Abstract
Persistent pulmonary hypertension of the newborn (PPHN) is a failure of pulmonary vascular resistance to decline at birth rapidly. One principal mechanism implicated in PPHN development is mitochondrial oxidative stress. Expression and activity of mitochondrial superoxide dismutase (SOD2) are decreased in PPHN; however, the mechanism remains unknown. Recently, OLA1, an Obg-like ATPase-1, was shown to act as a critical regulator of proteins controlling cell response to stress including Hsp70, an obligate chaperone for SOD2. Here, we investigated whether OLA1 is causally-linked to PPHN. Compared to controls, SOD2 expression is reduced in distal-pulmonary arteries (PAs) from PPHN-patients and fetal-lamb models. Disruptions of the SOD2 gene reproduced PPHN phenotypes, manifested by elevated right ventricular (RV) systolic pressure, PA-endothelial cells (PAEC) apoptosis, and PA-smooth muscle cells (PASMC) proliferation. Analyses of SOD2 protein dynamics revealed higher ubiquitinated-SOD2 protein levels in PPHN-lambs, suggesting dysregulated protein ubiquitination. OLA1 controls multiple proteostatic mechanisms and is overexpressed in response to stress. We demonstrated that OLA1 acts as a molecular chaperone, and its activity is induced by stress. Strikingly, OLA1 expression is decreased in distal-PAs from PPHN-patients and fetal-lambs. OLA1 deficiency enhanced CHIP affinity for Hsp70-SOD2 complexes, facilitating SOD2 degradation. Consequently, mitochondrial H2O2 formation is impaired, leading to X-linked inhibitor of apoptosis (XIAP) over-expression that suppresses caspase activity in PASMCs, allowing them to survive and proliferate, contributing to PA remodeling. In-vivo, ola1−/− downregulated SOD2 expression, induced distal-PA remodeling, and RV hypertrophy. We conclude that decreased OLA1 expression accounts for SOD2 downregulation and, therefore, a therapeutic target in PPHN treatments.
Keywords: Mitochondria, SOD2, Oxidative stress, Reactive oxygen species, OLA1
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a vascular remodeling disease characterized by elevated pulmonary vascular resistance (PVR) due to a dysfunctional and apoptosis-prone pulmonary artery (PA)-endothelial cells (PAEC) and a proliferative and apoptosis-resistant phenotype of PA-smooth muscle cells (PASMC). The elevated PVR leads to intracardiac shunting of deoxygenated blood and hypoxemia that is often unresponsive to supplemental oxygen. 1–3 Even when diagnosed early and therapy initiated promptly, PPHN increases the mortality and neurodevelopmental impairments among survivors.4, 5 PPHN is a multifactorial disorder and occurs as a primary disease6 or a secondary manifestation of lung diseases, including meconium aspiration syndrome_ENREF_7,7 respiratory distress syndrome (RDS),8 bronchopulmonary dysplasia (BPD),9 and lung hypoplasia.10, 11 Though the precise etiology is unknown, converging evidence implicates mitochondrial oxidative stress as the central unifying underlying mechanism of PPHN. 12–14 Therefore, the identification of the mechanism underlying mitochondrial redox imbalance in PPHN is a pressing need to improve the disease outcome.
The vascular dysfunction in PPHN evolves antenatally and impairs the adaptation of pulmonary circulation at birth. The PPHN model used in this study was induced by in-utero ligation of the ductus arteriosus (DA); therefore, a suitable model of the disease.15, 16 At birth, the ductal ligated lambs develop severe PPHN characterized by PAEC dysfunction and apoptosis, and excessive PASMC proliferation.17, 18 These pathological findings resemble those in clinical PPHN. Moreover, PPHN-lambs share with human patients a low superoxide dismutase-2 (SOD2) expression.14 (Fig.1) The many similarities shared by human and experimental models suggest that the patho-physiological mechanisms uncovered in PPHN-lambs could be applied to human.
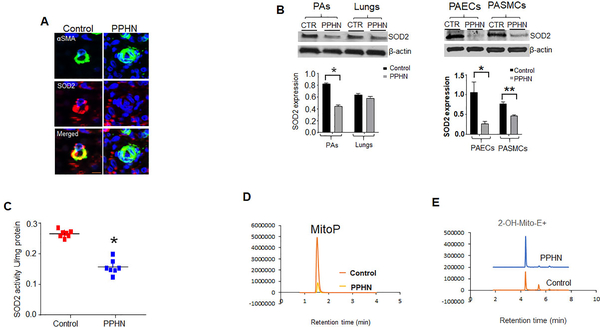
Figure 1:
SOD2 expression is decreased in human patients and animal models. (A) Human lung specimens were cut into 4μm sections and mounted slides were subjected to immunofluorescence (IF) staining for αSMA (green) and SOD2 (red) and DAPI (blue) nuclear staining. Representative IF images from infants without PPHN and PPHN patients (n=4), showing a lower immunoreactivity for SOD2 protein in the remolded distal-PAs (Scale bar=100μm). (B) Western blots and corresponding densitometry analysis of SOD2 protein in the lungs, distal-PAs, isolated PA-endothelial cells (PAEC), and PA-smooth muscle cells (PASMC) from controls and PPHN fetal-lambs (n=6). (C) Scattered plots showing decreased SOD2 activity in PA tissue homogenates from controls and PPHN lambs (n=7). (D) LC-MS/MS tracing of O2¯-specific mitoSOX oxidized product, 2-hydroethythidium (2-OH-Mito-E+) retention over time and (E) MitoP /MitoB ratio relative to deuterated internal standards (n=4). *’**p<.05.
