Abstract
Objective:
Older adults with knee osteoarthritis (OA) and comorbid subsyndromal depressive symptoms are at elevated risk for incidental major depression or anxiety disorders. Using an indicated prevention paradigm, the authors conducted a sequenced multiple assignment randomized trial (SMART) to: 1) evaluate the effect of cognitive behavioral therapy (CBT) and physical therapy (PT), together with the temporal ordering of these interventions, on patient-reported global impression of change (P-GIC), mood, anxiety, and pain, and 2) compare the strategies’ impact on incidence of common psychiatric disorders over 12-months.
Method:
This intervention development trial compared four adaptive strategies delivered in two stages (each up to 8 weeks), contrasted with Enhanced Usual Care (EUC). The strategies were CBT followed by an increased dose of CBT (CBT-CBT), CBT followed by PT (CBT-PT), PT followed by an increased dose of PT (PT-PT), and PT followed by CBT (PT-CBT). Participants (n=99) were ≥ 60 years old and met clinical criteria for knee OA and subthreshold depression. Response was defined as at least “much better” on the P-GIC. Participants were assessed quarterly for 12 months for incidence of psychiatric disorders.
Results:
Stage 1 response was higher for PT (47.5%) compared to CBT (20.5%). Non-responders receiving an additional dose of the same intervention experienced a response rate of 73%, higher than for switching to a different intervention. All strategies were superior to EUC (5%). Although not powered to detect effects on disorders, neither intervention strategy nor response status affected twelve-month incidence of depression and anxiety disorders.
Conclusions:
As response rates were similar for PT-PT and CBT-CBT, it may be dose and not type of these interventions that is necessary for clinical benefit. For non-responders, this finding may guide providers to stay the clinical course for up to 12 weeks before switching. These results support future trials of SMART designs in late-life depression prevention.
Keywords: knee arthritis, depression, anxiety, prevention, cognitive behavioral therapy, physical therapy, SMART trial
Introduction
Knee osteoarthritis (OA) is a leading cause of disability (1) among older adults in the US (2) and among the top 10 causes of disability worldwide (3, 4). The prevalence of symptomatic knee OA in adults aged 55–64 is estimated at 12%−16% (5).
There are both biological and psychological mechanisms through which the pain and disability of knee OA may contribute to depression and anxiety. Chronic pain shrinks brain areas specific for mood regulation (6), challenging mood homeostasis in response to stress (7), and the disability resulting from symptomatic knee OA may cause demoralization, isolation, and reduced self–efficacy for managing life stressors (8). It is estimated that depression is two to three times more prevalent among patients with OA (9, 10), and those with comorbid depressive symptoms are most at risk for the onset of major depressive episodes and/or anxiety disorders (11, 12).
Treatments for knee OA are often delivered as sequences of care and include weight loss, analgesics, ambulatory assistive devices, and arthroplasty (13). Learning–based interventions such as Cognitive Behavioral Therapy (CBT) or a knee-specific physical therapy (PT) (14, 15) are routinely prescribed along with analgesics for pain control and improved functioning. Both CBT and PT are behaviorally activating, improve self–efficacy, and reduce learned helplessness (16, 17). In knee OA patients living with subthreshold depression, these interventions may reduce pain, improve functioning, and decrease risk of conversion to syndromal depression or anxiety.
Most depression and anxiety prevention trials use a simple randomized controlled trial approach, in which one or more active prevention interventions are delivered to an at-risk sample (18). Participants are then followed to document onset of these disorders. However, this may not be the most efficient way to test prospectively–defined strategies of care. For example, a study may be designed using a strategy where each subject’s intervention may be adapted depending on whether that individual responds at a given point in time to their originally assigned treatment. These types of trials, called sequenced multiple assignment randomized trials (SMART) (19), reflects what happens in the clinic, as clinicians usually adapt or switch interventions based on initial response (20). It also provides the opportunity to compare treatment options among non-responders to early interventions (19).
The aims of this intervention–development SMART trial were to evaluate the clinical effect of CBT and PT, as well as the order effects of the interventions, on patient–reported global impression of change (P-GIC), mood, anxiety, and pain outcomes. We also compared both the strategies in response status (defined by P-GIC) on the prevention of any new-onset depression and anxiety disorder (i.e., major depression, minor depression, dysthymia, generalized anxiety disorder, social phobia, PTSD, and panic disorder) during one-year follow–up. Because CBT may target stress reduction, pain, and mood symptoms, we hypothesized that 1) CBT would be superior to PT as a first-line intervention, and 2) for initial non-responders, CBT followed by PT would be the superior strategy.
