Abstract
The BBSome, a complex consisting of 8 Bardet-Biedl syndrome (BBS) proteins, is involved in the regulation of various cellular processes. Recently, the BBSome complex has emerged as an important regulator of cardiovascular function with implications for disease. In this study, we examined the role of the BBSome in vascular smooth muscle and its effects on the regulation of cardiovascular function. Smooth muscle-specific disruption of the BBSome through tamoxifen-inducible deletion of Bbs1 gene, a critical component of the BBSome complex, reduces relaxation and enhances contractility of vascular rings and increases aortic stiffness independent of changes in arterial blood pressure. Mechanistically, we demonstrate that smooth muscle Bbs1 gene deletion increases vascular angiotensinogen gene expression implicating the renin-angiotensin system (RAS) in these altered cardiovascular responses. Additionally, we report that smooth muscle-specific Bbs1 knockout mice demonstrate enhanced endothelin-1 induced contractility of mesenteric arteries, an effect reversed by blockade of the angiotensin type 1 receptor with losartan. These findings highlight the importance of the smooth muscle BBSome in the control of vascular function and arterial stiffness through modulation of RAS signaling.
Keywords: BBSome, endothelial function, smooth muscle, renin-angiotensin system
Summary
Smooth muscle-specific disruption of the BBSome causes vascular dysfunction and arterial stiffening. These vascular changes involve activation of the renin-angiotensin system as indicated by the increase in angiotensinogen gene expression and the ability of losartan to restores vascular function in mice lacking the BBSome in smooth muscle. Thus, the smooth muscle BBSome plays an important role in the regulation of vascular function and the development of arterial stiffness.
Introduction
Despite considerable advances in understanding the underlying mechanisms and clinical treatment cardiovascular-related diseases remains a leading cause of mortality1. Vascular dysfunction and arterial stiffness are independent risk factors for the development of cardiovascular diseases including hypertension and stroke. Human and animal models of cardiovascular disease exhibit vascular dysfunction such as increased vascular contractility, decreased vasodilator function, and increased arterial stiffness2–4. Numerous underlying mechanisms of vascular dysfunction have been proposed such as signaling arising from the renin-angiotensin system (RAS), oxidative stress, and inflammatory pathways5–7. Indeed, altered RAS activity has been shown to be a major component in many cardiovascular diseases8. Additionally, blockade of the RAS has been demonstrated to restore vascular function in certain contexts9–11 highlighting its importance in the regulation of vascular function and homeostasis. However, the mechanisms connecting RAS signaling and vascular dysfunction remain unclear and represent a critical gap in our understanding of cardiovascular-related diseases.
Bardet-Biedl syndrome (BBS) proteins have emerged as critical regulators of various cellular processes including gene expression and trafficking of receptors to cilia and the plasma membrane. Eight BBS proteins (BBS1, 2, 4, 5, 7, 8, 9 and 18) interact together to form a complex called the BBSome12. The BBSome is assembly through a process that involves another protein complex that contains 3 BBS proteins (BBS6, 10 and 12). On the other hand, BBS3 mediates the recruitment of the BBSome to the cell membrane whereas BBS17 controls the trafficking of this protein complex in cilia. The interconnection between the various BBS proteins explain the overlapping phenotypes that arise from mutations in any of the 21 BBS genes identified so far13.
The high prevalence of hypertension and other cardiovascular risks in humans bearing mutations in BBS genes highlight the importance of these genes for cardiovascular regulation14,15, 16. Knockout mouse models of BBS recapitulate many of the phenotypes observed in BBS patients including hypertension supporting further the relevance of BBS genes for cardiovascular control17–19. Of note, variants in BBS genes have been associated with hypertension and other cardiovascular risks in non-BBS individuals20.
We previously reported the expression of BBS genes in various cardiovascular-related tissues including the vasculature18. We showed that components of the BBSome, including Bbs1, are expressed in vascular cells (endothelial and smooth muscle) as well as in vessels such as the aorta18. We also demonstrated that mice bearing deletion of various BBS genes display contrasting changes in vascular functions18. For example, Bbs6 null mice exhibit normal acetylcholine-induced relaxation responses compared to wildtype littermates whereas Bbs2 null mice display enhanced acetylcholine-induced vasorelaxation despite both mouse models being obese. Presence of obesity is a confounding factor in using the global null mice to dissect the role of the BBSome in the vasculature. In addition, the contribution of the BBSome in various cell types of the vasculature to the regulation of vascular function remains largely unknown.
In this study, we tested the hypothesis that the smooth muscle BBSome is required for the regulation of cardiovascular function. We generated a novel mouse model that allows temporal and selective disruption of the BBSome, through Bbs1 gene deletion, in smooth muscle cells. We examined the effects of smooth muscle-specific Bbs1 gene deletion on vascular function, blood pressure and arterial stiffening. Additionally, we provide evidence identifying the role of the RAS in the regulation of vascular function and arterial stiffening in response to smooth muscle BBSome disruption.
Methods
An expanded Methods section is available in the online-only Data Supplement.
The data that support the findings of this study are available from the corresponding author on reasonable request.
Animals
To generate a transgenic mouse line with smooth muscle-specific disruption of the BBSome, we crossed mice expressing a tamoxifen inducible smooth muscle-specific myosin heavy chain Cre promoter (Strain: B6.FVB-Tg(Myh11-cre/ERT2)1Soff/J; Jackson Labs #019079) with mice harboring floxed alleles of the Bbs1 gene21 from our colony. We then crossed these mice to a tdTomato reporter line (Strain: ROSA[StopF/F-tdTomato]) from our colony for visualization of Cre dependent tdTomato expression. The mice were maintained on a mixed background for this study (129SvEV/C57Bl/6J) as this breeding paradigm has been validated in our laboratory and represents consistency with previous studies22,23. All mice used in this study were males as the bacterial artificial chromosome transgene that encodes the inducible smooth muscle Cre recombinase (smMHCCreERT2) was inserted on the Y chromosome. Cre-dependent recombination was induced via Tamoxifen injection (IP; 75mg/kg) in corn oil, which was used as vehicle, for 5 consecutive days at ~8 weeks of age. Molecular and physiological analyses were performed ~4 weeks after tamoxifen injections. We found no differences between mice with genotypes: SMCre/Bbs1wt/wt + Tx and SMCre/Bbs1F/F + Corn Oil (vehicle) for vascular function assays and refer to both groups as wildtype controls throughout the studies. All studies were approved by the University of Iowa Animal Research Committee.
