Background.
While heart transplantation is a highly effective treatment in patients with advanced heart failure, the number of people waiting for a transplant exceeds the number of available donors. With the advent of direct acting antivirals (DAA) for the eradication of Hepatitis C, the heart transplant donor pool has been expanded to include donors with untreated Hepatitis C. To help with the development of future protocols for Hepatitis C–positive heart transplants, we performed a review of the literature on DAA therapy in the context of heart transplantation.
Methods.
We searched MEDLINE, EMBASE, OVIDE JOURNAL, and GOOGLE SCHOLAR for papers published between 01.01.2011 and 01.06.2019 using key words “heart transplantation” associated with “hepatitis C.”
Results.
After removing duplicates, we screened 78 articles and retained 16 for primary analysis and 20 for sustained virologic response 12 weeks after completion of the DAA therapy (SVR-12). The data from 62 patients were extracted from these publications. Fifty-six (90%) patients had donor-derived hepatitis C and 6 (10%) patients were chronically infected with hepatitis C before transplantation. All living transplanted patients achieved SVR-12, defined as hepatitis C virus RNA below the limit of detection 12 weeks after treatment completion. Treatment duration ranged from 4 to 24 weeks. Clinically relevant modification to the dosing of immunosuppressive mediations during DAA therapy was documented in only 1 patient (1.6%). Six (14%) patients experienced rejection during DAA therapy.
Conclusions.
Despite different timings of initiation of DAA therapy across the included studies, there were no differences in sustained viral clearance. Early commencement of DAA with a potentially shorter treatment duration (<8 wk) is appealing; however, further studies are required before recommending this approach.
While heart transplantation is a highly effective treatment in patients with advanced heart failure, the number of people waiting for transplant exceeds available donors. In the United States alone, up to 350 people die each year awaiting heart transplant,1 and it has been estimated that if the donor pool was widened to include those with hepatitis C, >140 extra heart transplants could be performed annually.2
Hepatitis C virus (HCV) is an enveloped flavivirus with a parenteral mode of transmission.3 There are 6 viral genotypes with >67 subtypes.4 Before 2001, screening for hepatitis C in donors and recipients was not routinely performed. This led to numerous donor-derived hepatitis C (DDHC) infections5 and increased morbidity and mortality.6,7 As a result, donors with HCV were routinely excluded.
With the arrival of highly-effective direct acting antiviral (DAA) therapy including pan-genotypic DAA, transplantation of hepatitis C–positive donor organs,8-10 including hearts,11 has become a viable option. A growing number of protocols addressing this topic are being established and a number of centers are currently following patients who have received organs from HCV-positive donors.12-16 The most recent International Society of Heart and Lung Transplantation (ISHLT) conference abstracts include the largest published cohorts of transplant recipients undergoing successful Hepatitis C treatment5,17-26; however, these studies are still on-going.
As expressed in the editorial by Givertz et al,27 transplantation of hearts from hepatitis C–positive donors (either RNA and/or antibody positive) has presented clinicians with an opportunity to expand the donor pool and close waitlist gaps. It is also an opportunity for marginal recipients to significantly shorten the waiting list times by accepting a heart from a hepatitis C–positive donor. In 1 center, the mean waitlist time was 329 days for those receiving an HCV negative graft and 78 days in those accepting a Hepatitis C–positive graft.28 In another cohort, the waitlist time for Hepatitis C–positive grafts was as little as 4 days.5 However, some questions remain unanswered that need to be addressed before heart transplantation from HCV-positive donors becomes the standard of care.
The objective of our review was to analyze the currently published literature to address the following questions: what is the efficacy of DAA in the setting of heart transplantation? What interactions between immunosuppression and DAA have been documented? Does DAA increase the risk of acute rejection? What is the optimal timing for the initiation of DAA? And what is the most favorable duration of therapy?
MATERIALS AND METHODS
Search Method
We utilized PRISMA flow-chart to plan our review.29 An electronic search was performed in Medline, Embase, Ovide journal, and Google Scholar to identify all articles and abstracts in English, French, and German published between 01.01.2011 and 01.06.2019. We chose this particular time frame to cover the era of direct DAA therapy.
In order to set up the search, we used keywords, automatic generated synonyms (in Ovid), and MESH terms. We searched for “heart transplantation” associated with “hepatitis C” or with “direct-acting antiviral” in the title of the publication. In an attempt to cover all the available literature, we use 3 predefined goals (see Appendix for complete list of terms used in the search and details of internal controls).
Study Identification
All publications, including cohort studies, clinical trials, reviews, case series, case reports, or conference abstracts, were eligible if they documented at least 1 patient being treated with an interferon-free hepatitis C regimen after a heart transplantation. After removing duplicates by comparison of DOI, all abstracts from identified publications were examined for eligibility.
Exclusion criteria were publications about pediatric patients (<18 y old at time of transplantation) and insufficient data completeness (defined as <50% of prespecified patient characteristics available in the full text article). References in the included articles were then searched to identify other studies for inclusion.
Data Extraction
We predefined 23 patient and treatment characteristics and searched for them manually in each of the retained publications. The main items were source of infection; time lapse between transplantation and start of DAA; regimen used; follow-up of the viral load; type of immunosuppressive therapy for initial induction and maintenance; reported interactions between DAA and immunosuppressive therapy; and rejection episodes described during treatment (see Appendix for a completed list of extracted data).
RESULTS
Publication Selection and Quality Control
An electronic search identified 146 publications. After removing duplicates, 78 publications were screened and 34 met inclusion criteria. After reading the papers, 14 publications were excluded—3 were in a pediatric population30 and the other 11 had insufficient data for analysis.
The PRISMA-chart is provided in Figure 1 and the list of publications included is detailed in Table 1.
FIGURE 1.