Emerging evidence suggests that PPHN is a disorder of protein ubiquitination, a post-translational modification (PTM) implicated in many diseases including cancer, neurodegenerative, and cardiovascular diseases.19, 20 The biological relevance of altered protein ubiquitination in PPHN is supported by high levels of ubiquitinated-SOD2 proteins in PPHN-lambs.21 This finding has led to a proposed new mechanism for mitochondrial oxidative stress in PPHN-lambs whereby following ductal ligation; the vascular cells react to pressure overload in pulmonary circulation by activating adaptive proteostatic mechanisms, allowing them to survive and adapt to stress. Central to this cytoprotective response is overexpression of molecular chaperones that promote refolding of misfolded proteins while minimizing aggregation events. However, if pressure overload persists beyond certain thresholds, the protein refolding arm of stress responses is lost, and overactivity of the ubiquitin-proteasomal degradation pathways ensues, leading to instability of a subset of proteins, such as SOD2. Consequently, newly-made SOD2 is no longer able to fold efficiently under stress precluding its mitochondrial import. The resulting increase in mitochondrial reactive oxygen species (ROS) formation negatively impacts developing vasculature and cells (ECs and SMCs) associated with the vasculature, culminating in PPHN.22, 23 Therefore, the identification of signaling molecules controlling the refolding arm of stress response will uncover the mechanism underlying PPHN.
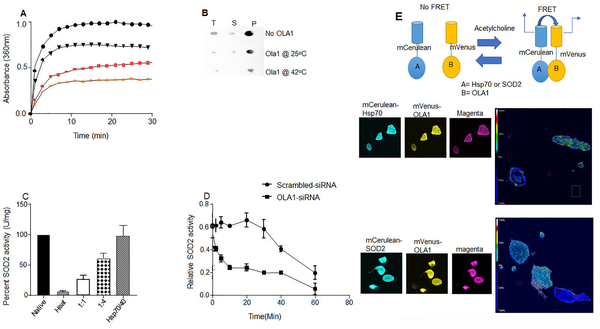
Increasing evidence points to OLA1, an Obg-like ATPase-1 as a central regulator of cellular stress response through its implication in multiple adaptive mechanisms critical at maintaining proteostasis during stress.24 OLA1 belongs to TRAFAC (the translation factor-related) class of Obg family, and YchF subfamily of P-loop GTPases that plays a crucial role in regulating diverse cellular processes including DNA repair subcellular localization and is overexpressed in cancer in response to stress.25 The observation that disruption of the OLA1 gene reduced the stability of Hsp70 during heat-shock further supports OLA1 roles in SOD2 regulation. 26 Moreover, OLA1 regulates cell cycle and proliferation, all of which are derranged in PPHN.27 Given decreased OLA1 expression in human-PPHN (Fig.4), and its enhancement of Hsp70 chaperone activity (Fig.6), we, therefore, hypothesized that OLA1 deficiency is causally-linked to PPHN.
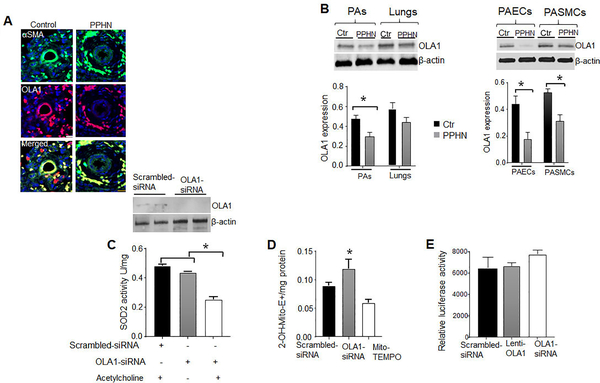
Figure 4.
Decreased OLA1 expression contributes to SOD2 deficiency in PPHN. (A) Dual immunofluorescence staining for αSMA (green), OLA1 and DAPI nuclear staining showing decreased OLA1 expression in distal-PAs from PPHN patients compared to infants without PPHN (n=4). (B) Immunoblottings showing decreased OLA1 expression in the lungs, PAs, isolated PAECs, and PASMCs from PPHN compared to control-lambs (n=7). (C) Western blots and bar charts showing decreased OLA1 expression and SOD2 activity OLA1-siRNA treated PAECs and controls, with or without acetylcholine treatments (n=5). (D) Bar charts showing increased O2¯-specific oxidized mitoSOX product, 2-OH-Mito-E+ formation in OLA1-silenced PAECs, scrambled-siRNA, and mito-TEMPO-treated OLA1 deficient PAECs (n=5). (E) Bar charts showing the effects of OLA1-siRNA, scrambled-siRNA, and Lenti-OLA1 on the SOD2 gene promoter activity in normal PAECs (n=4). *p<.05
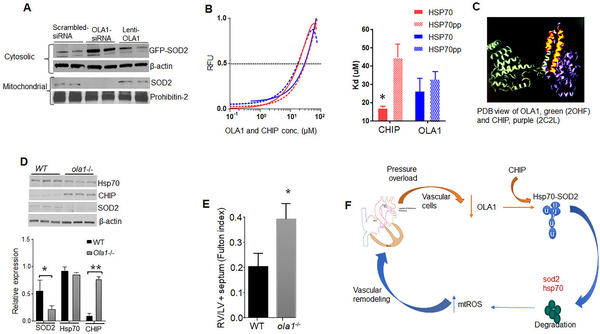
Figure 6.
OLA1 enhances SOD2 activity in a chaperone-dependent manner. (A) Representative immunoblots showing cytosolic and mitochondrial SOD2 protein contents in OLA1-silenced normal PAECs (n=4). (B) The equilibrium dissociation constants (Kd) between OLA1 and CHIP and phosphorylated (blue) or non-phosphorylated FAM-tagged C-terminal peptides of Hsp70 measured by fluorescence polarization binding assay (n=6). (C) Structural alignment of the CHIP-TPR, and OLA1. Alignments of PDB structures 2C2L (CHIP-TPR) and 2OHF (OLA1, green). OLA1 aligned segment is highlighted in orange and corresponds to the N-terminal TPR2 region of CHIP. (D) Bar charts showing decreased SOD2 and Hsp70 expression in lungs of ola1−/− compared to WT mice (n=5). (E) Disruptions of the OLA1 gene recapitulated PPHN phenotypes. Immunoblots showing changes in expression of SOD2, CHIP and Hsp70 in lungs of ola1−/− and WT mice (n=6). Bar charts showing increased RV hypertrophy assessed by Fulton index in ola1−/− compared to WT mice (n =7). (F) The schema for the proposed new mechanism for SOD2 deficiency in PPHN-lambs.*’** p<.05.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Human Lung Tissues
Human lung tissues used in this study were from infants that died from PPHN. PPHN was confirmed in these patients by echocardiogram and treated appropriately. PPHN and controls (age-matched infants that died from other diseases) specimens were obtained from the pathology at the Medical College of Wisconsin (MCW).