Vascular function
Vascular function was assessed in aortic and mesenteric arterial rings from SMCreERT2/Bbs1F/F and wildtype mice. In brief, the thoracic aorta (3mm length rings) and second-order mesenteric arteries (2mm length rings) were dissected and cleaned of debris before they were mounted on a wire myograph (DMT; Model 610M). Aortic rings were mounted on steel pins and mesenteric arteries were mounted on 2 tungsten wires (25μm diameter) and placed in a 95%O2/5%CO2 Krebs solution containing the following (in mM): 118.3 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 11 Glucose, pH 7.4, 37C. Resting tension was set at 0.5g for aortic rings and IC90 was applied to all mesenteric rings as previously described18, 24 for 45 minutes. Aortic and mesenteric arterial rings were sub-maximally contracted with PGF2α (aortic) or U-46619 (mesenteric) based on established protocols in our laboratory25 and others26 where smooth and sustained contraction was best achieved with these agonists, respectively. Vascular rings were then subjected to cumulative concentration response curves to acetylcholine (1nM-10μM) and sodium nitroprusside (1nM-10μM). Additionally, cumulative contractile responses to phenylephrine (1nM-100μM), PGF2α (1μM-100μM), U-46619 (1nM-10μM), serotonin (1nM-100μM), endothelin-1 (0.01nM-100nM) and KCl depolarization (100mM) were also assessed. A subset of arteries was pre-incubated with losartan (1μM; 30min) prior to contractile or relaxation function assessment. Data are reported as percentage relaxation, maximum contractility (g), or maximum tension (mN/mm) generated. All vascular reagents were purchased from Sigma except for U-46619 (Cayman Chemical).
Pulse wave velocity
Pulse wave velocity (PWV) measurements were performed using Doppler ultrasound (MouseDoppler, Indus Instruments, TX). Mice were anesthetized with isoflurane (2%) and ECG recorded for heart beat analysis. Ultrasound waveforms were collected using 20 MHz ultrasound probes placed on the descending aorta and abdominal aorta near the iliac bifurication. Waveforms were collected over 5–8 cardiac cycles and the distance was measured between the ultrasound probes. Pulse wave velocities were calculated dividing the distance (meters) by time (seconds).
Statistical Analysis
All data are expressed as means ± SEM. Data were analyzed and graphed using GraphPad 7 Prism software. Group comparisons were made via unpaired t-test. Blood pressure and vascular reactivity assays were analyzed using a Two-way ANOVA with or without repeated measures and a Bonferroni’s (2 groups) or Tukey’s (3 or more groups) post hoc test. Differences are considered statistically significant if p<0.05.
Results
Validation of smooth muscle-specific Bbs1 gene deletion
Consistent with our previous report18, we found that the Bbs1 gene is expressed in the smooth muscle cells isolated from the aorta (Figure S1 in the online-only Data Supplement). We also demonstrate the efficacy of using the SMCreERT2 promoter to delete Bbs1 in smooth muscle cells. Isolated primary aortic smooth muscle cells from SMCreERT2/Bbs1F/F mice were infected with an adenoviral Cre construct (Ad-Cre) to induce recombination of LoxP sites. Ad-Cre infection reduced Bbs1 gene expression by ~95% compared to Ad-GFP infected control smooth muscle cells (p<0.0001 via unpaired t-test; Figure S1).
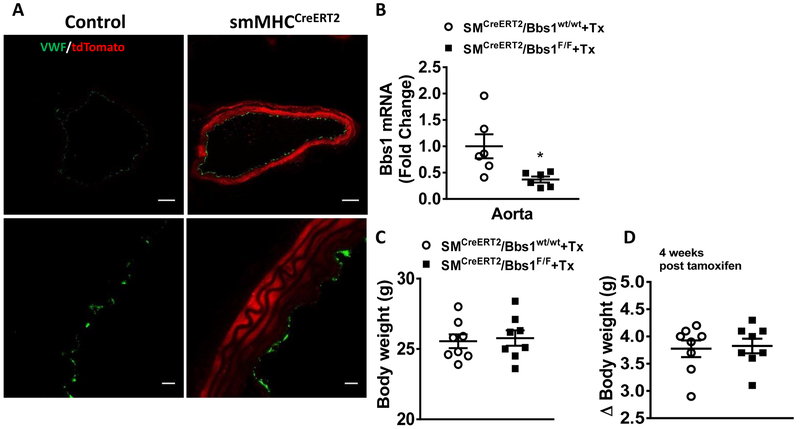
Next, we generated mice lacking the Bbs1 gene specifically in smooth muscle cells by treating the SMCreERT2/Bbs1F/F transgenic mice with tamoxifen. We validated this mouse model by visualizing Cre dependent tdTomato expression in the vascular smooth muscle layer of tamoxifen treated SMCreERT2/Bbs1F/F/STOPF/F-tdTomato mice. Of note, while tdTomato (red fluorescence) expression was found in smooth muscle cells of the aorta, endothelial cells (stained with von Willebrand Factor; green) did not express tdTomato (Figure 1A). To further validate the model, we isolated aortas from wildtype and SMCreERT2/Bbs1F/F mice and measured Bbs1 gene expression by quantitative real-time PCR in whole aortic lysates. SMCreERT2/Bbs1F/F mice demonstrated significantly reduced Bbs1 transcript levels compared to wildtype controls (p<0.05 via unpaired t-test; Figure 1B).
Figure 1 – Validation of smooth muscle-specific Bbs1 knockout mice.
(A) Representative confocal images of aortic rings from tamoxifen (Tx)-treated SMCreERT2/Bbs1F/F and wildtype mice visualized for td-Tomato (red) and von Willebrand factor (green) at low (top) and high (bottom) magnification. (B) Bbs1 mRNA expression of aortic rings from tamoxifen-treated SMCreERT2/Bbs1F/F and wildtype mice (n=6/group; *p<0.05 via unpaired t-test). (C) Total body weight and (D) weight gain after tamoxifen treatment in SMCreERT2/Bbs1F/F and wildtype mice (n=8/group), bar: 100μm.