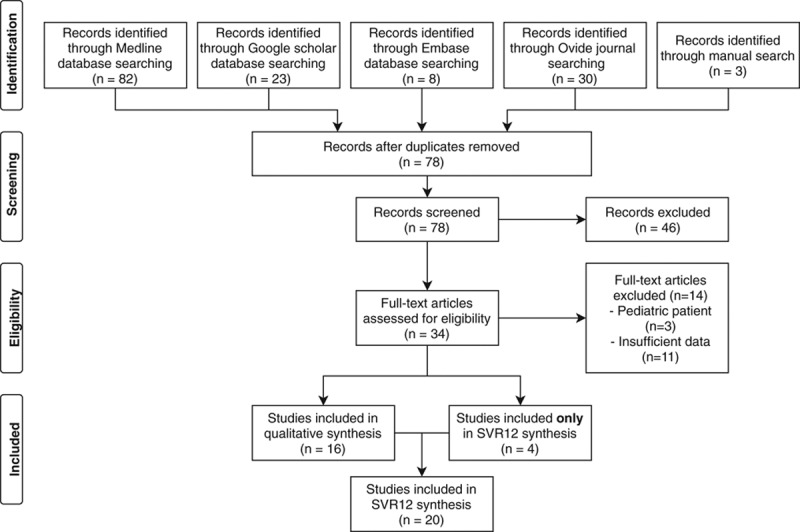
PRISMA flow-chart. SVR12, sustained viral response after 12 wk.
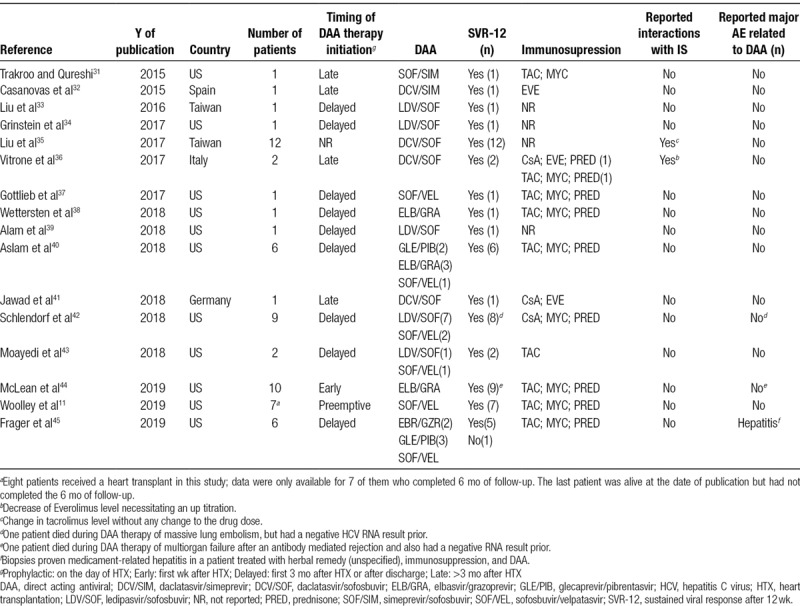
TABLE 1.
List of included publications
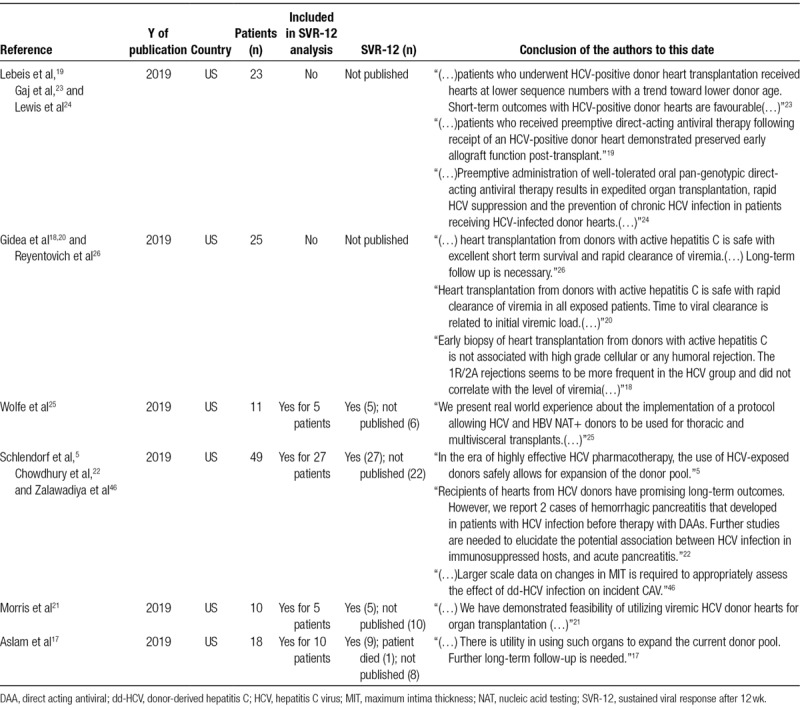
Some studies that had preliminary results presented as published abstracts at the 2019 ISHLT meeting represent important recent work, and a large number of patients who have been successfully treated. Though not suitable for our primary analysis, they provide important information about this topic and will be invaluable once the studies are fully completed. Four of them were integrated into the analysis of sustained viral response after 12 weeks (SVR-12). The summary of ongoing studies is shown in Table 2.
TABLE 2.
List of abstract from ongoing study
The 16 included studies consisted of 2 abstracts (Congress abstract/presentation), 7 case reports, 3 case series, and 4 cohort studies. The completeness of data provided was rated as sufficient in 5 (31%) publications and as good in the remaining 11 (69%).
Characteristics of the Publications
Eleven out of 16 publications (68%) were written by research groups working in institutions in the United States.