Animal Studies
The MCW Animal Care and Use Committee approved all protocols for animal use. The pregnant ewe was purchased from May Enterprises (Philadelphia).16 At 125-day gestation, PPHN was induced by DA ligation. Eight days later; fetal lambs were delivered by cesarean section, and age-matched sham ligated lambs were used as controls. Ola1 knockout mice (strain C57BL/6FVB/N Ola1 tm1.2Zshi/Ola1tm1.2Zshi were a generous gift from Dr. Shi (Houston Methodist Hospital Texas). Sod2 null mice were purchased from The Jackson Laboratory (strain B6.129S7Sod2tm1Leb/J), and the strain was maintained by mating heterozygotes (female/male) with C57BL/6J inbred mice. Age-matched wild-type C57BL/6J mice were used as controls.
Hemodynamic Measurements
Mice were anesthetized using 3–4% isoflurane and maintained with 2% immediately before measurements of the right ventricular systolic pressure (RVSP). RVSP was measured blindly by closed-chest RV catheterization using Millar’s probe (size 1F). RV hypertrophy (Fulton index-RV/LV+ septum) was measured after RVSP measurements.
Cell isolation, culture, and treatments
Controls and PPHN-PAECs were isolated from near-term fetal lambs of either gender.16
Mitochondrial ROS Quantification
Mitochondrial superoxide (O2¯) and hydrogen peroxide (H2O2) were measured using mitoSOX and mitoB staining. O2¯and H2O2 oxidation products were quantified by liquid chromatography mass spectrometry (LC-MS/MS, Shimadzu Nexera UHPLC system), 28 (details in the online supplemental file).
Statistics
Statistical analysis was done with GraphPad Prism 7.0. Values were expressed as fold change or mean ± standard error of the mean. Unpaired Student t-tests were used for comparison between two groups, and one-way analysis of variance was used for >2 groups. A P <0.5 was considered statistically significant.
Results
SOD2 expression is decreased in human-patients and PPHN fetal-lambs
The SOD2 protein level was assessed in lung specimens from PPHN-patients.
Immunofluorescence analysis revealed a lower immunoreactivity for SOD2 protein in distal-PAs of PPHN-patients compared to patients without PPHN (Fig.1a). To identify the source of decreased SOD2 protein, we used PPHN-lambs. SOD2 expression is reduced in distal-PAs, isolated PAECs, and PASMCs from PPHN compared to control-lambs (Fig.1b). However, we found no change in the lungs’ SOD2 expression between PPHN and control-lambs. In line with decreased expression, SOD2 activity and rates of mitochondrial H2O2 formation overtime were also lower while mitochondrial O2¯ levels were significantly higher in PPHN-PAs compared to controls (Figs.1c, 1d, and 1e).
Disruptions of the SOD2 gene reproduce PPHN phenotypes
Whereas PPHN-PAECs have higher apoptosis rates compared to control-lambs,29 PPHN-PASMCs exhibited increased proliferation (Fig.1a). To determine whether SOD2 deficiency accounts for changes in vascular cell phenotypes, we generated global sod2−/− mice. Mouse lung- ECs from sod2−/− have higher apoptosis rates compared to WT-mice (Fig.2a). Conversely, the PASMCs have higher proliferation rates (assessed by Ki67 labeling) in sod2−/− (Fig.2b). Functionally, sod2−/− mice have higher RVSP compared to WT-mice (Fig.2c), reinforcing the view that SOD2 deficiency contributes to PPHN development.
Figure 2:
Disruptions of the SOD2 gene recapitulate PPHN phenotypes. (A) Flow cytometry analysis plots showing higher apoptosis rates in isolated mouse lung microvascular endothelial cells from sod2−/− compared to WT-mice (n=6). (B) Dual immunofluorescence staining for αSMA (pink), and Ki-67 (green) and DAPI nuclear staining showing increased Ki-67 expression in the remodeled distal-PAs in sod2−/− compared to WT-mice (n=4). (C) Tracings showing elevated right systolic ventricular pressure (RSVP) in sod2−/− compared to WT mice (n=6). Scale bar=100μm.
SOD2 deficiency induces PPHN phenotypes
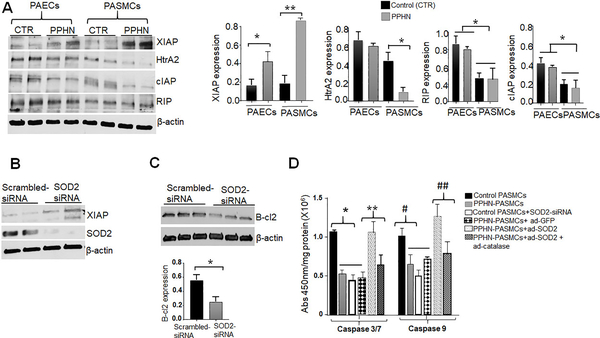
To identify the mechanism by which SOD2 induces PPHN phenotypes, we investigated the effects of SOD2 on several genes known to regulate cell survival, such as RIP (receptor-interacting protein kinases) and several inhibitors of apoptosis (IAPs), including cIAP (cytosolic inhibitor of apoptosis), XIAP (X-linked inhibitor of apoptosis) and HtrA2, whose anti or pro-apoptotic activity stems primarily from direct modulation of caspase activity.30–34 To pinpoint, which of these proteins are involved in regulating PPHN-cells survival, we measured their levels in fetal-lambs. Compared to PPHN-PAECs, the levels of XIAP protein were higher while RIP, cIAP, and HtrA2 protein levels were significantly lower in PPHN-PASMCs. These findings suggest that HtrA2, cIAP, and RIP counteract the anti-apoptotic activity of XIAP in PAECs, increasing their sensitivity to apoptosis stimuli. Since there were no significant differences in cIAP and RIP expression between controls and PPHN-PASMCs (Fig.3a), we, therefore, focused our attention on HtrA2 and XIAP and further investigated whether SOD2 deficiency accounts for their dysregulation in PPHN. Strikingly, SOD2-silencing increased XIAP expression in normal PASMCs compared to scrambled-siRNA (Fig.3b). This increase is associated with decreases in B-cl2 protein (Fig.3c) and caspase activity levels in normal PASMCs (Fig. 3d). Conversely, SOD2 overexpression increased caspase activity in PPHN-PASMCs, and this effect was abolished by catalase overexpression (Fig.3d), pointing to H2O2 as the redox signaling regulating XIAP protein. Together, these results indicate that SOD2 deficiency contributes to the apoptotic-proliferative imbalance in PA-wall in PPHN via differential regulation of XIAP and HtrA2 expression.