We have previously demonstrated that tissue-specific disruption of the Bbs1 gene can cause body weight effects such as obesity23. Thus, we sought to determine if smooth muscle deletion of the Bbs1 gene had any body weight effects in mice. Compared to wildtype controls, SMCreERT2/Bbs1F/F mice exhibited no change in body weight or weight gain after tamoxifen administration (Figure 1C–D).
Smooth muscle-specific Bbs1 gene deletion alters vascular relaxation responses in the aorta but not the mesenteric artery
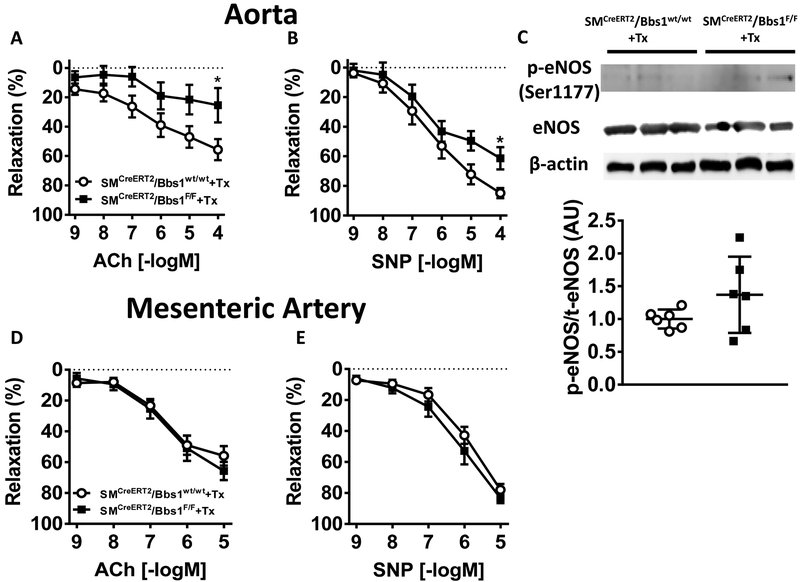
We next assessed the role of smooth muscle deletion of the Bbs1 gene in the regulation of vascular relaxation in the aorta and mesenteric artery. Aortic rings of SMCreERT2/Bbs1F/F mice displayed decreased endothelial-mediated vasorelaxation responses to acetylcholine compared to wildtype control mice (Figure 2A; pinteraction<0.05 via 2-way ANOVA with repeated measures). Endothelial-independent vasorelaxation responses to sodium nitroprusside (SNP) was also decreased in SMCreERT2/Bbs1F/F mice relative to wildtype controls (Figure 2B; pinteraction<0.05 via 2-way ANOVA with repeated measures). The reduced acetylcholine and SNP responses of the aorta suggest alterations in nitric oxide (NO) signaling. However, expression ratio of phospho-eNOS (Ser1177) to total eNOS protein was unchanged in the aorta of SMCreERT2/Bbs1F/F mice (Figure 2C).
Figure 2 – Vasorelaxation responses of aortic and mesenteric artery rings from smooth muscle-specific Bbs1 knockout mice.
Cumulative concentration response curves measured in aortic (top) and mesenteric arterial (bottom) rings from tamoxifen-treated SMCreERT2/Bbs1F/F and wildtype mice. Relaxation responses to acetylcholine (ACh, A,D) and SNP (B,E) (n=8/group; *pinteraction<0.05 via 2 way ANOVA with repeated measures). (C) Protein expression of aortic rings from tamoxifen-treated SMCreERT2/Bbs1F/F and wildtype mice for ratio of p-eNOS(Ser1177)/eNOS (n=6/group).
Next, we assessed the role of smooth muscle-specific deletion of the Bbs1 gene on mesenteric artery function, a more prototypical resistance vessel involved in the regulation of arterial pressure. We found that both endothelial-dependent acetylcholine and endothelial-independent SNP relaxation responses were intact in SMCreERT2/Bbs1F/F mice compared to wildtype controls (Figure 2D–E) diverging from our findings in the large conduit aorta. These data suggest that Bbs1 gene deletion in smooth muscle cells interferes with both endothelial and smooth muscle-mediated vasorelaxation responses in the aorta. SMCreERT2/Bbs1F/F mice treated with corn oil (vehicle) displayed no differences relative to tamoxifen treated wildtype controls for relaxation responses of the aorta and mesenteric artery (Figure S2A–D) indicating the specificity of the SMCreERT2 driver.
