Abstract
Hypertension is associated with increased sympathetic activity. A component of this sympatho-excitation may be driven by increased signaling from sensory endings from the heart to the autonomic control areas in the brain. This pathway mediates the so-called “cardiac sympathetic afferent reflex” (CSAR), which is also activated by coronary ischemia or other nociceptive stimuli in the heart. The CSAR has been shown to be enhanced in the heart failure state and in renal hypertension (HTN). However, little is known about its role in the development or progression of HTN or the phenotype of the sensory endings involved. To investigate this, we used the selective afferent neurotoxin, resiniferatoxin (RTX) to chronically abolish the CSAR in two models of HTN; the spontaneous hypertensive rats (SHR) and angiotensin II (AngII) infusion (240 ng/kg/min). Blood pressure (BP) was measured in conscious animals for two to eight weeks post RTX. Epidural application of RTX to the T1–T4 spinal segments prevented the further BP increase in 8-week-old SHR and lowered BP in 16-week-old SHR. RTX did not affect BP in WKY normotensive rats, nor in AngII-infused rats. Epicardial application of RTX (50 μg/ml) in 4-week-old SHR prevented the BP increase whereas this treatment does not lower BP in 16-week-old SHR. When RTX was administered into the L2–L5 spinal segments of 16-week-old SHR no change in BP was observed. These findings indicate that signaling via thoracic afferent nerve fibres may contribute to the HTN phenotype in the SHR but not in the Ang II infusion model of HTN.
Keywords: blood pressure, sympathetic nerve activity, sensory neurons, angiotensin
Summary:
Ablation of thoracic T1–T4 DRG reduced BP in SHR with established HTN and prevented any further BP rise in pre-hypertensive animals. The anti-hypertensive effects of sensory spinal nerve ablation in the SHR were site specific to the upper thoracic T1–T4 DRG, and no effect on BP was seen in an Ang II (240 ng/kg) infusion rat model of HTN. Neuro-modulation of BP at the level of the upper thoracic spinal sensory nerves may be beneficial in the treatment HTN, particularly etiologies with a high sympathetic drive.
Introduction
Increased sympathetic nervous system activity is a well-established hallmark1–3, and negative prognostic indicator of essential hypertension (HTN)4–6. Sympathetic dysfunction during HTN has been identified at several distinct anatomical sites, including sympathetic outflow to the kidney7–9, the cardiac stellate ganglia10–12, chemo- and baroreflex signalling13, 14, and brainstem control of autonomic output15. In addition, many currently prescribed therapeutics for the treatment of hypertension either directly or indirectly target the sympathetic nervous system16.
Hypertension is a highly complex and multifactorial disease. Different pathways are thought to contribute at different stages of HTN from the initial development, to progression and maintenance of the HTN phenotype. Factors such as life style and genetics are also thought to influence disease progression. While treatments that target the sympathetic nervous system are effective in some patients, their systemic actions can lead to a number of adverse side effects17, 18. An increased focus for more targeted treatments of HTN, in particular treatment for resistant hypertension, has led to the development of a number of neuromodulation therapies that alter sympathetic outflow to a single organ or reflex pathway. These include renal nerve denervation9 and baroreflex stimulation19, 20.
The increased cardiac sympathetic activity observed in HTN is similar to that seen in heart failure21–23. In both animal and clinical studies removal of the cardiac sympathetic input has been shown to improve prognosis in these individuals24–29. Moreover, recent studies have shown that chronic ablation of cardiac afferent nerve endings with the potent TRPV1 receptor agonist resiniferatoxin (RTX), prevents cardiac remodelling and preserves cardiac diastolic function in rats with heart failure induced by coronary artery ligation28.
Cardiac afferent neurons enter the central nervous system through both the vagus nerves via the nodose ganglia and through sympathetic pathways directly into the spinal cord where cell bodies reside in the thoracic dorsal root ganglia (DRG). Stimulation of the cardiac sensory endings mediate a sympatho-excitatory reflex, also called the Cardiac Sympathetic Afferent Reflex (CSAR)29, 30. Cardiac afferent nerves can be directly stimulated by application of, but not limited to, capsaicin, bradykinin and adenosine28, 30–32. In conditions of hypertrophy and myocardial ischemia, bradykinin and adenosine are released directly from the myocardium to act on these nerve terminals eliciting increased sympathetic outflow. Cardiac afferent fibres also release neuropeptides (e.g. substance P and Calcitonin Gene Related Peptide) and can initiate inflammatory processes that may mediate cardiac and vascular remodelling28.
The sensitivity of the CSAR has previously been shown to be increased in HTN31, although the role of this reflex in the development or maintenance of HTN is currently unknown. Therefore, in the current study we hypothesized that ablation of cardiac sensory neurons using RTX would reduce blood pressure (BP) or prevent the BP rise in two different models of HTN: the spontaneously hypertensive rat (SHR) and angiotensin II (AngII) infusion. Both animal models have previously been described to exhibit a neurogenic component11, 33–35. Ablation of transient receptor potential vanilloid 1 (TRPV1) cardiac afferents were targeted in areas containing cell bodies in the DRGs of the upper thoracic and lumbar spinal segments, and nerve terminals on the surface of the heart.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files
Animal models
Experiments were performed on male SHR at three different ages: 3–4 weeks old (n = 12), 8 weeks old (n = 12) and 16 weeks (n = 36). Male Wistar-Kyoto (WKY) rats aged, 3–4 weeks (n = 11), and 16 weeks (n = 10) were used as normotensive controls. Male Sprague-Dawley (SD) rats (200–250 g) at 16 weeks of age (n=31) were used as an Ang II treated model of HTN or normotensive controls (Charles River, USA). These experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were housed in an on-site facility and were allowed to acclimate to their new environment for at least 1 week following arrival. Water and laboratory rat chow were provided ad libitum, and animals were housed in 12-hour light/dark cycles. For chronic surgical procedures, Isoflurane (2%) was administered for induction and maintenance of anaesthesia. Analgesia using Buprenex (Buprenorphine - 0.05 mg/kg) was given on the day of surgery, and Carprofen (5 mg/kg) for three days post-surgery for post-procedure pain management. Rats were euthanized at the conclusion of the study (terminal experiments) under α-chloralose-urethane anaesthesia (urethane 800 mg/kg, ip; α-chloralose 40 mg/kg, ip). Euthanasia was confirmed by removal of vital organs (heart and lungs).
Detailed surgical and histochemical methods can be found in the Supplemental File.
Below is a brief description of the methods.
Spinal afferent denervation:
The upper thoracic spinal afferents were ablated in 8 - week old and 16 - week old SHR and WKY rats, and 16- week old SD rats. At the start of each experiment rats were randomized into RTX or vehicle treated groups. Briefly, rats were anesthetized (2% isoflurane) and placed in the prone position. A small midline incision was made in the region of the T13-L1 thoracic vertebrae. Following dissection of the superficial muscles, two small holes (approximately 2 mm × 2 mm) were made in the left and right sides of the T13 vertebrae. For thoracic sympathetic afferent denervation, a polyethylene catheter (PE-10) was inserted into the epidural space via one hole and gently advanced approximately 4 cm cranial to the T1 level. Resiniferitoxin or vehicle was injected bilaterally at T1 to T4, 10 ul per segment.