Figure 3.
SOD2 deficiency accounts for the apoptotic-proliferation imbalance in distal PAs of PPHN. (A) Western blottings and densitometry analyses showing differences in XIAP, HtrA2, RIP, and cIAP expression in PAECs and PASMCs between controls and PPHN (n=6). (B) Immunoblottings showing increased XIAP expression in SOD2-silenced normal PASMCs compared to controls (scrambled siRNA, n=5). (C) Immunoblottings showing decreased B-cl2 expression in SOD2-silenced PASMCs compared to scrambled-siRNA (n=6). (D) Bar charts showing levels of caspase 3/7 and 9 activities in PASMCs from controls and PPHN, SOD2-silenced PASMCs and in PPHN-PASMCs overexpressing SOD2, catalase or GFP (n=4). *’**,#, ## p<.05
Decreased OLA1 expression contributes to SOD2 deficiency in PPHN
To define the mechanism underlying SOD2 deficiency, we profiled proteins interacting with Hsp70, an obligate chaperone for SOD2 in human umbilical vein microvascular endothelial cells (HUVEC). Among the proteins identified, OLA1 was intriguing (table1 in the online supplemental file), given a recent report showing that OLA1 stabilizes Hsp70 during heat-shock, 26 To determine OLA1 roles in SOD2 regulation, we first measured its expression and found decreases in OLA1 expression in human and PPHN-lambs (Fig.4a). However, there is no change in the lungs’ OLA1 expression between controls and PPHN-lambs (Fig.4b). To determine whether OLA1 insufficiency accounts for SOD2 deficiency, siRNA-mediated knockdown of OLA1 was performed. OLA1-siRNA reduced OLA1 expression in PAECs compared to scrambled-siRNA (Fig.4c). Despite this decrease, lack of OLA1 did not affect SOD2 activity. However, when OLA1-deficient cells were stimulated with acetylcholine, SOD2 activity was significantly reduced (Fig. 3c). Mitochondrial O2¯ levels were also increased, validating decreased SOD2 antioxidant activity in OLA1-silenced cells (Fig.4d). Importantly, OLA1-siRNA induced PAEC apoptosis and a proliferative and apoptosis-resistant PASMCs (Figs.S1a and S1b). More importantly, pre-treatment with mito-TEMPO, a SOD mimetic rescued the cells from OLA1-siRNA induced apoptosis and proliferation.
OLA1 influences SOD2 activity at posttranscriptional levels
To determine whether OLA1 influences SOD2 at transcriptional levels, PAECs were transfected with OLA1-siRNA or lentivirus carrying human OLA1 and assessed for the SOD2 gene promoter activity using luciferase reporter assays. Neither silencing nor OLA1 overexpression has any effects on the SOD2 promoter activity (Fig.4e), indicating that OLA1 influences SOD2 activity at the post-transcriptional level.
OLA1 acts as a molecular chaperone
Previously, we reported that Hsp70 chaperones SOD2 to the mitochondria.21 Others have shown that OLA1 stabilizes Hsp70 during heat-shock.26 We, therefore, investigated OLA1’s ability to prevent SOD2 aggregation using in-vitro chaperone assays. Human SOD2 protein was expressed in E. coli and purified using standard methods. We first ensured that the purified SOD2 protein is functional by monitoring its secondary structure using circular dichroism (CD) spectroscopy. Far-UV spectra showed that purified SOD2 proteins were correctly folded (Fig.S2a). We then determined the temperature required to inactivate the purified SOD2 protein using fluorescence-based thermal melt assays and observed that SOD2 has a melting temperature (Tm) value of 65oC (Fig.S2b). When subjected to heat at 65oC, 90% of SOD2 activity was lost within 10 min. The addition of OLA1 inhibited aggregation of denatured-SOD2 proteins in a concentration-dependent manner (Fig.5a). Further, the presence of OLA1 increased the soluble fraction of SOD2 protein after heat treatments (Fig.5b). We then tested the effect of OLA1 on the aggregation of chemically denatured-SOD2 proteins using guanidine hydrochloride (GdnHCl) as the denaturant. Again, OLA1 dose-dependently inhibited aggregation of chemically denatured-SOD2 proteins (Fig. S2a). The ability of OLA1 to prevent SOD2 aggregation was enhanced by heat, indicating that OLA1 is heat responsive and that stress is required for OLA1 inhibition of SOD2 aggregation. Strikingly, OLA1 also refolds inactivated SOD2 proteins alone, and in cooperation with the Hsp70/Hsp40 chaperone system. Incubation of heat-denatured-SOD2 proteins in refolding buffered alone resulted in a low yield of SOD2 activity. However, when OLA1 was added to the buffer, SOD2 activity increased to 55%. In the presence of purified Hsp70/Hsp40 proteins in refolding buffer, 90% of SOD2 activity was recovered (Fig.5c). These data specify OLA1 as a molecular chaperone having an optimum activity at high temperatures. We then investigated whether OLA1 could suppress SOD2 aggregation in-vivo by subjecting PAECs to heat-shock at 42oC, in the presence or absence of OLA1. OLA1-silenced PAECs have a lower SOD2 activity upon heat-shock, indicating increased denaturation of SOD2 protein in the absence of OLA1 (Fig. 5d). At high temperatures, OLA1 overexpression enhanced SOD2 association with Hsp70 (Fig.S2d), suggesting that OLA1 promotes a complex formation between SOD2 and Hsp70. For verification, we used modified ELISA methods and observed that OLA1 promoted SOD2 binding to Hsp70 (Fig.S2e), confirming the in-vivo results.
