Highlights
-
•
The endosomal escapes of PEDV during the entry occur in 4 h after virus inoculation in the presence of exogenous proteases in the medium.
-
•
Without proteases in the medium, PEDV remained in the endosomes and degraded.
-
•
Inhibitors of endosome maturation or cathepsin B/L blocked PEDV entry by retaining viruses in the endosomes.
Keywords: Porcine epidemic diarrhea virus, Exogenous and endogenous proteases, Virus entry, Endosomes
Abstract
Exogenous and endogenous proteases play important roles in porcine epidemic diarrhea virus (PEDV) entry and replication. The roles of proteases in the viral endosomal escape and replication using trypsin (KD) or elastase (AA)-adapted US PEDV strains were studied. While PEDV KD and AA require different exogenous protease for efficient replication in cells, PEDV KD was more dependent on the protease than PEDV AA. There was no marked difference in viral trafficking between them during the entry events. Both PEDV were observed in the endosomes with or without protease at 1 h after virus inoculation. With protease, viral signals in the endosomes disappeared after 4 h, and newly synthesized viral proteins were detected in the ER after 6 h. However, without protease, viruses remained in the endosomes up to 24 h, which correlated with limited virus replication. Inhibitors of cathepsins, endogenous proteases, significantly reduced the replication of both PEDV by interfering with the viral endosomal escape.
1. Introduction
Coronavirus entry processes require complex interactions between multiple host and viral factors to enter the cells and initiate viral RNA translation and replication (Belouzard et al., 2012). The coronavirus spike (S) protein, a class I viral fusion protein, is a critical viral factor for coronavirus entry. The S protein contains two subunits: N-terminal S1 subunit which binds to cellular receptors and S2 subunit which is responsible for membrane fusion (Paul and Perlman, 2013). The S protein undergoes conformation changes exposing two major cleavage sites at the junction of S1 and S2 subunit (S1/S2) and just upstream of the fusion peptide (S2′) before and during virus entry into host cells (Belouzard et al., 2012). Cleavage at the S2′ site by proteases exposes the fusion peptide, which is mainly composed of hydrophobic amino acids (Belouzard et al., 2012). The fusion peptide is then introduced into the host cell membrane by conformational change of S2 subunit mediated by refolding of heptad repeat 1 (Belouzard et al., 2012). As the fusion protein folds back, viral and cellular membranes are drawn together to initiate the membrane fusion for virus entry (Kirchdoerfer et al., 2016).
The proteases involved in the proteolytic cleavage of S protein have been studied for several coronaviruses. It was shown that furin processes the S protein of mouse hepatitis virus (MHV) strain A59 and infectious bronchitis virus (IBV) targeting the junction of S1/S2 (Sturman et al., 1985; Yamada and Liu, 2009). Since furin is mainly expressed in the trans-golgi network and the newly produced viruses possess cleaved S protein, the S proteins of those coronaviruses are thought to be processed during the stage of viral assembly (Seidah and Prat, 2012; Sturman et al., 1985; Yamada and Liu, 2009). The S proteins of severe acute respiratory syndrome coronavirus (SARS-CoV), middle east respiratory syndrome coronavirus (MERS-CoV) and HCoV-229E are reported to be activated by a membrane-bound protease, serine 2 (TMPRSS2), which is widely distributed in the respiratory tracts (Bertram et al., 2011; Bugge et al., 2009; Shirato et al., 2013). Other cellular proteases such as cathepsins, which exist in the endosomes and lysosomes, have been reported to process coronavirus S protein of SARS-CoV, MERS-CoV, human coronavirus (HCoV)-229E and feline coronavirus (FCoV) and MHV-2 (Kawase et al., 2009; Kim et al., 2013; Qiu et al., 2006; Regan et al., 2008; Simmons et al., 2005; Wicht et al., 2014). While the replication of most coronaviruses does not require exogenous proteases in cell culture media, proteases in the medium have been reported to enhance the replication of some coronaviruses including SARS-CoV (Matsuyama et al., 2005). For instance, trypsin, dispase, thermolysin and elastase have been reported to activate the S protein of SARS-CoV and enhance viral replication in cells (Matsuyama et al., 2005). The mutations in S protein are reported to alter protease susceptibility, pathogenicity and host range (Millet and Whittaker, 2015). Therefore, the proteolytic activation of S protein and viral entry are potential targets for antiviral drug development (Du et al., 2017; Simmons et al., 2013).
Porcine epidemic diarrhea virus (PEDV), which belongs to the genus of Alphacoronavirus in the Coronaviridae family, causes porcine epidemic diarrhea (PED), a disease responsible for severe economic losses in the swine industry worldwide (Jung and Saif, 2015). PEDV infects the villous epithelium of the small intestines and causes diarrhea and vomiting in the affected pigs with up to 100% mortality in neonatal piglets (Jung and Saif, 2015). Unlike most coronaviruses, field isolation and efficient replication of most PEDV strains requires the presence of exogenous trypsin in culture medium (Li et al., 2016). Previous reports have shown that cleavage of PEDV S protein by trypsin occurs after viral receptor binding (Li et al., 2016; Park et al., 2011; Wicht et al., 2014). However, the detailed mechanism of proteolytic activation of PEDV S protein by proteases is not well understood. In this study, we aimed to investigate the role of proteases in PEDV entry focusing on the endosomal escape by confocal microscopy. Using two cell-culture-adapted PEDV strains that require trypsin (PEDV KD) or pancreatic elastase (PEDV AA) in cell culture for virus replication (Kim et al., 2017), we examined if exogenous proteases are involved in the endosomal escape of PEDV for efficient viral replication. We also investigated the roles of endogenous proteases (cathepsin B and L) in the endosomes/lysomes and endosome maturation in PEDV replication in correlation with the endosomal escape.
