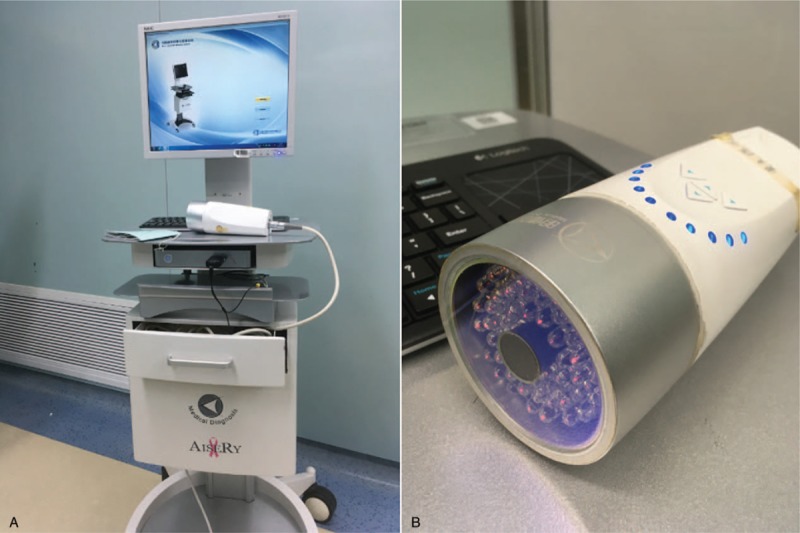
(A) NIR. (B) Fluorescence detecting probe. NIR = near-infrared fluorescence imaging systems.
Figure 2.
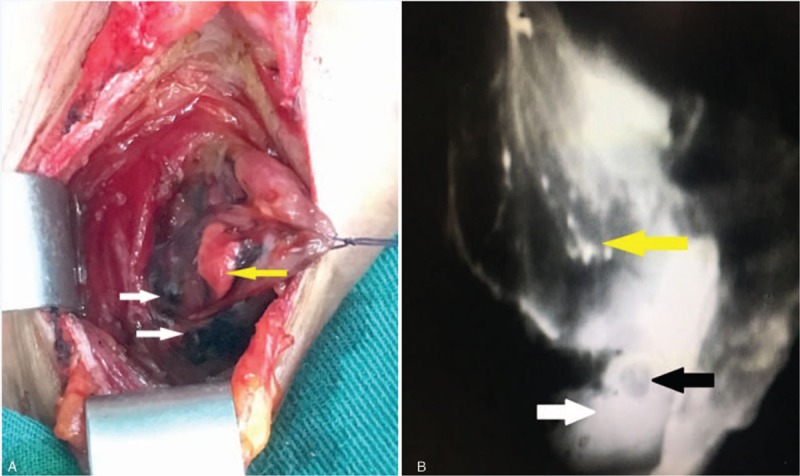
(A) White arrows show black-stained SLNs by carbon nanoparticles; yellow arrow shows the parathyroid gland not stained black. (B) The image was obtained using near-infrared fluorescence imaging systems. White arrow shows fluorescent SLNs; black arrow shows the parathyroid gland; yellow arrow shows the thyroid gland. SLNs = sentinel lymph nodes.
Figure 3.
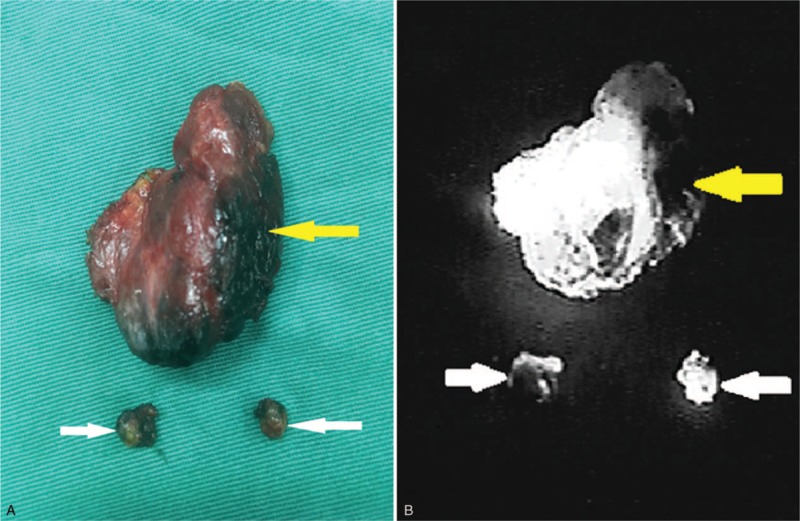
(A) Removed specimen. White arrows show SLNs; and the yellow arrow shows the thyroid gland. (B) An image of the same specimen was obtained using near-infrared fluorescence imaging systems. White arrows show SLNs and yellow arrow shows the thyroid gland. SLNs = sentinel lymph nodes.
