Metformin expands the neural precursor pool in adult females, but not males, and is correlated with cognitive recovery.
Abstract
Resident neural stem and progenitor cells, collectively termed neural precursor cells (NPCs), reside in a well-defined neurogenic niche in the subventricular zone (SVZ) and contribute to ongoing postnatal neurogenesis. It is well established that the NPC niche can alter the behavior of NPCs. NPC activation is a promising therapeutic strategy for brain repair. The drug metformin has been shown to activate neural stem cells, promote differentiation, and lead to functional motor recovery in a neonatal stroke model. We demonstrate that metformin-induced NPC expansion and functional recovery is sex hormone dependent. Metformin increases the size of the NPC pool in adult females, but not males, and promotes cognitive recovery in a model of brain injury in females, but not males. Our data demonstrate that metformin has age- and sex-dependent effects on NPCs that correlate with functional recovery, which has important implications for neural repair.
INTRODUCTION
The activation of endogenous neural stem and progenitor cells, collectively termed neural precursor cells (NPCs), shows promise as a potential repair strategy following brain injury (1, 2). NPCs are located in two distinct neurogenic regions of the brain: the subventricular zone (SVZ) of the lateral ventricles, and the dentate gyrus (DG) of the hippocampus. Previous studies have used drugs and growth factors to enhance resident NPCs following injury to regenerate tissue and promote functional recovery (3, 4). One such drug is metformin, commonly used to treat type 2 diabetes, which was shown to enhance NPC-derived neurogenesis and oligogenesis, as well as expand the size of the neural stem cell (NSC) pool in the SVZ from postnatal mice in vitro (5–7). Previous work demonstrated that 1 week of metformin treatment (beginning 1 day after injury) led to motor recovery in neonatal mice that received a hypoxic-ischemic (H-I) injury. This functional recovery was correlated with an expansion of the NPC pool, migration of NPCs to the parenchyma, and an increase in newly born neurons and oligodendrocytes. A number of studies have reported that metformin promotes functional improvements in rodent models of adult stroke (4, 8–10), attributing the metformin-induced recovery to enhanced neurogenesis and angiogenesis. Metformin has also been shown to have anti-inflammatory properties, highlighting the pleiotropic effects of metformin in the brain (11, 12). On the basis of these promising results, there is clear potential for metformin, a relatively safe and well-tolerated drug, to be used in a clinical setting to treat the injured brain. What remains unknown is how metformin affects NPCs through aging and between sexes. It is well established in both preclinical and clinical settings that sex and age are factors that affect both the extent of brain injury and recovery processes (13–16).
NPCs reside in a well-defined region in the developing and mature brain. This NPC niche is a dynamic structure that changes through aging and following injury (17). It is composed of different cell types, including ependymal and endothelial cells, microglia, and the factors released by these cells (18). Changes in the niche that occur with aging have been well characterized and include increased microglial activation, reduced NPC proliferation, and altered blood vessel morphology (17, 19, 20). Differences in the niche between sexes are less well characterized, but studies have confirmed the differential expression of estradiol receptors as well as CYP19, a cytochrome P450 enzyme, between male and female SVZ-derived NPCs (21–23). Sex hormones have also been shown to affect neurogenesis and proliferation (24–26). The interplay between NPC activation and niche-related effects is critical for understanding how metformin can affect cellular and functional outcomes between sexes and in the aging and/or injured brain.
Here, we investigated the effect of metformin on NPCs in females and males across ages and asked whether sex plays a role in the metformin-induced recovery in an H-I injury model. We found significant differences in the response of NPCs to metformin through aging (in neonatal, juvenile, and adult mice), which varied between sexes. The effect of metformin on NPC responsiveness was dependent on sex hormones, whereby estradiol in combination with metformin was sufficient to expand the NSC pool in females, and testosterone was inhibitory to the effect of metformin. Last, we assessed the efficacy of metformin administration in rescuing cognitive deficits following neonatal H-I injury and whether the effects were sex dependent. Adult males and females had similar cognitive impairments following neonatal H-I injury, but metformin was only effective at improving cognitive function in females. Hence, we have demonstrated age- and sex-dependent effects of metformin on NPCs that may correlate with improved functional recovery in adult female mice.
RESULTS
Metformin has age- and sex-dependent effects on the NSC pool in the SVZ
We performed an in vitro dose-response curve with SVZ-derived cells from females and males, from both neonatal and adult mice, using the neurosphere assay, where the total number of neurospheres reflects the size of the NSC pool (fig. S1, A to D). Neonatal SVZ cultures exposed to 1 μM metformin in vitro [Fig. 1, A (females) and B (males)] and mice treated with daily metformin injections from postnatal day 9 (P9) to P12 (in vivo) [Fig. 1, C (females) and D (males)] showed a significant increase in neurosphere formation in both sexes. Conversely, in separate experiments performed in juveniles, metformin did not affect the size of the NSC pool in vitro or in vivo in females [Fig. 1, A (in vitro) and C (in vivo)] or males [Fig. 1, B (in vitro) and D (in vivo)]. In young adult mice, metformin exposure expanded the size of the NSC pool in females [Fig. 1, A (in vitro) and C (in vivo)], but not males [Fig. 1, B (in vitro) and D (in vivo)]. To determine whether the lack of expansion in adult male mice was due to the dose or duration of metformin treatment, we administered metformin to adult male mice using the neonatal paradigm (20 mg/kg of metformin for 4 days), where males were responsive, as well as other permutations of dose and duration (20 mg/kg for 7 days and 200 mg/kg for 4 days). We found that the dose and duration of metformin treatment had no effect on the number of neurospheres from the adult male SVZ (fig. S2, A and B). These findings reveal that the ability of metformin to expand the size of the NSC pool in the SVZ is dependent on both sex and age.
Fig. 1. In vitro and in vivo metformin treatment leads to an age- and sex-dependent expansion of the NSC pool.