2. Materials and methods
2.1. Cells, viruses, and reagents
PEDV KD and AA were described in our previous publication (Kim et al., 2017). PEDV KD and AA were propagated in Vero (ATCC®-CCL-81™) cells in the presence of L-1-tosylamide-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin (Sigma-Aldrich, St Louis, MO) or elastase (Promega, Madison, WI), respectively, in Eagle’s Minimal Essential Medium (MEM) supplemented with 100U/ml penicillin and 100 μg /ml streptomycin and 5% fetal bovine serum (FBS). Concentrated (>100-fold) PEDV KD or AA was prepared by ultracentrifugation of viruses at 100,000×g through a 30% w/v sucrose cushion at 4 °C for 2 h. The pellet was resuspended in serum free MEM and stored at −80 °C.
2.2. Regents and antibodies
Leupeptin (trypsin inhibitor), Elastatinal (elastase inhibitor), CA074-Me (cathepsin B inhibitor) and chloroquine (endosomal acidification inhibitor) were obtained from Sigma-Aldrich (St. Louis, MO). Z-FL-COCHO (Cathepsin L inhibitor) was purchased from Calbiochem (San Diego, CA). Anti-PEDV polyclonal antibody was collected from a pig challenge study previously reported by us (Kim et al., 2017).
2.3. One-step growth kinetics
Confluent Vero cells were incubated for 1 h at 37 °C with PEDV KD or PEDV AA at a MOI of 0.1. After washing 3 times with PBS, fresh MEM containing mock-medium, trypsin (1 μg/ml) or trypsin (1 μg/ml) + leupeptin (5 μM) or elastase (1 μg/ml) was added to the cells infected with PEDV KD, and fresh MEM containing mock-medium, elastase (1 μg/ml), or elastase (1 μg/ml) + elastatinal (5 μM) was added to the cells infected with PEDV AA. Virus infected cells were further incubated at 37 °C and virus RNA titers were measured by real-time quantitative RT-PCR at various time points following incubation. For real-time quantitative RT-PCR, total RNA was extracted from the PEDV infected cells using the RNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Real-time quantitative RT-PCR was performed using One-Step Platinum RT-PCR kit (Invitrogen, Carlsbad, CA) with forward primer (5′-GCTATGCTCAGATCGCCAGT-3′), reverse primer (5′-TCTCGTAAGAGTCCGCTAGCTC-3′), and probe (5′-/56-FAM/TGCTCTTTG/ZEN/GTGGTAATGTGGC/3IABkFQ/-3′) targeting the PEDV N gene on a Rotor-Gene Q (Qiagen) (Kim et al., 2017). The condition for RT-PCR was 50 °C for 30 min (for RT) and 95 °C for 5 min, then 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 60 s and elongation at 72 °C for 30 s. The TCID50 equivalents/ml were calculated from the Ct values based on the equation derived from the standard curve generated with the serial dilution of cell culture-grown PEDV.
2.4. Leupeptin addition assay
To determine which step of the virus entry is dependent on trypsin, leupeptin (a trypsin inhibitor) addition assay were performed using PEDV KD. Confluent Vero cells were inoculated with PEDV KD at an MOI of 0.1 in the presence of trypsin, and leupeptin (1 μM) was added to the culture media at a binding (4 °C for 1 h), entry (37 °C for 1 h) or replication (37 °C for 10 h) stage. Between each stage, cells were washed extensively (3×) with MEM. PEDV KD infection with trypsin (but without leupeptin) was included a control. PEDV KD infection without trypsin also served as a control. Virus replication was assessed by real-time quantitative RT-PCR at the end of a replication stage, and TCID50 equivalents/ml was determined as described above. Virus RNA titers from each treatment were compared to those with trypsin (without leupeptin).
