Abstract
Objective:
To test the hypothesis that a functional polymorphism of the serotonin transporter gene (serotonin-transporter-linked polymorphic region [5-HTTLPR]), which is thought to be associated with differential environmental sensitivity, moderates the association between low levels of empathic accuracy (i.e., ability to recognize emotions in others) in patients with neurodegenerative disease and caregivers’ well-being.
Methods:
Participants were 54 patients with neurodegenerative disease and their caregivers. Patients’ empathic accuracy was measured using a dynamic tracking task in which they continuously rated the emotions of a character in a film; accuracy was determined by comparing patient ratings with those made by an expert panel. Caregivers provided a saliva sample for genotyping. Caregivers’ well-being was measured as a latent construct indicated by validated measures of depression, anxiety, and negative affect.
Results:
Lower levels of patients’ empathic accuracy were associated with lower levels of caregivers’ well-being. Importantly, caregivers’ 5-HTTLPR genotype moderated this association such that lower empathic accuracy in patients predicted lower well-being for caregivers with the short/short genotype (standardized β = 0.66), but not for caregivers with the short/long (standardized β = 0.66) or long/long genotypes (standardized β = 0.66).
Conclusion:
Consistent with previous findings that the short/short variant of 5-HTTLPR is associated with greater sensitivity to environmental influences, caregivers with the short/short variant manifest lower well-being when caring for a patient with low levels of empathic accuracy than caregivers with the other variants. This finding contributes to the authors’ understanding of biological factors associated with individual differences in caregiver vulnerability and resilience.
Keywords: Caregiver, depression, anxiety, empathic accuracy, 5-HTTLPR, gene-environment interaction
INTRODUCTION
Neurodegenerative diseases are incurable, debilitating conditions that result in deficits in cognitive, emotional, and motor functioning. With increasing incidence of these diseases, the number of close loved ones serving as caregivers is rising dramatically.1 Compared with non-caregiving adults and nondementia caregivers, caregivers of patients with dementia have considerably lower well-being.2,3 Not all caregivers experience similar declines in well-being. Therefore, it is important to identify the factors accounting for caregivers’ vulnerability and resilience.
Research implicates a number of factors associated with greater vulnerability to the adverse outcomes of caregiving: personality traits, such as neuroticism;4 demographic or external factors, including inadequate financial or social support;4 and patients’ behavioral and psychological symptoms, such as a lack of empathy.4 Indeed, emerging consensus from the literature suggests that patients’ behavioral and psychological symptoms are even worse for caregiver burden and health than patients’ cognitive or functional impairments.5–7 In particular, deficits in empathy have emerged as an important source of caregiver vulnerability.
Empathy is critical for close relationships. Having a more empathically accurate close relational partner is associated with lower depression,8 and patients with more empathic therapists and physicians often have better mental and physical health outcomes.9,10 Empathy is particularly important for those in high stress contexts in which social support is needed (such as caregiving). For example, a study using caregiver report of patient empathy found that lower patient empathy was related to poor relationship quality between the patient and caregiver.11 Moreover, patients with lower empathic accuracy (who are less able to track others’ changing emotions) have caregivers who experience greater burden, strain, loneliness, and depressive symptoms.6 These findings suggest patients’ inability to recognize and respond to others’ changing emotions may be particularly devastating for caregivers.
Important individual differences may exist in the ways that caregivers respond to environmental risk factors. Caregivers with genetic variations associated with heightened sensitivity to environmental influences may be more impacted by patients’ empathic accuracy deficits. A series of studies using a candidate gene approach has shown that short allele carriers of the serotonin-transporter-linked polymorphic region (5-HTTLPR) polymorphism manifest greater sensitivity to both positive and negative aspects of their environment. Examples include heightened amygdala reactivity to negative faces,12 greater empathic responding to distressing stimuli,13 and increased positive emotional expressions to amusing stimuli.14
Early studies on 5-HTTLPR demonstrated an association between the short allele and heightened risk for depression, anxiety, and suicide in the face of adversity.15 Findings from this approach were met with controversy, as replication issues arose and meta-analyses concluded that the 5-HTTLPR by environment interaction did not predict depression.16 However, more recent meta-analyses have found support for this prediction.17 These findings support hypotheses that caregivers who are 5-HTTLPR short allele carriers would be more sensitive to the negative stresses of patients’ empathic accuracy deficits, leading to worse outcomes for those caregivers.
The present study investigates whether caregivers’ 5-HTTLPR genotypemoderates the association between patients’ empathic accuracy and caregivers’ well-being. We hypothesized that caregivers with two copies of the short allele of 5-HTTLPR would be at greater risk for lowest levels of well-being when caring for a patient with lower empathic accuracy, compared with caregivers with the other genotypes. To our knowledge, this study is the first to investigate this relationship.