Epidemiology and Characteristics of the Patients/Transplant/HCV
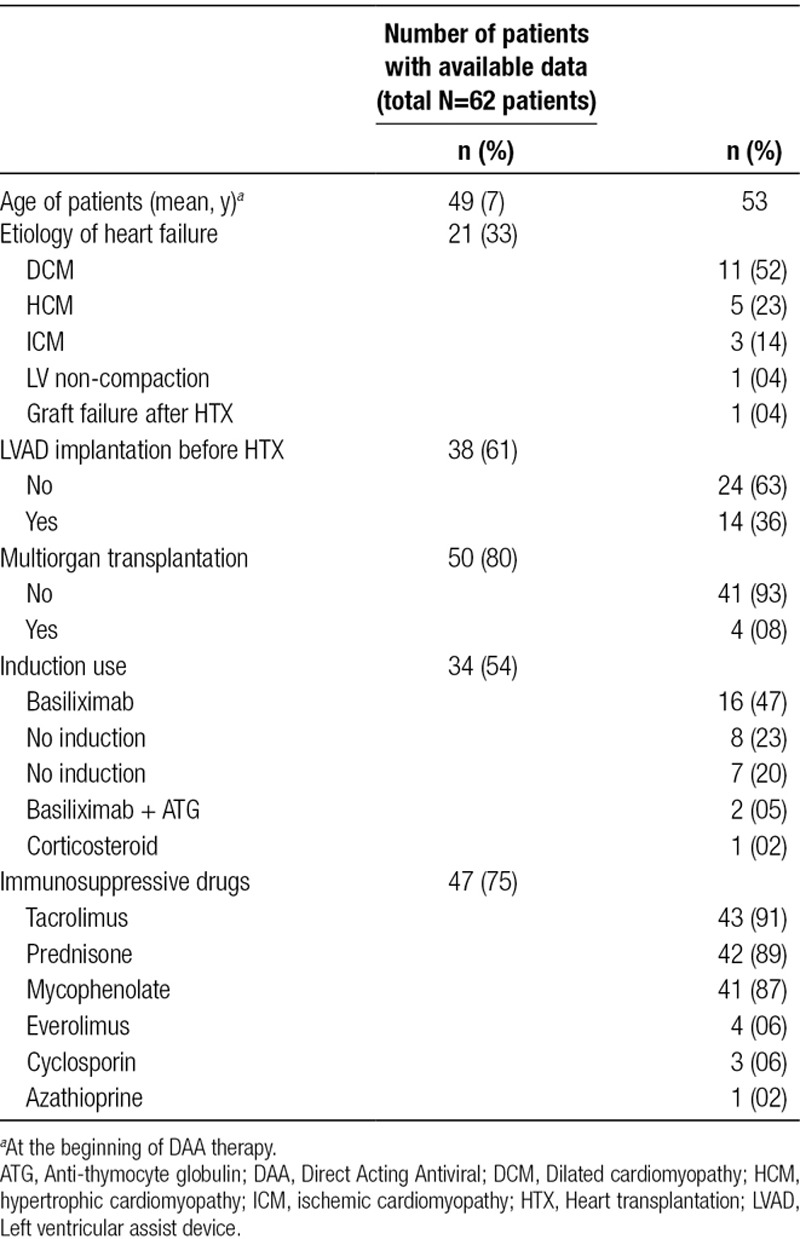
Selected epidemiological and descriptive characteristics of the transplant recipients are described in Table 3. Four patients (8%) underwent a multiorgan transplantation, 3 of these patients (6%) received a combined heart-kidney transplant38,40,45 and 1 patient (2%) received a heart-liver transplant.34 The majority of patients (n = 16, 47%) received basiliximab as induction. The most commonly used maintenance immunosuppression was a combination containing tacrolimus (91%). Most centers used the same induction and maintenance immunosuppression protocol as per their usual practice without changing the dose or the timing of the immunosuppressive drugs.44
TABLE 3.
Characteristic of patients and transplantation included in the analysis
Data relating to the nature of the hepatitis C infection are shown in Table 4. DDHC was the principal mode of infection (n = 56, 82%), including 5 patients previously infected with hepatitis C and cured (documented by negative nucleic acid testing [NAT], then reinfected by a NAT-positive heart during transplantation [DDHC*]). Another 6 patients (10%) were transplanted while having known, active HCV. None of the patients received DAA prophylactically. Seven patients (14%) were treated preemptively (starting day 1 posttransplant) and 10 patients (20%) received therapy during the first week posttransplant. The majority were treated after the first week posttransplant; 28 (56%) in the first 3 months after transplantation and five (10%) >3 months after transplant (ranging from 6 mo to 14 y).
TABLE 4.
