Abstract
Objective
Determine sarcomere length (Ls) operating ranges of the superficial masseter and temporalis in vitro in a macaque model and examine the impact of position-dependent variation on Ls and architectural estimates of muscle function (i.e., fiber length, PCSA) before and after Ls-normalization.
Design
Heads of adult Macaca fascicularis (n=4) and M. mulatta (n=3) were bisected postmortem. One side of the jaw was fixed in occlusion, the other in maximum gape. Ls was measured bilaterally using laser diffraction and these measurements were used to estimate sarcomere-length operating ranges. Differences in fiber length and PCSA between sides were tested for significance prior to and following Ls-normalization.
Results
Sarcomere-length operating ranges were widest for the anterior superficial masseter and narrowest for the posterior temporalis. Compared with other mammals, macaque operating ranges were wider and shifted to the right of the descending limb of a representative length-tension curve. Fibers were significantly stretched by as much as 100%, and PCSAs reduced by as much as 43%, on the maximally gaped compared with occluded sides. Ls-normalization substantially reduced position-dependent variance.
Conclusions
The superficial masseter ranges between 87–143% and the temporalis between 88–130% of optimal Ls from maximum gape to occlusion, indicating maximum relative Ls for these macaque muscles exceeds the upper end range previously reported for the jaw muscles of smaller mammals. The wider macaque operating ranges may be functionally linked to the propensity for facially prognathic primates to engage in agonistic canine display behaviors that require jaw-muscle stretch to facilitate production of wide jaw gapes.
Keywords: masseter, temporalis, sarcomere length, fiber length, macaque
1. Introduction
A theoretical tradeoff exists between force production and excursion in muscle based on the inverse relationship between a muscle’s ability to generate force and the length of its fibers (Gans, 1982). In prior work, we have examined this potential tradeoff in several comparisons of primate jaw muscles, finding that some species exhibit this tradeoff (e.g., tree-gouging common marmosets; Taylor, Eng, Anapol, & Vinyard, 2009) while others do not (e.g., crab-eating macaques; Taylor & Vinyard, 2009; Terhune, Hylander, Vinyard, & Taylor, 2015). Multiple factors can potentially intervene to moderate this tradeoff such as increasing muscle weight, which increases a muscle’s physiologic cross-sectional area (PCSA) and thus its force-generating capacity.
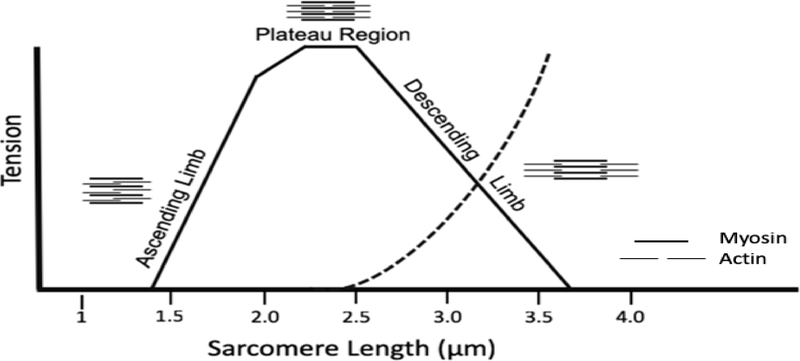
Another factor that impacts muscle force production is the lengths of constituent sarcomeres during contraction. Sarcomeres have the capacity to shorten (concentric) and lengthen (eccentric) during muscle contraction; the range over which sarcomeres actively shorten and are lengthened within a muscle fiber characterizes the sarcomere-length operating range (LsOR). It is well appreciated that changes in sarcomere length (Ls) impact the amount of force that a muscle can generate (Gordon, Huxley, & Julian, 1966; Podolsky & Shoenberg, 1983; Williams & Goldspink, 1978). The structural basis for the relationship between Ls and muscle force is described by the sarcomere length-tension (L-T) curve (Fig. 1). Every sarcomere has a length at which the magnitude of overlap between myosin and actin is optimal for producing force – the plateau region of the L-T curve (Fig. 1). When sarcomeres are lengthened beyond their optimal length during muscle stretch (the descending limb of the L-T curve), the overlap between actin and myosin filaments is decreased and muscle force diminishes (Fig. 1). If a muscle is sufficiently stretched to the point where no myosin cross-bridges can interact with actin filaments, no force can be generated. Stretch beyond the sarcomere operating range, whether via trauma or fatigue, can disrupt myofibril architecture (Fridén & Lieber, 1992; Lieber & Fridén, 1993). As sarcomeres shorten during muscle contraction, more cross-bridges are formed and active force increases until the plateau region is reached. With continued shortening (the ascending limb of the L-T curve), the actin filaments begin overlapping one another. This overlap impedes the formation of additional cross bridges, again diminishing muscle force production (Fig. 1). At sufficiently short lengths, the myosin filament reaches the z-disk, which prohibits any further shortening of the sarcomere. At this point, no more force can be generated. Thus, where sarcomeres (and by extension, muscle fibers) operate on the length-tension (L-T) curve has important implications for muscle function.
Figure 1.
Schematic of a representative sarcomere length-tension curve for skeletal muscle. Myosin (thick) and actin (thin) filaments show varying amounts of overlap in different regions of the length-tension curve. Tension diminishes as myofilament overlap increases (Ascending Limb) or decreases (Descending Limb) relative to the Plateau Region. Dotted line represents passive tension.
The influence of sarcomere length changes on muscle function during contraction leads to consideration of a problem frequently encountered in studies of muscle fiber architecture; namely, the variance that is introduced into architectural estimates of muscle force when joints are fixed in different positions. It is widely appreciated that joint position contributes significantly to variation in sarcomere and, by extension, fiber length in limb (Felder, Ward, & Lieber, 2005; Sacks & Roy, 1982; Williams & Goldspink, 1978) and jaw-closing (Nordstrom, Bishop, & Yemm, 1974; Nordstrom & Yemm, 1972) muscles. Fixation of limbs in either flexed or extended positions results in shortened or lengthened muscles, respectively, and subsequent changes in fiber lengths (Felder et al., 2005; Nordstrom & Yemm, 1972; Williams & Goldspink, 1978). Because fiber length (Lf) is used in the equation to estimate muscle PCSA (Gans, 1982; Powell, Roy, Kanim, Bello, & Edgerton, 1984; Weber, 1851), and PCSA is proportional to a muscle’s maximum force-generating capacity (Powell et al., 1984), positional variation impacting Lf necessarily impacts architectural estimates of muscle force. Moreover, stretch-related changes in fiber architecture have physiological consequences as they result in muscles operating on different portions of the L-T curve (e.g., Williams & Goldspink, 1978).
One way to minimize positional variation in fiber length within a given muscle across individuals is to select a similar joint position of interest either experimentally at the time of fixation (Anapol & Jungers, 1986; Felder et al., 2005; Sacks & Roy, 1982) or opportunistically in a comparative sample (Perry & Wall, 2008; Taylor & Vinyard, 2004). However, in practice, cadaveric specimens are often obtained from museums, zoological institutions and research centers, where fixation of cadavers in desired joint positions is generally not possible. In such instances, it is common practice to normalize fiber length estimates using a standard sarcomere length (Ls) to facilitate comparisons among individuals and species (Anapol & Barry, 1996; Anapol & Gray, 2003; Anapol, Shahnoor, & Gray, 2004; Anapol, Shahnoor, & Ross, 2008; Eng, Ward, Vinyard, & Taylor, 2009; Huq, Wall, & Taylor, 2015; Lieber & Blevins, 1989; Lieber & Fridén, 2001; Organ, Teaford, & Taylor, 2009; Taylor et al., 2009; Ward, Hentzen, Smallwood, Eastlack, Burns, Fithian, Fridén, & Lieber, 2006).