Activation of cardiac spinal afferents
Epicardial application of bradykinin (BK) has been demonstrated to effectively stimulate cardiac spinal afferents via the BK2 receptor36, 37. Therefore, a similar approach was employed to activate cardiac spinal afferents in this study. The chest was opened through the fourth intercostal space. A square of filter paper (3 × 3 mm) saturated with BK (10 μg/ml), was applied to the left ventricle. Hemodynamics were continuously recorded. After the responses peaked, the heart was rinsed three times with 10 ml of warm normal saline.
Statistical Analysis
Statistical analyses were designed to test the hypotheses that the ablation of cardiac afferent neurons treated with RTX would reduce BP in an HTN rat model compared to vehicle control. Animals were randomized to vehicle or RTX treatment groups. All animals that started the study completed the study, no animals were excluded. Number of samples ‘n’ equals the number of animals in each group. Statistics were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA. Version 8). Differences between treatments were determined using a Mixed-effects model for repeated-measures ANOVA. Due to the length of the study, most data sets contained some missing values that were Missing at Random (MAR). For example, technical issues resulting in failure to acquire data from one animal on one day. Therefore, a Mixed-effects model was used. For comparison between two groups both Sidak and Bonferroni corrections for multiple comparisons were used, and when comparing between four groups (AngII experiments) both Turkey and Bonferroni corrections for multiple comparisons were used. Mean AP and HR are reported as absolute changes. Changes in MAP, HR after BK application and echocardiographic parameters comparing pre- vs post-treatment were determined by a paired t-test. All values are expressed as mean ± standard error of the mean (SEM). p<0.05 was considered statistically significant.
Results
The efficacy of epidural T1–T4 DRG application of RTX in ablating the CSAR.
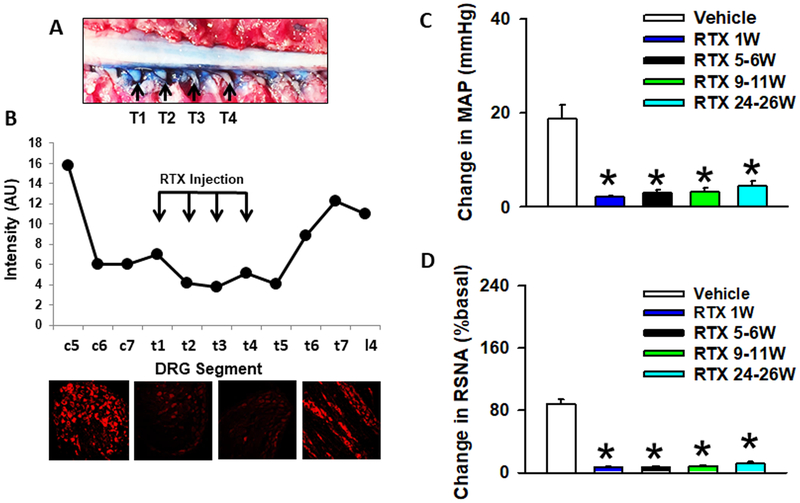
In the current study, we performed T1–T4 epidural application of the selective afferent neurotoxin RTX in order to ablate the CSAR. Our previous study showed that this approach significantly 1) reduced the number of both TRPV1-positive and IB4-positive DRG neurons at the thoracic levels and 2) abolished the pressor and sympatho-excitatory responses to epicardial application of BK ~10 weeks post RTX administration28. Here, we further provide additional detailed information regarding 1) the range of thoracic DRGs that were affected by RTX; and 2) the time course of CSAR ablation. As shown in (Figure 1A and 1B), both Evans Blue dye and immunofluorescence staining indicates that due to drug diffusion, the actual thoracic DRGs affected by epidural T1–T4 DRG application of RTX ranged from levels C6 to T5. In functional experiments (Figure 1C and 1D), we confirmed that epidural T1–T4 DRG application of RTX successfully abolished CSAR activation evoked by epicardal application of BK in normotensive rats for more than 6 months, demonstrating the long term efficacy of CSAR ablation. In addition, we carried out a small pilot study in SHR rats showing that epidural T1–T4 application of RTX can also abolish the CSAR in SHR rats 2 months post RTX (SHR+RTX vs. SHR+Vehicle: ΔMAP 2.2±0.8 vs. 20.7±5.6 mmHg, P=0.01; ΔHR 1.3±1.4 vs. 27.3±1.9 bpm, P=0.01; ΔRSNA 3.1±0.9% vs. 126.5±21.7% baseline, P=0.01; n=4/RTX group and n=3/vehicle group).
Figure 1.
Model validation. A) Upper panel shows a view of the thoracic spinal cord from the dorsal surface showing the distribution of RTX (stained by Evans Blue dye) after epidural administration of 10 ul into each segment. B) Immunofluorescent images of TRPV1 in the DRGs from the lower cervical to upper lumbar segments 5–6 weeks post T1–T4 application of RTX. TRPV1 staining is largely abolished 2 segments above and 2 segments below the injection sites (T1–T4). C) MAP and D) RSNA absolute change from baseline, in response to CSAR stimulation with bradykinin (10 μg/ml) in Sprague Dawley rats treated with vehicle or at increasing time points (1 week – 26 weeks) post RTX treatment.
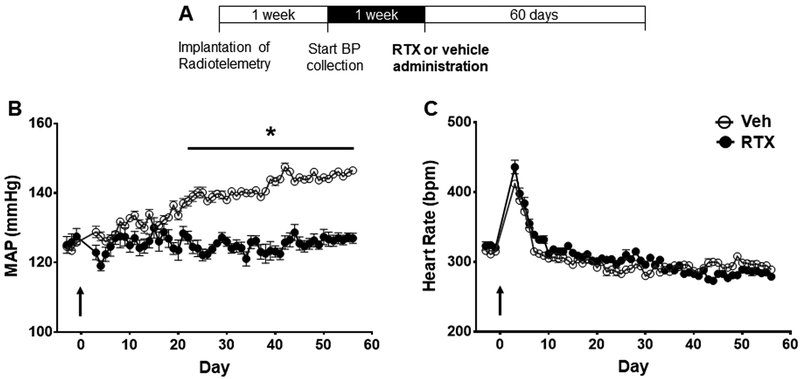
Effect of upper thoracic RTX on conscious MAP and HR in 8-week-old SHR.