Hepatitis C characteristics and treatment
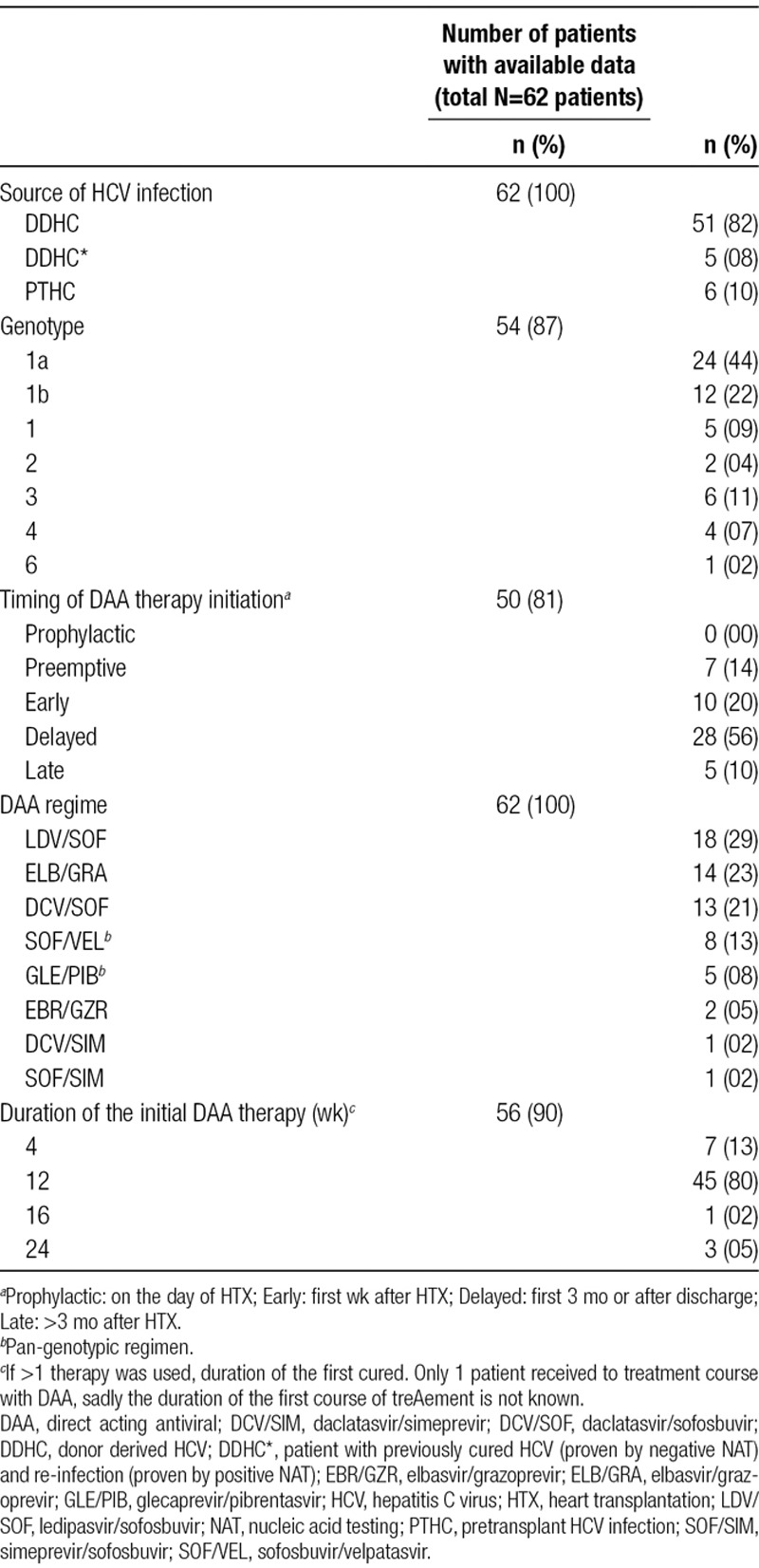
Eight different DAA regimes were used. A pan-genotypic regimen was used in 15 (21%) of all treated patients. Forty-five (80%) were treated for 12 weeks. In 7 patients (13%), 4 weeks of treatment was used.11
Type of DAA Therapy
The types of DAA regimens are shown in Table 3. The different regimens reflect differences in local policies and timing of availability of various DAA. Despite the heterogeneity of DAA regimens no difference in HCV clearance was demonstrated, with all patients clearing the virus.
Efficacy of DAA Therapy
All patients treated with a complete course of DAA achieved RNA clearance between 1 and 12 weeks of therapy (Table 5).
TABLE 5.
Results of HCV therapy in all published data
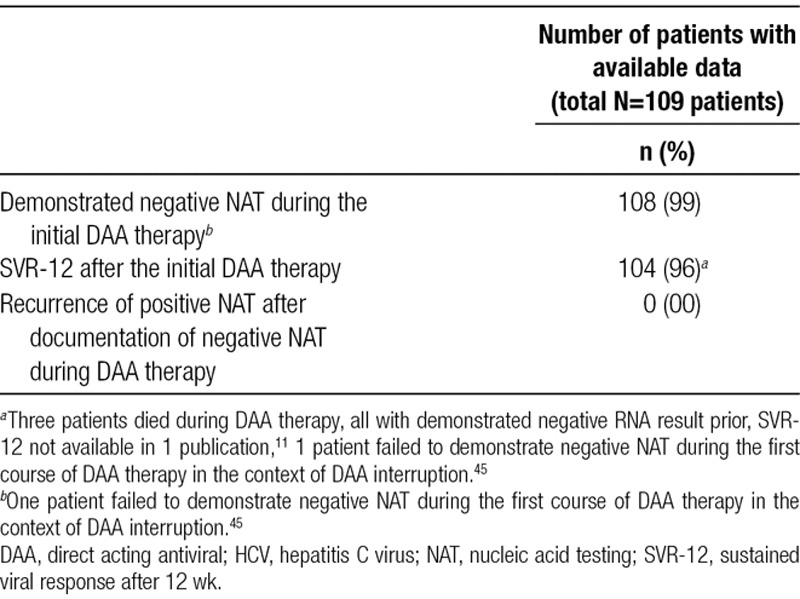
Median time to clearance was 4 weeks. All surviving patients with available data achieved as SVR-12, which is an accepted criterion to determine HCV cure.47 One patient failed to reach viral clearance after cessation of the DAA drug in the context of a medication induced hepatitis.45 The longest follow-up was 18 months after DAA therapy with persistently negative RNA. No relapses were documented.
Complications and Drug Interactions During DAA Therapy
The presence of non-life-threatening complications was not systematically reported. In the publications that did report non-life threatening morbidity data, the adverse event rate was ~60% of patients,35 which is slightly lower than the adverse event rates reported in the Phase 3 DAA treatment studies.48
Major complications are shown in Table 6. Three patients died during follow-up: 1 (1%) due to a massive pulmonary embolus, 1 (1%) due to multiorgan failure after antibody-mediated rejection, and 1 (1%) due to disseminated bacterial infection. All events were adjudicated by the study teams as not related to HCV infection or DAA therapy. Six (16%) patients suffered from rejection during DAA therapy; all occurred <3 months following transplantation. Changes in immunosuppressive drug levels were reported in 2 (7%) patients. In 1 patient, the DAA was ceased secondary to medication-induced hepatitis. This patient was concomitantly on a DAA, a herbal supplement and azole therapy for an opportunistic infection. We cannot exclude the role of the DAA in this scenario; however, both herbal supplements and azole therapy can provoke a clinical picture of hepatitis. The cessation of the DAA resulted in an ongoing DDHC and this patient needed to be treated with 3 different DAA regimes over a prolonged period to be cured of the infection.45
TABLE 6.