Figure 5.
OLA1 acts as a molecular chaperone. (A) Heat-dependence of OLA1 chaperone activity. Comparisons of inhibition of SOD2 aggregation by a 4-fold molar excess of OLA1 at indicated temperatures (saturation signal was normalized to 100) black circles (inactivated SOD2 without OLA1), black triangles (0.3μM OLA1), pink squares (0.6μM OLA1), and orange triangles (1.2μM) (inactivated SOD2+OLA1 at 37°C. (B) Dot blot analysis showing that the presence of OLA1 at a ratio that conveyed half-maximum or total aggregation suppression in light scattering assays prevented precipitation of SOD2 protein after heat-shock (1 hr at 65°C) (T=Total, S=Supernatant, P=Pellet. (C) Bar charts showing refolding of inactivated SOD2 protein in the presence of OLA1 and human Hsp70/40 system. (D) Line graphs showing decreased SOD2 activity in OLA1-silenced cells compared to scrambled-siRNA (n=4). (E) Schema for the FRET assay principle. Confocal images and analyses for FRET by photobleaching the acceptor, and the heat map shows the degree of interactions.
OLA1 physically interacts with SOD2 and Hsp70
We then investigated whether OLA1 is just part of the complex or physically interacts with Hsp70 and SOD2. Using purified proteins and pulldown assays, we saw that OLA1 bound to SOD2 and Hsp70 (Fig.S3a and S3b), however, we observed no direct interactions between OLA1 and CHIP. In live cells, FRET analysis by acceptor photobleaching showed a higher percent FRET efficiency in cells expressing mVenus-OLA1 and mCerulean-Hsp70 (33%) as well as mCerulean-SOD2 and mVenus-OLA1 (30%), confirming that OLA1 interacts with SOD2 and Hsp70 (Fig.5d). To identify the domains where OLA1 bind in Hsp70 and SOD2, we used a series of deletion mutants. HEK-293T cells were co-transfected with cDNAs encoding YFP-tagged OLA1, GST-tagged Hsp70 mutants (1–400, 400–538, 538–638, 638–641) and EGFP-tagged SOD2 deletion mutants (1–100,100–180,180–222). OLA1 co-precipitated with region 638–641 in Hsp70 (Fig.S3c) and 1–100 in SOD2 (Fig.S3d).
Decreased OLA1 activity reduces SOD2 activity
Fig.S2d suggests that OLA1 enhances Hsp70 interaction with SOD2. Since Hsp70 chaperones SOD2 to the mitochondria, we investigated whether OLA1 influences this critical step in SOD2 activation processes. Strikingly, OLA1-silencing decreased mitochondrial SOD2 protein contents, and re-expression of OLA1 restored the mitochondrial SOD2 contents (Fig.6a).
Decreased OLA1 expression synergizes with dephosphorylated Hsp70 to downregulate SOD2 in PPHN
Fig.S3c shows that OLA1 and CHIP compete for binding in the C-terminus of Hsp70. Whereas CHIP decreases SOD2 activity, OLA1 enhances SOD2 import (Fig.6a). Given that phosphorylation alters Hsp70 binding to CHIP. 28, 35, we, therefore, sought to determine the effect of phosphorylation on Hsp70 binding to OLA1 using fluorescence polarization assay. The calculated dissociation constant (Kd) indicated that CHIP has a stronger affinity for non-phosphorylated than phosphorylated-Hsp70, while phosphorylated-Hsp70 has a higher affinity for OLA1 (Fig.6b). For verification, we used ELISA methods and found that OLA1 bound more strongly to phosphorylated than non-phosphorylated-Hsp70 (Fig.S3e). Phosphorylation has similar effects on Hsp70 binding to OLA1 and CHIP in PAECs (Fig.S3f). To determine the biological relevance of this observation, we quantified phosphorylated Hsp70 levels in PPHN-lambs and found reduced phosphorylated-Hsp70 levels in PPHN compared to control PAECs (Fig.S4a). Since phosphorylation decreases Hsp70 affinity for CHIP, we hypothesized that OLA1 insufficiency enhances CHIP-mediated degradation of SOD2 protein (Fig.S4b). Indeed, the levels of ubiquitinated SOD2 and Hsp70 proteins were significantly higher in PPHN compared to control-PAECs (Fig.S4c). Further, CHIP-silencing restored SOD2 expression in PPHN-PAECs (Fig.S4d). Importantly, OLA1 dose-dependently increased SOD2 activity in PPHN-PAECs (Figs.S4e). These findings indicate that both SOD2 and Hsp70 are direct targets of OLA1.
Structural alignment between OLA1 and CHIP
To analyze further how phosphorylation influences OLA1 and CHIP interactions with the C-terminus of Hsp70, we compared the crystal structures of the OLA1 (PDB:2OHF) and CHIP-TPR (PDB:2C2L) domains. While we found a limited amino-acid sequence similarity, alignments of the CHIP-TPR with OLA1 showed high structural similarity, mainly, the N-terminal part of OLA1 that binds Hsp70 (Fig.6c). Importantly, phosphorylation of the C-terminal serine and threonine in Hsp70 prevented the formation of H-bonds with the CHIP-TPR domain and disrupted protein binding. 28These findings suggest that Hsp70 phosphorylation increases SOD2 activity by enhancing Hsp70 interactions with OLA1 through conformational changes in Hsp70 that favor OLA1 binding.
