Background.
New-onset stage 4–5 chronic kidney disease (CKD) after liver transplantation (LT) is associated with high morbidity, mortality, and economic burden. In 2010, we instituted an early renal sparing immunosuppression (RSI) protocol for LT recipients with severe renal dysfunction (pre-LT dialysis/estimated glomerular filtration rate (eGFR)<30mL/min/1.73 m2 or post-LT acute kidney injury) consisting of 2 doses of basiliximab for induction and delaying tacrolimus to post-LT day 4–7. We examined the effect of early RSI on post-LT renal outcomes.
Methods.
Data on all adults who had LT between January 1, 2010, and December 12, 2014 were collected. We calculated the renal risk index (RRI) score for each LT recipient (https://rri.med.umich.edu). Primary outcome was new-onset post-LT stage 4–5 CKD.
Results.
Of 214 LT recipients, 121 (57%) received early RSI and 93 (43%) received standard immunosuppression. Cumulative incidence of new-onset stage 4–5 CKD was higher in early RSI compared with standard immunosuppression (P = 0.03). Female sex and RRI score were the significant risk factors for development of post-LT stage CKD in the entire study cohort as well as the LT recipients with RRI ≥ sixth decile (high-risk group).
Conclusions.
Delaying tacrolimus initiation combined with basiliximab induction did not have a durable effect on long-term renal outcomes in high-risk LT recipients. Further studies are needed to identify the effective strategies to preserve renal function by targeting patients at high risk for CKD progression.
INTRODUCTION
Renal dysfunction after liver transplantation (LT) is a recognized complication that negatively impacts patient and graft survival and increases morbidity, resource utilization, and healthcare costs.1-4 Calcineurin inhibitors (CNIs) are the mainstay of transplant immunosuppression. Although there are multiple recipient, donor, and operative factors that contribute to development of post-LT renal dysfunction, CNIs have been implicated as one of the major causes for post-LT renal dysfunction.1,3,5-7 Data from randomized clinical trials have suggested that early renal protective strategies employing interleukin (IL)-2 induction agents to allow the delay of initiation of CNIs in the early post-LT period resulted in better renal outcomes and comparable graft survival at 6–12 months posttransplant compared with a conventional-dose tacrolimus regimen that was initiated immediately post-LT.7-9 Since most of these studies largely excluded LT recipients with severely diminished renal function, the role of these strategies in renal protection among such patients is not firmly established.7,8
We examined our renal outcomes from the early model for end stage liver disease (MELD) era (2002–2008). The overall 5 year cumulative incidence of stage 4–5 chronic kidney disease (CKD) was 22% and the 1-year cumulative incidence among those with estimated glomerular filtration rate (eGFR) <30 mL/min/m2 was as high as 20%.10 This prompted us to establish a protocol of early renal sparing immunosuppression (RSI) in 2010 for LT recipients with significant pre-LT renal insufficiency (eGFR<30 mL/min/1.73 m2 or dialysis) or those who developed post-LT acute kidney injury (AKI) defined as urine output<0.5 mL/kg/h or ≥50% increase in serum creatinine on post-op day (POD) 1. The early RSI protocol consisted of IL-2 induction with basiliximab on POD 1 and POD 4 and delayed introduction of tacrolimus until POD 4 to POD 7 with a tacrolimus trough goal of 6–10 ng/mL within the first 3 months following LT.
The primary aim was to retrospectively examine the effect of early RSI on the development of stage 4 or 5 CKD following LT. Our secondary aims were to identify risk factors associated with posttransplant stage 4–5 CKD and compare biopsy-proven acute rejection and patient survival between recipients of early RSI and standard immunosuppression (SI).
MATERIALS AND METHODS
Study Design and Patient Population
This was a retrospective observational cohort study of adult patients (≥18 y old) who underwent deceased donor LT between January 1, 2010, and December 31, 2014 at the University of Michigan Transplant Center and subsequently received a tacrolimus-based immunosuppression therapy according to the center protocol. Patients were excluded if they met at least one of the following criteria: on renal replacement therapy (RRT) >3 months prior to LT, previous transplant, multi-organ transplant including combined kidney-liver transplant, or previous exposure to tacrolimus or cyclosporine. The patients were followed until June 30, 2017. This retrospective cohort study was approved by University of Michigan Institutional Review Board. The patients involved in this study were treated in a manner in accordance with the Declaration of Helsinki and Declaration of Istanbul.
