Exceptional fossils reveal annelid nervous system evolution.
Abstract
Annelid worms are a disparate, primitively segmented clade of bilaterians that first appear during the early Cambrian Period. Reconstructing their early evolution is complicated by the extreme morphological diversity in early diverging lineages, rapid diversification, and sparse fossil record. Canadia spinosa, a Burgess Shale fossil polychaete, is redescribed as having palps with feeding grooves, a dorsal median antenna and biramous parapodia associated with the head and flanking a ventral mouth. Carbonaceously preserved features are identified as a terminal brain, circumoral connectives, a midventral ganglionated nerve cord and prominent parapodial nerves. Phylogenetic analysis recovers neuroanatomically simple extant taxa as the sister group of other annelids, but the phylogenetic position of Canadia suggests that the annelid ancestor was reasonably complex neuroanatomically and that reduction of the nervous system occurred several times independently in the subsequent 500 million years of annelid evolution.
INTRODUCTION
Features of nervous systems, including brains, nerve cords and appendage, and sense organ innervation, have been described from a number of taxa from Burgess Shale–type fossil Lagerstätten, primarily from arthropods [e.g., (1, 2)]. These fossils have provided critical comparative data regarding the purported plesiomorphic state of aspects of the nervous system, character evolution, and the homology of appendages, revealing aspects of arthropod nervous system evolution that would be untraceable using data from extant taxa alone (1, 2). The fossilized nervous tissues described thus far in Cambrian Burgess Shale–type deposits are preserved as carbonaceous compressions [e.g., (3)] allowing visualization using scanning electron microscopy (SEM) backscatter imaging and elemental mapping (3, 4).
However, the interpretation of these features is not without controversy, and they have alternatively been identified as decayed remains of internal features (5) or as the result of differential shrinkage of internal features relative to the cuticle during decay (6), particularly in ecdysozoans. A major caveat of this view is that decay resistance is an imperfect predictor of preservation potential and that features preserved in fossil specimens do not always mirror decay stages of living analogs (7). Currently, the precise preservation pathway of nervous tissue is poorly understood, although some studies implicate the enrichment of lipids in nervous tissue (2, 3), which are more stable during diagenesis than other abundant macromolecules such as proteins (8).
We describe carbonaceously preserved internal anatomical features from Canadia spinosa, a fossil annelid from the Burgess Shale (9–11) that we identify as remnants of the cephalic, axial, and appendicular nervous system. These features are bilaterally symmetrical and share common morphologies and topological relationships with external anatomical features across numerous specimens. Our findings suggest that nervous system preservation may be widespread in fossil bilaterians, having been positively identified in ecdysozoans, chordates, and now a lophotrochozoan. Phylogenetic analyses incorporating new nervous system data (12, 13) and recent fossil discoveries (14, 15) resolve Canadia in the annelid stem group (9, 10), and we infer that the annelid ancestor had a reasonably complex nervous system that has been reduced numerous times independently during transitions in lifestyle and/or extensive modifications of the annelid body plan.
RESULTS
C. spinosa has circa 23 chaetigers, each with prominent biramous parapodia and fascicles of flattened chaetae (Fig. 1, A and B). The notochaetae are longer and broader than the neurochaetae, have serrated tips and form a near continuous dorsolateral covering (Figs. 1, A and B, and 3A) (11). The distal portions of the neurochaetae are curved ventrally, shown in specimens in lateral view (Fig. 1B). The head bears flexible, contractile (10), paired appendages that are palps (e.g., Fig. 1 and fig. S2) (10, 16). Canadia specimens lack evidence of any type of eyes. The gut is rarely preserved but is straight along its length (fig. S4S) with an eversible proboscis (Fig. 3A) and occupies approximately one-third of the body width when visible (fig. S4S). The presence of a median antenna, similar to that of Kootenayscolex (15), is now recognized in four specimens (Fig. 1, E to G, and fig. S1, B, C, and E). The median antenna is approximately the length of the first chaetiger and ca. 40% the width of the adjacent palps (Fig. 1, E to G) and attaches dorsally at the terminus of the head. The mouth is ventral, triangular in outline (Figs. 1D and 2A), and flanked by a pair of parapodia with chaetae (Figs. 1D and 2, J and K). The dorsal chaetal fascicle had previously been considered dorsal (10, 11) or ventral (15, 16). New specimens preserved in lateral view confirm that the broader paleae are dorsal in Canadia and that the newly found smaller fascicle is ventral (Fig. 1D), consistent with the chaetation and ramus morphology of the chaetigers along the remainder of the trunk.
Fig. 1. General anatomy of C. spinosa.
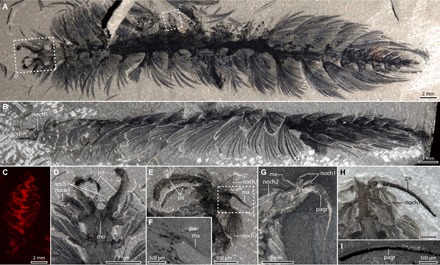
(A) USNM83929C, counterpart in dorsal view, polarized light, specimen underwater. (B) USNM277933, lateral view, note ventrally curved tips of notochaetae, polarized light, dry. (C) Elemental map for carbon of gill from USNM83929C part, position of equivalent gill of counterpart shown in (A). (D) Close-up view of head of USNM83929C, and anterior appendages, polarized light, dry. (E) Close-up view of anterior region of USNM83929D, polarized light, dry. (F) SEM backscatter image of median antenna of USNM83929D [area outlined in (E)]. (G) ROMIP65172.1 showing median antenna, reflected light, specimen under alcohol. (H and I) ROMIP65173, dorsal view. (H) Close-up view of palp and thick carbon band in palp, polarized light, specimen under ethanol. (I) SEM backscatter image showing carbon in the palp. Dashed boxes indicate positions of close up images. ma, median antenna; mo, mouth; nech, neurochaetae; noch, notochaetae; pa, palps; pagr, palp groove.
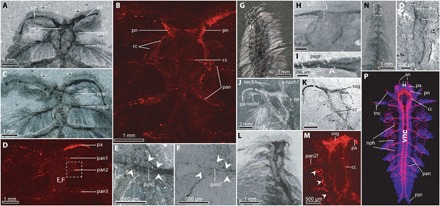
Fig. 3. Preservation of nervous tissue in C. spinosa in specimens preserved in ventral view.