Methods
Overview and Rationale for Study Design:
We have previously published a detailed description of the protocol used in this intervention development study (11). The trial compared four adaptive strategies delivered in 2 stages, using Enhanced Usual Care (EUC) as the control (group 5). For EUC, information was mailed to each participant’s primary care physician (PCP) describing the best practice approach for prescribing analgesics for knee OA (21). Incidental findings during scheduled blinded assessments (i.e., new onset symptoms or worsened pain) were related to their PCP. Each of the four adaptive strategies, that also included EUC, were:
Eight weekly sessions of CBT; if no response, deliver another 4 weekly sessions [CBT-CBT].
Eight weekly sessions of CBT; if no response, switch to 8 weekly sessions of PT [CBT-PT].
Eight weekly sessions of PT; if no response, deliver another 4 weekly sessions [PT-PT].
Eight weekly sessions of PT; if no response, switch to 8 weekly sessions of CBT [PT-CBT].
Participants:
Recruitment utilized a blended approach to depression and anxiety prevention by including those individuals with knee OA (selective prevention) who also have subsyndromal depressive symptoms (indicated prevention) (see published methodology paper (11)). Subjects were ≥ 60 years old; met clinical criteria for knee OA (22); scored at least “1” on 9-item Patient Health Questionnaire (PHQ-9) (23) (including at least one of the cardinal symptoms of depression [low mood or anhedonia]) endorsed for at least several days for the past 2 weeks; scored 7–15 on pain subscale of Western Ontario and McMaster University Arthritis Index (24); and Modified Mini Mental State (3MS) Examination ≥ 80 (25). Exclusion criteria stipulated current or partially remitted major depressive disorder (MDD or history of MDD within last 12 months) as determined by the Structured Clinical Interview for DSM-IV (SCID) (26); currently taking an antidepressant or anti-anxiety medication (the latter for > 4 days/week for the past 4 weeks); lifetime history of bipolar disorder or psychotic-spectrum disorder; or involved in knee pain-related workers compensation or litigation. Written informed consent was obtained from all participants after the procedures had been fully explained. Participants could enter the study meeting SCID criteria for minor depression or an anxiety disorder, but at follow-up assessments, if they continued to meet syndromal criteria, they were not considered new–onset conditions.
Interventions:
Randomization and Sequence of Interventions:
Stage 1 participants were randomized to 8 weeks of either CBT, PT, or EUC. Each CBT and PT session lasted 45–60 minutes. Participants were randomized in 2:1:2 manner at the beginning of the study to receive CBT, EUC, or PT, respectively. A permuted block scheme was used to balance intervention assignments according to prior history of MDD. Blinding was maintained by the data manager, and independent evaluators and the biostatisticians were masked to random assignment until outcomes analysis was complete. Participants were instructed to not divulge the condition to which they had been assigned.
At Stage 2, Stage 1 non-responders (defined below) were randomized to an additional 4 sessions of the same intervention or 8 sessions of the alternative intervention (Figure 1). All participants, regardless of response status, were followed for 12 months for incident episodes of major depression, another depressive disorder, or an anxiety disorder.
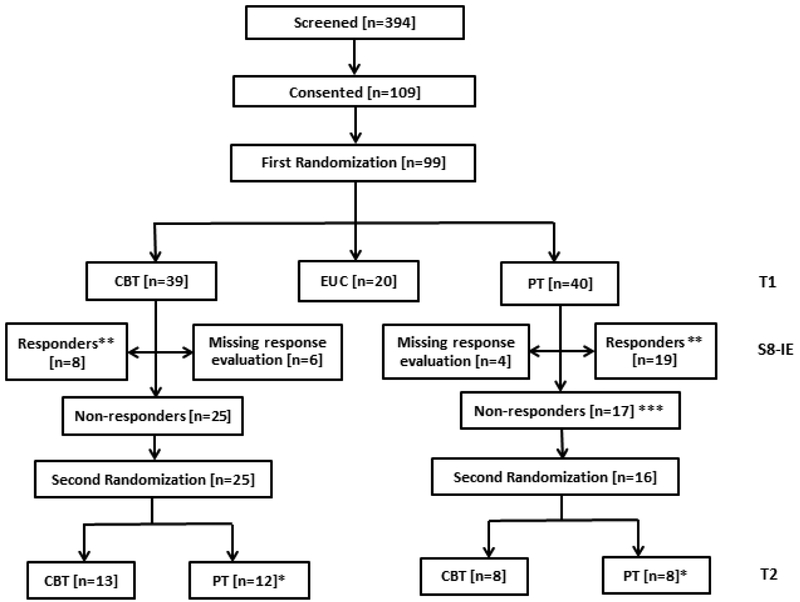
Figure 1.
Consort Diagram
*: 5 out of 12 who were randomized to receive PT (after receiving CBT in the first stage) at the second stage declined intervention. 1 out of 8 who are randomized to receive PT (after receiving PT in the first stage) declined intervention.
