Abstract
Bladder cancer (BC), the most common malignancy of the urinary tract, accounts for 90–95% of all urothelial carcinomas (UCs), while upper urinary tract UC (UUTUC) accounts for only 5–10%. Radical nephroureterectomy with excision of bladder cuff, and radical cystectomy with pelvic lymph node dissection and a urinary diversion (UD) are the gold standard treatments for UUTUC and muscle-invasive bladder cancer (MIBC), respectively. These two treatments can be performed simultaneously when a bilateral or unilateral UUTUC is present with a MIBC, and are called complete urinary tract extirpation (CUTE) and hemi-CUTE, respectively. This complex surgery can help the patient by avoiding multi-staged surgeries, repeated anesthesia, and delay in completion of treatment. Herein, we report the first cases of a hemi-CUTE and CUTE in our department and share our experience with this aggressive and complex surgical treatment.
Keywords: Bladder cancer, nephroureterectomy, radical cystectomy, upper urinary tract, urinary tract extirpation, urothelial carcinoma
Introduction
Urothelial carcinoma (UC) is the fourth most common tumor among all malignancies.[1] Bladder cancer (BC), the most common malignancy of the urinary tract, is the 9th most common cancer worldwide and accounts for 90–95% of UCs.[2,3] However, upper urinary tract (UUT) UCs account for only 5–10% of UCs.[3] Recurrence of disease in the bladder occurs in 22–47% of UUTUC patients, whereas concurrent BC is present in 17% of the cases.[4]
Radical nephroureterectomy with excision of the bladder cuff and radical cystectomy (RC) with a urinary diversion (UD) are the gold standard treatments for UUTUC, and muscle-invasive and recurrent high-risk non–muscle-invasive BC, respectively.[5,6] The combination of these treatments is performed for a BC with a simultaneous bilateral or unilateral UUTUC, which is called complete urinary tract extirpation (CUTE) and hemi-CUTE, respectively. Dialysis-dependent end-stage renal disease (ESRD) and non-functioning kidney(s) are the other relative indications for (hemi-)CUTE. Herein, we present the first cases of hemi-CUTE and CUTE performed in our department for a male patient with simultaneous muscle-invasive BC (MIBC) and left renal pelvis tumor, and a male patient with MIBC and dialysis-dependent ESRD, respectively.
Case presentations
Case-1
A 66-year-old male patient admitted to our outpatient clinic with macroscopic hematuria that persisted for the last two months in March 2017. Suprapubic ultrasound revealed a papillary mass on the left lateral bladder wall, and contrast-enhanced abdominopelvic computed tomography (CT) confirmed the diagnosis of a 4×3 cm solid-papillary tumoral lesion on the left and another papillary tumor with a dimension of 2×1 cm on the right lateral walls of the bladder. Urinalysis showed 57 RBC/hpf and 3 WBC/hpf, where the results of other preoperative workup tests, including kidney function tests, were within normal limits. The patient underwent a transurethral resection of bladder tumor (TUR-BT), and the pathological evaluation showed a high-grade (HG) MIBC. Afterwards, he had a full evaluation for staging purposes, in which no signs of locally advanced or metastatic disease were identified. With the diagnosis of an organ-confined MIBC, he was offered to undergo a RC with a UD; however, he refused this operation. Therefore, a bladder-sparing treatment modality with cisplatin-based chemotherapy and definitive radiotherapy was planned in our multidisciplinary uro-oncology meeting.
The patient received six cycles of gemcitabine (1000 mg/m2) + cisplatin (70 mg/m2) chemotherapy and external radiotherapy (180 cGy × 28 fractions to pelvic region and additional 200cGy × 5 fractions to bladder; total dosage of 6040 cGy) without any major adverse events. During the evaluation of response to chemotherapy after the fourth cycle, left hydroureteronephrosis was identified and a nephrostomy tube was placed percutaneously. After completion of the chemo-radiotherapy scheme, he was consulted to the urology department because of hematuria coming from the nephrostomy tube. Magnetic resonance imaging–urography revealed a urothelial tumor extending from left renal pelvis to lower ureter (Figure 1). A second staging evaluation, including thoraco-abdominopelvic CT and whole body bone scintigraphy, did not show any metastasis, therefore, an extirpative surgery was offered to the patient according to the advice of our multidisciplinary uro-oncology team.