(A to H) ROMIP56972, specimen in ventral view. (A) Complete specimen, reflected light, specimen underwater. (B) Anterior region, SEM-EDX elemental map for carbon. (C) SEM backscatter image showing ventral and parapodial nerves. (D) SEM backscatter image showing palp and right circumoral connective. (E) SEM backscatter image showing palp nerve and left circumoral connective. (F) Posterior segments, photographed in reflected light underwater. (G) SEM EDX map for carbon of region shown in (F). (H) SEM backscatter of boxed region in (G), arrowheads demark the outline of the gut superimposed on the ventral nerve cord. (I to K) GSC8232A, specimen in ventral view. (J) SEM backscatter showing ventral nerves and ventral nerve cord of boxed region in (I). (K) Close-up view of parapodial nerve and parapodial outlines preserved carbonaceously, arrowheads indicate the body margin. cc, circumoral connective; ga, ganglion; pa, palp; pan, parapodial nerve; pn, palp nerve; sn, segmental nerve; vnc, ventral nerve cord.
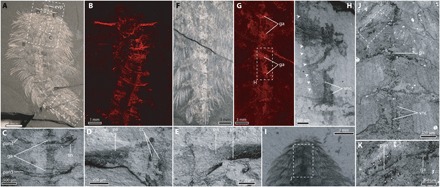
Fig. 2. Anterior nervous system of C. spinosa.
(A and B) USNM83929B, part in dorsal view. (A) Anterior region, polarized light, specimen under alcohol. (B) SEM-EDX map for carbon. (C) USNM83929B, counterpart, anterior region, cross-polarized light, specimen under alcohol. (D) USNM83929B, counterpart, SEM-EDX map for carbon. (E and F) Close-up view of region shown in box with dashed outline shown in (D). White arrowheads indicate carbonaceous preservation of the parapodial margin. (E) Cross-polarized light under ethanol. (F) SEM backscatter. (G to I) USNM275517, specimen in dorsal view. (G) Anterior segments, reflected light underwater. (H) Close-up view of head, SEM backscatter. (I) Close-up view of palp showing feeding groove/innervation. (J and K) Close-up view of anterior region of ROMIP65174, specimen in dorsal view. (J) Reflected light under ethanol. (K) SEM backscatter. (L and M) ROMIP65175, part, specimen in dorsal view. (L) Anterior region, polarized light, photographed under ethanol. (M) Close-up view of head, SEM-EDX map for carbon. (N and O) ROMIP65176, juvenile in dorsal view. (N) Anterior segments, cross-polarized light under ethanol. (O) Close-up view of head and three anterior segments, SEM backscatter [area outlined in (N)]. (P) Confocal microscope image of Neanthes arenaceodentata (Aciculata) acetylated α-tubulin immunoreactivity is red, and cell nuclei are blue, refigured and modified from (50). Dashed boxes indicate positions of close-up images. an, antenna; cc, circumoral connective; cs, commissure; mo, mouth; nech, neurochaetae; noch, notochaetae; nop, notopodium (numbered by chaetiger); nph, nephridia; pa, palp; pan, parapodial nerve (numbered by chaetiger); pn, palp nerve; pyc, pygidial cirrus; sog, supraesophogeal ganglion; tnc, tentacular cirrus.
Branchiae (Fig. 1, A and C, and fig. S3), which are not known in other Cambrian polychaetes, occupy the space between adjacent segments (fig. S3, B to D) but were previously reconstructed as attaching between the rami (11). The branchiae occur from chaetiger four onward, are largest in the midbody, are conical in shape, and consist of a central axis with paired lobes with an offset symmetry.
Canadia was previously considered nektonic or nektobenthic (11); however, an epibenthic lifestyle is more likely based on morphological and taphonomic grounds. The relative stiffness and fusiform shape of the body are poorly adapted for undulating swimming based on comparison with extant annelids (11), and the chaetal morphology is similar to that of certain epibenthic aciculates, such as amphinomids or aphroditaceans (17), where the notochaetae are protective and the neurochaetae are used for crawling. The preservation of mostly complete [or slightly disarticulated in situ (e.g., Fig 1A)] specimens implies rapid burial near the sediment-water interface, limited transport (18), and a lifestyle proximal to the benthos.
Carbonaceous preservation and nervous system
Carbonaceous remains identified inside the body are visualized as dark structures in SEM backscatter and in brighter colors in energy-dispersive x-ray (EDX) elemental maps for carbon and also appear dark with cross-polarized light (Figs. 2 and 3 and figs. S4 to S6). We identify some of these carbonaceously preserved features as traces of the nervous system in both dorsal (Fig. 2) and ventral (Fig. 3) views.
Approximately half of the palp width is occupied by a thick carbon band [Figs. 1 (H and I) and 2 (B and D) and fig. S2 (A to G)], and similar structures occur in Kootenayscolex (fig. S2, H to K) (15). These carbon films are preserved along the margins of the palps or switch from a median to a marginal position in twisted palps (fig. S2), suggesting a feature that has both an internal and an external expression. This feature conforms in dimensions and position to the ciliated groove of feeding palps of sedentary polychaetes, suggesting a feeding, in addition to sensory (10, 16), function. The carbon film within the palps likely includes other tissues, such as nerves, as palps are prominently innervated (19). This carbon film is continuous with discrete carbon films around the oral region. These structures conform in morphology and topological relationships to other features to the circumoral connectives (Fig. 2, A, B, K, M, and O) with a carbonaceous mass consistent with a supraesophageal ganglion at its anterior (Fig. 2, K to O). The circumoral nerves join midventrally at the third chaetiger (Fig. 2, A, B, and J to M). Nerves branch from the circumoral connectives into the palps (Figs. 2, A to D and J to M, and 3, B and D) and parapodia (Fig. 2, A to D). A complete transverse commissure across the left and right connectives at chaetiger two is likely present (Fig. 2B).
The body cuticle contains only small amounts of carbon that are generally not detectable in our elemental maps, except along body outlines and at the margin of certain structures, such as some parapodia (Figs. 2, E and F, and 3K) where the thickness of the cuticle has been enhanced by compaction. In all cases, however, the structures identified as parapodial nerves are central and distinct from the margins of the parapodia and also extend toward the ventral midline, beyond the limits of the parapodia and their margins (e.g., Figs. 2, E and F, and 3, J and K).