**: All 8 CBT responders at S8-IE (independent evaluation at week 8) have data at T2; all 19 PT responders at S8-IE have data at T2
***: One of the 17 non-responders didn’t complete 1st intervention and didn’t want any further therapy.
Cognitive Behavioral Therapy (CBT) for Pain:
Intervention modules included: 1) combating demoralization; 2) teaching coping skills and problem-solving techniques; 3) shifting self–view to that of an active, resourceful, and competent person and encouraging behavioral activation; 4) learning to alter associations between thoughts, feelings, and behaviors that do not promote analgesia and identifying how to change automatic, maladaptive thoughts; 5) learning relaxation skills; and 6) facilitating maintenance and generalization of skills. All CBT sessions were audio taped and 20% more randomly selected for fidelity ratings, with corrective feedback given as needed. We used weekly group supervision to enhance one-on-one feedback.
Physical Therapy (PT):
PT combined supervised exercises and manual therapy techniques. The supervised PT components represents state–of–the–art evidence–based practice guidelines (14, 27, 28) and combined aerobic and strengthening exercises. The manual therapy techniques involve the application of manual force from the therapist (29). These techniques included a series of motions of the tibia with respect to the femur that are needed for normal knee flexion and extension. The manual therapy techniques also included lower extremity stretching delivered by the therapist (30). All participants were instructed in a home exercise program with the goal to be independent in the exercises by week 8.
Assessments, Criteria for Response, and Booster Sessions:
Initial response was elicited at the end of stage I, and if participants were randomized a second time, they had response assessed again after completing the second randomized intervention. Clinically significant response to the interventions was defined as 1) much better or 2) very much better on the P-GIC that ranged from 1–7. The wording of the P-GIC is: “Check the circle that best describes how you (the patient) have felt overall since you began participating in this research study.” We selected the P-GIC as criteria for response because participants endorsed both knee pain and mild emotional distress, and only using percent improvement of pain as the response criterion could miss other improvements valued by participants, such as improvement in insomnia, psychological stress, or self-efficacy
Both responders and non-responders were then assessed at six time points by independent evaluators (IE) blind to randomized intervention assignment. These were T1: Baseline; after the first intervention (measured 8 weeks after baseline for those assigned to active intervention and referred to as S8-IE; T2: after the 2nd intervention (measured 16 weeks after baseline for all, including those randomized to EUC); T3-T6: three, six, nine, and twelve months after the end of the second intervention. In addition to the P-GIC, pain (using a 0–10 numeric rating scale), depression (using the PHQ-9), and anxiety (using the GAD-7) (31) were assessed at T1-T6. We assessed new-onset depression and anxiety syndromes at these time points using the MINI Neuropsychiatric Interview (32). Responders received booster sessions at T4 and T5 following the end of the prevention intervention(s). If responders received both interventions, they selected the type of booster they preferred. Non-responders were referred to their PCP with pain and mood treatment recommendations (21).
Statistical Analyses:
We estimated early response rates for the first stage interventions (CBT and PT) with proportions and corresponding 95% confidence intervals (CIs). Subjects assigned to EUC were not included in the early response analysis (i.e., at S8-IE) since outcomes for EUC were not assessed until T2. Equality was tested using chi–square test for two proportions. Then we estimated the response rates at the end of stage two among the first stage responders stratified by the initial intervention. We also estimated the response rates for the first stage non-responders stratified by first and second stage interventions. The results are reported as proportions and 95% CIs.
For each of the five strategies (CBT-CBT, CBT-PT, PT-PT, PT-CBT, EUC), response rates at T2 were estimated using a G-Computation approach (33). This method observes that for any adaptive treatment strategy, there are two intervention paths associated with it. For example, a patient following the CBT-CBT strategy may respond in two ways – respond to initial CBT and stay as responder at time T2, or not respond to initial CBT but respond after receiving more CBT during stage 2. Therefore, response rates under this strategy would be estimated by combining the response rates at the end of stage 2 for these two paths using a total probability formula. More explicitly, if p1 is the proportion of early responders among those who received CBT at stage 1, p2 is the proportion of responders at time T2 among the responders at stage 1, and p3 is the proportion of responders at time T2 among those who received CBT in both stages, then the proportion of responders at time T2 for the strategy CBT-CBT is estimated as p1*p2 + (1-p1)*p3. The CIs for these estimates are calculated using the delta method (34). The equality of the response rates across these strategies are tested using Wald’s Chi-square test.