The following information from the time of transplant were extracted from the electronic medical records: demographics including age, sex, race, height, and weight, etiology of liver disease, serum creatinine, serum bilirubin, prothrombin time/International normalized ratio of prothrombin time, serum albumin and serum sodium, laboratory MELD score at LT, pre-LT dialysis status, body mass index (BMI), diabetes, history of transjugular intrahepatic portosystemic shunt, and organ procurement and transplantation network status-1 transplant listing. From the postoperative phase, patient and graft survival, length of stay, hypertension, diabetes, and renal function variables were collected at POD 7 and then at 1 month, 6 months, 1 year, and at last follow-up. Serum creatinine, eGFR, urine output, RRT type, and duration were collected to classify both acute and chronic renal outcomes according to National Kidney Foundation kidney disease improving global outcomes (KDIGO) criteria.11,12
Posttransplant Immunosuppression
The posttransplant immunosuppression regimen for individual patients was identified by review of the medication administration record. Early RSI was defined as 2 doses of 20 mg basiliximab with initiation of tacrolimus on POD 4 or later. SI was defined as no basiliximab induction and initiation of tacrolimus on POD 0–1. Tacrolimus trough target was 6–10 ng/mL for the first 3 months after transplant and 4–8 ng/mL after 3 months for all LT recipients. During the follow-up period, if a patient’s tacrolimus was switched to cyclosporine, they were included in the study.
All the LT recipients received 1000 mg of mycophenolate mofetil twice daily and corticosteroids. Steroids were tapered and discontinued by the end of the second month of LT in all but those with cirrhosis related to autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis. Mycophenolate mofetil was typically discontinued by 6–12 months of LT based upon the etiology of liver disease and the primary hepatologists’ decision. Patients who experienced episodes of acute rejection had individualized adjustments of their immunosuppression, including re-initiation of corticosteroids or increased CNI trough target levels.
Renal Outcome
Outcomes including serum creatinine and eGFR, calculated using Modification of Diet in Renal Disease Study-4 equation, and dialysis status were recorded at baseline, posttransplant day 7, month 1, month 6, year 1, and last follow-up. Post-LT stage 4–5 CKD was defined as either the need for chronic RRT or eGFR using Modification of Diet in Renal Disease Study-4 <30 mL/min/1.73 m2. The date of initiation of dialysis was recorded. AKI during the first 7 days following LT was graded according to the KDIGO classification: no AKI (Stage 0), Stage 1, Stage 2, or Stage 3.11
Renal Risk Index (RRI) Score and RRI Decile
The RRI was derived from a cohort of 43 514 adult recipients who underwent deceased donor LT alone between February 28, 2002, and December 12, 2010. In an adjusted Cox model, 14 recipient factors at LT were independently associated with post-LT end-stage renal disease (ESRD).13 This RRI has been validated with a C-statistic of 0.76 (95% confidence interval [CI], 0.75-0.78).
 |
RRI score and RRI decile were retrospectively calculated using the RRI calculator (https://rri.med.umich.edu).
Subgroup of LT Recipients at High Risk for Developing Stage 4–5 Chronic Kidney Disease
In order to delineate the effect of immunosuppression (early RSI vs SI), we identified a subgroup of LT recipients at high risk for developing stage 4–5 CKD based on RRI decile. Our previous study showed that every decile increase in RRI was associated with higher risk of post-LT ESRD.13 Therefore, we dichotomized RRI decile at the sixth decile.
Statistical Analysis
The continuous variables were presented as median and interquartile range (IQR), and categorical variables were presented as proportions. The primary outcome was post-LT stage 4–5 CKD. The secondary outcomes were biopsy-proven acute rejection and survival. Kaplan-Meier analysis was used to estimate the incidence of post-LT stage 4–5 CKD and 1-, 3-, and 5-year survival. Acute rejection rates were compared using Chi squared statistics. Multi-collinearity diagnostics for the independent covariates were performed using tolerance and variance inflation factor.
