Abstract
The ibogaine derivative 18-methoxycoronaridine (18-MC) has been found to decrease self-administration of morphine, nicotine and alcohol in rats after systemic injection. However oral dosing is the preferred route clinically. The current study evaluated the effect of oral 18-MC dosing in rats on alcohol and nicotine self-administration. For the nicotine study, young adult female Sprague-Dawley rats were fitted with IV jugular infusion catheters and trained for nicotine self-administration in 45 min. sessions. At weekly intervals they were administered by oral gavage doses of 18-MC (0, 10, 20 and 40 mg/kg) following a repeated measures counterbalanced design twice. Acute oral 18-MC, at the 40 mg/kg dosage, significantly reduced nicotine self-administration. There was a differential effect of 18-MC with rats above or below the median level of nicotine self-administration during the pretreatment baseline performance. Rats with lower baseline performance showed a significant reduction in nicotine self-administration with the 40 mg/kg dosage, while those in the higher baseline group did not show a significant effect of 18-MC. In alcohol studies, the effects of the same doses of 18-MC were tested in both male and female alcohol preferring (P) rats that had free access to water and alcohol (10% v/v) 6 hr/day. The results show that 18-MC dose-dependently reduced alcohol intake in both male and female rats. All doses caused significant reductions in alcohol self-administration. These data reinforce previous findings that 18-MC is significantly effective in reducing alcohol intake and nicotine self-administration. The finding that 18-MC is also effective orally makes it advantageous for further development as a possible new therapy for treating alcoholism as well as smoking addiction.
Keywords: Smoking, Treatment, Addiction, Alcoholism, Drinking, Alcohol Preferring Rat
Introduction
18-methoxycoronaridine (18-MC) is a synthetic iboga alkaloid congener, derived from ibogaine, a naturally occurring indole alkaloid found in the root bark of the West African shrub, Tabernanthe iboga (Bandarage et al. 1999). 18-MC has been shown to decrease the reinforcing efficacy of a variety of drugs of abuse including nicotine, morphine, cocaine, methamphetamine and alcohol after systemic injection (Glick et al. 1998; Maisonneuve and Glick 1999; Rezvani et al. 1997). 18-MC does not produce the same unwanted behavioral side effects and neuronal toxicity associated with ibogaine (Glick et al. 2000; Molinari et al. 1996). To help further determine the possible clinical use of 18-MC, it is important to see if it is effective in reducing nicotine and alcohol self-administration after oral administration.
Many drugs of abuse exert their reinforcing properties through a common neural substrate, enhancing dopamine release in the nucleus accumbens (Gessa et al. 1985; Koob et al. 1994; Tizabi et al. 2002). There is a dynamic relationship between both alcohol and nicotine in the mesolimbic dopaminergic system. Acute systemic administration of nicotine has been demonstrated to increase the firing rate of dopaminergic neurons and increase dopamine release in the nucleus accumbens of rats (Imperato et al. 1986; Tizabi et al. 2002). Similarly, systemic administration of alcohol has been shown to increase dopamine release in the nucleus accumbens in rats (Tizabi et al. 2002). It is possible that a shared neuronal pathway accounts for this phenomenon. It has been demonstrated that pretreatment (19h beforehand) with a systemic administration of 40 mg/kg 18-MC significantly attenuated nicotine-induced dopamine release in the nucleus accumbens of rats (Glick et al. 1998). Interestingly, the increase in dopamine release was associated with a significant and prolonged reduction in oral nicotine self-administration (Glick et al. 1998).
The current study was conducted to determine the efficacy of orally administered 18-MC for reducing alcohol and nicotine self-administration. Furthermore, rats with higher and lower baseline levels of nicotine self-administration were also assessed to determine possible differential 18-MC effects in lighter or heavier nicotine users. It was hypothesized that, similar to systemic administration, oral administration of 18-MC would reduce both alcohol and nicotine intake.