To investigate how PHQ9 scores change over time under various intervention strategies, we used mixtures of linear mixed effect models (LMEM). Essentially, separate LMEM were fit to the data with common intervention paths and then the slopes from the appropriate paths were combined to estimate the slope of PHQ9 scores over time under a given strategy (35). For example, to estimate the CBT-CBT strategy effect on PHQ9 scores over time, we fit LMEM of PHQ9 scores over time (as the outcome) and time (as a covariate) to the data from two groups: 1) participants who started CBT, achieved early responses and had no further intervention; and 2) participants who started CBT, did not achieve early response, and continued CBT in the second stage. We estimated the slopes of PHQ9 scores over time for these two groups from the LMEM fit. With these estimated slopes and the proportion of participants who had early responses with initial CBT, we then estimated the slope under the CBT-CBT strategy in a similar manner as to how we estimated the response rates described above. The same approach was taken to estimate the slopes for the other three strategies. We tested the equality of slopes using a Wald Chi-Square test. A similar approach was used for anxiety severity (using the GAD-7) and pain (using a 0–10 numeric rating scale).
As can be seen from the CONSORT diagram (Figure 1 and supplemental Table 1 ), missing data were minimal in this study and the rate of missing data were similar across the randomized intervention groups. Therefore, we assumed that the data were missing at random (MAR), and hence the above likelihood-based methods appropriately handled missing data under this assumption.
To investigate whether response, assessed with the PGI-C, at the end of the intervention was associated with the incidence of any depressive or anxiety disorder diagnosis during 12-month follow-up, we used response at time T2 as the predictor and time from T2 to the diagnosis as the survival time in a Cox proportional hazard model. The results are presented using Kaplan-Meier survival curves by response status, and hazard ratios (HRs) and the 95% CIs from the Cox model. Censoring was assumed to be random and proportional hazard assumption was tested using Grambsch and Therneau’s weighted residuals method (36).
The effect of strategies on the time to any diagnosis was estimated with survival curves and corresponding CIs. In this analysis, the time to any diagnosis was defined as time from randomization to any diagnosis. The equality of survival curves was tested using log-rank tests modified for SMART trials (37).
Results:
The first participant entered the trial on February 16, 2012, and the last participant exited on February 10, 2016. At entry, 99 participants were randomized to PT (n=40), CBT (n=39), and EUC (n=20) (Table 1). Six CBT subjects and four PT subjects had response evaluation data missing at the end of Stage 1. At the end of Stage 1, 25 participants who had not responded to CBT were re-randomized, with equal probability, to four more sessions of CBT (n=13) or eight sessions of PT (n=12). Similarly, 16 participants who had not responded to PT were rerandomized to eight sessions of CBT (n=8) or four more sessions of PT (n=8). The attrition rate (at any time during the trial) for CBT, EUC, and PT was 20.5% (8/39), 30% (6/20), and 15% (6/40), respectively, and these rates were not significantly different among the three conditions (χ2(2) = 1.86, p = 0.394). After the second randomization, there was no attrition among responders assigned to either CBT-CBT or PT-PT, or among those receiving PT-CBT. Only one responder each from participants receiving CBT-CBT, CBT-PT, or PT-PT did not have complete data.
Table 1:
Baseline Descriptive Characteristics
| Variable | Category | ALL [n=99] |
EUC [n=20] |
CBT [n=39] |
PT [n=40] |
P-value |
|---|---|---|---|---|---|---|
| Gender | Female | 61.62% | 60.00% | 58.97% | 65.00% | 0.85a |
| Male | 38.38% | 40.00% | 41.03% | 35.00% | 0.85a | |
| Race | African American | 16.16% | 15.00% | 20.51% | 12.50% | 0.83b |
| Caucasian | 81.82% | 85.00% | 79.49% | 82.50% | 0.83b | |
| Age | 71.00 (7.58) | 69.85 (7.12) | 71.77 (7.36) | 70.83 (8.10) | 0.65c | |
| Education | 14.95 (2.45) | 14.65 (2.92) | 14.87 (2.58) | 15.18 (2.09) | 0.72c | |
| 3MS Score | 95.60 (3.90) | 95.60 (3.59) | 95.51 (3.67) | 95.68 (4.33) | 0.98c | |
| Body Mass Index | 31.49 (8.53) | 31.24 (7.42) | 30.67 (7.45) | 32.42 (10.01) | 0.66c | |