METHODS
Participants
Patients with neurodegenerative disease and their caregivers (N dyads = 54) were recruited from the Memory and Aging Center at the University of California, San Francisco (UCSF). Patients were evaluated at UCSF and diagnosed based on current consensus criteria.18–23 The sample included patients with frontotemporal dementia, Alzheimer disease, neurodegenerative diseases that impact motor functioning, and those at high risk for a neurodegenerative disease (e.g., mild cognitive impairment). Caregivers were predominantly spouses, and all self-identified as playing a primary role in providing care to the patient. Demographic characteristics of patients and caregivers are presented in Table 1.
TABLE 1.
Demographic Characteristics of Caregivers and Patients
| S/S (n = 14) | S/L (n = 26) | L/L (n = 14) | Testa | p | |
|---|---|---|---|---|---|
| Caregiver | |||||
| Sex | 8M; 6F | 6M; 20F | 7M; 7F | 4.98 | 0.083 |
| Age | 61.44(8.25) | 59.82 (10.40) | 62.05 (11.96) | 0.23 | 0.79 |
| Race (n) | 1.98 | 0.37 | |||
| White/European American | 10 | 23 | 12 | ||
| Non-white | 4 | 3 | 2 | ||
| Caregiver type (n) | 2.47 | 0.65 | |||
| Friend | 0 | 1 | 0 | ||
| Other relative | 1 | 1 | 2 | ||
| Spouse | 13 | 24 | 12 | ||
| Depressive symptoms (CES-D) | 14.50 (12.08) | 11.58(8.45) | 7.14(3.35) | 3.29b | 0.19 |
| Anxiety symptoms (BAI) | 8.36 (10.09) | 6.54(7.41) | 5.36(4.27) | 0.56 | 0.58 |
| Negative affect (PANAS) | 19.29(8.45) | 19.04(7.11) | 17.21 (5.10) | 0.39 | 0.68 |
| Positive affect (PANAS) | 30.37 (7.72) | 34.81 (8.60) | 33.12(6.02) | 1.48 | 0.24 |
| Patient | |||||
| Sex | 6M; 8F | 18M; 8F | 7M; 7F | 4.53 | 0.10 |
| Age | 61.89(11.58) | 62.98 (9.96) | 64.41 (8.39) | 0.22 | 0.80 |
| Race (n) | 4.02 | 0.13 | |||
| White/European American | 9 | 17 | 13 | ||
| Non-white | 5 | 9 | 1 | ||
| Diagnosis (n) | 11.80 | 0.30 | |||
| Alzheimer disease | 2 | 2 | 3 | ||
| Frontotemporal dementia (FTD) | 3 | 11 | 6 | ||
| Mild cognitive impairment | 2 | 0 | 2 | ||
| Motor neurodegenerative disease | 4 | 10 | 2 | ||
| Other neurodegenerative disease | 2 | 0 | 1 | ||
| Primary relative of FTD patient | 1 | 3 | 0 | ||
| Empathic accuracy | 0.65 (0.33) | 0.69(0.19) | 0.63(0.18) | 0.41 | 0.67 |
| Disease severity (CDR-Box) | 4.36(2.91) | 3.65 (2.85) | 2.64 (2.00) | 1.46 | 0.24 |
Notes. Mean (with standard deviation in parentheses) for participant characteristics listed by caregiver 5-HTTLPR genotype group, unless otherwise noted. BAI: Beck Anxiety Inventory; CDR-Box: Clinical Dementia Rating Scale Box Score; CES-D: Center for Epidemiologic Studies Depression Scale; F: female; FTD: frontotemporal dementia; L/L: long/long; M: male; PANAS: Positive and Negative Affect Schedule; S/L: short/long; S/S: short/short.
Reporting F-statistic from one-way analysis of variance for continuous variables and the Pearson χ2 test for categorical variables to test for significant differences across the three genotype groups.
Kruskal-Wallis one-way analysis of variance because of heteroskedasticity across genotype groups.
Procedure
Patients underwent detailed neurologic, neuropsy-chological, and neuroimaging assessments at UCSF. Within 3 months of their UCSF visit, patients and caregivers came to the Berkeley Psychophysiology Laboratory at the University of California, Berkeley. Informed consent was obtained from both patients and caregivers. All procedures were approved by the University of California, Berkeley Committee for Protection of Human Subjects. Patients completed emotional functioning tasks,24 including an objective measure of empathic accuracy.6,25 Caregivers completed questionnaires and provided a saliva sample for genetic testing.