Cox regression analysis was performed to assess the predictors of post-LT stage 4–5 CKD. The model was stratified by immunosuppression status (early RSI vs SI). The model was adjusted for sex, etiology of liver disease, preoperative eGFR and MELD score, RRI, and AKI status.
For the subgroup analysis, we fitted 3 separate models using Cox regression analysis to examine the predictors of stage 4–5 CKD in each specific subgroup. Hypertension and diabetes were not significant in the univariate analysis; therefore, they were not included in the final model. In addition to immunosuppression status, each model was adjusted for pre-LT eGFR, post-LT AKI stage, RRT administered within first 7 days of LT, sex, and RRI score.
All analyses were conducted using IBM SPSS version 25 (IBM Corp., Armonk, NY). P value <0.05 was considered statistically significant.
RESULTS
Cohort Characteristics
The characteristics of the cohort (N = 214) were shown in Table 1. The median age of the cohort was 56 years (IQR: 49–62), 68.7% were males, 36.4% had hepatitis C, 13.6% had nonalcoholic steatohepatitis (NASH), 10.3% had alcoholic liver disease, and 7.5% had cryptogenic cirrhosis. Hepatocellular carcinoma was diagnosed in 30.4% of patients and transplantation was performed with an exception MELD score. The median lab MELD score was 17 (IQR: 12–24) and median RRI was 1.45 (IQR: 0.75–2.47) at the time of LT. The median follow-up time was 4.1 years (IQR: 2.7–5.7 y). Post-LT hypertension was present in 60% and post-LT diabetes was present in 32% of the recipients at the end of follow-up.
TABLE 1.
Baseline characteristics of the cohort
Immunosuppression Groups
Based upon our protocol, 121 LT recipients received early RSI and 93 received SI. Baseline characteristics in both groups were similar except for higher MELD score (P < 0.001) and BMI (P = 0.003) at LT in the early RSI group (Table 1). The median time from LT to tacrolimus initiation was 4 days in early RSI group and 1 day in SI group. The median tacrolimus trough levels at 1 month, 6 months, 1 year after LT, and at the end of follow-up were 7.7, 6.6, 6.2, and 5.4 ng/mL, respectively. There was no significant difference in the mean tacrolimus trough levels at 1 month, 6 months, and at the end of the follow-up between early RSI and SI groups (Table 1). The proportion of LT recipients with post-LT hypertension (SI: 57%; RSI: 63%) and diabetes (SI: 35%; RSI: 29%) were similar in both the groups. As expected, the eGFR at LT was significantly lower in early RSI group (60.2 vs 96.4 mL/min/1.73 m2: P<0.001).