Variation in masseter and temporalis sarcomere lengths from occlusion to maximum mouth opening (jaw gape) has been documented in a handful of species (Table 1). However, we currently do not know the impact of this variation on jaw-adductor fiber architecture (e.g., fiber length, PCSA). To better understand this system, we report on estimates of LsOR measured in vitro for the masseter and temporalis muscles in a moderately large-bodied, facially prognathic Old World monkey (Macaca) that generates relatively wide jaw gapes as a component of their social display behavior (Deputte, 1994). We then use these data to experimentally test the extent to which maximum jaw gape impacts sarcomere and fiber lengths and how these changes influence muscle force production. Finally, we quantitatively test the ability of Ls-normalization to effectively eliminate joint-angle dependent variation in fiber length in jaw adductors.
Table 1.
Sarcomere length ranges reported for the masseter and temporalis muscles in various species.1
| Species | Muscle | Ls Min (μm) | Ls Max (μm) | Reference |
|---|---|---|---|---|
| Human | Masseter | 1.90 | 4.80 | Van Eijden & Raadsheer, 1992 |
| Human | Temporalis | 2.20 | 3.80 | Van Eijden & Raadsheer, 1992 |
| Rabbit | Masseter | 2.13 | 3.27 | Weijs & Van Der Wielen-Drent, 1983 |
| Rabbit | Temporalis | 2.10 | 2.80 | Weijs & Van Der Wielen-Drent, 1982 |
| Rat | Anterior masseter | 1.94 | 2.98 | Nordstrom et al., 1974 |
| Rat | Posterior masseter | 2.09 | 2.45 | Nordstrom et al., 1974 |
| Rat | Temporalis | 1.77 | 2.29 | Nordstrom et al., 1974 |
| Common marmoset | Anterior superficial masseter | 1.63 | 2.87 | Eng et al., 2009 |
| Common marmoset | Posterior superficial masseter | 1.63 | 1.73 | Eng et al., 2009 |
| Common marmoset | Anterior temporalis | 1.59 | 2.47 | Eng et al., 2009 |
| Common marmoset | Middle temporalis | 1.59 | 2.44 | Eng et al., 2009 |
| Common marmoset | Posterior temporalis | 1.59 | 2.29 | Eng et al., 2009 |
| Cotton-top tamarin | Anterior superficial masseter | 1.51 | 3.39 | Eng et al., 2009 |
| Cotton-top tamarin | Posterior superficial masseter | 1.51 | 1.92 | Eng et al., 2009 |
| Cotton-top tamarin | Anterior temporalis | 1.77 | 3.47 | Eng et al., 2009 |
| Cotton-top tamarin | Middle temporalis | 1.77 | 3.44 | Eng et al., 2009 |
| Cotton-top tamarin | Posterior temporalis | 1.77 | 2.67 | Eng et al., 2009 |
Ls minimum (Min) and maximum (Max) were measured at occlusion and a maximum linear jaw gape of 10.5 mm, respectively, in the rabbit, and at occlusion and a maximum linear gape of 16.5 mm in the rat. Ls Min was measured at occlusion in the common marmoset and cotton-top tamarin and Ls Max was modeled in both species to a maximum active jaw gape of 24.2 mm. Ls Min in humans was measured from a Caucasian cadaveric sample with the jaws fixed in the mouth-closed position and an average interincisal distance of 4.2 mm; the Ls Max for human masseter and temporalis are modeled estimates.
2. Materials and Methods
2.1. Samples
We took sarcomere and architectural measurements of the superficial masseter and temporalis muscles on four cadaveric adult female Macaca fascicularis (7.6–10.6 years; 3.0–4.4 kg) and three adult male M. mulatta (9.0–15.0 years; 9.1–12.0 kg). The heads were bisected postmortem and one side of the jaw was fixed in centric occlusion while the other side was fixed in maximum jaw gape. All macaque cadavers were without obvious craniofacial or dental pathologies. No animals were sacrificed for purposes of this study.
2.2. Fiber architecture measurements
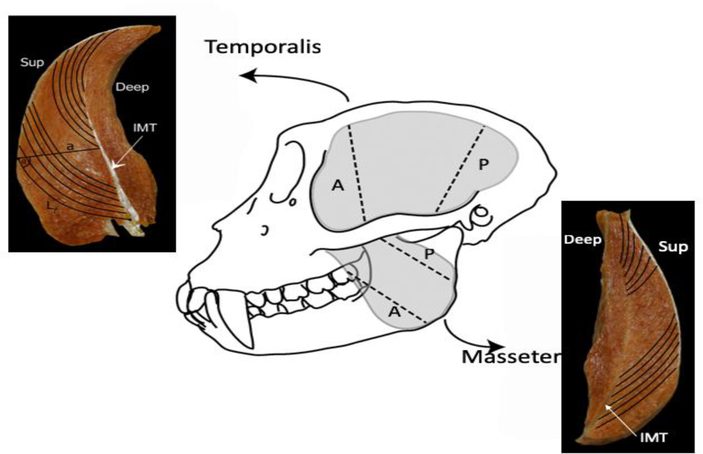
For each fixed specimen, the skin and overlying fascia were removed and the right and left masseter and temporalis muscles harvested en masse from the skull. Muscles were blotted dry, trimmed of fat and fascia and weighed to the nearest 1.0 g or 0.1 g depending on size. The deep and superficial portions of the masseter muscles were separated and the superficial masseter was used for analysis. Following previously published protocols (Anapol & Barry, 1996; Anapol & Jungers, 1986; Anapol et al., 2008; Taylor et al., 2009; Terhune et al., 2015) we separated the superficial masseter and temporalis muscles into anterior and posterior regions, and sectioned these muscle regions along their lengths, from superficial to deep (perpendicular to the plane of bony attachment), to prepare each region for in situ fiber length measurements (Fig. 2).
Figure 2.
Schematic of a macaque skull (M. fascicularis). The gray-shaded areas represent the temporalis and masseter muscles in situ. Once removed en masse from the skull, each muscle was sectioned along its length into anterior and posterior regions (black hatched lines) for fiber length measurements. Cross sections of each muscle depict measurements taken at each section: Lf, fiber length; a, perpendicular distance from the intramuscular tendon [IMT] to the superficial tendon. Pinnation angle [θ] was computed as the arcsine of a/NLf.
For each muscle region, we measured fiber length (Lf) as the distance between the proximal and distal myotendinous junctions (IMT; Fig. 2). We measured six adjacent fibers from each of two locations for a total of 12 fibers per muscle region and a maximum of 24 fibers per muscle. For each fiber, we measured the perpendicular distance (a) from the distal myotendinous junction to the central tendon (Fig. 2) and used this measurement to estimate pinnation angle. Care was taken to measure only intact, uncut fibers running from tendon attachment to tendon attachment to ensure that Lf measurements were sampled from comparable sites from right and left sides of the skull and across specimens.
Upon completion of fiber length measurements, a small chunk of muscle was removed from each Lf sampling site and digested at room temperature in 30% HNO3 to facilitate isolation of fiber bundles. Small fiber bundles then were manually separated under a dissecting stereomicroscope (Nikon SMZ 1500), mounted on glass slides and cover-slipped. We measured in situ sarcomere lengths (±0.01 μm) directly from these measured fibers using laser diffraction (Lieber, Yeh, & Baskin, 1984) and normalized Lf of the measured fibers to a standardized Ls of 2.41 μm.1 Normalized Lf (NLf) was computed as
We calculated average Lf and NLf for the anterior and posterior superficial masseter and temporalis. We computed pinnation angle for both raw (Lf) and normalized (NLf) fibers as the arcsine of a/fiber length. We estimated physiologic cross-sectional area (PCSA) for the superficial masseter and temporalis following (Gans & Bock, 1965; Powell et al., 1984):
where 1.0564 is muscle-specific density (Murphy & Beardsley, 1974). We employed the same equation using NLf to estimate normalized PCSA (NPCSA).
2.3. Data analysis
We measured in situ sarcomere lengths bilaterally for each specimen from muscles fixed in occlusion on one side and from muscles fixed in maximum gape on the other side. These values provide estimates of minimum and maximum sarcomere lengths to yield estimates of LsOR for each muscle region. We superimposed the LsORs on a representative L-T curve derived from rhesus macaque myofilament lengths (Walker and Schrodt, 1974). We estimated Lf and NLf for each muscle region (i.e., anterior and posterior) and PCSA and NPCSA for each muscle (i.e., superficial masseter and temporalis) for the occluded and maximally gaped sides.