2.5. Confocal microscopy
Recently, we demonstrated that PEDV utilizes the endocytic pathway, and successful viral endosomal escape and subsequent virus replication requires the presence of proteases such as trypsin or elastase. Therefore, PEDV cellular trafficking with or without protease (and its inhibitor) was investigated by confocal microscopy. Vero cells were seeded onto Lab-Tek™ II CC2™ chamber slide (Fisher Scientific, Pittsburgh, PA), treated with 5% FBS and grown to 70% confluency. Mock or PEDV KD or AA at an MOI of 50 were inoculated into the confluent cells on the chamber slides, and the virus-infected cells were incubated at 4 °C for 1 h. After washing with PBS for 3 times, the cells were subject to the following treatment for 1 h, 4 h, 6 h or 8 h prior to confocal microscopy. The treatments are: 1) Cells infected with PEDV KD were incubated with Mock (no trypsin), TPCK-treated trypsin (1 μg/ml) or TPCK-treated trypsin (1 μg/ml) + trypsin inhibitor (leupeptin, −1 μM); 2) Cells infected with PEDV AA strain were incubated with Mock (no trypsin), elastase (1 μg/ml) or elastase (1 μg/ml) + Elastatinal (5 μM); 3) Cells infected with KD were incubated with TPCK-treated trypsin with or without cathepsin inhibitors [Z-FL-COCHO (10 μM) or CA074-Me (40 μM)] or chloroquine (75 μM). To prepare cells for confocal microscopy, cells were fixed in 4% formaldehyde (Sigma-Aldrich) in PBS (pH 7.4) at room temperature (RT) for 15 min, permeabilized with 0.1% Triton x-100 (Fisher Scientific) in PBS for 10 min at RT. The cells were then washed three times with PBS, and incubated in PBS containing 0.5% bovine serum albumin for 15 min. After washing with PBS for three times, cells were further incubated with primary antibody to PEDV (1:200) at 37 °C for 2 h. After washing three times with PBS, the slides were incubated with FITC labeled secondary antibodies diluted 1:100 in PBS. Cell nucleus was stained with sytox orange (0.5 μM in 0.9% NaCl). To determine the intracellular location of PEDV, the KD-infected cells fixed at 6 and 12 h PI were incubated with the PEDV antibody and mouse monoclonal antibody against VPS26A (endosome marker) or PDIA3 (ER marker), followed by the appropriate secondary antibodies with FITC and AlexFluor®594. Coverslips were mounted with ProLong® Gold antifade reagent (Molecular Probes), and the cells were scanned with a confocal microscope LSM 510 (Zeiss, Oberkochen, Germany) using a 100x oil-immersion objective lens. The images were processed by Image J software 1.51 (http://imagej.nih.gov/ij/). The colocalization analysis was performed using JACoP and colocalization-MBF plugins for ImageJ software.
2.6. Statistical analysis
The effects of cathepsin or chloroquine in PEDV replication were statistically analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Statistical analysis was performed using student t-test. P value of <0.05 was considered as statistically significant. Data were from at least three independent experiments.
3. Results
3.1. Efficient replication of PEDV requires the addition of protease
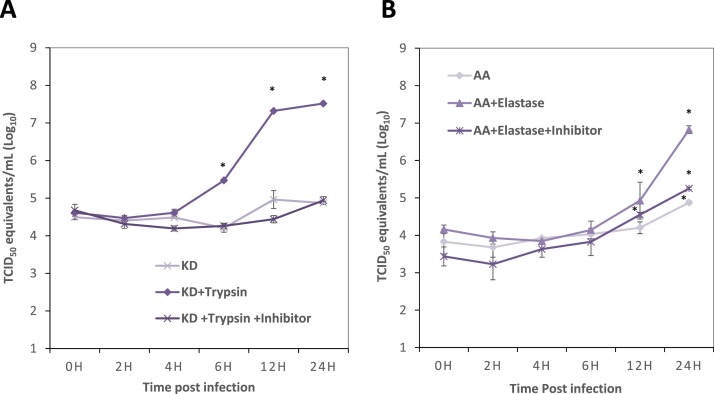
To determine the effect of protease in PEDV entry and replication, one-step growth kinetics of PEDV KD and AA were investigated in the presence or absence of trypsin or elastase, respectively. Trypsin inhibitor or elastase inhibitor was also used as additional controls for the study. The RNA levels (or viral titers) at each time point were compared to those at 0 h. During 0–4 h post infection, the titers of PEDV KD in all three groups remained unchanged and statistically not different (Fig. 1 A). The virus RNA titers of trypsin-treated group increased rapidly after 4 h post-infection (PI) and reached 7.5 log10 TCID50/ml at 24 h PI (Fig. 1A). However, the virus RNA titiers of mock-medium treatment (without trypsin) or trypsin plus inhibitor group did not significantly increase during 4–24 h post-infection (PI) (Fig. 1A). For PEDV AA, virus RNA titiers remained unchanged during 0–6 h PI in all three groups (Fig. 1A). The virus RNA titiers of PEDV AA with elastase significantly increased after 6 h PI, reaching 6.9 log10 TCID50/ml at 24 h PI. Interestingly, virus RNA titiers of PEDV AA also significantly increased at 24 h with mock-medium (without elastase) or at 12 h and 24 h with elastase plus inhibitor compared to those at 0 h (Fig. 1B). Although virus RNA titiers of PEDV AA also significantly increased without elastase or with elastase plus inhibitor, the increments are only moderate up to 5.0 log10 TCID50/ml (Fig. 1B).
Fig. 1.
Replication kinetics of PEDV KD & AA. Confluent Vero cells were inoculated with PEDV KD (A) or AA (B) at an MOI of 5 and incubated at 37 °C for 1 h. After thorough washing with PBS, fresh MEM containing (A) Mock-medium, TPCK-treated trypsin (1 μg/ml) or TPCK-treated trypsin (1 μg/ml)+inhibitor or (B) elastase (1 μg/ml), elastase (1 μg/ml)+inhibitor were added to the virus infected Vero cells. Viral RNA was extracted from the cells at 0, 2, 4, 6, 12 or 24 h post-inoculation (PI) for real-time qRT-PCR, and genome copy numbers were calculated by plotting Ct values against a standard curve gene-rated using a series of dilutions of in-vitro transcribed PEDV RNA genome. Error bars show standard deviations, and asterisks indicate significant difference (p < 0.05) in virus RNA titiers, compared to those at 0 h.