| CIRS-G TOTAL | 8.58 (3.38) | 8.05 (3.05) | 8.54 (3.32) | 8.88 (3.63) | 0.67c | |
| Pain NRS | 9.52 (4.10) | 9.35 (3.59) | 9.77 (4.21) | 9.35 (4.31) | 0.89c | |
| Did not meet DSM-IV criteria for minor depressive disorder or any anxiety disorder at baseline | 86.87% | 80.00% | 87.18% | 90.00% | 0.44b | |
| PHQ-9 | 5.60 (2.11) | 5.60 (2.58) | 5.33 (2.14) | 5.85 (1.83) | 0.56c | |
| GAD-7 | 3.17 (2.68) | 3.70 (3.50) | 2.62 (2.41) | 3.45 (2.42) | 0.24c | |
| History of MDD | 33.33% | 35.00% | 33.33% | 32.50% | 0.98a | |
| History of GAD | 17.17% | 15.00% | 15.38% | 20.00% | 0.83a |
Chi-square test, df=2
Fisher Exact test
ANOVA F test, df=2, 96
3MS: Modified Mini Mental State Exam; CIRS-G: Cumulative Illness Rating Scale for Geriatrics; NRS: Numeric Rating Scale; PHQ-9: Patient Health Questionnaire; GAD-7: Generalized Anxiety Disorder Questionnaire; MDD: Major Depressive Disorder; GAD: Generalized Anxiety Disorder
At the time of second randomization among CBT non-responders, GAD-7 scores were lower for those assigned to CBT (n=13, 1.08 (1.12) compared to those randomized to PT (n=12, 2.92 (2.47) (ANOVA F(df=1, 23)= 5.94, p=0.02
In the first stage, 8 of 39 (20.5%; 95% CI: 7.8%−33.2%) of the CBT participants and 19 of 40 (47.5%; 95% CI: 32%−63%) of the PT participants responded to the initial intervention (27 percentage point difference, χ2(1) = 6.39, p = 0.012). There was no statistically significant difference (Fisher’s exact test, p >0.99) in the proportion of participants maintaining response eight weeks after the first stage: 7 of 8 initial CBT responders; (87.5%; 95% CI: 47.4%−99.7%) and 16 of 19 initial PT responders (84.2%; 95% CI: 60.4%−96.6%).
Comparing Strategies of Care for Patient Reported Global Impression of Change.
Our primary aim was to compare CBT-CBT, CBT-PT, PT-PT, PT-CBT, and EUC based on Stage 2 response. The response rates for these strategies are shown in Figure 2. Participants who received the CBT-CBT strategy had two potential ways to achieve response – respond to initial CBT and maintain response at T2, or fail to respond to initial CBT but respond to CBT during the second stage as assessed at T2. Since 20.5% initially responded to CBT of whom 87.5% remained as responders in the second stage; and since 69.2% for whom CBT failed to produce a response in the first stage subsequently achieved response with CBT in the second stage, the combined response rate for the CBT-CBT strategy is 0.205*0.875+(1–0.205)*0.692 = 73%. Using the same method, we calculated the response rates for CBT-PT, PT-CBT, and PTPT strategies, respectively, as 37.8%, 53.1%, and 72.9%, compared to 5% with EUC. Overall equality of strategy effects was rejected (χ2(4) = 63.12, p < 0.0001). Figure 2 shows that EUC is inferior to all the adaptive strategies (χ2(1) = 53.27, p < 0.0001). The a priori hypothesis that CBT-PT strategy would be significantly better than the other strategies was not supported by the data ( χ2(1) = 1.24, p = 0.265)
Figure 2: Forest plot for proportion of early response at stage 1 by interventions (a), and proportion for response at stage 2 by strategies (b).
Sample size of CBT-CBT group = 13+8=21, in which 8 people are responders in stage 1 and 13 people are non-responders who get CBT for their 2nd intervention. Sample size of CBT-PT group = 12+8=20, in which 8 people are responders in stage 1 and 12 people are non-responders who get PT for their 2nd intervention. Sample size of PT-CBT and PT-PT groups are both = 19+8, in which 19 people are responders in stage 1 and 8 people are assigned to either PT or CBT for their 2nd intervention.
Time to First Onset of Depressive or Anxiety Disorders During 12-Month Follow-Up
Among the 99 people randomized, 10 people received a psychiatric diagnosis before T2 and one person did not have an evaluation of response at T2. Of the remaining 88, 12 did not have any event records after T2 (EUC: 4; CBT: 5; PT: 3). This left 76 participants (40 responders and 36 non-responders) for time to first onset of any diagnosis after T2.
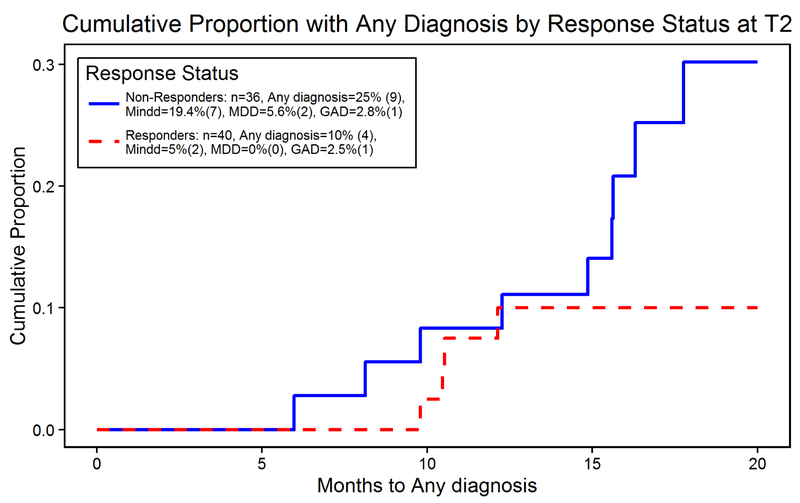
Four responders and nine non-responders developed an incident (i.e., new-onset) psychiatric disorder. Despite longer time without any diagnosis for responders compared to non-responders, the difference in the cumulative proportion with any diagnosis was not statistically significant (Figure 3). The cumulative proportion with any diagnosis by strategy were not statistically significant across strategies (Supplemental Figure 1).
