Abstract
Interleukin (IL) −22, a member of the IL-10 family, plays a role in antiviral immune responses to a number of viral infections. However, it is unclear whether IL-22 is involved in the mucosal immunity against herpes simplex virus 2 (HSV-2) infection in the female reproductive tract (FRT). In this study, we studied whether IL-22 could inhibit HSV-2 infection of human cervical epithelial cells (End1/E6E7 cells). We showed that End1/E6E7 cells express the functional IL-22 receptor complex (IL-22R1 and IL-10R2). When treated with IL-22, End1/E6E7 cells expressed the higher levels of IFN-stimulated genes (ISGs: ISG15, ISG56, OAS-1, OAS-2, and Mx2) than untreated cells. In addition, IL-22-treated cells produced higher levels of the tight junction proteins (ZO-1 and Occludin) than untreated cells. Mechanistically, IL-22 could activate the JAK-STAT signaling pathway by inducing the phosphorylation of STAT1 and STAT3. These observations indicate the potential of IL-22 as an anti-HSV-2 agent in the FRT mucosal innate immunity against HSV-2 infection.
Keywords: Human cervical epithelial cells, IL-22, IFN-stimulated gene, Signal transducers and activators of transcription, Tight junction proteins, HSV-2
1. Introduction
Genital herpes is one of the most common sexually transmitted diseases [1], in which ulcerative and vesicular lesions appear on the genitals with lifelong latency [2]. Herpes simplex virus type 2 (HSV-2) infection, the primary cause of genital herpes [3], significantly augments the likelihood of developing cervical cancer and human immunodeficiency virus (HIV) infection [4, 5]. Currently, no vaccine has been developed for preventing genital HSV-2 infection [6, 7]. Although there are several broad-spectrum antiviral drugs (acyclovir, valaciclovir and famciclovir) clinically available for treatment of people infected with HSV [6–8], these medications can only control virus reactivation and do not eliminate latent virus [9, 10]. In addition, drug resistance [11] and mutation of the virus [12] diminish effectiveness of the treatment. Thus, developing novel and more effective drugs to control the spread of HSV-2 becomes urgent.
The mucosal immunity in the female reproductive tract (FRT) functions as a first-line and primary innate defense mechanism against HSV-2 infection [11]. Physical barriers of the FRT include the vulva, cervix, epithelial surface of the mucosa, basement membrane of ovarian follicles, and the zona pellucid of the oocyte [12]. Among these barriers, epithelial cells regulate the movement of the molecules across the epithelium, thereby blocking the entry of microbes and playing a vital role in the FRT defense mechanism against pathogens [13–15]. Studies have shown that immunological activation of human cervical epithelial cells (HCEs) can induce the expression of specific mucosal proteins, cytokines, antiviral factors and adhesion molecules to inhibit viral infections [16, 17]. As the outermost layer of cells in the FRT, HCEs have direct contact with HSV-2 [17]. Thus, the interactions of HSV-2 with HCEs in the FRT are likely to be the first step and crucial part of HSV-2 sexual transmission.
Interleukin (IL)-22, originally called IL-TIF for IL-10-related T cell-derived inducible factor [18], is a member of the IL-10 family of cytokines. Many immune cells, including T cells, monocytes, dendritic cells, natural killer (NK) cells and innate lymphoid cells can produce IL-22 [19–21]. However, the primary source of IL-22 is activated T cells, particularly Th22, Th1 and Th17 T cells [20]. IL-22 mediates its biological functions through a heterodimeric transmembrane receptor complex including IL-22R1 and IL-10R2 [22]. The R1 chain is an IL-22 specific subunit, whereas IL-10R2 serves in the signaling of IL-10, IL-26, IL-28a/p and IL-29 [23–26]. The ubiquitous expression of IL-10R2 explains why it serves as part of several cytokine receptors [27]. The expression of the IL-22R1 chain determines whether a cell is a target for IL-22. Studies of different laboratories have shown that several types of epithelial cells express the IL-22R1 chain and, therefore, are the target cells of IL-22 [28, 29].
The interaction with IL-22 receptor results in signal transduction via JAK (Janus kinases)/STAT (Signal transducers and activators of transcription) pathway, leading to activation of Jak1, Tyk2, and STATs [29, 30]. When activated by IL-22, STATs become phosphorylated on specific tyrosine residues by JAKs and form homodimers or heterodimers with other STAT proteins and then translocate to the nucleus where they regulate the transcription of target genes [31]. Studies [29, 30] have shown that the antiviral activities of IL-22 are mediated through the activation of STATs, as the activation of JAK/STAT signaling pathway results in the production of multiple antiviral ISGs. It has been documented that IL-22 could inhibit several viruses, including HIV [32], hepatitis B virus [33], rotavirus [28], influenza [34] and HSV-2 [35]. However, it is unclear whether IL-22 has the ability to suppress HSV-2 infection of human cervical epithelial cells (HCEs), an important component of the mucosal immunity in the FRT. We also studied the cellular and molecular mechanisms underlying IL-22-mediated HSV-2 inhibition in HCEs.
2. Materials and methods
2.1. Virus and reagents
HSV-2 G strain was provided by Dr. Qinxue Hu (State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences, China). PE Annexin V Apoptosis Detection Kit I was purchased from BD (Pharmingen, USA). Recombinant human IL-22 was purchased from Peprotech Inc. (Frankfurt, Germany). Anti-IL-22 receptor antibodies (anti-IL-22R1 and anti-IL-10R2) were purchased from R&D (Minneapolis, MH, USA). Rabbit antibodies against ISGs, STATs and tight junction proteins were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against HSV-1 + HSV-2 gD were purchased from Abcam (Cambridge, UK). Antibody against GAPDH was purchased from Proteintech (Chicago USA). All culture plasticwares were obtained from Corning (Corning, NY). Unless otherwise specified, all other culture reagents were purchased from Invitrogen (San Diego, CA).
2.2. Cell culture
The HCE cell line (End1/E6E7 cells) is derived from normal human cervical epithelium, which is immortalized by human papillomavirus type 16 (HPV16) E6/E7 [36]. This cell line has been extensively used and accepted as an in vitro HCE model [37–39]. The cells were cultured in keratinocyte growth medium (Gibco, Grand Island, NY) supplemented with the provided recombinant epidermal growth factor (0.1 ng/ml) and bovine pituitary extract (50 |ig/ml). Cells were grown at 37°C in a 5% CO2 incubator.
2.3. Cell viability assay
The cytotoxic effect of IL-22 was evaluated by the MTT assay based on the manufacturer’s instructions. End1/E6E7 cells seeded in 96-well plate (5×104 cells/well) were treated with different concentrations of IL-22 for 72 h. Cells were then exposed to 3-(4, 5-Dimethylthiazol-2-yl) −2, 5-diphenyltetrazolium bromide (MTT) (20 μl/well), and incubated for 4 h at 37C in darkness. The formation of soluble formazan from MTT was measured by spectrophotometric determination of absorption at 490 nm using a plate reader (SpectraMax i3, Molecular Devices, Sunnyvale, CA, USA).