The axial nervous system of posterior segments (along with associated ventral peripheral nerves) is preserved in two specimens (Fig. 3). Preservation of ventral features is rare, consistent with preferential splitting of specimens along the imbricated notopodial paleae that form a plane of weakness. Ventral specimens reveal a prominent nerve innervating the parapodia (as in the anterior region in dorsal view) and at least one other segmental nerve (Fig. 3C), but more may have been present, given the small diameter of some of these nerves among extant taxa (20). The parapodial nerves cross the ventral midline (Fig. 3, B, C, and J), suggesting that these nerves form a continuous commissure across the ventral cords and innervating the parapodia. The parapodial nerves are accompanied by swellings in the ventral nerve cord (Fig. 3, G and J), which appear brighter in carbon maps (Fig. 3G). These features are situated between the parapodia in both the anterior (Fig. 3, C and J) and posterior (Fig. 3G) regions and are preserved continuously with carbon films that insert into the parapodia themselves (Fig. 3, C and J). Although the preservation of the ventral nerve cord is often diffuse, with carbon more concentrated in peripheral nerves and ganglia [similar to that observed in Chengjiangocaris (2)], the nerve cord appears paired (rather than a single medullary bundle) in well-preserved regions (e.g., Fig. 3, H and J). The gut margins are sometimes preserved along with the ventral nerve cord (Fig. 3H). When well preserved (e.g., fig. S4S), the gut forms a straight tube, wholly unlike the branched morphology visible in the ventral nerve cord.
Bayesian phylogenetic analyses (fig. S7) recover C. spinosa in the annelid stem group, both with and without a topological constraint (see the Supplementary Materials for details). We recover palaeoannelids (21) as the sister groups of all other annelids in the unconstrained analyses but cannot distinguish between palaeoannelid monophyly and paraphyly, both of which have been recovered in recent phylogenomic analyses (13). Our analysis without a topological constraint recovers Sipuncula as the annelid sister lineage, as in previous morphological analyses (9) but in conflict with most molecular analyses (21). Chaetopteriformia (a clade of Apistobranchus, Chaetopteridae, and Psammodrilidae) is monophyletic, and these taxa are united in having a type of heteronomous segmentation with a differentiated anterior region where the notopodia are cirriform and supported by internal chaetae (13, 17). Both analyses recover most of the Cambrian fossil polychaetes as a paraphyletic grade that subtends the annelid crown node (i.e., as stem group annelids). The recently described Ipoliknus is recovered as the deepest branch in the annelid stem group in the constrained analysis (fig. S7A) but is in a polytomy in the unconstrained analysis (fig. S7B).
DISCUSSION
Taphonomy and preservation
In specimens from the Burgess Shale, so-called dark stains are often associated with the fossils, extending some distance from the specimen with a typically diffused aspect. These stains are thought to represent decay fluids that are often concentrated around the mouth or anus (7) but can also extend along various parts of the body outline (22). These dark stains appear similar to carbon films when fossils are visualized using cross-polarized light but do not contain detectable amounts of carbon using elemental maps [Fig. 1B, fig. S6, and map of Marrella splendens in (7)]. Specimens of Canadia preserve both types of dark stain in the same specimen (e.g., USNM83929B; Fig. 1, A and B), indicating that the carbon films and decay-derived dark stains have different taphonomic histories. In Fuxianhuia, dark regions associated with the head have recently been reinterpreted as preservation of biofilms (5) without any accompanying compositional data. Therefore, it is unknown whether the suite of morphologies observed in this recent study (5) share the same taphonomic pathway as structures interpreted as fossil brains (which are, at least in part, carbonaceously preserved) in Fuxianhuia (3).
The carbonaceous preservation that we observe is highly selective and is restricted to particular tissues and morphological features, which are mainly internal. Although chaetae are the elements of the body that preserve more readily, even after decay and complete dissociation of the body (18), carbon films associated with chaetae are rarely observed using SEM imaging (including elemental maps). When they are present, they preserve microscopic ultrastructural details such as the impressions of microvilli (fig. S1D). The fragmentary nature of these films (e.g., fig. S1D) suggests that the carbon films were thin, fragile, and may have become lost during splitting.
Evolutionary significance
Along with other exceptionally preserved Palaeozoic taxa, Canadia has featured heavily in discussions of the early evolution of Trochozoa (10, 16, 23, 24), and the annelids of the Burgess Shale have long been recognized as an important source of evidence regarding the morphology of the annelid ancestor (9, 16, 25). Our new reconstruction of Canadia (Fig. 4A, fig. S8, and movie S1) refines details of gross morphology, including the location of the gills, the presence of a median antenna, and the morphology of the head. Evolutionary insights from annelid fossils have thus far been limited to external characters, but discovery of well-preserved nervous tissue in a Cambrian polychaete supplements prior discoveries of exceptionally preserved nervous tissues in Burgess Shale–type fossils (1, 2, 15) and allows us to augment recent investigations into extant annelid nervous systems (13) with evidence from the fossil record.
Fig. 4. Reconstruction and schematic reconstructions of annelid nervous systems plotted on a simplified phylogeny.
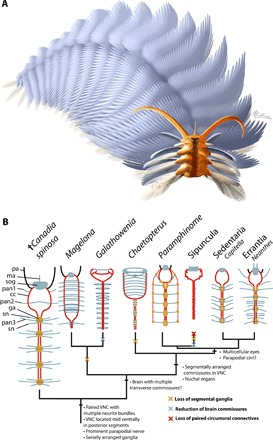
(A) Reconstruction by M. Collins © Royal Ontario Museum, all rights reserved (see also movie S1). (B) Simplified phylogeny of Annelida with C. spinosa recovered in the annelid stem group (see also the Supplementary Materials for the full phylogenetic results). Topology is based on the analysis using phylogenetic constraints based on recent phylogenomic trees. Proposed nervous system synapomorphies are shown at nodes, and inferred losses are shown along branches as cross marks. cc, circumoral connectives; ga, segmental ganglion; ma, median antenna; pan, parapodial nerve; pa, palp; sn, segmental nerve; sog, supraesophegeal ganglion; vnc, ventral nerve cord.