Figure 1.
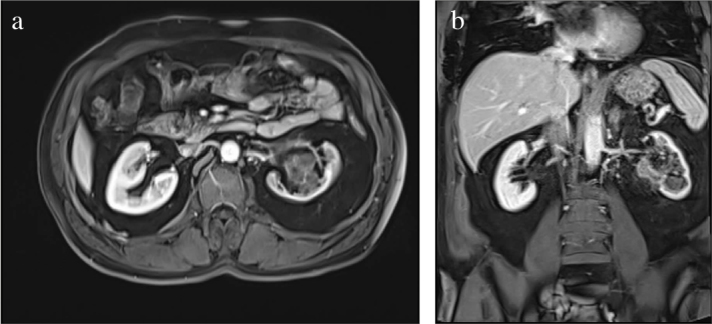
a, b. Magnetic resonance imaging scan showing the UUTUC in left kidney. (a) Axial section, (b) Coronal section
At this time, the patient agreed to undergo hemi-CUTE procedure, defined as left nephroureterectomy plus cystoprostatectomy plus urethrectomy (Figure 2). As a UD for the remaining functioning right kidney, a Bricker ileal conduit was created with isolation of an ileal segment of approximately 10–15 cm, located 20 cm proximal of the ileocecal valve; and an extended pelvic lymph node dissection (ePLND) was performed in May 2018. In order to decrease the morbidity of the operation, left transperitoneal laparoscopic nephrectomy was performed, while cystoprostatectomy plus ePLND part of the operation was completed with open surgery via a subumbilical median incision, and urethrectomy was performed via a prepubic approach.[7] The total operative time was 440 min and the estimated blood loss (EBL) was 1300 mL, which was replaced with intraoperatively delivered 3 units of erythrocyte suspension (ES) and two more units after the operation (Grade 2 according to the modified Clavien-Dindo classification [CDC]).
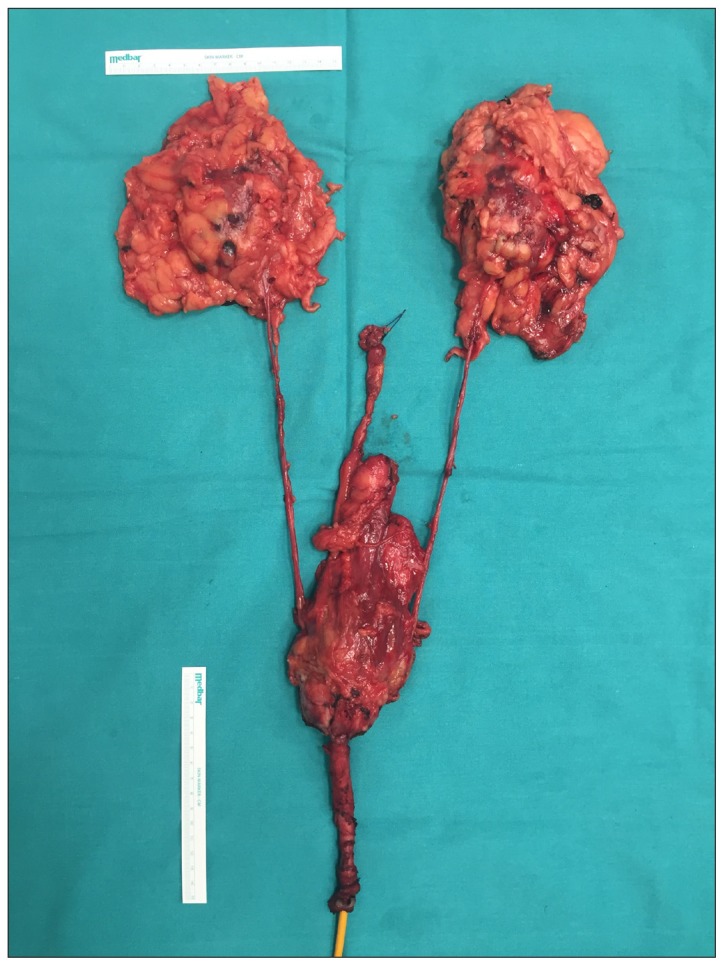
Figure 2.