Methods
Subjects
Young adult female Sprague-Dawley rats (N=18; Taconic Farms, Germantown, NY, USA) were used for the nicotine study. For the alcohol study adult male (N=10) and female (N=9) alcohol preferring rats bred in our laboratory were used. The rats were housed individually on a 12:12 reversed light-dark cycle (lights on at 6 PM) so that experimental sessions occurred during the active part of their diurnal cycle. Animals in the nicotine study had ad libitum access to water at all times excluding experimental sessions, and were fed approximately 18 g food daily 20–30 minutes after the completion of their experimental session. With this feeding regime, animals were maintained at approximately 85% of free-feeding weight. Their weights ranged from 150–250 g. At the end of the study, IV catheter patency tests were conducted. Only the data of those animals that passed the patency test were included in the data analysis. Animals for the alcohol study had ad libitum access to water and food but only 6 hr/day access (10 a.m. to 4 p.m.) to a solution of 10% (v/v) alcohol. All studies were conducted in accordance with the regulations outlined by the Duke University Animal Care and Use Committee.
Preparation of Drugs
Solutions of 18-MC were prepared daily in saline and were given by oral gavage in 10, 20 and 40 mg/kg dosages. Saline solution was used as the control. All solutions were given 3 ml/kg body weight 30 min before testing. Solutions of nicotine bitartrate were prepared weekly in sterilized isotonic saline and kept refrigerated in the dark between experiments. The pH of the nicotine solution was adjusted to 7.0 using NaOH solution and then the solution was passed through a 0.22 μm Nalgene filter (Nalgene Nunc International, Rochester, NY) for sterilization. The doses of nicotine used were calculated as a function of the nicotine base weight. Solutions of 10% (v/v) alcohol were prepared twice weekly from a solution of 100% ethanol diluted with tap water (Rezvani et al., 2013).
Experimental Protocol
1. Nicotine Study
Surgery
Before the start of nicotine self-administration sessions, all animals were trained to lever press in a standard dual-lever experimental chamber (Med Associates, St. Albans, VT, USA) for food reinforcement. Each chamber was equipped with two levers (one active, one inactive), two cue lights located directly above each lever, a house light, and a tone generator. After lever pressing was established, animals experienced three sessions of lever pressing for food under a fixed ratio (FR) 1 schedule of reinforcement. Following the completion of their final training session with food reinforcement, animals were anesthetized with ketamine (60 mg/kg) and dormitor (15 mg/kg) and a catheter (Strategic Application Inc., Libertyville, IL, USA) was implanted into their jugular vein. The jugular catheter was attached to a harness that could be tethered to the infusion pump during experimental sessions. Animals were given a minimum of 24 hours to recover from surgery before experiencing nicotine self-administration sessions (Rezvani et al. 2010; 2013).
Nicotine Self-Administration Training
Before beginning nicotine self-administration, rats were trained for three sessions on lever pressing for food reinforcement. Then, they were fitted with i.v. catheters and received nicotine infusions (0.03 mg/kg/infusion) under a fixed ratio (FR) 1 schedule of reinforcement for ten sessions. The rats were trained to self-administer nicotine (0.03 mg/kg/infusion, IV) via operant lever response with a visual secondary reinforcer. Two levers were available to be pressed and only one caused the delivery of nicotine. Pressing the lever on the active side resulted in the activation of the feedback tone for 0.5 second and the immediate delivery of one 50-μl infusion of nicotine in less than 1 second. Each infusion was immediately followed by a one-minute period of timeout in which the cue lights went out, the house light came on and responses were recorded but not reinforced (Rezvani et al., 2010; 2013).
Oral 18-MC Treatment
At weekly intervals, rats were administered by oral gavage doses of 18-MC (0, 10, 20 and 40 mg/kg) following a repeated measures counterbalanced design twice. The drug was given in a volume of 3 ml/kg 30 min before each nicotine self-administration session.
2. Alcohol Study
Following the standard procedure of the two-bottle choice and after the rats established stable and reliable intakes of alcohol and water for several weeks with 24 hr access to water and alcohol, they were switched to 6-hr/day limited access (10 a.m. to 4 p.m.) to alcohol (10% v/v) and 24hr free access to water and food. Following establishing a reliable baseline, rats were given an oral dose of 18-MC (0, 10, 20 and 40 mg/kg) 30 min before exposure to alcohol following a cross-over design with random assignment. Alcohol and water intakes were recorded at 2, 4 and 6 h after the alcohol exposure. The interval between drug administrations was at least 2 days.