Smooth muscle-specific Bbs1 gene deletion alters contractile responses in the aorta and mesenteric artery
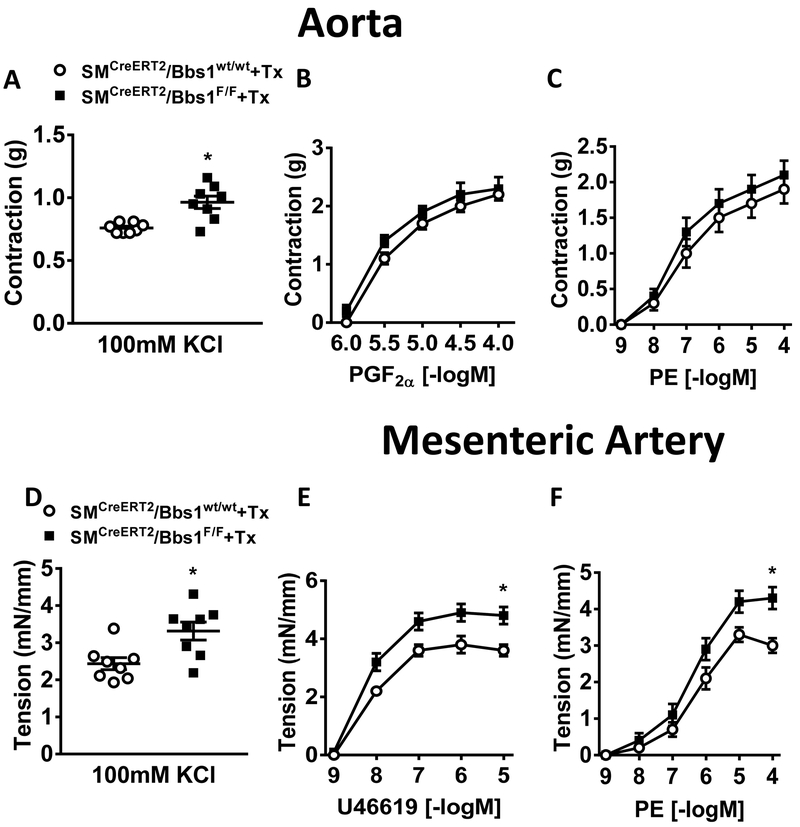
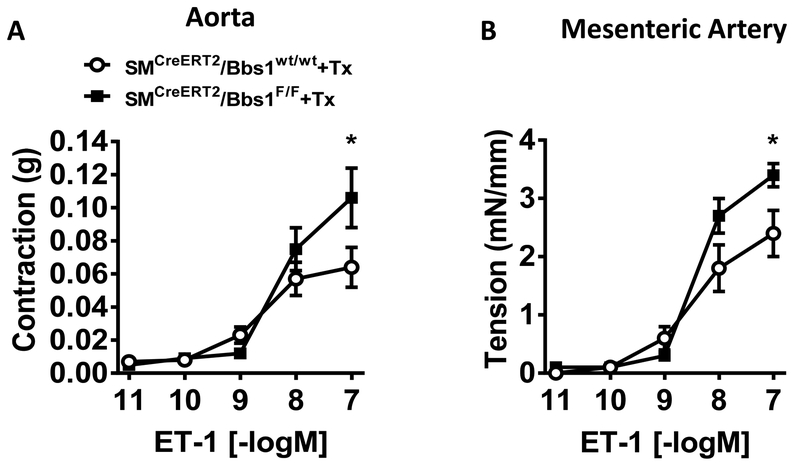
We next assessed whether smooth muscle-specific Bbs1 gene deletion affects the ability of the aorta and mesenteric artery to contract in response to activating calcium. Maximal depolarization to KCl (100mM) was significantly elevated in SMCreERT2/Bbs1F/F mice compared to wildtype controls in both the aorta (Figure 3A) and mesenteric artery (p<0.05 via unpaired t-test, Figure 3D). However, maximal aortic responses to prostaglandin (PGF2α) and phenylephrine (PE) were not different between SMCreERT2/Bbs1F/F and wildtype mice (Figure 3B–C). Strikingly, the mesenteric artery of SMCreERT2/Bbs1F/F mice displayed increased agonist induced contraction to thromboxane (U-46619) and PE (pinteraction<0.05 via 2-way ANOVA with repeated measures, Figure 3E–F). No difference was detected in the contractile response evoked by serotonin (5-HT) in the aorta and the mesenteric artery of SMCreERT2/Bbs1F/F mice (Figure S3). Interestingly, a significant increase in contractility of both the aorta and mesenteric artery for endothelin-1 (ET-1) was noted in SMCreERT2/Bbs1F/F mice (Figure 4A–B; pinteraction<0.05 via 2-way ANOVA with repeated measures). These data indicate selectivity and vascular bed specificity in altered contractile responses after Bbs1 gene deletion in smooth muscle cells. It should be noted that aorta and mesenteric artery contractile function were not altered in mice receiving vehicle (corn oil) injections (Figure S4A–J).
Figure 3 – Contractile responses of aortic and mesenteric artery rings from smooth muscle-specific Bbs1 knockout mice.
Cumulative concentration response curves measured in aortic (top) and mesenteric artery (bottom) rings from tamoxifen-treated SMCreERT2/Bbs1F/F and wildtype mice. Contractile responses to KCl (A,D), PGF2α (B), U-46619 (E), phenylephrine (PE; C,F). n=8/group; (A,D): *p<0.05 via unpaired t-test; (E,F): *pinteraction<0.05 via 2 way ANOVA with repeated measures.
Figure 4 – Contractile responses to endothelin-1 of aortic and mesenteric artery rings from smooth muscle-specific Bbs1 knockout mice.
Cumulative concentration response curves in aortic (A) and mesenteric (B) arterial rings to endothelin-1 (ET-1). (n=6–7/group; *pinteraction<0.05 via 2-way ANOVA with repeated measures).
Smooth muscle-specific Bbs1 gene knockout does not alter blood pressure but increases arterial stiffening of the aorta
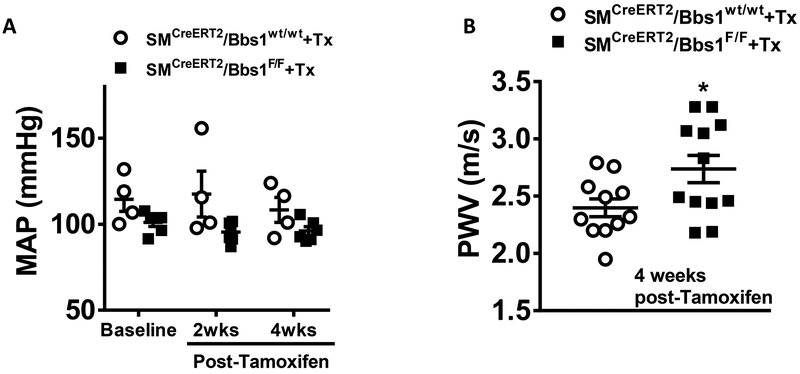
We measured mean arterial pressure by radiotelemetry and found no differences between SMCreERT2/Bbs1F/F and wildtype mice at both 2- and 4-weeks post-Tamoxifen administration (Figure 5A). Additionally, there was no differences between wildtype and SMCreERT2/Bbs1F/F mice for systolic, diastolic or pulse pressure (Figure S5A–C) as well as heart rate or motor activity (Figure S5D–E). Interestingly, we found a significant increase in aortic pulse wave velocity measured 4 weeks post-Tamoxifen (p<0.05 via unpaired t-test; Figure 5B) indicating smooth muscle-specific Bbs1 gene deletion causes aortic stiffening.
Figure 5 – Mean arterial pressure and aortic stiffness of smooth muscle specific Bbs1 knockout mice.