Patient Empathic Accuracy
Apparatus and procedures
Patients sat in front of a television monitor with a rating dial located near their dominant hand. The dial consisted of a small metal box with a rotating pointer that traversed a 180° path. The path overlaid a 9-point scale anchored by the legends “very bad” on the left, “neutral” in the middle, and “very good” on the right. Patients moved the rating dial continuously to reflect the feelings of a target character in an 80-second film clip, which depicted an actress experiencing a range of positive and negative emotions. Patients demonstrated that they understood the instructions. During the task, a voltage was generated reflecting the dial position; a computer sampled the voltage every 3 milliseconds and computed the average dial position for every second.
Accuracy score calculation
Empathic accuracy was calculated using time-lagged cross-correlations between each patient’s ratings and those obtained from an expert panel of healthy individuals. To allow for differences in information processing and motoric speed that is common in neurodegenerative disease, the maximum correlation coefficient was selected for lags between −10 and +10 seconds.
Clinical Measures for Patients and Caregivers
Patient disease severity
At UCSF, the Clinical Dementia Rating Scale was completed using a semi-structured interview by clinicians,26 assessing functional performance in six domains: memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care. For each domain, a score was given ranging from 0 (none) to 3 (severe) based on a description of functioning. Scores were summed across domains to create a score (Clinical Dementia Rating Scale Box Score), ranging from 0–18. Higher scores indicated greater disease severity.
Caregiver well-being
Caregiver well-being was conceptualized as a latent variable indicated by low levels of depressive symptoms, anxiety symptoms, and negative affect.
Caregiver depression
Caregivers’ depressive symptoms for the past week were assessed using the Center for Epidemiologic Studies Depression Scale27 20-item questionnaire. Caregivers rated themselves on a 4-point scale from 0 (rarely or none of the time) to 3 (most or all of the time) for each item (e.g., “I felt lonely”). Four items were reverse scored, then all items were summed. Higher scores indicated greater levels of depressive symptoms.
Caregiver anxiety
Caregivers’ anxiety symptoms were assessed using the Beck Anxiety Inventory,28 a 21-item questionnaire. Caregivers rated themselves on a 4-point scale from 0 (not at all) to 3 (severely) for each symptom (e.g., “unable to relax”). Scores were summed. Higher scores indicated greater levels of anxiety symptoms.
Caregiver affect
Caregivers’ trait positive and negative affect were assessed using the Positive and Negative Affect Schedule,29 a 20-item questionnaire that evaluates levels of positive affect (e.g., enthusiastic, interested, determined) and negative affect (e.g., scared, afraid, upset). Caregivers rated the extent to which they experienced each of 20 emotions in the past month on a 5-point scale from 1 (very slightly or not at all) to 5 (extremely). Scores were summed, with higher scores indicating greater levels of affect. Four caregivers did not complete one item on the Positive and Negative Affect Schedule. For them, scores were imputed by taking the average of the nine completed items and multiplying that value by 10.
Caregiver 5-HTTLPR Genotyping
Caregivers were invited to participate in a DNA assessment during the laboratory session. DNA were collected and extracted from saliva using Oragene kits (DNA Genotek, Kanata, Ontario, Canada) according to manufacturer’s protocol. Anonymized DNA samples were extracted and purified by Creative Genomics (Port Jefferson Station, NY). The extracted DNA were genotyped at the University of California, Los Angeles. Amplification was performed using the AccuPrime Taq High Fidelity DNA Polymerase kit (Invitrogen, Carlsbad, California). The reaction contained a 6-FAM labeled forward primer (/56-FAM/GGCGTTGCCGCTCTGAATGC) and a reverse primer (GAGGGACTGAGCTGGACAACCA).30 The polymerase chain reactions were then sent for fragment analysis on an AB3730XL with a LIZ1200 size standard. Data quality was assessed by duplicating a sub-set of random DNA samples; genotype data reproducibility was 100%. The genotyping yielded three groups, individuals with two short alleles (S/S; n= 14), one short and one long allele (S/L; n = 26), or two long alleles (L/L; n= 14). This genotype distribution was consistent with previous studies and did not deviate from Hardy-Weinberg equilibrium: χ2(1) = 0.07; p = 0.791. The 5-HTTLPR genotype was coded using an additive coding scheme (1 = L/L, 2 = S/L, and 3 = S/S), as in previous studies.13,15
Analytic Plan
First, we conducted bivariate correlations to evaluate associations between patients’ empathic accuracy and dementia severity, and caregivers’ depression, anxiety, negative affect, and positive affect (Table 2). In addition, we evaluated internal consistency among the indicators of well-being and tested for main effects among the indicator variables as a function of caregiver genotype groups.
