Abstract
Background:
The blood glucose response to moderate-intensity exercise remains unclear for patients with type 2 diabetes (T2DM). In addition, little is known about determinants of blood glucose response to a 12-week moderate-intensity exercise training. Therefore, this study aimed to explore trends in blood glucose in response to a 12-week moderate-intensity exercise training in patients with T2DM and to explore the predictors of post-exercise blood glucose (PEBG) and exercise-induced glucose response (EIGR).
Methods:
A prospective longitudinal study was conducted. Of the 66 participants with T2DM recruited from outpatient clinics of a medical center, 20 were eligible to enroll in a 12-week moderate-intensity exercise training. Participants were randomly assigned to 1 of 3 exercise times (morning, afternoon, or evening). Blood glucose were measured pre- and post-exercise. The EIGR was calculated by subtracting the PEBG from the before-exercise blood glucose (BEBG). Generalized estimating equations were used to examine the trends and predictors of PEBG and EIGR.
Results:
The BEBG declined progressively (β = −1.69, P < .001); while the PEBG (β = −0.18, P = .08) remained stable over time during the 12-week exercise training. Higher BEBG predicted higher (β = 0.53, P < .001) PEBG. Higher baseline maximum oxygen uptake (VO2max) contributed to a larger magnitude of EIGR; higher HgbA1c and BEBG predicted higher EIGR (β = 0.27, P = .02; β = 0.45, P < .001); afternoon or evening exercise predicted lower (β = −13.2, P = .04; β = −5.96, P = .005) EIGR than did morning exercise.
Conclusions:
A 12-week moderate-intensity exercise training appears safe for patients with T2DM. Time of day for exercise, baseline VO2max, and baseline metabolic control may influence the impact of exercise for individuals with T2DM. These findings provide considerations for design of optimal exercise training for T2DM patients.
Keywords: blood glucose response, exercise, moderate-intensity exercise, type 2 diabetes
1. Introduction
Exercise, an important treatment component of type 2 diabetes (T2DM), improves insulin sensitivity, glycemic control, and contributes to long-term prevention of cardiovascular disease and improved psychological well-being.[1–5] Participation in exercise training is safe for most patients, and its health benefits usually outweigh associated risks including hypoglycemia.[6]
Exercise stimulates metabolism, promotes glucose uptake, increases insulin sensitivity, and contributes to a blood glucose reduction.[7] As a result of these effects, long-term exercise training can reduce blood glucose levels over time in T2DM patients.[4,7,8] However, specific exercise protocols characterized by moderate-to-high intensity might result in episodes of hypoglycemia.[9] This is most likely to occur in those treated with insulin and/or agents, such as sulfonylureas and glinides, which increase insulin release from pancreas beta cells.[7,9,10] As a result, exercise can potentially decrease blood glucose, albeit uncommonly, to hypoglycemic levels. Although the likelihood is uncommon,[9] it can be fatal[11] and as such, affect an individual's willingness to participate in moderate-to-high intensity exercise training.[12,13] To date, there appears to be little in the literature regarding the impact of moderate-high intensity exercise on blood glucose patterns in patients with T2DM.[4] Therefore, we aimed explore trends in blood glucose levels in response to a 12-week, moderate-intensity exercise training in patients with T2DM.
In patients with T2DM, a variety of factors can influence blood glucose levels. These include exercise (mode, time, duration, frequency, and intensity), dietary patterns, and individuals’ clinical characteristics, for example, age, gender, maximum oxygen uptake (VO2max), baseline metabolic control, duration of diagnosed T2DM.[7,14–16] Way and colleagues found that regardless of exercise intensity, the effect of exercise on insulin sensitivity persisted for 2 to 3 days.[17] Duration of exercise may also be a crucial factor contributing to the improvement in insulin sensitivity.[18] In another study, regular, moderate-high intensity exercise training for at least 12 weeks resulted in greater metabolic improvement compared to lower-intensity exercise.[19] Therefore, to investigate predictors of exercise-induced blood glucose response without adjustment for the potential covariates might have bias. In addition, there is no evidence regarding predictors of blood glucose response to a 12-week moderate-intensity exercise training, especially when the potential covariates are controlled. We conducted a prospective longitudinal design study to explore predictors of blood glucose response to a 12-week moderate-intensity exercise training after adjustment for potential covariates, such as clinical characteristics, exercise characteristics, and metabolic control.
Medication use is a major clinical factor that directly and indirectly regulates blood glucose levels. Exogenous forms of insulin and agents that stimulate insulin secretion in a glucose-independent manner (e.g., sulfonylureas and glinides) increase the propensity for hypoglycemia during moderate-to-vigorous intensity exercise.[9] An understanding of blood glucose response to moderate-to-vigorous exercise is essential for minimizing the potential hypoglycemia in T2DM patients treated with antidiabetic medications. Because of this gap, we enrolled T2DM patients on antidiabetic medication treatment to explore the patterns of exercise-induced blood glucose response and to examine the effects of antidiabetic medication on this response. Further, little is known about the glucose response to exercise under adjustment for other covariates, such as baseline hemoglobin A1c (HgbA1c), antidiabetic medication, and diabetes duration. Therefore, this study aimed to investigate the association between antidiabetic medication and exercise-induced blood glucose response to a 12-week moderate-intensity exercise training after controlling for potential clinical confounders (i.e., HgbA1c, diabetes duration).