3.2. Trypsin is required after viral attachment/entry of PEDV KD
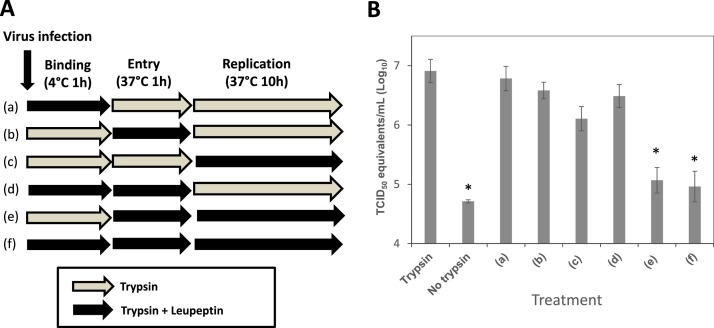
To determine which stage of PEDV replication is dependent on trypsin, we designed and conducted leupeptin (serine protease inhibitor) inhibition assay as described in Fig. 2 A. Absence of trypsin in the media led to a significant reduction of virus RNA titiers, as expected, compared to those with trypsin. Significant changes in virus RNA titiers were observed when leupeptin was present in the media during both entry and replication stages (treatments e and f), indicating trypsin activity is required for both stages (Fig. 2B). We also tested whether pre-incubation of cells or virus with trypsin induces viral replication. Pre-incubation of concentrated PEDV KD with TPCK-treated trypsin (up to 5 μg /ml) or pre-incubation of Vero cells with TPCK-treated trypsin (up to 5 μg /ml) for 1 h at 37 °C did not lead to virus replication (data not shown).
Fig. 2.
Addition of leupeptin at different virus replication stages (PEDV KD). Confluent Vero cells were inoculated with PEDV KD at an MOI of 5 in the presence of trypsin (1 μg/ml) or trypsin + inhibitor (leupeptin, 1 μM) at 4C for 1 h (binding stage). After thorough washing with PBS, virus infected cells were transferred to 37C and incubated for 1 h with trypsin or trypsin + inhibitor (entry stage). After another thorough washing, cells were incubated for additional 10 h with trypsin or trypsin + inhibitor before viral replication was assessed. (A) A schematic drawing shows trypsin or trypsin + leupeptin treatment of cells at various stages of virus replication. (B) Virus replication was quantified by real time qRT-PCR at 12 h PI. Error bars show standard deviations. Asterisks indicate significant difference (p < 0.05) in virus genome levels, compared to those of PEDV infection with trypsin.
3.3. Protease was required for the endosomal escape of PEDV
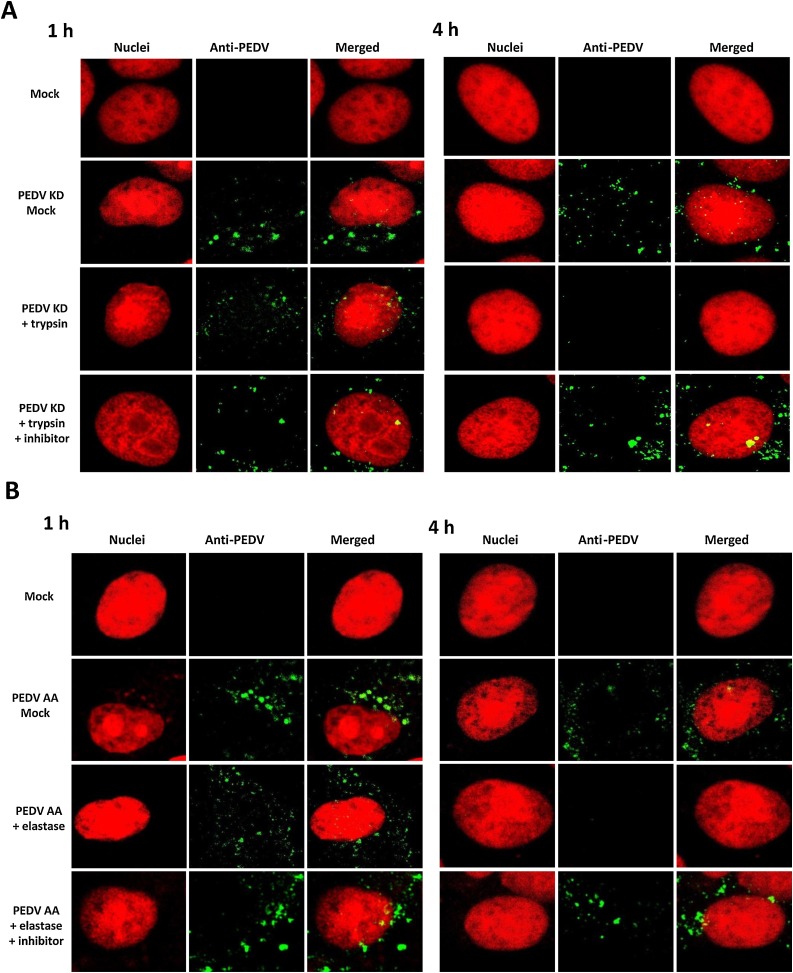
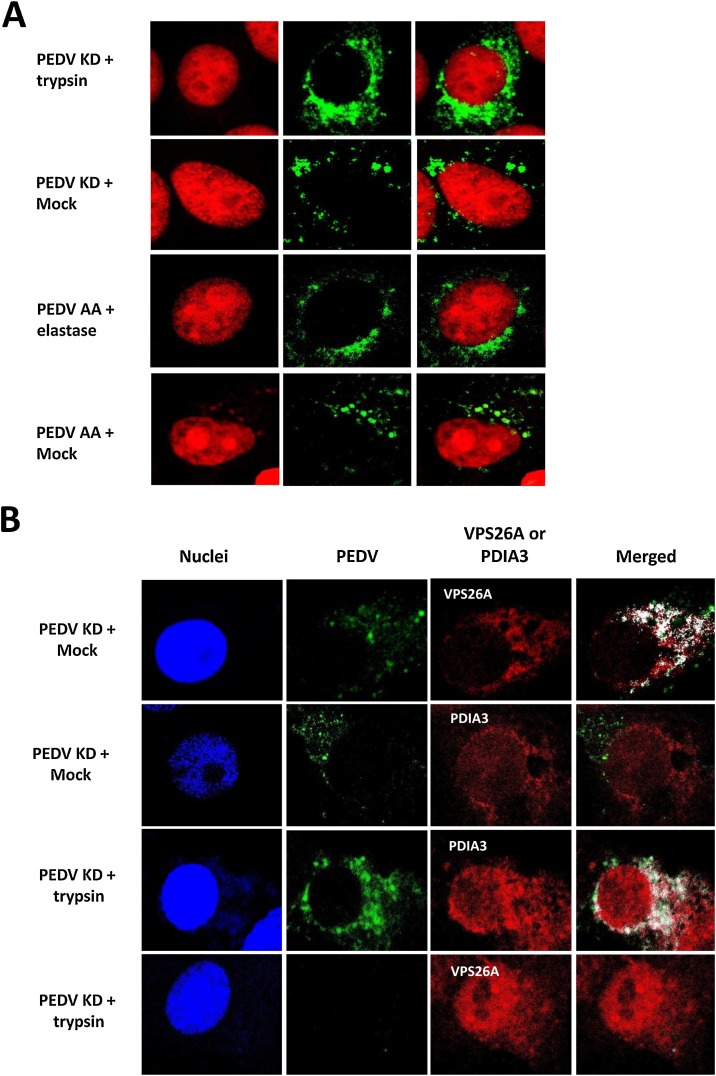