Figure 3: Cumulative proportion with any diagnosis comparing responders to non-responders.
Despite observed longer time without any diagnosis for responders compared to non-responders, the difference in the cumulative proportion with any diagnosis was not statistically significant (Logrank test z = −1.43, p = 0.153; HR for responders = 0.42; 95% CI 0.13−1.37)
Changes in Depressive Symptoms, Anxiety Symptoms, and Pain During 12-Month Follow-Up
Depression (PHQ9).
Scores declined for all strategies (overall mean score declined from 5.60 at randomization to 3.66 at T6), however, the rates of decline over time, which ranged from 0.05 points per month for those receiving EUC to 0.12 points per month for those receiving PTCBT, were not significantly different across the five strategies (Chi-square test from LMEM, χ2(4) = 4.68, p = 0.322).
Anxiety (GAD7).
The rates of decline over time were significantly different across five strategies (χ2(4) = 22.05, p < 0.0001). Over time, GAD7 scores declined significantly only for PT-CBT (0.07 points per month, χ2(1) = 7.15, p = 0.008) and PT-PT (0.09 points per month, χ2(1) = 13.49, p = 0.0002).
Pain (Numeric Rating Scale).
The pain scores improved for all strategies. However, the rates of decline over time, ranging from 0.12 points per month for EUC to 0.18 points per month for CBT-CBT, were not significantly different across the strategies.
Discussion
In this intervention development trial using a SMART design, we observed higher rates of response in stage 1 for PT compared to CBT. The difference in response rate at this stage was 27 percentage points, suggesting a clinically meaningful superiority of PT versus CBT. Stage 1 non-responders who were re-randomized to an increased dose of the same intervention experienced a response rate of 73%, higher than for those strategies which involved switching. All the strategies of care were superior to EUC, which had a response rate of only 5%. There was no difference at twelve-month follow-up for incident cases of depression or anxiety disorders by either intervention strategy or response status.
Because CBT may improve pain and mood, we predicted that teaching participants active coping skills would prepare them to better engage in PT and in-between session home exercises (27). Our hypothesis that CBT-PT would be superior to the other strategies was not supported, and may be explained by the superiority during Stage 1 of PT to CBT. For initial non-responders to either PT or CBT, those who received longer duration of either PT or CBT experienced response rates of 73%. This observation challenges the frequently followed practice of switching or augmenting treatment if response is not observed after 8 weeks of an intervention (38, 39). It is possible that patients may need to practice the techniques learned in CBT and PT longer than 8 weeks to benefit from the acquired psychological skills and physical gains (40). Our findings lend credence to reports that: 1) additional visits for PT and supervised exercise adds greater symptomatic relief for older adults with knee OA (27), and 2) maximizing completed CBT sessions improves psychiatric outcomes (41, 42). The observation that response rates were similar for PT-PT and CBT-CBT suggests it may be the dose, not the type, of these behaviorally-activating interventions that is necessary for benefit. This finding may guide providers to stay the clinical course for up to 12 weeks before switching.
We observed that 10 people experienced incident episodes of depressive or anxiety disorders before T2 (10%) and 4 of the responders and 9 of the non-responders (17%) received a new diagnosis during 1-year follow-up. Compared with published rates of incident major depression in persons with subsyndromal symptoms receiving usual care (1 in four to five/year) (43–45), the potential protective effect against depression and anxiety is noteworthy because we used both depression and anxiety as outcomes of interest.
During 12-months of follow-up, anxiety was the only symptom which significantly improved, and only for PT-CBT and PT-PT. The observation that both strategies included PT suggest that PT may have an anxiety-specific effect. We have previously reported that in patients with knee OA, higher anxiety is related to worse physical function, and that both high anxiety and fearavoidance beliefs were associated with impairments in lower extremity-related activities of daily living (46). To our knowledge this is the first report of an anti-anxiety effect of PT.