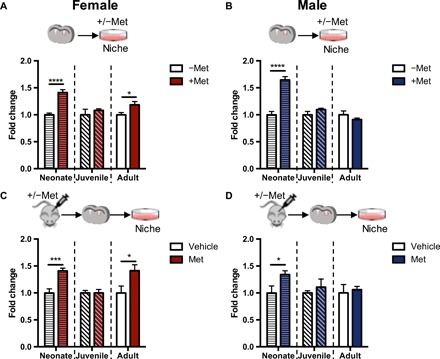
(A and B) Fold change in the number of neurospheres from the SVZ of neonatal (P8), juvenile (P17), and adult (7 weeks) females [(A) neonate: −Met, n = 16 mice; +Met, n = 16 mice; P < 0.001; juvenile: −Met, n = 7 mice; +Met, n = 7 mice; adult: −Met, n = 15 mice; +Met, n = 15 mice; over three to seven independent experiments per age group, P < 0.05; Student’s t test] and males [(B) neonate: −Met, n = 15 mice; +Met, n = 15 mice; P < 0.001; juvenile: −Met, n = 10 mice; +Met, n = 10 mice; adult: −Met, n = 11 mice; +Met, n = 11 mice; over four to five independent experiments per age group, Student’s t test] following in vitro metformin administration (1 μM). (C and D) Fold change in the number of neurospheres from the SVZ of neonatal (P8), juvenile (P17), and adult (7 weeks) females [(C) neonate: −Met, n = 4 mice; +Met, n = 5 mice; P < 0.05; juvenile: −Met, n = 9 mice; +Met, n = 8 mice; adult: −Met, n = 10 mice; +Met, n = 8 mice; P < 0.05; over three to five independent experiments per age group, Student’s t test] and males [(D) neonate: −Met, n = 6 mice; +Met, n = 6 mice; P < 0.05; juvenile: −Met, n = 6 mice; +Met, n = 5 mice; adult: −Met, n = 7 mice; +Met, n = 5 mice; over three to four independent experiments per age group, Student’s t test] following in vivo administration with vehicle or metformin (20 or 200 mg/kg). Experiments across different ages were analyzed using a Student’s t test. *P < 0.05, ***P < 0.005, ****P < 0.001. Met, metformin.
The size of the NSC pool and responsiveness to sex hormones are similar in males and females in the absence of metformin
Given the observed sex-dependent differences in the response of NSCs to metformin, we hypothesized that sex hormones may play a role in mediating the differential response between females and males. First, we determined that the number of NSCs from the age-matched SVZ of male and female mice was similar under baseline conditions (fig. S3, A to C). Furthermore, the exposure of primary adult-derived SVZ cultures to estradiol or testosterone (female and male sex hormones, respectively) had no effect on the number of neurospheres from adult mice of either sex (fig. S4, A and B). Since primary cultures contain niche cells, we next asked whether pure populations of NSCs (i.e., passaged neurosphere-derived cells, effectively removing the niche) were responsive to sex hormones. Neurospheres from male and female cultures were dissociated and replated in estradiol or testosterone. Estradiol exposure led to a significant increase in the number of passaged neurospheres from both sexes (fig. S4C). Testosterone had no effect on the number of passaged neurospheres in either sex (fig. S4D). Together, these findings demonstrate that the differential expansion of the NSC pool in adult males and females in response to metformin is not due to baseline differences in the size of the NSC pool, or the effects of hormones on their own.
The estradiol-exposed niche is sufficient for metformin to expand the NSC pool in females
To test whether the metformin-mediated expansion of the NSC pool is hormone dependent, we administered estradiol to female juvenile mice, an age when female NSCs are not responsive to metformin. Mice received estradiol treatment for 10 days beginning at P17, and metformin for 7 days beginning at P21 (n = 8 to 9; Fig. 2A). We observed a significant increase in the number of neurospheres from the SVZ of juvenile female mice treated with estradiol and metformin, revealing that estradiol is sufficient to enable the metformin-induced increase in the NSC pool, similar to what is seen in adult female mice (Fig. 2A). In a loss-of-function experiment, we performed the neurosphere assay from adult female mice that were ovariectomized on P28, effectively removing circulating estradiol (27), and that received 7 days of metformin beginning at P50 (Fig. 2B). As predicted, no expansion of the NSC pool was observed in ovariectomized mice in response to metformin administration (Fig. 2B). Together, these findings support the hypothesis that estradiol is sufficient for the metformin-mediated expansion of the NSC pool.
Fig. 2. Estradiol in the female niche is sufficient for the metformin-induced expansion of the NSC pool.
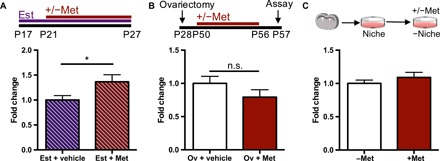
(A) Experimental paradigm and fold change in the number of neurospheres from the SVZ of female juvenile mice that received estradiol (5.6 mg/kg) or vehicle for 11 days and metformin (200 mg/kg) or vehicle for 7 days in vivo (Est + vehicle, n = 9 mice; Est + Met, n = 8 mice; over five independent experiments; P < 0.05; Student’s t test). (B) Experimental paradigm and fold change in the number of neurospheres from control and ovariectomized mice following administration of vehicle or metformin (200 mg/kg) for 1 week in vivo (Ov + vehicle, n = 8 mice; Ov + Est, n = 8 mice; over three independent experiments; Student’s t test). (C) Fold change in the number of neurospheres passaged in the presence or absence of 1 μM metformin in vitro (−Met, n = 7 mice; +Met, n = 7 mice; over four experiments; Student’s t test). *P < 0.05. Est, estradiol; Met, metformin; Ov, ovariectomized; n.s., not significant.
The expansion of the NSC pool was seen following metformin administration in vivo and in vitro in primary SVZ cultures from female mice in the presence of metformin. To ask whether the female niche is necessary for the observed expansion, we removed the niche by plating passaged neurosphere-derived cells in the presence of metformin. Notably, metformin did not lead to an increase in neurospheres in the absence of the niche (Fig. 2C), revealing that metformin acts through the female niche to mediate the expansion of the NSC pool.