At 8 weeks of age SHR rats exhibit moderately elevated BP and were used as a model for the developing phase of HTN. Administration of epidural RTX to the T1–T4 thoracic regions resulted in maintenance of BP near the baseline level compared to vehicle - treated controls (Figure 2B and Figure 1S, n = 6) Over the 60-day observation period MAP increased between 20–25 mmHg in the vehicle group compared to no further increase in the RTX treated group. There was no difference in HR between the two groups (Figure 2C). Post RTX or vehicle surgery there was a transient increase in HR that stabilized one-week post-surgery. This surgical stress on HR was seen in all groups. RTX treatment had no effect on cardiac hypertrophy in 8-week-old SHR (Table S1).
Figure 2.
A) Timeline for SHR and WKY radiotelemetry experiments. Effect of T1–T4 epidural RTX (10 μg/ml) or vehicle on B) mean arterial pressure (MAP), and C) heart rate (HR) in young 8-week-old SHR (n = 6 both groups). *p< 0.05 SHR treated with RTX compared to Vehicle. Arrow indicates when RTX or Vehicle was administered.
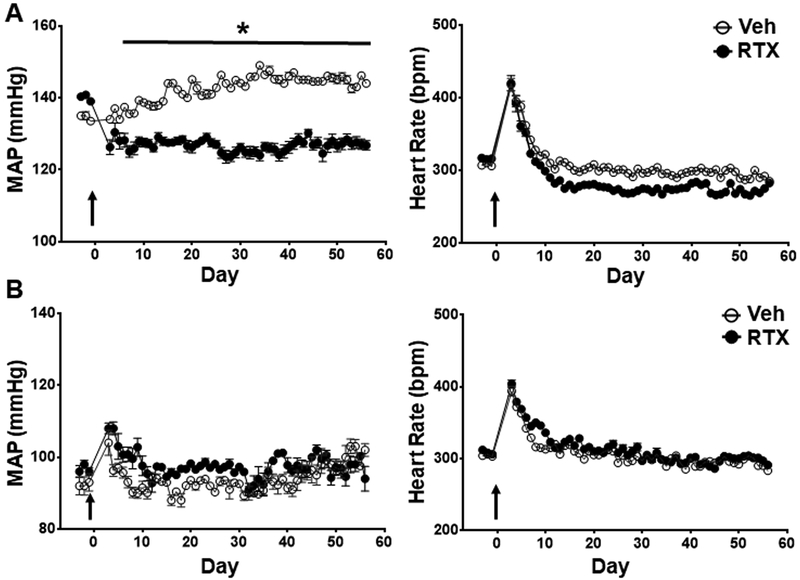
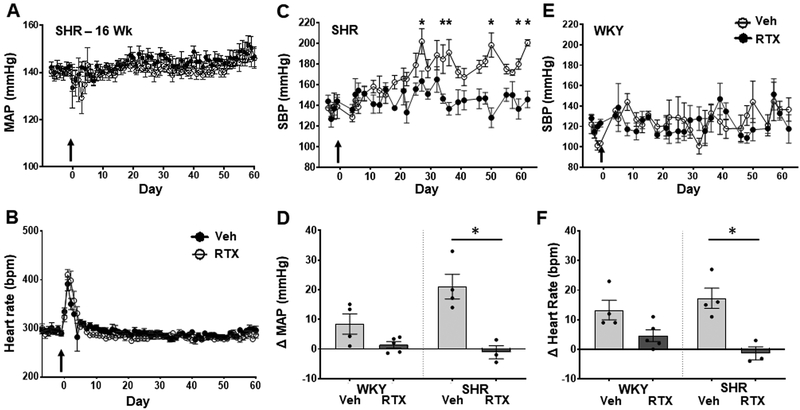
Effect of upper thoracic RTX on conscious MAP and HR in 16-week-old SHR and WKY rats.
At 16 weeks of age SHR rats have established HTN. Administration of epidural RTX to the T1–T4 upper thoracic region resulted in an immediate and significant reduction in MAP compared to vehicle control SHR (Figure 3A, n=7 per group). In vehicle treated animals, there was a further increase of approximately 10 mmHg in pressure over the observation period whereas BP decreased by approximately 15 mm Hg and remained at this level for the duration of the study (Figure 3 and Figure 2S). Resiniferitoxin had no effect on ejection fraction compared to vehicle in the 16-week-old SHR, indicting that the reduction in blood pressure was not due to reduced cardiac function (data supplement: Table S7). There were no changes in MAP or HR after T1–T4 administration of RTX in 16-week-old WKY (Figure 3B, n=5 per group). Conscious HR declined with age in both SHR vehicle and SHR RTX treated groups. RTX did not change baseline HR in both WKY and SHR rats compared to vehicle control. RTX treatment had no effect on cardiac hypertrophy in 16-week-old SHR (Table S2).
Figure 3.
Effect of T1–T4 epidural RTX (10 μg/ml) or vehicle on MAP (MAP), and heart rate (HR) in A) 16-week-old SHR (n = 7 both groups) and B) WKY rats (n = 5 both groups). *p < 0.05 SHR treated with RTX compared to Vehicle. Arrow indicates when RTX or Vehicle was administered.
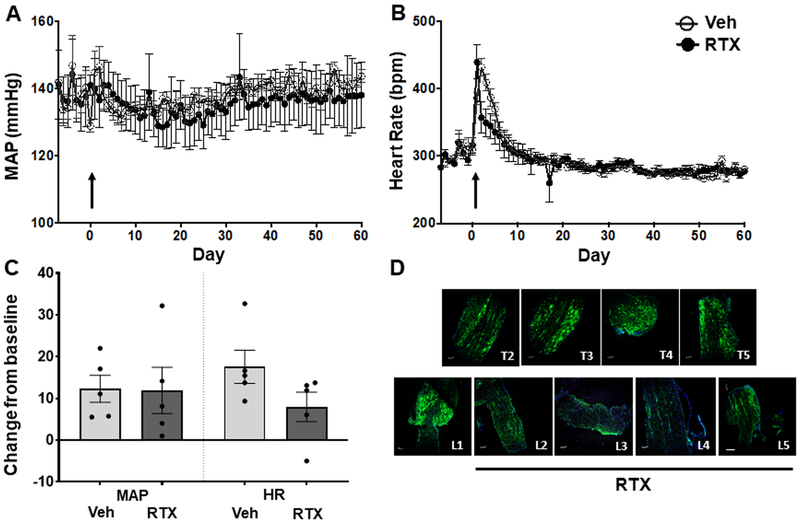
Effect of lumber RTX on conscious MAP and heart rate in 16-week-old SHR.
To test the site specificity of the antihypertensive effect of RTX, we administered it in the epidural space at the lumber L2–L5 vertebrae in 16-week-old SHR with established HTN. There were no changes in BP or HR after RTX administration compared to vehicle controls (Figure 4A and 4B and Figure S3, n=5 per group). Stimulation of the CSAR was performed under anaesthesia at the end of the study. Bradykinin (10 μg/ml) elicited an increase in MAP, and HR that was comparable between the two groups (Figure 4C, n=5 per group. Baseline MAP: Veh, 136.9±7.6. RTX, 143.7±1.9. HR: Veh 373±20. RTX: 366±7). This demonstrated that lumber application of RTX had no effect on the CSAR. TRPV1 immunohistochemistry showed a pronounced loss of TRPV1 positive cells in the L1–L5 DRG that received RTX compared to the upper thoracic DRG (Figure 4D). Resiniferitoxin had no effect on cardiac function, or hypertrophy (Tables S3 and S8). Therefore, the reduction in BP observed in the SHR is site specific to ablation of TRPV1 expressing neurons in the upper thoracic DRG.