Mean arterial pressure (MAP), measured via radiotelemetry, (A, n=4–6/group) and pulse wave velocity (PWV), measured via Doppler ultrasound, (B, n=11–12/group) in tamoxifen-treated SMCreERT2/Bbs1F/F and wildtype mice at 4 weeks post-Tamoxifen treatment. *p<0.05 via unpaired t-test.
Smooth muscle-specific Bbs1 gene deletion increases vascular angiotensinogen gene expression
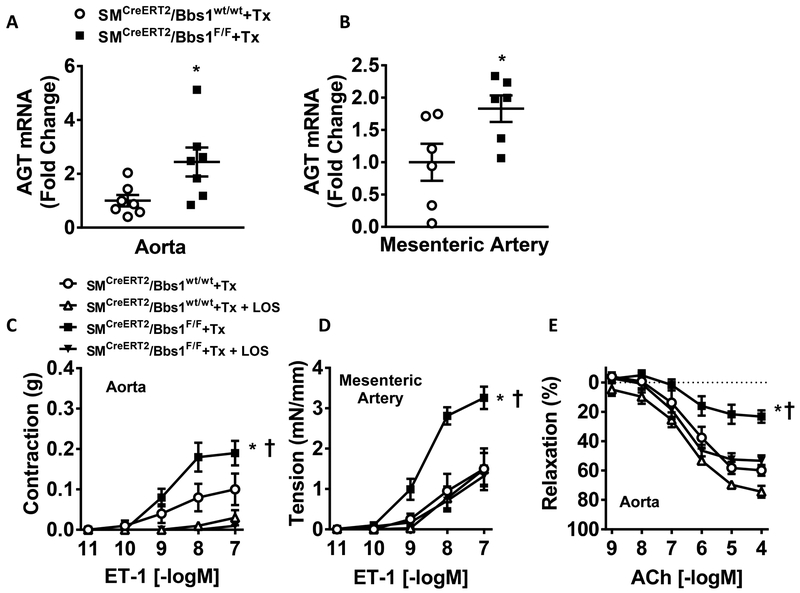
The reduced acetylcholine and SNP responses paired with increased arterial stiffening in the aortas from SMCreERT2/Bbs1F/F mice compared to wildtype controls suggests alterations in the RAS. Indeed, we found increased mRNA levels for angiotensinogen in the aorta (Figure 6A; p<0.05 via unpaired t-test) and mesenteric artery (Figure 6B; p<0.05 via unpaired t-test) of SMCreERT2/Bbs1F/F mice compared to wildtype controls. No differences were observed in the expression of renin, ACE, angiotensin type 1a or 1b receptor, or the angiotensin type 2 receptor in the aorta (Figure S6A–E).
Figure 6 – Vascular effects of the renin-angiotensin system in aorta of smooth muscle specific Bbs1 knockout mice.
Angiotensinogen expression in aorta (A) and mesenteric artery (B) of tamoxifen-treated SMCreERT2/Bbs1F/F and wildtype mice (n=7/group; *p<0.05 via unpaired t-test). Cumulative concentration response curves in aortic (C) and mesenteric arterial (D) rings to endothelin-1 (ET-1) with or without losartan pre-incubation (1μM; 30min) (n=6–8/group). Cumulative concentration response curves in aortic rings (E) to acetylcholine (ACh) with or without losartan pre-incubation (1μM; 30min) (n=6/group); *†p<0.05 via 2-way ANOVA with repeated measures.
We also examined the expression of a variety of other genes involved in the regulation of vascular function. However, there were no significant changes in aortic expression of IL-1β, V-CAM1, SOD2, NOX1, NOX2 or NOX4 mRNAs in SMCreERT2/Bbs1F/F mice compared to wildtype controls (Figure S7A–F). Similarly, we found no differences in protein expression and the activities of eNOS, Akt, ERK, and PKCα signaling in aortas of SMCreERT2/Bbs1F/F mice compared to wildtype controls (Figure S8A–D).
Cross-talk between the RAS and ET-1 has been previously implicated in the regulation of contractile function27, 28. Thus, we tested whether activation of vascular RAS in SMCreERT2/Bbs1F/F mice contributes to the increased ET-1 constriction of the aortic and mesenteric arterial rings. Consistent with such possibility, pre-incubating aortic rings with losartan (1μM; 30min) abolished the contractile responses to ET-1 in both SMCreERT2/Bbs1F/F and wildtype mice (Figure 6C; pinteraction<0.0001 via 2-way ANOVA with repeated measures). Interestingly, in the mesenteric artery pre-incubation with losartan reduced ET-1 contraction in SMCreERT2/Bbs1F/F mice without changing contractile response in wildtype mice (Figure 6D; pinteraction<0.0001 via 2-way ANOVA with repeated measures). Restoration of ET-1 contractile function by losartan led us to test whether losartan treatment could also restore endothelial-mediated relaxation responses in aortic rings of SMCreERT2/Bbs1F/F mice. Indeed, pre-incubation of aortic rings with losartan restored acetylcholine-mediated relaxation responses in SMCreERT2/Bbs1F/F mice to the level of wildtype mice (Figure 6E; pinteraction<0.0001 via 2-way ANOVA with repeated measures).
Discussion
In this study, we identified an important role for the smooth muscle BBSome in the regulation of vascular function and the development of arterial stiffness. Smooth muscle-specific deletion of the Bbs1 gene, a critical component of the BBSome complex, results in impaired endothelial–dependent and –independent vascular relaxation responses, enhances contractile function, and increases arterial stiffening independent of changes in arterial pressure or body weight. Mechanistically, we demonstrate that smooth muscle Bbs1 gene deletion lead to an increase in vascular angiotensinogen mRNA in two different vascular beds. Furthermore, blockade of the angiotensin type 1 receptor with losartan restores normal contractile function to endothelin-1 of smooth muscle-specific Bbs1 gene null mice implicating the RAS in mediating the enhanced contractile responses evoked by smooth muscle BBSome disruption. These results highlight a complex and highly integrated pathway connecting the smooth muscle BBSome, the RAS, and cardiovascular function. Taken together, these data suggest that the smooth muscle BBSome is a novel regulator of vascular function and arterial stiffening.