Macaca fascicularis are smaller in body (Smith & Jungers, 1997) and jaw (Hylander, 2013) size compared with M. mulatta and both species are sexually dimorphic. Although each individual served as its own control, males and females differed in maximum gape and, thus, in their LsORs. Maximum gape at time of fixation ranged between 56.0–58.8 mm for female M. fascicularis and between 87.4–103.2 mm for male M. mulatta. Given this, we treated the female M. fascicularis and male M. mulatta separately in all statistical analyses.
We used one-tailed paired Student’s t-tests to evaluate differences in Ls, Lf, and PCSA between the occluded and gaped sides. We predicted significantly longer sarcomeres, longer fibers, and smaller PCSAs for the muscles fixed in maximum jaw gape. The majority of the heads fixed in centric occlusion showed a nominal amount of variation in incisor gape (between 0–4.0 mm for M. fascicularis and 0–4.5 mm for M. mulatta). However, one male M. mulatta had a linear gape on the occluded side of 8.9 mm (~10% of maximum gape). Thus, we normalized the muscles from both the occluded and gaped sides and tested for significant side-to-side differences. We predicted no significant differences between the occluded and maximally gaped sides in fiber length or PCSA following sarcomere-length normalization of the muscles. We used an a priori α=0.05.
3. Results and Discussion
3.1. Macaque masseter and temporalis sarcomere length operating ranges
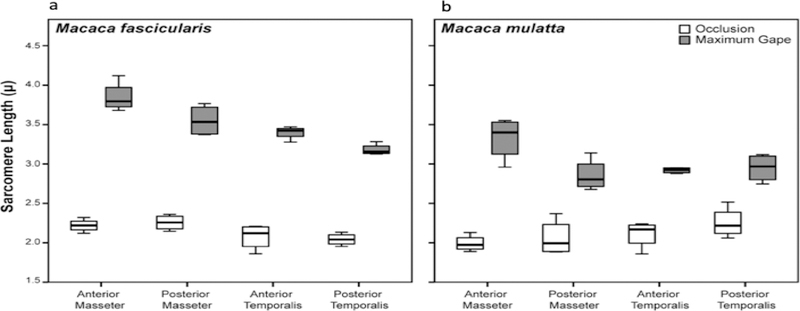
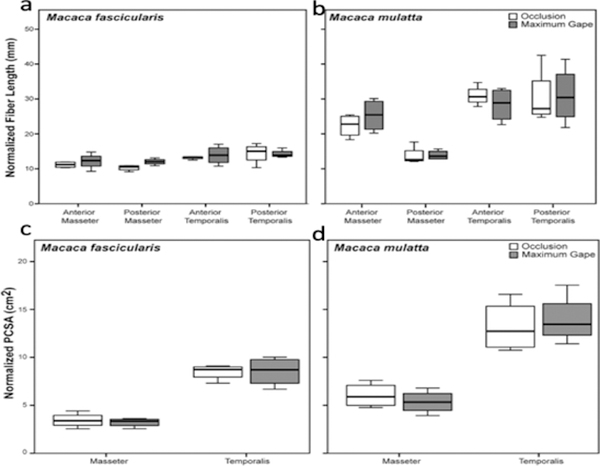
Sarcomere length operating ranges varied by individual, species, muscle and muscle region but all fell within the theoretical physiological range of 1.3–4.1 μm (Walker and Schrodt, 1974; Table 2 and Supplementary Table S1). On the occluded side, minimum Ls ranges between 1.96–2.26 μm for the superficial masseter and between 2.04–2.28 μm for the temporalis (Table 2 and Fig. 3). Maximum Ls for the gaped side ranges between 2.92–3.85 μm for the superficial masseter and 2.93–3.40 μm for the temporalis (Table 2 and Fig. 3). These values represent percentage increases (i.e., from minimum Ls to maximum Ls) of between 49–73% for the superficial masseter and 31–63% for the temporalis. The anterior superficial masseter operated over the widest LsOR in both species/sexes, while the posterior temporalis operated over the narrowest range (Table 2 and Fig. 3). These results are consistent with previous findings for similar muscle regions in other mammals (Table 1). However, in M. fascicularis, there was a clear antero-posterior gradient with Ls Max being greatest in the anterior superficial masseter, followed by the posterior superficial masseter, anterior temporalis and posterior temporalis (Table 2 and Fig. 3a). No such gradient was observed for M. mulatta (Fig. 3b).
Table 2.
Sarcomere-length operating ranges for the anterior and posterior superficial masseter and temporalis muscles and results of tests for significant differences in Ls Min and Ls Max.1
| Muscle | Species | Ls Min (μm) | Ls Max (μm) | % Increase | P-value |
|---|---|---|---|---|---|
| Anterior superficial masseter (ASM) | M. fascicularis | 2.22 | 3.85 | 73.4 | 0.0006 |
| M. mulatta | 1.99 | 3.45 | 73.4 | 0.0012 | |
| Posterior superficial masseter (PSM) | M. fascicularis | 2.26 | 3.55 | 57.1 | 0.0015 |
| M. mulatta | 1.96 | 2.92 | 49.0 | 0.0155 | |
| Anterior temporalis (AT) | M. fascicularis | 2.08 | 3.40 | 63.5 | 0.0005 |
| M. mulatta | 2.08 | 2.93 | 40.9 | 0.0064 | |
| Posterior temporalis (PT) | M. fascicularis | 2.04 | 3.18 | 55.9 | 0.0002 |
| M. mulatta | 2.28 | 2.98 | 30.7 | 0.0074 |
Ls Min and Ls Max are based on species means.
Figure 3.
Box plots of masseter and temporalis sarcomere length measurements taken at occlusion and maximum jaw gape for a) M. fascicularis and b) M. mulatta. Sarcomere lengths at maximum jaw gape are significantly (P < 0.05) greater compared with sarcomere lengths at occlusion in both species for all muscle regions (see Table 2). Sarcomere-length operating range is greatest for the anterior superficial masseter in both species. In this and all subsequent box plots, line within box denotes median. Boundaries of the box represent 25th and 75th percentiles. Whiskers indicate 10th and 90th percentiles.
3.2. Position-dependent variation and sarcomere-length normalization
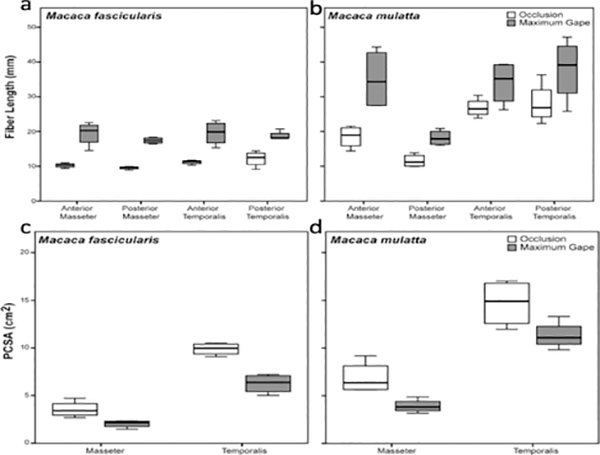
Fibers from the gaped side of the jaws were significantly longer compared with the occluded side for both sexes/species (Table 3 and Fig. 5). Fibers were stretched by as much as 100% for the superficial masseter and by as much as 75% for the temporalis (Table 3). PCSAs were also significantly reduced in the gaped compared with the occluded sides, by as much as 43% for the masseter and 37% for the temporalis (Table 3 and Fig. 5). The reduction in PCSAs is the result of significantly elongated fibers (Table 3) combined with decreased pinnation angles in comparison with the occluded side (Supplementary Table S2). Following Ls normalization of fibers, there were no significant differences between the occluded and gaped sides in Lf or PCSA (Table 4 and Fig. 6), indicating that Ls normalization effectively eliminated the joint-dependent variation in Lf and PCSA.