Testosterone inhibits the metformin-induced expansion of the NSC pool
Unlike what was observed in juvenile females, estradiol and metformin treatment did not expand the size of the NSC pool in juvenile male mice (Fig. 3A). One possible explanation is that an inhibitory environment in the male SVZ prevents the NSCs from responding to metformin. We asked whether testosterone was sufficient to inhibit the effect of metformin on NSC expansion by administering testosterone, in the presence or absence of metformin, to neonatal mice of both sexes from P9 to P12, an age when metformin expands the NSC pool in both sexes (Fig. 1, C and D). The coadministration of metformin and testosterone led to a loss of this expansion in males (Fig. 3B) and females (fig. S5). We predicted that if testosterone was inhibitory to the effect of metformin, the administration of metformin to castrated adult males (that lack testosterone) would result in an expansion of the NSC pool. We found that 1 week of metformin delivery to castrated adult male mice resulted in a significant 1.2-fold increase in neurospheres (Fig. 3C). These findings support the hypothesis that testosterone inhibits the metformin-induced expansion of the NSC pool.
Fig. 3. Testosterone inhibits the metformin-induced expansion of the stem cell pool.
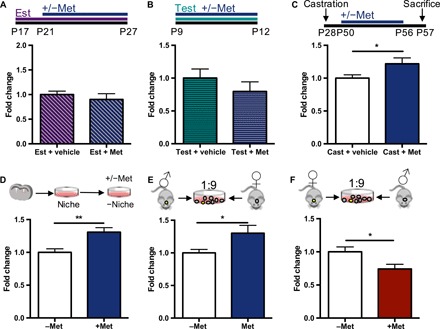
(A) Experimental paradigm and fold change in the number of neurospheres from the SVZ of male juvenile mice that received estradiol (5.6 mg/kg) or vehicle for 11 days and metformin (200 mg/kg) or vehicle for 7 days in vivo (Est + vehicle, n = 5 mice; Est + Met, seven mice from three to four independent experiments, Student’s t test). (B) Experimental paradigm and fold change in the number of neurospheres from male neonatal mice that received testosterone only or testosterone and metformin for 4 days in vivo (Test + vehicle, n = 7 mice; Test + Met, n = 7 mice over seven independent experiments; Student’s t test). (C) Experimental paradigm and fold change in the number of neurospheres from control and castrated males following vehicle or metformin (200 mg/kg) administration for 1 week in vivo (Cast + vehicle, n = 9 mice; Cast + Met, n = 9 mice; over three independent experiments; P < 0.05; Student’s t test). (D) Fold change in the number of neurospheres passaged in the presence or absence of 1 μM metformin in vitro (−Met, n = 7 mice; +Met, n = 7 mice; over four experiments; P < 0.01; Student’s t test). (E) Coculture paradigm and fold change in the number of neurospheres from YFP male cells plated in an overwhelmingly female environment, in the presence or absence of 1 μM metformin, in vitro (n = 9 independent experiments, P < 0.05, Student’s t test). (F) Coculture paradigm and fold change in the number of neurospheres from YFP female cells plated in an overwhelmingly male environment in the presence or absence of 1 μM metformin in vitro (n = 8 independent experiments, P < 0.05, Student’s t test). *P < 0.05, **P < 0.01. Cast, castrated; Est, estradiol; Met, metformin; Test, testosterone.
Similar to the passaging experiments using neurosphere-derived cells from females, we plated passaged neurosphere-derived cells from males (in the absence of the niche) in the presence of metformin. We observed a significant 1.3-fold increase in the number of passaged neurospheres (Fig. 3D), revealing that male-derived NSCs are able to respond to metformin.
We next performed a series of cell coculture experiments using primary SVZ dissections (containing niche cells) from male and female mice. We made the strong prediction that male-derived cells plated in the presence of metformin and an overwhelmingly female environment would lead to an increase in the number of male-derived neurospheres (Fig. 3E). To distinguish between cells from opposite sexes, we performed coculture experiments using control and yellow fluorescent protein (YFP)–positive mice. Neurospheres from the YFP mice (male or female) were counted after being plated in the environment of wild-type mice of the opposite sex. We observed a significant increase in the number of male-derived neurospheres in the presence of metformin in an overwhelmingly female environment. Conversely, the coculture of cells from the female SVZ in an overwhelmingly male environment led to a loss of the NSC expansion (Fig. 3F), supporting an inhibitory signal from the male environment that prevents the metformin-mediated expansion.
Metformin has sex-dependent effects on proliferation but increases neurogenesis in both sexes
Previous studies have shown that metformin treatment leads to an increase in proliferation within the SVZ (5). On the basis of our sex-dependent observations, we predicted that we would observe a differential effect of metformin on proliferation in the SVZ between females and males. Adult females and males received metformin for 1 week, and proliferating cells were labeled with bromodeoxyuridine (BrdU) for 2 days before perfusion (Fig. 4A). We observed a significant 1.37-fold increase in the number of BrdU+ cells in females (Fig. 4, B and C), but not males (Fig. 4, D and E). Metformin has also been shown to enhance neurogenesis (5), so we assessed whether there were sex-dependent effects of metformin on differentiation. One week of metformin treatment (Fig. 4F) led to an increase in the number of Dcx+Ki67+ cells in both females (Fig. 4, G and H) and males (Fig. 4, I and J), suggesting increased neurogenesis. These findings suggest that metformin is able to promote neurogenesis in both sexes, irrespective of the expansion in the NSC pool.
Fig. 4. Metformin has sex-dependent effects on proliferation but enhances neurogenesis in both females and males.