Post-LT Stage 4–5 CKD and Evolution of eGFR Over Time
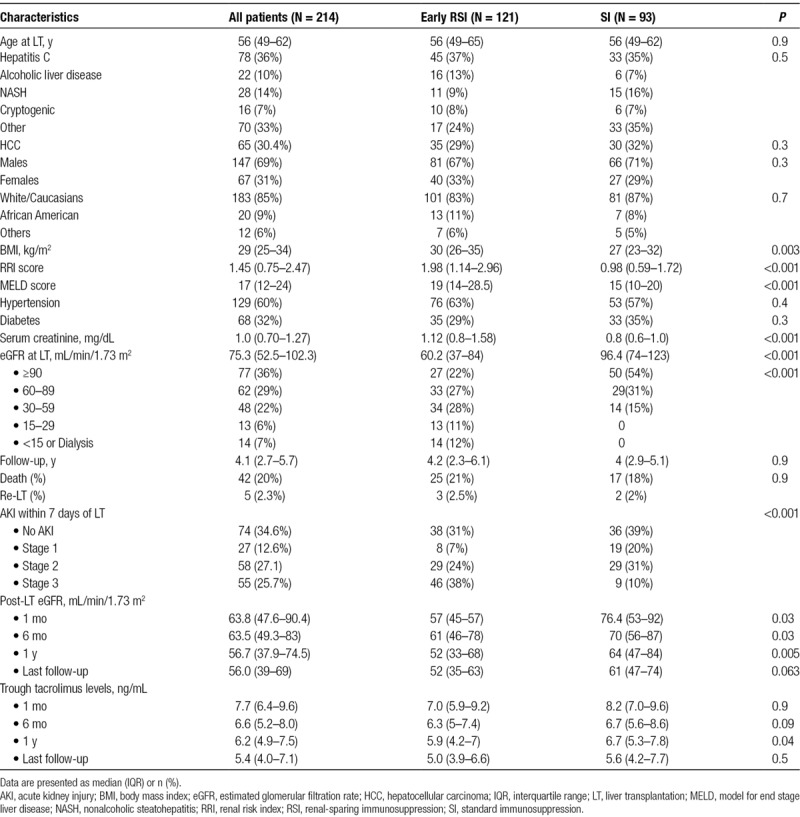
Thirty-six patients developed CKD stage 4–5 during a median follow-up of 4.1 years (IQR: 2.5–5.7). Only 7 of the 93 (7.5%) in the SI group developed post-LT stage 4–5 CKD compared with 29 (23.9%) in the RSI group (P = 0.002). The incidence rate of new-onset stage 4–5 CKD was 2% per patient-year in the SI group and 7% per patient-year in the early RSI group. A significantly higher proportion of patient in RRI ≥ sixth decile (25/106, 23.6%) developed post-LT stage 4–5 CKD compared with those in RRI < sixth decile group (11 of 108, 10.2%) (Figure 1).
FIGURE 1.
Post-LT stage 4–5 CKD stratified by immunosuppression. CKD, chronic kidney disease; LT, liver transplantation; RRI, renal risk index; RSI, renal-sparing immunosuppression; SI, standard immunosuppression.
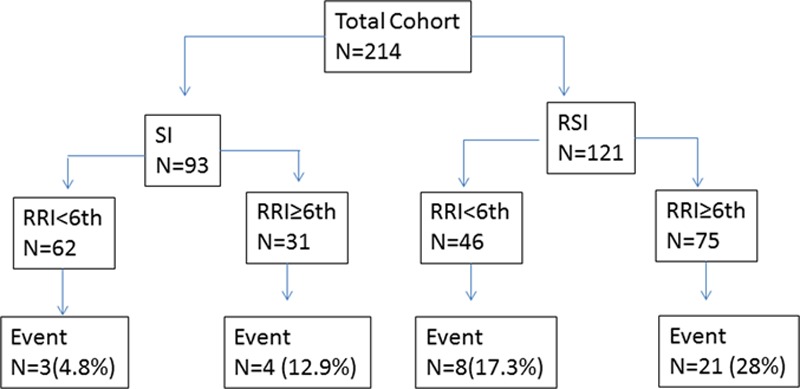
The unadjusted overall 1-, 3-, and 5-year cumulative incidence of post-LT stage 4–5 CKD was 8.6%, 13.5%, and 19.7% for the entire cohort. The unadjusted 1-, 3-, and 5-year cumulative incidence of stage 4–5 CKD in early RSI group was 14.5%, 21.5%, and 27.5%, respectively. Figure 2 shows the adjusted cumulative incidence of stage 4–5 CKD stratified by immunosuppression status.
FIGURE 2.
Adjusted cumulative incidence of stage 4–5 CKD stratified by immunosuppression. CKD, chronic kidney disease; LT, liver transplantation; RSI, renal sparing immunosuppression; SI, standard immunosuppression.
A total of 55 patients met the definition of “Stage 3” on the KDIGO AKI criteria within first week of LT. Of these 55, 14 developed stage 4–5 CKD: 7 within 1 year of LT, 5 in the second year, and 2 after 2 years of LT. The median eGFR at the last follow-up was 52.2 mL/min/1.73 m2 in this group.
A total of 27 patients had eGFR<30 mL/min/1.73 m2 at LT. Of those, only 8 developed stage 4–5 CKD after a mean follow-up of 5.4 ± 0.6 years: 4 within 1 year of LT, 1 in the second year, 2 in the third year, and 1 after 3 years of LT.