Table 3.
Means (± standard deviations) and one-tailed significance tests for differences in superficial masseter and temporalis fiber lengths and PCSAs between occluded and gaped sides.1
| M. fascicularis | M. mulatta | |||||||
|---|---|---|---|---|---|---|---|---|
| Measurements | Occluded side | Gaped side | %Change | P-value | Occluded side | Gaped side | %Change | P-value |
| Superficial Masseter | ||||||||
| Anterior Lf (mm) | 10.2±0.7 | 19.4±3.5 | 88.7 | 0.006 | 18.8±3.9 | 37.7±8.9 | 100.1 | 0.013 |
| Posterior Lf (mm) | 9.6±0.5 | 17.4±0.9 | 81.9 | 0.002 | 11.4±2.2 | 18.9±2.1 | 66.5 | 0.016 |
| PCSA (cm2) | 3.6±0.9 | 2.0±0.4 | 42.8 | 0.016 | 7.3±1.8 | 4.2±0.6 | 43.0 | 0.027 |
| Temporalis | ||||||||
| Anterior Lf (mm) | 11.2±0.6 | 19.6±3.5 | 74.6 | 0.006 | 26.8±3.3 | 36.6±4.6 | 36.6 | 0.041 |
| Posterior Lf (mm) | 12.2±2.3 | 18.8±1.3 | 54.3 | 0.005 | 30.0±5.5 | 41.8±5.5 | 39.2 | 0.006 |
| PCSA (cm2) | 9.9±0.7 | 6.3±1.0 | 36.7 | 0.004 | 15.6±2.1 | 11.4±1.8 | 27.2 | 0.012 |
%Change represents the % increase in fiber length, and the % decrease in PCSA, in the gaped compared with the occluded sides.
Figure 5.
Box plots of masseter and temporalis fiber lengths (a-b) and PCSAs (c-d) at occlusion and maximum jaw gape. Fibers are significantly longer (P < 0.05) and PCSAs significantly smaller (P < 0.05) at maximum jaw gape compared with fibers and PCSAs at occlusion in both species for all muscle regions (see Table 3). As fibers are stretched (and pinnation angles decrease; Supplementary Table S2), muscle PCSAs decrease as a function of change in jaw position.
Table 4.
Means (± standard deviations) and significance tests for differences in architectural estimates normalized for sarcomere length between occluded and gaped sides.1
| M. fascicularis | M. mulatta | |||||||
|---|---|---|---|---|---|---|---|---|
| Measurements | Occluded side | Gaped side | %Change | P-value | Occluded side | Gaped side | %Change | P-value |
| Superficial Masseter | ||||||||
| Anterior NLf (mm) | 11.2±0.9 | 12.2±2.3 | 9.0 | 0.340 | 22.8±3.9 | 26.3±5.3 | 15.3 | 0.052 |
| Posterior NLf (mm) | 10.3±0.8 | 12.0±0.9 | 16.7 | 0.087 | 14.1±3.1 | 13.8±1.7 | 2.6 | 0.700 |
| NPCSA (cm2) | 3.4±0.8 | 3.2±0.5 | 6.7 | 0.541 | 6.3±1.4 | 5.8±0.9 | 7.8 | 0.574 |
| Temporalis | ||||||||
| Anterior NLf (mm) | 13.2±0.5 | 13.9±2.7 | 5.3 | 0.660 | 31.2±3.5 | 30.3±3.9 | 3.3 | 0.620 |
| Posterior NLf (mm) | 14.4±2.9 | 14.3±1.2 | 1.2 | 0.921 | 32.3±8.8 | 34.1±6.8 | 5.1 | 0.700 |
| NPCSA (cm2) | 8.5±0.8 | 8.5±1.5 | 0.6 | 0.956 | 14.0±2.6 | 14.2±3.1 | 1.4 | 0.679 |
%Change represents the % increase in fiber length, and the % decrease in PCSA, in the gaped compared with the occluded sides.
Figure 6.
Box plots of masseter and temporalis fiber lengths (a-b) and PCSAs (c-d) at occlusion and maximum jaw gape, normalized to a standard sarcomere length of 2.41 μm. The absence of significant differences (P > 0.05) in fiber lengths and PCSAs between the occluded and maximally-gaped sides indicates that Ls-normalization has effectively eliminated the impact of position-dependent variation in fiber lengths and PCSAs in these jaw muscles (see Table 4).
3.3. Comparison of LsORs between female M. fascicularis and male M. mulatta
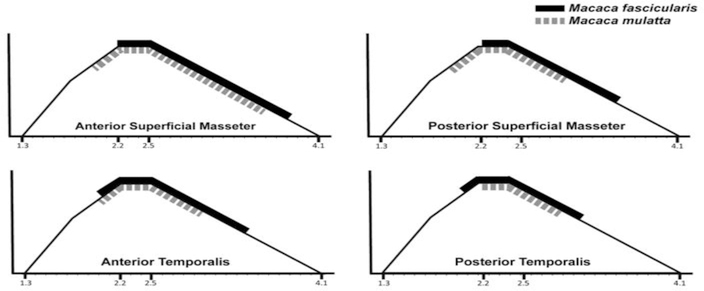
For all muscle regions, smaller-bodied female M. fascicularis showed wider LsORs compared with larger-bodied male M. mulatta, though this difference for the anterior superficial masseter is minimal (Table 2; Figs. 3 and 4). Macaca fascicularis also displayed absolutely shorter fibers compared with M. mulatta (Table 3). When we statistically evaluated these differences in fiber length with the jaws in occlusion (using one-tailed Mann-Whitney U-tests), we found that M. fascicularis had significantly shorter fibers for the anterior (p=0.017) and posterior (p=0.039) masseter and the anterior (p=0.017) and posterior (p=0.017) temporalis, compared with M. mulatta. The wider LsORs observed for M. fascicularis can be explained by their significantly shorter fibers. Shorter fibers indicate fewer sarcomeres in series, resulting in a greater amount of stretch per sarcomere. The wider LsOR of M. fascicularis were also shifted further to the right of the L-T curve such that they operate over a greater portion of the descending limb compared with male M. mulatta (Fig. 4).
Figure 4.
Masseter and temporalis sarcomere-length operating ranges for M. fascicularis (solid black line) and M. mulatta (hatched grey line) superimposed on a length-tension curve for rhesus macaque myofilament lengths (Walker and Schrodt, 1974). Operating range is interpolated from Ls values at occlusion and maximum gape. Male Macaca mulatta sarcomere lengths operate over narrower ranges and are shifted to the left compared with female M. fascicularis, reflecting the longer male fibers. With more sarcomeres in series to minimize the amount of stretch during angular rotation of the joint, fibers remain closer to the peak of the length-tension curve, minimizing loss of force.
Both male M. fascicularis and M. mulatta have significantly wider maximum jaw gapes compared with female conspecifics (Hylander, 2013) and the internal architecture of their jaw adductors likely mitigates the stretch-related loss of muscle force at wide jaw gapes. Specifically, the longer fibers of males with more sarcomeres in series minimize the amount of muscle stretch needed to achieve a given amount of angular rotation at a joint. This, in turn, presumably minimizes loss of force as fibers would remain closer to the peak of the L-T curve. Because the longer fibers of male M. mulatta distribute lengthening over more sarcomeres, their fibers operate over a narrower LsOR, and are shifted to the left of the L-T curve, compared with female M. fascicularis. As an important first approximation, these in vitro LsOR estimates justify future in vitro and in vivo studies to determine the operating ranges of the jaw adductors and where these muscles operate along the L-T curve during feeding and active gape display behaviors.