2.4. Outcomes
The primary endpoints were the number of SLNs, the number of metastatic SLNs, and CLNs after surgery. Secondary outcomes included:
-
(1)
The number of retrieved CLNs,
-
(2)
Metastasis rate of lymph nodes,
-
(3)
Operative time (minutes),
-
(4)
The number of identified parathyroid glands (PGs),
-
(5)
Postoperative complications: bleeding, hypoparathyroidism, and recurrent laryngeal nerve palsy, and
-
(6)
Tumor stage according to the American Joint Committee on Cancer (AJCC) Staging 8th edition.[18]
2.5. Statistical analysis
Continuous data are expressed as the mean ± standard. Independent-samples t tests were performed to compare continuous variables and chi-square tests were applied to analyze categorical data. SPSS17.0 (SPSS, Inc, Chicago, IL) was used to perform the statistical analysis; P < .05 was considered to indicate a statistically significant difference.
3. Results
3.1. Patients’ demography
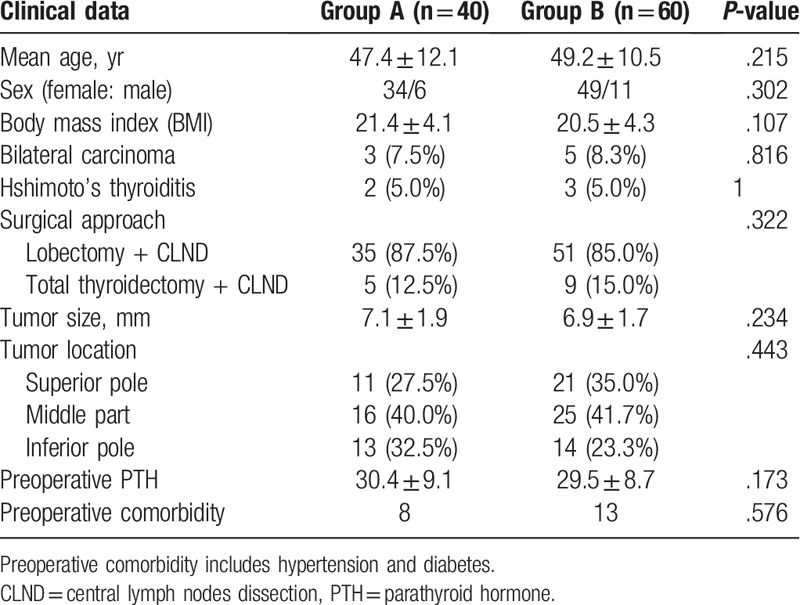
A total of 100 consecutive PTMC patients with cN0 were included in this study. There were 83 women and 17 men with an average age of 48.1 ± 9.1 years old. No case allergy to ICG and CNs was recorded. Forty patients were in Group A. Sixty patients were in Group B. Preoperative patients’ data were recorded and compared; no significant differences were found between the groups and 2 groups of patients were comparable (Table 1).
Table 1.
Patients’ characteristics in 2 groups.
3.2. SLN and CLN dissection
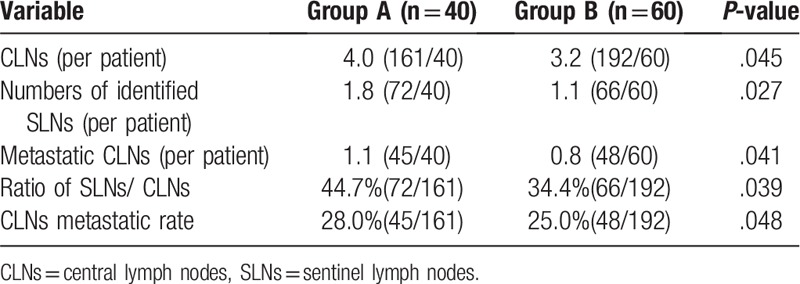
A total of 138 SLNs were identified.
