Abstract
Erythrina corallodendron L., a kind of landscape tree, has long been used as a traditional medicine. In this study, the composition of essential oil extracted from the leaves was analysed by GC-MS (gas chromatograph-mass spectrometer), with linalool identified as the main compound. Its cytotoxicity against MDA-MB-231, MCF-7 and HMLE cells was examined by MTT and cloning assays. Transwell and wound-healing assays were used to examine the inhibition of migration and invasion. Western blot, qRT-PCR and immunofluorescence staining were used to measure the mRNA and protein expression of factors related to EMT (snail, slug, E-cadherin, N-cadherin and vimentin). The essential oil of Erythrina corallodendron leaves was found to inhibit the proliferation, migration and invasion of breast cancer cells in a dose-dependent manner. The findings of this study suggest that the essential oil of E. corallodendron leaves may merit further investigation as a potential clinical or adjuvant drug for treating breast cancer migration and invasion.
Keywords: breast cancer, epithelial-mesenchymal transition, erythrina corallodendron, essential oil, metastasis
1. Introduction
Breast cancer is one of the most harmful diseases to women worldwide, second only to lung cancer.[1] Its features of easy metastasis and poor prognosis make it even more lethal.[2–4] The distal metastasis sites of breast cancer include the bones, lungs, brain and liver, and some of the subtypes are more malignant than others.[5–8] During the process of migration and invasion, the tightly integrated epithelial cells of breast cancers become loose, produce pseudopodium, and become mesenchymal cells.[9–11] This process is called the epithelial-mesenchymal transformation (EMT). The EMT process occurs following alterations in a series of pathways, with characteristic features at the molecular level. Most of these processes begin with the activation of transcription factors, such as the activation of snail and slug.[12–14] Once cancer cells transform into mesenchymal cells, they can easily penetrate the vascular endothelium and move from the primary site to distal secondary sites, thereby enhancing the risk of recrudescence.[15]
The genus Erythrina, which includes almost 200 species, is mainly distributed in tropical and subtropical areas.[16] One of them, Erythrina corallodendron L., the national flower of Argentina, is widely used for urban landscaping due to its attractive flowers. Recent years, researchers had put their attention into the chemical composition of E. corallodendron. Many studies have reported the function and molecular dynamics of EcorL, which is the lectin isolated from E. corallodendron.[17–19] The proteinase inhibition of E. corallodendron seeds had also been investigated in early evidences.[20,21] Essential oils are always present in high amounts in odorous leaves, seeds and flowers, especially in colourful organs.[22,23] In addition, essential oils are widely used in treating lung cancer and metastatic cancers, such as breast cancer and leukemia.[24–27] In traditional medicine, the bark of E. corallodendron has been used for anesthesia and acesodyne. However, the action and molecular mechanisms of E. corallodendron on cancer cells remains unknown. In this study, we analyzed the chemical composition of E. corallodendron leaf essential oil (ECEO) and investigated the underlying anti-breast cancer properties.
2. Materials and methods
2.1. Chemical reagents
DMSO and MTT (3-(4,5-Dimethylthiazzol)-2,5-diphenyltetrazolium bromide) were purchased from Sigma (Darmstadt, Germany). All primary and secondary antibodies were bought from Cell Signaling Tech. (Danvers). Matrigel used for the Transwell assay was bought from Sigma (Darmstadt, Germany), and Transwell chambers were bought from PALL (New York). The ReverTra Aoe qPCR RT Kit was purchased from TOYOBO (Tokyo, Japan).
2.2. Preparation of essential oil and GC-MS analysis
Erythrina corallodendron L. specimens were collected from Zhangzhou, Fujian, China in August 2018 and identified by Prof. Yu-Xin Ma (Zhejiang Ocean University). A type specimen was deposited in the specimen room of Zhejiang Ocean University. Essential oil was obtained from the fresh leaves of E. corallodendron (500 g) by a hydrodistillation method using a Clevenger apparatus for 3 hours. The highest essential oil yield reached 8.01%. The GC-MS analysis was performed by Disha Corporation using a ThermoFisher TSQ 8000 Evo GC-MS system.
2.3. Cell culture
All breast cancer cells were purchased from the Shanghai Institute for Biological Sciences (SIBS) and the Chinese Academy of Sciences (Shanghai, China). Human breast cancer cells (MDA-MB-231 and MCF-7) were cultured in RPMI-1640 medium (Hyclone Laboratories, Inc., Logan, UT) and non-cancerous mammary epithelial cells (HMLE) were cultured in 1:1 DMEM:F-12 medium (Hyclone Laboratories, Inc.). All media contained 10% fetal bovine serum (FBS) supplemented with 100 units/mL of penicillin and 100 μg/mL of streptomycin. All cells were incubated in 5% CO2 at 37°C. All experiments were reviewed and approved by the Ethics Committee of Shandong University, and were conducted according to the committees’ guidelines.