3.4. Comparison of masseter and temporalis LsORs with other species
Many skeletal muscle fibers likely operate over a wide range of sarcomere lengths during contraction (Burkholder & Lieber, 2001; Eng et al., 2009; Herring, Grimm, & Grimm, 1984; Nordstrom et al., 1974; Son, Indresano, Sheppard, Ward, & Lieber, 2018; Ward et al., 2006; Weijs & van der Wielen-Drent, 1982, 1983). A survey of LsOR across a variety of vertebrates (Burkholder & Lieber, 2001) reported a substantial amount of variation in LsOR among limb and jaw muscles within and among species. Portions of the ascending and descending limbs of the L-T curve commonly were included in the LsOR in different species’ muscles, indicating that many species have the ability to operate over a wide range of the L-T curve. The functional consequences of operating over a wide LsOR are enhanced fiber (and presumably whole muscle) excursion while at some expense to a muscle’s force-generating capacity. Synergistic muscle groups are one way to counteract the reduction in force of any single muscle.
Sarcomere-length operation range has been estimated in vitro for the masseter and temporalis muscles in humans (van Eijden, Korfage, & Brugman, 1997; Van Eijden & Raadsheer, 1992) and in a handful of small mammals (Eng et al., 2009; Hertzberg, Muhl, & Begole, 1980; Nordstrom et al., 1974; Nordstrom & Yemm, 1972; Weijs & van der Wielen-Drent, 1982, 1983) (Table 1). Collectively, these LsOR estimates suggest that the jaw-closing muscles have the capacity to operate over a wide range of sarcomere lengths and well into the descending limb of the L-T curve, where sarcomeres are stretched and active force is diminished (Fig. 1). This configuration translates into the ability to generate a relatively wide jaw gape, a performance that is essential for feeding (Vinyard, Wall, Williams, & Hylander, 2003), predatory (Kiltie, 1984; Slater & Van Valkenburgh, 2009; Williams, Peiffer, & Ford, 2009) and gape display behaviors (Deputte, 1994; Verheyen, 1954). From a functional standpoint, wide gapes likely move jaw-closing muscle fibers onto the descending limb of the L-T curve, where the overlap of actin-myosin filaments is decreasing, and active force is declining, relative to the plateau region (Fig. 1). Given that where muscles operate along the L-T curve has implications for force production, an understanding of how sarcomere length changes during movement can provide important insights into how muscle-joint complexes are structured to facilitate muscle function. For the masticatory apparatus, this includes performance at extreme joint postures, such as generating adequate bite forces at wide jaw gapes. We note that, due to the absence of sensory feedback (e.g., muscle spindles, golgi tendon organs), maximum LsORs estimated in vitro are likely to be greater than maximum active or passive ranges estimated in vivo and we currently lack data on LsOR throughout the gape cycle during active behaviors such as chewing or biting.
Minimum Ls estimates for the macaque superficial masseter and temporalis muscles are similar to those previously measured for humans, rabbits, and rats (with the exception of rat temporalis). Alternatively, minimum Ls fell well above those reported for other anthropoid primates such as common marmosets and cotton-top tamarins (Tables 1 and 2), suggesting that in occlusion, the marmoset and tamarin jaw muscles were markedly shortened (Eng et al., 2009). By contrast, maximum Ls for macaque anterior and posterior superficial masseter and temporalis exceed those reported for all other species with the exception of humans (Tables 1 and 2). We note that maximum Ls for human (Van Eijden and Raadsheer, 1992), common marmoset, and cotton-top tamarin (Eng et al., 2009) masseter and temporalis are modeled estimates. Given that at ~4.3 μm, human actin and myosin filaments have likely reached the point of no overlap (Lieber, Loren, & Fridén, 1994), the modeled maximum Ls for human masseter of 4.80 μm may be an overestimate.
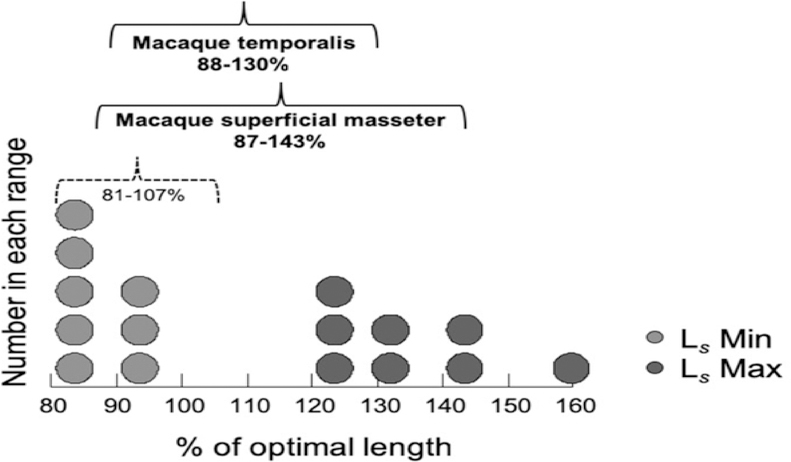
Minimum and maximum relative Ls (computed as percent optimal length, L0), have an average range of 81–107% across a sample of 51 vertebrate limb and jaw muscles (Burkholder & Lieber, 2001). Following Burkholder & Lieber (2001), we estimated minimum and maximum relative Ls for our macaque data by dividing our in situ Ls measurements from the occluded and gaped sides by an optimal Ls estimate of 2.41 μm. Results showed that the superficial masseter has an average range of between 87–143% and the temporalis between 88–130% of optimal Ls (Fig. 7). These findings indicate that maximum relative Ls for these macaque jaw muscles falls well above the upper end of the range for limb and jaw muscles (Burkholder & Lieber, 2001), and above the upper end of the range for relative masseter (88–127%) and temporalis (84–108%) sarcomere lengths reported for rabbit and rat masseter and temporalis (Burkholder & Lieber, 2001).
Figure 7.
Dot density histogram of relative sarcomere lengths (measured sarcomere length divided by a standard sarcomere length of 2.41 μm; reported as % of optimal length, L0) for macaque anterior and posterior superficial masseter and anterior and posterior temporalis muscles. Light grey dots represent relative Ls Min (at occlusion) and dark grey dots represent relative Ls Max (at maximum jaw gape), for each muscle region, averaged by species. The average range is 87–143% L0 for macaque masseter and 88–130% L0 for the temporalis (solid black brackets). The range for relative sarcomere lengths for these macaque jaw-adductors includes a large portion of the descending limb of the L-T curve, and is both wider and shifted to the right of the L-T curve in comparison with the range reported for jaw and limb muscles (81–107%; dotted black bracket) across a variety of vertebrates (Burkholder & Lieber, 2001). The macaque pattern may be characteristic of facially prognathic primates and other mammals that generate relatively wide maximum jaw gapes.
There are several possible explanations for why our in vitro maximum Ls estimates are longer in comparison with those in the same muscles in other species. One possibility is that small mammals like rabbits and rats do not generate relatively wide maximum jaw gapes akin to those of facially prognathic macaques, with the net effect being that less strain is imposed on the sarcomeres when stretched during wide-mouth opening. That said, maximum sarcomere length estimates compiled in Burkholder & Lieber (2001) for these mammals do not appear to have been measured with the jaws in maximum gape. Maximum angular gapes estimated in vivo for rabbits range between 20–25° (Weijs & Dantuma, 1981) but the Ls estimate used in Burkholder & Lieber (2001) was from a maximum angular gape of 8.5° (Weijs & van der Wielen-Drent, 1983). Likewise, the rat data are from jaws fixed in linear gapes up to 16.5 mm (Nordstrom et al., 1974) but in Nordstrom & Yemm (1974) rats achieved maximum linear gapes of up to 20.9 mm. Thus, it seems likely that the maximum Ls reported in the literature for these muscles in these species are underestimates, resulting in underestimates of their LsORs.
Facial configuration influences the position of the jaw adductors on the skull and their capacity for muscle stretch (Herring & Herring, 1974). Long, prognathic faces, like those of macaques, are correlated with the production of relatively wide maximum jaw gapes (Hylander, 2013), which is facilitated by muscle stretch. Similar to other sexually dimorphic and prognathic Old World monkeys, macaques generate relatively wide jaw gapes as part of their behavioral repertoire of engaging in agonistic canine display (or ‘yawning’) (Deputte, 1994). Thus, macaque facial morphology may also contribute to their greater maximum Ls. Our current findings indicate that their passive superficial masseter and temporalis LsORs are both wider and shifted to encompass more of the descending limb of the L-T curve as compared with other mammals. Additional work is needed on other primates and nonprimate mammals to determine whether this finding reflects a macaque morphotype or whether this pattern is characteristic of moderate- to large-bodied prognathic mammals that generate relatively wide maximum jaw gapes.