In Group A, 72 SLNs were identified in 40 patients (1.8 nodes per patient) with a total of 161 CLNs. SLNs were successfully detected in 32 patients; out of these, SLNs of 10 patents were positive with frozen biopsy and of 22 patients were negative with frozen biopsy. CLNs were positive in 2 patients with permanent biopsy. The sensitivity was 83.3% (10/12) with a false negative rate of 16.7% (2/12). In Group B, 66 SLNs were identified in 60 patients (1.1 nodes per patient) with a total of 192 CLNs. SLNs were successfully detected in 42 patients; out of these, SLNs of 13 patents were positive and 29 patients were negative with frozen biopsy. CLNs were positive in 6 patients with permanent biopsy. The sensitivity was 68.4% (13/19) with a false negative rate of 31.6% (6/19). The number of metastatic CLNs (per patient) was 1.1 (45/40) and 0.8 (48/60) in Groups A and B, respectively (P = .041). The ratio of SLNs/CLNs was 44.7% and 34.4% in Groups A and B, respectively (P = .039). The CLN metastatic rate was 28.0% and 25.0% Group A and B, respectively (P = .048). The above data showed statistical significance (P < .05) (Table 2).
Table 2.
Comparison of the dissected SLNs and CLNs in 2 groups.
3.3. Surgery duration and postoperative complications
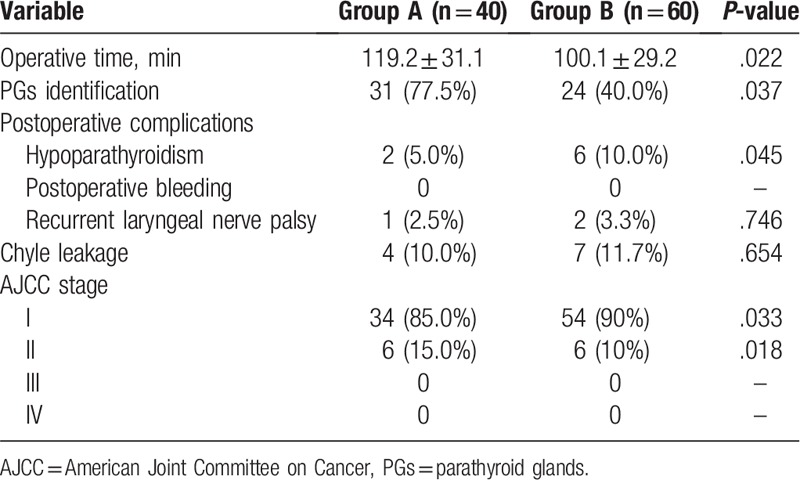
Complications after surgery included postoperative chyle leakage, postoperative bleeding, hypoparathyroidism, and laryngeal nerve palsy (Table 3). Surgery duration was 119.2 ± 31.1 minutes in Group A and 100.1 ± 29.2 minutes in Group B (P = .022). PGs were identified in 31 (77.5%) patients in Group A versus 24 (40.0%) in Group B (P = .037). Hypothyroidism as a postoperative complication occurred less frequently in Group A 2 (5.0%) versus 6 (10.0%) in Group B (P = .045). No significant differences were observed in the frequency of postoperative bleeding or recurrent laryngeal nerve palsy. Comparing 2 groups’ AJCC stage, more stage II (P = .018) and less stage I (P = .033) carcinomas were diagnosed in Group A than that in Group B.
Table 3.
Variables compared between 2 groups.
4. Discussion
More people are being diagnosed with PTMC due to the growing incidence of this disease and greater use of high-frequency ultrasound, other imaging modalities, and FNAC, which have increased the rate of detection.[19] Despite the good outlook of PTMC, lymph node metastasis can affect the overall prognosis and survival of patients,[20–22] especially when distant metastasis occurs.[23] CLND significantly reduces locoregional recurrence.[24] It is noteworthy that the CLN metastatic rate in our study was over 20%, and though slightly lower than that in other studies,[4–6] it still necessitated routine CLND.
Tracers of lymph nodes, methylene blue, and other dyes have certain advantages in the detection of SLNs in thyroid cancer surgery. They can help to detect more potentially malignant lymph nodes and reduce the recurrence rate of tumors. However, the detection rate of lymph nodes with such dyes is low, and the accidental coloration of the surrounding tissues and recurrent laryngeal nerves makes differentiation during surgery difficult, which may lead to mistakes such as unintended cuts or injuries.[25] In previous studies, the detection rate of SLNs using CNs was higher than when using the methylene blue dye technique.[26–29] However, Yan et al[30] reported that no increase in the number of SLN metastasis-positive cases was observed when CNs were used, which is a significant limitation of the method.
The main aim of this study was to assess the feasibility of imaging using ICG combined with CNs to intraoperatively identify SLNs in PTMC patients. Our study showed that the use of ICG combined with CNs did not increase the likelihood of complications after surgery. Meanwhile, a higher average number of dissected SLNs and CLNs was confirmed and more metastatic lymph nodes were found when using ICG combined with CNs. Furthermore, a higher sensitivity and lower false-negative rate was observed. These findings might be explained by a number of phenomena.