2.4. Flow cytometry analysis of apoptosis
In addition, we also used Annexin V/7-AAD assay to detect IL-22’s cytotoxicity in End1/E6E7 cells. End1/E6E7 cells seeded in 24-well plate (2× 105 cells/well) were treated with different concentrations of IL-22 for 72 h. Wash cells twice with cold phosphate-buffered saline (PBS) and then resuspend cells in 1 × Binding Buffer at a concentration of 1 × 105 cells/ml. Annexin V- PE (2.5 μl) and 7-AAD (5 μl) were added and incubated for 15 min at RT (25°C) in the dark, and then analyzed by FCM (FACScan, Becton Dickinson, San Jose, CA).
2.5. RNA extraction and real-time PCR
Total cellular RNA from End1/E6E7 cells was extracted using TRI-Reagent (Molecular Research Center, Cincinnati, OH) as described previously [17]. Reverse transcription was performed using the random primer, dNTPs, M-MLV reverse transcriptase and RNase inhibitor (Promega Co., Madison, WI) according to the manufacturer’s instructions. Real-time PCR was performed with IQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA) as described previously [40]. The level of GAPDH mRNA were used as an endogenous reference to normalize the quantities of target mRNA. The primers were synthesized by Invitrogen Inc. The sequences of oligonucleotide primers are shown in Table 1.
Table 1.
Primer Sets for Real-time PCR
| Primer | Accession No. | Orientation | Sequences | Product (bp) |
|---|---|---|---|---|
| GAPDH | NM_002046 | Sense | 5’-GGTGGTCTCCTCTGACTTCAACA-3’ | 127 |
| Antisense | 5’-GTTGCTGTAGCCAAATTCGTTGT-3’ | |||
| IL-22R1 | NM_021258.3 | Sense | 5’-TCTGCTCCAGCACGTGAAAT-3’ | 124 |
| Antisense | 5’-GTCCCTCTCTCCGT ACGTCT-3 | |||
| IL-10R2 | NM_000628.4 | Sense | 5’-GGCTGAATTTGCAGATGAGCA-3’ | 427 |
| Antisense | 5’-GAAGACCGAGGCCATGAGG-3’ | |||
| ICP0 | D10471.1 | Sense | 5’-GTGCATGAAGACCTGGATTCC-3’ | 82 |
| Antisense | 5’-GGTCACGCCCACT ATCAGGTA-3’ | |||
| ICP27 | D10471.1 | Sense | 5’-TTCTGCGATCCATATCCGAGC-3’ | 101 |
| Antisense | 5’-AAACGGCATCCCGCCAAA-3’ | |||
| ICP8 | D10658.1 | Sense | 5’-AGGACATAGAGACCATCGCGTTCA-3’ | 99 |
| Antisense | 5’-TGGCCAGTTCGCTCACGTTATT-3’ | |||
| HSV-2 gC | AJ297389.1 | Sense | 5’-AAATCCGATGCCGGTTTCCCAA-3’ | 120 |
| Antisense | 5’-TTACCATCACCTCCTCTAAGCTAGGC-3’ | |||
| HSV-2 gD | K02373.1 | Sense | 5’-ATCCGAACGCAGCCCCGC-3’ | 142 |
| Antisense | 5’-TCTCCGTCCAGTCGTTTAT-3’ | |||
| DNA polymerase | M16321.1 | Sense | 5’-GCTCGAGTGCGAAAAAACGTTC-3’ | 215 |
| Antisense | 5’-CGGGGCGCTCGGCTAAC-3’ | |||
2.6. Agarose gel electrophoresis
Total cellular RNA extracted from End1/E6E7 cells was subjected to the RT-PCR with the primers specific for IL-22 receptor complex consisting of IL-22R1 and IL-10R2. Amplified PCR products were displayed on 2% agarose gel. Human hepatoma- derived hepatic cell lines HepG2 and L02 cells, in which others previously demonstrated functional IL-22R1 and IL-10R2 expression [41], served as positive controls.
2.7. Virus propagation and infection
HSV-2 was propagated and purified from Vero cells by the standard sucrose gradient procedure and used for infection studies at a multiplicity of infection (MOI) of 0.001. After the addition of HSV-2, plates were incubated at 37°C for 90 min, and then washed with DMEM. The cell cultures were then maintained in fresh keratinocyte growth medium for up to 72 h. Cellular DNA was extracted from HSV-2-infected cells with DNA lysis buffer containing100 mM KCl, 20 mM Tris, pH 8.4, 500 μg/ml proteinase K (Sigma-Aldrich, St. Louis, MO, USA), and 0.2% (v/v) NP-40 (BDH, Poole, UK). Lysates were incubated at 60°C for 2 h and then at 100°C for 15 m i n. HSV-2 gD was analyzed by real-time PCR, using the specific primers (Table 1), and was quantified by using serial dilutions of HSV-2 gD standards with known copy numbers.
2.8. Antiviral assay
End1/E6E7 cells were seeded in a 48-well microtiter plate (105 cells/well) and allowed to achieve confluence overnight at 37°C. Then End1/E6E7 cells were pretreated with IL-22 for 24 h and infected with HSV-2 (at a low MOI of 0.001) for 2 h. The cells were then washed to remove unattached viruses and subsequently cultured in the presence of IL-22. To study the roles of IL-22-IL-22R signaling pathway in anti-HSV-2 activity, End1/E6E7 cells were first incubated with the antibodies to IL-22 receptor. End1/E6E7 cells were then treated with IL-22 for 24 h and infected with HSV-2. HSV-2 genome DNA from HSV-2-infected cells or culture supernatant were extracted with DNA lysis buffer as described above.
2.9. Plaque forming assay
The production of infectious HSV-2 was measured by a plaque forming assay as previously described [42]. Briefly, End1/E6E7 cells were seeded in 24-well plates for 24 h prior to infection at a density of 2 × 105 cells per well in complete medium. Supernatant collected from the cell cultures was series-diluted and used for titration of infectious virus. Plaque formation was assessed 48 h after fixation with cold absolute methanol for 30 min followed by the addition of 1% crystal violet. After rinsing off crystal violet with PBS, the number of plaques was counted manually. The infection was performed in triplicate samples.
2.10. Western blotting
Total cell lysates were prepared by the cell extraction buffer (Invitrogen, Shanghai, China) with 1% protease inhibitor cocktail (Sigma, MO) according to the manufacturer’s instructions. Equal amounts of protein lysates were separated on 4–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis precast gels an d transferred to an Immunobiolon-P membrane (Millipore, Germany). Nonspecific sites were blocked with 5% nonfat dried milk before being incubated with antibody. Blots were developed with SuperSignal West Pico Chemiluminescent Substrated (Thermo Fisher Scientific, Waltham). Densitometric analysis was performed by using ImageJ 1.44 software (National Institutes of Health).