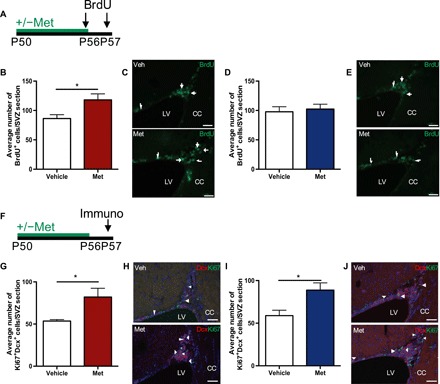
(A) Experimental paradigm. (B) Average number of BrdU+ cells in the SVZ of adult females treated with vehicle or Met (200 mg/kg) for 1 week (Vehicle, n = 4 mice; Met, n = 4 mice; P < 0.05; Student’s t test). (C) BrdU+ cells (arrows) in the SVZ of vehicle- or Met-treated female mice. (D) Average number of BrdU+ cells in the SVZ of adult males treated with vehicle or Met (200 mg/kg) for 1 week (Vehicle, n = 3 mice; Met, n = 3 mice; Student’s t test). (E) BrdU+ cells (arrows) in the SVZ of vehicle- or Met-treated male mice. (F) Experimental paradigm. (G) Average number of Ki67 + Dcx + cells in the SVZ of female mice that received Met (200 mg/kg) for 1 week in vivo (Vehicle, n = 5 mice; Met, n = 5 mice; P < 0.05; Student’s t test). (H) Ki67+Dcx+ cells (arrowheads) in the SVZ of vehicle- or Met-treated female mice. (I) Average number of Ki67 + Dcx + cells in the SVZ of male mice that received Met (200 mg/kg) for 1 week in vivo (Vehicle, n = 4 mice; Met, n = 3 mice; P < 0.05; Student’s t test). (J) Ki67+Dcx+ cells (arrowheads) in the SVZ of vehicle- or Met-treated male mice. *P < 0.05. Scale bars, 50 μm. CC, corpus callosum; Dcx, doublecortin; LV, lateral ventricle; Met, metformin; Veh, vehicle.
Metformin treatment following H-I rescues cognitive deficits in adult females
Metformin treatment promotes recovery of motor function following neonatal H-I injury (6). To determine whether the sex-dependent effects of metformin on the NPC population differentially affect functional recovery, we assessed cognitive function, which is impaired in adult mice following neonatal H-I insult (28, 29). Following H-I, both males and females demonstrated a significant impairment in trial 8 of the puzzle box task, with an increased latency to complete the trial (Fig. 5, A to C); this reflects a deficit in the acquisition of a novel task (removing the cardboard plug to access the goal box). Five weeks of metformin treatment, beginning 1 day after injury and extending into adulthood, led to a significant improvement in trial 8 in females (Fig. 5B). Conversely, males who received metformin showed no significant improvement compared to H-I–injured animals (Fig. 5C). In addition, H-I–injured females had a slightly higher pass rate than males (61.5% versus 45.5%, respectively), with the female pass rate even higher and more similar to controls in females that received metformin compared with males (fig. S6, E and F; H-I + Met females, 80%; H-I + Met male, 46.2%). Mice that were able to learn the task in trial 8 were able to complete trial 9 as quickly as controls (fig. S6, A and B), suggesting that a motor impairment does not account for the increased latency on trial 8 in H-I–injured animals. Using the foot fault task, we demonstrated that by 5 weeks after H-I, injured animals showed no significant deficit in motor function (fig. S6, C and D). These results suggest that the sex-dependent effects of metformin on the NPC population may play a role in functional recovery following injury.
Fig. 5. Long-term metformin treatment leads to cognitive improvements in females, but not males, following early postnatal H-I injury.
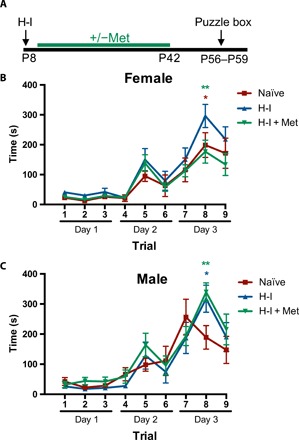
(A) Experimental paradigm. (B) Trial times in the puzzle box task for female mice comparing naïve (uninjured), H-I injured, and H-I+ metformin-treated cohorts (Naïve, n = 11 mice; H-I, n = 13 mice; H-I + Met, n = 15 mice; *P < 0.05, **P < 0.01, F8,288 = 31.73) repeated-measures two-way ANOVA). (C) Trial times in the puzzle box task for male mice comparing naïve (uninjured), H-I injured, and H-I + metformin-treated cohorts, which resulted in a trial effect (Naïve, n = 8 mice; H-I, n = 11 mice; H-I + Met, n = 13 mice; *P < 0.05, **P < 0.01, F8,232 = 32.93 repeated-measures two-way ANOVA). *P < 0.05, **P < 0.01. Met, metformin.
DISCUSSION
Metformin has garnered much attention in the field of neural regeneration, owing to its capacity to activate the endogenous stem cell pool in the brain and enhance neurogenesis. Here, we have demonstrated that metformin has age- and sex-dependent effects on SVZ-derived NPCs and that circulating sex hormones play a role in the response of NPCs to metformin. Furthermore, we show that these sex differences may affect cognitive recovery after injury. These findings highlight the importance of understanding the role of aging and sex with a view toward therapeutic applications.
While early postnatal mice respond robustly to metformin treatment, regardless of sex, the effect is attenuated in juveniles and diverging in adulthood into responsive females and nonresponsive males. Using ovariectomized and castrated animals, as well as exogenously delivered estradiol, we showed that this differential effect is linked to hormones. Hormone levels fluctuate throughout the life span of the animal. Early in postnatal development, there is a short period when both male and female rats experience a small peak in estradiol, whereas a peak in testosterone occurs only in males within hours of birth (30). When male and female mice go through puberty (~5 and 6 weeks of age), there are increases in testosterone and estradiol, respectively (31). The changes in sex hormones coincide with the timeline of responsiveness of NPCs to metformin, consistent with the observation that estradiol and testosterone play permissive and inhibitory roles, respectively, in regulating NPC behavior in response to metformin.