2.4. MTT assay
In vitro cytotoxicity was determined by the MTT assay (Sigma). All cells were seeded into 96-well plates at a density of 5 × 103 cells/well. After 24 hours of culture, ECEO was added at various concentrations (0.25, 0.5, 1, 2, 4, 8, or 16 μg/mL). Cells were subsequently treated with 10 μL MTT (5 g/L) for 4 h, then the medium was replaced by 150 μL DMSO. The absorbance was determined at 570 nm using a microplate reader. Cell viability was calculated as the percentage absorbance relative to the absorbance of the control group.
2.5. Cell cloning assay
The cell cloning assay was performed on MDA-MB-231 cells. Cells were seeded in a growth disk at a density of 250 cells per disk. After 24 hours of growth, ECEO was added to the disks at different concentration. Cells were cultured in 5% CO2 at 37°C for 1 week. Cells were stained with 4% methanol for 30 minutes, washed, then stained with crystal violet (Sigma). Cells clones were captured with an Olympus CKX31 microscope (Tokyo, Japan) and counted the number of clones.
2.6. Would-healing assay
The migration capacity of cells in vitro was measured using a wound-healing assay. MDA-MB-231 and MCF-7 cells were cultured with RPMI-1640 (10% FBS) in a 24-well plate at a concentration of 5 × 105 cells/mL. Micropipette tips were used to scratch the surface to create linear gaps. When the cell destiny reached 90%, 3 washes with 3 mL of PBS were performed to flush out any suspended cells. The cells left on the plate were starved for 12 hours to eliminate the interference of proliferation. The experiment was independently repeated 3 times. The rate of wound healing (%) was calculated as [1 - (scratch width of the ECEO group / scratch width of the control group)] × 100% to evaluate the migration ability of cells.
2.7. Transwell assay
Transwell assays were performed to measure the invasion capacity of cells. Matrigel was mixed with serum-free RPMI-1640 (1:8), then spread onto the upper chamber membrane of the insert. The solution was kept at 37°C for 1 hour to allow it to solidify into a gel. Cells with a destiny of 5 × 105 cells/mL were suspended in 200 μL serum-free RPMI-1640, then the suspension was poured into the upper chamber of the insert, and the lower chamber was filled with 600 μL RPMI-1640 containing 20% FBS. Cells were treated with ECEO for 24 hour, with 3 replicate inserts for each concentration. For each concentration, treated cells were incubated with 5% CO2 at 37°C for 24 hours. The medium was discarded, then the chamber was washed twice with PBS. Cells remaining in the upper chamber were cleaned using cotton swabs. Cells in the lower chamber were fixed with methanol for 15 minutes, stained with 0.5% cresyl violet, washed 3 times with double distilled water, then dried. Cells were counted in 5 fields (100× magnification) that were randomly caught by a Nikon TE2000 microscope. For the Transwell migration assay, Matrigel was not added into the wells, but the remaining procedure was the same as the invasion assay.
2.8. Western blot assay
The cell expression of EMT-related proteins was measured by western blot analysis. Cells treated with ECEO were incubated with 5% CO2 at 37°C for 24 hours. Cells were then lysed using RIPA buffer (Beyotime Biotech, Shanghai, China) at 4°C for 30 minutes. The total cell protein content was separated by 12% SDS-polyacrylamide gel electrophoresis, then proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% non-fat milk in TBS-T buffer for 1 hour, then the membrane was then incubated with primary antibodies against GAPDH, E-cadherin, N-cadherin, vimentin, snail and slug (diluted 1:800, Cell Signaling Technology) overnight at 4°C. After washing 3 times with TBS-T buffer and once with TBS buffer for 6 minutes, the membranes were incubated with the secondary HRP-conjugated goat anti-mouse IgG secondary antibody for 1.5 hours at room temperature. Membranes were washed using the same process described above. Proteins on the membranes were visualized using the enhanced chemiluminescence detection system (ECL, Amersham Biosciences). Protein expression was quantified by gray analysis using Image J 2.0 software.
2.9. Quantitative real-time PCR
Total RNA was extracted from cells using the ReverTra Aoe qPCR RT Kit, and RNA was converted into cDNA using the ReverTra Aoe qPCR RT Kit. The mRNA sequences were downloaded from NCBI and primers were designed using primer premier 5. Pair of primers were listed as below:

The GAPDH gene was used as a control for mRNA. For each PCR reaction, a master mix that included SYBR Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan), forward primer, reverse primer, and 10 ng of template cDNA was prepared. The PCR conditions were 45 cycles of sequential denaturation (95°C for 2 minutes), annealing (60°C for 15 seconds), and extension (72°C for 20 seconds). The ΔΔCT value was used to analyse the qPCR data. All samples were measured in triplicate in independent experiments.
2.10. Immunofluorescence staining (IMC) assay
For IMC staining assays, cells were seeded in 24-well plates and cultured with particular conditions for 6 hours. Then, cells were washed 3 times with PBS and fixed with 4% paraformaldehyde at room temperature for 20 minutes. All cells were washed and permeated by 0.5% Triton-X-100 3 times at room temperature for 20 minutes and incubated with 3% BSA blocking for 30 minutes. Primary antibodies were added to each sample and incubated at 4°C overnight. After that, samples were washed using PBS-Triton 3 times, and IMC secondary antibodies were added in the dark room for 1 hour. DAPI was used for locating the nuclei. Then, images were taken by Nikon TE2000 (Tokyo, Japan) microscope.