3.5. Divergence in LsOR between the superficial masseter and temporalis muscles
In both macaque species, LsORs are greatest for the anterior superficial masseter compared to other muscle regions (Table 2). In M. fascicularis the posterior superficial masseter also has a wider LsOR compared with the anterior and posterior temporalis (Table 2). These findings suggest that when fibers are stretched during mouth opening, sarcomere strain is greatest in the superficial masseter, particularly the anterior region of this muscle. Likewise, in both species the anterior superficial masseter operates over a greater portion of the descending limb of the L-T curve compared with the temporalis, facilitating muscle stretch and the production of wide mouth opening. Alternatively, the temporalis operates over a more favorable region of the L-T curve for generating muscle and bite force.
The differences in LsOR between the superficial masseter and temporalis can be added to a suite of physiological and morphological features that support functional divergence between these two muscles. Previous work evaluating jaw adductor muscle-activity patterns during chewing suggests the superficial masseter may have evolved diverse functional roles in different groups of primates, while temporalis muscle function appears more evolutionarily conserved for generating vertical bite force (Hylander, Ravosa, Ross, Wall, & Johnson, 2000; Hylander, Wall, Vinyard, Ross, Ravosa, Williams, & Johnson, 2005; Vinyard, Ravosa, Wall, Williams, Johnson, & Hylander, 2007; Vinyard, Wall, Williams, & Hylander, 2008; Ram & Ross, 2018). Work relating masseter and temporalis fiber architecture and electromyography in primates show similar functional relationships (Vinyard & Taylor, 2010). Our findings of a wider sarcomere-length operating range for the masseter while the temporalis is restricted closer to the plateau region of the L-T curve where sarcomere overlap is optimal for maximizing muscle and bite force support these EMG patterns. Architectural studies of the jaw-adductors in anthropoid primates (e.g., common marmosets, crab-eating macaques) likewise indicate a functional partitioning of the masseter and temporalis (Taylor et al., 2009; Terhune et al., 2015).
3.6. The impact of position-dependent variation on fiber architecture
Our findings demonstrate that variation in joint position at the time of fixation has the potential to introduce substantial variation in architectural estimates of muscle excursion/contraction velocity (Lf) and muscle force (PCSA) (Table 3). Previous reports of fiber length increases in rat limb muscles from neutral to extreme plantarflexion (160°) fall well within these ranges (~53%−75% for the tibialis anterior, extensor digitorum longus and soleus; Felder et al., 2005). In our sample, we estimated the variance in Lf between the occluded and gaped sides (Table 3) as ranging between 30.2–106.1 mm2 across the four muscle regions examined. Sarcomere-length normalization substantially reduces this variance to 1.6–4.9 mm2 (Table 4). We likewise observe reduced variance for PCSA (3.2–8.1 cm for raw PCSA compared with 0.4–0.9 cm2 for PCSA normalized for Ls) (Tables 3 and 4).
In this study, our focus has been on position-dependent variation that translates into error for estimating fiber lengths at a consistent position on the L-T curve. We recognize that there are other sources of error associated with estimating fiber architecture. For example, beyond standard measurement error, estimates of pinnation are a source of error. This is because fibers rotate during muscle contraction and this rotation impacts force generation and fiber contraction velocity that cannot be accounted for in static estimates of pinnation (Azizi, Brainerd, & Roberts, 2008; Azizi & Roberts, 2014). Additionally, architectural estimates of muscle force do not take into account parallel or series elastic components, such as connective tissues and tendons, which can increase force following stretch of an active muscle (i.e., residual force enhancement; Rode, Siebert, Herzog, & Blickham, 2009).
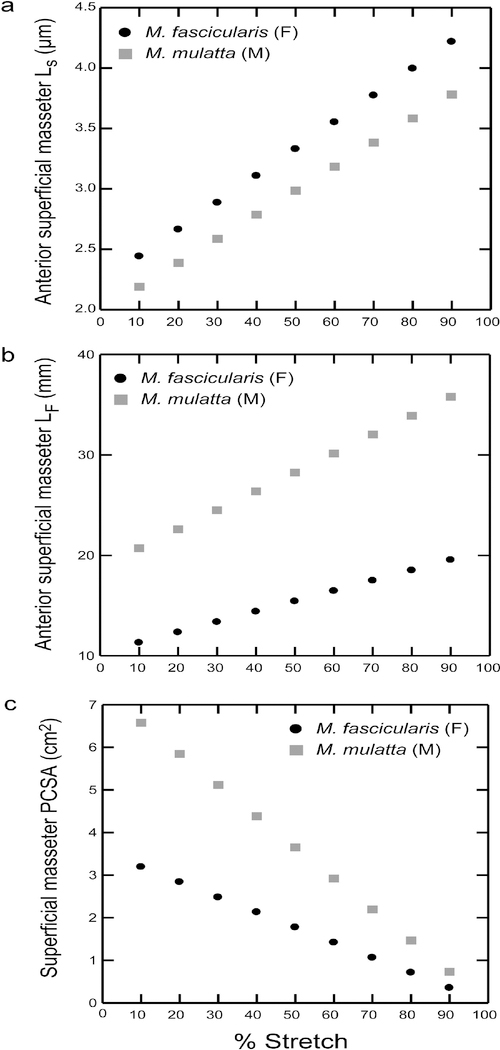
Although the variances reported here are associated with joints fixed in extreme positions, our data show that even with incremental fiber stretching (e.g., 10% increments), sarcomeres show moderate increases in length (Supplementary Table S3). These incremental increases vary across muscle regions, ranging between 0.204–0.226 μm for M. fascicularis and 0.199–0.225 μm for M. mulatta (Fig. 8a). This equates to approximately 8–9% of resting length per sarcomere (assuming an average resting length of 2.41~2.5μm (Huxley, 1957; Huxley, 1972; Walker & Schrodt, 1974). These sarcomere-length increases translate into increases in fiber length and decreases in PCSAs. For example, anterior superficial masseter fibers undergo an average increase in length of ~1 mm in M. fascicularis and ~1–2 mm in M. mulatta per 10% fiber stretch (Fig. 8b). For the anterior and posterior temporalis, the average increase in fiber length per 10% stretch is greater, ranging between ~1–3 mm. Notably, our sarcomere lengths measured in vitro at maximum jaw gapes fall within these estimated ranges for sarcomeres stretched by as much as 70–80% for the anterior superficial masseter, 40–50% for the posterior superficial masseter, 40–60% for the anterior temporalis and 30–50% for the posterior temporalis. Our fiber lengths measured at maximum gape fall within the estimated ranges for fibers stretched by as much as 90% for the anterior superficial masseter and our superficial masseter PCSAs measured at maximum gape fall within the estimated ranges for fibers stretched by 40–50% (Fig. 8c; Supplementary Table S3). Given that maximizing an opportunistic sample is likely to require including specimens with jaws fixed in a variety of positions, these findings demonstrate the importance of a correction factor for position-dependent variation, such as normalizing fiber length measurements to a standard sarcomere length.
Figure 8.
Plots of changing a) sarcomere lengths (Ls); b) fiber length (Lf); and c) PCSA as a function of percent fiber stretch for the anterior superficial masseter. Fiber stretch is interpolated in 10% increments from occlusion to 90%. For every 10% increase in stretch from occlusion, sarcomeres lengthen by ~8–9%, and fibers lengthen between 1–3 mm, depending on muscle region. Sarcomere and fiber lengths measured in vitro at maximum jaw gapes fall within our estimated ranges for sarcomeres stretched by as much as 70–80%, and fiber stretched by as much as 90%, respectively, from occlusion. (Supplementary Table S3).