Due to the known circadian variation in glucose-regulating hormones (e.g., cortisol) and subsequent glucose levels,[20] it is important to examine the impact of time when exercise is performed. However, time of exercise is often not considered when exercise interventions for T2DM patients are tested.[21] In the current study, we sought to describe blood glucose response in patients randomized to 3 different times of moderate-intensity exercise periods (morning, afternoon, or evening) for 12 weeks. Specifically, we evaluated the trends in blood glucose levels (i.e., before-exercise blood glucose [BEBG], post-exercise blood glucose [PEBG], and exercise-induced blood glucose response [EIGR]) in response to a 12-week exercise training in T2DM patients. In addition, we explored predictors of PEBG and EIGR after adjusting for potential covariates (i.e., clinical characteristics, exercise time of day and BEBG).
2. Methods
2.1. Study design and procedure
A prospective longitudinal design using purposive sampling was conducted. All eligible Taiwanese participants with T2DM at a medical center were invited to enroll in the exercise-training program that included 3 sessions per week, 30 minutes per session for 12 weeks (total 36 sessions). Each participant's exercise sessions were equally randomized into 3 times of day (morning/08:00–10:00, afternoon/14:00–16:00, evening/18:00–20:00) using permuted-block randomization. Capillary blood glucose samples were obtained before and after each 30-minute moderate-intensity exercise session. The EIGR, described as the difference between pre-exercise (BEBG), and post-exercise (PEBG) blood glucose values, was calculated.
2.2. Participants and setting
Patients with T2DM being seen at an outpatient clinic in a northern Taiwan medical center were recruited by an endocrinology/metabolism physician to enroll in an exercise study. Patients, 40 to 60 years of age, diagnosed as having T2DM based on the criterion of the American Diabetes Association,[22] and being treated with only oral antidiabetic medications were eligible to enroll. Other inclusion criteria were
-
(1)
able to speak and understand Mandarin;
-
(2)
able to walk without assistance;
-
(3)
had no regular exercise habits; and
-
(4)
agreed to join in a 12-week moderate-intensity exercise training after passing a graded exercise test (GXT) described below.
Exclusion criteria included insulin therapy, a history of cancer, end-stage renal disease with dialysis, an inability to participate in exercise training due to comorbid neurological and musculoskeletal conditions, severe comorbidity or complications, such as heart failure, autonomic neuropathy, and recent stroke within 6 months.
Initially 66 T2DM patients were invited to participate. Thirty-nine declined to participate due to lack of time, and 3 were excluded. Of those excluded participants, 1 did not meet the criteria of T2DM and 2 had moderate physical disability. Of the remaining 24 participants, who were invited to receive GXT, 2 had evidence of autonomic neuropathy (e.g., no elevation in blood pressure and heart rate regardless of exercise intensity during GXT) and 2 dropped out after the exercise test (Fig. 1). Twenty-two passed the GXT based on the guidelines of the American College of Sports Medicine (ACSM) for exercise testing.[6] A second research nurse measured the baseline characteristics (e.g., self-reported demographics and lifestyle patterns, blood analyses, and anthropometric measures) of the participants. Participants were then invited to perform 36 exercise sessions (3 sessions/week for 12 weeks), which were randomly assigned to morning, afternoon, or evening using permuted-block randomization. Two participants dropped out after exercise test.
Figure 1.
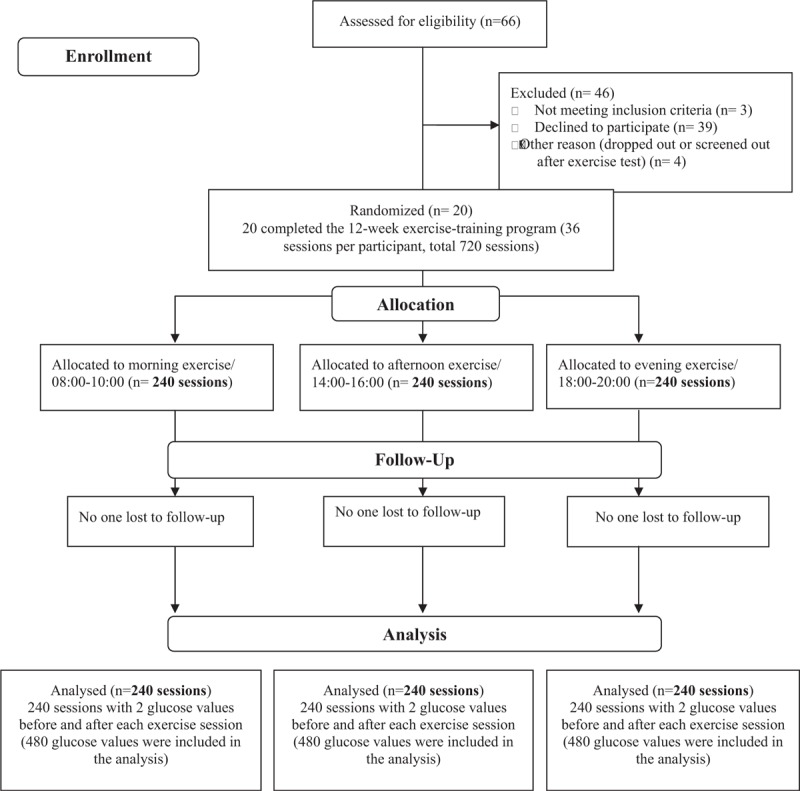
Flowchart of recruiting participants and analysis.