2.11. Statistical analysis
Data are shown as mean ± standard deviation from triplicate experiments. All data were analyzed using SPSS 16.0 (SPSS Inc., Chicago, IL), and 1-way ANOVA was used for multiple comparisons. Significant differences are indicated as follows: ∗P < .05; ∗∗P < .01.
3. Results
3.1. Composition of Erythrina corallodendron L. leaf essential oil
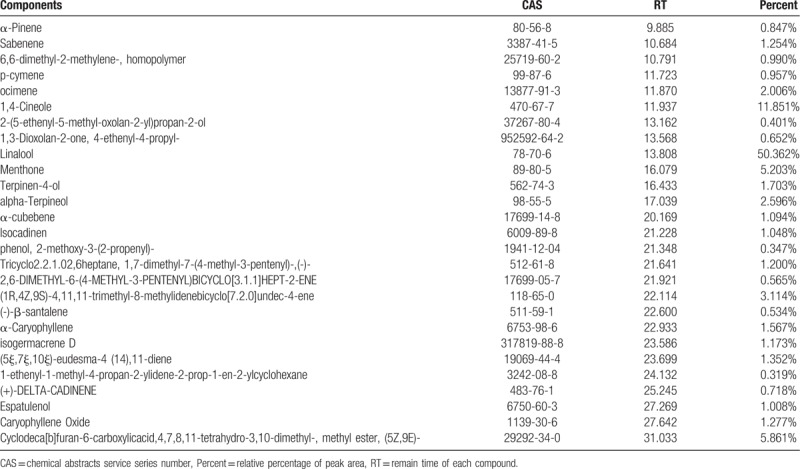
The essential oil of Erythrina corallodendron L. leaves was a clear, pale yellow liquid. The essential oil yield was 8.01% and the density was 0.78 g/mL. The identified components are listed in Table 1. In total, 27 compounds of 99% of essential oil were identified by GC-MS analysis. Among the 27 compounds, linalool, 1,4-cineole, cyclodeca[b]furan-6-carboxylicacid,4,7,8,11-tetrahydro-3,10-dimethyl-,methylester,(5Z,9E), menthone, (1R,4Z,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene and α-terpineol were the top 6 compounds, each of which was present at a percentage higher than 2.5%. It is undeniable that the compounds contained in ECEO differ greatly from essential oils of other species of Erythrina, meaning it may have unique uses or mechanisms.
Table 1.
Identified chemical compounds (%) of the ECEO.
3.2. Erythrina corallodendron L. leaf essential oil inhibits the proliferation of breast cancer cells
In order to examine the proliferation-inhibiting effects of ECEO, we performed a MTT assay and compared the results with drugs that have a proven positive effect in breast cancer cells (MDA-MB-231 and MCF-7) and non-cancerous mammary epithelial cells (HMLE). The results are presented in Table 2. We found that ECEO could inhibit the proliferation of breast cancer cells in a dose-dependent manner. In addition, compared with the normal human breast cell line, HMLE, we found that ECEO had grater cytotoxicity to breast cancer cells than normal cells, which is interesting from a clinical perspective. The proliferation-inhibiting effect of ECEO was not the same as the positive drugs, but ECEO still possesses the potential for treating breast cancers. Furthermore, in order to eliminate the influence of apoptosis, we performed a cell cloning assay. As shown in Figure 1, ECEO could, in part, inhibit the proliferation of breast cancer, not only due to reduced cell viability.
Table 2.
IC50 values of cytotoxic activity against MDA-MB-231, MCF-7 and HMLE. Cells were treated with ECEO and positive drugs (Doxorubicin, Capecitabine) in different concentrations.

Figure 1.
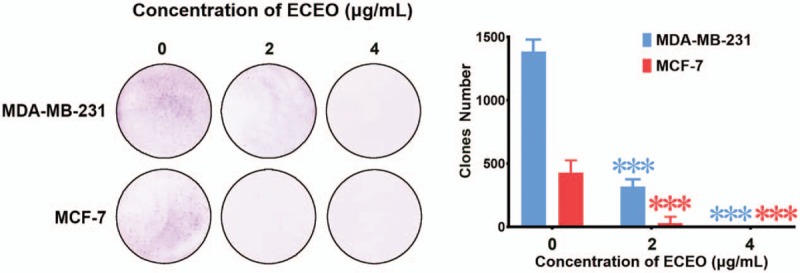
Cell cloning assay performed on MDA-MB-231 and MCF-7 cells treated with Erythrina corallodendron L. leaf essential oil (ECEO). Left, photo captured after culture with ECEO and subsequent staining. Right, quantification of the number of clones.