TABLE 2.
Zero-Order Correlations of Key Study Variables
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. Caregiver depressive symptoms (CES-D) | – | ||||
| 2. Caregiver anxiety symptoms (BAI) | 0.68d | – | |||
| 3. Caregiver negative affect (PANAS) | 0.82d | 0.76d | – | ||
| 4. Caregiver positive affect (PANAS) | −0.41c | −0.17 | −0.24a | – | |
| 5. Patient empathic accuracy | −0.31b | −0.29b | −0.26a | 0.11 | – |
| 6. Patient dementia severity (CDR-Box) | 0.59d | 0.31b | 0.40c | −0.38c | −0.41c |
Notes. BAI: Beck Anxiety Inventory; CDR-Box: Clinical Dementia Rating Scale Box Score; CES-D: Center for Epidemiologic Studies Depression Scale; PANAS: Positive and Negative Affect Schedule.
p <0.10.
p <0.05.
p <0.01.
p <0.001.
Structural equation modeling analyses proceeded in three steps. First, we used confirmatory factor analyses (CFA) to test a measurement model of caregiver wellbeing, a latent variable indicated by caregivers’ depressive symptoms, anxiety symptoms, and negative affect, using the lavaan package in R software version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Next, we created a path model with patient empathic accuracy predicting caregiver well-being and used multi-group modeling to examine whether caregivers’ 5-HTTLPR genotype moderated this association. We report standardized β and z-statistic (similar to t-statistic in linear regression) for these associations, which are measures of effect size. Finally, we probed the stability of the findings when controlling for patients’ dementia severity and caregivers’ age, sex, and race.
Nonsignificant χ2 values (ps >0.05) indicated satisfactory model fit. We also inspected the comparative fit index (CFI) and standardized root mean square of residual (SRMR), following established guidelines.31 For CFI, values greater than 0.90 indicated reasonable fit and values greater than 0.95 indicated good fit. For SRMR, values less than 0.08 indicated good fit.
To rule out the possibility that observed effects were driven by individuals at “high risk” for a neuro-degenerative disease, we repeated the structural equation modeling analyses, removing those with mild cognitive impairment diagnoses and primary relatives of patients with frontotemporal dementia (Supplementary Tables S1–S3).
RESULTS
Preliminary Analyses
The Pearson correlations revealed that lower patients’ empathic accuracy was associated with greater caregivers’ depression (r =−0.31; p = 0.021), anxiety (r =−0.29; p = 0.036), and negative affect (r =−0.26; p = 0.060). Patients’ empathic accuracy was not associated with caregivers’ positive affect (r = 0.11; p = 0.45), consistent with its exclusion from the well-being latent construct. As expected, patients’ empathic accuracy and dementia severity were also correlated (r =−0.41; p = 0.002).
Next, we examined reliability among caregivers’ depressive symptoms, anxiety symptoms, and negative affect. Reliability was high (Cronbach’s α = 0.89), supporting the possibility that the observed scores for these variables are influenced by an underlying, latent construct.
We also tested whether there were significant differences in caregivers’ levels of depression, anxiety, and negative affect across genotype groups. Using the Levene test, we found that there was inequality in the variances of caregivers’ depressive symptoms (but not the other measures) across genotype groups. The Kruskal-Wallis one-way analysis of variance (a nonparametric method that allows for heteroskedasticity) revealed no differences in caregivers’ depressive symptoms and one-way analysis of variance revealed no differences in caregivers’ anxiety symptoms and negative affect across caregivers’ genotype groups (Table 1).
Structural Equation Modeling
We used CFA to test a measurement model of caregiver well-being, indicated by caregivers’ depressive symptoms, anxiety symptoms, and negative affect. For clarity of interpretation, we reversed the directions of the factor loadings (such that greater loadings of depression, anxiety, and negative affect reflect lower well-being). In the initial CFA, the residual variance for caregivers’ negative affect was not significantly different from zero (δ = 0.079; p = 0.26). The χ2 analyses comparing a measurement model with caregivers’ negative affect residual variance fixed to zero to the initial measurement model (without negative affect residual variance fixed to zero) demonstrated that the models were not significantly different (Δχ2(1) = 1.15; p = 0.28). Therefore, we repeated the CFA, fixing the residual variance for caregivers’ negative affect to zero. The CFA for caregiver well-being indicated excellent fit: χ2(1) = 1.15; p = 0.28; CFI = 1.00; SRMR= 0.02.