Data Analysis
For the nicotine study, the results were assessed by analysis of variance for repeated measures with dose of 18-MC and phase one and two of repeated testing of the drugs. A between subjects factor was high or low responding based on rats being above or below the median of pre-drug treatment nicotine self-administration. For the alcohol study, there was a two-way repeated measures analysis of variance with the factors being 18-MC dose and time after dosing. Planned comparisons of control vs. each of the dose conditions were made. Interactions with p<0.10 were followed-up with tests of the simple main effects (Snedecor and Cochran 1967). Dunnett’s tests were used to compare effects of 18-MC dose conditions to the control vehicle. A p-value of 0.05 (two-tailed) was used as the threshold for significance.
Results
1. Nicotine Study
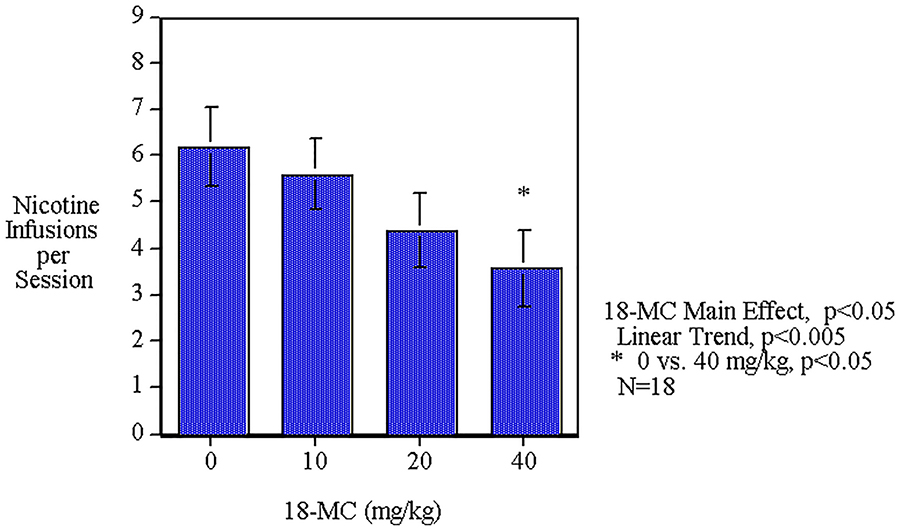
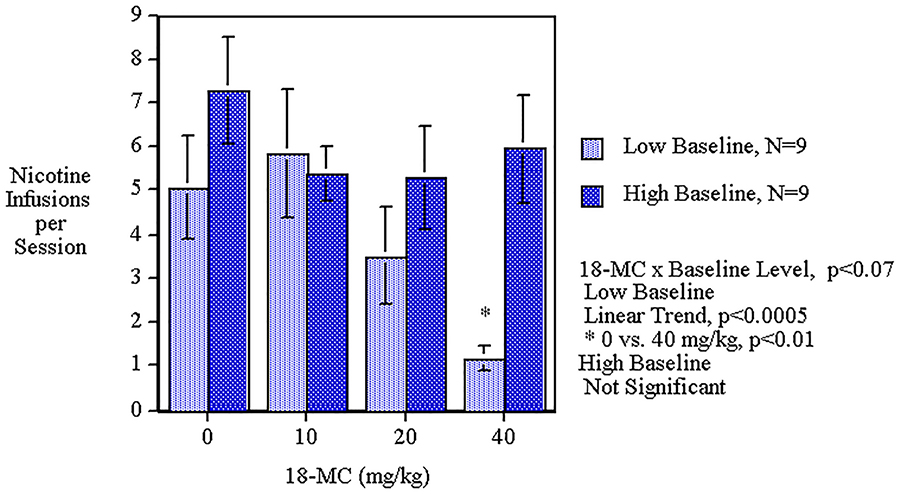
Acute oral administration of 18-MC caused a significant main effect (F(3,48)=3.06, p<0.05) by decreasing nicotine self-administration. The linear trend of decreasing nicotine self-administration over the dose range was significant (p<0.005). The 40 mg/kg dose caused a significant (p<0.05) decrease in nicotine self-administration. (Fig. 1). No differential effect was seen with the first and second phase of the drug administration. The analysis included a median split between those above or below the median level of nicotine self-administration during the pretreatment baseline performance. The low response group averaged 2.82±0.48 nicotine infusions per session and the high response group averaged 8.20±0.86 nicotine infusions per session during sessions 1–5 of nicotine self-administration training before 18-MC treatment (p<0.0005). Analysis of the data showed that throughout the baseline training the rats in the high baseline group had higher nicotine self-administration than those in the low baseline group. In the last baseline session the high group averaged 8.9±0.8 infusions whereas the low group averaged 3.7±0.9, which was a significant difference (p<0.0005). All the other baseline sessions showed significant differences except for session 1 during which the groups were not significantly different from each other. The regression of pellet self-administration was not significantly correlated with the nicotine infusion. The groups with high and low nicotine self-administration did not have significant differences in food pellet self-administration. The interaction of 18-MC x baseline level of performance (F(3,48)=2.52, p<0.07) prompted tests of the simple main effects of 18-MC in each group. As demonstrated in Figure 2, the rats with lower baseline performance showed a significant (F(3,24)=7.89, p<0.005) main effect of 18-MC decreasing in nicotine self-administration. The rats with lower baseline performance showed a significant (p<0.01) decrease in nicotine self-administration by the 40 mg/kg dose while those with the higher baseline did not show a significant effect of 18-MC.