3.3. Erythrina corallodendron L. leaf essential oil inhibits the migration and invasion of breast cancer cells
Migration and invasion are generally considered crucial factors in the process of breast cancer metastasis. When migration and invasion of cancer cells occurs, cells undergo significant morphological changes. After treating breast cancer cells with ECEO, we observed that cells showed a clear change in morphology, where mesenchymal cells became round and grew tightly, as shown in Figure 2. Such changes indicate that an EMT process occurred, which is discussed below. We then performed a wound-healing assay to check whether ECEO could inhibit the migration ability of breast cancers. As shown in Figure 3, even low doses of ECEO could significantly inhibit the migration rate of breast cancer cells. We subsequently performed a Transwell assay to simulate the extracellular environments and investigate the invasion-inhibiting ability of breast cancer cells by ECEO. The results presented in Figure 4 indicate that treatment with ECEO decreased both the migration and invasion ability of breast cancer cells in a dose-dependent way.
Figure 2.
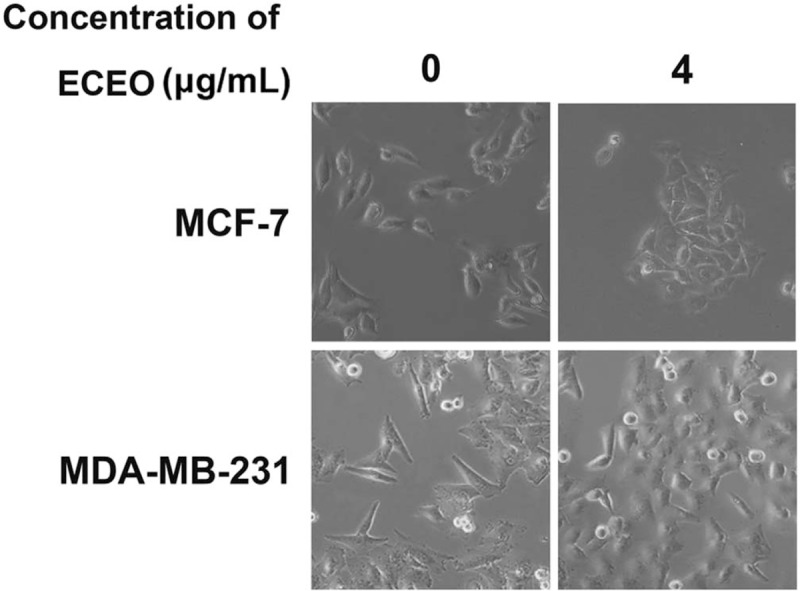
Treatment with Erythrina corallodendron L. leaf essential oil (ECEO) causes morphological changes in breast cancer cells. Normal breast cancer cells were spindle-like with a scattered distribution. After ECEO treatment, cells become round and aggregated, indicating significant inhibition of the epithelial-mesenchymal transition (EMT) process.
Figure 3.
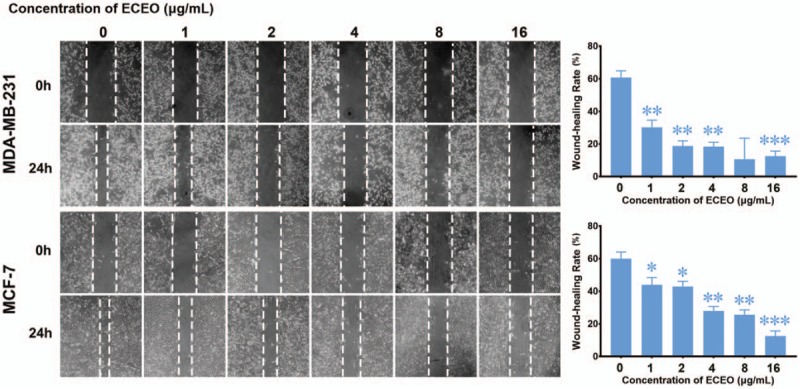
Treatment with Erythrina corallodendron L. leaf essential oil (ECEO) for 24 hours inhibits the migration of breast cancer cells in a wound-healing assay in a dose-dependent manner. Left, images of the wound-healing assay (100× magnification). Right, quantification of the wound-healing rate. ∗P < .05 compared to the control.
Figure 4.
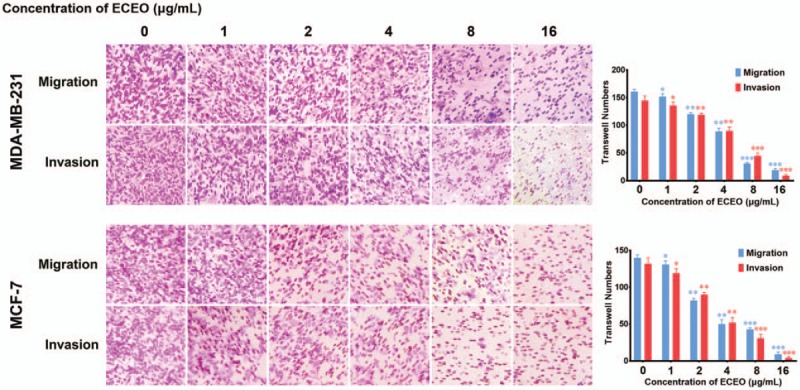
Treatment with Erythrina corallodendron L. leaf essential oil (ECEO) for 24 hours inhibits the invasion and migration of breast cancer cells in a dose-dependent manner. Cell migration and invasion were assessed by Transwell assay after 24 hours of incubation. Left, crystal violet staining (100× magnification). Right, quantification of the number of cells in the Transwell assay. Values are expressed as the mean ± SD of 3 independent experiments. ∗P < .05, ∗∗P < .01 compared to the untreated control.