The effect of protease in the entry events of PEDV KD or PEDV AA in Vero cells was examined by confocal microscopy. While PEDV AA and KD require different protease for replication, there was no difference in the overall viral trafficking between these strains (Fig. 3 A and B). In the cells incubated with protease (trypsin or elastase), aggregated fluorescence signals of PEDV KD or AA were observed within the cell cytoplasm at 1 h PI, but the signals disappeared at 4 h PI. However, fluorescence signals of PEDV KD or AA remained at 4 h or 6 h PI in the cells incubated without protease or with protease + inhibitor (Fig. 3A, and 4). These signals were colocalized with VPS26A (an endosome marker) but not with PDIA3 (an ER marker) (Fig. 4 B). At 6 h PI, diffuse fluorescence signals of PEDV KD or AA were observed around the nucleus in the cells incubated with protease (Fig. 4, PEDV KD + trypsin and PEDV AA + elastase Panels), and these florescence colocalized with PDIA3, which suggests newly synthesized PEDV proteins were detected in ER (Fig. 4B). However, in the cells incubated without trypsin or elastase (Mock), aggregated fluorescence signals were still visible in the endosomes at 6 h PI (Fig. 4, PEDV KD + Mock and PEDV AA + Mock Panels) and 24 h PI (data not shown).
Fig. 3.
Confocal microscopy of PEDV entry. Confluent Vero cells grown on Lab-Tek II CC2 chamber slides were infected either with Mock (medium) or PEDV KD (A) or AA (B) at an MOI of 50, and incubated with Mock-medium, trypsin 1 μg/ml (elastase 1 μg/ml) or trypsin + inhibitor (elastase + inhibitor) for 1 h or 4 h. Fixed cells were probed with swine polyclonal anti-PEDV primary antibodies, followed by FITC-labelled goat-anti-swine antibody (green). Nuclei were stained with sytox orange (5 μM) (red), and merged images for PEDV and nuclei were prepared by using Image. J.
Fig. 4.
Confocal microscopy of KD or PEDV with or without protease. A. Virus infected cells were prepared at 6 h after PEDV KD or PEDV AA with or without (Mock) trypsin or elastase, respectively. Virus infection, treatment and procedures are same as Fig. 3. B. Co-localization of PEDV KD with the endosomes (VPS26A) or ER (PDIA3) marker. PEDV KD infected cells without (Mock) or with trypsin were fixed at 6 h and PEDV, PEDV (green), VPS26A (red), PDIA3 (red) or merged images were presented.