2.11. Data analysis
When appropriate, data were obtained from at least three independent experiments and expressed as mean ± SD. For comparison of the mean of two groups, the statistical significance was measured by Student’s t-test. To compare the difference between multiple groups, statistical significance was analyzed using a one-way analysis of variance (ANOVA), followed by Newman-Keul’s test. Calculations were performed with GraphPad Prism Statistical Software (GraphPad Software Inc., San Diego, CA). Statistical significance was defined as P < 0.05 or P < 0.01.
3. Results
3.1. End1/E6E7 cells express IL-22 receptor complex
We first determined whether human cervical epithelial cells (End1/E6E7 cells) express the IL-22 receptor complex. As demonstrated in Fig. 1A, similar to the positive control cells (HepG2 and L02), End1/E6E7cells expressed both IL-22R1 and IL-10R2. We then examined the effect of IL-22 on the viability of End1/E6E7 cells. As shown in Fig. 1B and 1C, little cytotoxic effect was observed in End1/E6E7 cells treated with IL-22 at the dose as high as 1000 ng/ml.
Fig. 1. End1/E6E7 cells express IL-22 receptor complex.
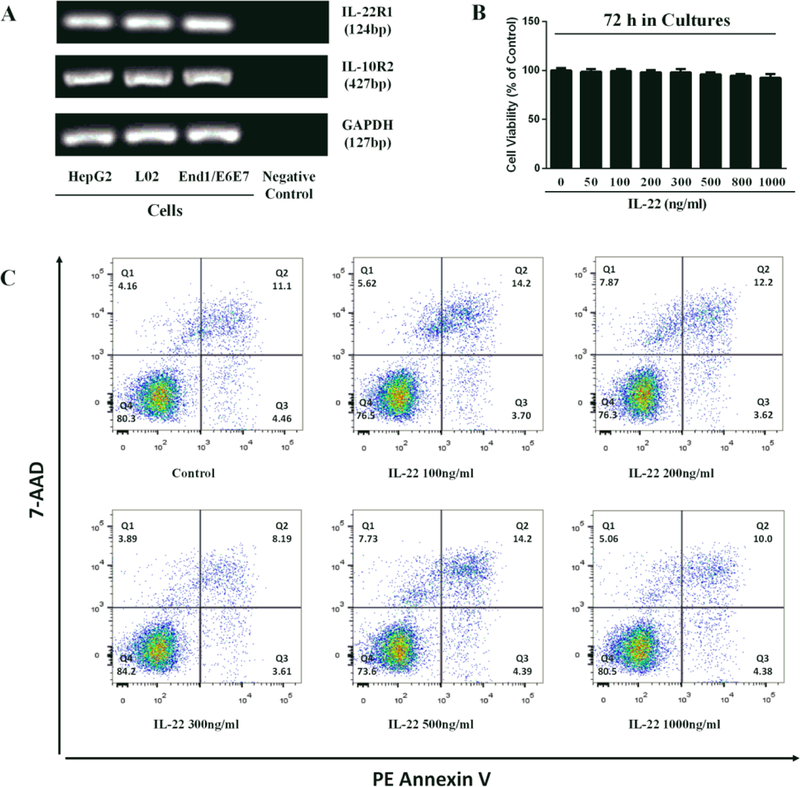
(A) Total cellular RNA extracted from HepG2, L02 and End1/E6E7 cells was subjected to the RT-PCR with the primers specific for IL-22R (IL-22R1 and IL-10R2). Amplified PCR products were displayed on 2% agarose gel. Negative control: no cellular RNA but water was used as a template. (B, C) End1/E6E7 cells were treated with IL-22 at the indicated concentrations. Cells viability was assessed by MTT assay (B) and Annexin V/7-AAD assay (C) 72 h post IL-22 treatment.
3.2. IL-22 inhibits HSV-2 replication
We next determined whether IL-22 can suppress HSV-2 replication in End1/E6E7 cells. To assay the antiviral effect of IL-22, we measured HSV-2 genes and viral titration. As shown in Fig. 2A and 2B, IL-22 pretreatment of the cells inhibited intracellular and extracellular HSV-2 gD gene expression. In addition, IL-22 could inhibit the production of infectious HSV-2 as demonstrated in the plaque forming assay (Fig. 3). To investigate whether the IL-22-IL-22R signaling is responsible for the anti-HSV-2 activity, End1/E6E7 cells were cultured in the media containing the anti-IL-22 receptor subunit (anti-IL-22R1 and anti-IL-10R2) antibodies prior to the addition of IL-22 to the cell cultures. As demonstrated in Fig. 2C and 2D, the antibodies to IL-22 receptor complex largely blocked the effect of IL-22 on HSV-2 inhibition in End1/E6E7 cells. To further determine the anti-HSV-2 activity of IL-22, we examined the effect of IL-22 on several key HSV-2 genes, including immediate early genes (ICP0 and ICP27), early genes (DNA polymerase and ICP8), and late genes (HSV gC, and HSV gD). As shown in Fig. 4, IL-22 suppressed HSV-2 IE (Fig. 4A–B), E (Fig. 4C–D) and L (Fig. 4E–F) genes expression in HSV-2-infected End1/E6E7 cells.
Fig. 2. IL-22 inhibits HSV-2.
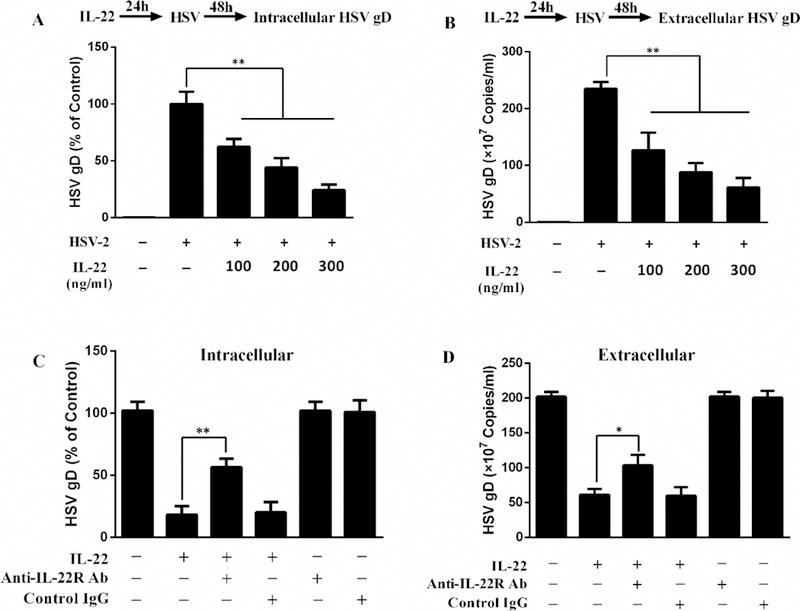
(A, B) End1/E6E7 cells were treated with or without IL-22 at the indicated concentrations for 24 h prior to HSV-2 infection. Intracellular and extracellular HSV-2 gD gene expression was measured at 48 h post HSV-2 infection. (C, D) For IL-22 receptor pretreatment, the anti-IL-22R1 antibody and anti-IL-10R2 antibody (10 μg/mL) were added to End1/E6E7 cell cultures for 1 h prior to IL-22 treatment. Intracellular and extracellular HSV-2 gD gene expression was measured at 48 h post HSV-2 infection. The results are the mean ± SD of triplicate cultures, representative of three independent experiments (*P < 0.05, **P < 0.01).