These results suggest that treatment with ECEO could be an efficient clinical adjuvant drug, in combination with positive drugs, for controlling the metastasis of breast cancer.
3.4. Erythrina corallodendron L. leaf essential oil inhibits the EMT process of breast cancer cells
In order to examine whether ECEO inhibited the migration and invasion of breast cancer cells through EMT pathways, we examined the mRNA and protein expression of EMT markers. First, as shown in Figure 5, the mRNA expression levels of snail and slug were dose-dependently inhibited by ECEO, which could directly result in lower expression of E-cadherin and suppression of the EMT process.
Figure 5.
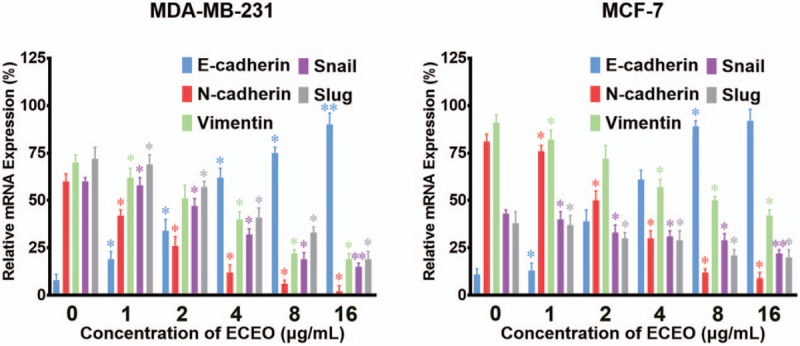
Breast cancer cells treated with Erythrina corallodendron L. leaf essential oil (ECEO) for 24 hours showed altered expression of epithelial-mesenchymal transition (EMT)-related mRNA in a dose-dependent manner. Expression of E-cadherin was upregulated while slug, snail, vimentin and N-cadherin were downregulated. All data were analyzed using a qPCR system. ∗P < .05, ∗∗P < .01 compared to the control.
Furthermore, the mRNA expression of vimentin and N-cadherin was decreased by ECEO in a dose-dependent manner. Naturally, the protein expression showed a similar decrease, as shown in Figure 6. As the essential marker of EMT, the expression of E-cadherin represents direct evidence to determine the occurrence and degree of the EMT process. Therefore, we used IMC to further assess the expression level and site of E-cadherin expression. The results of IMC are shown in Figure 7. With an increasing concentration of ECEO, expression of E-cadherin in breast cancer cells increased and changed location into the peripheral cytoplasm. All of the results discussed above suggest that ECEO could inhibit the migration and invasion of breast cancer through suppressing the EMT process.
Figure 6.
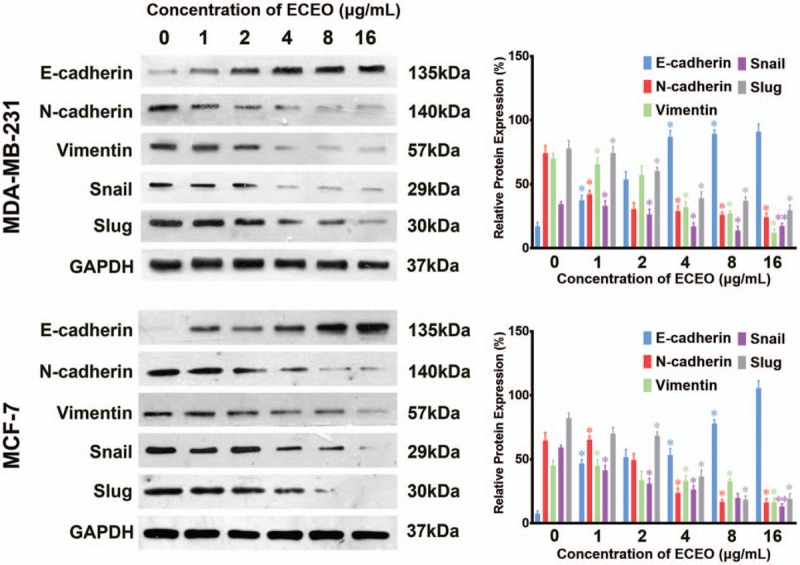
Breast cancer cells treated with Erythrina corallodendron L. leaf essential oil (ECEO) for 24 hours showed altered expression of epithelial-mesenchymal transition (EMT)-related protein in a dose-dependent manner. Expression of E-cadherin was upregulated while slug, snail, vimentin and N-cadherin were downregulated. ∗P < .05, ∗∗P < .01 compared to the control.
Figure 7.