We acknowledge that this intervention development study (11) was not powered for confirmatory testing of these strategies of care. The fundamental purpose of conducting an intervention development study, however, is to examine the feasibility of an approach for use in a confirmatory trial (47), and this goal was achieved based on our ability to: 1) successfully complete this project in both rehabilitation and late-life mental health clinics; 2) implement a SMART design with minimal attrition that did not differ across intervention assignments; and 3) observe preliminary outcomes consistent with a prevention effect. Given the scope of the project, follow-up was limited to 12 months. In our next trial, we plan to follow participants for 2 years; our observation of a longer time to incident depressive or anxiety disorders for responders supports the need for longer follow-up. Acknowledging that time to event, in relation to response status, was not significantly different, a possible implication is that it may not matter HOW a patient reduces symptoms that increase vulnerability to depression, but improvement per se, that is important for preventing new onset depression and anxiety. This inference is supported by another project in which we described that BOTH problem solving therapy (a depression-specific intervention) and coaching in healthy eating practices (a health-promoting but not depression-specific intervention) both reduced new onset major depression over the course of two years (48). Future work which also assesses beliefs about treatment of chronic pain and personality characteristics which may be linked with outcomes may also inform future prevention research.
To our knowledge, this is the first trial in older adults that included a focus on depression and anxiety prevention that 1) used a SMART design, and 2) included physical therapy as one of the prevention strategies. No other late-life depression prevention studies have used a physical activity intervention (i.e., exercise, PT), but have instead used watchful waiting, psychological/behavioral interventions, or antidepressant pharmacotherapy (48–52). While these results need to be confirmed in an adequately powered trial, they suggest that 1) all the adaptive strategies were superior to EUC; 2) a SMART trial may be an efficient and clinically relevant approach for testing prevention strategies for late-life psychiatric disease, and 3) increasing the duration of exposure of an intervention may be superior to switching for improving patient-reported impression of change in older adults living with knee OA and subthreshold depression.
Supplementary Material
Highlights.
What is the primary question addressed by this study?
Older adults with knee osteoarthritis (OA) and comorbid subsyndromal depressive symptoms are at elevated risk for incidental major depression or anxiety disorders. The aims of this intervention–development SMART trial were to evaluate the clinical effect of CBT and PT, as well as the order effects of the interventions, on patient–reported outcomes. We also compared both the strategies and response status on the prevention of common psychiatric disorders during one-year follow–up.
What is the main finding of this study?
Stage 1 response was higher for PT (47.5%) compared to CBT (20.5%). Nonresponders re-randomized to an increased dose of the same intervention experienced a response rate of 73%, higher than for switching. All strategies were superior to enhanced usual care (5%). Neither intervention strategy nor response status affected twelve-month incidental depression and anxiety disorders.
What is the meaning of the finding?
it may be dose and not type of these interventions that is necessary for clinical benefit. This finding may guide providers to stay the clinical course for up to 12 weeks before switching. a SMART trial may be an efficient and clinically relevant approach for testing prevention strategies for late-life psychiatric disease.
Disclosures and Acknowledgements:
This work was funded by P30 MH090333 (Reynolds). Dr. Karp reports receipt of medication supplies from Pfizer and Indivior for investigator initiated studies. He receives compensation for service on the editorial boards of the American Journal of Geriatric Psychiatry and Journal of Clinical Psychiatry. Dr. Butters served as a consultant for GlaxoSmithKline from whom she received remuneration for participating in cognitive diagnosis consensus conferences for a clinical trial. Dr. Reynolds reported receiving pharmaceutical support for National Institutes of Health-sponsored research studies from Bristol-Myers-Squibb, Forest, Pfizer, and Lilly; and serving on the American Association for Geriatric Psychiatry editorial review board. He has received an honorarium as a speaker from MedScape/WebMD. The other authors have no potential conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas E, Peat G, Mallen C, et al. Predicting the course of functional limitation among older adults with knee pain: do local signs, symptoms and radiographs add anything to general indicators? Ann Rheum Dis. 2008;67:1390–1398. [DOI] [PubMed] [Google Scholar]
- 2.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 5.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 2007;34:172–180. [PubMed] [Google Scholar]
- 6.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vachon-Presseau E, Roy M, Martel MO, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136:815–827. [DOI] [PubMed] [Google Scholar]
- 8.Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain. 2002;100:55–64. [DOI] [PubMed] [Google Scholar]