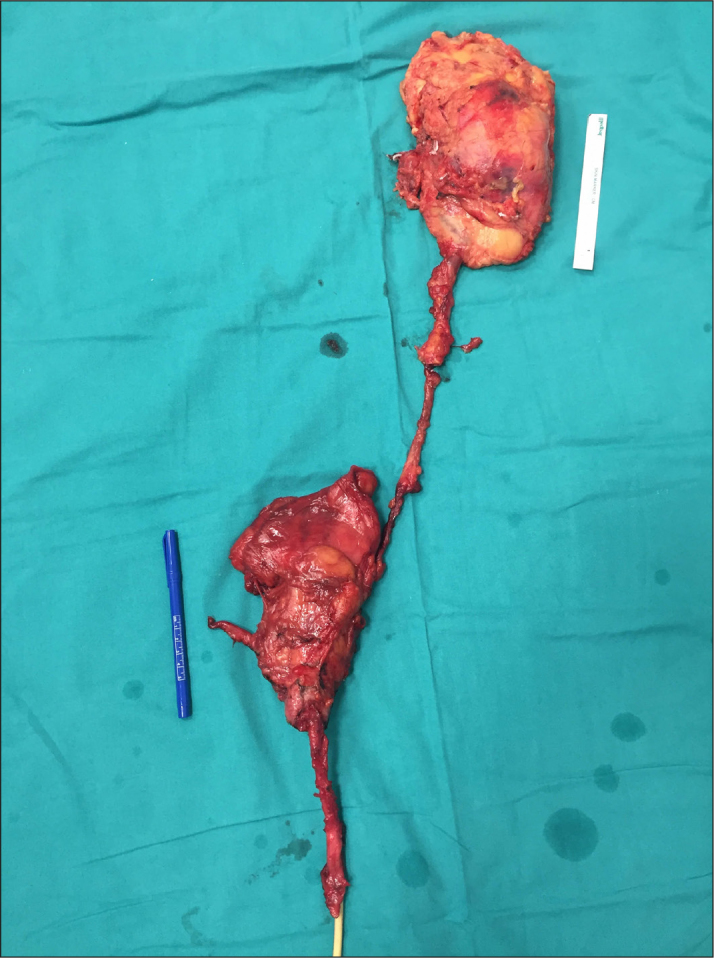
Image of the hemi-CUTE specimen
The pathological evaluation of the surgical specimens revealed a HG, renal parenchyma-infiltrating UUTUC that was filling the renal pelvis and upper two-thirds of the ureter without an extra-ureteric extension, two HG deep muscle layer-infiltrating tumors on the bladder dome and neck (2.3 cm and 2.5 cm, respectively), and a prostate adenocarcinoma (less than 1% of the whole prostate tissue) with a Gleason score of 3+3=6 (Figure 3). No tumor was observed in the dissected pelvic lymph nodes, the urethra and the surgical margins; however, a metastatic lymph node was found on the hilum of the kidney. The final pathological stages were T3N1M0 for UUTUC, T2bN0M0 for BC, and T2N0M0 for prostate cancer, according to the Tumor-Node-Metastasis (TNM) staging. As the UUTUC was identified during definitive chemo-radiotherapy, adjuvant chemotherapy was not advised to the patient. He has been followed up uneventfully for five months.
Figure 3.