Figure 4.
Effect of L2–L5 epidural RTX (10 μg/ml) or vehicle on A) mean arterial pressure (MAP), and B) heart rate (HR) in 16-week-old SHR rats (n = 6 both groups). C) MAP and HR absolute change from baseline, in response to CSAR stimulation with bradykinin (10 μg/ml). D) TRPV1 immunofluorescence in thoracic (T2–T5) and lumber (L1–L5). Arrow indicates when RTX or Vehicle was administered
Effect of epicardial RTX on blood pressure in 16-week-old and 4-week-old SHR.
In order to determine if the spinal sensory input from the heart was responsible for mediating the HTN in SHR we applied RTX (50 μg/ml) to the surface of the left and right ventricles, and recorded pressure by radiotelemetry in 16-week-old rats, and by tail-cuff volume pressure recording (VPR) in 4-week-old SHR. While there was no effect on MAP or HR in 16-week-old SHR (Figure 5 A and 5B, Figures S4 and Figure S5. n = 6, both groups), epicardial RTX resulted in reduced left ventricular wall thickness and increased ejection fraction compared to vehicle treated animals (Table S8). In 4-week-old SHR there was an abrogation of the development of HTN following epicardial RTX treatment compared to vehicle (Figure 5C), which was not observed in 4-week-old WKY rats (Figure 5E). At the end of the study RTX treated young SHR had a slightly reduced body weight and heart weight compared to vehicle treated, there was no difference between RTX and vehicle treated WKY rats (Table S4).
Figure 5:
Panel A and B, effect of epicardial application of RTX (50 μg/ml) or Vehicle in 16-week-old SHR on conscious A) MAP and B) HR measured with radio-telemetry (n=5 both groups). Panel C and E, effect of epicardial application of RTX (50 μg/ml) or Vehicle on conscious systolic blood pressure (SBP) measured by VPR tail-cuff in 4-week-old SHR (C, Veh, n=5 and RTX, n= 5 ) and WKY rats (E, Veh, n=4 and RTX, n= 5). Panel D and F, change in MAP (D) and HR (F) in response to cardiac sympathetic afferent reflex (CSAR) stimulation with bradykinin (10 μg/ml) in SHR (Veh, n=5 and RTX, n= 3) and WKY rats (Veh, n=4 and RTX, n= 5). *P < 0.05 SHR compared to WKY. Arrow indicates when RTX or Vehicle was administered.
Application of RTX (50 μg/ml) to the surface of the left and right ventricles at 4-weeks of age resulted in a clear loss of the CSAR, when tested 60 days post RTX treatment (approximately 12-weeks-old) in both SHR and WKY (Figure 5D and 5F) (SHR: Veh n=5, RTX n= 3. Baseline MAP: Veh=175±12.9, RTX=109.8±18.9; HR: Veh=447±21, RTX=329±50. WKY: Veh n=4 and RTX n= 5. Baseline MAP: Veh=79.3±21.2, RTX=89.3±30.7; HR: Veh=393±33, RTX=328±51). Vehicle treated SHR had an increased CSAR compared to WKY at this age (P = 0.0571).
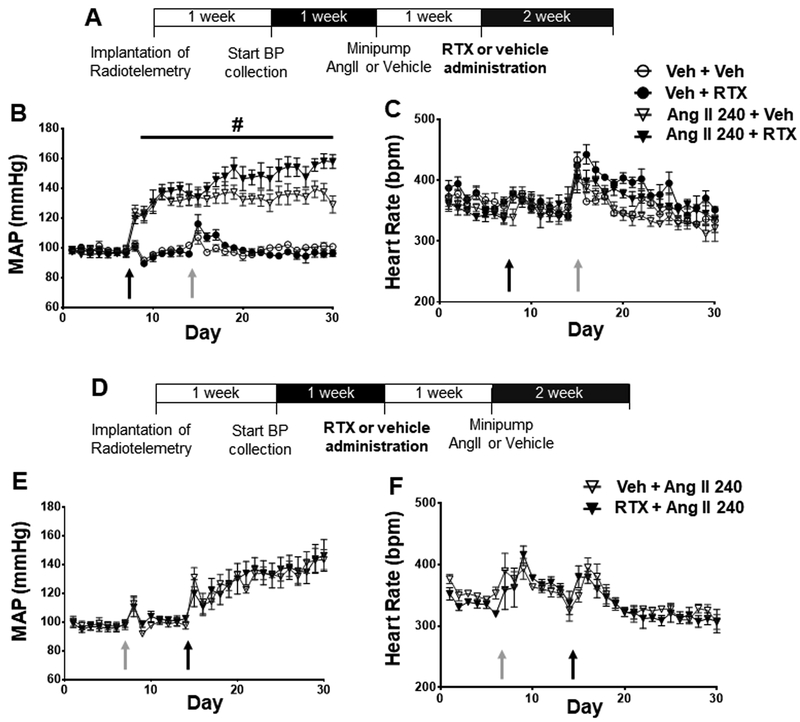
Angiotensin II-Induced Hypertension
To further evaluate the effect of thoracic afferent TRPV1 denervation on the genesis of HTN we evaluated epidural administration of RTX or vehicle at the T1–T4 thoracic levels in rats infused with Ang II subcutaneously (Figure 6 and Figure S6). Angiotensin II infusion increased BP in SD rats immediately after minipump implantation (Figure 6B). There was no effect on HR (Figure 6C). RTX treatment performed 1-week post-minipump implant did not alter the course of MAP or HR change. In a second group of rats, we determined if RTX administration prevented or attenuated the increase in MAP when administered prior to Ang II infusion. RTX treatment did not alter MAP (Figure 6E) or HR (Figure 6F) responses when given before Ang II. Hypertrophy was induced by Ang II infusion which was not affected by RTX treatment (Table S5). At the end of the conscious telemetry recording experiments, a subset of RTX-treated Ang II rats underwent terminal experiments where the BP and HR responses to Bradykinin (BK) were evaluated to confirm the ablation of TRPV1 by RTX. In these animals who received RTX, there was no increase in BP and HR after BK application (Figure S7).
Figure 6.
Effect of RTX treatment in HTN model induced by Ang II infusion A) Timeline for experimental protocol. B) The mean arterial pressure (MAP) and C) heart rate (HR) responses to RTX after chronic Ang II infusion (240 ng/kg/min) (Veh + Veh, n = 4, Veh + RTX, n = 4, Ang II + Veh, n = 9, Ang II + RTX, n = 6), D) Timeline for experimental protocol, E) MAP and F) HR responses to RTX before chronic Ang II infusion (Ang II + Veh, n = 4, Ang II + RTX, n = 4) # P<0.05 Ang II compared to Veh groups, * P<0.05 RTX Ang II compared to Veh Ang II.