Despite being classically presented as a rope-ladder arrangement such as that of arthropods (13, 26), the annelid nervous system displays broad anatomical variations, from loop-like brains and single medullary nerve cords (26, 27) to differentiated, subdivided brains with ganglionated nerve cords with multiple neurite bundles (20, 26). The most complex nervous systems occur in aciculates (Amphinomida, Eunicida, and Phyllodocida), a clade (9, 28) or grade (29) that includes active and motile animals, many of which are carnivores and predators (30). The morphology of the nervous system of the last common ancestor of annelids has been difficult to reconstruct based on uncertainties in relationships, divergent nervous system morphologies in extant annelid subgroups, and close correlations between nervous system morphology and mode of life (20, 27, 31). Phylogenomic trees suggest that the sessile (30) Oweniidae and Magelonidae [Palaeoannelida (21)] form the sister group of all other annelid lineages (32) and notably have relatively simple nervous systems, lacking features such as segmental ganglia (13, 27). A recent investigation of the oweniid nervous system demonstrated that the presence of only a loop-like brain [first shown in Galathowenia (27)] is widespread in the group (12). On this basis, a tube dwelling annelid ancestor with a structurally simple nervous system has been proposed (12).
C. spinosa occupies a critical position for inferring the sequence of character transformations that led to the body plan of crown group annelids (9, 10, 25). The terminal position of the brain confirms that the head of Cambrian taxa is poorly differentiated from the trunk, as has been proposed previously (15, 16). There is no anterior prostomial lobe, with the body terminating with the brain and mouth region, with the prostomium consisting only of the brain and palps. This is the likely condition in taxa with similar head morphologies, such as Kootenayscolex and Burgessochaeta (15). The anteriormost parapodia are innervated from the circumoral nerve ring, posterior to the palps and brain, which is consistent with an interpretation that the peristomium is chaetigerous (15). Alternatively, this is concordant with a condition where the peristomium is limited to the lips surrounding the mouth [potentially the plesiomorphic condition for annelids (29)] and is not visible dorsally and where segments crowd the head. Kootenayscolex likewise preserves carbonaceous features in the head that are concordant with nervous tissue (15), showing a similar overall topology to the tissue preserved in Canadia, including the presence of a supraesophageal ganglion and innervation of the palps.
Our ancestral state reconstruction recovers the highest posterior probability for a crown annelid ancestor with a moderately complex nervous and sensory system, regardless of use of the constrained or unconstrained topology and incorporating topological uncertainty (Fig. 4B and fig. S7). The annelid ancestor is inferred to have been equipped with a pair of prostomial palps and bicellular eyes and lacked nuchal organs. Although the innervation of nuchal organs is conspicuous when these structures are enlarged [e.g., Spionidae (19)], the ancestral nuchal organ likely consisted of only pits/grooves and therefore had a low fossilization potential. Evidence for fossilized nuchal organs has only ever been recognized in an amphinomid species, where there is an enlarged caruncle, which is preserved by pervasive phosphatization (33). The ancestral annelid brain had multiple transverse commissures [as in many polychaetes, except some taxa such as chaetopterids, terebelliforms (26), and adult oweniids (12)]. The ancestral nervous system consisted of a supraesophageal ganglion with transverse commissures, connected to the ventral nerve cord by paired connectives. The ventral nerve cord contained multiple neurite bundles, segmental ganglia, and enlarged parapodial nerves. While the complexity of this ancestral nervous system exceeds that of paleoannelidans, it is less developed than that of aciculates (Errantia and Amphinomidae), revealing divergent trends in nervous system evolution during the subsequent >500 million years of annelid evolution. This differs from previous reconstructions in the presence of serially repeated ganglia, which was previously uncertain (13), and the presence of a median nerve in the ventral nerve cord, which was previously considered part of the annelid “ground pattern” (31). Canadia does share some neuroataomical similarity with early diverging taxa however. For example, the circumoral connectives converge to form a midventral nerve cord in anterior segments, which also occurs in Oweniidae, Magelonidae, and Chaetopteridae (Fig. 4) (13).
In extant errant annelid taxa, the nervous system has been variously supplemented with nerves that innervate the parapodial, tentacular, and peristomial cirri, as well as prostomial antennae and multicellular eyes. These features are present in both Errantia and Amphinomidae, which morphological trees have grouped together as Aciculata (28), with aforementioned features regarded as homologs, but this clade is not currently supported by phylogenomic data (32). If these features do share a common origin, then they have subsequently been lost independently at least twice in Sedentaria and Sipuncula, the latter of which must also have reduced most of the hallmarks of the annelid nervous system and body plan more generally. Amphinomids are also notable for having unique nervous system features, such as prominent peripheral longitudinal nerve cords with additional ganglia (34).
In contrast, some infaunal, sessile, tube dwelling, and parasitic taxa have lost several plesiomorphic features, reducing the brain and sensory organs and appendages, losing segmental ganglia, and reducing the ventral nerve cord to a single neurite bundle. This includes taxa in Sedentaria, such as Terebelliformia, where the transverse brain commissures are absent and palps have been lost (26), and the tube dwelling Maldanidae, where segmental ganglia are absent and there is a single ventral neurite bundle (35). The most extreme cases of reduction would be found in Echiura [where the nervous system only shows metamery early in ontogeny (36)] and Sipuncula. If Sipunculans are ingroup (fig. S7A) (29, 32) rather than outgroup (fig. S7B) (9, 25) taxa, then sipunculans have lost brain commissures, segmental ganglia, paired ventral nerve cords, paired circumoral connectives, and segmental nerves. Such a reduction in the segmental arrangement of the nervous system is indicated by studies of the developing nervous system (37) in some sipunculan genera. Multicellular eyes are present in Amphinomidae, Errantia, and some groups in Sedentaria [e.g., Orbiniidae (38)], and consequently, they may have been present in the clade that is the sister group of Palaeoannelida, regardless of the competing topologies from phylogenomics and morphological data, which differ primarily in the placement of Amphinomidae and Sipuncula (fig. S7).