Complications during HCV therapy
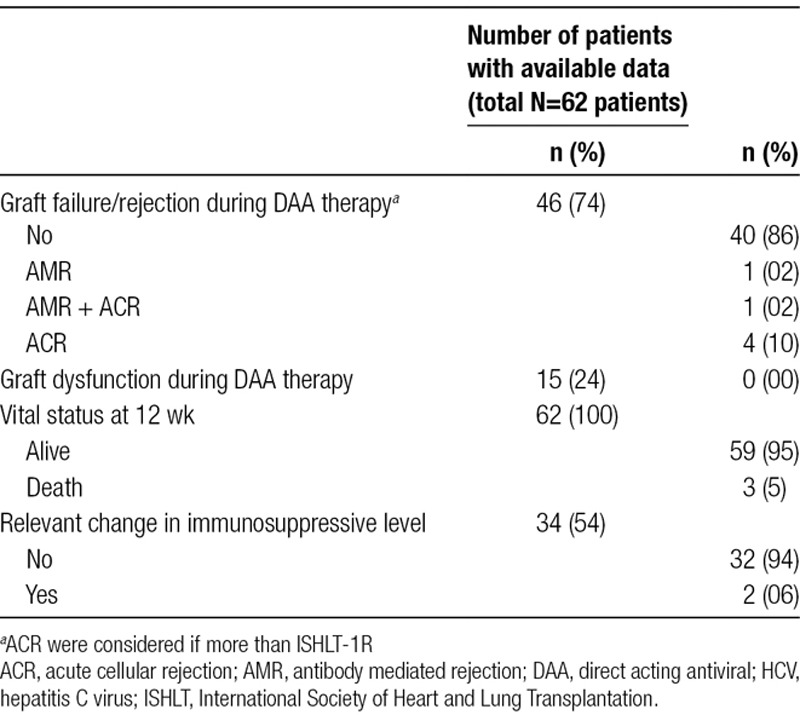
One study reported an interaction between ledipasvir/sofosbuvir and everolimus that required a dose reduction of the everolimus.35 Another study reported an interaction between daclatasvir/sofosbuvir(DCV/SOF) and both mycophenolate and tacrolimus resulting in a slightly decreased level of tacrolimus and an increased level of mycophenolate without requiring any dose adjustments.36
There were no documented cases of an interaction between DAA and the induction regime in our series.
DISCUSSION
What Is the Efficacy of DAA in the Setting of Transplantation?
In the setting of transplantation, DAA therapy appears to be safe and effective for the treatment of HCV. Despite the heterogeneity of the studies, there was no reported HCV relapse following complete DAA therapy. Nevertheless, it is worth noting that continued monitoring of viral loads posttreatment was reported in most series. In the THINKER trial that reported on kidney transplants from HCV-viremic donors to HCV-negative recipients, 1 patient had increased HCV viral loads on follow-up measurements during therapy because of DAA resistance and required a change in his DAA therapy.
Due to geographic variations in the prevalence of HCV genotypes, differences exist in national guidelines on the use of DAA for HCV. American guidelines recommend the use of a genotype-guided therapy,49 whereas in Australia a pan-genotypic combination is recommended as first-line treatment.50 Despite the differences in choice of DAA combinations, timing of initiation and duration of DAA therapy between studies, all studies reported 100% cure rate. None of the studies identified in this review reported prophylactic administration of DAA (commencing pretransplant) and only 1 study reported the use of a preemptive protocol in the first week posttransplant. Most studies documented HCV infection of the recipient (by NAT testing) before commencement of DAA therapy. This likely reflects the high cost of DAA therapy and the reimbursement arrangements that exist in countries where there is a requirement to prove HCV infection before commencing DAA therapy.
In the case of transplantation the use of a pan-genotypic is probably the optimal approach for prophylactic and preemptive treatment because of the expected delay in obtaining the genotype of the donor. It is worth noting that a pan-genotypic treatment is being utilized in 2 ongoing studies of DAA after transplantation as well as the most recent publication on the topic.11,12,16,24
What Interactions Between Immunosuppression, Induction Regime and DAA Have Been Observed?
The data regarding pharmacokinetic interactions between DAA and immunosuppression are also reassuring. Initiation of the DAA resulted in a significant change to biochemical drug levels in only 2 of 62 patients (3%); however, neither of these patients developed rejection. In an abstract addressing this question, no adjustment of immunosuppression was needed after DAA therapy was started.51 A recently published review on immunosuppression levels in patients undergoing DAA therapy confirmed this probable lack of interaction. No change in the drug levels were observed retrospectively when DAA were started.51
Moreover, if DAA therapy is administered early after transplantation, the risk of persistent sub- or supra-therapeutic levels of immunosuppression drugs is greatly reduced as immunosuppressive drug levels are routinely checked and titrated at regular intervals during this time period.
In this series, most of the patients (16 patients, 47%) received basiliximab as induction and no interaction between induction and DAA were reported, but it is worth mentioning that most of the patients (n = 33, 66%) did not receive DAA during the first week after transplantation. With the half-life of 30 days for Anti-thymocyte globulin (ATG)52 and 7 days for basiliximab, the absence of interaction between DAA and induction regime must be reviewed with some caution.
Does DAA Therapy Increase the Risk of Acute Rejection?