3.4. Cathepsin activity is required for efficient replication of PEDV
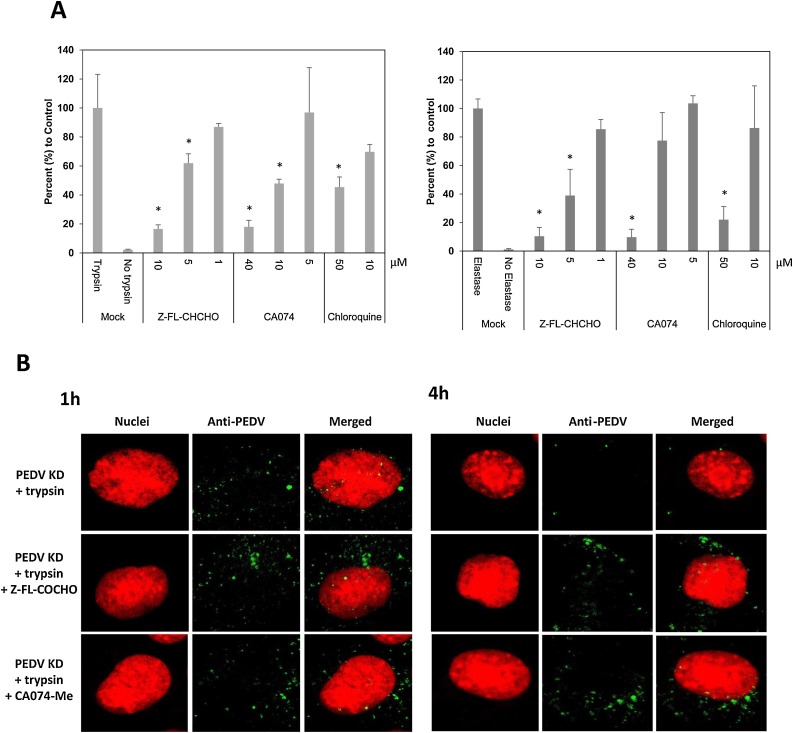
As the PEDV KD and PEDV AA travel through the endosomes, the roles of endosomal proteases, cathepsin B and L, in virus replication were examined using inhibitors. The cytotoxicity of each inhibitor was determined in Vero cells, and the concentrations showing minimal toxicity was used (up to 10 μM for Z-FL-COCHO and up to 40 μM for CA074-Me). Treatment of cells with cathepsin L inhibitor (Z-FL-COCHO) significantly reduced PEDV KD or AA replication at 10 and 5 μM (Fig. 5 A). Cathepsin B inhibitor (CA074-Me) also significantly reduced the replication of PEDV KD at 40 and 10 μM (Fig. 5A) and PEDV AA at 40 μM. Chloroquine which inhibits acidification of endosomes significantly reduced the replication of PEDV KD or AA at 50 μM (Fig. 5A). In confocal microscopy, there was no difference in the fluorescence signals of PEDV KD between trypsin only and trypsin + cathepsin B or L inhibitor at 1 h PI. At 4 h PI, the fluorescence signals were greatly reduced in cells without inhibitors, while there is still no change in the fluorescence signals in the cells with cathepsin B or L inhibitor (Fig. 5B).
Fig. 5.
Effect of Cathepsin inhibitors (Z-FL-CHCHO or CA074-Me) or chloroquine in PEDV entry into the cells. (A). Confluent Vero cells were pre-treated with mock (medium), Z-FL-CHCHO, CA074-Me or chloroquine for 1 h before PEDV inoculation (MOI 10). Following virus infection, cells were incubated with same inhibitor in the presence of TPCK-treated trypsin(1 μg/ml) for PEDV KD (left panel) or elastase (1 μg/ml) for PEDV AA (right panel) at 37 °C, and total RNAs were collected at 12 h PI. Viral replication was assessed by real time qRT-PCR. (B) Confluent Vero cells grown on Lab-Tek II CC2 chamber slides were pre-treated with mock(medium), Z-FL-CHCHO, CA074-Me, or chloroquine for 1 h prior to PEDV inoculation (MOI 50). Then PEDV KD was inoculated to the cells with TPCK-treated trypsin (1 μg/ml). The cells were incubated at 37 °C for 1 or 4 h, then fixed and stained for confocal laser scanning microscopy. Error bars show standard deviations. Asterisks indicate significant difference (p < 0.05) compared to the control (trypsin or elastase treatment only).
4. Discussion
Many viruses, including PEDV, utilize receptor-mediated endocytosis to gain entry into the host cells (Park et al., 2014). During the endocytic entry processes, viruses or viral genome translocate to cytoplasm (endosomal escapes) and initiate virus replication. Because the endosomal escape of virus is critical for successful virus replication, viruses utilize various mechanisms for the events (Grove and Marsh, 2011; Gruenberg and van der Goot, 2006; Marsh and Helenius, 2006). Early endosomes become progressively acidic as they mature into late endosomes and eventually into lysosomes. Endosomal proteases have been implicated in the crucial roles by digesting and processing of proteins transported in the endosomal/lysosomal compartments [reviewed in (Muller et al., 2012)]. The S protein of SARS coronavirus and the Glycoprotein (GP) of Ebola virus are cleaved by endosomal proteases, such as cathepsin L and B, to expose a putative fusion domain (Chandran et al., 2005; Cote et al., 2011; Ebert et al., 2002; Grove and Marsh, 2011). For reovirus, a non-enveloped virus, endosomal proteases remove the outer-capsid protein σ3, which exposes the protein μ1. The μ1protein is involved in viral membrane-penetration, which allows the reovirus core particles to be delivered into cytoplasm for transcription of viral genome (Schiff, 1998). Recently we demonstrated that porcine enteric calicivirus (PEC) requires endosomal cathepsins and bile acids that are supplemented in the medium for viral endosomal escape (Shivanna et al., 2014a, b; Shivanna et al., 2015).