The gills of Canadia (Fig. 1C and fig. S3) are distinct from those of extant annelids (11, 17) and could be autapomorphic, although they share intriguing similarities in both morphology and attachment with molluscan ctenidia (11, 23), which are also repeated on along the A-P axis in stem molluscs [e.g., Odontogriphus (39)]. It is likely that multiple repeated gills were present in the ancestral mollusc (24), and consequently, the branchiae could represent a deep homolog shared with molluscs (11, 23). Branchiae are phylogenetically widespread among annelids in both sedentary and errant taxa, and it is likely that they have multiple independent origins. If the serialized branchiae in Canadia are truly a homolog shared with molluscs, this would necessitate losses of ctenidia in some other trochozoan phyla, as most recent phylogenomic trees do not recover annelids and molluscs as sister taxa (40). In tube-dwelling or burrowing taxa, they are often localized to one end of the body (17), whereas they are present along the body in many active and epibenthic forms [e.g., Eunicidae, where, like Canadia, they are absent from the anterior segments (17)].
The evolution of serialization of internal and external characters has featured heavily in discussions of bilaterian origins, with multiple animal phyla and classes displaying serial repetition of one or more organ system. Such an organization ranges from segmentation in annelids, arthropods, and chordates (41) to serial repetition of one or only a few features, such as gills and shell plates in aculiferan and monoplacophoran molluscs (24). Annelids display a combination of serial repetition of parapodia, chaetal bundles, cirriform appendages, coelomic cavities, nephridia, branchiae, and features of the nervous system, some or all of which may be absent in some annelid subclades (9, 28). Although our current understanding of bilaterian evolution strongly suggests that many serialized features shared by animal phyla are the result of notable convergence (e.g., segmentation in annelids and arthropods), the fossil polychaetes discussed herein demonstrate that many of the serial features that characterize polychaetes (including in the nervous system) evolved early in the evolutionary history of annelids. Although the sister phylum of annelids is not segmented, members of candidate sister taxa share serial repetition of some anatomical features with annelids (although not features pertaining to the nervous system), such as serially repeated paired coelomic cavities and chaetae in craniid brachiopods (42) and iterated chaetal rows in the stem-group mollusc Wiwaxia (43). It would therefore be difficult to infer the total absence of serialization of features in the ancestors of all supraphyletic groups of trochozoans, and iteration of chaetal bundles at least may have had a broader distribution in the past than it does among extant phyla. Serialization of features of the nervous system have also featured prominently in discussions of panarthropod evolution, and some analyses of fossil panarthropods preserving nervous tissue have likewise identified convergent loss of serialization of particular features, such as multiple intersegmental nerves (2), as an important factor in determining the distribution of characters observed in extant taxa.
Given the presence of large locomotory parapodia in Canadia and other Cambrian stem annelid taxa, we consider it likely that the ancestral annelid had an active lifestyle crawling on the benthos. Furthermore, our ancestral state reconstruction does not support a tube-dwelling crown annelid ancestor (fig. S7). Although many early branching annelids have sedentary and tube-dwelling lifestyles (Palaeoannelida, Chaetopteriformia, and Sipuncula), their modes of life are dissimilar, and their morphology is highly heterogeneous (9, 32). Oweniids live in agglutinated tubes and exhibit suspension and deposit feeding, magelonids live in burrows [or more rarely in buried tubes (44)] and engage in subsurface deposit feeding and microcarnivory, whereas chaetopterids are suspension feeders, using various methods such as mucous nets and water-pumping using specialized segments (30). It is possible that these sessile modes of life have evolved independently and that the annelid ancestor was characterized by a motile and active mode of life and consequently a moderately complex nervous system. The morphology of the nervous system of Canadia indicates that some annelid novelties [such as a differentiated bipartite prostomium/peristomium head complex (15, 16), pygidial cirri (45), and nuchal organs] originated after the evolution of a typical annelid nervous system, including the positioning of the ventral nerve cord at the ventral midline, serially repeated parapodial/segmental nerves, and a supraesophageal brain.
MATERIALS AND METHODS
Fossil material
All materials analyzed are deposited at the Royal Ontario Museum, Toronto, Canada (ROMIP), National Museum of Natural History, Washington DC, USA (USNM), and Geological Survey of Canada, Ottawa, Canada (GSC). See table S1 for a full list of material analyzed.
Imaging
Photographs were taken using Canon camera bodies using MP-E 65-mm and EF 10-mm macro lenses or using a Leica M205C stereomicroscope. To enhance contrast, specimens were illuminated at high angle underwater or under ethanol with or without cross-polarized light. Secondary electron micrographs and backscatter SEM images were obtained in the Department of Earth Sciences at the University of Toronto using a JEOL 6610LV SEM and at the Smithsonian National Museum of Natural History using a Zeiss EVO MA15 SEM. Elemental maps were obtained using an environmental scanning electron microscope (FEI Quanta 200 FEG) equipped with an energy scanning spectroscopy x-ray detector and octane plus silicon drift detector (using TEAM software, version 4.1) under low vacuum conditions (70 Pa, 5 to 15 kV, 400-μs dwell time) at the University of Windsor Great Lakes Institute for Environmental Research, Canada.
Phylogenetic analysis
Our phylogenetic data matrix contains 80 taxa and 226 characters and is described in detail in the Supplementary Materials. Unstable taxa were identified using concatabominations (46) and pruned from phylogenetic analyses. These were Guanshanchaeta and Arkonips, which were identified as equivalent with Pygocirrus and Fossundecima, respectively. Guanshanchaeta and Arkonips were selected for removal as their character data contained more missing data. Phylogenetic analyses were performed using MrBayes 3.2.6 (47) using the mkv + gamma model using the Lewis (48) correction for the absence of extensive coding of autapomorphies in the matrix. Analyses ran for 10,000,000 generations with the first 50% of samples discarded as burn in. Convergence was assessed using the average deviation of split frequencies (convergence at <0.01) estimated sample size (ESS) scores (ESS > 200) and potential scale reduction factor (PSRF, ~1.00) in MrBayes and graphically using Tracer v1.6.
The data matrix contains 80 taxa and 226 characters. Analyses were conducted using MrBayes 3.2.6 for Bayesian analyses with the mkv + gamma model. Ancestral states for nervous system morphology were estimated using Bayestraits (49). We estimated ancestral states using two topologies: One was derived from analysis of the morphological matrix and another was derived from an analysis with a backbone constraint based on recent phylogenomic results. This constraint was based on the topology of Helm et al. (13), which is the most recent phylogenomic analysis and also contains the most comprehensive taxon sample of annelids that are positioned close to the root. Although some clades recovered by Helm et al. (13) were also recovered and strongly supported in the unconstrained morphological phylogeny (such as clitellate monophyly and annelid monophyly), the monophyly of these clades was also constrained to ensure that the constrained analysis would not sample trees in conflict with those by Helm et al. (13).