2.3. Graded exercise test
The GXT was performed using a motorized treadmill (Trackmaster 400, JAS Fitness System, USA) for identification of maximum oxygen uptake (VO2max), maximum heart rate (HRmax), and the risk for potentially life-threatening cardiovascular disease (e.g., myocardial infarction).[6] VO2max, the maximum rate of oxygen consumption, is measured during incremental exercise testing. Participants’ ventilation, oxygen and carbon dioxide concentration in both inhaled and exhaled air were analyzed to detect arterial-venous oxygen differences (oxygen consumption). Each participant's VO2max, calculated by the maximal amount of blood the heart pumps per minute (cardiac output) and the amount of oxygen utilization, was determined when oxygen consumption remained at a steady state despite an increase in intensity.[6] Simultaneously, the HRmax was determined when participants’ heart rate stayed constant, despite the intensity of exercise increasing. GXT procedures followed a modified Balke protocol.[23] Female participants were invited to receive GXT at their available time except during menstruation. Two observers (a medical/rehabilitation doctor and a research nurse) were involved with each participant throughout the GXT. The GXT was immediately stopped if the participants complained of exhaustion; reached maximum heart rate or VO2max; had a respiratory exchange ratio >1.15; developed symptoms, such as chest pain, dyspnea, pallor, diaphoresis, or dizziness; had systolic pressure >250 mm Hg or diastolic pressure >120 mm Hg; had a decrease in systolic pressure of >10 mm Hg compared to the systolic pressure at rest; or requested stopping the test.[6]
2.4. Training protocol
The training protocol followed the “FITT” principle according to ACSM's Guidelines including Frequency, Intensity, Time, and Type.[6]
-
(1)
Frequency: all participants received supervised exercise training three times per week on alternate days for 12 weeks at the medical center.
-
(2)
Intensity: the intensity of exercise training (target heart rate) was set at 70% heart rate reserve (Karvonen method = resting heart rate (HRrest) + 70% [HRmax–HRrest], equals to 70% VO2max) obtained from the GXT. Appropriate speed and grade were used to achieve the target heart rate being monitored by the heart rate monitor (Polar S510, Electro Oy, Finland) and to accurately control the required exercise intensity.[6]
-
(3)
Time: according to the ACSM's Guidelines,[6] healthy adults should do at least 150 minutes of moderate-intensity aerobic activity per week, and these recommendations can be adjusted for each individual based on their exercise capacity and specific health risks. Therefore, the duration of each training session in our study included warm-up phase (5–10 minutes), endurance phase (30 minutes), and cool-down phase (5–10 minutes). The training took place 1 to 2 hours after a meal and.
-
(4)
Type: treadmills (928ME2C, Takasuma, Japan) were used for the aerobic exercise training. All participants followed this training protocol and maintained their usual lifestyles.
2.5. Measures of blood glucose response
The research nurse performed capillary blood glucose testing before and after each exercise session. The 12-week exercise training time, corresponding to a total 36 sessions of exercise, was used as a continuous variable for evaluating the trend of blood glucose response, including the patterns of PEBG and EIGR. Moreover, we appraised the blood glucose response according to the different exercise times of day (i.e., morning/08:00–10:00, afternoon/14:00–16:00, and evening/18:00–20:00).
A reliable and well-valid one-touch glucometer (FreeStyle Blood Glucose Monitoring System, TheraSense, USA), which has been previously reported,[24] was used to measure capillary blood glucose. Prior to the study start, a glucose analyzer (YSI model 1500 glucose analyzer, Yellow Springs Instrument Company Inc., USA) validated the one-touch glucometer. Values from the glucometer and glucose analyzer were highly correlated (r = .89, P < .001).
2.6. Ethical consideration
Institutional review board approval (Reference number: 097–05–157) was obtained from the local medical center in Taiwan. This trial has been registered on the “ClinicalTrials.gov” (NCT03335930). All participants gave written informed consent when invited to join the study.
2.7. Statistical analysis
Statistical analyses were performed by SPSS version 16.0 (SPSS Inc., Chicago, IL). Descriptive data are presented as mean/standard deviation (SD) and numbers/percentage (%). Before evaluating the trend in blood glucose response during the 12-week/36-session exercise-training program, 2 variables including “training month (1st, 2nd, and 3rd month of the training)” and “timing of exercise sessions (1st to 36th session of exercise)” were coded. With generalized estimating equations (GEE),[25] we examined the change patterns of BEBG, PEBG, and EIGR over time during the training program. The trend analysis of for these three variables were adjusted for covariates (i.e., age, gender, baseline body mass index, VO2max, HgbA1c, antidiabetic medication (Metformin, Sulfonylureas, Repaglinide), duration of diagnosed diabetes, and exercise time of day (morning, afternoon, or evening). When evaluating the predictors of EIGR and PEBG, univariate analysis was applied first and, following the multivariate analysis, incorporated with those significant covariates from univariable analysis. All statistical analyses were 2-tailed and considered significant at P < .05.