The addition of exogenous trypsin (or other proteases) is absolutely required for the isolation of PEDV from field samples as well as efficient replication of most PEDV strains (Li et al., 2016). It has been shown that proteases activity is required for efficient PEDV replication at the post-viral attachment stage, but where and how they work during virus entry is not clearly understood. Using two different PEDV strains KD or AA that were adapted to grow in the presence of trypsin or elastase, respectively, we investigated the roles of proteases in PEDV replication focusing on the viral entry events. One-step growth kinetic study showed that viral replication (or RNA synthesis) occurs between 4–12 h PI (Fig. 1) in the presence of trypsin or elastase. Without trypsin, no significant replication of PEDV KD occurred, indicating that this strain is heavily dependent on the presence of trypsin for replication. PEDV AA replication was significantly hampered by the absence of elastase but, interestingly, limited viral replication occurred even without elastase (or elastase with its inhibitor) (Fig. 1). It is unclear how this limited viral replication occurs in the absence of elastase, but it is possible that it is associated with the fact that protease independent PEDV such as PEDV 8aa in our previous report (Kim et al., 2017) can be generated by passaging the virus in the absence of any protease. It is possible that minor population of the protease-independent PEDV may be generated spontaneously during the infection of PEDV KD or AA with trypsin or elastase, respectively, and elastase provides more favorable conditions for producing the protease-independent PEDV.
The leupeptin inhibition assay (Fig. 2A) showed that trypsin is required for the post-viral attachment stage (Fig. 2A and B), which is consistent with a previous study by Wicht et al (Wicht et al., 2014) where they reported that PEDV replication is significantly reduced when trypsin is inhibited after viral attachment to cellular receptors. During viral entry through receptors and endosomes, escaping of viral genomes from endosomes is critical for viral replication. Viral proteins in endosomes undergo conformational changes by decreasing pH and/or cleavage of viral proteins by host proteases in the endosomes, which leads to exposure of fusion domains or other mechanisms for membrane fusion and viral genome translocation to cytoplasm (Grove and Marsh, 2011; Marsh and Helenius, 2006). Recently, our group has demonstrated the crucial events of the endosomal escape of PEDV and caliciviruses using the confocal microscopy (Kim et al., 2017; Shivanna et al., 2014b, 2015). In that confocal microscopy study, the fluorescence signals for PEDV and caliciviruses co-localized with the endosome marker Rab7, and viral escape from the endosomes occurred at 3 h PI (Kim et al., 2017; Shivanna et al., 2014b, 2015).
While PEDV KD and AA require different protease for efficient replication, there was no difference in viral trafficking in the early stages of viral replication (Fig. 3). Both PEDV KD and AA were detected in the endosomes at 1 h PI regardless of the presence or absence of trypsin or elastase, but viral florescence signals disappeared at 4 h PI only in the presence of trypsin or elastase (Fig. 3A for PEDV KD and 3B for PEDV AA). These suggested both PEDV KD and AA were able to escape from the endosomes at 4 h PI only in the presence of protease. At 6 h PI, viral signals were detected on perinuclear areas for both PEDV KD and AA in the presence of trypsin and elastase, respectively (Fig. 4A), and the signals were co-localized with ER marker (PDI3A) (only PEDV KD was shown in Fig. 4B), suggesting active viral protein synthesis (with replication). Without proteases, viral signals remained at endosomes (co-localized with an endosome marker, VPS26A) at 6 h PI (Fig. 4A and 4B) or later time points up to 24 h PI (data not shown). This is consistent with our previous report with viral trafficking through endosomes, and interference with the proper viral escapes led to significantly reduction of viral replication (Kim et al., 2017; Shivanna et al., 2014b, 2015).
Cathepsins are host proteases that are usually found in the endosomal compartments. The cathepsin family is classified into cysteine (cathepsins B, L, H, K, S, and O), aspartyl (cathepsin D and E) and serine (cathepsin G) proteases (Vasiljeva et al., 2007). Cathepsin B and L have been reported to be involved in virus fusion and/or uncoating of some coronaviruses including SARS coronavirus, feline corona virus and murine hepatitis virus (MHV) (Bosch et al., 2008; Kim et al., 2013; Qiu et al., 2006). A previous study using the pseudovirus of PEDV reported that PEDV S protein is activated by cathepsins, and a lysosomal acidification inhibitor, or lysosomal cysteine protease inhibitors including cathepsin B (CA074) and L (Z-FY-CHO) inhibitors significantly reduced pseudovirus entry and PEDV replication (Liu et al., 2016). The results from this study are consistent with the previous study (Liu et al., 2016) demonstrating that cathepsins are required PEDV entry. We further demonstrated that cathepsin L and B inhibitors (Z-FL-CHCHO or CA074, respectively) inhibited the replication of PEDV KD and AA strain via interfering with the endosomal viral escapes (Fig. 5A and B).
Based on the results, we established a proposed model for protease-mediated PEDV replication in Vero cells. In this model, PEDV binds to its receptor (APN), enters the cells via the endocytic pathway to reach the late endosomes, which is independent of exogenous protease in the medium. In the presence of exogenous protease, the activation of S protein can lead to virus escape from the late endosomes into the cytoplasm to initiate virus replication. In addition to exogenous protease, cathepsins in the endosomes are required for successful endosomal escape of PEDV. In the absence of protease, PEDV remains in the late endosomes/lysosomes and is destined to be degraded.
Acknowledgement
We would like to thank David George for technical assistance. This work was supported by NIH Grant, <GN1>R01AI130092</GN1>.