Subsequent analyses showed that a structural equation model (Fig. 1) with patient empathic accuracy as a predictor of caregiver well-being had excellent fit: χ2(3) = 3.82; p = 0.28; CFI = 0.99; SRMR = 0.05. Factor loadings and residual variances are reported in Table 3.
FIGURE 1.
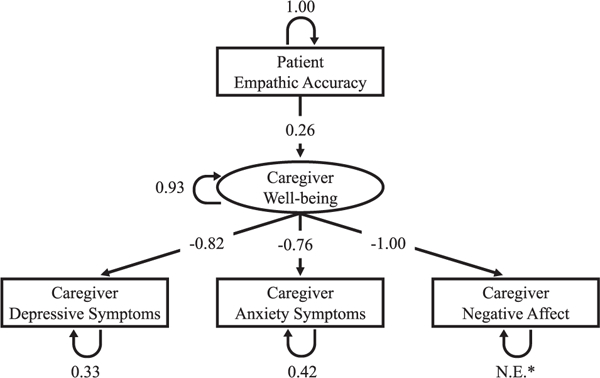
Structural equation model with patient empathic accuracy predicting caregiver well-being.
* N.E. = not estimated; fixed to 0
TABLE 3.
Caregiver Well-Being Factor Loadings and Residual Variances
| Indicator | Measurement Model | Multi-Group Model | Multi-Group Model with Covariatesa | ||||
|---|---|---|---|---|---|---|---|
| S/S | S/L | L/L | S/S | S/L | L/L | ||
| Depressive symptoms (CES-D) | −0.82 | −0.93 | −0.79 | −0.68 | −0.93 | −0.79 | −0.68 |
| (0.33) | (0.13) | (0.37) | (0.54) | (0.13) | (0.36) | (0.54) | |
| Anxiety symptoms (BAI) | −0.76 | −0.86 | −0.68 | −0.81 | −0.86 | −0.68 | −0.81 |
| (0.42) | (0.26) | (0.53) | (0.35) | (0.26) | (0.54) | (0.35) | |
| Negative affect (PANAS) | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | |
Notes. Factor loadings (with residual variances in parentheses). BAI: Beck Anxiety Inventory; CES-D: Center for Epidemiologic Studies Depression Scale; L/L: long/long; PANAS: Positive and Negative Affect Schedule; S/L: short/long; S/S: short/short.
Covariates include caregiver age, sex, race, and patient dementia severity.
Multi-Group Modeling
Next, we examined whether the inclusion of 5-HTTLPR genotype groups improved model fit using multi-group modeling (i.e., by comparing a model in which the association between patients’ empathic accuracy and caregivers’ well-being was constrained to be equal across caregiver 5-HTTLPR genotype variants to a model in which this association was not constrained using Δχ2 tests). Results showed that including caregivers’ 5-HTTLPR genotype group significantly improved model fit: Δχ2(2) = 8.76; p = 0.013. The multi-group model also indicated excellent fit, χ2(9) = 9.30; p = 0.41; CFI = 1.00; SRMR = 0.04.
Consistent with our hypothesis, 5-HTTLPR moderated the association between patient empathic accuracy and caregiver well-being. Specifically, lower patient empathic accuracy predicted lower caregiver well-being—only for those with the short/short genotype (β = 0.66; z = 2.81; p = 0.002)—but not for those with the short/long (β =0.05; z = 0.23; p = 0.82) or long/long (β = −0.21; z = −0.79; p = 0.44) genotypes. These associations are depicted in Figure 2, and the simple correlations for each genotype group show the same pattern of findings (Supplementary Table S4).
FIGURE 2.
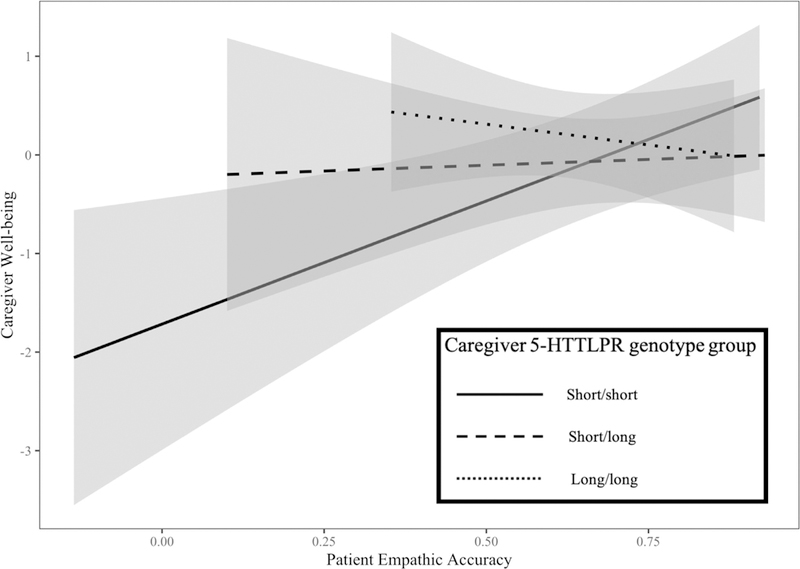
Simple slopes and 95% confidence intervals by 5-HTTLPR genotype group.