As our phylogenies were not fully resolved and contain uncertainty regarding the placement of fossil taxa, we carried out Bayesian ancestral state reconstruction, integrating over competing topologies by sampling topologies in proportion to their posterior probability. Our analyses used topologies and branch lengths from the last 1000 trees sampled during the morphological phylogenetic analyses (both with and without topological constraints). Ancestral states were reconstructed using the multistate model in Bayestraits, in a Bayesian framework with 8,000,000 generations with the first 50% of samples discarded as burn-in. Analyses used an exponential hyperprior using the command “exp 0 10” in Bayestraits. The ancestral states reconstructed on fig. S7 are mean estimates from the post burn-in samples.
Supplementary Material
Acknowledgments
We thank D. Erwin and M. Florence for access to the Smithsonian Institution Burgess Shale collections (National Museum of Natural History), M. Collins and L. Fields for reconstructions, and S. Lackie for elemental maps. The Canadia material from the Royal Ontario Museum was collected under several Parks Canada Research and Collections permits to Desmond Collins. Funding: Funding comes from Barbara Polk Milstein foundations, J.-B.C.’s NSERC Discovery grant (#341944), the Dorothy Strelsin Foundation, and a Royal Ontario Museum Research grant. This is Royal Ontario Museum Burgess Shale project number 78. Author contributions: J.-B.C. and L.A.P. conceived the study and imaged the fossil specimens. J.-B.C. prepared specimens. L.A.P. interpreted the fossils with substantial input from J.-B.C. L.A.P. assembled the morphological data and performed the phylogenetic analyses. L.A.P. wrote the initial draft of the manuscript with input from J.-B.C. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax5858/DC1
Supplementary Text
Fig. S1. Additional specimens of C. spinosa showing details of overall anatomy.
Fig. S2. Morphology of the palps of Canadia spinosa and Kootenayscolex barbarensis.
Fig. S3. Gill morphology and attachment in Canadia spinosa.
Fig. S4. Additional images and camera lucida drawings of anterior and axial nervous system of C. spinosa.
Fig. S5. Elemental map of USNM83929C.
Fig. S6. Additional elemental maps of USNM83929B showing the same region as Fig. 2A.
Fig. S7. Phylogenetic position of C. spinosa based on morphological character matrix using the mkv + gamma model and ancestral state reconstruction.
Fig. S8. Three-dimensional reconstructions of C. spinosa by Lars Fields.
Table S1. Details of fossil material analyzed.
Movie S1. Movie of three-dimensional reconstruction of C. spinosa by Lars Fields.
Data file S1. Character taxon matrix in NEXUS format.
REFERENCES AND NOTES
- 1.Ma X., Hou X., Edgecombe G. D., Strausfeld N. J., Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Yang J., Ortega-Hernández J., Butterfield N. J., Liu Y., Boyan G. S., Hou J.-b., Lan T., Zhang X.-g., Fuxianhuiid ventral nerve cord and early nervous system evolution in Panarthropoda. Proc. Natl. Acad. Sci. U.S.A. 113, 2988–2993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma X., Edgecombe G. D., Hou X., Goral T., Strausfeld N. J., Preservational pathways of corresponding brains of a Cambrian euarthropod. Curr. Biol. 25, 2969–2975 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Park T.-Y. S., Kihm J.-H., Woo J., Park C., Lee W. Y., Smith M. P., Harper D. A. T., Young F., Nielsen A. T., Vinther J., Brain and eyes of Kerygmachela reveal protocerebral ancestry of the panarthropod head. Nat. Commun. 9, 1019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., Steiner M., Dunlop J. A., Shu D., Microbial decay analysis challenges interpretation of putative organ systems in Cambrian fuxianhuiids. Proc. Biol. Sci. 285, 20180051 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sansom R. S., Preservation and phylogeny of Cambrian ecdysozoans tested by experimental decay of Priapulus. Sci. Rep. 6, 32817 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry L. A., Smithwick F., Nordén K. K., Saitta E. T., Lozano-Fernandez J., Tanner A. R., Caron J.-B., Edgecombe G. D., Briggs D. E. G., Vinther J., Soft-bodied fossils are not simply rotten carcasses–toward a holistic understanding of exceptional fossil preservation: Exceptional fossil preservation is complex and involves the interplay of numerous biological and geological processes. Bioessays 40, 1700167 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Eglinton G., Logan G. A., Molecular preservation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 333, 315–328 (1991). [DOI] [PubMed] [Google Scholar]
- 9.Parry L. A., Edgecombe G. D., Eibye-Jacobsen D., Vinther J., The impact of fossil data on annelid phylogeny inferred from discrete morphological characters. Proc. Biol. Sci. 283, 20161378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eibye-Jacobsen D., A reevaluation of Wiwaxia and the polychaetes of the Burgess Shale. Lethaia 37, 317–335 (2004). [Google Scholar]
- 11.Conway Morris S., Middle Cambrian polychaetes from the Burgess Shale of British Columbia. Philos. Trans. R. Soc. Lond. B Biol. Sci. 285, 227–274 (1979). [Google Scholar]
- 12.Beckers P., Helm C., Purschke G., Worsaae K., Hutchings P., Bartolomaeus T., The central nervous system of Oweniidae (Annelida) and its implications for the structure of the ancestral annelid brain. Front. Zool. 16, 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helm C., Beckers P., Bartolomaeus T., Drukewitz S. H., Kourtesis I., Weigert A., Purschke G., Worsaae K., Struck T. H., Bleidorn C., Convergent evolution of the ladder-like ventral nerve cord in Annelida. Front. Zool. 15, 36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J., Conway Morris S., Cuthill J. F. H., Shu D., Sclerite-bearing annelids from the lower Cambrian of South China. Sci. Rep. 9, 4955 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanglu K., Caron J.-B., A new Burgess Shale polychaete and the origin of the annelid head revisited. Curr. Biol. 28, 319–326.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Parry L., Vinther J., Edgecombe G. D., Cambrian stem-group annelids and a metameric origin of the annelid head. Biol. Lett. 11, 20150763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.G. Rouse, F. Pleijel, Polychaetes (Oxford Univ. Press, 2001), pp. 354.