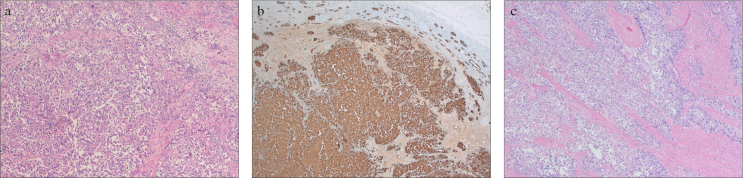
a–c. Histopathological evaluation of the hemi-CUTE specimen. (a) Infiltrating urothelial carcinoma of the kidney. The tumor is composed of invasive malignant urothelial cell groups (hematoxylin & eosin, 100X). (b) Infiltrating urothelial carcinoma of the kidney. In immunohistochemistry, the tumor cells were positive for CK7 (CK7, ×50). (c) Infiltrating urothelial carcinoma of the urinary bladder. The tumor is composed of high-grade urothelial cell groups arranged in a papillary pattern, which infiltrated the muscular layer of the bladder (hematoxylin & eosin, 100X)
Case-2
A 65-year-old man presenting with macroscopic hematuria had undergone a TUR-BT in May 2016 with an initial diagnosis of bladder tumor, and the pathological examination revealed a T1 HG tumor without muscle layer in the specimen. Two months later he had undergone a second TUR-BT, in which a T2 HG tumor was identified. He refused to undergo a RC because of his comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease, ESRD [hemodialysis 3 times a week], left foot amputation due to diabetic foot and osteomyelitis); therefore, bladder-sparing modality with a re-TUR-BT plus chemotherapy (gemcitabine [1250 mg] + cisplatin [75 mg]) but without radiotherapy (as he refused) was performed. After completion of the chemotherapy in April 2017, he did not come to his regular follow-up visits. During his admission to our department in July 2018 with a macroscopic hematuria, a solid-papillary tumor was found covering the whole bladder dome (Figure 4). The pathology showed a T2 HG tumor, and the patient was offered an extirpative surgery. With no local or distant metastases detected during the staging evaluation, although he was in American Society of Anesthesiologists (ASA) Class 3 risk group based on preoperative workup, he accepted to undergo a CUTE operation, defined as bilateral nephroureterectomy + RC + urethrectomy + ePLND without a UD for this patient (Figure 5). The total operative time was 280 min and the EBL was 400 mL, and ES transfusion was not required.
Figure 4.
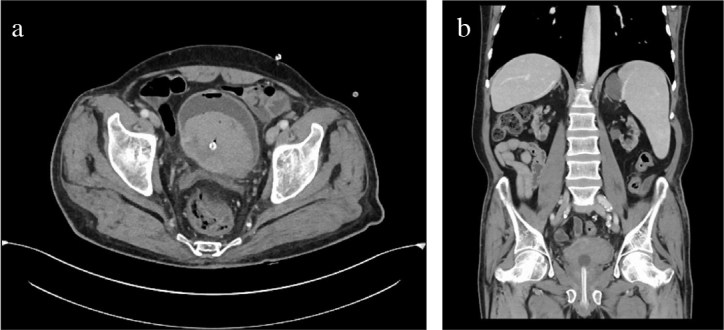
a, b. Computed tomography image showing the tumor in bladder dome. (a) Axial section. (b) Coronal section; bilateral atrophic kidneys with numerous cysts suggestive of end-stage renal disease are shown
Figure 5.
Image of the CUTE specimen
The pathological evaluation of the operation specimen revealed a perivesical fat layer-infiltrating tumor measuring 5.5×5.5 cm, and arising from the bladder trigone, chronic pyelonephritis and numerous renal cysts (Figure 6). No tumor was observed in the dissected pelvic lymph nodes, the urethra and the surgical margins. The final pathological stage was T3aN0M0 according to the TNM staging. Although the patient was fine on the first few days after the operation, he died due to a massive pulmonary embolism on the postoperative fifth day.
Figure 6.