3. Results
3.1. Baseline characteristics of participants
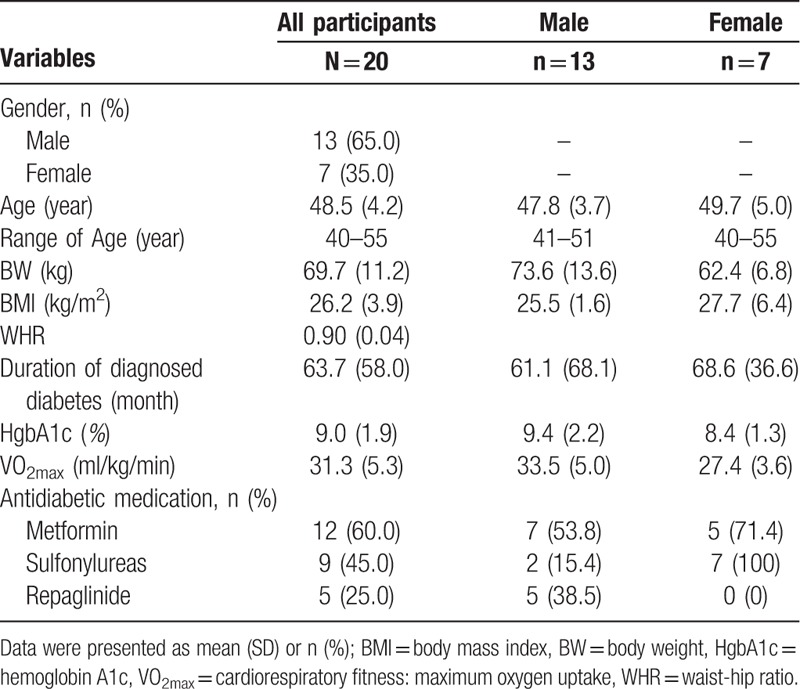
Twenty participants completed 100% of the exercise training sessions and data collection. There were no demographic differences (age, gender) between those initially approached and those completed the study. Table 1 presents the demographics, medication, and baseline physical characteristics including body weight, body mass index (BMI), waist-hip ratio, HgbA1c level, and VO2max. The 20 randomized participants, mean age 48.5 years (range: 40–55 years), had been diagnosed with T2DM for 5.3 years. Men composed more than half (65%) of the sample. All of the participants were treated with at least 1 type of oral antidiabetic medication and 60% (n = 12) were treated with two types of oral antidiabetic medications. Most of the participants received Metformin (n = 12, 60%), Sulfonylureas (n = 9, 45%), or Repaglinide (n = 5, 25%), and had a mean HgbA1c of 9.0 (Table 1).
Table 1.
Demographic, medication, and baseline physical characteristics of participants.
3.2. Blood glucose value before and after each exercise session
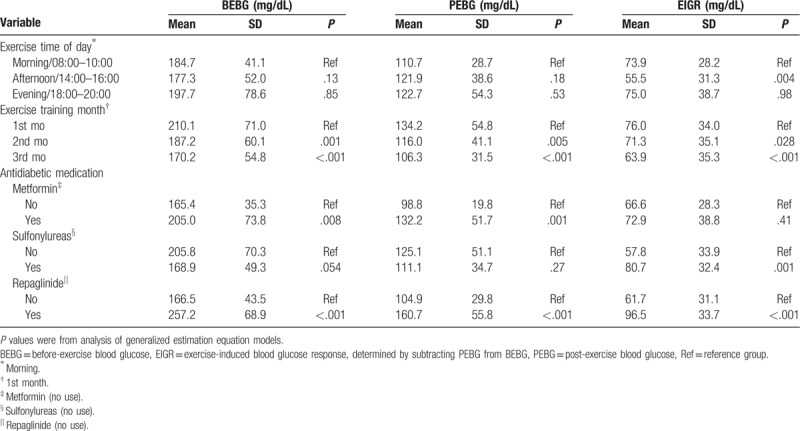
Compared to morning exercise there was a small albeit significant (P = .004) increase in EIGR after exercise in the afternoon (Table 2). There were no differences in the BEBG and PEBG between the 3 different exercise times of day (morning, afternoon, and evening). The BEBG at both the 2nd and 3rd training months was significantly lower (P = .001; P < .001) than the 1st training month; similarly, the PEBG and EIGR at both the 2nd and 3rd training months were significantly lower (P < .05) than those at the 1st training month. Comparisons of blood glucose response (BEBG, PEBG, EIGR) to exercise training between those with and without treatment of antidiabetic medicine (Metformin, Sulfonylureas, Repaglinide) are presented as Table 2.
Table 2.
Analysis of blood glucose response during a 12-week/36-session moderate-intensity exercise training and comparisons of blood glucose response between those with and without antidiabetic medicine treatment.