Figure 1:
Acute oral 18-MC effects on nicotine self-administration (mean±sem) in female rats. Data represent means±sem (n=18).
Figure 2:
Acute oral 18-MC effects on nicotine self-administration in low and high baseline groups (means±sem) in female rats.
Response on the active vs inactive lever was also analyzed. During baseline training there was a significant (p<0.0005) difference between active (13.74±2.75) and inactive (4.69±2.10) lever pressing. Lever pressing during the two phases of 18-MC testing showed a significant (p<0.01) interaction of correct-incorrect side x MC-18 dose x test phase. Follow-up tests of the simple main effects showed that during Phase 1 in the control condition there continued to be a side of response effect with significantly (p<0.001) more correct (10.61±2.31) than incorrect (5.17±1.14) lever pressing. During the Phase 1 of testing the correct side lever pressing was significantly decreased by the 20 mg/kg (p<0.01) and the 40 mg/kg (p<0.01) 18-MC doses to 5.61±1.30 and 4.06±1.20 respectively. In Phase 1, 18-MC did not significantly affect performance on the incorrect lever. In Phase 2, after the cumulated added testing under the influence of 18-MC correct side lever pressing in the control condition (7.06±1.42) fell significantly (p<0.05) from response in Phase 1. In Phase 2 18-MC did not significantly affect correct side lever pressing, but did significantly (p<0.05) reduce responding on the incorrect side lever with the 40 mg/kg dose (3.61±0.84 compared with control 7.61±1.61).
2. Alcohol Study
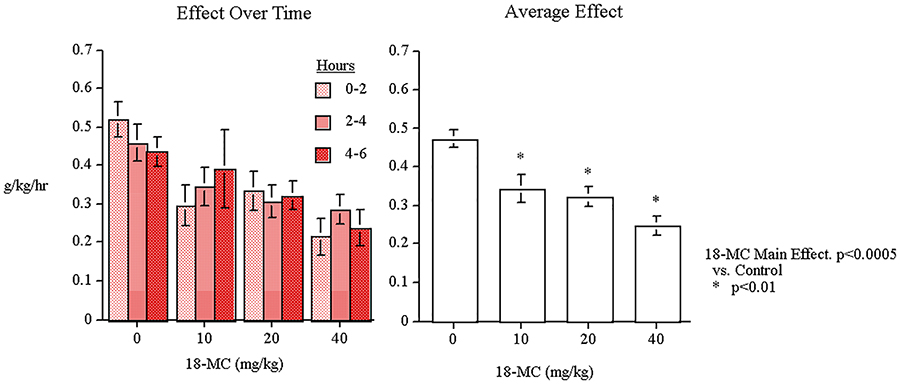
The alcohol study showed that oral administration of 18-MC caused significant decreases in the amount of alcohol consumed and the preference for alcohol vs. water. With the measure of g/kg of alcohol consumed, there was a significant main effect of 18-MC (F(3,51)=10.94, p<0.0005). As shown in Figure 3, planned comparisons of the dose groups vs. control showed that all the doses caused significant decreases in the g/kg of alcohol consumed over the six hours after oral administration (10 mg/kg p<0.01, 20 mg/kg p<0.01 and 40 mg/kg p<0.01). There were not a significant main effects of sex or hours or interactions with 18-MC. 18-MC did not have any significant effects on water intake. The percent preference data showed that this was not merely due to an overall decrease in total fluid intake.