Fig. 3. IL-22 reduced HSV-2 production in End1/E6E7 cells.
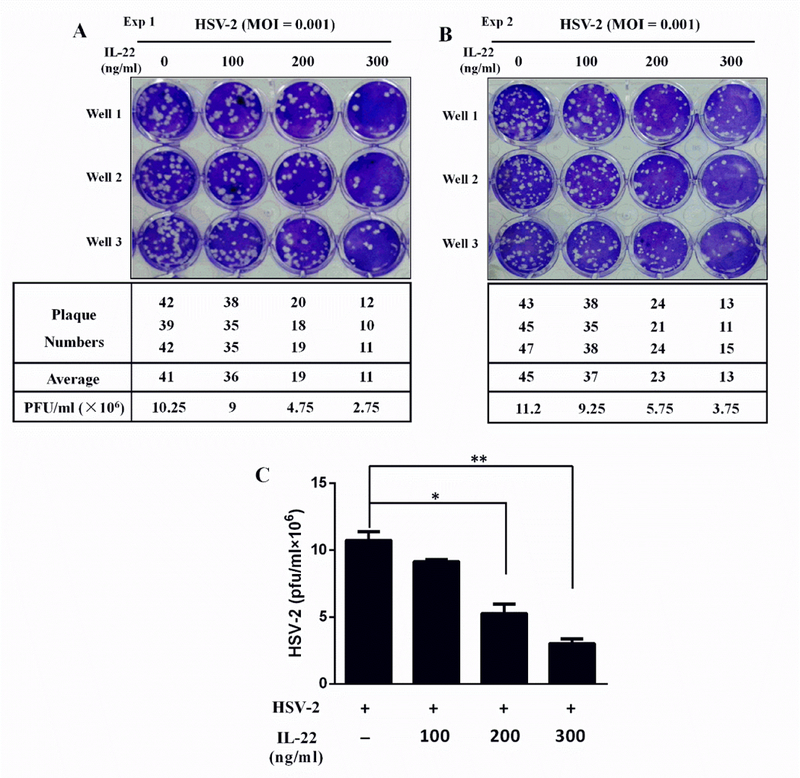
End1/E6E7 cells were treated with or without IL-22 for 24 h and then infected with HSV-2 at an MOI of 0.001 for 48 h. (A, B) Culture supernatant was collected and assayed for production of infectious virus by plaque forming assay. Plaque forming units (PFU) were assessed 48 h after fixation with cold methanol for 30 min followed by the addition of crystal violet. After rinsing off crystal violet with PBS, the plaque numbers were counted under a microscope. (C) This figure was generated based on the data (plaque numbers) shown in Fig. 3A and 3B.
Fig. 4. Effects of IL-22 on the gene expression of HSV-2.
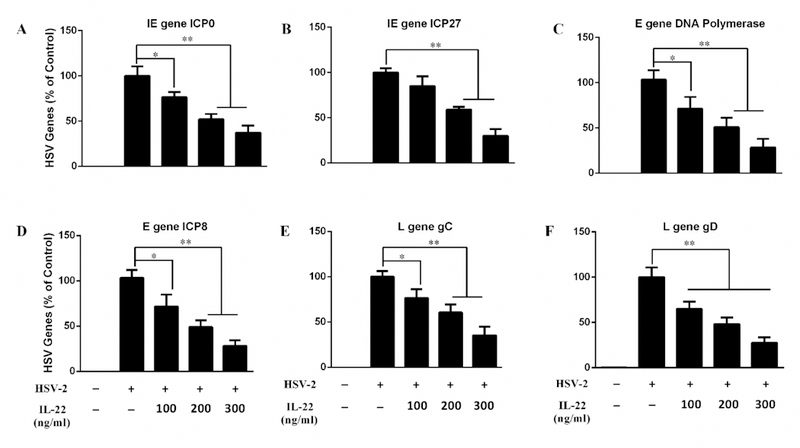
End1/E6E7 cells were pretreated for 24 h with or without IL-22 and then infected with HSV-2 (MOI = 0.001). Cellular RNA was subjected to real-time PCR for HSV-2 IE (A, B), E (C, D) and L (E, F) genes and GAPDH RNA. The results were expressed as HSV-2 genes levels relative (%) to control (without treatment, which is defined as 100%). Data are shown as mean ± SD for three independent experiments (*P < 0.05, **P < 0.01).
3.3. IL-22 induces phosphorylation of STAT1 and STAT3
Studies [29, 30] have shown that the biological effects of IL-22 on viral infections are primarily mediated by the activation of STAT signaling pathway. To understand the mechanism(s) of IL-22-mediated HSV-2 inhibition, we thus investigated the effect of IL-22 on the induction of STAT1 and STAT3 in End1/E6E7 cells. As shown in Fig. 5A and 5B, although IL-22 treatment had little effect on the expression of both STAT1 and STAT3, it significantly enhanced the phosphorylation of STAT1 and STAT3.
Fig. 5. Effect of IL-22 activation on STATs, ISGs and tight junction proteins.