Echocardiographic data (data supplement: Table S9) show that RTX treatment increased left ventricular posterior wall (LVPW) thickness in rats treated before Ang II infusion but not in vehicle treated rats.
Discussion
Because sympatho-excitation is a major component of essential HTN38 we investigated a potential mechanism that has heretofore been neglected as a factor in initiating and sustaining increased levels of sympathetic nerve activity in HTN, namely enhanced excitatory input from cardiac spinal afferents, also called the CSAR. Previous findings by our group showed an exaggerated CSAR in chronic heart failure and that ablation of TRPV1 expressing cardiac spinal afferents resulted in a reduction in arterial pressure28, 29. These data provided support and rationale for the current study. Furthermore, acute experiments in hypertensive rats were suggestive of an enhanced CSAR31. The main findings of the current study were: 1) Ablation of TRPV1 expressing neurons in the thoracic T1–T4 DRG reduced BP in SHR with established HTN and prevented BP from increasing in younger SHR; 2) Ablation of TRPV1 expressing neurons in the lumbar L2–L5 DRG had no effect on BP in SHR with established HTN; 3) Ablation of TRPV1 expressing neurons in thoracic DRG had no effect on Ang II-mediated increase in BP when RTX was given pre or post Ang II infusion.
Previous studies by Zhu et.al. demonstrated that acute intrapericaridal administration of RTX in the two-kidney one-clip (2K1C) rat model of HTN resulted in rapid reduction of baseline RSNA, and a near complete abolishment of the CSAR 120 minutes after RTX administration31. These experiments were performed acutely in vagotomized rats under anaesthesia and provide evidence that ablation of cardiac afferent nerves can reduce sympathetic tone in an acute HTN model. In the current study, we extended this evidence to show that chronic ablation of upper thoracic sympathetic afferent neurons results in a long-lasting reduction in BP in conscious freely moving HTN animals. Importantly, these data also show that this phenomenon is model dependent.
Excessive sympatho-excitation is well established in HTN, with increasing evidence suggesting there may be role for sensory input from organs and vascular beds in driving this phenotype39–41. The visceral afferent hypothesis suggests that activation of visceral sensory nerves induces activation of the sympathetic nervous system to preserve blood flow to a given organ. Visceral afferents can be activated by several paracrine or endocrine factors, including but not limited to, hypoxia, metabolic factors, redox signalling, and inflammation13, 42–45. Although these conditions are present and altered in HTN, it remains unclear how sensory nerve fibres are activated or sensitized in HTN. This is likely to be specific to the location and type of sensory neuron in question. For instance, evidence suggests that targeting visceral afferents with renal nerve denervation40, or carotid body denervation41, 46 lowers BP in HTN animal models. One potential hypothesis for spinal afferent sensitization in HTN may be low grade inflammation. Renal inflammation is observed in many models, including salt sensitive HTN47. IL-6 concentration has previously been shown to correlate with systolic BP in apparently healthy men48.
The current study indicates that signalling via cardiac spinal afferent neurons contribute to the development and maintenance of HTN in the SHR model. While in the older SHR (16 weeks at the start of the study) RTX resulted in a drop in BP, in young (8 week and 4 week) SHR there was no immediate drop, but a failure for the BP to rise in line with the vehicle. This suggests that signalling via spinal afferents may be involved in the progression and maintenance of HTN but are not involved in basal BP regulation. The lack of effect on BP following lumbar administration of RTX, and reduction in SBP after epicardial RTX in young SHR indicate a site-specific effect following ablation of cardiac sensory nerves in SHR. The SHR has previously been shown to exhibit increased cardiac sympathetic nerve activity, even before the development of HTN11, 21, 49. Here, we present the first evidence to our knowledge supporting a role for cardiac sensory neurons that contribute to the HTN in this model. Previous studies from our laboratory have shown that RTX administration to the epicardial surface of the heart ablates TRPV1 positive nerves on the surface of the left ventricle28, 29 and reduced the CSAR for up to six months after epidural (T1–T4) administration. While upper thoracic ablation of sensory nerve fibres prevented the BP rise in 8-week-old SHR and chronically reduced the MAP in 16-week-old SHR, we cannot conclude from these studies that the reduction in BP was exclusively due to the specific loss of cardiac sensory endings. Sensory nerve fibers from the heart, lungs and other areas of the thoracic cavity travel through the upper thoracic DRGs. To establish if the response seen was specific to the loss of TRPV1 expressing cardiac sensory nerve fibres, RTX was painted onto the surface of the left and right ventricles. This should result in the loss of TRPV1 expressing nerve fibers on the surface of the ventricles. Cardiac sensory nerve fibers that do not express TRPV1 should be unaffected. Care was taken to insure that only the ventricles, and not the atria, were painted with RTX. In 16-week-old SHR, no change in MAP was seen compared to vehicle control. This finding is curious since in normotensive Sprague Dawley rats epicardial RTX completely abolished the CSAR and ablated surface sensory nerves28, 29. The rationale for epicardial RTX application is based on a fact that the majority of cardiac spinal afferent nerve fibres innervate the surface or superficial layers of the ventricles in normotensive animals50, 51. However, it is possible that the innervation pattern of cardiac spinal afferents could change due to chronic HTN-induced cardiac hypertrophy. Increased sympathetic nerve activity to the heart is associated with hypertensive left ventricular remodeling and hypertrophy2. As SHRs age they develop cardiac hypertrophy52 (see heart weight and echocardiographic data in supplemental Tables S1, S4, S7 and S8). Little is known about the localization and distribution of sensory nerve fibers during this process, or at different stages of HTN in SHR. We hypothesized that the lack of response to epicardial RTX at 16 weeks of age could be due to the lack of sufficient ablation of the cardiac sensory nerves because of changes in their distribution within the heart in advanced HTN with hypertrophy. During late stage HTN cardiac spinal afferents may innervate, or exhibit hyperinnveration, of both surface and deeper layers of the ventricle. Compared to our epidural strategy where the neuronal soma are targeted, epicardial application of RTX may only target a subset of these fibers, not enough to significantly abolished cardiac sensory spinal signaling. Further anatomical studies need to be performed to compare the cardiac spinal afferent innervation pattern at different stages of SHR compared to similar time points in the WKY rats.
Since RTX may not have reached the nerve endings due to significant cardiac hypertrophy or cardiac hyperinnervation into the deeper layer of the LV in the late stage of HTN, we applied RTX to the epicardial surface of young, 4-week-old SHR to retest the potential anti-hypertensive effect of selective cardiac spinal afferent denervation after epicardial RTX in SHR. The aim of using very young SHR was to apply the treatment at a stage before there was likely to be significant cardiac hypertrophy and HTN. However, even at this age the SBP in the SHR was slightly higher than that of age matched WKY rats (Figure 5). The prevention of SBP rise following epicardial RTX in this group indicates that sensory nerve fibers originating from the heart are indeed involved at least in the developmental stage of HTN in the SHR. Furthermore, in 16-week thoracic epidural application of RTX and in 4-week epicardial RTX there was a significant reduction in BP in SHR animals, however no change in BP was observed between RTX and vehicle-treated WKY rats. These data suggest that in the normotensive conditions cardiac afferent signalling does not contribute to baseline BP.