- 9.Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Zhang M, Lin EH, et al. Mental disorders among persons with arthritis: results from the World Mental Health Surveys. Psychol Med. 2008;38:1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karp JF, Dew MA, Wahed AS, et al. Challenges and Solutions for Depression Prevention Research: Methodology for a Depression Prevention Trial for Older Adults with Knee Arthritis and Emotional Distress. Am J Geriatr Psychiatry. 2016;24:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262–268. [DOI] [PubMed] [Google Scholar]
- 13.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. [DOI] [PubMed] [Google Scholar]
- 14.Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173–181. [DOI] [PubMed] [Google Scholar]
- 15.Creamer P, Flores R, Hochberg MC. Management of osteoarthritis in older adults. Clin Geriatr Med. 1998;14:435–454. [PubMed] [Google Scholar]
- 16.Maciejewski PK, Prigerson HG, Mazure CM. Self-efficacy as a mediator between stressful life events and depressive symptoms. Differences based on history of prior depression. Br J Psychiatry. 2000;176:373–378. [DOI] [PubMed] [Google Scholar]
- 17.Seligman ME. Learned helplessness. Annu Rev Med. 1972;23:407–412. [DOI] [PubMed] [Google Scholar]
- 18.Buntrock C, Ebert D, Lehr D, et al. Effectiveness of a web-based cognitive behavioural intervention for subthreshold depression: pragmatic randomised controlled trial. Psychother Psychosom. 2015;84:348–358. [DOI] [PubMed] [Google Scholar]
- 19.Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24:1455–1481. [DOI] [PubMed] [Google Scholar]
- 20.Bruyere O, Cooper C, Pelletier JP, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2014;44:253–263. [DOI] [PubMed] [Google Scholar]
- 21.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older P. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. [DOI] [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 26.First M, Spitzer R, Gibbon M, et al. : Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, D.C., American Psychiatric Press; 1997. [Google Scholar]
- 27.Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301–1317. [PubMed] [Google Scholar]
- 28.Ottawa panel evidence-based clinical practice guidelines for therapeutic exercises and manual therapy in the management of osteoarthritis. Phys Ther. 2005;85:907–971. [PubMed] [Google Scholar]
- 29.Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther. 2007;12:109–118. [DOI] [PubMed] [Google Scholar]
- 30.Maitland G: Peripheral Manipulation. Boston, Butterworth-Heinemann; 1991. [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20: 22–33;quiz 34–57. [PubMed] [Google Scholar]
- 33.Bembom O, van der Laan MJ. Statistical methods for analyzing sequentially randomized trials. Journal of the National Cancer Institute. 2007;99:1577–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wahed AS. Inference for two-stage adaptive treatment strategies using mixture distributions. Journal of the Royal Statistical Society: Series C (Applied Statistics). 2010;59:1–18. [Google Scholar]
- 35.Miyahara S, Wahed AS. Assessing the effect of treatment regimes on longitudinal outcome data: application to REVAMP study of depression. Journal of Statistical Research. 2012;46:233–254. [Google Scholar]
- 36.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 37.Kidwell KM, Wahed AS. Weighted log-rank statistic to compare shared-path adaptive treatment strategies. Biostatistics. 2013;14:299–312. [DOI] [PubMed] [Google Scholar]
- 38.Gore M, Sadosky A, Leslie D, et al. Patterns of therapy switching, augmentation, and discontinuation after initiation of treatment with select medications in patients with osteoarthritis. Clin Ther. 2011;33:1914–1931. [DOI] [PubMed] [Google Scholar]
- 39.Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30:517–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almirall D, Nahum-Shani I, Sherwood NE, et al. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014;4:260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hundt NE, Amspoker AB, Kraus-Schuman C, et al. Predictors of CBT outcome in older adults with GAD. J Anxiety Disord. 2014;28:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of Online Collaborative Care for Treating Mood and Anxiety Disorders in Primary Care: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79:71–79. [DOI] [PubMed] [Google Scholar]
- 44.Munoz RF, Ying YW, Bernal G, et al. Prevention of depression with primary care patients: a randomized controlled trial. Am J Community Psychol. 1995;23:199–222. [DOI] [PubMed] [Google Scholar]
- 45.Smit F, Ederveen A, Cuijpers P, et al. Opportunities for cost-effective prevention of late-life depression: an epidemiological approach. Arch Gen Psychiatry. 2006;63:290–296. [DOI] [PubMed] [Google Scholar]
- 46.Scopaz KA, Piva SR, Wisniewski S, et al. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2009;90:1866–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds CF 3rd, Thomas SB, Morse JQ, et al. Early intervention to preempt major depression among older black and white adults. Psychiatr Serv. 2014;65:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van’t Veer-Tazelaar PJ, van Marwijk HW, van Oppen P, et al. Stepped-care prevention of anxiety and depression in late life: a randomized controlled trial. Arch Gen Psychiatry. 2009;66:297–304. [DOI] [PubMed] [Google Scholar]
- 50.Robinson RG, Jorge RE, Moser DJ, et al. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA. 2008;299:2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rovner BW, Casten RJ, Hegel MT, et al. Low vision depression prevention trial in age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2014;121:2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albert SM, King J, Dew MA, et al. Design and Recruitment for a Randomized Controlled Trial of Problem-Solving Therapy to Prevent Depression among Older Adults with Need for Supportive Services. Am J Geriatr Psychiatry. 2016;24:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.