- 18.Caron J.-B., Jackson D. A., Taphonomy of the greater phyllopod bed community, Burgess Shale. Palaios 21, 451–465 (2006). [Google Scholar]
- 19.Forest D. L., Lindsay S. M., Observations of serotonin and FMRFamide-like immunoreactivity in palp sensory structures and the anterior nervous system of spionid polychaetes. J. Morphol. 269, 544–551 (2008). [DOI] [PubMed] [Google Scholar]
- 20.G. Purschke, 24 Annelida: Basal groups and Pleistoannelida. Structure and evolution of invertebrate nervous systems, 768 (2015).
- 21.Weigert A., Bleidorn C., Current status of annelid phylogeny. Organ. Divers. Evolution 2016, 1–18 (2016). [Google Scholar]
- 22.Conway Morris S., Caron J.-B., Pikaia gracilens Walcott, a stem group chordate from the Middle Cambrian of British Columbia. Biol. Rev. 87, 480–512 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Conway Morris S., Peel J. S., Articulated halkieriids from the Lower Cambrian of North Greenland and their role in early protostome evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 347, 305–358 (1995). [Google Scholar]
- 24.Vinther J., Parry L., Briggs D. E. G., Van Roy P., Ancestral morphology of crown-group molluscs revealed by a new Ordovician stem aculiferan. Nature 542, 471 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Eibye-Jacobsen D., Vinther J., Reconstructing the ancestral annelid. J. Zoolog. Syst. Evol. Res. 50, 85–87 (2012). [Google Scholar]
- 26.L. Orrhage, M. C. Müller, in Morphology, Molecules, Evolution and Phylogeny in Polychaeta and Related Taxa (Springer, 2005), pp. 79–111.
- 27.Rimskaya-Korsakova N. N., Kristof A., Malakhov V. V., Wanninger A., Neural architecture of Galathowenia oculata Zach, 1923 (Oweniidae, Annelida). Front. Zool. 13, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rouse G. W., Fauchald K., Cladistics and polychaetes. Zool. Scr. 26, 139–204 (1997). [Google Scholar]
- 29.Struck T. H., Golombek A., Weigert A., Franke F. A., Westheide W., Purschke G., Bleidorn C., Halanych K. M., The evolution of annelids reveals two adaptive routes to the interstitial realm. Curr. Biol. 25, 1993–1999 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Jumars P. A., Dorgan K. M., Lindsay S. M., Diet of worms emended: An update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 7, 497–520 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Müller M. C. M., Polychaete nervous systems: Ground pattern and variations—cLS microscopy and the importance of novel characteristics in phylogenetic analysis. Integr. Comp. Biol. 46, 125–133 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Weigert A., Helm C., Meyer M., Nickel B., Arendt D., Hausdorf B., Santos S. R., Halanych K. M., Purschke G., Bleidorn C., Struck T. H., Illuminating the base of the annelid tree using transcriptomics. Mol. Biol. Evol. 31, 1391–1401 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Parry L. A., Wilson P., Sykes D., Edgecombe G. D., Vinther J., A new fireworm (Amphinomidae) from the Cretaceous of Lebanon identified from three-dimensionally preserved myoanatomy. BMC Evol. Biol. 15, 256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T. H. Bullock, G. A. Horridge, Structure and Function in the Nervous Systems of Invertebrates (W. H. Freeman, 1965).
- 35.Brinkmann N., Wanninger A., Capitellid connections: Contributions from neuromuscular development of the maldanid polychaete Axiothella rubrocincta (Annelida). BMC Evol. Biol. 10, 168 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessling R., Metameric organisation of the nervous system in developmental stages of Urechis caupo (Echiura) and its phylogenetic implications. Zoomorphology 121, 221–234 (2002). [Google Scholar]
- 37.Kristof A., Wollesen T., Wanninger A., Segmental mode of neural patterning in Sipuncula. Curr. Biol. 18, 1129–1132 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Wilkens V., Purschke G., Pigmented eyes, photoreceptor-like sense organs and central nervous system in the polychaete Scoloplos armiger (Orbiniidae, Annelida) and their phylogenetic importance. J. Morphol. 270, 1296–1310 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Caron J.-B., Scheltema A., Schander C., Rudkin D., A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature 442, 159–163 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Marlétaz F., Peijnenburg K. T. C. A., Goto T., Satoh N., Rokhsar D. S., A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr. Biol. 29, 312–318.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Chipman A. D., Parallel evolution of segmentation by co-option of ancestral gene regulatory networks. Bioessays 32, 60–70 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Nielsen C., The development of the brachiopod Crania (Neocrania) anomala (O. F. Müller) and its phylogenetic significance. Acta Zool. 72, 7–28 (1991). [Google Scholar]
- 43.Yang J., Smith M. R., Lan T., Hou J.-b., Zhang X.-g., Articulated Wiwaxia from the Cambrian Stage 3 Xiaoshiba Lagerstätte. Sci. Rep. 4, 4643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills K., Mortimer K., Observations on the tubicolous annelid Magelona alleni (Magelonidae), with discussions on the relationship between morphology and behaviour of European magelonids. J. Mar. Biol. Assoc. U.K. 99, 715–727 (2019). [Google Scholar]
- 45.Vinther J., Eibye-Jacobsen D., Harper D. A. T., An Early Cambrian stem polychaete with pygidial cirri. Biol. Lett. 7, 929–932 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siu-Ting K., Pisani D., Creevey C. J., Wilkinson M., Concatabominations: Identifying unstable taxa in morphological phylogenetics using a heuristic extension to safe taxonomic reduction. Syst. Biol. 64, 137–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., Huelsenbeck J. P., MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis P. O., A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001). [DOI] [PubMed] [Google Scholar]
- 49.M. Pagel, A. Meade, BayesTraits. Computer program and documentation (2007); http://www.evolution.rdg.ac.uk/BayesTraits.html.