Interestingly, in 8-week and 16-week SHR in which RTX was administred to the T1–T4 DRG and a reduction in BP was observed, RTX caused no significant change in HR compared to control. SHR showed a continual reduction in HR with age in both treated and vehicle groups. The mechanisim for this phenomenon is unknown but may be due to input from baroreceptors in the SHR, or impairment of intrinsic HR in HTN. Since baseline HR was continuely declining, the effects of RTX on HR in SHR may have been masked by other intrinsic factors. Furthermore, RTX had no effect on diurnal BP or HR variations in any study and caused a similar reduction in BP in SHR with T1–T4 RTX in both the day and night periods during 24 hour recordings (data not shown).
Our data also showed that epidural T1–T4 DRG delivery of RTX in SHR lowered blood pressure but did not change any cardiac functional or structural parameter analyzed by echocardiography or weight, which excludes a cardiac factor contributing to the anti-hypertensive effect of cardiac afferent denervation in SHR. On the other hand, one could also expect that this treatment should attenuate cardiac hypertrophy in SHR rats because it decreases afterload. However, did not occur. It should be noted that RTX only moderately lowered BP in the 16-week old SHR rats or prevented the further increase in BP in the 8-week SHR rats. In other words, these rats were still hypertensive (systolic BP >160 mmHg) at the end of RTX treatment. Therefore, it is possible that this moderate anti-hypertensive effect might not be sufficient to prevent cardiac hypertrophy or reverse the established cardiac hypertrophy in these SHR rats.
The SHR is an inbred genetic model that has been used in HTN research for the past 60 years. While it has been characterized as a sympatho-excitatory model, there is still debate as to its relevance to human essential HTN53, 54. Therefore, in an attempt to determine if ablation of thoracic spinal afferents contributed to a reduction or prevention of HTN associated with a non-genetic model, we evaluated the effects of RTX in response to chronic infusion of Ang II. This model of HTN exhibits a sympatho-excitatory component and a vascular component55–57. While Ang II infusion resulted in a prompt and sustained increase in MAP, administration of epidural RTX either prior to or following Ang II did not result in attenuation of the pressor response (Figure 6). The mechanism for the lack of effect of RTX in the AngII model is unclear. Obviously, it is possible that spinal afferents are not activated in this model and thus would have little effect on MAP. Although the two hypertensive rat models used here were both previously described to be characterized by a neurogenic component to their HTN11, 33–35, the origin of the increased blood pressure in response to Ang II infusion is multifactorial. Ang II has previously been shown to alter central neurotransmission55, 57–59 and have a direct action on post-ganglionic sympathetic neurons60 to cause sympathoexcitation. Furthermore, Ang II has a direct vasoconstrictor effect on blood vessels61–63. It is possible that the lack of efficacy of RTX on BP in the Ang II model is due to the site of action of Ang II occurring at higher levels, downstream of visceral afferents. It is also possible that the balance between sympatho-excitation and direct vasoconstriction by Ang II favors the vasoconstrictor pathway. The current study was not designed to dissect these mechanisms. A limitation of this study is that while RTX is likely to cause a reduction in basal sympathetic tone, changes in sympathetic tone over time in conscious animals was not recorded in this study. Therefore, we cannot draw any direct conclusions linking the effects observed with T1–T4 application of RTX, to a reduction in sympathetic tone. Another modest limitation is that similar to most published animal studies to date, the present study was completed using only male SHR and Ang II-infused rats. However, growing evidence indicates that hypertension pathophysiology significantly differs between the sexes, including within the neural-hormonal axis64–66. Whether cardiac spinal afferent denervation in female SHR results in similar effects remains unknown. Further studies need to be peformed to address this question.
Clearly, the neuro-modulation of BP is not the same in all etiologies of HTN. Experience with therapy for resistant HTN has established this fact67, 68. The translational impact of this study is consistent with the increased interest in elucidating additional targets for the treatment of HTN9, 14, 69, especially resistant HTN19, 70, 71. The recent development of neural therapies for the treatment of HTN have demonstrated some positive and encouraging results, particularly within the treatment of resistant and non-compliant patient populations. The results presented here raises an additional possibility of afferent modulation of efferent signaling originating in the periphery and at the level of the DRGs.
The current study also addresses the specificity of this antihypertensive therapy. The clinical application of this therapy would require an appropriate pre-treatment screen to identify a suitable patient population for RTX therapy. Lack of suitable inclusion/exclusion criteria may have contributed to the inconclusive results seen with other neuromodulation therapies, and in general most current large scale clinical trials72. Because increased cardiac afferent signalling may only contribute to the increased sympathetic drive and elevation in BP, a combination of direct neuromodulation and classical pharmacological therapies may be most effective as previously observed for the treatment of heart failure where combing standard heart failure medication with device therapy exhibited a synergistic therapeutic effect73.
In summary, increased sympathetic nervous system activity is observed in all clinical and animal models of HTN, and at all stages of disease progression, from pre-hypertensive to treatment resistant38, 74. In combination, our findings demonstrate that ablation of cardiac afferent nerves at the level of the upper thoracic DRG can prevent the development of HTN in young SHR, and chronically lower the BP in SHR with established HTN. While not examined in the current study, our previous data in rats with chronic heart failure also suggest that this therapy can result in reduced cardiac fibrosis and an improvement in diastolic function. In contrast, no effect on BP was seen with thoracic afferent ablation in AngII-induced HTN. These results highlight the importance and potential of a new neuromodulation target dependent on thoracic/cardiac spinal afferent nerves in the treatment of HTN.
Perspectives
Synmpatho-excitation in HTN is a well known phenomenon. Multiple mechanisms and pathways have been described that could potentially contribute to sympatho-excitation. The role of cardiac spinal sensory afferent input in the pathogenesis of HTN in the SHR is unique and documented in these studies using the ultrapotent TRPV1 agonist and neurotoxin, RTX. While these studies cleary show that the contribution of cardiac spinal afferents is model specific (e.g. SHR vs Angiotensin II infusion) it points to a potential for these afferents to be a therapeutic target in some forms of HTN that are characterized by increased sympathetic nerve activity.
Supplementary Material
Novelty and Significance: 1) What Is New, 2) What Is Relevant?
What Is New?
The first study to demonstrate that ablation of spinal thoracic sensory nerves can chronically lower blood pressure (BP) in a hypertensive animal model.
What is Relevant?
Hypertension (HTN) is associated with increased activity of the sympathetic nervous system. A potential mechanism for initiating and sustaining this increased sympathetic tone is by increased signaling through sensory spinal afferents - inline with the visceral afferent hypothesis of HTN.