Finally, we probed the stability of the results by controlling for patients’ dementia severity and caregivers’ age, sex (coded 0 = female, 1 = male), and race (coded 0 = non-white, 1= white). Multi-group modeling revealed that including caregivers’ 5-HTTLPR genotype significantly improved model fit: Δχ2(2) = 7.96; p = 0.019. Overall, the multi-group model with the addition of these covariates indicated suboptimal fit: χ2(33) = 66.37; p = 0.001; CFI = 0.80; SRMR = 0.08. Again, patient empathic accuracy was positively associated with caregiver well-being for those with the short/short genotype (β = 0.47; z = 2.21; p = 0.018), but not for those with the short/long (β = 0.07; z = 0.52; p = 0.61) or long/long (β = −0.28; z = −1.07; p = 0.30) genotypes.
DISCUSSION
The present study sought to increase understanding of individual differences in caregiver well-being by evaluating the influence of caregivers’ 5-HTTLPR genotype on their vulnerability to patients’ empathic accuracy deficits. We measured patients’ empathic accuracy objectively through a dynamic tracking task and used this measure to predict caregivers’ well-being as indicated by measures of depression, anxiety, and negative affect. We explored differences in this relationship across caregivers’ 5-HTTLPR genotypes.
Findings revealed a significant positive relationship between patients’ empathic accuracy and caregivers’ well-being for caregivers with the short/short genotype (even after controlling for caregivers’ age, sex, and race and patients’ dementia severity), but not for caregivers with the short/long and long/long genotypes. Given the study’s relatively small sample size and the inclusion of multiple covariates in the model, independent replication will be necessary before these results can be considered conclusive. Nevertheless, these findings provide preliminary evidence that caregivers with the short/short genotype may be at heightened risk for low well-being when caring for a patient with neurodegenerative disease who has deficits in empathic accuracy. To our knowledge, this is the first study to identify a specific genetic polymorphism that may play a role in moderating the association between patient deficits and caregiver outcomes.
Our results are consistent with prior work suggesting that the short/short 5-HTTLPR genotype acts as a susceptibility factor to environmental stimuli, amplifying negative outcomes in negative contexts12 (i.e., stressful caregiving environment) and positive outcomes in positive contexts.14 Given this bidirectional sensitivity, one might expect caregivers with short/short to be particularly healthy (i.e., high well-being) when caring for a patient with high empathic accuracy. However, because neurodegenerative disease overwhelmingly produces declines in patient functioning, associated environmental effects will pre-dominately be reflected in negative outcomes.
Prior research has documented short allele carriers’ heightened sensitivity both on distal or long-term measures (e.g., psychopathology and marital satisfaction)15,32 as well as on more proximal measures (e.g., reactivity to an emotional stimulus).13,14 Extending this model further, heightened emotional reactivity may be a proximal mechanism through which the short allele contributes to distal out-comes.33 Longitudinal studies are needed to test whether caregivers’ heightened negative emotional reactivity (a proximal mechanism), perhaps in response to patients’ increasing empathic accuracy deficits, mediates the relationship between caregivers’ genotype and changes in caregivers’ well-being over time (a distal outcome).
It is also important to understand the biological basis of caregivers’ vulnerability. The short allele is associated with lower levels of serotonin uptake and lower transcriptional efficiency of the serotonin transporter protein.34 It has been argued that increased available synaptic serotonin in short allele carriers may result in increased amygdala excitability to external stimuli,12 which may result in heightened emotional reactivity. The efficiency of the serotonin system has been shown to decline with age both in terms of central serotonin transporter availability and postsynaptic serotonin receptors.35 Therefore, it is possible that the effects of serotonin-related genes such as 5-HTTLPR are magnified with age, which may shed light on how this single allele could have such a powerful effect in our sample of older adults. Although brain imaging data were not collected in this study, white matter pathology has been similarly implicated in the stress exposure-outcome pathway in caregivers.36 Future studies that evaluate multiple pathways could disentangle the relative contributions of these mechanisms to caregivers’ increased vulnerability.