4. Conclusion
Sarcomere-length operating ranges for macaque jaw adductors vary between 1.96–2.26 μm and 2.92–3.85 μm for the masseter, and between 2.04–2.28 μm and 2.93–3.40 μm for the temporalis. Macaque operating ranges were wider and shifted to the right of the descending limb of a representative length-tension curve compared with other mammals. These wider operating ranges may be functionally linked to agonistic canine display behaviors in male macaques that require jaw-muscle stretch to facilitate production of wide jaw gapes. Significant position-dependent variation in architectural estimates of muscle excursion and muscle force were effectively eliminated by normalizing fibers to a standard sarcomere length.
Supplementary Material
Highlights.
In vitro jaw-adductor sarcomere lengths increase from occlusion to maximum gape
Superficial masseter sarcomere lengths ranged between 1.96–2.26 μm and 2.92–3.85 μm
Temporalis sarcomere lengths ranged between 2.04–2.28 μm and 2.93–3.40 μm
Fibers at maximum gape were significantly stretched compared with occlusion
Normalizing sarcomere length effectively eliminates position-based length variation
Acknowledgements
We thank Dr. William Hylander, Duke University, and Dr. Michael Nader and the Nader Lab, Wake Forest School of Medicine, for providing the cadaveric specimens. The comments of two reviewers and the Editor improved the quality of this manuscript.
Funding
This work was supported by grants from the National Science Foundation (BCS 1723041) and the National Skeletal Muscle Research Center at UCSD (NIH R24 HD050837).
Footnotes
Conflict of interest
None declared.
Ethics approval
Not required. No animals were sacrificed for purposes of this study. All specimens were used secondarily to other research projects pertaining to drug addiction and cognitive function that were approved by the 2003 National Research Council Guidelines for the Care and the Use of Mammals in Neuroscience and Behavioral Research and with approval by the Animal Care and Use Committee of Wake Forest University.
Various standards have been used to normalize raw muscle fibers to a standard sarcomere length to minimize positional variation in architectural estimates of Lf in limb (Powell et al., 1984; Felder et al., 2005) and jaw (Anapol et al., 2008) muscles. As optimal Ls has not been empirically determined for any jaw adductor in any primate species, we use a standard of 2.41 μm, which falls within the range of optimal Ls for mammalian striated muscle (Huxley, 1957, 1972) and is the empirically determined optimal Ls for macaque hindlimb muscle (Walker & Schrodt, 1974). The use of this standard should not be construed to indicate fibers are being restored to their optimal Ls.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anapol F, & Barry K (1996). Fiber architecture of the extensors of the hindlimb in semiterrestrial and arboreal guenons. American Journal of Physical Anthropology, 99, 429–447. [DOI] [PubMed] [Google Scholar]
- Anapol F, & Gray JP (2003). Fiber architecture of the intrinsic muscles of the shoulder and arm in semiterrestrial and arboreal guenons. American Journal of Physical Anthropology, 122, 51–65. [DOI] [PubMed] [Google Scholar]
- Anapol F, & Jungers WL (1986). Architectural and histochemical diversity within the quadriceps femoris of the brown lemur (Lemur fulvus). American Journal of Physical Anthropology, 69, 355–375. [DOI] [PubMed] [Google Scholar]
- Anapol F, Shahnoor N, & Gray JP (2004). Fiber architecture, muscle function, and behavior: Gluteal and hamstring muscles of semiterrestrial and arboreal guenons In Anapol F, German RZ & Jablonski NG (Eds.), Shaping Primate Evolution (pp. 99–133). Cambridge: Cambridge Studies in Biological and Evolutionary Anthropology. [Google Scholar]
- Anapol F, Shahnoor N, & Ross CF (2008). Scaling of reduced physiologic cross-sectional area in primate muscles of mastication In Vinyard CJ, Ravosa MJ & Wall CE (Eds.), Primate Craniofacial Function and Biology (pp. 201–215). New York: Springer. [Google Scholar]
- Azizi E, Brainerd EL, & Roberts TJ (2008). Variable gearing in pennate muscles. Proc Natl Acad Sci U S A, 105, 1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, & Roberts TJ (2014). Geared up to stretch: pennate muscle behavior during active lengthening. Journal of Experimental Biology, 217, 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder TJ, & Lieber RL (2001). Sarcomere length operating range of vertebrate muscles during movement. Journal of Experimental Biology, 204, 1529–1536. [DOI] [PubMed] [Google Scholar]
- Deputte BL (1994). Ethological study of yawning in primates. I. Quantitative analysis and study of caucsation in two species of Old World monkeys (Cercocebus albigena and Macaca fascicularis). Ethology, 98, 221–245. [Google Scholar]
- Eng CM, Ward SR, Vinyard CJ, & Taylor AB (2009). The morphology of the masticatory apparatus facilitates muscle force production at wide jaw gapes in tree-gouging common marmosets (Callithrix jacchus). Journal of Experimental Biology, 212, 4040–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder A, Ward SR, & Lieber RL (2005). Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. Journal of Experimental Biology, 208, 3275–3279. [DOI] [PubMed] [Google Scholar]
- Fridén J, & Lieber RL (1992). Structural and mechanical basis of exercise-induced muscle injury (Review). Medicine and Science in Sports & Exercise, 24, 521–530. [PubMed] [Google Scholar]
- Gans C (1982). Fiber architecture and muscle function. Exercise and Sport Sciences Reviews, 10, 160–207. [PubMed] [Google Scholar]
- Gans C, & Bock WJ (1965). The functional significance of muscle architecture: a theoretical analysis. Ergeb Anat Entwicklungsgesch, 38, 115–142. [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, & Julian FJ (1966). The variation in isometric tension with sarcomere length in vertebrate muscle fibres. Journal of Physiology (London), 184, 143–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, & Herring SE (1974). The superficial masseter and gape in mammals. The American Naturalist, 108, 561–576. [Google Scholar]
- Herring SW, Grimm AF, & Grimm BR (1984). Regulation of sarcomere numer in skeletal muscle: A comparison of hypotheses. Muscle & Nerve, 7, 161–173. [DOI] [PubMed] [Google Scholar]
- Hertzberg SR, Muhl ZF, & Begole EA (1980). Muscle sarcomere length following passive jaw opening in the rabbit. The Anatomical Record, 197, 435–440. [DOI] [PubMed] [Google Scholar]
- Huq E, Wall CE, & Taylor AB (2015). Epaxial muscle fiber architecture favors enhanced excursion and power in the leaper Galago senegalensis. Journal of anatomy, 227, 524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF (1957). Muscle structure and theories of contraction. Progress in Biophysics and Biophysical Chemistry, 4, 255–312. [PubMed] [Google Scholar]
- Huxley HE (1972). Molecular basis of contraction in cross-striated muscles In Bourne GH (Ed.), The Structure and Function of Muscle, 2nd edition. (pp. 301–387). New York: Academic Press. [Google Scholar]
- Hylander WL (2013). Functional links between canine height and jaw gape in catarrhines with special reference to early hominins. American Journal of Physical Anthropology, 150, 247–259. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Ravosa MJ, Ross CF, Wall CE, & Johnson KR (2000). Symphyseal fusion and jaw-adductor muscle force: an EMG study. American Journal of Physical Anthropology, 112, 469–492. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Wall CE, Vinyard CJ, Ross CF, Ravosa MJ, Williams SH, & Johnson KR (2005). Temporalis function in anthropoids and strepsirrhines: an EMG study. American Journal of Physical Anthropology, 128, 35–56. [DOI] [PubMed] [Google Scholar]