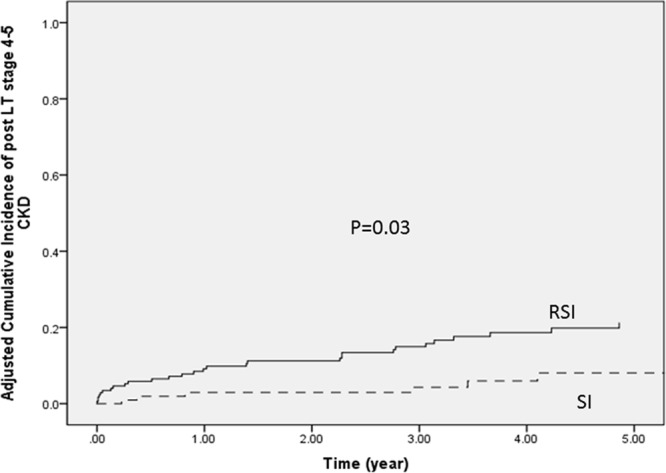
Figure 3 showed the evolution of eGFR over time after LT stratified by immunosuppression. In the SI group, the eGFR declined significantly during the first month after LT (median eGFR 96.4 mL/min/1.73 m2 at LT vs 76.4 mL/min/1.73 m2 at 1 mo; P < 0.001). This decline was not observed in the early RSI group (median eGFR 60.2 mL/min/1.73 m2 at LT vs 57.4 mL/min/1.73 m2 at 1 mo; P = NS). The eGFR in the early RSI group remained lower than that in the SI group throughout the first year post-LT, and the trend continued until the last follow-up.
FIGURE 3.
Median eGFR at LT, 1 mo, 6 mo, and 1 y and at the end of last follow-up stratified by early RSI and SI. eGFR, estimated glomerular filtration rate; LT, liver transplantation; RSI, renal sparing immunosuppression; SI, standard immunosuppression.
Independent Predictors of Post-LT Stage 4–5 CKD
We knew a priori that LT recipients who met the early RSI protocol guidelines would have high incidence of post-LT stage 4–5 CKD because of the prevalent risk factors in this group (lower eGFR and RRT at LT, and higher proportion of AKI within 24 h of LT). Therefore, we stratified the model by SI and early RSI groups for the entire cohort and high risk subgroup (RRI ≥ sixth decile).
Entire Cohort
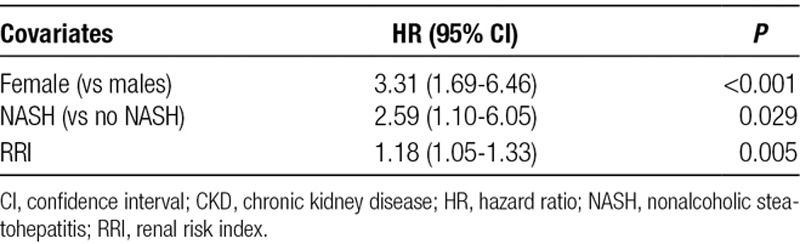
In an adjusted stratified model, NASH (hazard ratio [HR] = 2.59 [95% CI, 1.10-6.05]; P = 0.029), female sex (HR = 3.31 [95% CI, 1.69-6.46]; P < 0.001), and RRI (HR = 1.18 per unit increase in RRI score [95% CI, 1.05-1.33]; P = 0.005) were the independent risk factors of stage 4–5 CKD (Table 2). Post-LT AKI (P = 0.12) and post-LT RRT within 7 days (P = 0.09) were not associated with stage 4–5 CKD. Compared with males, females had significantly lower eGFR (females: 62.1 mL/min/1.73 m2 vs males: 79 mL/min/1.73 m2; P = 0.003) before LT, despite similar serum creatinine levels (females: 1.0 mg/dL vs males: 0.97 mg/dL; P = 0.22) and lab MELD scores (females: 18 vs males: 17; P = 0.21). This difference persisted at 1 and 6 months after LT and at the last follow-up. Since the diagnosis of NASH is the leading cause of LT among females,14 we tested the interaction between females and NASH. We fitted a separate model to see the effect of sex by diagnosis of NASH on post-LT stage 4–5 CKD. In this model, the interaction term (Sex*NASH) was not significant.
TABLE 2.