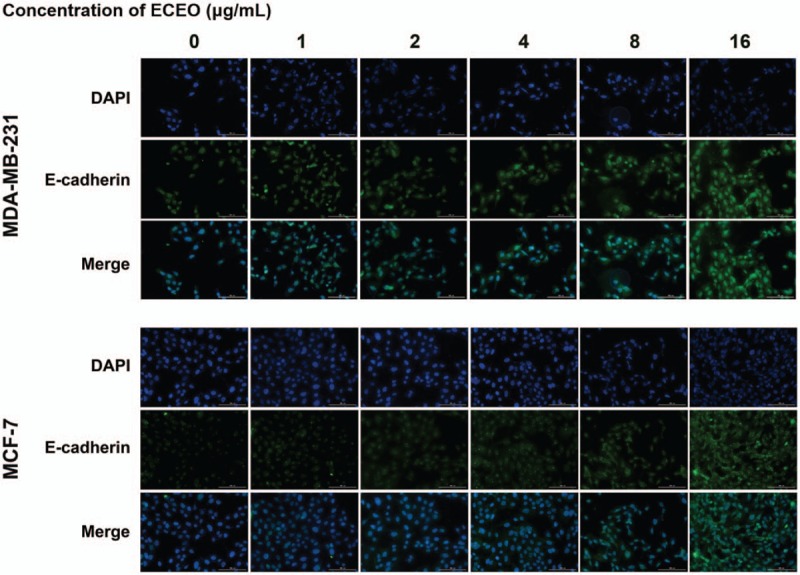
Breast cancer cells treated with Erythrina corallodendron L. leaf essential oil (ECEO) for 24 hours showed altered expression of E-cadherin in the immunofluorescence staining assay (IMC). The expression of E-cadherin was upregulated as the ECEO concentration increased.
4. Discussion
Erythrina corallodendron L. has been used as a traditional Chinese medicine for a long time. Some alkaloids and lectins have been isolated from this plant.[28,29] Moreover, there is emerging evidence of glycosylation effects.[30,31] However, the essential oil of E. corallodendron L. leaves and its anti-cancer effects had never been studied before now. First, our study reported the composition of ECEO and identified 27 compounds. Among these compounds, linalool, a chain terpene alcohol, was the predominant compound of ECEO. Regarding its traditional use, linalool has been used as an anti-bacterial compound, and also has a sedation effect. Researchers have reported that linalool could be used as a treatment for oral cancers by acting through the PI3K/Akt pathway.[32] Meanwhile, α-terpineol, another compound identified in ECEO, has been studied in hepatocellular carcinoma cells, and was found to have an effect on the inhibition on cancer migration.[33,34] Menthone, commonly present in essential oils, has not been reported to have anti-cancer effects so far, instead, it has a slight toxicity. These major compounds may play a critical role in treating breast cancer, and we believe that traces of these compounds might also act through unique mechanisms; however, this requires further research.
Breast cancer, due to its poor prognosis and easily metastasis, is one of the most harmful cancers worldwide.[2] One of the initial reasons for its poor prognosis is its rapid proliferation.[9] There are now several drugs that are used clinically to treat breast cancer, especially capecitabine and doxorubicin. These drugs, although they have a strong effect on cancer cells, possess a series of side effects including injury to normal tissues and cells. In our research, ECEO inhibited the proliferation of breast cancer with a much lower injury to normal cells, showing maximum inhibition on MDA-MB-231 cell lines. Therefore, the cytotoxic activity of ECEO merits further research.
The migration and invasion activities of cancer cells are the leading reason for poor prognosis. During the process of metastasis, the epithelioid cancer cells are transformed into mesenchymal cells, which can then separate from the primary tumor tissue and pass through the blood vessels to secondary tissues.[35–38] One way to treat the metastasis of breast cancer is to suppress this process by inhibiting migration and invasion.[5] The migration and invasion inhibition activities of ECEO were tested using a series of in vitro experiments, and the results showed a positive inhibitory tendency. Among the numerous causes of breast cancer migration and invasion, EMT is one of the most important. There are a series of notable phenomena related to EMT. First, slug and snail, the activators of EMT, were obviously decreased by ECEO treatment. Some of these EMT factors are known to change significantly, especially the overexpression of E-cadherin and the decreased expression of N-cadherin and vimentin.[15] By investigating the changes in expression of EMT markers after ECEO treatments, we found that ECEO could inhibit the EMT process, and thereby inhibit the migration and invasion of breast cancer.
For now, researchers only have superficial knowledge about the EMT process, and none of the drugs that specifically target EMT have been used clinically. Due to a lack of specific noninvasive markers, attempts to target EMT always cause more damage to healthy cells or lead to recrudescence, even drugs that target EGFR, PDGFR, or FGFR.[39–42] Essential oils are natural products with a lower cytotoxicity to healthy cells, which could be used as adjuvant drugs for cancer treatments.[25,26] Our results suggest that ECEO inhibits proliferation and EMT-mediated migration and invasion in vitro, which supports its potential use as an adjuvant therapy for the treatment of breast cancer. However, further research on the in vivo toxicity and action is required.
5. Conclusion
We reported the composition of ECEO and found that linalool is the main compound, which merits further investigation. Subsequent assays demonstrated that ECEO has a high cytotoxicity toward breast cancer cells, and only causes minor damage to normal cells. Through a cloning assay, we found that the inhibition effects of ECEO are, at least in part, due to an effect on proliferation. Furthermore, the Transwell and wound-healing assays demonstrated that ECEO inhibits the migration and invasion of breast cancer cells. By measuring expression levels with qRT-PCR, IMC and western blot assays, it was found that this inhibition effect is mediated by suppression of the EMT process through the slug/snail pathways. Based on the findings discussed above, we can conclude that ECEO could be used as a clinical drug or adjuvant drug to help treat breast cancer metastasis.