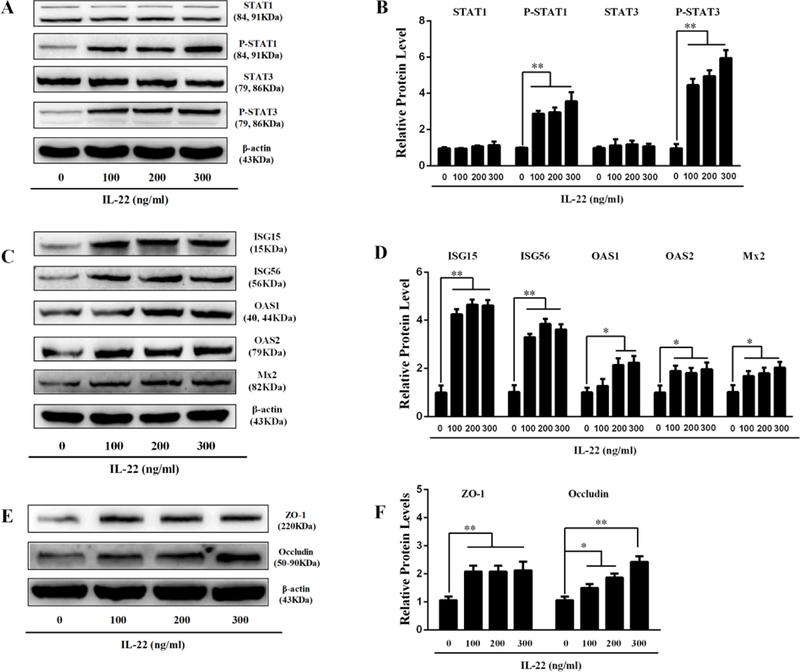
End1/E6E7 cell cultures treated with indicated concentrations of IL-22 for 12 h . Cellular proteins were subjected to Western blot assay using antibodies to STATs (A), ISGs (C) and TJs (E). (B, D, F) Densitometry analysis of relative STATs, ISGs and TJ protein levels compared with β-actin was performed by ImageJ 1.44 software. Data are shown as mean ± SD for three independent experiments (*P < 0.05, **P < 0.01).
3.4. IL-22 induces ISGs
The action of STATs on virus-infected cells is to elicit an antiviral state, which is characterized by the induction of ISGs [43]. To further determine the mechanism(s) by which IL-22 inhibited HSV-2 infection, we investigated whether IL-22 can induce the expression of the antiviral ISGs in End1/E6E7 cells. As shown in Fig. 5C and 5D, IL-22 treatment of the cells upregulated the expression of ISG15, ISG56, OAS-1, OAS-2, and Mx2. To determine the role of STAT1/STAT3 in the IL-22 action on ISGs, we performed the experiments using the JAK inhibitor (JAK Inh), STAT1 inhibitor (Fludarabine) or STAT3 inhibitor (Stattic). As shown in Fig 6, both JAK/STAT inhibitor and STAT1 inhibitor could diminish the effect of IL-22 on the ISGs (Fig. 6A–B). However, the STAT3 inhibitor did not diminish the IL-22 effect on the ISGs (Fig. 6C).
Fig. 6. Effect of STAT1, STAT3 or JAK/STAT inhibitor on the expression of ISGs and tight junction proteins in End1/E6E7 cells.
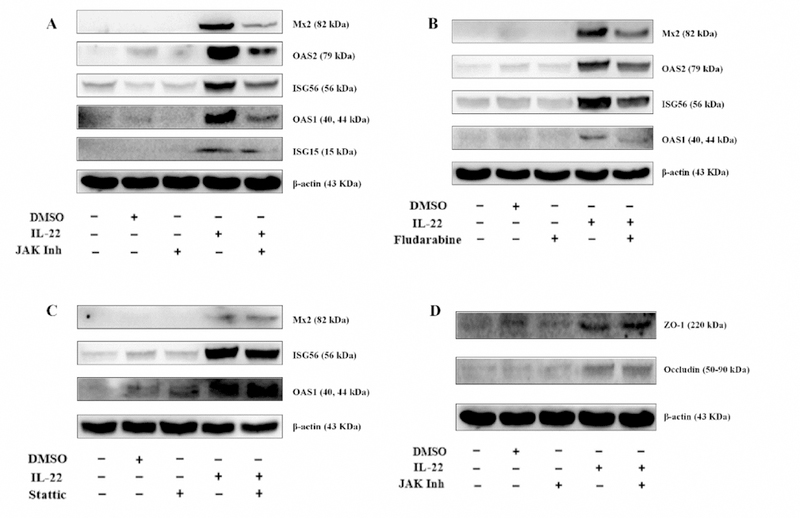
End1/E6E7 cells were treated with or without JAK inhibitor (JAK Inh), STAT1 inhibitor (Fludarabine) or STAT3 inhibitor (Stattic) for 1h and then incubated with IL-22 for additional 12 h. Cellular proteins were subjected to Western blot assay using the antibodies to the ISGs and the TJs.
3.5. IL-22 upregulates tight junction proteins
Tight junction proteins (TJs), including ZO-1 and Occludin, are the key cellular components for integrity of epithelial cells. We wanted to determine whether IL-22 can impact the expression of the TJs. When added to End1/E6E7 cell cultures, IL-22 upregulated the expression of ZO-1 and Occludin (Fig. 5E–F). However, TJs expression was independent of JAK/STAT pathway (Fig. 6D).
4. Discussion
During HSV-2 sexual transmission, human cervical epithelial cells (HCEs) are in direct contact with the virus in female reproductive tract (FRT). Our early work demonstrated that HCEs possess functional TLR3/RIG-I signaling pathway, which could be immunologically activated by Poly I:C to exert the anti-HSV-2 responses [17]. In the present study, we focused on the effect of IL-22 on HSV-2 replication in HCEs (End1/E6E7 cells). Several types of epithelial cells, such as intestinal epithelial cells (IECs) [28], have been shown to express the IL-22R and are targets for IL-22. The IL-22 treatment of epithelial cells results in greater antimicrobial defense, protection against damage, and regeneration of respective tissues [29]. Through binding to the dimeric IL-22R (including IL-22R1 and IL-10R2), IL-22 could trigger phosphorylation of the kinases Jak1 and Tyk2, resulting in the activation of the transcription factor STATs [29, 30]. We showed that End1/E6E7 cells express the functional IL-22-IL-22R signaling system (Fig. 1A), activation of which by IL-22 induced the production of the antiviral factors.
End1/E6E7 cells are originally derived from the primary human cervical epithelium [39], and have been extensively used as an excellent in vitro model of HCEs [37–39]. We demonstrated that the treatment of End1/E6E7 cells with IL-22 inhibited HSV-2 infection/replication (Fig. 2A–B and Fig. 3). This finding supports a recent study [35] showing that treatment of cultured human vaginal epithelial cells (VK2 cells) with IL-22 resulted in diminished HSV-2 replication. The inhibitory effect of IL-22 on HSV-2 was specific receptor-mediated as the antibodies to IL-22 receptors (IL-22R1 and IL-10R2) could largely blocked IL-22-mediated HSV-2 inhibition in End1/E6E7 cells (Fig. 2C–D). Our further experiments showed that IL-22 had the ability to inhibit the expression of all three key HSV-2 genes (Fig. 4). HSV-2 genes are grouped in three temporal classes: immediate early (IE), early (E) and late (L) genes. IE genes are transcribed first during HSV-2 replication, which can activate transcription of the E gene, whose gene products replicate the viral DNA [44]. Viral DNA replication stimulates the expression of the L genes, encoding the structural proteins [44]. The ability of IL-22 to inhibit all three key HSV-2 genes is clinically significant, as it would be extremely difficult for the virus to mutate and become resistant to IL-22.