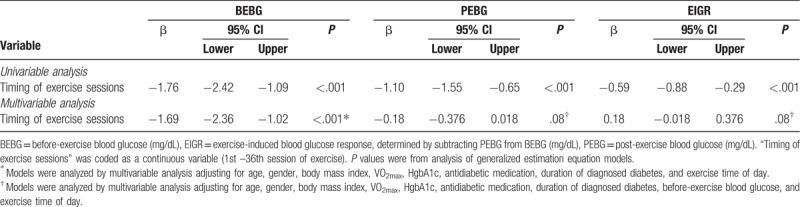
The changes in blood glucose response over time during a 3-month moderate-intensity exercise training are shown in Figure 2 and Table 3. In univariable analysis (Table 3), BEBG, PEBG, and EIGR significantly declined (β = −1.76, P < .001; β = − 1.10, P < .001; β = −0.59, P < .001) over time as the number of exercise session increased. In multivariable analysis, the participants’ BEBG declined as exercise session number increased (β = −1.69, P < .001); while the PEBG (β = −0.18, P = .08) and EIGR (β = −0.18, P = .08) were stable after adjusting for age, gender, BMI, VO2max, baseline HgbA1c, antidiabetic medication, and BEBG, and timing of exercise sessions (1st –36th).
Figure 2.
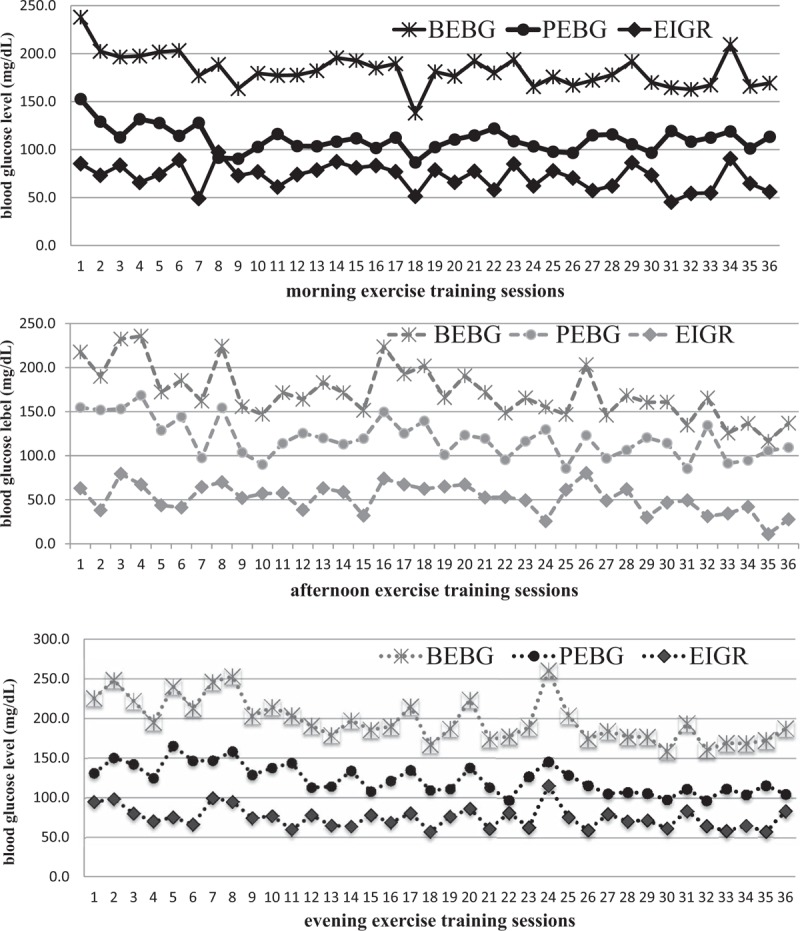
Trends of blood glucose response in different times of day (morning, afternoon, evening) during a 3-month/ 36-session moderate-intensity exercise training. BEBG = before-exercise blood glucose, EIGR = exercise-induced blood glucose response, determined by subtracting PEBG from BEBG, PEBG = post-exercise blood glucose.
Table 3.
Trend analysis of blood glucose response during a 12-week/36-session moderate-intensity exercise training.
3.3. Predictors of exercise-induced blood glucose response
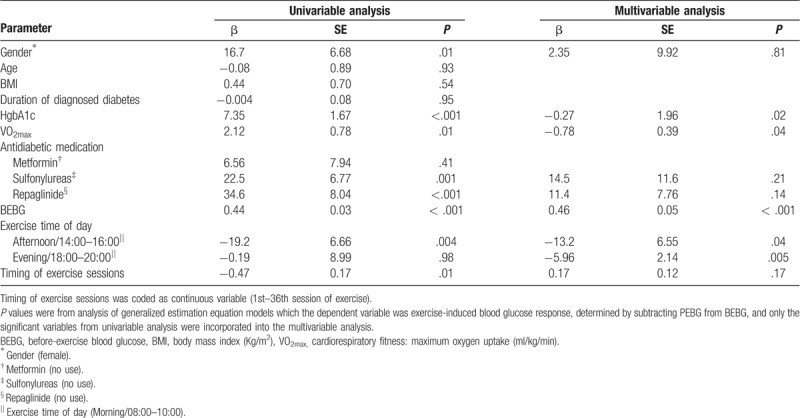
Tables 4 and 5 show predictors of PEBG and EIGR using univariable and multivariable analysis in GEE models. In univariable analysis, some factors (gender, VO2max, baseline HgbA1c, antidiabetic medication, BEBG, exercise time of day, and timing of exercise sessions) predicted exercise-induced blood glucose response (Table 5). Independent of the significant variables from univariable analysis, higher BEBG (β = 0.53, P < .001) contributed to higher PEBG (Table 4). After controlling for the significant covariates from univariable analysis, higher VO2max contributed to a larger magnitude of EIGR; higher HgbA1c and BEBG predicted greater EIGR (β = 0.27, P = .02; β = 0.45, P < .001); afternoon exercise (β = −13.2, P = .04) or evening exercise (β = −5.96, P = .005) significantly predicted lower EIGR as compared to morning exercise (Table 5).