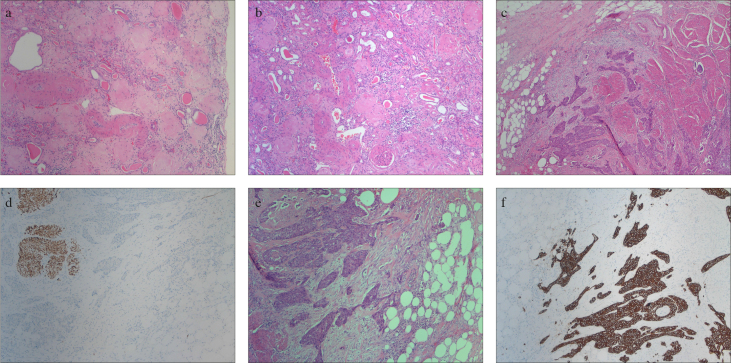
a–f. Histopathological evaluation of the CUTE specimen. (a) Glomerulosclerosis, right kidney (hematoxylin & eosin 100X). (b) Glomerulosclerosis, left kidney (hematoxylin & eosin, 100X). (c) Infiltrating urothelial carcinoma of the urinary bladder (hematoxylin & eosin, 50X). (d) Tumor-muscle interaction (desmin, 100X). (e) Tumor-muscle interaction (hematoxylin & eosin, 100X). (f) Tumor-muscle interaction (pancytokeratin, 100X)
Discussion
Due to frequent recurrences and rapidly progressive course of UC, an aggressive treatment with a strict follow-up is recommended not only for prolonging the survival, but also for improving the quality of life of the patients with UC. CUTE is advantageous as it avoids multi-staged surgeries and recurrent application of anesthesia. Besides it does not cause a delay in completion of treatment, lowers the risk of intra-and post-operative complications, and decreases use of (narcotic) analgesics.[8,9] In their series of 30 UC patients who were receiving hemodialysis for ESRD, Wu et al. concluded that one-stage CUTE would be the choice of treatment for this specific patient group, which has higher rates of multifocality and recurrences, increased anesthesia risk, susceptibility of fluid overload, poor cardiovascular system, coagulopathy diathesis, unsuitability for use of contrast agents in imaging studies, and decreased tolerance to chemotherapy and/or radiotherapy, by avoiding repeated surgeries and anesthetic procedures.[8] Various authors have reported that this complex and invasive surgery could be performed by open, laparoscopic or robotic surgery with acceptable morbidity and mortality rates.[9–11]
Previously indications of CUTE have been defined to treat patients with BC and bilateral UUTUC. Papers from some experienced centers have also reported the results of CUTE performed for ESRD patients.[8–15] As the complication rates were expected to be high in uremic patients, physicians were often reluctant to perform this invasive surgery. This complex surgery has recently begun to be performed more often, and most of the centers present results similar to each other.[9–15] Ou and Yang[14] have published their CUTE series consisting of 5 male and 5 female ESRD patients with indicated range of operative times (176–458 min), EBL (300–2500 mL), hospital stay (9–20 days)s and a complication rate of 10%. Chen et al.[15] presented one of the largest series with 14 female ESRD patients who had undergone mini-incision CUTE. They reported a median operative time of 242.5 min, a median hospital stay of 11 days and a complication rate of 21.4%. Ou and Yang[16] recently published their updated CUTE series with 42 ESRD patients (16 males, 26 females) with a mean age of 58.2 years. The mean EBL was 1370 mL and the mean duration of hospitalization was 26.1 days, while the complication rate was reported as 47.6%. Huang et al.[17] evaluated complication rates in the largest CUTE series on 81 Taiwanese UC patients with ESRD. They found major complication rate as 36.4%, while 65.4% of the cohort had no major complications. The patient subgroup with major complication rates were those with younger age, lower Charlson Comorbidity Index (CCI) scores, higher preoperative serum albumin level, and shorter hospital stay. In multivariate logistic regression analysis, CCI≥5 and surgery performed by a low-volume surgeon (≤3/years) were found to be independent predictors for major complications.[17] Our results for the two cases were in accordance with previously published series.
To the best of our knowledge, these cases are the first case reports for hemi-CUTE and CUTE performed in our institution, in our region and as well as in our country. Our initial experience demonstrates that, with transfer of the technique and experience after a dedicated uro-oncology fellowship in a tertiary center with high case load, this complex surgery can be performed safely and efficiently in well-selected and well-informed patients.
Acknowledgments
We would like to thank Prof. Dr. Steven Joniau and Prof. Dr. Hein Van Poppel for teaching the technique and transfer of their experience to our department.