To understand the mechanism(s) of IL-22-mediated the HSV-2 inhibition, we investigated the effect of IL-22 on the induction of STATs and the ISGs. We observed that IL-22 enhanced the phosphorylation of STAT1/STAT3 and induced the multiple antiviral ISGs (ISG15, ISG56, OAS-1, OAS-2, and Mx2) (Fig. 5). In addition, we examined the impact of IL-22 on the expression of the tight junction proteins, including ZO-1 and Occludin, the key proteins for the integrity and permeability of the epithelial barrier. It has been reported [45] that disruption of epithelial tight junction proteins increases the probability of HSV binding to nectin-1, a cellular receptor for HSV entry. We observed that IL-22 treatment could upregulate the expression of both ZO-1 and Occludin in End1/E6E7 cells (Fig. 5E–F). We also found that the JAK/STAT pathway plays a role in IL-22-mediated anti-HSV-2 activity, as the JAK/STAT inhibitor and STAT1 inhibitor could effectively block the induction of ISGs (Fig. 6A–B), and HSV-2 inhibition by IL-22 (Fig. 7A–D). However, IL-22-mediated HSV-2 control appears to be independent of STAT3, as the STAT3 inhibitor could not block the IL-22 action on HSV-2 (Fig. 7E–F) and ISGs (Fig. 6C). These observations are clinically relevant and significant, as they suggest that IL-22 has potential as a therapeutic agent for protecting epithelial cells from HSV-2 infection and/or infection-associated damages.
Fig. 7. Effect of STAT1, STAT3 or JAK/STAT inhibitor on the IL-22-mediated HSV-2 inhibition.
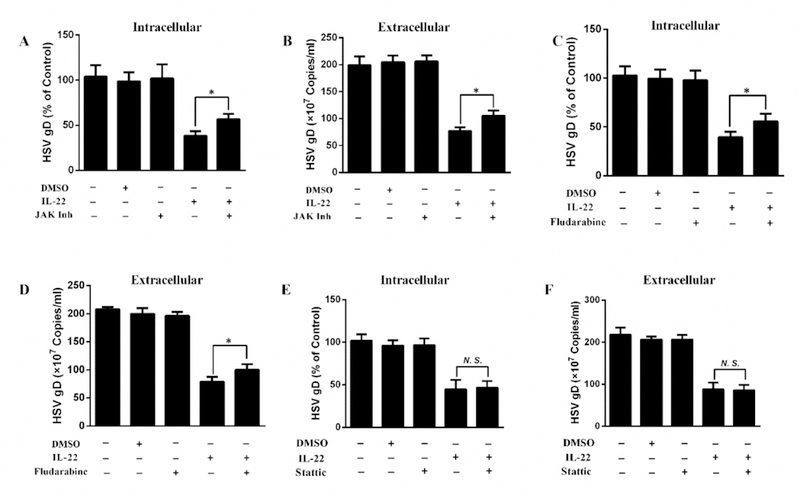
End1/E6E7 cells were treated with or without JAK inhibitor (JAK Inh), STAT1 inhibitor (Fludarabine) or STAT3 inhibitor (Stattic) for 1h and incubated with IL-22 for additional 24 h prior to HSV-2 infection. Intracellular (A, C, E) and extracellular (B, D, F) HSV-2 gD gene expression was measured at 48 h post HSV-2 infection. Data are shown as mean ± SD for three independent experiments (*P < 0.05, **P < 0.01, N. S., not significant).
In conclusion, we have experimentally demonstrated that IL-22 treatment of End1/E6E7 cells could induce the intracellular antiviral factors and enhance the expression of the tight junction proteins (Fig. 8). These findings support the notion that HCEs are involved in the FRT mucosal innate immune defense against HSV-2 infection [17]. However, further investigations with suitable animal models and human specimens are necessary in order to confirm that IL-22R signaling of HCEs is indeed effective in producing the antiviral factors and inhibiting HSV-2 infection/replication in FRT. These future studies will be crucial for the development of IL-22-based intervention strategies to control HSV-2 mucosal transmission in FRT.
Fig. 8. Schematic diagram of mechanisms for IL-22-mediated HSV-2 inhibition in human cervical epithelial cells.
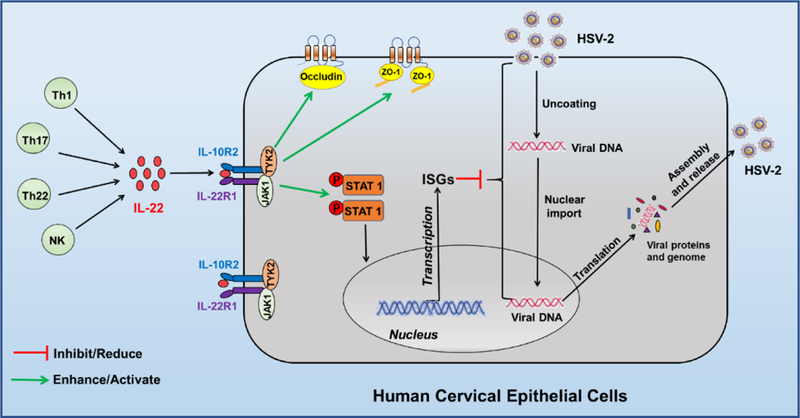
Activated Th cell and NK cell subsets are the primary sources of IL-22 which can bind to the dimeric IL-22R complex (both IL- 22R1 and IL-10R2), resulting in the signal transduction of JAK-STAT pathway in human cervical epithelial cells. Through facilitating the phosphorylation of STAT1, IL-22 induces IFN-stimulated genes (ISG15, ISG56, OAS-1, OAS-2, and Mx2) that have the ability to block HSV-2 DNA transport to cell nuclear and inhibit viral translation, assembly and release. In addition, through the binding to the receptors on human cervical epithelial cells, IL-22 induces the expression of the tight junction proteins (ZO-1 and Occludin), the key cell membrane components for the cell integrity.
Highlights.
Human cervical epithelial cells (HCEs) express the functional IL-22 receptor.
IL-22 inhibits HSV-2 replication in HCEs.
IL-22 induces the expression of ISGs and the tight junction proteins in HCEs.
IL-22 has potential as a therapeutic agent to inhibit HSV-2 infection.
Acknowledgements
We thank Dr. Qinxue Hu (State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences) for providing the HSV-2 G strain.
Funding
This work was supported by the National Natural Sciences Foundation of China (81571962 to W-Z.H.), and the National Institutes of Health (DA041302, DA042373 and DA045568 to W-Z.H.).