References
- Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Glowacka I., Muller M.A., Lavender H., Gnirss K., Nehlmeier I., Niemeyer D., He Y., Simmons G., Drosten C., Soilleux E.J., Jahn O., Steffen I., Pohlmann S. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., Bartelink W., Rottier P.J. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82(17):8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge T.H., Antalis T.M., Wu Q. Type II transmembrane serine proteases. J. Biol. Chem. 2009;284(35):23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran K., Sullivan N.J., Felbor U., Whelan S.P., Cunningham J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308(5728):1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote M., Misasi J., Ren T., Bruchez A., Lee K., Filone C.M., Hensley L., Li Q., Ory D., Chandran K., Cunningham J. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477(7364):344–U122. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Yang Y., Zhou Y., Lu L., Li F., Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin. Ther. Targets. 2017;21(2):131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D.H., Deussing J., Peters C., Dermody T.S. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 2002;277(27):24609–24617. doi: 10.1074/jbc.M201107200. [DOI] [PubMed] [Google Scholar]
- Grove J., Marsh M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011;195(7):1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., van der Goot F.G. Mechanisms of pathogen entry through the endosomal compartments. Nature reviews. Mol. Cell Biol. 2006;7(7):495–504. doi: 10.1038/nrm1959. [DOI] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204(2):134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase M., Shirato K., Matsuyama S., Taguchi F. Protease-mediated entry via the endosome of human coronavirus 229E. J. Virol. 2009;83(2):712–721. doi: 10.1128/JVI.01933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Mandadapu S.R., Groutas W.C., Chang K.O. Potent inhibition of feline coronaviruses with peptidyl compounds targeting coronavirus 3C-like protease. Antiviral Res. 2013;97(2):161–168. doi: 10.1016/j.antiviral.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Oh C., Shivanna V., Hesse R.A., Chang K.O. Trypsin-independent porcine epidemic diarrhea virus US strain with altered virus entry mechanism. BMC Vet. Res. 2017;13(1):356. doi: 10.1186/s12917-017-1283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531(7592):118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., van Kuppeveld F.J.M., He Q., Rottier P.J.M., Bosch B.J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016;226:117–127. doi: 10.1016/j.virusres.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Ma Y., Yang Y., Zheng Y., Shang J., Zhou Y., Jiang S., Du L., Li J., Li F. Cell entry of porcine epidemic diarrhea coronavirus is activated by lysosomal proteases. J. Biol. Chem. 2016;291(47):24779–24786. doi: 10.1074/jbc.M116.740746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124(4):729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Ujike M., Morikawa S., Tashiro M., Taguchi F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. U. S. A. 2005;102(35):12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S., Dennemarker J., Reinheckel T. Specific functions of lysosomal proteases in endocytic and autophagic pathways. Biochim. Biophys. Acta. 2012;1824(1):34–43. doi: 10.1016/j.bbapap.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Cruz D.J., Shin H.J. Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion. Arch. Virol. 2011;156(10):1749–1756. doi: 10.1007/s00705-011-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Cruz D.J., Shin H.J. Clathrin- and serine proteases-dependent uptake of porcine epidemic diarrhea virus into Vero cells. Virus Res. 2014;191:21–29. doi: 10.1016/j.virusres.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.M., Perlman S. 6th ed. Lippincot Williams & Willkins; Philadelphia, PA: 2013. Coronaviridae, Fields Virology; pp. 825–858. [Google Scholar]
- Qiu Z., Hingley S.T., Simmons G., Yu C., Das Sarma J., Bates P., Weiss S.R. Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry. J. Virol. 2006;80(12):5768–5776. doi: 10.1128/JVI.00442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan A.D., Shraybman R., Cohen R.D., Whittaker G.R. Differential role for low pH and cathepsin-mediated cleavage of the viral spike protein during entry of serotype II feline coronaviruses. Vet. Microbiol. 2008;132(3–4):235–248. doi: 10.1016/j.vetmic.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L.A. Reovirus capsid proteins sigma 3 and mu 1: interactions that influence viral entry, assembly, and translational control. Curr. Top. Microbiol. Immunol. 1998;233(Pt 1):167–183. doi: 10.1007/978-3-642-72092-5_8. [DOI] [PubMed] [Google Scholar]
- Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11(5):367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- Shirato K., Kawase M., Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J. Virol. 2013;87(23):12552–12561. doi: 10.1128/JVI.01890-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna V., Kim Y., Chang K.O. The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology. 2014;456-457:268–278. doi: 10.1016/j.virol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna V., Kim Y., Chang K.O. Endosomal acidification and cathepsin L activity is required for calicivirus replication. Virology. 2014;464-465:287–295. doi: 10.1016/j.virol.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna V., Kim Y., Chang K.O. Ceramide formation mediated by acid sphingomyelinase facilitates endosomal escape of caliciviruses. Virology. 2015;483:218–228. doi: 10.1016/j.virol.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Zmora P., Gierer S., Heurich A., Pohlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100(3):605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L.S., Ricard C.S., Holmes K.V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: activation of cell-fusing activity of virions by trypsin and separation of two different 90K cleavage fragments. J. Virol. 1985;56(3):904–911. doi: 10.1128/jvi.56.3.904-911.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljeva O., Reinheckel T., Peters C., Turk D., Turk V., Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr. Pharm. Des. 2007;13(4):387–403. doi: 10.2174/138161207780162962. [DOI] [PubMed] [Google Scholar]
- Wicht O., Li W., Willems L., Meuleman T.J., Wubbolts R.W., van Kuppeveld F.J., Rottier P.J., Bosch B.J. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. J. Virol. 2014;88(14):7952–7961. doi: 10.1128/JVI.00297-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Liu D.X. Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells. J. Virol. 2009;83(17):8744–8758. doi: 10.1128/JVI.00613-09. [DOI] [PMC free article] [PubMed] [Google Scholar]