We have demonstrated through the manipulation of systemic estradiol and testosterone that sex hormones affect the impact of metformin on NPCs in the SVZ. Notably, local estradiol synthesis also occurs within the brain and may play a role in our findings. Studies have demonstrated that aromatase, an enzyme that converts testosterone to estradiol, is expressed in various regions in the male and female brain, particularly in the hypothalamus and amygdala (2–4, 32, 33). In the SVZ, aromatase is more highly expressed in males than females (5). The extent of conversion of testosterone to estradiol in the male niche in the SVZ, and how this estradiol may affect surrounding cells, is not known, but our data reveal that the removal of testosterone by castration leads to an expansion of the NSC pool. Hence, testosterone has inhibitory effects of NSCs irrespective of whether it is being converted to estradiol in the male SVZ niche. We would hypothesize that the level of conversion of testosterone to estradiol through aromatase is low in the male SVZ, which is consistent with the interpretation that estradiol, or the removal of testosterone, creates a permissive environment.
Our findings reveal that the adult female and male niche exerts opposing effects on the responsiveness of NSCs to metformin. In females, estradiol creates a permissive environment, as demonstrated by the lack of NSC expansion in ovariectomized females, combined with the attenuation of responsiveness to metformin in vitro when cells are passaged out of the niche, and lastly, the increase in NSCs following estradiol treatment in juveniles. Conversely, testosterone is inhibitory in the male environment, as evidenced by the responsiveness of NSCs in castrated mice and the expansion of passaged NSCs in the absence of the niche. While some studies have shown the direct effects of hormones on NSCs and neurogenesis, there is little known about how hormones affect other cells in the niche or the composition of the niche (25, 26). Our findings support the hypothesis that hormones regulate factors released by niche cells, leading to differential effects on NSCs and how they respond to drugs. Estradiol has been shown to induce the expression of growth factors, such as the BDNF (brain-derived neurotrophic factor), as well as act as a transcription factor to activate several pathways such as the MAP (mitogen-activated protein) kinase pathway. These could be potential mechanisms through which estradiol enables the effects of metformin on the NPC pool (34, 35). Moreover, hormones can alter estradiol receptor expression and levels of cytochrome p450 enzymes in NPCs (21, 22). Hence, hormones may differentially affect the expression of other proteins implicated in the response to metformin exposure, such as the transporter through which metformin enters cells, or the proteins involved in the downstream pathways, such as TAp73 and aPKC-CBP (atypical protein kinase C - CREB binding protein).
We found that the sex-dependent effects of metformin on NPCs may be correlated with functional recovery after injury, as we observed a sex-dependent improvement in cognitive function following an H-I injury. This was an intriguing finding as the H-I injury itself is known to alter NPCs and the niche, so it was unknown what effect these injury-induced changes would have on the ability of metformin to expand the population (35, 36). We assessed cognition using the puzzle box task, as it allowed us to examine different aspects of cognition, such as learning, problem solving, and short- and long-term memory (37). While metformin-induced expansion of the NPC pool and neurogenesis following H-I have been correlated with functional motor recovery, whether metformin would be effective at rescuing cognitive deficits was not clear (6). It is noteworthy that while our findings demonstrate a correlation between metformin-induced NPC expansion and functional recovery in the puzzle box task, it does not rule out the possibility that enhanced hippocampal neurogenesis may also be contributing to the observed cognitive improvement. In addition, our findings are consistent with studies demonstrating that sex and age can affect the severity of the injury, as well as functional outcomes. For example, over the age of 75, women have more strokes than men and exhibit poorer poststroke outcomes (38). Sexual dimorphisms also exist with regard to postinjury mechanisms of cell death, and age plays a role in the severity of stroke infarct (39). This highlights the importance of assessing preclinical drugs in both sexes, as sex and age may lead to differential therapeutic outcomes.
While our study focused primarily on the effects of metformin on NPCs and how these effects are mediated by hormones and the NPC niche, metformin has pleiotropic effects that could also affect neural recovery. It will be important to investigate the role of angiogenesis and relative contribution of NSC activation in the recovery process and whether these effects are sex dependent. It is possible that the pleiotropic effects of metformin could alter the behavior of other cells in the NSC niche, such as endothelial cells and microglia, which may, in turn, alter NPC behavior. Furthermore, while we explored the impact of estradiol in mediating the effects of metformin on NPCs, we cannot rule out the possibility that progesterone may also play a role in creating a permissive environment, as progesterone is effectively reduced following an ovariectomy.
While we have determined that there are age- and sex-dependent effects of metformin on NPCs in the uninjured brain, it is unclear whether similar effects would be seen in the injured brain in terms of the expansion of the stem cell pool. Our findings reveal a correlation with NPC expansion and cognitive recovery in a neonatal model of stroke, which further supports a role for metformin in brain repair; however, its application to other injury models remains unclear. Our studies demonstrating the role of the niche highlight the need to explore the effects of metformin in different models of injury, which lead to diverse changes in the cellular microenvironment. We are currently pursuing these studies using a juvenile brain irradiation injury model as an important step to determine whether the combination of injury and metformin, at an age when the NPC pool is not responsive in the uninjured state, can lead to NPC expansion and functional recovery. When considering the impact of the sex- and age-dependent effects of metformin in humans, a comprehensive understanding of hormonal regulation in disease/aging and injury states will be critical to ensure benefit.
Here, we have demonstrated hormone-mediated sex- and age-dependent effects of metformin on the NPC population. These findings may have significant clinical implications, as the therapeutic effects of metformin are being investigated in numerous clinical trials, including brain injury. This study could inform the selection criteria for who may benefit most from metformin treatment for brain injury, specifically with respect to the age and sex of patients.
MATERIALS AND METHODS
Animals
All experiments were performed in accordance with institutional guidelines approved by the Animal Care Committee at the University of Toronto. C57Bl/6 mice were purchased from Charles River Laboratories (Montreal, QC) or bred in-house. Castrated and ovariectomized mice were purchased from the Jackson laboratory (Maine, USA). Rosa-YFP mice used for the coculture experiments were bred in-house. Following weaning, mice were group housed in a 12-hour light/dark cycle, and food and water were provided ad libitum. Throughout these experiments, neonatal mice were defined as P8 to P12, juvenile mice were P17 to P28, and adults were over 6 weeks of age.