Figure 3:
Acute oral 18-MC effects on alcohol self-administration (g/kg/h) in P-rats (means±sem) (n=19).
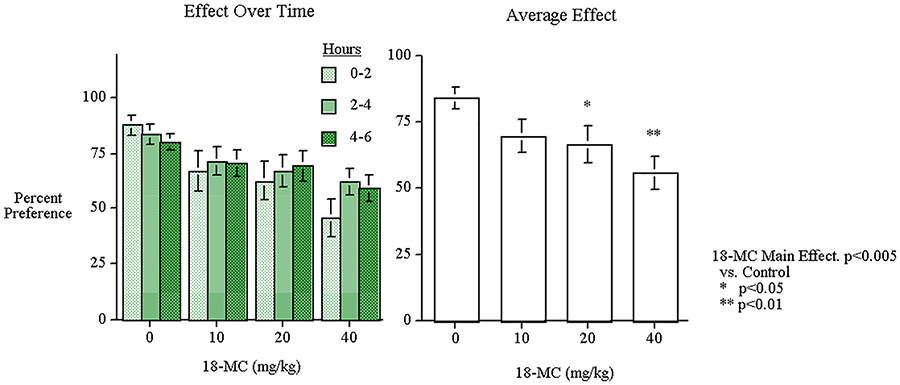
With percent alcohol preference relative to water consumption, there was a significant main effect of 18-MC (F(3,51)=5.65, p<0.005); there was a decrease in alcohol preference relative to water with two of the 18-MC drug doses (20 mg/kg p<0.05 and 40 mg/kg p<0.01). As shown in Figure 4, there was no effect of time or time x 18-MC interaction. The main effect of sex was significant (F(1,17))=14.05, p<0.005) with males having a higher preference for alcohol (80.08±3.71) than females (56.16±4.38). There was no significant interaction of sex with 18-MC treatment. The selective effect of 18-MC on alcohol consumption was reinforced by the fact that no significant 18-MC effects were seen with regard to water consumption. 18-MC did not have any significant effects on water intake. In fact there was a slight though non-significant increase in water consumption after 18-MC administration (control=1.92±0.55 ml/hr, 10 mg/kg=2.40±0.65 ml/h, 20 mg/kg=2.65±0.72 ml/h, and 40 mg/kg=2.83±0.56 ml/h). Neither the g/kg nor the percent preference measures showed any interaction of low and high baseline response with 18-MC drug effects as had been seen with nicotine self-administration.
Figure 4:
Acute oral 18-MC effects on alcohol preference vs. water in P-rats (mean±sem) (n=19).
Discussion
The current study was conducted to evaluate the effects of acute oral administration of 18-MC on nicotine and alcohol intake in rats. The present data show that oral 18-MC significantly reduced both alcohol intake and alcohol preference in selectively-bred alcohol preferring (P) rats and intravenous (IV) nicotine self-administration in Sprague Dawley rats. These findings replicate and extend earlier findings that acute systemic injection of 18-MC significantly reduced alcohol intake in alcohol preferring (P) rats (Rezvani et al. 1997) and IV nicotine self-administration in Sprague Dawley rats (Glick et al. 2000). For development of 18-MC for possible clinical use it was important to demonstrate its oral efficacy. Previously, only morphine self-administering rats had been administered 18-MC orally (Maisonneuve and Glick, 1999).