Table 4.
Predictors of post-exercise blood glucose during a 12-week/36-session moderate-intensity exercise training.
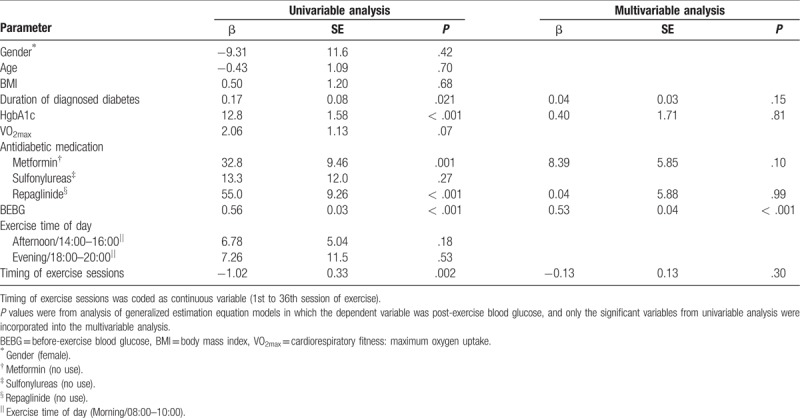
Table 5.
Predictors of exercise-induced blood glucose response during a 12-week/36-session moderate-intensity exercise training.
4. Discussion
A major non-pharmacological treatment strategy in management of T2DM is moderate-intensity exercise training. Our 12-week moderate exercise program was found to be feasible and to progressively reduce blood glucose levels. In this study, we attribute blood glucose finding to improved metabolic control of participants produced by the exercise intervention. Our results suggest that a 12-week moderate-intensity exercise training is feasible and safe for T2DM patients. Time of exercise (i.e., morning, afternoon, or evening) also predicted exercise-induced blood glucose levels. Together these preliminary findings provide information for the design of future optimal exercise training for persons with T2DM.
Our results, albeit in a small sample, adds evidence to support that a moderate-intensity exercise is safe for T2DM patients.[7,26] No participant experienced an exercise-induced hypoglycemia episode after exercise. Thus, these preliminary findings call into question the notion that moderate to strenuous exercise in persons with T2DM will result in hypoglycemic events either during or after exercise.[26] We conjecture that performing a moderate-intensity exercise at 1 to 2 hours post-meal for those receiving only oral antidiabetic medication treatment may be safe and beneficial but requires validation in a larger randomized controlled trial. Given that the sample size of this study was small, we applied G∗Power analysis (version 3.1) to conduct Post hoc analysis (Repeated measures, within factors) with an alpha set at 0.05, sample size of 20, number of group: 1, number of measurements: 36, and a median effect size of 0.25, resulting in a power of 0.99. In addition, the power calculation using SPSS version 16.0 (General linear model, repeated measures) for BEBG, PEBG, and EIGR were 1.0, 1.0 and 0.99, respectively. Therefore, our findings remain valuable for considerations of designing exercise training among patients with T2DM in clinical settings.
All participants after controlling for potential confounders (age, gender, BMI, VO2max, HgbA1c, antidiabetic medication, duration of diagnosed diabetes, BEBG level, and exercise time of day) had stable post-exercise glucose levels and exercise-induced glucose response during the 12 weeks study period. We coded the 1st to 36th exercise training sessions as a continuous variable (timing of exercise sessions), to investigate the patterns of blood glucose over time and found that as the number of exercise session increased, the BEBG decreased after adjusting for all potential confounders measured. However, as the number of exercise sessions increased, the PEBG did not decrease and the EIGR did not change. Our results are consistent with the findings of Liubaoerjijin et al[19] who found moderate-high intensity exercise produced improvements in metabolic control. We add new information to support the use of moderate-intensity exercise in T2DM management. Our findings based on a permuted block randomization design also suggest that it can be safely performed at different times of day (morning, afternoon, or evening) for 12 weeks.
Antidiabetic medications work through several mechanisms. Metformin use predicted a higher PEBG level than those not receiving metformin, but did not predict EIGR. This is consistent with Boule et al, who found that the effect of metformin on glycemic reduction can be attenuated by exercise.[27] Although Sulfonylureas and Repaglinide have the potential to increase the risk of hypoglycemic episodes during exercise[9] and we found that patients treated with Sulfonylureas or Repaglinide had greater EIGR than those without. None of our participated patients suffered from hypoglycemic episodes during exercise. Given that antidiabetic medication can influence the blood glucose response (Metformin and Repaglinide predicted higher PEBG; Sulfonylureas and Repaglinide predicted greater EIGR), none of them had significant effects on PEBG or EIGR after adjustment for significant covariates. Our findings supported Hallsten's research that noted there was no synergic effect with insulin-like medication on glucose reduction during exercise in T2DM patients.[28] Future more studies could focus on examining the effects of antidiabetic medications on PEBG levels and EIGR to elucidate the interaction of moderate-intensity exercise and anti-diabetic medicine use in patients with T2DM.