- 50.Winchell C. J., Valencia J. E., Jacobs D. K., Confocal analysis of nervous system architecture in direct-developing juveniles of Neanthes arenaceodentata (Annelida, Nereididae). Front. Zool. 7, 17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin N., Anctil M., The nervous system of the tube-worm Chaetopterus variopedatus (Polychaeta). J. Morphol. 181, 161–173 (1984). [DOI] [PubMed] [Google Scholar]
- 52.Jones M. L., On the morphology, feeding, and behavior of Magelona sp. Biol. Bull. 134, 272–297 (1968). [DOI] [PubMed] [Google Scholar]
- 53.Meyer N. P., Carrillo-Baltodano A., Moore R. E., Seaver E. C., Nervous system development in lecithotrophic larval and juvenile stages of the annelid Capitella teleta. Front. Zool. 12, 15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orrhage L., On the microanatomy of the brain and the innervation and homologues of the cephalic appendages of Hesionidae and Syllidae (Polychaeta). Acta Zool. 77, 137–151 (1996). [Google Scholar]
- 55.Orrhage L., On the innervation and homologues of the cephalic appendages of the Aphroditacea (Polychaeta). Acta Zool. 72, 233–246 (1991). [Google Scholar]
- 56.Orrhage L., Eibye-Jacobsen D., On the anatomy of the central nervous system of Phyllodocidae (Polychaeta) and the phylogeny of phyllodocid genera: A new alternative. Acta Zool. 79, 215–234 (1998). [Google Scholar]
- 57.Orrhage L., On the microanatomy of the supraoesophageal ganglion of some amphinomids (Polychaeta Errantia), with further discussion of the innervation and homologues of the polychaete palps. Acta Zool. 71, 45–59 (1990). [Google Scholar]
- 58.Orrhage L., On the anatomy of the central nervous system and the morphological value of the anterior end appendages of Ampharetidae, Pectinariidae and Terebellidae (Polychaeta). Acta Zool. 82, 57–71 (2001). [Google Scholar]
- 59.Orrhage L., On the innervation and homologues of the anterior end appendages of the Eunicea (Polychaeta), with a tentative outline of the fundamental constitution of the cephalic nervous system of the polychaetes. Acta Zool. 76, 229–248 (1995). [Google Scholar]
- 60.Orrhage L., On the structure and homologues of the anterior end of the polychaete families Sabellidae and Serpulidae. Zoomorphology 96, 113–167 (1980). [Google Scholar]
- 61.A. V. Filippova, A. B. Tzetlin, G. Purschke, in Advances in Polychaete Research (Springer, 2003), pp. 215–223.
- 62.Wells G., The anatomy of the body wall and appendages in Arenicola marina L., Arenicola claparedii Levinsen and Arenicola ecaudata Johnston. J. Mar. Biol. Assoc. U.K. 29, 1–44 (1950). [Google Scholar]
- 63.Clark M. E., Histochemical localization of monoamines in the nervous system of the polychaete Nephtys. Proc. R. Soc. Lond. B Biol. Sci. 165, 308–325 (1966). [DOI] [PubMed] [Google Scholar]
- 64.Tzetlin A. B., Dahlgren T., Purschke G., Ultrastructure of the body wall, body cavity, nephridia and spermatozoa in four species of the Chrysopetalidae (Annelida,“Polychaeta”). Zool. Anz. 241, 37–55 (2002). [Google Scholar]
- 65.Zattara E. E., Bely A. E., Fine taxonomic sampling of nervous systems within Naididae (Annelida: Clitellata) reveals evolutionary lability and revised homologies of annelid neural components. Front. Zool. 12, 8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weidhase M., Bleidorn C., Helm C., Structure and anterior regeneration of musculature and nervous system in Cirratulus cf. cirratus (Cirratulidae, Annelida). J. Morphol. 275, 1418–1430 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Thompson I., Johnson R. G., New fossil polychaete from Essex, Illinois. Fieldiana Geol. 33, 471–487 (1977). [Google Scholar]
- 68.Bartolomaeus T., Secondary monociliarity in the Annelida: Monociliated epidermal cells in larvae of Magelona mirabills (Magelonida). Microfauna Mar. 103, 27–332 (1995). [Google Scholar]
- 69.Parker A. R., Colour in Burgess Shale animals and the effect of light on evolution in the Cambrian. Proceedings of the Royal Society of London B: Biological Sciences 265, 967–972 (1998). [Google Scholar]
- 70.Butterfield N. J., A reassessment of the enigmatic Burgess Shale fossil Wiwaxia corrugata (Matthew) and its relationship to the polychaete Canadia spinosa Walcott. Paleobiology 16, 287–303 (1990). [Google Scholar]
- 71.Zhang Z., Smith M. R., Shu D., New reconstruction of the Wiwaxia scleritome, with data from Chengjiang juveniles. Sci. Rep. 5, 14810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merz R. A., Woodin S. A., Polychaete chaetae: Function, fossils, and phylogeny. Integr. Comp. Biol. 46, 481–496 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Randel N., Jékely G., Phototaxis and the origin of visual eyes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Metalnikoff S., Sipunculus nudus. Z. Wiss. Zool 68, 261–322 (1900). [Google Scholar]
- 75.A. Kristof, A. S. Maiorova, 23 Annelida: Sipuncula. Structure and evolution of invertebrate nervous systems, (2015).
- 76.Sumner-Rooney L., Sigwart J. D., Do chitons have a brain? New evidence for diversity and complexity in the polyplacophoran central nervous system. J. Morphol. 279, 936–949 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax5858/DC1
Supplementary Text
Fig. S1. Additional specimens of C. spinosa showing details of overall anatomy.
Fig. S2. Morphology of the palps of Canadia spinosa and Kootenayscolex barbarensis.
Fig. S3. Gill morphology and attachment in Canadia spinosa.
Fig. S4. Additional images and camera lucida drawings of anterior and axial nervous system of C. spinosa.
Fig. S5. Elemental map of USNM83929C.
Fig. S6. Additional elemental maps of USNM83929B showing the same region as Fig. 2A.
Fig. S7. Phylogenetic position of C. spinosa based on morphological character matrix using the mkv + gamma model and ancestral state reconstruction.
Fig. S8. Three-dimensional reconstructions of C. spinosa by Lars Fields.
Table S1. Details of fossil material analyzed.
Movie S1. Movie of three-dimensional reconstruction of C. spinosa by Lars Fields.
Data file S1. Character taxon matrix in NEXUS format.