Previous studies have shown that systemic injections (IP or SC) of 18-MC effectively reduce nicotine preference and nicotine-induced dopamine release (Glick et al. 1998). Treatment with 18-MC has also been shown to reduces morphine (Maisonneuve and Glick 1999), cocaine (Glick et al. 1996), methamphetamine (Glick et al. 2000) and alcohol self-administration in rats (Rezvani et al. 1997).
The rats with lower baseline performance showed a significant (p<0.01) decrease in nicotine self-administration by the 40 mg/kg dose while those with the higher baseline performance did not show a significant effect of 18-MC. The fact that the 18-MC treatment was selectively effective with rats in the lower, but not higher than median nicotine self-administration in the pre-treatment baseline sessions, may have been due to any of the several reasons. The higher avidity of nicotine self-administration in high performers may have presented a tougher target for therapy, requiring a higher dose than was tested in this study. Alternatively, it may be the case that rats with higher levels of nicotine self-administration have a neurobehavioral substrate that is different enough from the low responders to obviate the therapeutic effect of 18-MC. This may have been due to pre-existing differences in rats that self-administer greater amounts of nicotine or to the persisting effects of the greater amounts of nicotine self-administered or a combination of these two. These data reinforce our finding that 18-MC is significantly effective in reducing nicotine self-administration, with particular effectiveness in lighter users of nicotine. Previously, we have seen a similar effect with D-cycloserine significantly lowering nicotine self-administration in rats with a low baseline level of nicotine self-administration but not being effective with the rats above the median level of baseline responding (Levin et al. 2011). The groups with high and low nicotine self-administration did not have significant differences in food pellet self-administration. The regression of pellet self-administration was not significantly correlated with the nicotine infusion, indicating that the differences in response were not merely the carryover from the lever press training for food reinforcement.
Previous findings have shown that the systemic administration of 20 and 40 mg/kg 18-MC significantly reduced the consumption of sucrose and saccharin without affecting water intake in rats (Taraschenko et al., 2008). It has been suggested that the mechanism of 18-MC’s action on consumption of palatable fluids may involve an alteration in taste perception or modulation of central neurotransmitters mediating reward. However, the precise mechanism of 18-MC’s effect on palatable solution such as sucrose consumption remain to be determined (Taraschenko et al., 2008). At this point, it is not known and it remains to be seen if the oral administration of 18-MC would induce the same effect on palatable solutions as the systemic administration.
One interesting finding was that oral 18-MC was more effective in reducing alcohol intake than nicotine intake as all three oral doses of 18-MC significantly reduced alcohol intake but only 40 mg/kg dosage significantly reduced nicotine self-administration. In addition, 18-MC was effective in reducing alcohol self-administration regardless of low or high baseline self-administration level unlike with nicotine which was only significant with the lower baseline group. This difference in effectiveness might be due to using two different strains of rats for nicotine and alcohol studies. Selectively-bred alcohol preferring (P) rats have unique neurochemical profiles for dopaminergic and other neurochemical systems which are involved directly or indirectly in reinforcing effects of alcohol (Bell et al., 2011). It is possible that 18-MC has specific effects on one of these systems. It is also possible that oral 18-MC may interfere with alcohol metabolism. Further studies are needed to conclusively understand the mechanism of action and the differential effects of 18-MC on nicotine and alcohol intake.
Several neuronal mechanisms may be involved in the suppressant effects of 18-MC on alcohol and nicotine self-administration. The reinforcing properties of most drugs of abuse including nicotine and alcohol are partly related to their interactions with the mesolimbic dopaminergic system. Both alcohol and nicotine administration have been shown to increase dopamine release in the nucleus accumbens (DiChiara and Imperato, 1988; Tizabi et al., 2002). In alcohol preferring rats, administration of low doses of alcohol has been shown to activate dopaminergic neurons in the ventral tegmental area (Gessa et al, 1985). Interestingly, 18-MC has been shown to reduce nicotine-induced dopamine release in the nucleus accumbens of rats (Glick et al. 1998). Thus, it is possible that 18-MC exerts its effects on both alcohol and nicotine intake by blunting their effects on the mesolimbic dopaminergic system and consequently reducing their rewarding effects, which leads to reduced intake. Indeed it has been shown that 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens (Taraschenko et al. 2007).