It is well documented that vascular dysfunction is an important contributor to the development and progression of hypertension and that mitigation of these vascular deficits reduces cardiovascular risks4. To our knowledge, clinical measurements of vessel function in BBS patients have not been performed. However, using mouse models of BBS our laboratory has previously demonstrated contrasting changes in vascular relaxation and contractile responses depending on which BBS gene was globally deleted. Additionally, we have demonstrated expression in endothelial and smooth muscle cells as well as vascular tissues of several BBS genes including the Bbs1 gene18. In BBS patients, the Bbs1 gene is the most commonly mutated gene with a single missense mutation in methionine to arginine (M390R) accounting for ~80% of mutations in this gene29. We and others have previously demonstrated that deletion of the Bbs1 gene recapitulates many of the clinical features of BBS.21, 23
To understand the contribution of the BBSome to the regulation of cardiovascular function, we developed a tamoxifen-inducible smooth muscle-specific Bbs1 gene deletion mouse model. Our data demonstrate both impaired endothelial-dependent and -independent relaxation responses in aortic rings from smooth muscle-specific Bbs1 knockout mice measured at 4 weeks post-Tamoxifen administration. Interestingly, these findings are unique to the aorta as we found no differences in relaxation responses in mesenteric arterial rings suggesting both regional specificity and predominance of other vasodilator signals (EDHF or prostacyclin) for these responses depending on the vascular bed studied.
The activity and bioavailability of NO is a primary determinant of vascular relaxation particularly in large conduit vessels such as the aorta4. In this study, we found no changes in the expression of phospho-eNOS (Ser117) or total eNOS protein in aortic rings lacking the Bbs1 gene in smooth muscle cells. NO levels can be modulated through the production of reactive oxygen species and proinflammatory cytokines4. We found no differences in mRNA expression for a variety of ROS-producing/degrading and inflammatory genes in smooth muscle Bbs1 gene deleted mice compared to wildtype controls. These findings suggest that aortic endothelial relaxation function may be intact while the smooth muscle is unresponsive to the relaxing effects of NO in smooth muscle Bbs1 gene deleted mice. We did not measure the expression of guanylate cyclase, cGMP levels, or eNOS uncoupling in this study. Additionally, we found no differences in expression of several ROS-related genes in the vasculature of smooth muscle Bbs1 gene deleted mice. However, this was not an exhaustive investigation and the overall contribution of ROS signaling to the observed phenotypes cannot be excluded. Therefore, additional studies are needed to elucidate the role of smooth muscle BBSome disruption on impaired vascular relaxation responses and the signaling pathways involved.
Enhanced agonist-induced contractility contributes to and predicts the development of hypertension in a variety of human and animal models30–32. In this study, we found differentially enhanced contractile responses in the aorta and mesenteric artery to both receptor–dependent and –independent activation of the smooth muscle contractile apparatus indicating a pro-contractile vascular state. Specifically, the aorta of smooth muscle Bbs1 null mice displayed enhanced contraction to membrane-induced depolarization with KCl, but not to agonist-induced contraction. The reason for this is unclear but may be due to differential triggering thresholds to activating calcium by these signaling pathways or inhibition of myosin phosphatase in response to smooth muscle Bbs1 gene deletion. Interestingly, resistance arteries of the mesenteric circulation demonstrated increased contraction to all agonists studied except for serotonin (5-HT). This finding of no contractile effect of 5-HT in aortic and mesenteric vascular rings is consistent with our finding of no changes in expression and activity of the MAP kinase signaling pathway. Contraction to endothelin-1 was significantly elevated in both aortic and mesenteric arterial rings of smooth muscle Bbs1 gene deleted mice compared to wildtype. This finding is intriguing as endothelin-1 has been implicated in hypertension in both human33, 34 and animal models35. Indeed, blockade of endothelin-1 receptor signaling improves vascular function in several models of hypertension36–38. This also translates to human hypertension as blockade of endothelin-1 receptors have been shown to improve acetylcholine-induced vasodilation in hypertensive humans33.
We previously demonstrated that global deletion of individual genes that encode the BBSome subunits (Bbs2 or Bbs4), a subunit of the BBS chaperonin complex that assembles the BBSome (Bbs6) or a component involved in the transport and localization of the BBSome (Bbs3), all result in obesity. Interestingly, Bbs4, Bbs6 and Bbs3 null mice exhibit elevated blood pressure while Bbs2 null mice display normal blood pressure17, 39. These findings are supported by human studies where variants of Bbs4 and Bbs6 but not Bbs2 were associated increased risk of for the development of hypertension in the general population20, 40 highlighting a complex connection between BBS proteins and regulation of blood pressure. We have previously reported that a global Bbs1M390R knock-in mouse model of BBS demonstrates several characteristics of BBS including obesity41. Interestingly, Bbs1M390R mice exhibit normal arterial pressure despite obesity41. In our current study, smooth muscle-specific deletion of the Bbs1 gene did not affect body weight or arterial pressure at 4 weeks post-tamoxifen administration confirming the normal arterial pressure phenotype in the Bbs1M390R model. However, it remains to be determined if long-term chronic smooth muscle-specific BBSome disruption affect adiposity and blood pressure.
Arterial stiffening is a well-known predictor of cardiovascular disease3 and has been demonstrated to precede the development of hypertension in human and animal studies2, 42. Of note, arterial stiffness displays regional variability depending on which vascular bed likely due to the timing and local gene environment within the specific vascular bed43. Using Doppler ultrasound pulse wave velocity, we demonstrate increased aortic stiffness in smooth muscle Bbs1 deletion mice. This finding is in concert with the reduced relaxation responses of the aortic rings measured ex vivo suggests a direct effect of BBSome disruption in smooth muscle on arterial compliance. We did not measure collagen or elastin deposition in the extracellular matrix (ECM) of smooth muscle Bbs1 gene deleted mice. Thus, further studies are warranted to elucidate the composition of the ECM and its contribution to the increased arterial stiffness in this model.