Disruptions of the OLA1 gene recapitulated PPHN phenotypes
To test the clinical relevance of OLA1 in PPHN, we generated global ola1−/− mice. We found lower levels of SOD2 mRNA (Fig.S5a), protein (Fig.6d), and activity (Fig.S5b) in the lungs of ola1−/− compared to WT-mice. One possible explanation for decreased SOD2 mRNA levels is the instability of SOD2 transcript mediated by CHIP overactivity, given that OLA1 does not affect the SOD2 promoter activity (Fig.4e). We found CHIP overexpression (Fig.6d), and higher amounts of CHIP bound to Hsp70 in ola1−/− (Fig.S5c). Contrary to findings in PPHN-lambs,21 Hsp70 levels were lower in ola1−/− mice (Fig.6d). These findings are associated with a lower SOD2 activity (Fig.S5b), and higher mitochondrial O2¯levels (Fig.S5d). Structurally, ola1−/− provoked RV (Fig.6e) and PA remodeling, assessed by Fulton indices and αSMA labeling in ola1−/− mice (Fig.S5e).
Discussion
We report here that proteostatic downregulation of SOD2 is a potential mechanism contributing to PPHN in the ductal ligation lamb model. Our data identify decreased OLA1 expression as the basis for SOD2 downregulation in PPHN-lambs. We demonstrated that pressure overload of pulmonary circulation following DA ligation decreased OLA1 expression. OLA1 insufficiency enhanced CHIP-mediated degradation of SOD2 and Hsp70, a cytosolic molecular chaperone that promotes folding of newly-made SOD2 and transports properly folded SOD2 to mitochondria for subsequent import, prompting a rapid drop in SOD2 activity. As a consequence, ROS metabolism by the mitochondria is impaired, creating an oxidative stress milieu that induced changes in vascular cell phenotypes: PAEC apoptosis and a proliferative PASMC phenotype. The pathological findings in ola1−/− mice parallel those in PPHN-lambs and are clinically relevant since both patients and PPHN-lambs have a low OLA1 expression
Given the critical role of mitochondrial ROS in vascular formation, the activity of SOD2 must be tightly regulated for a healthy lung vessel growth, a developmental process characterized by vasculogenesis and angiogenesis, two critical steps that operate cooperatively to ensure normal blood vessel patterning and are regulated by redox signaling. 23, 36,37 Under normal intrauterine conditions, hypoxia increases ROS formation by the mitochondria that induce the activation of growth factors that regulate new vessel growth from the existing one. 38 However, developing fetuses are often challenged by extreme physiological and abnormal environmental conditions that can increase mitochondrial ROS generation and negatively impacts developing vasculature and cells (endothelial and smooth muscle cells) associated with the vasculature. The contributory role of OLA1 in regulation of the mitochondrial redox balance so far is unknown, 26 our data demonstrated that OLA1 acts as a molecular chaperone to promote folding of misfolded SOD2 proteins and prevent its aggregation during stress. Though the detailed mechanism is not fully understood, our data demonstrated that OLA1 stabilizes Hsp70-SOD2 complexes and drives the assembly to the mitochondria for subsequent import. This is supported by the following observations: 1) In cells over-expressing OLA1, the amounts of immunoprecipitable Hsp70-SOD2 complexes were higher, 2) the addition of purified OLA1 proteins to refolding buffer containing Hsp70 /Hsp40 increased reactivation of inactivated SOD2 proteins, and 3) OLA1 enhanced Hsp70-dependent mitochondrial SOD2 import. Therefore, SOD2 deficiency in PPHN-lambs is a consequence of OLA1 insufficiency (Fig.6f).
Loss of OLA1 chaperone activity induces changes in vascular cell phenotypes, patho-gnomonic of PPHN. These changes are due to SOD2 deficiency, and in this regard, CHIP plays a pivotal role. Recently, we reported that CHIP acts as the gatekeeper preventing SOD2 overactivation by targeting SOD2 for degradation.28 In the lungs of ola1−/− and PPHN-lambs, we found CHIP overexpression. Our data identify decreased OLA1 expression as the factor driving CHIP overactivity in PPHN. In addition to increased degradation of SOD2 transcripts and translated proteins, CHIP also reduced Hsp70’s ability to effectively fold translated SOD2 proteins as well as its delivery to the mitochondria for import. Thus, OLA1 insufficiency provides a convincing mechanism for proteostatic SOD2 downregulation in PPHN. However, the mechanism underlying impaired OLA1 response to stress is unknown and warrant further investigations.
In summary, we report proteostatic downregulation of SOD2 as a new mechanism of PPHN. Our data show that reduced OLA1 expression enhanced CHIP-mediated degradation of SOD2. OLA1, therefore, has emerged as both an etiologic and therapeutic targets in PPHN.
Perspectives
PPHN is a developmental disorder of pulmonary circulation affecting term and near- term infants. Many infants affected by PPHN have a variety of underlying lung diseases. However, 20% of these infants have no known identifiable cause. Findings of decreased SOD2 expression both in human and animal models identified SOD2 defect as an etiologic factor common to all PPHN. The present work shows that increased CHIP-mediated degradation of SOD2 transcript and translated protein decreases SOD2 expression and activity in PPHN-cells. We provide evidence that reduced OLA1 activity enhanced CHIP-mediated degradation of SOD2 and occurred early in the disease process. Not surprisingly, OLA1 inhibition downregulated SOD2 and recapitulated PPHN phenotypes. While the therapeutic benefit of SOD2 in PPHN is widely acknowledged, how to increase the de novo SOD2 synthesis with the necessary check-points that prevent SOD2 overactivation has remained a challenge. The observations that are increasing OLA1 expression or reducing CHIP expression restored SOD2 function suggests that proteostatic inactivation of SOD2 is reversible and that OLA1 and CHIP are potential drug targets in PPHN.