Independent predictors of stage 4–5 CKD
Since every unit increase in RRI score was associated with the 18% increased risk of post-LT stage 4–5 CKD, we examined the cumulative incidence of stage 4–5 CKD by dichotomizing the RRI decile at sixth decile. The incidence of stage 4–5 CKD was significantly higher in those with RRI ≥ sixth decile compared with those with RRI < sixth decile (Figure 4).
FIGURE 4.
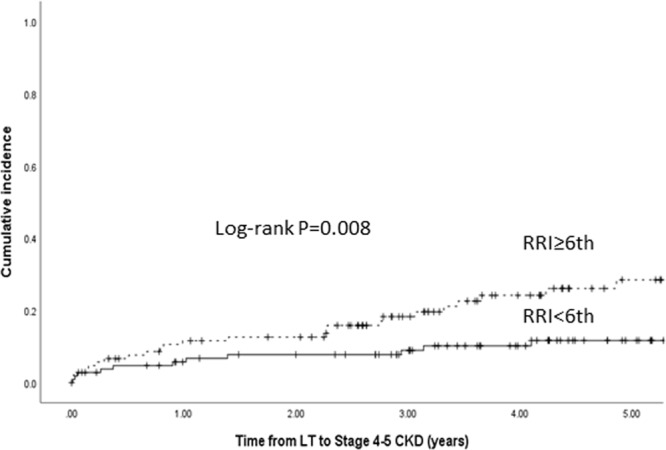
Cumulative incidence of post-LT stage 4–5 CKD stratified by RRI decile. CKD, chronic kidney disease; LT, liver transplantation; RRI, renal risk index.
LT Recipients With RRI ≥ Sixth Decile
This subgroup consisted of 106 (SI: 31; RSI: 75) LT recipients. Out of 106, 25 developed stage 4–5 CKD after a median follow-up of 4.1 years (IQR: 3.1–5.4). The unadjusted 1-, 3-, and 5-year cumulative incidence of stage 4–5 CKD was 12%, 18%, and 28% in this subgroup (Figure 4). There was a non-significant trend towards lower unadjusted 1-, 3-, and 5- year cumulative incidence of stage 4–5 CKD in SI compared with early RSI groups (SI: 6.6%, 6.6%, and 12.4% vs RSI: 13.7%, 21.3%, and 34%, respectively; P = 0.12).
Female sex (HR = 4.13 [95% CI, 1.86-9.2]; P<0.001) and RRI score (HR = 1.18 [95% CI, 1.02-1.36]; P = 0.023) were the independently associated risk factors of post-LT stage 4–5 CKD in this subgroup. This model was adjusted for immunosuppression status, pre-LT eGFR, post-LT AKI, and RRT within first 7 days of LT. None of these factors reached statistical significance.
Acute Rejection and Post-LT Survival
There was no significant difference in the overall acute rejection (22.5% vs 21.4%; P = 0.26) and acute rejection within 1 year (15% vs 18%; P = 0.55) in the SI and RSI group, respectively. There was no significant difference in the proportion acute rejection by sex, race, and etiology of liver disease (data not shown).
There was a total of 42 deaths and 9 re-LT during the median follow-up of 4.1 years (IQR: 3.3–6.6). The overall 1-, 3-, and 5-year patient survival for the cohort was 92%, 84%, and 79%, respectively. There were no significant differences in 1-, 3-, and 5-year survival stratified by sex, immunosuppression protocol (RSI vs SI), and RRI ≥ sixth decile.
DISCUSSION
Renal dysfunction, acute or chronic, is a major source of morbidity among LT recipients. In this cohort study, the adjusted cumulative probability of post-LT stage 4–5 CKD remained high among LT recipients with severe renal dysfunction at LT despite the use of early RSI. Female sex, diagnosis of NASH, and high RRI were the independent risk factors of new-onset post-LT stage 4–5 CKD in the SI as well as RSI group. The incidence of acute rejection and patient survival remained similar in both groups. Notably, a significant deterioration in renal function occurred during the first month of LT in the SI group despite the baseline median eGFR of 96.4 mL/min/1.73 m2 and use of low tacrolimus trough target of 6–10 ng/mL. This decline in eGFR was not observed in the early RSI group; however, this group started at a lower pretransplant baseline eGFR (60.2 mL/min/1.73 m2).