Another possible mechanism for suppressant effects of 18-MC on nicotine and alcohol intake is its effect on specific nicotinic receptors. It has been shown that nicotinic α3β4 receptors are blocked by 18-MC (Glick et al, 2002; Pace et al. 2004). These receptors, particularly in the medial habenula, play key roles in the neural basis of drug reinforcement (Quick et al. 1999). Thus, it is possible, as indicated by many previous results (Glick et al. 2006, 2008, 2011), that 18-MC exerts its “anti-addictive” effects by blocking these receptors in the medial habenula. However, regardless of exactly how 18-MC acts, it is important to know that it can exerts its effects via the oral route of administration. This certainly enhances the likelihood that an oral formulation suitable for clinical use could be developed.
In summary, our findings demonstrated that acute oral administration of 18-MC can significantly reduce both alcohol intake and preference as well nicotine self-administration in rats. Although the exact neuronal mechanism of action is not fully understood, it may exert its suppressant effect on alcohol and nicotine intake by reducing their reinforcing properties by damping dopaminergic activity in the mesolimbic system.
Acknowledgement
This research was supported by P50 grant DA027840 from NIDA.
References
- Bandarage UK, Kuehne ME, Glick SD (1999) Total syntheses of racemic albifloranine and its anti-addictive congeners, including 18-methoxycoronaridine. Tetrahedron 55: 9405–9424 [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L (2012) Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol. Biochem. Behav 103:119–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U S A 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G (1985) Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Research 348: 201–203 [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA (2000) 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport 11: 2013–2015 [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Visker KE, Fritz KA, Bandarage UK, Kuehne ME (1998) 18-methoxycoronardine attenuates nicotine-induced dopamine release and nicotine preferences in rats. Psychopharmacology 139: 274–280 [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH (1986) Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand 128: 151–158 [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G (1986) Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Euro. J. Pharmacol 132: 337–338 [DOI] [PubMed] [Google Scholar]
- Koob GF, Rassnick S, Heinrichs S, Weiss F (1994) Alcohol, the reward system and dependence. EXS 71: 103–114 [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Petro A, Rose JE (2011) D-cycloserine selectively decreases nicotine self-administration in rats with low baseline levels of response. Pharmacology, Biochemistry and Behavior 98: 210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD (1999) Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. European Journal of Pharmacology 383: 15–21 [DOI] [PubMed] [Google Scholar]
- Molinari HH, Maisonneuve IM, Glick SD (1996) Ibogaine neurotoxicity: A re-evaluation. Brain Research 737: 255–262 [DOI] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA (1999) Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology 38: 769–783 [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Slade S, Wells C, Petro A, Lumeng L, Li TK, Xiao Y, Brown ML, Paige MA, McDowell BE, Rose JE, Kellar KJ, Levin ED (2010) Effects of sazetidine-A, a selective alpha4beta2 nicotinic acetylcholine receptor desensitizing agent on alcohol and nicotine self-administration in selectively bred alcohol-preferring (P) rats. Psychopharmacology (Berl) 211:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Ying Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD (1997) Attenuation of alcohol consumption by a novel non-toxic ibogaine analog (18-Methoxycoronaridine) in alcohol preferring rats. Pharmacol. Biochem. Behav 58: 615–619 [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Sexton HG, Johnson J, Wells C, Gordon K and Levin ED (2013). Effects of caffeine on alcohol consumption and nicotine self-administration in rats. Alcoholism: Clinical and Experimental Research 37: 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG (1967) Statistical Methods Iowa State University Press, Iowa State University Press [Google Scholar]
- Taraschenko OD, Rubbinaccio HY, Maisonneuve IM, Glick SD (2008) 18-Methoxycoronaridinie: a potential new treatment for obesity in rats? Psychopharmacology 201:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD (2007) 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse 61: 547–560 [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RJ, Louis VA, Taylor RE (2002) Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcoholism: Clinical and Experimental Resesearch 26: 394–399 [PubMed] [Google Scholar]