Abbreviations:
- IL-22
Interleukin 22
- IL-TIF
IL-10-related T cell-derived inducible factor
- HSV-2
Herpes simplex virus 2
- HPV16
Human papillomavirus type 16
- HIV
Human immunodeficiency virus
- HBV
Hepatitis B virus
- FRT
Female reproductive tract
- HCEs
Human cervical epithelial cells
- DMEM
Dulbecco’s modified eagle medium
- FBS
Fetal bovine serum
- PBS
Phosphate-buffered saline
- JAK
Janus kinases
- STAT
Signal transducers and activators of transcription
- ISG
IFN-stimulated gene
- TJs
Tight junction proteins
- MOI
Multiplicity of infection
- PFU
Plaque forming unit
- IE genes
Immediate early genes
- E genes
Early genes
- L genes
Late genes
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Nevzorova Y, Tschaharganeh DF, Sicinski P, Trautwein C, Liedtke C, Differential functions of cyclin El and E2 for cell cycle control and endoreplication during liver regeneration in mice, Hepatology 46(4) (2007) 788a–788a. [Google Scholar]
- [2].Szabo E, Lodi C, Korpos E, Batmunkh E, Rottenberger Z, Deak F, Kiss L, Tokes AW, Lotz G, Laszlo V, Kiss A, Schaff Z, Nagy M, Expression of matrilin-2 in oval cells during rat liver regeneration, Matrix Biol 26(7) (2007) 554–560. [DOI] [PubMed] [Google Scholar]
- [3].Gupta R, Warren T, Wald A, Genital herpes, Lancet 370(9605) (2007) 2127–37. [DOI] [PubMed] [Google Scholar]
- [4].Lehtinen M, Koskela P, Jellum E, Bloigu A, Anttila T, Hallmans G, Luukkaala T, Thoresen S, Youngman L, Dillner J, Hakama M, Herpes simplex virus and risk of cervical cancer: a longitudinal, nested case-control study in the nordic countries, American journal of epidemiology 156(8) (2002) 687–92. [DOI] [PubMed] [Google Scholar]
- [5].Wald A, Link K, Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis, The Journal of infectious diseases 185(1) (2002) 45–52. [DOI] [PubMed] [Google Scholar]
- [6].McMahon MA, Stivers JT, Siliciano RF, Kohli RM, Acyclovir, the anti-herpes drug, has direct anti-HIV activity and selects for HIV reverse transcriptase mutants, Abstr Pap Am Chem S 237 (2009) 482–482. [Google Scholar]
- [7].Evidence-based drug reviews - Valacyclovir - New indication: for genital herpes, simpler administration, Can Fam Physician 45 (1999) 1697–1700. [PMC free article] [PubMed] [Google Scholar]
- [8].Degreef H, Andrejevic L, Aoki F, Arend J, Ashton R, Debacker W, Bartlett K, Vanblokland WB, Bishop S, Boon R, Borbujo J, Calz AM, Candaele M, Collins P, Crawford G, Cvijetic O, Decroix J, Decuyper C, Delescluse J, Demaubeuge J, Duschet P, Fransen H, Frenk E, Fritsch P, Gheeraert P, Goetijn M, Gonzalez A, Goossen J, Grcic R, Griffin D, Gschnait F, Hanssens Y, Harms M, Hosang M, Ilic V, Isenberg Y, Jansen A, Jones S, Jovovic D, Krafft T, Kranendonk H, Lalosevic J, Leen C, Marcias M, Mcgougall B, Mckendrick M, Milojevic M, Naber F, Nelemans F, Nye F, Ona MB, Parent D, Portnoy J, Prak H, Ranin J, Roelfsema J, Rol H, Rooyakkers A, Sacks S, Shafran S, Sleyffers BCMB, Stalder H, Steenhuisen W, Steichen V, Stevancevic Z, Stratenus M, Takic C, Twynholm M, Vandencamp M, Vanderendt J, Vanhecke E, Verhelst A, Vujaklija V, Wade A, White J, Wood M, Zeijav S, Famciclovir, a New Oral Antiherpes Drug - Results of the First Controlled Clinical- Study Demonstrating Its Efficacy and Safety in the Treatment of Uncomplicated Herpes-Zoster in Immunocompetent Patients, Int J Antimicrob Ag 4(4) (1994) 241–246. [DOI] [PubMed] [Google Scholar]
- [9].Chao CT, Chen YC, Chiang CK, Huang JW, Hu FC, Fang CC, Chang CC, Yen CJ, Sequence Variants of Peroxisome Proliferator-Activated Receptor-Gamma Gene and the Clinical Courses of Patients with End-Stage Renal Disease, Dis Markers (2015) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chiang C, Beljanski V, Yin K, Olagnier D, Ben Yebdri F, Steel C, Goulet ML, DeFilippis VR, Streblow DN, Haddad EK, Trautmann L, Ross T, Lin RT, Hiscott J, Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists, Journal of virology 89(15) (2015) 8011–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Roizman B, The strategy of conquest: herpes simplex virus (HSV) versus the host cell, Febs J 275 (2008) 46–46. [Google Scholar]
- [12].Sheldon IM, Owens SE, Turner ML, Innate immunity and the sensing of infection, damage and danger in the female genital tract, Journal of reproductive immunology 119 (2017) 67–73. [DOI] [PubMed] [Google Scholar]
- [13].Blaskewicz CD, Pudney J, Anderson DJ, Structure and Function of Intercellular Junctions in Human Cervical and Vaginal Mucosal Epithelia, Biol Reprod 85(1) (2011) 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wira CR, Grant-Tschudy KS, Crane-Godreau MA, Epithelial cells in the female reproductive tract: A central role as sentinels of immune protection, Am J Reprod Immunol 53(2) (2005) 65–76. [DOI] [PubMed] [Google Scholar]
- [15].Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R, Bacterial Modulation of Human Fetal Membrane Toll-like Receptor Expression, Am J Reprod Immunol 69(1) (2013) 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L, Innate and adaptive immunity in female genital tract: cellular responses and interactions, Immunol Rev 206 (2005) 306–335. [DOI] [PubMed] [Google Scholar]
- [17].Zhou L, Li JL, Zhou Y, Liu JB, Zhuang K, Gao JF, Liu S, Sang M, Wu JG, Ho WZ, Induction of interferon-lambda contributes to TLR3 and RIG-I activation-mediated inhibition of herpes simplex virus type 2 replication in human cervical epithelial cells, Molecular human reproduction 21(12) (2015) 917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dumoutier L, Louahed J, Renauld JC, Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9, Journal of immunology 164(4) (2000) 1814–9. [DOI] [PubMed] [Google Scholar]
- [19].Dezso K, Jelnes P, Laszlo V, Baghy K, Bodor C, Paku S, Tygstrup N, Bisgaard HC, Nagy P, Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver regeneration, Am J Pathol 171(5) (2007) 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen ER, Cen Y, Lu DH, Luo W, Jiang HX, IL-22 inactivates hepatic stellate cells via downregulation of the TGF-1/Notch signaling pathway, Mol Med Rep 17(4) (2018) 5449–5453. [DOI] [PubMed] [Google Scholar]