Since the cumulative incidence of post-LT stage 4–5 CKD was significantly higher among LT recipients with RRI ≥ sixth decile compared with those with RRI decile <6, we examined the factors associated with stage 4–5 CKD among those with RRI decile ≥6 (high-risk subgroup). Female sex and every unit increase in RRI score calculated at the time of LT were independently associated with high risk of post-LT stage 4–5 CKD, regardless of immunosuppression status. These results, although requiring validation in larger dataset, have implications in risk stratification based upon RRI decile and use of personalized immunosuppression especially in female sex to attenuate the risk of CKD progression. RRI is a simple, objective, and validated tool that could be used to identify LT recipients at varying risk of developing post-LT ESRD.
There is very little guidance on RSI for LT recipients with severe renal dysfunction going into transplant. The objective of implementing an early RSI protocol for select LT recipients (delayed introduction of tacrolimus along with IL-2 induction for patients with severe renal dysfunction/AKI within 24 h of LT) was to avoid further renal injury due to high levels of tacrolimus while giving renal function time to recover from post-LT AKI.7,15,16 Many studies have shown patients with NASH have high prevalence and incidence of CKD, and patients with NASH cirrhosis needing LT are significantly increasing.17-21 Our study validated that the NASH LT recipients are at a higher risk for CKD progression compared with non-NASH LT recipients. The higher risk of CKD progression associated with NASH in the posttransplant setting coupled with the increase in NASH patients undergoing LT will add to the rising burden of advanced CKD among LT recipients.
Fussner et al22 found an association between post-LT stage 3 CKD and female sex at 1 year after LT. In the same study, females with the diagnosis of NASH were associated with stage 3 CKD within 5 years of LT. Our study found that females had a 3-fold higher relative risk of stage 4–5 CKD compared with males. Since NASH is the leading indication of LT among females,14 we speculated that the increased incidence of post-LT stage 4–5 CKD might be driven by NASH among female LT recipients. The interaction between NASH and sex was not significant, suggesting that the sex effect was independent of diagnosis of NASH. Moreover, female sex was also one of the independent risk factors of stage 4–5 CKD in the high-risk subgroup (RRI ≥ sixth decile). This finding highlights the role of sex as a biological factor influencing the pathogenesis of post-LT stage 4–5 CKD.
The utilization of basiliximab increased significantly after the implementation of the early RSI protocol in our institution with greater than half of the cohort receiving early RSI. This increased utilization of basiliximab is associated with a significant increase in medication costs. Using the average wholesale price for basiliximab of $4301.22 per 20 mg dose,23 the cost for basiliximab induction is estimated to be $860 200.44 per 100 patients. We believe that our data provide guidance in proactive identification of LT candidates at the highest risk for CKD progression. This would enable risk-stratified administration of RSI and hence cost saving.
Many multicenter, prospective, randomized clinical trials that examined the safety and efficacy of immunosuppression regimen (eg, IL-2 induction and delay in CNI initiation or use of low dose CNI and mammalian target of rapamycin inhibitors) excluded LT patients who had preexisting renal dysfunction.7,8,24 The DIAMOND study examined the effect of different dosing regimens of prolonged-release tacrolimus using tacrolimus trough target of 5–15 ng/mL on renal function after LT and did not exclude patients with preexisting renal dysfunction.9 Our RSI protocol was similar to their delayed initiation arm except that we used immediate-release tacrolimus and a lower tacrolimus trough target of 6–10 ng/mL. The rejection rates were similar in both the groups in our study cohort. This finding suggests that early RSI is safe and efficacious. However, this group had high cumulative incidence of post-LT stage 4–5 CKD. This high incidence in the early RSI group is attributed to selection bias associated with the implementation of the early RSI protocol for those who were already had compromised renal function going into LT: patients with pre-LT renal insufficiency and post-LT AKI. However, none of these factors were statistically significant in the multivariate analysis. This could be because of small sample size.
The main limitation of our study is that it is a retrospective experience from a single center, which results in the potential for bias due to unmeasured patient selection and center-specific processes. In addition, the findings of this study may not apply at centers with a different patient population. Another limitation is that our study did not examine perioperative factors, such as hemodynamic instability, prolonged use of vasopressor, post-reperfusion syndrome, blood loss/blood transfusions, or colloids used for fluid replacement given the retrospective nature. However, this is an important study to show a real-world experience among LT recipients with severe renal dysfunction, a population usually excluded from most randomized controlled trials that examine the efficacy of early renal sparing strategies.