Implications
Patients’ empathic accuracy deficits may render them incapable of identifying and therefore responding to others’ emotions. When patients’ empathic accuracy declines and caregivers lose a vital source of social support, caregivers with the short/short genotype might feel particularly lonely and isolated. If these findings replicate, they suggest that evaluating caregivers’ genotype in conjunction with patient deficits may facilitate identification of caregivers at greatest risk for low well-being. These caregivers may be good candidates for preventative interventions and supportive resources, such as emotion regulation skills (to mitigate heightened emotional reactivity) or referrals to caregiver support groups (to address feelings of loneliness). There are also important implications for pharmacological interventions. Short allele carriers show promising response and remission rates for depression treatment using selective serotonin reuptake inhibitors.37 Additional work is needed to clarify the specific biological pathways through which selective serotonin reuptake inhibitors may mitigate depressive symptoms for short allele carriers. In light of recent evidence showing that low levels of mental health in caregivers are associated with greater patient mortality,38 the implications of these findings are important for patients and caregivers alike.
Strengths and Limitations
Strengths of this study include the dyadic design and a specific measure of stress. Previous research on 5-HTTLPR has typically studied individual participants and assessed overall levels of stress without regard to the particular contributing stressors (e.g., stressful life event checklist).17 In addition, patients’ empathic accuracy was measured objectively and separately from the caregiver; therefore, we reduced common method variance that could inflate found associations.
There are also important limitations to note. Our sample size (N = 54 dyads) was small by the standards of candidate gene studies.39 Therefore, the results should be interpreted cautiously awaiting future replication in larger samples. A larger sample size would also have enabled us to detect possible codominant effects of the 5-HTTLPR alleles (i.e., differences between one and two long alleles). In addition, it will be important to evaluate the boundary conditions of these findings, such as whether they are generalizable across all types of caregiving relationships (e.g., professional caregivers, friends) and other types of patient deficits in emotional functioning (e.g., problems with emotion regulation).
In general, candidate gene approaches have been met with criticism.16 Our decision to focus on 5-HTTLPR as a candidate genetic vulnerability factor in caregivers was based on documented biological pathways linking 5-HTTLPR and well-being (with 5-HTTLPR encoding a direct target for antidepressant medication)37 and well-established links between 5-HTTLPR and heightened reactivity to emotional stimuli.12–14,32 Other approaches (e.g., genome-wide association studies) have clear benefits, although we note that they typically do not assess tandem repeat genetic variants, such as 5-HTTLPR.40
CONCLUSION
Caregiving can exact a heavy toll, but there is huge variability in how caregivers react to the challenges of caregiving. As the number of older adults in the population rises, there is an urgent need to identify factors that contribute to individual differences in caregiver outcomes. Evaluating the role of specific patient deficits (e.g., empathic accuracy) in the context of caregiver vulnerabilities (e.g., 5-HTTLPR polymorphism) provides valuable information regarding which caregivers are susceptible to a particular kind of environmental stressor. Future research should continue to examine the pathways through which genes interact with the social environment to alter caregivers’ quality of life. Uncovering patient and caregiver factors that undermine caregiver well-being will be critical for developing effective interventions that promote the health of caregivers and the patients in their care.
Supplementary Material
Acknowledgments
This work was supported by NIA grants 2P01AG019724-11 and 1R01AG041762-01A1 awarded to Robert W. Levenson and NIMH Affective Science Training grant 5T32MH020006-20 awarded to Jenna L. Wells.
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.jagp.2019.04.009.
Contributor Information
Jenna L. Wells, Department of Psychology, University of California, Berkeley, CA
Casey L. Brown, Department of Psychology, University of California, Berkeley, CA
Alice Y. Hua, Department of Psychology, University of California, Berkeley, CA
Peter D. Soyster, Department of Psychology, University of California, Berkeley, CA
Kuan-Hua Chen, Department of Psychology, University of California, Berkeley, CA
Deepika R. Dokuru, Department of Neurology, University of California, Los Angeles
Giovanni Coppola, Department of Neurology, University of California, Los Angeles
Claudia M. Haase, Department of Psychology, Northwestern University, Evanston, IL.