The risk of rejection due to the HCV viral load or treatment with DAA cannot be formally determined from published studies due to the heterogeneity of the data. However, 6 (14%) patients suffered from acute cellular or antibody-mediated rejection over the course of the DAA therapy. The rate of acute rejections in these studies is comparable with the available data on rejection in the absence of HCV or DAA.53
In a cohort of 25 patients, there was no significant difference in the observed rate of rejection (ISHLT grad >1R) between patients with DDHC and a control group. No correlation between viral load and rejection could be found.18 The long-term risk of chronic rejection is for the moment mostly unknown. One group has shown that patients who are viremic before initiation of DAA treatment have more marked intimal thickening shown on intravascular ultrasound of the left anterior descending coronary artery.46
What Is the Optimal Timing of the Initiation and Duration of DAA Therapy?
The optimal timing of initiation of DAA and the duration of the therapy in the setting of transplantation of a noninfected patient with a HCV-infected heart is still not established.
In the patients reported in this review, most commenced treatment at first documentation of viremia. Seven patients (14%) received preemptive therapy (first day after transplantation) and none received prophylactic treatment as has been described in a recently published kidney transplant protocol9,10 and ongoing heart transplant study.24 As seen in the current review, the success of DAA therapy does not seem to be affected by the timing of initiation. Nevertheless, the long-term consequences of the initial viremic period are unknown, particularly the risk of hepatitis C-induced coronary arteriosclerosis,46,54 and the possibility of accidental transmission to medical staff should also be considered. In the context of immunosuppression, initial viremia may be extremely high and this could have negative consequences. Some data suggest a deleterious effect of the viremic load on the incidence of ISHLT-1R mild rejection (but not moderate and severe rejection)18 and there are 2 case reports of possible DDHC-associated pancreatitis in patient with high viral load before commencing DAA therapy.22
As demonstrated with kidney transplantation in the same setting, a prophylactic dose or a preemptive dose (given few h after transplant) could diminish or even completely suppress viral load.9-11
Regarding duration of therapy, international guidelines on DAA for HCV treatment recommend a duration of 8–12 weeks depending on the DAA regimen.55,56 The most recently published study on heart (and lung) transplantation from HCV-infected donors utilized a 4-week protocol of preemptive therapy with a pan-genotype combination commencing within hours of transplantation.11 Interestingly, nearly all recipients had detectable hepatitis C viremia immediately posttransplant but all patients achieved sustained viral clearance with no late relapses. While this study suggests that preemptive initiation of treatment may allow a shorter course of DAA to be administered, only 8 heart transplants were included in the study. A confirmatory study in a larger cohort of patients would be desirable before routinely advocating this regimen.
Limitations
Given the observational nature of all the studies included in this review, there is a possible publication bias in favor of studies with positive outcomes. Nonetheless, the highly consistent conclusions of all published studies in relation to the efficacy of DAA and favorable clinical outcomes of heart transplant recipients with DDHC provides compelling evidence to support the use of NAT-positive hepatitis C donors for heart transplantation. Our review did not address the safety of heart transplantation from NAT-negative hepatitis C–seropositive donors; however, published data show that the risk of transmission of hepatitis C from these donors is very low.57
CONCLUSIONS AND RECOMMENDATIONS
Based on this systematic review, we make the following conclusions and propose the following recommendations regarding protocols for heart transplantation from HCV viremic donors to HCV-negative recipients:
-
-
While all DAA regimens achieve excellent cure rates for HCV infection, in view of the high rate of acute kidney injury in the first weeks after heart transplantation, we recommend the use of a pan-genotypic drug combination that is eliminated by the liver.
-
-
Despite different timings of initiation of DAA therapy across the included studies, there were no differences in sustained viral clearance. Early commencement of DAA with a potentially shorter treatment duration (< 8 wk) is appealing; however, further studies are required before recommending this approach.
-
-
No patients developed viral resistance or treatment failure when DAA was used according to the protocol. One patient failed therapy after an interruption of therapy. We recommend that all patients undergo serial NAT testing during the first weeks of treatment until their HCV RNA is below the limit of detection to either confirm viral clearance or detect treatment failure. Twelve weeks after cessation of the treatment, regardless of the duration of therapy, a last NAT should be performed to confirm SVR-12.
-
-
No preemptive changes in induction and maintenance immunosuppression are needed. Interactions between DAA and immunosuppression do exist but appear rare. Data on the interaction between induction and DAA are scarce, but the literature on the use of Basiliximab with DAA suggests it is safe to use. When DAA is initiated during the early phase after transplantation, immunosuppressive drug levels are routinely measured until a stable dose of immunosuppression is found. In this context, the risk of dangerously low or high levels of immunosuppression is minimal. However, when DAA therapy is completed, immunosuppressive medication levels should be monitored in case of changes related to cessation of the therapy.
-
-
For recipients of NAT-negative hepatitis C-seropositive (ie, antibody positive) donors, a “watchful waiting” protocol with serial NAT testing up to 3 months posttransplant is recommended.
-
-
Because the risk of coronary intimal thickening is known for patient with chronic hepatitis C and because some data suggest that patient with DDHC and initial viral load have some intimal thickening on intravascular ultrasound at 1 years, a careful assessment of vasculopathy in this population is suggested.
Footnotes
Published online 23 August, 2019.
B.S. participated in research design, the performance of the research, and the writing of the article. N.B., N.D., G.M., J.N., P.S.M., and C.S.H. participated in the writing of the article.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.National Data - OPTN. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed April 24, 2019.