In conclusion, post-LT stage 4–5 CKD is common and delaying tacrolimus introduction combined with basiliximab induction may not prevent stage 4–5 CKD in LT recipients who are at high risk of developing post-LT stage 4–5 CKD. Further studies are needed to identify the role of personalized renal sparing strategies based upon sex in preserving renal function among LT recipients. Use of the RRI may offer additional value in designing individualized immunosuppression protocols aimed at renal protection among LT recipients.
Footnotes
Published online 08 August, 2019.
P.S., C.J.S., and J.M.P. participated in research design, performance of research, data analysis, writing, editing, and revision. Y.S., J.N., J.E., and J.S. participated in the performance of research, data collection, and writing of the manuscript. S.T. participated in performance of research, data analysis, writing, editing, and revision.
The authors declare no funding or conflicts of interest.
Previous Presentation: This study was presented in part at the annual AASLD meeting; November 9–13, 2018; San Francisco, CA.
REFERENCES
- 1.Sharma P, Bari K. Chronic kidney disease and related long-term complications after liver transplantation. Adv Chronic Kidney Dis. 2015;22:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojo AO. Renal disease in recipients of nonrenal solid organ transplantation. Semin Nephrol. 2007;27:498–507. [DOI] [PubMed] [Google Scholar]
- 3.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Schaubel DE, Guidinger MK, et al. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group USMFLS. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med 1994;331:1110–1115. [DOI] [PubMed] [Google Scholar]
- 6.Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18:3031–3041. [DOI] [PubMed] [Google Scholar]
- 7.Levitsky J, O’Leary JG, Asrani S, et al. Protecting the kidney in liver transplant recipients: practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant. 2016;16:2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuberger JM, Mamelok RD, Neuhaus P, et al. ; ReSpECT Study Group. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the ‘ReSpECT’ study. Am J Transplant. 2009;9:327–336. [DOI] [PubMed] [Google Scholar]
- 9.TruneČka P, Klempnauer J, Bechstein WO, et al. ; DIAMOND† Study Group. Renal function in de novo liver transplant recipients receiving different prolonged-release tacrolimus regimens-the DIAMOND study. Am J Transplant. 2015;15:1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P, Welch K, Eikstadt R, et al. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15:1142–1148. [DOI] [PubMed] [Google Scholar]
- 11.(KDOQI) TNKFKDOQI. Section 2: AKI definition. Kidney International Suppl. 2012;2:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.disease KCPGfteamock. Chapter 1: definition and classification of CKD. Kidney International 2012;3(Suppl 1):19–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Goodrich NP, Schaubel DE, et al. Patient-specific prediction of ESRD after liver transplantation. J Am Soc Nephrol. 2013;24:2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Perálvarez M, Germani G, Darius T, et al. Reducing early exposure to calcineurin inhibitors: the key factor for a successful renal sparing strategy. Am J Transplant. 2013;13:239. [DOI] [PubMed] [Google Scholar]
- 16.Textor SC, Burnett JC, Jr, Romero JC, et al. Urinary endothelin and renal vasoconstriction with cyclosporine or FK506 after liver transplantation. Kidney International. 1995;47:1426–1433. [DOI] [PubMed] [Google Scholar]
- 17.Sirota JC, McFann K, Targher G, et al. Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988–1994. Am J Nephrol. 2012;36:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Targher G, Bertolini L, Rodella S, et al. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5:2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444–450. [DOI] [PubMed] [Google Scholar]
- 20.Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624–633. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312:189–190. [DOI] [PubMed] [Google Scholar]
- 22.Fussner LA, Charlton MR, Heimbach JK, et al. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34:1259–1266. [DOI] [PubMed] [Google Scholar]
- 23.Micromedex I. IBM Watson Health, Greenwood Village,; Colorado, USA.: IBM Micromedex® RED BOOK® (electronic version). [Google Scholar]
- 24.De Simone P, Nevens F, De Carlis L, et al. ; H2304 Study Group. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]