- Kiltie RA (1984). Size ratios among sympatric neotropical cats. Oecologia, 61, 411–416. [DOI] [PubMed] [Google Scholar]
- Lieber RL, & Blevins FT (1989). Skeletal muscle architecture of the rabbit hindlimb: Functional implications of muscle design. Journal of Morphology, 199, 93–101. [DOI] [PubMed] [Google Scholar]
- Lieber RL, & Fridén J (1993). Muscle damage is not a function of muscle force but active muscle strain. Journal of Applied Physiology, 74(2), 520–526. [DOI] [PubMed] [Google Scholar]
- Lieber RL, & Fridén J (2001). Clinical significance of skeletal muscle architecture. Clinical Orthopedics and Related Research, 383, 140–151. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Loren GJ, & Fridén J (1994). In vivo measurement of human wrist extensor muscle sarcomere length changes. Journal of Neurophysiology 71, 874–881. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Yeh Y, & Baskin RJ (1984). Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophysics Journal, 45, 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RA, & Beardsley AC (1974). Mechanical properties of the cat soleus muscle in situ. American Journal of Physiology, 227, 1008–1013. [DOI] [PubMed] [Google Scholar]
- Nordstrom SH, Bishop M, & Yemm R (1974). The effect of jaw opening on the sarcomere length of the masseter and temporal muscles of the rat. Archives of Oral Biology, 19, 151–155. [DOI] [PubMed] [Google Scholar]
- Nordstrom SH, & Yemm R (1972). Sarcomere length in the masseter muscle of the rat. Archives of Oral Biology, 19, 895–902. [DOI] [PubMed] [Google Scholar]
- Organ JM, Teaford MF, & Taylor AB (2009). Functional correlates of fiber architecture of the lateral caudal musculature in prehensile and nonprehensile tails of the platyrrhini (primates) and procyonidae (carniovora). Anatomical Record A Discoveries in Molecular and Cellular Evolution and Biology, 292, 827–841. [DOI] [PubMed] [Google Scholar]
- Perry JMG, & Wall CE (2008). Scaling of the chewing muscles in Prosimians In Vinyard CJ, Ravosa MJ & Wall CE (Eds.), Primate Craniofacial Biology and Function (pp. 217–240). New York: Springer. [Google Scholar]
- Podolsky RJ, & Shoenberg M (1983). Force generation and shortening in skeletal muscle In Peachey LD, Adrian RH & Geiger SR (Eds.), Skeletal Muscle (pp. 173–187). Baltimore, MD: American Physiological Society. [Google Scholar]
- Powell PL, Roy RR, Kanim P, Bello MA, & Edgerton VR (1984). Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. Journal of Applied Physiology, 57, 1715–1721. [DOI] [PubMed] [Google Scholar]
- Ram Y, & Ross CF (2018). Evaluating the triplet hypothesis during rhythmic mastication in primates. Journal of Experimental Biology, 221, 165985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode C, Siebert T, Herzog W, & Blickham R (2009). The effects of parallel and series elastic components on the active cat soleus force-length relationship. Journal of Mechanics in Medicine and Biology, 9, 105–122. [Google Scholar]
- Sacks RD, & Roy RR (1982). Architecture of hind limb muscles of cats: Functional significance. Journal of Morphology, 173, 185–195. [DOI] [PubMed] [Google Scholar]
- Slater GJ, & Van Valkenburgh B (2009). Allometry and performance. The evolution of skull form and function in felids. Journal of Evolutionary Biology, 22, 2278–2287. [DOI] [PubMed] [Google Scholar]
- Smith RJ, & Jungers WL (1997). Body mass in comparative primatology. Journal of Human Evolution, 32, 523–559. [DOI] [PubMed] [Google Scholar]
- Son J, Indresano A, Sheppard K, Ward SR, & Lieber RL (2018). Intraoperative and biomechanical studies of human vastus lateralis and vastus medialis sarcomere length operating range. Journal of Biomechanics, 67, 91–97. [DOI] [PubMed] [Google Scholar]
- Taylor AB, Eng CM, Anapol FC, & Vinyard CJ (2009). The functional correlates of jaw-muscle fiber architecture in tree-gouging and nongouging callitrichid monkeys. American Journal of Physical Anthropology, 139, 353–367. [DOI] [PubMed] [Google Scholar]
- Taylor AB, & Vinyard CJ (2004). Comparative analysis of masseter fiber architecture in tree-gouging (Callithrix jacchus) and nongouging (Saguinus oedipus) callitrichids. Journal of Morphology, 261, 276–285. [DOI] [PubMed] [Google Scholar]
- Taylor AB, & Vinyard CJ (2009). Jaw-muscle fiber architecture in tufted capuchins favors generating relatively large muscle forces without compromising jaw gape. Journal of Human Evolution, 57, 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhune CE, Hylander WL, Vinyard CJ, & Taylor AB (2015). Jaw-muscle architecture and mandibular morphology influence relative maximum jaw gapes in the sexually dimorphic Macaca fascicularis. Journal of Human Evolution, 82, 145–158. [DOI] [PubMed] [Google Scholar]
- van Eijden TMGJ, Korfage JA, & Brugman P (1997). Architecture of the human jaw-closing and jaw-opening muscles. Anatomical Record A Discoveries in Molecular and Cellular Evolution and Biology, 248, 464–474. [DOI] [PubMed] [Google Scholar]
- Van Eijden TMGJ, & Raadsheer MC (1992). Heterogeneity of fiber and sarcomere length in the human masseter muscle. The Anatomical Record, 232, 78–84. [DOI] [PubMed] [Google Scholar]
- Verheyen R (1954). Monographie éthologique de l’hippopotame. Brussels: Institut des Parcs Nationaux du Congo Belge. [Google Scholar]
- Vinyard CJ, Ravosa MJ, Wall CE, Williams SH, Johnson KR, & Hylander WL (2007). Jaw-muscle function and the origin of primates In Ravosa MJ & Dagosto M (Eds.), Primate Origins and Adaptations (pp. 179–231). New York: Kluwer. [Google Scholar]
- Vinyard CJ, & Taylor AB (2010). A preliminary analysis of the relationship between jaw-muscle architecture and jaw-muscle electromyography during chewing across primates. The Anatomical Record, 293, 572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinyard CJ, Wall CE, Williams SH, & Hylander WL (2003). Comparative functional analysis of skull morphology of tree-gouging primates. American Journal of Physical Anthropology, 120, 153–170. [DOI] [PubMed] [Google Scholar]
- Vinyard CJ, Wall CE, Williams SH, & Hylander WL (2008). Patterns of variation across primates in jaw-muscle electromyography during mastication. Integrative and Comparative Biology, 48, 294–311. [DOI] [PubMed] [Google Scholar]
- Walker SM, & Schrodt GR (1974). I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. The Anatomical Record A Discoveries of Molecular and Cellular Evolution and Biology, 178, 63–81. [DOI] [PubMed] [Google Scholar]
- Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, Friden J, & Lieber RL (2006). Rotator cuff muscle architecture. Clinical Orthopedics and Related Research, 448, 157–163. [DOI] [PubMed] [Google Scholar]
- Weber EF (1851). Uber die Langenverhaltnisse der Fleischfasern der Muskeln im allgemeinen. Ber. K. Sachs. Ges. Wiss. Nat. Phys, K1, 64–86. [Google Scholar]
- Weijs WA, & Dantuma R (1981). Functional anatomy of the masticatory apparatus of the rabbit. Netherlands Journal of Zoology, 31, 99–147. [Google Scholar]
- Weijs WA, & van der Wielen-Drent TK (1982). Sarcomere length and EMG-activity in some jaw muscles of the rabbit. Acta Anatomica, 113, 178–188. [DOI] [PubMed] [Google Scholar]
- Weijs WA, & van der Wielen-Drent TK (1983). The relationship between sarcomere length and activation pattern in the rabbit masseter muscle. Archives of Oral Biology, 28, 307–315. [DOI] [PubMed] [Google Scholar]
- Williams PE, & Goldspink G (1978). Changes in sarcomere length and physiological properties in immobilized muscle. Journal of anatomy, 127, 459–468. [PMC free article] [PubMed] [Google Scholar]
- Williams SH, Peiffer E, & Ford S (2009). Gape and bite force in the rodents Onychomys leucogaster and Peromyscus maniculatus: does jaw-muscle anatomy predict performance? Journal of Morphology, 270, 1338–1347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.