First, after the injection of CNs into the thyroid parenchyma, CNs enter lymphatic vessels rapidly, accumulate in the lymph nodes, and stain them black. However, lymphatic vessels partially blocked by cancer cells can impact the staining process. As ICG is much smaller, it can map lymph networks where the CNs would not show.
Second, in our study, CNs accidentally stained both the lymph nodes and the surroundings in several cases, thus causing confusion during the SLN resection. In cases where CNs were combined with ICG, lymph nodes were more fluorescent than neighboring tissue, which facilitated the dissection.
Third, in combined imaging cases, surgeons were more likely to spend more time finding the SLNs, which helped to position those not so easily observed, especially when they were not at the expected anatomical site.
As a result, the ICG combined with CNs method extended the duration of surgery, equipment setup, and the search for SLNs. Nevertheless, the mapping accuracy provided by this method offset the risks associated with more time spent in the operating theatre. Accurate staging of cancer allows for the development of better treatment plans, which reduces the risk of relapse.
Regarding postoperative complications, in this study, the group that underwent imaging using ICG combined with CNs had fewer cases hypoparathyroidism after surgery. This is an unexpected finding which warrants further investigation.
Although our study showed the feasibility of ICG combined with CNs in mapping SLNs of PTMC patients, in some patients, we noticed that the surrounding tissue was as fluorescent as SLNs, which impacted identification. The injection point, timing of injection, concentration of ICG, and the speed at which it was administered may play a role; these factors require further study. Therefore, how to administer these 2 agents is a key point in imaging SLNs. Meanwhile, because of the small number of patients enrolled in this study, further research is necessary to ascertain to what extent this combined method has clinical applications.
5. Conclusion
Imaging using ICG combined with CNs is feasible and safe for SLN identification in PTMC patients. Compared with using only CNs, more SLNs can be removed and more metastatic lymph nodes can be confirmed when using the combined method. The combined method, therefore, allows to stage tumors more accurately, which benefits patients in the long-term.
Acknowledgments
We acknowledge the work of the nurses and doctors of Yinzhou Hospital of Ningbo University Medical College involved in the surgeries.
Author contributions
Formal analysis: Yan-Ping Shen.
Funding acquisition: Li Jia-Gen.
Investigation: Xing Zhang, Gun Chen.
Methodology: Xing Zhang.
Project administration: Li Jia-Gen.
Resources: Li Jia-Gen.
Software: Yan-Ping Shen.
Supervision: Li Jia-Gen.
Writing – original draft: Xing Zhang.
Writing – review and editing: Xing Zhang.
Footnotes
Abbreviations: CLN = central lymph node, CLND = central lymph node dissection, CNs = carbon nanoparticles, FNAC = fine needle aspiration cytology, ICG = indocyanine green, NIR = fluorescence imaging systems, PTMC = papillary thyroid microcarcinoma, SLNs = sentinel lymph nodes.
How to cite this article: Zhang X, Shen YP, Li JG, Chen G. Clinical feasibility of imaging with indocyanine green combined with carbon nanoparticles for sentinel lymph node identification in papillary thyroid microcarcinoma. Medicine 2019;98:36(e16935).
This study is supported by the Foundation of Ministry of Health of Zhejiang Province, Hangzhou, China (No. 2017KY613).
The authors have no conflicts of interest to disclose.