- 2.Kling CE, Perkins JD, Landis CS, et al. Utilization of organs from donors according to hepatitis C antibody and nucleic acid testing status: time for change. Am J Transplant. 2017;17:2863–2868. [DOI] [PubMed] [Google Scholar]
- 3.Gastaminza P, Dryden KA, Boyd B, et al. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 2010;84:10999–11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakano T, Lau GM, Lau GM, et al. An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region. Liver Int. 2012;32:339–345. [DOI] [PubMed] [Google Scholar]
- 5.Schlendorf K, Zalawadiya S, Shah A, et al. Successful transplantation of 58 hepatitis C-exposed donor hearts in the era of direct-acting antiviral therapies J Heart Lung Transplant. 2019;38(Suppl 4):S48. [Google Scholar]
- 6.Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23:277–283. [DOI] [PubMed] [Google Scholar]
- 7.Gasink LB, Blumberg EA, Localio AR, et al. Hepatitis C virus seropositivity in organ donors and survival in heart transplant recipients. JAMA. 2006;296:1843–1850. [DOI] [PubMed] [Google Scholar]
- 8.Bushyhead D, Goldberg D. Use of hepatitis C-positive donor livers in liver transplantation. Curr Hepatol Rep. 2017;16:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco A, Moreso F, Merino E, et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: a prospective study. Transpl Int. 2019;32:710–716. [DOI] [PubMed] [Google Scholar]
- 10.Durand CM, Bowring MG, Brown DM, et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized trial. Ann Intern Med. 2018;168:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolley AE, Singh SK, Goldberg HJ, et al. ; DONATE HCV Trial Team. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Transplant of Redeemed Organs by Judicious Administration of New Direct-Acting Antivirals for Hepatitis-C Heart Recipients - Tabular View - ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/record/NCT03383419?term=Hepatitis+C&cond=Heart+Transplant&rank=6. Accessed April 2, 2019.
- 13.HCV Positive Heart Donors - Tabular View - ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/record/NCT03382847?term=Hepatitis+C&cond=Heart+Transplant&rank=5. Accessed April 2, 2019.
- 14.DAA Treatment in Donor HCV-positive to Recipient HCV-negative Heart Transplant - Tabular View - ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/record/NCT03208244?term=Hepatitis+C&cond=Heart+Transplant&rank=4. Accessed April 2, 2019.
- 15.Zepatier For Treatment Of Hepatitis C-Negative Patients Who Receive Heart Transplants From Hepatitis C-Positive Donors (HCV) - Tabular View - ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/record/NCT03146741?term=Hepatitis+C&cond=Heart+Transplant&rank=2. Accessed April 2, 2019.
- 16.Expanding the Pool in Orthotopic Heart Transplantation - Full Text View - ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT03222531?term=Hepatitis+C&cond=Heart+Transplant&rank=1. Accessed April 2, 2019.
- 17.Aslam S, Yumul I, Mariski M, et al. Outcomes of heart and heart-kidney transplantation from hepatitis C virus (HCV) positive donors into HCV negative recipients. J Heart Lung Transplant. 2019;38:S66–S67. [DOI] [PubMed] [Google Scholar]
- 18.Gidea CG, Narula N, Reyentovich A, et al. The impact of HCV viremia in heart transplant recipients from donors with HCV infection on acute and humoral cellular rejection J Heart Lung Transplant. 2019Suppl 4):S66. [Google Scholar]
- 19.Lebeis TA, Afari ME, Bethea ED, et al. Evaluation of early allograft function in donor HCV-positive to recipient HCV-negative cardiac transplantation managed with preemptive direct acting antiviral therapy J Heart Lung Transplant. 2019Suppl 4):S275–S276. [Google Scholar]
- 20.Gidea CG, Reyentovich A, Smith D, et al. Magnitude of recipient viremia after heart transplantation from HCV viremic donors and time to clearance with therapy J Heart Lung Transplant. 2019Suppl 4):S65–S66. [Google Scholar]
- 21.Morris KL, Adlam JP, Bochan M, et al. Single center experience of hepatitis C donor viremic cardiac transplantation J Heart Lung Transplant. 2019Suppl 4):S265. [Google Scholar]
- 22.Chowdhury M, Schlendorf K, Zalawadiya S, et al. Incidence of pancreatitis in recipients of hepatitis C donor hearts J Heart Lung Transplant. 2019Suppl 4):S311. [Google Scholar]
- 23.Gaj KJ, D'Alessandro DA, Bethea ED, et al. Acceptance of HCV-positive donor hearts improves organ acceptance selectivity: single center experience. J Heart Lung Transplant. 2019Suppl 4):S49–S50. [Google Scholar]
- 24.Lewis GD, Bethea ED, Gaj K, et al. Preemptive pan-genotypic direct acting antiviral therapy in donor HCV-positive to recipient HCV-negative cardiac transplantation produces viral clearance and is associated with favorable outcomes J Heart Lung Transplant. 2019Suppl 4):S65. [Google Scholar]
- 25.Wolfe CR, Sen S, Kappus MR, et al. Hepatitis and thoracic transplantation - no more virus non-grata J Heart Lung Transplant. 2019;38:S308. [Google Scholar]
- 26.Reyentovich A, Gidea C, Smith D, et al. Clinical experience with heart transplantation from hepatitis C positive donors J Heart Lung Transplant. 2019;38(Suppl 4):S48. [Google Scholar]
- 27.Givertz MM, Woolley AE, Baden LR. Expanding the donor pool with use of hepatitis C infected hearts. Circ Heart Fail. 2018;11:e005656. [DOI] [PubMed] [Google Scholar]
- 28.Real World Impact of HCV Viremic Solid Organs on Waitlist Times - ATC Abstracts. Available at https://atcmeetingabstracts.com/abstract/real-world-impact-of-hcv-viremic-solid-organs-on-waitlist-times/. Accessed June 21, 2019.