Surgeries
The H-I injury was performed as previously described (6). Briefly, P8 mice were anesthetized with isoflurane, and the left common carotid artery was permanently ligated with 6-0 silk sutures and then transected. Mice were returned to their home cage to recover for 1.5 hours. Subsequently, mice were placed in a hypoxic chamber (8% oxygen) for 1 hour while the body temperature was maintained at 37°C using a heating pad. Castration and ovariectomy surgeries were performed by the Jackson laboratory at P28 before arrival.
Drug administration
For in vitro experiments, a fresh stock solution of metformin (1,1-dimethylbiguanide hydrochloride; Sigma-Aldrich, Oakville Ontario) was prepared (0.167 mg/ml) in serum-free media (SFM), containing 10X Dulbecco’s modified Eagle’s medium/F12, 30% glucose, 7.5% NaHCO3, 1 M Hepes, l-glutamine, hormone mix, and penicillin and streptomycin. Dilutions were made in SFM to reach the desired end concentration. For in vivo experiments, metformin was prepared fresh daily in sterile 1X phosphate-buffered saline (PBS). For experiments in naïve early postnatal preweaning animals, mice received subcutaneous injections of metformin (20 mg/kg) once daily from P9 to P12. In juveniles and young adults, mice received intraperitoneal injections of metformin (200 mg/kg) once daily from P21 to P27 and from P50 to P56, respectively. When investigating the ideal dose of metformin in males, mice received 20 or 200 mg/kg of metformin via intraperitoneal injections for 4 or 7 days. For experiments investigating the effects of hormones, estradiol (5.6 mg/kg, Sigma-Aldrich, Missouri, USA) or vehicle (sesame oil, Sigma-Aldrich, Missouri, USA) was administered subcutaneously from P17 to P27, along with concurrent intraperitoneal metformin (200 mg/kg) or PBS injections from P21 to P27, as mentioned above. For testosterone administration, testosterone propionate (100 μg, Toronto Research Chemicals, Ontario, Canada) or vehicle (sesame oil) was injected subcutaneously from P9 to P12, along with concurrent intraperitoneal injections of metformin (20 mg/kg) or PBS. To investigate the effect of post–H-I metformin treatment, mice received daily subcutaneous injections (20 mg/kg) from P9 to P27, followed by drug delivery through an implanted subcutaneous osmotic pump (20 mg/kg per day; Durect Corporation, California, USA) from P28 to P41.
BrdU treatment and staining
BrdU (65 mg/kg, Sigma-Aldrich, Missouri, USA) was administered via intraperitoneal injection once daily on P56 and P57. One hour after the last injection, mice were anesthetized with 250 mg/kg tribromoethanol (Sigma-Aldrich, Missouri, USA) and then transcardially perfused with cold 1X PBS followed by 4% paraformaldehyde (PFA; Sigma-Aldrich, Missouri, USA). The brains were extracted and transferred to 4% PFA to postfix for 1 hour and then stored at 4°C in 20% sucrose for cryoprotection. The brains were cryosectioned (20-μm sections) on superfrost slides (Fisher Scientific, Pennsylvania, USA) and maintained at 4°C until staining.
Slides were thawed for 10 min at room temperature and rehydrated with 1X PBS followed by permeabilization with 0.03% Triton X-100 (Sigma-Aldrich, Missouri, USA). Slides were incubated in 1 N HCl at 65°C for 30 min and then in 10% normal donkey serum (NDS; Sigma-Aldrich, Missouri, USA) for 1 hour at room temperature. Next, slides were incubated with primary rat BrdU antibody (1:100, Abcam, Cambridge, UK) overnight followed by a donkey anti-rat Alexa Fluor 488 secondary antibody (1:400, Invitrogen, Oregon, USA) for 2 hours at 37°C and then coverslipped with Vectamount mounting media (Vector, California). Quantification was performed at ×20 magnification by counting one section per every 200 μm beginning at the crossing of the corpus callosum and ending at the appearance of the anterior commissure. Approximately four to six sections were counted per brain. BrdU+ cells were counted on a Zeiss microscope (Axiovert 200 M, Zeiss, Germany).
Immunohistochemistry
Dcx/Ki67 staining
Slides were thawed for 10 min at room temperature then rehydrated with 1X PBS. Antigen retrieval was performed by incubating the slides in citrate buffer at 95°C for 15 min. Slides were cooled to room temperature before incubation with 5% NDS in 0.03% Triton X-100 for 1 hour at room temperature and then incubated with goat anti-Ki67 (1:500, Abcam, Cambridge, UK) and mouse anti-doublecortin (Dcx) (1:200, Santa Cruz Biotechnology, Texas, USA) antibodies in 5% NDS in 0.03% Triton X-100 at 4°C overnight. Slides were incubated in secondary antibodies (donkey anti-goat Alexa Fluor 488 and donkey anti-mouse Alexa Fluor 568; 1:400 in PBS, Invitrogen, Oregon, USA) for 1.5 hours at room temperature and then incubated with DAPI (1:10,000, Invitrogen, Oregon, USA) for 10 min. Slides were coverslipped using Vectamount mounting media (Vector, California). Sections were imaged every 200 μm beginning at the crossing of the corpus callosum and ending at the appearance of the anterior commissure. Approximately four to five sections were imaged per brain at a ×20 magnification.
Neurosphere assay
For in vivo experiments, brain tissue was collected on the day of the last injection or 1 day after the last vehicle or metformin injection. Mice were anesthetized with isoflurane (Fresenius Kabi, Ontario, Canada) and euthanized by cervical dislocation. The brains were removed, and NPCs were isolated from the SVZ of mice at various ages as previously described (6, 40). Following the microdissection of the SVZ, cells were incubated in an enzyme solution [containing trypsin (1.3 mg/ml), hyaluronidase (0.76 mg/ml), kynurenic acid (0.12 mg/ml); Sigma-Aldrich, Missouri, USA] for 30 min at 37°C and then centrifuged at 1500 revolutions per minute (RPM) for 5 min. Cells were resuspended in a trypsin inhibitor solution (0.67 mg/ml; Worthington Biochemical Corporation, New Jersey, USA) and triturated, and then centrifuged at 1500 RPM for 5 min. Cells were resuspended in SFM, triturated five times, centrifuged at 1500 RPM for 3 min, resuspended in 1 ml of SFM, and then counted on a hemocytometer. Cells were plated at clonal density (10 cells/μl) (41) in the presence of fibroblast growth factor (20 ng/ml, Gibco, New York, USA), epidermal growth factor (20 ng/ml, PeproTech, Quebec, Canada), and heparin (2 μg/ml, Sigma-Aldrich, Missouri, USA) in SFM (neurosphere media) for 7 days in the presence or absence of metformin, and the number of neurospheres was counted. For experiments with early postnatal mice, cells were plated at 5 cells/μl. For passaging, neurospheres were collected, dissociated into a single cell suspension, and replated at 2.5 cells/μl in neurosphere media for 7 days. For coculture experiments, primary YFP cells were plated at 1 cell/μl as a control and cocultured with 9 cells/μl from C57Bl/6 mice of the opposite sex.