Acknowledgments
We thank Bryan Hackfort of the UNMC Echo Core for performing and analyzing the echocardiograms.
Sources of Funding These studies were supported by funds from Sorrento Therapeutics, Inc. and NIH grant R01 HL-116608, R01 HL-121012 and PO1HL62222. Julia Shanks was supported, in part, by an American Heart Association Post-Doctoral Fellowship (#18POST34030046). Dr. Zucker was also supported, in part, by the Claire and Theodore Hubbard Foundation.
Footnotes
Conflicts of Interest/Disclosures
None
References
- 1.Greenwood JP, Stoker JB and Mary DA. Single-unit sympathetic discharge : quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–10. [DOI] [PubMed] [Google Scholar]
- 2.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F and Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–5. [DOI] [PubMed] [Google Scholar]
- 3.Grassi G Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension (Dallas, Tex : 1979). 2009;54:690–7. [DOI] [PubMed] [Google Scholar]
- 4.Anderson EA, Sinkey CA, Lawton WJ and Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–83. [DOI] [PubMed] [Google Scholar]
- 5.Davrath LR, Goren Y, Pinhas I, Toledo E and Akselrod S. Early autonomic malfunction in normotensive individuals with a genetic predisposition to essential hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H1697–704. [DOI] [PubMed] [Google Scholar]
- 6.Flaa A, Mundal HH, Eide I, Kjeldsen S and Rostrup M. Sympathetic activity and cardiovascular risk factors in young men in the low, normal, and high blood pressure ranges. Hypertension (Dallas, Tex : 1979). 2006;47:396–402. [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. [DOI] [PubMed] [Google Scholar]
- 8.Schlaich MP, Sobotka PA, Krum H, Lambert E and Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–4. [DOI] [PubMed] [Google Scholar]
- 9.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT and Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81. [DOI] [PubMed] [Google Scholar]
- 10.Larsen HE, Lefkimmiatis K and Paterson DJ. Sympathetic neurons are a powerful driver of myocyte function in cardiovascular disease. Sci Rep. 2016;6:38898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanks J, Manou-Stathopoulou S, Lu CJ, Li D, Paterson DJ and Herring N. Cardiac sympathetic dysfunction in the prehypertensive spontaneously hypertensive rat. American journal of physiology Heart and circulatory physiology. 2013;305:H980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Nikiforova N, Lu CJ, Wannop K, McMenamin M, Lee CW, Buckler KJ and Paterson DJ. Targeted neuronal nitric oxide synthase transgene delivery into stellate neurons reverses impaired intracellular calcium transients in prehypertensive rats. Hypertension. 2013;61:202–7. [DOI] [PubMed] [Google Scholar]
- 13.Malliani A, Pagani M and Bergamaschi M. Positive feedback sympathetic reflexes and hypertension. Am J Cardiol. 1979;44:860–5. [DOI] [PubMed] [Google Scholar]
- 14.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L and Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Zimmerman MC, Biswal S and Zucker IH. Selective Nrf2 Gene Deletion in the Rostral Ventrolateral Medulla Evokes Hypertension and Sympathoexcitation in Mice. Hypertension (Dallas, Tex : 1979). 2017;69:1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Colle S, Morello F, Rabbia F, Milan A, Naso D, Puglisi E, Mulatero P and Veglio F. Antihypertensive drugs and the sympathetic nervous system. J Cardiovasc Pharmacol. 2007;50:487–96. [DOI] [PubMed] [Google Scholar]
- 17.Tedla YG and Bautista LE. Drug Side Effect Symptoms and Adherence to Antihypertensive Medication. American journal of hypertension. 2016;29:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JS. Adverse drug effects, compliance, and initial doses of antihypertensive drugs recommended by the Joint National Committee vs the Physicians’ Desk Reference. Arch Intern Med. 2001;161:880–5. [DOI] [PubMed] [Google Scholar]
- 19.Papademetriou V, Doumas M, Faselis C, Tsioufis C, Douma S, Gkaliagkousi E and Zamboulis C. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens. 2011;2011:964394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancia G, Parati G and Zanchetti A. Electrical carotid baroreceptor stimulation in resistant hypertension. Hypertension (Dallas, Tex : 1979). 2010;55:607–9. [DOI] [PubMed] [Google Scholar]
- 21.Shanks J and Herring N. Peripheral cardiac sympathetic hyperactivity in cardiovascular disease: role of neuropeptides. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florea VG and Cohn JN. The autonomic nervous system and heart failure. Circulation research. 2014;114:1815–26. [DOI] [PubMed] [Google Scholar]
- 23.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G and Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. [DOI] [PubMed] [Google Scholar]
- 24.Olde Nordkamp LR, Driessen AH, Odero A, Blom NA, Koolbergen DR, Schwartz PJ and Wilde AA. Left cardiac sympathetic denervation in the Netherlands for the treatment of inherited arrhythmia syndromes. Neth Heart J. 2014;22:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R and Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome. A worldwide report. Circulation. 1991;84:503–11. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, Zareba W, Robinson JL, Hall WJ, Brink PA, Toivonen L, Epstein AE, Li C and Hu D. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–33. [DOI] [PubMed] [Google Scholar]
- 27.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A and Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–9. [DOI] [PubMed] [Google Scholar]
- 28.Wang HJ, Wang W, Cornish KG, Rozanski GJ and Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014;64:745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HJ, Rozanski GJ and Zucker IH. Cardiac sympathetic afferent reflex control of cardiac function in normal and chronic heart failure states. The Journal of physiology. 2017;595:2519–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malliani A and Montano N. Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension. 2002;39:63–8. [DOI] [PubMed] [Google Scholar]
- 31.Zhu GQ, Xu Y, Zhou LM, Li YH, Fan LM, Wang W, Gao XY and Chen Q. Enhanced cardiac sympathetic afferent reflex involved in sympathetic overactivity in renovascular hypertensive rats. Experimental physiology. 2009;94:785–94. [DOI] [PubMed] [Google Scholar]
- 32.Du YH and Chen AF. A “love triangle” elicited by electrochemistry: complex interactions among cardiac sympathetic afferent, chemo-, and baroreflexes. Journal of applied physiology. 2007;102:9–10. [DOI] [PubMed] [Google Scholar]
- 33.Gorbea-Oppliger VJ and Fink GD. Clonidine reverses the slowly developing hypertension produced by low doses of angiotensin II. Hypertension (Dallas, Tex : 1979). 1994;23:844–7. [DOI] [PubMed] [Google Scholar]
- 34.Osborn JL and Camara AK. Renal neurogenic mediation of intracerebroventricular angiotensin II hypertension in rats raised on high sodium chloride diet. Hypertension (Dallas, Tex : 1979). 1997;30:331–6. [DOI] [PubMed] [Google Scholar]
- 35.Coote JH and Sato Y. Reflex regulation of sympathetic activity in the spontaneously hypertensive rat. Circulation research. 1977;40:571–7. [DOI] [PubMed] [Google Scholar]