Limitations
Although the use of human lungs is a great resource, there are limitations in using human tissues; notably, we are unsure of maternal exposure to factors that can alter SOD2 levels including smoking and steroids both of which can induce SOD2 expression Further, PPHN infants received medical treatments before their demise that may have impacted SOD2 functions. However, this is unlikely to be an important confounder, as the rodent and human data are comparable. The human lung specimens available are post-mortem samples; therefore, they were not prepared for immunofluorescence.
Supplementary Material
Novelty and Significance.
What is new?
We demonstrate that OLA1 stabilizes SOD2 protein and facilitates Hsp70-dependent mitochondrial SOD2 import during stress. Decreased OLA1 expression induces proteostatic SOD2 downregulation and enhances mitochondrial oxidative stress, causally-linked to PPHN.
We demonstrate that SOD2 deficiency contributes to PPHN development through induction of mitochondrial oxidative stress that causes differential activation of inhibitors of apoptosis (XIAP and HtrA2).
What is relevant?
Perturbations of developing vasculature and cells (endothelial and smooth muscle cells) associated with the vasculature by mitochondrial ROS induce changes in vascular cell phenotypes, culminating in PPHN.
Our study demonstrates the importance of OLA1-ubiquitin-proteasomal signaling axis in the regulation of mitochondrial redox balance; thus, determining whether lung vascular growth is physiological or pathological.
Summary
Decreased OLA1 expression resulted in SOD2 inactivation that causes changes in vascular cell phenotypes, pathognomonic of PPHN. The mechanisms of SOD2 deficiency and how SOD2 deficiency induces PPHN were characterized for the first time.
Acknowledgments
We thank the scholarly committee members, Kirkwood Pritchard Jr., Ph.D., Ramani Ramchandran, Ph.D., Camara Amadou, Ph.D., and Neil Hogg, Ph.D. for their mentorship. We also thank Dr. Hill’s lab, Dept of Biochemistry at MCW for their help with circular dichroism, melting assays, and fluorescence polarization.
Sources of Funding. The authors acknowledge financial support from the NIH (K08-AJA080271), CRI and department of pediatrics at MCW (AJA), 1R01HL136597–01A1 and 1R01HL144519–01 from NHLBI and a multiyear innovation research grant from CRI (GGK).
Footnotes
Disclosure: None
References
- 1.Abman SH; Ivy DD; Ziegler JW; Kinsella JP, Mechanisms of abnormal vasoreactivity in persistent pulmonary hypertension of the newborn infant. J Perinatol 1996, 16 (2 Pt 2 Su), S18–23. [PubMed] [Google Scholar]
- 2.Storme L; Aubry E; Rakza T; Houeijeh A; Debarge V; Tourneux P; Deruelle P; Pennaforte T; French Congenital Diaphragmatic Hernia Study, G., Pathophysiology of persistent pulmonary hypertension of the newborn: impact of the perinatal environment. Arch Cardiovasc Dis 2013, 106 (3), 169–77. [DOI] [PubMed] [Google Scholar]
- 3.Lakshminrusimha S; Swartz DD; Gugino SF; Ma CX; Wynn KA; Ryan RM; Russell JA; Steinhorn RH, Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 2009, 66 (5), 539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipkin PH; Davidson D; Spivak L; Straube R; Rhines J; Chang CT, Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr 2002, 140 (3), 306–10. [DOI] [PubMed] [Google Scholar]
- 5.Berti A; Janes A; Furlan R; Macagno F, High prevalence of minor neurologic deficits in a long-term neurodevelopmental follow-up of children with severe persistent pulmonary hypertension of the newborn: a cohort study. Ital J Pediatr 2010, 36, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cua CL; Blankenship A; North AL; Hayes J; Nelin LD, Increased incidence of idiopathic persistent pulmonary hypertension in Down syndrome neonates. Pediatr Cardiol 2007, 28 (4), 250–4. [DOI] [PubMed] [Google Scholar]
- 7.Davis RO; Philips JB 3rd; Harris BA Jr.; Wilson ER; Huddleston JF, Fatal meconium aspiration syndrome occurring despite airway management considered appropriate. Am J Obstet Gynecol 1985, 151 (6), 731–6. [DOI] [PubMed] [Google Scholar]
- 8.Kabra NS; Kluckow MR; Powell J, Nitric oxide in a preterm infant with pulmonary hypoplasia. Indian J Pediatr 2004, 71 (5), 427–9. [DOI] [PubMed] [Google Scholar]
- 9.Steurer MA; Nawaytou H; Guslits E; Colglazier E; Teitel D; Fineman JR; Keller RL, Mortality in infants with bronchopulmonary dysplasia: Data from cardiac catheterization. Pediatr Pulmonol 2019, 54 (6), 804–813. [DOI] [PubMed] [Google Scholar]
- 10.Peters EA; Engle WA; Yoder MC, Pulmonary hypoplasia and persistent pulmonary hypertension: favorable clinical response to high-frequency jet ventilation. J Perinatol 1992, 12 (1), 21–4. [PubMed] [Google Scholar]
- 11.Makanga M; Maruyama H; Dewachter C; Da Costa AM; Hupkens E; de Medina G; Naeije R; Dewachter L, Prevention of pulmonary hypoplasia and pulmonary vascular remodeling by antenatal simvastatin treatment in a nitrofen-induced congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol 2015, 308 (7), L672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrow KN; Wedgwood S; Lee KJ; Czech L; Gugino SF; Lakshminrusimha S; Schumacker PT; Steinhorn RH, Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 2010, 174 (3), 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hori M; Nishida K, Oxidative stress and left ventricular remodeling after myocardial infarction. Cardiovasc Res 2009, 81 (3), 457–64. [DOI] [PubMed] [Google Scholar]
- 14.Afolayan AJ; Eis A; Teng RJ; Bakhutashvili I; Kaul S; Davis JM; Konduri GG, Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 2012, 303 (10), L870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover TR; Parker TA; Zenge JP; Markham NE; Kinsella JP; Abman SH, Intrauterine hypertension decreases lung VEGF expression, and VEGF inhibition causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 2003, 284 (3), L508–17. [DOI] [PubMed] [Google Scholar]