- [21].Sonnenberg GF, Fouser LA, Artis D, Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22, Nat Immunol 12(5) (2011) 383–390. [DOI] [PubMed] [Google Scholar]
- [22].Hines IN, Kremer M, Isayama F, Perry AW, Milton RJ, Black AL, Byrd CL, Wheeler MD, Impaired liver regeneration and increased oval cell numbers following T cell-mediated hepatitis, Hepatology 46(1) (2007) 229–241. [DOI] [PubMed] [Google Scholar]
- [23].Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, Pestka S, Donnelly RP, Kotenko SV, Cloning, expression and initial characterisation of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10), Genes Immun 1(7) (2000) 442–450. [DOI] [PubMed] [Google Scholar]
- [24].Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, Haugen H, Jelinek L, Kelly JD, Madden K, Maurer MF, Parrish- Novak J, Prunkard D, Sexson S, Sprecher C, Waggie K, West J, Whitmore TE, Yao L, Kuechle MK, Dale BA, Chandrasekher YA, Interleukin 20: Discovery, receptor identification, and role in epidermal function, Cell 104(1) (2001) 9–19. [DOI] [PubMed] [Google Scholar]
- [25].Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen ML, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP, IFN-lambda s mediate antiviral protection through a distinct class II cytokine receptor complex, Nat Immunol 4(1) (2003) 69–77. [DOI] [PubMed] [Google Scholar]
- [26].Sheppard P, Kindsvogel W, Xu WF, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM, IL-28, IL-29 and their class II cytokine receptor IL-28R, Nat Immunol 4(1) (2003) 63–68. [DOI] [PubMed] [Google Scholar]
- [27].Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R, IL-22 increases the innate immunity of tissues, Immunity 21(2) (2004) 241–254. [DOI] [PubMed] [Google Scholar]
- [28].Hernandez PP, Mahlakoiv T, Yang I, Schwierzeck V, Nguyen N, Guendel F, Gronke K, Ryffel B, Holscher C, Dumoutier L, Renauld JC, Suerbaum S, Staeheli P, Diefenbach A, Interferon-lambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection, Nat Immunol 16(7) (2015) 698-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wolk K, Witte E, Witte K, Warszawska K, Sabat R, Biology of interleukin-22, Semin Immunopathol 32(1) (2010) 17–31. [DOI] [PubMed] [Google Scholar]
- [30].Gimeno Brias S, Stack G, Stacey MA, Redwood AJ, Humphreys IR, The Role of IL-22 in Viral Infections: Paradigms and Paradoxes, Frontiers in immunology 7 (2016) 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Levy DE, Darnell JE, STATs: Transcriptional control and biological impact, Nat Rev Mol Cell Bio 3(9) (2002) 651–662. [DOI] [PubMed] [Google Scholar]
- [32].Misse D, Yssel H, Trabattoni D, Oblet C, Lo Caputo S, Mazzotta F, Pene J, Gonzalez JP, Clerici M, Veas F, IL-22 participates in an innate Anti-HIV-1 host-resistance network through acute-phase protein induction, Journal of immunology 178(1) (2007) 407–415. [DOI] [PubMed] [Google Scholar]
- [33].Feng DC, Kong XN, Weng HL, Park O, Wang H, Dooley S, Gershwin ME, Gao B, Interleukin-22 Promotes Proliferation of Liver Stem/Progenitor Cells in Mice and Patients With Chronic Hepatitis B Virus Infection, Gastroenterology 143(1) (2012) 188–U375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kumar P, Thakar MS, Ouyang W, Malarkannan S, IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection, Mucosal Immunol 6(1) (2013) 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stanfield BA, Rider PJF, Caskey J, Del Piero F, Kousoulas KG, Intramuscular vaccination of guinea pigs with the live-attenuated human herpes simplex vaccine VC2 stimulates a transcriptional profile of vaginal Th17 and regulatory Tr1 responses, Vaccine 36(20) (2018) 2842–2849. [DOI] [PubMed] [Google Scholar]
- [36].Hazuda DJ, Wolfe AL, Hastings JC, Robbins HL, Graham PL, Lafemina RL, Emini EA, Viral Long Terminal Repeat Substrate-Binding Characteristics of the Human-Immunodeficiency-Virus Type-1 Integrase, J Biol Chem 269(6) (1994) 3999–4004. [PubMed] [Google Scholar]
- [37].Govender Y, Avenant C, Verhoog NJD, Ray RM, Grantham NJ, Africander D, Hapgood JP, The Injectable-Only Contraceptive Medroxyprogesterone Acetate, Unlike Norethisterone Acetate and Progesterone, Regulates Inflammatory Genes in Endocervical Cells via the Glucocorticoid Receptor, PloS one 9(5) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hijazi K, Cuppone AM, Smith K, Stincarelli MA, Ekeruche-Makinde J, De Falco G, Hold GL, Shattock R, Kelly CG, Pozzi G, Iannelli F, Expression of Genes for Drug Transporters in the Human Female Genital Tract and Modulatory Effect of Antiretroviral Drugs, PloS one 10(6) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sathe A, Reddy KVR, TLR9 and RIG-I Signaling in Human Endocervical Epithelial Cells Modulates Inflammatory Responses of Macrophages and Dendritic Cells In Vitro, PloS one 9(1) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cheng K, Wang XH, Yin H, Small-Molecule Inhibitors of the TLR3/dsRNA Complex, J Am Chem Soc 133(11) (2011) 3764–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg MHJ, Weiss TS, Prufer T, Olszak T, Steib CJ, Storr M, Goke B, Diepolder H, Bilzer M, Thasler WE, Auernhammer CJ, IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro, Am J Physiol-Gastr L 292(4) (2007) G1019–G1028. [DOI] [PubMed] [Google Scholar]
- [42].Chen XQ, Wang ZX, Yang ZY, Wang JJ, Xu YX, Tan RX, Li EG, Houttuynia cordata blocks HSV infection through inhibition of NF-kappa B activation, Antivir Res 92(2) (2011) 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ejercito PM, Nebrija KGE, Feria RP, Lara-Figueroa LL, Traffic Simulation Software Review, Int Conf Inform Inte (2017) 381–384. [Google Scholar]
- [44].Lafemina RL, Graham PL, Legrow K, Hastings JC, Wolfe A, Young SD, Emini EA, Hazuda DJ, Inhibition of Human-Immunodeficiency-Virus Integrase by Bis-Catechols, Antimicrob Agents Ch 39(2) (1995) 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lafemina RL, Schneider CL, Robbins HL, Callahan PL, Legrow K, Roth E, Schleif WA, Emini EA, Requirement of Active Human-Immunodeficiency-Virus Type-1 Integrase Enzyme for Productive Infection of Human T-Lymphoid Cells, Journal of virology 66(12) (1992) 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]