- 36.Soukhova-O’Hare GK, Zhang JW, Gozal D and Yu J. Bradykinin B2 receptors mediate pulmonary sympathetic afferents induced reflexes in rabbits. Life sciences. 2006;78:1990–7. [DOI] [PubMed] [Google Scholar]
- 37.Mazzone SB and Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiological reviews. 2016;96:975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grassi G and Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–66. [DOI] [PubMed] [Google Scholar]
- 39.Koeners MP, Lewis KE, Ford AP and Paton JF. Hypertension: a problem of organ blood flow supply-demand mismatch. Future Cardiol. 2016;12:339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foss JD, Wainford RD, Engeland WC, Fink GD and Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA and Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395. [DOI] [PubMed] [Google Scholar]
- 42.Gordan R, Gwathmey JK and Xie LH. Autonomic and endocrine control of cardiovascular function. World journal of cardiology. 2015;7:204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor-Clark TE. Oxidative stress as activators of sensory nerves for cough. Pulmonary pharmacology & therapeutics. 2015;35:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habler HJ, Janig W and Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. The Journal of physiology. 1990;425:545–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Giorgio R, Barbara G, Blennerhassett P, Wang L, Stanghellini V, Corinaldesi R, Collins SM and Tougas G. Intestinal inflammation and activation of sensory nerve pathways: a functional and morphological study in the nematode infected rat. Gut. 2001;49:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV and Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. The Journal of physiology. 2012;590:4269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD and Manning RD, Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3388–95. [DOI] [PubMed] [Google Scholar]
- 48.Chae CU, Lee RT, Rifai N and Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension (Dallas, Tex : 1979). 2001;38:399–403. [DOI] [PubMed] [Google Scholar]
- 49.Li D, Lee CW, Buckler K, Parekh A, Herring N and Paterson DJ. Abnormal intracellular calcium homeostasis in sympathetic neurons from young prehypertensive rats. Hypertension. 2012;59:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zahner MR, Li DP, Chen SR and Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. The Journal of physiology. 2003;551:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barber MJ, Mueller TM, Davies BG and Zipes DP. Phenol topically applied to canine left ventricular epicardium interrupts sympathetic but not vagal afferents. Circulation research. 1984;55:532–44. [DOI] [PubMed] [Google Scholar]
- 52.Doggrell SA and Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39:89–105. [DOI] [PubMed] [Google Scholar]
- 53.Pinto YM, Paul M and Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res. 1998;39:77–88. [DOI] [PubMed] [Google Scholar]
- 54.Doggrell SA and Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovascular Research. 1998;39:89–105. [DOI] [PubMed] [Google Scholar]
- 55.de Morais SDB, Shanks J and Zucker IH. Integrative Physiological Aspects of Brain RAS in Hypertension. Curr Hypertens Rep. 2018;20:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leenen FHH, Blaustein MP and Hamlyn JM. Update on angiotensin II: new endocrine connections between the brain, adrenal glands and the cardiovascular system. Endocrine connections. 2017;6:R131–R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Sullivan MJ, Dale WE, Hasser EM, Blaine EH and Cunningham JT. Fos-like immunoreactivity in the medulla after acute and chronic angiotensin II infusion. J Pharmacol Exp Ther. 1998;284:1165–73. [PubMed] [Google Scholar]
- 58.Shinohara K, Kishi T, Hirooka Y and Sunagawa K. Circulating angiotensin II deteriorates left ventricular function with sympathoexcitation via brain angiotensin II receptor. Physiol Rep. 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biancardi VC and Stern JE. Compromised blood-brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. The Journal of physiology. 2016;594:1591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez SF, Huang MH, Davidson BA, Knight PR, 3rd and Izzo JL, Jr. Modulation of angiotensin II responses in sympathetic neurons by cytosolic calcium. Hypertension (Dallas, Tex : 1979). 2003;41:56–63. [DOI] [PubMed] [Google Scholar]
- 61.Fujii AM and Vatner SF. Direct versus indirect pressor and vasoconstrictor actions of angiotensin in conscious dogs. Hypertension (Dallas, Tex : 1979). 1985;7:253–61. [DOI] [PubMed] [Google Scholar]
- 62.Inscho EW, Imig JD and Cook AK. Afferent and efferent arteriolar vasoconstriction to angiotensin II and norepinephrine involves release of Ca2+ from intracellular stores. Hypertension (Dallas, Tex : 1979). 1997;29:222–7. [DOI] [PubMed] [Google Scholar]
- 63.Schnackenberg CG, Wilkins FC and Granger JP. Role of nitric oxide in modulating the vasoconstrictor actions of angiotensin II in preglomerular and postglomerular vessels in dogs. Hypertension (Dallas, Tex : 1979). 1995;26:1024–9. [DOI] [PubMed] [Google Scholar]
- 64.Fortes ZB, Nigro D, Scivoletto R and Carvalho MH. Influence of sex on the reactivity to endothelin-1 and noradrenaline in spontaneously hypertensive rats. Clinical and experimental hypertension Part A, Theory and practice. 1991;13:807–16. [DOI] [PubMed] [Google Scholar]
- 65.Kahonen M, Tolvanen JP, Sallinen K, Wu X and Porsti I. Influence of gender on control of arterial tone in experimental hypertension. The American journal of physiology. 1998;275:H15–22. [DOI] [PubMed] [Google Scholar]
- 66.Reckelhoff JF, Zhang H and Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension (Dallas, Tex : 1979). 2000;35:480–3. [DOI] [PubMed] [Google Scholar]
- 67.Lohmeier TE and Hall JE. Device-Based Neuromodulation for Resistant Hypertension Therapy. Circ Res. 2019;124:1071–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li P, Nader M, Arunagiri K and Papademetriou V. Device-Based Therapy for Drug-Resistant Hypertension: An Update. Curr Hypertens Rep. 2016;18:64. [DOI] [PubMed] [Google Scholar]
- 69.Foss JD, Fink GD and Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol. 2016;310:R262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alnima T, de Leeuw PW and Kroon AA. Baropacing as a new option for treatment of resistant hypertension. European journal of pharmacology. 2015;763:23–7. [DOI] [PubMed] [Google Scholar]
- 71.Schmieder RE. Managing Treatment-Resistant Patients. High blood pressure & cardiovascular prevention : the official journal of the Italian Society of Hypertension. 2015;22 Suppl 1:S11–3. [DOI] [PubMed] [Google Scholar]
- 72.Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018;11:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hai OY, Mentz RJ, Zannad F, Gasparini M, De Ferrari GM, Daubert JC, Holzmeister J, Lam CS, Pochet T, Vincent A and Linde C. Cardiac resynchronization therapy in heart failure patients with less severe left ventricular dysfunction. Eur J Heart Fail. 2015;17:135–43. [DOI] [PubMed] [Google Scholar]
- 74.Grassi G Sympathetic neural activity in hypertension and related diseases. Am J Hypertens. 2010;23:1052–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.