- 16.Konduri GG; Bakhutashvili I; Eis A; Afolayan A, Antenatal betamethasone improves postnatal transition in late preterm lambs with persistent pulmonary hypertension of the newborn. Pediatr Res 2013, 73 (5), 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng RJ; Du J; Welak S; Guan T; Eis A; Shi Y; Konduri GG, Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2012, 302 (7), L651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinhorn RH; Morin FC 3rd; Fineman JR, Models of persistent pulmonary hypertension of the newborn (PPHN) and the role of cyclic guanosine monophosphate (GMP) in pulmonary vasorelaxation. Semin Perinatol 1997, 21 (5), 393–408. [DOI] [PubMed] [Google Scholar]
- 19.Fu HY; Mukai M; Awata N; Sakata Y; Hori M; Minamino T, Protein Quality Control Dysfunction in Cardiovascular Complications Induced by Anti-Cancer Drugs. Cardiovasc Drugs Ther 2017, 31 (1), 109–117. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X; Ding S; Qiu C; Shi Y; Song L; Wang Y; Wang Y; Li J; Wang Y; Sun Y; Qin L; Chen J; Simons M; Min W; Yu L, SUMOylation Negatively Regulates Angiogenesis by Targeting Endothelial NOTCH Signaling. Circ Res 2017, 121 (6), 636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afolayan AJ; Teng RJ; Eis A; Rana U; Broniowska KA; Corbett JA; Pritchard K; Konduri GG, Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. Am J Physiol Lung Cell Mol Physiol 2014, 306 (4), L351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldosari S; Awad M; Harrington EO; Sellke FW; Abid MR, Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology. Antioxidants (Basel) 2018, 7 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harel S; Mayaki D; Sanchez V; Hussain SNA, NOX2, NOX4, and mitochondrial-derived reactive oxygen species contribute to angiopoietin-1 signaling and angiogenic responses in endothelial cells. Vascul Pharmacol 2017, 92, 22–32. [DOI] [PubMed] [Google Scholar]
- 24.Chen H; Song R; Wang G; Ding Z; Yang C; Zhang J; Zeng Z; Rubio V; Wang L; Zu N; Weiskoff AM; Minze LJ; Jeyabal PV; Mansour OC; Bai L; Merrick WC; Zheng S; Shi ZZ, OLA1 regulates protein synthesis and integrated stress response by inhibiting eIF2 ternary complex formation. Sci Rep 2015, 5, 13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Z; Liu Y; Rubio V; He J; Minze LJ; Shi ZZ, OLA1, a Translational Regulator of p21, Maintains Optimal Cell Proliferation Necessary for Developmental Progression. Mol Cell Biol 2016, 36 (20), 2568–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao RF; Rubio V; Chen H; Bai L; Mansour OC; Shi ZZ, OLA1 protects cells in heat shock by stabilizing HSP70. Cell Death Dis, 2013, 4, e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JW; Rubio V; Zheng S; Shi ZZ, Knockdown of OLA1, a regulator of the oxidative stress response, inhibits motility, and invasion of breast cancer cells. J Zhejiang Univ Sci B 2009, 10 (11), 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zemanovic S; Ivanov MV; Ivanova LV; Bhatnagar A; Michalkiewicz T; Teng RJ; Kumar S; Rathore R; Pritchard KA Jr.; Konduri GG; Afolayan AJ, Dynamic Phosphorylation of the C Terminus of Hsp70 Regulates the Mitochondrial Import of SOD2 and Redox Balance. Cell Rep 2018, 25 (9), 2605–2616 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng RJ; Du J; Xu H; Bakhutashvili I; Eis A; Shi Y; Pritchard KA Jr.; Konduri GG, Sepiapterin improves angiogenesis of pulmonary artery endothelial cells with in utero pulmonary hypertension by recoupling endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol 2011, 301 (3), L334–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimm S; Stanger BZ; Leder P, RIP and FADD: two “death domain”-containing proteins can induce apoptosis by convergent, but dissociable, pathways. Proc Natl Acad Sci U S A 1996, 93 (20), 10923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guicciardi ME; Werneburg NW; Bronk SF; Franke A; Yagita H; Thomas G; Gores GJ, Cellular inhibitor of apoptosis (cIAP)-mediated ubiquitination of phosphofurin acidic cluster sorting protein 2 (PACS-2) negatively regulates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytotoxicity. PLoS One 2014, 9 (3), e92124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin S; Kalkum M; Overholtzer M; Stoffel A; Chait BT; Levine AJ, CIAP1, and the serine protease HTRA2 are involved in a novel p53-dependent apoptosis pathway in mammals. Genes Dev 2003, 17 (3), 359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelliher MA; Grimm S; Ishida Y; Kuo F; Stanger BZ; Leder P, The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998, 8 (3), 297–303. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y; Imai Y; Nakayama H; Takahashi K; Takio K; Takahashi R, A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell 2001, 8 (3), 613–21. [DOI] [PubMed] [Google Scholar]
- 35.Muller P; Ruckova E; Halada P; Coates PJ; Hrstka R; Lane DP; Vojtesek B, C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene 2013, 32 (25), 3101–10. [DOI] [PubMed] [Google Scholar]
- 36.Mori K; Uchida T; Yoshie T; Mizote Y; Ishikawa F; Katsuyama M; Shibanuma M, A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS J 2019, 286 (3), 459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole TJ; Coffin JD, Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish an embryonic vascular pattern. J Exp Zool 1989, 251 (2), 224–31. [DOI] [PubMed] [Google Scholar]
- 38.Shafique E; Torina A; Reichert K; Colantuono B; Nur N; Zeeshan K; Ravichandran V; Liu Y; Feng J; Zeeshan K; Benjamin LE; Irani K; Harrington EO; Sellke FW; Abid MR, Mitochondrial redox plays a critical role in the paradoxical effects of NAPDH oxidase-derived ROS on coronary endothelium. Cardiovasc Res 2017, 113 (2), 234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.