Footnotes
Informed Consent: Written informed consents were obtained from two patients who participated in this case report.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.A.; Design – M.A., Ö.K.; Supervision – M.A.; Data Collection and/or Processing – M.A., Ö.K., İ.H., P.K., Ç.Y., Ö.A.; Analysis and/or Interpretation – M.A., Ö.K., İ.H., P.K., Ç.Y., Ö.A.; Literature Search – M.A., Ö.K.; Writing Manuscript – M.A.; Critical Review – M.A., Ö.K., İ.H., P.K., Ç.Y., Ö.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Consentino M, Palou J, Gaya JM, Breda A, Rodriguez-Faba O, Villavicencio-Mavrich H. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol. 2013;31:141–5. doi: 10.1007/s00345-012-0877-2. [DOI] [PubMed] [Google Scholar]
- 5.Margulis V, Shariat SF, Martin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. 2009;115:1224–33. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Consensus Conference in Bladder Cancer. Hautmann RE, Abol-Enein H, Hafez K, Haro I, Mansson W, Mills RD, et al. Urinary diversion. Urology. 2007;69(Suppl 1):17–49. doi: 10.1016/j.urology.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 7.Van Poppel H, Joniau S, Bogaert G. Preventive prepubic urethrectomy. Aktuelle Urol. 2010;41:137–44. doi: 10.1055/s-0029-1233502. [DOI] [PubMed] [Google Scholar]
- 8.Wu CF, Shee JJ, Ho DR, Chen WC, Chen CS. Different treatment strategies for end stage renal disease in patients with transitional cell carcinoma. J Urol. 2004;171:126–9. doi: 10.1097/01.ju.0000101758.41635.28. [DOI] [PubMed] [Google Scholar]
- 9.Holton MR, Van Zijl PS, Oberle WT, Jacobs SC, Sklar GN. Complete urinary tract extirpation: the University of Maryland experience. Urology. 2006;68:65–9. doi: 10.1016/j.urology.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 10.Barros R, Frota R, Stein RJ, Turna B, Gill IS, Desai MM. Simultaneous laparoscopic nephroureterectomy and cystectomy: a preliminary report. Int Braz J Urol. 2008;34:413–21. doi: 10.1590/S1677-55382008000400003. [DOI] [PubMed] [Google Scholar]
- 11.Ou YC, Yang CR, Yang CK, Cheng CL, Hemal AK. Simultaneous robot-assisted nephroureterectomy and cystectomy in patients with uremia and multifocal urothelial carcinoma. J Endourol. 2011;25:979–84. doi: 10.1089/end.2010.0602. [DOI] [PubMed] [Google Scholar]
- 12.Li CC, Wang HS, Wu WJ, Chou YH, Liu CC, Long CY, et al. Laparoscopic complete urinary tract exenteration with the specimen withdrawn transvaginally. BJU Int. 2009;104:82–6. doi: 10.1111/j.1464-410X.2008.08339.x. [DOI] [PubMed] [Google Scholar]
- 13.Lin VCH, Hung KC, Chen MJ, Lu K, Chen Y, Weng HC, et al. Single-session laparoscopic total urinary tract exenteration without repositioning for multifocal urothelial carcinoma in dialysis-dependent patients. Urology. 2011;77:98–103. doi: 10.1016/j.urology.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Ou CH, Yang WH. Complete urinary tract exenteration for a dialysis patient with urothelial cancer: lower midline and extraperitoneal approach in a supine position. Urology. 2011;77:1244–7. doi: 10.1016/j.urology.2010.07.499. [DOI] [PubMed] [Google Scholar]
- 15.Chen IH, Lin JT, Tsai JY, Wu T, Yu CC. A modified single mini-incision complete urinary tract exenteration for urothelial carcinoma in dialysis patients. Biomed Res Int. 2014 doi: 10.1155/2014/649642. 649642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou CH, Yang WH. Long-term outcomes of complete urinary tract exenteration for dialysis patients with urothelial cancer. Int Urol Nephrol. 2017;49:623–7. doi: 10.1007/s11255-017-1522-1. [DOI] [PubMed] [Google Scholar]
- 17.Huang YC, Chang YH, Shindel AW, Chang YL, Lin JH, Ho DR, et al. Perioperative complications and mortality in patients with urothelial carcinoma and end-stage renal disease undergoing one-stage complete urinary tract extirpation. Ann Surg Oncol. 2018;25:573–81. doi: 10.1245/s10434-017-6251-2. [DOI] [PubMed] [Google Scholar]