Behavioral tests
The puzzle box task was performed 7 weeks after injury as described previously (37, 42). The task assesses various domains of cognitive function, including short- and long-term memory and problem solving. Briefly, mice were acclimated to the brightly lit behavioral testing room for 10 min on each of the three consecutive testing days. On each day, mice performed three trials (37). For each trial, mice were placed in the brightly lit start box and required to solve tasks of increasing difficulty to enter a darkened goal box. The trials on the first day served as habituation trials. On trial 1, the underpass to the goal box was clear and there was an open door to the goal box. On trials 2 and 3, the underpass was clear, but there was no longer a door; therefore, mice had to go under the partition and through the underpass to enter the goal box. This was repeated the next day on trial 4. Trial 5 required mice to dig through bedding in the underpass, and this was repeated for trial 6 and again the next day for trial 7. On trial 8, mice were introduced to a cardboard plug that they were required to remove from the underpass to enter the goal box. This was repeated for trial 9. All trials were marked complete once the animal’s hind paws entered the goal box or after a maximum of 7 min. Mice were excluded from the study if it took them more than 3.5 min to complete any of the habituation trials on the first day.
The foot fault task was performed 5 weeks after injury to assess motor function. Mice were acclimated to the behavior room for 10 min before testing. Mice were placed on an enclosed wire grid and were free to explore for 3 min. A blinded observer watched the recorded tests and scored the mice based on the number of times their forepaws slipped through the wire grid. The percent fault difference was calculated as the number of slips per total steps with the right paw minus the number of slips/total steps with the left paw multiplied by 100.
Statistics
All data were analyzed using GraphPad Prism version 6 software. For comparisons of the two groups, an unpaired Student’s t test was used (Figs. 1 to 4). For grouped analyses in Fig. 5, a multiple comparisons test after two-way repeated-measures analysis of variance (ANOVA) with post hoc Tukey’s correction was performed. A multiple comparisons test after one-way ANOVA with post hoc Tukey’s correction was performed in figs. S1 and S2. A multiple comparisons test after two-way repeated-measures ANOVA with post hoc Tukey’s correction was used for figs. S4 and S6. A Student’s t test was used for figs. S3 and S5. A P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank J. Livingston, E. Gilbert, D. Derkach, A. Azimi, and N. Sachewsky for the help and expertise with some technical aspects of the research and in the preparation of the manuscript. Funding: This research was funded by an operating grant awarded to C.M.M. and the Doctoral Research Award awarded to R.M.R. from the Canadian Institutes of Health Research, as well as funding from the Ontario Institute for Regenerative Medicine, Brain Canada, the Stem Cell Network, and the Ontario Brain Institute. Author contributions: R.M.R. planned and performed experiments; collected, analyzed, and interpreted the data; and prepared and approved the manuscript. K.V.A. performed experiments and collected and analyzed the data. C.M.M. planned experiments, analyzed and interpreted the data, provided financial support, and prepared and approved the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax1912/DC1
Fig. S1. Metformin at 1 μM increases the number of neurospheres in an age- and sex-dependent manner.
Fig. S2. Metformin has no effect on the number of neurospheres from the adult male SVZ regardless of metformin dose or duration of treatment.
Fig. S3. The absolute number of neurospheres is the same in male and female C57 mice at each age examined.
Fig. S4. Lack of sex-dependent effects of estradiol and testosterone on primary and secondary neurospheres from the SVZ of adult female and male mice.
Fig. S5. Testosterone and metformin eliminate the effect of metformin on NPCs in the female SVZ.
Fig. S6. Motor impairments do not account for the deficits in task acquisition in females and males.