A higher baseline HgbA1c or BEBG level also contributes to a greater magnitude exercise-induced blood glucose decline. A similar response in EIGR found in a study by Nguyen.[29] Combined these results highlight that not only does energy expenditure during exercise initially increases the utilization of glucose[7,9] but also that a protective mechanism of glucose homeostasis exerts itself even though exercise results in reduction of blood glucose through increased glucose utilization.[26] This may explain why patients with higher plasma glucose or higher HgbA1c have a larger magnitude of EIGR.
A strong link exists between circadian rhythms and energy/glucose metabolism[21,30]; however, the fundamental mechanisms remain unclear. Our study showed that the EIGR in the afternoon or evening was significantly lower than in the morning independent of gender, VO2max, HgbA1c, antidiabetic medication, duration of diagnosed diabetes, and timing of exercise sessions. Similar to a study of type 1 diabetic patients,[31] our results suggest that performing exercise training in the morning may result in improved metabolic control when compared to exercise in afternoon or evening. This might be possibly associated with diet patterns at different times of day or the circadian rhythm of insulin and cortisol.[32] As the number of exercise session increased, the PEBG and EIGR did not significantly decrease. This suggests that the blood glucose response to moderate-intensity exercise reaches a threshold as exercise training time increases. A larger randomized trial is needed to validate both of these findings in T2DM patients.
Various factors including gender, VO2max, antidiabetic medication (use of sulfonylureas and Repaglinide), baseline metabolic control (HgbA1c and BEBG), and exercise time of day can influence participants’ blood glucose response to exercise in our univariable analysis. This explained why we found it necessary to control for these factors when examining the patterns and predictors of blood glucose response.
Our study results conducted a small sample size and only Taiwanese participants may not be generalizable to other racial or regional groups. Thus, the findings should be interpreted with caution. Future more rigorous studies with a larger sample size are recommended. Particularly, a fixed number of participants on each time of day are suggested to be included in future studies to reduce the bias regarding the participants’ chronotype. In addition, we only advised participants to control the mealtime before exercise. Diet including before exercise influence EIGR.[33] Further studies could standardize or include measures of diet patterns, for example, food frequency, nutrient composition, and meal timing. Despite these limitations, there are several strengths including the random allocation design for comparing the blood glucose response between different exercise times of day, the provision of individual and supervised exercise training with pre- and post-exercise blood glucose testing in a medical center, the high rate of study completion (100%) by the participants, and a statistical observed power of 0.9 to 1.0. Future studies could focus on both blood glucose and biomarkers that might elucidate how moderate-intensity exercise causes the blood glucose changes in this population.
5. Conclusion
A 12-week moderate-intensity exercise training is safe and improves metabolic control over time for patients with T2DM. A higher BEBG level or poor metabolic control leads to a larger magnitude of blood glucose reduction through a moderate-intensity exercise program among patients with T2DM, given that relatively higher PEBG was also found. Exercise in the morning resulted in a larger magnitude of blood glucose reduction than did afternoon or evening exercise among T2DM patients without changing medication regimens. Whether this is due the influence of circadian rhythms is unknown. This provides consideration for future optimal exercise-training design for patients with T2DM.
Acknowledgment
We thank all participants in the study.
Author contributions
Conceptualization: Shang-Lin Chiang, Wen-Chii Tzeng, Chia-Huei Lin.
Data curation: Chia-Huei Lin.
Formal analysis: Meei-Shyuan Lee, Chia-Huei Lin.
Funding acquisition: Chia-Huei Lin.
Investigation: Yi-Jen Hung.
Methodology: Wen-Chii Tzeng, Meei-Shyuan Lee, Chia-Huei Lin.
Project administration: Yi-Jen Hung, Wen-Chii Tzeng, Chia-Huei Lin.
Resources: Yi-Jen Hung.
Supervision: Shang-Lin Chiang.
Validation: Meei-Shyuan Lee.
Writing – original draft: Chia-Huei Lin.
Writing – review & editing: Shang-Lin Chiang, Margaret McLean Heitkemper.
Footnotes
Abbreviations: BEBG = before-exercise blood glucose, EIGR = exercise-induced glucose response, PEBG = post-exercise blood glucose, T2DM = type 2 diabetes.
How to cite this article: Chiang SL, Heitkemper MM, Hung YJ, Tzeng WC, Lee MS, Lin CH. Effects of a 12-week moderate-intensity exercise training on blood glucose response in patients with type 2 diabetes. Medicine 2019;98:36(e16860).
This study was funded by the Tri-Service General Hospital (TSGH-C98-78; TSGH-C105-144; TSGH-C108-127) and Ministry of Science and Technology (MOST107-2314-B016-068), Taipei, Taiwan.
The authors have no conflicts of interests to disclose.