Acknowledgments
We would like to thank Disha Corporation and Professor Yu-xin Ma for their generous help.
Author contributions
Conceptualization: Xia Li.
Data curation: Jia-hui Ma, Yao Fu, Hang Zhao.
Formal analysis: Xiang Xing, Jia-hui Ma, Fu-juan Jia.
Funding acquisition: Xiang Xing, Xia Li.
Investigation: Jia-hui Ma, Zhuo Han.
Methodology: Xiang Xing, Jia-hui Ma, Xiao-xuan Ye, Zhuo Han.
Project administration: Jia-hui Ma, Xia Li.
Resources: Yao Fu, Hang Zhao, Xiao-xuan Ye.
Software: Zhuo Han.
Visualization: Jia-hui Ma, Hang Zhao, Xiao-xuan Ye, Fu-juan Jia.
Writing – original draft: Jia-hui Ma.
Writing – review & editing: Jia-hui Ma, Xia Li.
Footnotes
Abbreviations: DMEM = Dulbecco modified eagle medium, DMSO = dimethyl sulfoxide, ECEO = Erythrina corallodendron L. leaf essential oil, EMT = epithelial-mesenchymal transition, GC-MS = gas chromatography-mass spectrometry, HMLE = normal human mammary epithelial cell lines, IMC = immunofluorescence staining assay, MTT = 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, qRT-PCR = quantitative real-time polymerase chain reaction, RPMI-1640 = Roswell Park Memorial Institute 1640 medium.
How to cite this article: Xing X, Ma JH, Fu Y, Zhao H, Ye XX, Han Z, Jia FJ, Li X. Essential oil extracted from erythrina corallodendron L. leaves inhibits the proliferation, migration, and invasion of breast cancer cells. Medicine 2019;98:36(e17009).
This work was supported, in part, by grants from the Shandong Provincial Natural Science Foundation (no. ZR2019MH001) and the Fundamental Research Funds for the Central Universities (no. 2019ZRJC004).
The authors have no conflicts of interest to disclose.
References
- [1].Veronesi U, Boyle P. Breast cancer - reply. Lancet 2005;366:1606–1606.16271640 [Google Scholar]
- [2].DeSantis CE, Ma J, Sauer AG, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. Ca-Cancer J Clin 2017;67:439–48. [DOI] [PubMed] [Google Scholar]
- [3].Pfeiler G, Bartsch R, Fitzal F. Highlights from the 2017 St. Gallen Breast Cancer Consensus. MEMO 2017;10:173–4. [Google Scholar]
- [4].Gray JM, Rasanayagam S, Engel C, et al. State of the evidence 2017: an update on the connection between breast cancer and the environment. Environ Health 2017;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005;5:591–602. [DOI] [PubMed] [Google Scholar]
- [6].Bos PD, Zhang XHF, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009;459:1005–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001;7:192–8. [DOI] [PubMed] [Google Scholar]
- [8].Kang YB, Siegel PM, Shu WP, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003;3:537–49. [DOI] [PubMed] [Google Scholar]
- [9].Negrini M, Calin GA. Breast cancer metastasis: a microRNA story. Breast Cancer Res 2008;10:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Welch DR, Steeg PS, Rinker-Schaeffer CW. Molecular biology of breast cancer metastasis - genetic regulation of human breast carcinoma metastasis. Breast Cancer Res 2000;2:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014;25:605–20. [DOI] [PubMed] [Google Scholar]
- [12].Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Bio 2002;3:155–66. [DOI] [PubMed] [Google Scholar]
- [13].Nieto MA, Sargent MG, Wilkinson DG, et al. Control of cell behavior during vertebrate development by slug, a zinc-finger gene. Science 1994;264:835–9. [DOI] [PubMed] [Google Scholar]
- [14].Peinado H, Olmeda D, Cano A. Snail, ZEB and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007;7:415–28. [DOI] [PubMed] [Google Scholar]
- [15].Sulaiman A, Yao Z, Wang L. Re-evaluating the role of epithelial-mesenchymal-transition in cancer progression. J Biomed Mater Res 2018;32:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bruneau A. Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae). Am J Bot 1997;84:54–71. [Google Scholar]
- [17].Adar R, Richardson M, Lis H, et al. The amino-acid sequence of erythrina-corallodendron lectin and its homology with other legume lectins. FEBS Lett 1989;257:81–5. [DOI] [PubMed] [Google Scholar]
- [18].Adar R, Sharon N. Mutational studies of the amino acid residues in the combining site of Erythrina corallodendron lectin. Eur J Biochem 1996;239:668–74. [DOI] [PubMed] [Google Scholar]
- [19].Gupta D, Arango R, Sharon N, et al. Differences in the cross-linking activities of native and recombinant Erythrina corallodendron lectin with asialofetuin - evidence for carbohydrate-carbohydrate interactions in lectin-glycoprotein complexes. Biochemistry 1994;33:2503–8. [DOI] [PubMed] [Google Scholar]