- 29.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radzi Y, Shezad MF, Danziger-Isakov L, et al. Using hepatitis C and B virus-infected donor organs for pediatric heart transplantation. J Thorac Cardiovasc Surg. 2019;158:548–553. [DOI] [PubMed] [Google Scholar]
- 31.Trakroo S, Qureshi K. Successful treatment of chronic hepatitis C infection with direct-acting antivirals in a heart transplant recipient: a case report. Transplant Proc. 2015;47:2295–2297. [DOI] [PubMed] [Google Scholar]
- 32.Casanovas T, Roca J, Niubó J. Successful treatment of hepatitis C virus infection combining daclatasvir and simeprevir in a heart transplant recipient with decompensated cirrhosis. J Heart Lung Transplant. 2016;35:949–951. [DOI] [PubMed] [Google Scholar]
- 33.Liu CH, Chen YS, Wang SS, et al. Treatment of de novo hepatitis C virus-related fibrosing cholestatic hepatitis after orthotopic heart transplantation by ledipasvir and sofosbuvir. J Formos Med Assoc. 2017;116:407–409. [DOI] [PubMed] [Google Scholar]
- 34.Grinstein J, Lourenco LM, Te HS, et al. Accepting hearts from hepatitis C-positive donor: can we expand the donor pool? J Card Fail. 2017;23:762–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu CH, Chen YS, Wang SS, et al. Sofosbuvir-based interferon-free direct acting antiviral regimens for heart transplant recipients with chronic hepatitis C virus infection. Clin Infect Dis. 2018;66:289–292. [DOI] [PubMed] [Google Scholar]
- 36.Vitrone M, Andini R, Mattucci I, et al. Direct antiviral treatment of chronic hepatitis C in heart transplant recipients Transpl Infect Dis. 2018;20:e12813. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb RL, Sam T, Wada SY, et al. Rational heart transplant from a hepatitis C donor: new antiviral weapons conquer the Trojan horse. J Card Fail. 2017;23:765–767. [DOI] [PubMed] [Google Scholar]
- 38.Wettersten N, Tran H, Mekeel K, et al. Successful heart-kidney transplantation from a hepatitis C viremic donor to negative recipient: one year of follow-up. Transpl Infect Dis. 2019;21:e13002. [DOI] [PubMed] [Google Scholar]
- 39.Alam A, Mukherjee A, James D, et al. Successful transplantation of a hepatitis C donor heart of unknown genotype to a hepatitis C seropositive recipient J Am College Cardiol. 2019;71(Suppl 11):A2381. [Google Scholar]
- 40.Aslam S, Mariski M, Pretorius G, et al. (170) - Heart and heart-kidney transplantation from hepatitis C virus (HCV) positive donors into HCV-negative recipients J Heart Lung Transplant. 2018;37(Suppl 4):S76–S77. [Google Scholar]
- 41.Jawad K, Feder S, Barten M, et al. Curative therapy of a hepatitis C infection due to an infected heart donor: 5-year outcomes after heart transplantation. Eur J Cardiothorac Surg. 2018;54:400–401. [DOI] [PubMed] [Google Scholar]
- 42.Schlendorf KH, Zalawadiya S, Shah AS, et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant. 2018;37:763–769. [DOI] [PubMed] [Google Scholar]
- 43.Moayedi Y, Gulamhusein AF, Ross HJ, et al. Accepting hepatitis C virus-infected donor hearts for transplantation: multistep consent, unrealized opportunity, and the Stanford experience. Clin Transplant. 2018;32:e13308. [DOI] [PubMed] [Google Scholar]
- 44.McLean RC, Reese PP, Acker M, et al. Transplanting hepatitis C virus-infected hearts into uninfected recipients: a single-arm trial. Am J Transplant. 2019; 1–10. 00:Available at 10.1111/ajt.15311. [DOI] [PubMed] [Google Scholar]
- 45.Frager SZ, Dhand A, Gass A, et al. Heart transplantation for hepatitis C virus non-viremic recipients from hepatitis C virus viremic donors. Cardiol Rev. 2019;27:179–181. [DOI] [PubMed] [Google Scholar]
- 46.Zalawadiya S, Lindenfeld J, Haddad E, et al. Intracoronary intimal thickness in transplant recipients of hepatitis C-positive donor hearts J Heart Lung Transplant. 2019;38:S281. [Google Scholar]
- 47.Burgess SV, Hussaini T, Yoshida EM. Concordance of sustained virologic response at weeks 4, 12 and 24 post-treatment of hepatitis c in the era of new oral direct-acting antivirals: a concise review. Ann Hepatol. 2016;15:154–159. [DOI] [PubMed] [Google Scholar]
- 48.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. ; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. [DOI] [PubMed] [Google Scholar]
- 49.Recommendations for Testing, Managing, and Treating Hepatitis C | HCV Guidance. Available at https://www.hcvguidelines.org/. Accessed February 15, 2019.
- 50.Gastroenterological Society of Australia. Available at https://www.gesa.org.au/resources/hepatitis-c-treatment/. Accessed May 6, 2019.
- 51.Boyle K, Fowler RE, Pollack A, et al. Appropriate management of drug interactions results in safe use of hepatitis C therapies in heart transplant recipients J Heart Lung Transplant. 2019;38:S200–S201. [Google Scholar]
- 52.Bunn D, Lea CK, Bevan DJ, et al. The pharmacokinetics of anti-thymocyte globulin (ATG) following intravenous infusion in man. Clin Nephrol. 1996;45:29–32. [PubMed] [Google Scholar]
- 53.Khush KK, Cherikh WS, Chambers DC, et al. ; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37:1155–1168. [DOI] [PubMed] [Google Scholar]
- 54.Prasad A, Zhu J, Halcox JP, et al. Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation. 2002;106:184–190. [DOI] [PubMed] [Google Scholar]
- 55.Australian recommendations for the management of hepatitis C virus infection: a consensus statement (September 2018) ASHM. Available at https://www.ashm.org.au/products/product/01032016. Accessed February 21, 2019. [DOI] [PubMed]
- 56.AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67:1477–1492. doi:10.1093/cid/ciy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel SR, Madan S, Saeed O, et al. Cardiac transplantation from non-viremic hepatitis C donors. J Heart Lung Transplant. 2018;37:1254–1260. [DOI] [PubMed] [Google Scholar]