REFERENCES AND NOTES
- 1.Bellenchi G. C., Volpicelli F., Piscopo V., Perrone-Capano C., Di Porzio U., Adult neural stem cells: An endogenous tool to repair brain injury? J. Neurochem. 124, 159–167 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Miller F. D., Kaplan D. R., Mobilizing endogenous stem cells for repair and regeneration: Are we there yet? Cell Stem Cell 10, 650–652 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Erlandsson A., Lin C.-H. A., Yu F., Morshead C. M., Immunosuppression promotes endogenous neural stem and progenitor cell migration and tissue regeneration after ischemic injury. Exp. Neurol. 230, 48–57 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Jeffers M. S., Hoyles A., Morshead C., Corbett D., Epidermal growth factor and erythropoietin infusion accelerate functional recovery in combination with rehabilitation. Stroke 45, 1856–1858 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Gallagher D., Devito L. M., Cancino G. I., Tsui D., He L., Keller G. M., Frankland P. W., Kaplan D. R., Miller F. D., Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11, 23–35 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Dadwal P., Mahmud N., Sinai L., Azimi A., Fatt M., Wondisford F. E., Miller F. D., Morshead C. M., Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Rep. 5, 166–173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatt M., Hsu K., He L., Wondisford F., Miller F. D., Kaplan D. R., Wang J., Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Rep. 5, 988–995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Q., Cheng J., Liu Y., Wu J., Wang X., Wei S., Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 40, 131–142 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Venna V. R., Li J., Hammond M. D., Mancini N. S., Mccullough L. D., Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke 39, 2129–2138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Tang G., Zhang Z., Wang Y., Yang G.-Y., Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci. Lett. 579, 46–51 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Tang G., Li Y., Wang Y., Chen X., Gu X., Zhang Z., Wang Y., Yang G.-Y., Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflammation 11, 177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao L., Li D., Liu H., Jiang F., Xu Y., Cao Y., Gao R., Chen G., Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res. Bull. 140, 154–161 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Gargano J. W., Reeves M. J., Sex differences in stroke recovery and stroke-specific quality of life: Results from a statewide stroke registry. Stroke 38, 2541–2548 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Petrea R. E., Beiser A. S., Seshadri S., Kelly-hayes M., Kase C. S., Wolf P. A., Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 40, 1032–1037 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkayed N. J., Harukuni I., Kimes A. S., London E. D., Traystman R. J., Hurn P. D., Gender-linked brain injury in experimental stroke. Stroke 29, 159–166 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Gillies G. E., McArthur S., Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 62, 155–198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruddy R. M., Morshead C. M., Home sweet home: The neural stem cell niche throughout development and after injury. Cell Tissue Res. 371, 125–141 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Doetsch F., A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 13, 543–550 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Solano Fonseca R., Mahesula S., Apple D. M., Raghunathan R., Dugan A., Cardona A., O’Connor J., Kokovay E., Neurogenic niche microglia undergo positional remodeling and progressive activation contributing to age-associated reductions in neurogenesis. Stem Cells Dev. 25, 542–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J., Daniels S. B., Lennington J. B., Notti R. Q., Conover J. C., The aging neurogenic subventricular zone. Aging Cell 5, 139–152 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Waldron J., McCourty A., Lecanu L., Neural stem cell sex dimorphism in aromatase (CYP19) expression: A basis for differential neural fate. Stem Cells Cloning 3, 175–182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecanu L., Sex, the underestimated potential determining factor in brain tissue repair strategy. Stem Cells Dev. 20, 2031–2035 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Waldron J., McCourty A., Lecanu L., Aging differentially affects male and female neural stem cell neurogenic properties. Stem Cells Cloning Adv Appl. 3, 119–127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanapat P., Hastings N. B., Reeves A. J., Gould E., Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 19, 5792–5801 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatar C., Bessert D., Tse H., Skoff R. P., Determinants of central nervous system adult neurogenesis are sex, hormones, mouse strain, age, and brain region. Glia 61, 192–209 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Barker J. M., Galea L. A. M., Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience 152, 888–902 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Kato A., Hojo Y., Higo S., Komatsuzaki Y., Murakami G., Yoshino H., Uebayashi M., Kawato S., Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front. Neural. Circuits 7, 149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Kooij M. A., Ohl F., Arndt S. S., Kavelaars A., van Bel F., Heijnen C. J., Mild neonatal hypoxia-ischemia induces long-term motor- and cognitive impairments in mice. Brain Behav. Immun. 24, 850–856 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Ten V. S., Wu E. X., Tang H., Bradley-Moore M., Fedarau M. V., Ratner V. I., Stark R. I., Gingrich J. A., Pinsky D. J., Late measures of brain injury after neonatal hypoxia–ischemia in mice. Stroke 35, 2183–2188 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Konkle A. T. M., McCarthy M. M., Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152, 223–235 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetter-O’Hagen C. S., Spear L. P., Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol. 54, 523–535 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelgadir S. E., Resko J. A., Ojeda S. R., Lephart E. D., Mcphaul M. J., Roselli C. E., Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology 135, 395–401 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Roselli C. E., Liu M., Hurn P. D., Brain aromatization: Classic roles and new perspectives. Semin. Reprod. Med. 27, 207–217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharfman H. E., MacLusky N. J., Estrogen-growth factor interactions and their contributions to neurological disorders. Headache 48 ( suppl. 2), S77–S89 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoff R. P., Bessert D. A., Barks J. D. E., Song D., Cerghet M., Silverstein F. S., Hypoxic–ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice. Int. J. Dev. Neurosci. 19, 197–208 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Rothstein R. P., Levison S. W., Damage to the choroid plexus, ependyma and subependyma as a consequence of perinatal hypoxia/ischemia. Dev. Neurosci. 24, 426–436 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Ben Abdallah N. M.-B., Fuss J., Trusel M., Galsworthy M. J., Bobsin K., Colacicco G., Deacon R. M. J., Riva M. A., Kellendonk C., Sprengel R., Lipp H.-P., Gass P., The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp. Neurol. 227, 42–52 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Reeves M. J., Bushnell C. D., Howard G., Gargano J. W., Duncan P. W., Lynch G., Khatiwoda A., Lisabeth L., Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 7, 915–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manwani B., McCullough L. D., Sexual dimorphism in ischemic stroke: Lessons from the laboratory. Women’s Health 7, 319–339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morshead C. M., Benveniste P., Iscove N. N., van der Kooy D., Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat. Med. 8, 268–273 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Coles-Takabe B. L., Brain I., Purpura K. A., Karpowicz P., Zandstra P. W., Morshead C. M., van den Kooy D., Don’t look: Growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 26, 2938–2944 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Nusrat L., Livingston-Thomas J. M., Raguthevan V., Adams K., Vonderwalde I., Corbett D., Morsheed C. M., Cyclosporin A-mediated activation of endogenous neural precursor cells promotes cognitive recovery in a mouse model of stroke. Front. Aging Neurosci. 10, 93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax1912/DC1
Fig. S1. Metformin at 1 μM increases the number of neurospheres in an age- and sex-dependent manner.
Fig. S2. Metformin has no effect on the number of neurospheres from the adult male SVZ regardless of metformin dose or duration of treatment.
Fig. S3. The absolute number of neurospheres is the same in male and female C57 mice at each age examined.
Fig. S4. Lack of sex-dependent effects of estradiol and testosterone on primary and secondary neurospheres from the SVZ of adult female and male mice.
Fig. S5. Testosterone and metformin eliminate the effect of metformin on NPCs in the female SVZ.
Fig. S6. Motor impairments do not account for the deficits in task acquisition in females and males.