References
- [1].Duclos M, Virally ML, Dejager S. Exercise in the management of type 2 diabetes mellitus: what are the benefits and how does it work? Phys Sportsmed 2011;39:98–106. [DOI] [PubMed] [Google Scholar]
- [2].Karjalainen JJ, Kiviniemi AM, Hautala AJ, et al. Effects of physical activity and exercise training on cardiovascular risk in coronary artery disease patients with and without type 2 diabetes. Diabetes Care 2015;38:706–15. [DOI] [PubMed] [Google Scholar]
- [3].Koivula RW, Tornberg AB, Franks PW. Exercise and diabetes-related cardiovascular disease: systematic review of published evidence from observational studies and clinical trials. Curr Diab Rep 2013;13:372–80. [DOI] [PubMed] [Google Scholar]
- [4].Rohling M, Herder C, Roden M, et al. Effects of long-term exercise interventions on glycaemic control in type 1 and type 2 diabetes: a systematic review. Exp Clin Endocrinol Diabetes 2016;124:487–94. [DOI] [PubMed] [Google Scholar]
- [5].Sardar MA, Boghrabadi V, Sohrabi M, et al. The effects of aerobic exercise training on psychosocial aspects of men with type 2 diabetes mellitus. Glob J Health Sci 2014;6:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 10th ed.Philadelphia: PA: Lippincott Williams & Wilkins; 2016. [Google Scholar]
- [7].Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Adams OP. The impact of brief high-intensity exercise on blood glucose levels. Diabetes Metab Syndr Obes 2013;6:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gulve EA. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther 2008;88:1297–321. [DOI] [PubMed] [Google Scholar]
- [10].Koivisto VA, Felig P. Effects of leg exercise on insulin absorption in diabetic patients. N Engl J Med 1978;298:79–83. [DOI] [PubMed] [Google Scholar]
- [11].Cha SA, Yun JS, Lim TS, et al. Severe hypoglycemia and cardiovascular or all-cause mortality in patients with type 2 diabetes. Diabetes Metab J 2016;40:202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alvarado MM, Kum HC, Gonzalez Coronado K, et al. Barriers to remote health interventions for type 2 diabetes: a systematic review and proposed classification scheme. J Med Internet Res 2017;19:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sohal T, Sohal P, King-Shier KM, et al. Barriers and facilitators for type 2 diabetes management in south Asians: a systematic review. PloS One 2015;10:e0136202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bacchi E, Negri C, Tarperi C, et al. Relationships between cardiorespiratory fitness, metabolic control, and fat distribution in type 2 diabetes subjects. Acta Diabetol 2014;51:369–75. [DOI] [PubMed] [Google Scholar]
- [15].Jun EH, Choi BY, Lee DC, et al. Cardiopulmonary fitness is independently associated with insulin resistance in non-diabetes mellitus patients of a university hospital in Korea. Korean J Fam Med 2013;34:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schwingshackl L, Missbach B, Dias S, et al. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia 2014;57:1789–97. [DOI] [PubMed] [Google Scholar]
- [17].Way KL, Hackett DA, Baker MK, et al. The effect of regular exercise on insulin sensitivity in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab J 2016;40:253–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Houmard JA, Tanner CJ, Slentz CA, et al. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 2004;96:101–6. [DOI] [PubMed] [Google Scholar]
- [19].Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol 2016;53:769–81. [DOI] [PubMed] [Google Scholar]
- [20].Sonnier T, Rood J, Gimble JM, et al. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabetes Complications 2014;28:836–43. [DOI] [PubMed] [Google Scholar]
- [21].Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A 2015;112:E2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kenney WL, Kamon E, Buskirk ER. Effect of mild essential hypertension on control of forearm blood flow during exercise in the heat. J Appl Physiol Respir Environ Exerc Physiol 1984;56:930–5. [DOI] [PubMed] [Google Scholar]
- [24].Lin CH, Ho CW, Chen LC, et al. Effects of a 12-week exercise training on insulin sensitivity, quality of life, and depression status in patients with type 2 diabetes. J Med Sci 2017;37:227–36. [Google Scholar]
- [25].Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- [26].Galbo H, Tobin L, van Loon LJ. Responses to acute exercise in type 2 diabetes, with an emphasis on metabolism and interaction with oral hypoglycemic agents and food intake. Appl Physiol Nutr Metab 2007;32:567–75. [DOI] [PubMed] [Google Scholar]
- [27].Boule NG, Robert C, Bell GJ, et al. Metformin and exercise in type 2 diabetes: examining treatment modality interactions. Diabetes Care 2011;34:1469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hallsten K, Virtanen KA, Lonnqvist F, et al. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 2002;51:3479–85. [DOI] [PubMed] [Google Scholar]
- [29].Nguyen NH, Rahme E, Dasgupta K. Acute exercise-induced glucose change during an exercise program in type 2 diabetes. J Cardiopulm Rehabil Prev 2008;28:122–7. [DOI] [PubMed] [Google Scholar]
- [30].Tseng HL, Yang SC, Yang SH, et al. Hepatic circadian-clock system altered by insulin resistance, diabetes and insulin sensitizer in mice. PloS One 2015;10:e0120380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gomez AM, Gomez C, Aschner P, et al. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J Diabetes Sci Technol 2015;9:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fernandes AL, Lopes-Silva JP, Bertuzzi R, et al. Effect of time of day on performance, hormonal and metabolic response during a 1000-M cycling time trial. PLoS One 2014;9:e109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ferland A, Brassard P, Lemieux S, et al. Impact of high-fat /low-carbohydrate, high-, low-glycaemic index or low-caloric meals on glucose regulation during aerobic exercise in Type 2 diabetes. Diabet Med. 2009; 26:589–95. [DOI] [PubMed] [Google Scholar]