Aberrant activity of the RAS has been associated with a variety of cardiovascular diseases such as obesity and hypertension44. Numerous studies have previously demonstrated a direct relationship between RAS activation and abnormal vascular function in various contexts11, 45, 46. RAS-induced vascular impairment is primarily mediated through the angiotensin type 1 receptor (AT1) as administration of losartan, a selective inhibitor of AT1 receptors, improves vascular reactivity in a variety of cardiovascular settings28, 47–49. Our observation of impaired vascular endothelial and smooth muscle mediated relaxation responses as well as enhanced contractility to different contractile agonists led us to investigate the RAS as a potential mediator of these responses. Indeed, our data demonstrate elevated mRNA levels of angiotensinogen in vascular segments spanning large and small arteries (aortic and mesenteric). Interestingly, we found no changes in expression of other RAS genes such as RAS enzymes (renin or ACE) or RAS receptors (AT1 or AT2). We did not measure local vascular RAS activity or circulating angiotensin peptides in vivo in response to smooth muscle Bbs1 gene deletion. However, consistent with the concept of increased local vascular RAS activity we found that blockade of the angiotensin type 1 receptor with losartan incubation of mesenteric arteries restored normal contractile function to endothelin-1 from smooth muscle Bbs1 gene deleted mice. Furthermore, losartan incubation of aortic rings restored acetylcholine-mediated endothelial function demonstrating the protective effect of RAS blockade against smooth muscle BBSome disruption. These data suggest that elevated RAS in response to smooth muscle Bbs1 gene deletion is an important regulatory pathway in the control of vascular function and may provide a targeting point for the cardiovascular disease associated with BBS patients. Future studies are needed to determine how disruption of the BBSome leads to an increase in angiotensinogen gene expression and whether in vivo RAS blockade may protect against the increased arterial stiffness associated with smooth muscle BBSome disruption.
Perspectives
The current study demonstrates for the first time a critical role for the smooth muscle BBSome in the regulation of vascular function and arterial stiffening in a manner independent of blood pressure and body weight. These findings demonstrate a link between basic cell biological mechanisms conserved in all organisms (i.e. intracellular transport and cilia function) and complex phenotypes such as cardiovascular dysfunction that result when these mechanisms are perturbed. Additional investigations into the role of the BBSome in mediating cardiovascular dysfunction are warranted. These investigations may help further our understanding of the basic molecular mechanisms that underlie vascular diseases associated with various conditions as well as provide novel therapeutic targets for the clinical treatment of vascular dysfunction and arterial stiffening, two major and independent risk factors for cardiovascular events.
Supplementary Material
Novelty and Significance.
What Is New?
Smooth muscle-specific BBSome disruption interferes with the aortic vascular relaxation responses.
Smooth muscle-specific BBSome disruption alters contractile responses in the aorta and mesenteric artery.
Smooth muscle-specific BBSome disruption increases arterial stiffening and vascular angiotensinogen gene expression.
What Is Relevant?
The smooth muscle BBSome plays an important role in the regulation of vascular function and the development of arterial stiffness.
Dysregulation of this protein complex may contribute to the vascular dysfunction associated with various conditions.
Acknowledgments
The authors thank Dr. Val C. Sheffield (University of Iowa) for providing the Bbs1F/F mice.
Sources of Funding
This work was supported by NIH grant HL084207, VA grant BX004249, AHA grants 14EIA18860041 and 16POST30830004, and the University of Iowa Fraternal Order of Eagles Diabetes Research Center.
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 2.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241 [DOI] [PubMed] [Google Scholar]
- 4.Reho JJ, Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci (Lond). 2017;131:1689–1700 [DOI] [PubMed] [Google Scholar]
- 5.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34:665–673 [DOI] [PubMed] [Google Scholar]
- 7.Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–975 [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324 [DOI] [PubMed] [Google Scholar]
- 9.Aznaouridis KA, Stamatelopoulos KS, Karatzis EN, Protogerou AD, Papamichael CM, Lekakis JP. Acute effects of renin-angiotensin system blockade on arterial function in hypertensive patients. J Hum Hypertens. 2007;21:654–663 [DOI] [PubMed] [Google Scholar]
- 10.Cherney DZ, Scholey JW, Jiang S, Har R, Lai V, Sochett EB, Reich HN. The effect of direct renin inhibition alone and in combination with ace inhibition on endothelial function, arterial stiffness, and renal function in type 1 diabetes. Diabetes Care. 2012;35:2324–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair AR, Agbor LN, Mukohda M, Liu X, Hu C, Wu J, Sigmund CD. Interference with endothelial ppar (peroxisome proliferator-activated receptor)-gamma causes accelerated cerebral vascular dysfunction in response to endogenous renin-angiotensin system activation. Hypertension. 2018;72:1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of bbs proteins cooperates with the gtpase rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213 [DOI] [PubMed] [Google Scholar]
- 13.Heon E, Kim G, Qin S, Garrison JE, Tavares E, Vincent A, Nuangchamnong N, Scott CA, Slusarski DC, Sheffield VC. Mutations in c8orf37 cause bardet biedl syndrome (bbs21). Hum Mol Genet. 2016;25:2283–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo DF, Rahmouni K. Molecular basis of the obesity associated with bardet-biedl syndrome. Trends Endocrinol Metab. 2011;22:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbedour K, Zucker N, Zalzstein E, Barki Y, Carmi R. Cardiac abnormalities in the bardet-biedl syndrome: Echocardiographic studies of 22 patients. Am J Med Genet. 1994;52:164–169 [DOI] [PubMed] [Google Scholar]
- 16.Green JS, Parfrey PS, Harnett JD, Farid NR, Cramer BC, Johnson G, Heath O, McManamon PJ, O’Leary E, Pryse-Phillips W. The cardinal manifestations of bardet-biedl syndrome, a form of laurence-moon-biedl syndrome. N Engl J Med. 1989;321:1002–1009 [DOI] [PubMed] [Google Scholar]