- [20].Joubert FJ. Purification and properties of proteinase inhibitors from Erythrina corallodendron seeds. Phytochemistry 1988;27:1297–300. [Google Scholar]
- [21].Joubert FJ, Sharon N. Proteinase inhibitors from Erythrina corallodendron and Erythrina cristagalli seeds. Phytochemistry 1985;24:1169–79. [Google Scholar]
- [22].Bakkali F, Averbeck S, Averbeck D, et al. Biological effects of essential oils - a review. Food Chem Toxicol 2008;46:446–75. [DOI] [PubMed] [Google Scholar]
- [23].Isman MB. Plant essential oils for pest and disease management. Crop Prot 2000;19:603–8. [Google Scholar]
- [24].Asif M, Yehya AHS, Al-Mansoub MA, et al. Anticancer attributes of Illicium verum essential oils against colon cancer. S Afr J Bot 2016;103:156–61. [Google Scholar]
- [25].Bayala B, Bassole IHN, Scifo R, et al. Anticancer activity of essential oils and their chemical components - a review. Am J Cancer Res 2014;4:591–607. [PMC free article] [PubMed] [Google Scholar]
- [26].Gautam N, Mantha AK, Mittal S. Essential oils and their constituents as anticancer agents: a mechanistic view. Biomed Res Int 2014;2014:154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Upadhyay RK. Essential oils: anti-microbial, antihelminthic, antiviral, anticancer and anti-insect properties. J Appl Biosci 2010;36:1–22. [Google Scholar]
- [28].Zhao H-E, Wu J, Xu F-Q, et al. Alkaloids from flowers of Erythrina corallodendron. Nat Prod Res 2018;1–6. [DOI] [PubMed] [Google Scholar]
- [29].Yang Z, Tsai M-S, Wu JH, et al. Defining the carbohydrate specificities of Erythrina corallodendron lectin (ECorL) as polyvalent Galbeta1-4GlcNAc (II) > monomeric II > monomeric Gal and GalNAc. Chang Gung Med J 2008;31:26–43. [PubMed] [Google Scholar]
- [30].Marcelo F, Javier Canada F, Andre S, et al. Alpha-N-linked glycopeptides: conformational analysis and bioactivity as lectin ligands. Org Biomol Chem 2012;10:5916–23. [DOI] [PubMed] [Google Scholar]
- [31].Kaushik S, Mohanty D, Surolia A. Role of glycosylation in structure and stability of Erythrina corallodendron lectin (EcorL): a molecular dynamics study. Protein Sci 2011;20:465–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pan W, Zhang G. Linalool monoterpene exerts potent antitumor effects in OECM 1 human oral cancer cells by inducing sub-G1 cell cycle arrest, loss of mitochondrial membrane potential and inhibition of PI3K/AKT biochemical pathway. J Buon 2019;24:323–8. [PubMed] [Google Scholar]
- [33].Hassan SB, Gali-Muhtasib H, Goransson H, et al. Alpha terpineol: a potential anticancer agent which acts through suppressing NF-kappa B signalling. Anticancer Res 2010;30:1911–9. [PubMed] [Google Scholar]
- [34].Oliveira MGB, Brito RG, Santos PL, et al. Alpha-terpineol, a monoterpene alcohol, complexed with beta-cyclodextrin exerts antihyperalgesic effect in animal model for fibromyalgia aided with docking study. Chem-Biol Interact 2016;254:54–62. [DOI] [PubMed] [Google Scholar]
- [35].Hazan RB, Phillips GR, Qiao RF, et al. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000;148:779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Helbig G, Christopherson KW, Bhat-Nakshatri P, et al. NF-kappa B promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 2003;278:21631–8. [DOI] [PubMed] [Google Scholar]
- [37].Luga V, Zhang L, Viloria-Petit AM, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012;151:1542–56. [DOI] [PubMed] [Google Scholar]
- [38].Price JT, Tiganis T, Agarwal A, et al. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3’-kinase and phospholipase C-dependent mechanism. Cancer Res 1999;59:5475–8. [PubMed] [Google Scholar]
- [39].Caric D, Raphael H, Viti J, et al. EGFRs mediate chemotactic migration in the developing telencephalon. Development 2001;128:4203–16. [DOI] [PubMed] [Google Scholar]
- [40].Cristofanilli M, Morandi P, Krishnamurthy S, et al. Imatinib mesylate (Gleevec (R)) in advanced breast cancer-expressing C-Kit or PDGFR-beta: clinical activity and biological correlations. Ann Oncol 2008;19:1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huijts PEA, Vreeswijk MPG, Kroeze-Jansema KHG, et al. Clinical correlates of low-risk variants in FGFR2, TNRC9, MAP3K1, LSP1 and 8q24 in a Dutch cohort of incident breast cancer cases. Breast Cancer Res 2007;9:R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rosty C, Aubriot M-H, Cappellen D, et al. Clinical and biological characteristics of cervical neoplasias with FGFR3 mutation. Mol Cancer 2005;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]