References
- [1].McCarthy M. US thyroid cancer rates are epidemic of diagnosis not disease, study says. BMJ 2014;24:1743. [DOI] [PubMed] [Google Scholar]
- [2].Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid 2014;24:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol 2018;14:670–83. [DOI] [PubMed] [Google Scholar]
- [4].Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1999;114:1050–8. [PubMed] [Google Scholar]
- [5].Shaha AR. Thyroid cancer: extent of thyroidectomy. Cancer Control 2000;7:240–5. [DOI] [PubMed] [Google Scholar]
- [6].Henry JF, Gramatica L, Denizot A, et al. Morbidity of prophylactic lymph node dissection in the central neck area in patients with papillary thyroid carcinoma. Langenbecks Arch Surg 1998;383:167–9. [DOI] [PubMed] [Google Scholar]
- [7].Jozaghi Y, Richardson K, Anand S, et al. Frozen section analysis and sentinel lymph node biopsy in well differentiated thyroid cancer. J Otolaryngol Head Neck Surg 2013;42:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kelley MC, Hansen N, McMasters KM. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Am J Surg 2004;188:49–61. [DOI] [PubMed] [Google Scholar]
- [9].Gipponi M, Di Somma C, Peressini A, et al. Sentinel lymph node biopsy in patients with Stage I/II melanoma: clinical experience and literature review. J Surg Oncol 2004;85:133–40. [DOI] [PubMed] [Google Scholar]
- [10].Garau LM, Rubello D, Ferretti A, et al. Sentinel lymph node biopsy in small papillary thyroid cancer. A review on novel surgical techniques. Endocrine 2018;62:340–50. [DOI] [PubMed] [Google Scholar]
- [11].Hao RT, Chen J, Zhao LH, et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillarythyroid microcarcinoma. Eur J Surg Oncol 2012;38:718–24. [DOI] [PubMed] [Google Scholar]
- [12].Ji YB, Lee KJ, Park YS, et al. Clinical efficacy of sentinel lymph node biopsy using methylene blue dye in clinically node-negative papillary thyroid carcinoma. Ann Surg Oncol 2012;19:1868–73. [DOI] [PubMed] [Google Scholar]
- [13].Wang L, Yang D, Lv JY, et al. Application of carbon nanoparticles in lymph node dissection and parathyroid protection during thyroid cancer surgeries: a systematic review and meta-analysis. Onco Targets Ther 2017;10:1247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Valente SA, Al-Hilli Z, Radford DM, et al. Near infrared fluorescent lymph node mapping with indocyanine green in breast cancer patients: a prospective trial. J Am Coll Surg 2018;S1072-7515:32225–7. [DOI] [PubMed] [Google Scholar]
- [15].Ahmed M, Purushotham A, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014;15:351–62. [DOI] [PubMed] [Google Scholar]
- [16].van den Bos J, van Kooten L, Engelen SME, et al. Feasibility of indocyanine green fluorescence imaging for intraoperative identification of parathyroid glands during thyroid surgery. Head Neck 2019;41:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vidal Fortuny J, Sadowski SM, Belfontali V, et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine greenfluorescence predicting parathyroid function after thyroid surgery. Br J Surg 2018;105:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tam S, Boonsripitayanon M, Amit M, et al. Survival in differentiated thyroid cancer: comparing the AJCC Cancer staging seventh and eighth editions. Thyroid 2018;28:1301–10. [DOI] [PubMed] [Google Scholar]
- [19].Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017;317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zaydfudim V, Feurer ID, Griffin MR, et al. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008;144:1070–7. [DOI] [PubMed] [Google Scholar]
- [21].Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid 2009;19:683–9. [DOI] [PubMed] [Google Scholar]
- [22].Lundgren CI, Hall P, Dickman PW, et al. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer 2006;106:524–31. [DOI] [PubMed] [Google Scholar]
- [23].Mercante G, Frasoldati A, Pedroni C, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillarymicrocarcinoma of the thyroid: results of a study in 445 patients. Thyroid 2009;19:707–16. [DOI] [PubMed] [Google Scholar]
- [24].Chen L, Wu YH, Lee CH, et al. Prophylactic central neck dissection for papillary thyroid carcinoma with clinically uninvolved central neck lymph nodes: a systematic review and meta-analysis. World J Surg 2018;42:2846–57. [DOI] [PubMed] [Google Scholar]
- [25].Hao RT, Chen J, Zhao LH, et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroidmicrocarcinoma. Eur J Surg Oncol 2012;38:718–24. [DOI] [PubMed] [Google Scholar]
- [26].Balasubramanian SP, Harrison BJ. Systematic review and meta-analysis of sentinel node biopsy in thyroid cancer. Br J Surg 2011;98:334–44. [DOI] [PubMed] [Google Scholar]
- [27].Raijmakers PG, Paul MA, Lips P. Sentinel node detection in patients with thyroid carcinoma: a meta-analysis. World J Surg 2008;32:1961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dzodic R, Markovic I, Inic M, et al. Sentinel lymph node biopsy may be used to support the decision to perform modified radical neck dissection in differentiated thyroid carcinoma. World J Surg 2006;30:841–6. [DOI] [PubMed] [Google Scholar]
- [29].Cunningham DK, Yao KA, Turner RR, et al. Sentinel lymph node biopsy for papillary thyroid cancer: 12 years of experience at a single institution. Ann Surg Oncol 2010;17:2970–5. [DOI] [PubMed] [Google Scholar]
- [30].Yan X, Zeng RC, Ma Z, et al. The utility of sentinel lymph node biopsy in papillary thyroid carcinoma with occult lymph nodes. PLoS One 2015;10:e129304. [DOI] [PMC free article] [PubMed] [Google Scholar]