- 17.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of bardet-biedl syndrome. J Clin Invest. 2008;118:1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer AM, Guo DF, Sheffield VC, Rahmouni K. Contrasting vascular effects caused by loss of bardet-biedl syndrome genes. Am J Physiol Heart Circ Physiol. 2010;299:H1902–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo DF, Beyer AM, Yang B, Nishimura DY, Sheffield VC, Rahmouni K. Inactivation of bardet-biedl syndrome genes causes kidney defects. Am J Physiol Renal Physiol. 2011;300:F574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benzinou M, Walley A, Lobbens S, Charles MA, Jouret B, Fumeron F, Balkau B, Meyre D, Froguel P. Bardet-biedl syndrome gene variants are associated with both childhood and adult common obesity in french caucasians. Diabetes. 2006;55:2876–2882 [DOI] [PubMed] [Google Scholar]
- 21.Carter CS, Vogel TW, Zhang Q, Seo S, Swiderski RE, Moninger TO, Cassell MD, Thedens DR, Keppler-Noreuil KM, Nopoulos P, Nishimura DY, Searby CC, Bugge K, Sheffield VC. Abnormal development of ng2+pdgfr-alpha+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med. 2012;18:1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starks RD, Beyer AM, Guo DF, Boland L, Zhang Q, Sheffield VC, Rahmouni K. Regulation of insulin receptor trafficking by bardet biedl syndrome proteins. PLoS Genet. 2015;11:e1005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo DF, Cui H, Zhang Q, Morgan DA, Thedens DR, Nishimura D, Grobe JL, Sheffield VC, Rahmouni K. The bbsome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet. 2016;12:e1005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reho JJ, Zheng X, Benjamin JE, Fisher SA. Neural programming of mesenteric and renal arteries. Am J Physiol Heart Circ Physiol. 2014;307:H563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reho JJ, Guo DF, Rahmouni K. Mechanistic target of rapamycin complex 1 signaling modulates vascular endothelial function through reactive oxygen species. J Am Heart Assoc. 2019;8:e010662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agbor LN, Ibeawuchi SC, Hu C, Wu J, Davis DR, Keen HL, Quelle FW, Sigmund CD. Cullin-3 mutation causes arterial stiffness and hypertension through a vascular smooth muscle mechanism. JCI Insight. 2016;1:e91015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YJ, Kwok CF, Juan CC, Hsu YP, Shih KC, Chen CC, Ho LT. Angiotensin ii enhances endothelin-1-induced vasoconstriction through upregulating endothelin type a receptor. Biochem Biophys Res Commun. 2014;451:263–269 [DOI] [PubMed] [Google Scholar]
- 28.Maeso R, Rodrigo E, Munoz-Garcia R, Navarro-Cid J, Ruilope LM, Lahera V, Cachofeiro V. Losartan reduces constrictor responses to endothelin-1 and the thromboxane a2 analogue in aortic rings from spontaneously hypertensive rats: Role of nitric oxide. J Hypertens. 1997;15:1677–1684 [DOI] [PubMed] [Google Scholar]
- 29.Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, Fulton AB, Carmi R, Luleci G, Chandrasekharappa SC, Collins FS, Jacobson SG, Heckenlively JR, Weleber RG, Stone EM, Sheffield VC. Identification of the gene (bbs1) most commonly involved in bardet-biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31:435–438 [DOI] [PubMed] [Google Scholar]
- 30.Stauffer BL, Westby CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol. 2008;23:350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018;114:529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardillo C, Campia U, Kilcoyne CM, Bryant MB, Panza JA. Improved endothelium-dependent vasodilation after blockade of endothelin receptors in patients with essential hypertension. Circulation. 2002;105:452–456 [DOI] [PubMed] [Google Scholar]
- 34.Touyz RM, Schiffrin EL. Role of endothelin in human hypertension. Can J Physiol Pharmacol. 2003;81:533–541 [DOI] [PubMed] [Google Scholar]
- 35.Schiffrin EL. Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens. 2001;14:83S–89S [DOI] [PubMed] [Google Scholar]
- 36.Barton M, d’Uscio LV, Shaw S, Meyer P, Moreau P, Luscher TF. Et(a) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension. 1998;31:499–504 [DOI] [PubMed] [Google Scholar]
- 37.Amiri F, Ko EA, Javeshghani D, Reudelhuber TL, Schiffrin EL. Deleterious combined effects of salt-loading and endothelial cell restricted endothelin-1 overexpression on blood pressure and vascular function in mice. J Hypertens. 2010;28:1243–1251 [DOI] [PubMed] [Google Scholar]
- 38.Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. Eta receptor blockade decreases vascular superoxide generation in doca-salt hypertension. Hypertension. 2003;42:811–817 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, Bugge K, Stone EM, Rahmouni K, Sheffield VC. Bardet-biedl syndrome 3 (bbs3) knockout mouse model reveals common bbs-associated phenotypes and bbs3 unique phenotypes. Proc Natl Acad Sci U S A. 2011;108:20678–20683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, Beales PL, Katsanis N, Bassett AS, Davidson WS, Parfrey PS. Clinical and genetic epidemiology of bardet-biedl syndrome in newfoundland: A 22-year prospective, population-based, cohort study. Am J Med Genet A. 2005;132A:352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A knockin mouse model of the bardet-biedl syndrome 1 m390r mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–19427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in western diet-induced obesity. Am J Physiol Heart Circ Physiol. 2015;309:H574–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabandugama PK, Gardner MJ, Sowers JR. The renin angiotensin aldosterone system in obesity and hypertension: Roles in the cardiorenal metabolic syndrome. Med Clin North Am. 2017;101:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Silva TM, Hu C, Kinzenbaw DA, Modrick ML, Sigmund CD, Faraci FM. Genetic interference with endothelial ppar-gamma (peroxisome proliferator-activated receptor-gamma) augments effects of angiotensin ii while impairing responses to angiotensin 1–7. Hypertension. 2017;70:559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin ii signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeso R, Navarro-Cid J, Rodrigo E, Ruilope LM, Lahera V, Cachofeiro V. Differential effects of losartan and doxazosin on vascular function in senescent spontaneously hypertensive rats. Am J Hypertens. 1999;12:1105–1108 [DOI] [PubMed] [Google Scholar]
- 48.Papadopoulos P, Tong XK, Imboden H, Hamel E. Losartan improves cerebrovascular function in a mouse model of alzheimer’s disease with combined overproduction of amyloid-beta and transforming growth factor-beta1. J Cereb Blood Flow Metab. 2017;37:1959–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He DH, Lin JX, Zhang LM, Xu CS, Xie Q. Early treatment with losartan effectively ameliorates hypertension and improves vascular remodeling and function in a prehypertensive rat model. Life Sci. 2017;173:20–27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.