Robert W. Levenson, Department of Psychology, University of California, Berkeley, CA
References
- 1.World Health Organization: Dementia: A Public Health Priority 2012 [Google Scholar]
- 2.Joling KJ, van Hout HP, Schellevis FG, et al. : Incidence of depression and anxiety in the spouses of patients with dementia: a naturalistic cohort study of recorded morbidity with a 6-year follow-up. Am J Geriatr Psychiatry 2010; 18:146–153 [DOI] [PubMed] [Google Scholar]
- 3.Ory MG, Hoffman RR III, Yee JL, et al. : Prevalence and impact of caregiving: a detailed comparison between dementia and nonde-mentia caregivers. Gerontologist 1999; 39:177–186 [DOI] [PubMed] [Google Scholar]
- 4.Brodaty H, Donkin M: Family caregivers of people with dementia. Dialogues Clin Neurosci 2009; 11:217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ornstein K, Gaugler JE: The problem with “problem behaviors”: a systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient—caregiver dyad. Int Psychogeriatrics 2012; 24:1536–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CL, Lwi SJ, Goodkind MS, et al. : Empathic accuracy deficits in patients with neurodegenerative disease: association with caregiver depression. Am J Geriatr Psychiatry 2018; 26:484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K-H, Wells JL, Otero MC, et al. : Experience of non-target negative emotions in patients with neurodegenerative diseases is related to lower emotional well-being in caregivers. Dement Geriatr Cogn Disord 2017; 44:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon AM, Tuskeviciute R, Chen S: A multimethod investigation of depressive symptoms, perceived understanding, and relationship quality. Pers Relatsh 2013; 20:635–654 [Google Scholar]
- 9.Hojat M, Louis DZ, Markham FW, et al. : Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011; 86:359–364 [DOI] [PubMed] [Google Scholar]
- 10.Lambert MJ, Barley DE: Research summary on the therapeutic relationship and psychotherapy outcome. Psychother Theory Res Pract Train 2001; 38:357–361 [Google Scholar]
- 11.Hsieh S, Irish M, Daveson N, et al. : When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol 2013; 26:174–184 [DOI] [PubMed] [Google Scholar]
- 12.Hariri AR, Mattay VS, Tessitore A, et al. : Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297:400–403 [DOI] [PubMed] [Google Scholar]
- 13.Gyurak A, Haase CM, Sze J, et al. : The effect ofthe serotonin transporter polymorphism (5-HTTLPR) on empathic and self-conscious emotionalreactivity. Emotion 2013; 13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haase CM, Beermann U, Saslow LR: et al. : Short alleles, bigger smiles? The effect of 5-HTTLPR on positive emotional expressions. Emotion 2015; 15:438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspi A, Sugden K, Moffitt TE, et al. : Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389 [DOI] [PubMed] [Google Scholar]
- 16.Risch N, Herrell R, Lehner T, et al. : Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA 2009; 301:2462–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karg K, Burmeister M, Shedden K, et al. : The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry 2011; 68:444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong MJ, Litvan I, Lang AE, et al. : Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorno-Tempini ML, Hillis AE, Weintraub S,et al. : Classification of primary progressive aphasia and its variants. Neurology 2011; 76:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litvan I, Agid Y, Calne D, et al. : Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996; 47:1–9 [DOI] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, et al. : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 2011; 7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rascovsky K, Hodges JR, Knopman D, et al. : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijesekera LC, Leigh PN: Amyotrophic lateral sclerosis. Orphanet J Rare Dis 2009; 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levenson RW, Ascher E, Goodkind M, et al. : Laboratory testing of emotion and frontal cortex. Handb Clin Neurol 2008; 88:489–498 [DOI] [PubMed] [Google Scholar]
- 25.Goodkind MS, Sturm VE, Ascher EA, et al. : Emotion recognition in frontotemporal dementia and Alzheimer’s disease: a new film- based assessment. Emotion 2015; 15:416–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JC: The Clinical Dementia Rating (CDR): current version and scoringrules. Neurology 1993; 43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS: The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401 [Google Scholar]
- 28.Beck AT, Epstein N, Brown G: et al. : Beck Anxiety Inventory. J Consult Clin Psychol 1988; 56:893. [DOI] [PubMed] [Google Scholar]
- 29.Watson D, Clark LA, Tellegen A: Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54:1063. [DOI] [PubMed] [Google Scholar]
- 30.Assal F, Alarcon M, Solomon EC, et al. : Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol 2004; 61:1249–1253 [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Bentler PM: Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct EquModeling 1999; 6:1–55 [Google Scholar]
- 32.Haase CM, Saslow LR, Bloch L, et al. : The 5-HTTLPR polymorphism in the serotonin transporter gene moderates the association between emotional behavior and changes in marital satisfaction overtime. Emotion 2013; 13:1068–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carver CS, Johnson SL, Joormann J: Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychol Bull 2008; 134:912–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesch K-P, Bengel D, Heils A, et al. : Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531 [DOI] [PubMed] [Google Scholar]
- 35.Van Dyck CH, Malison RT, Seibyl JP, et al. : Age-related decline in central serotonin transporter availability with [123I]β-CIT SPECT. Neurobiol Aging 2000; 21:497–501 [DOI] [PubMed] [Google Scholar]
- 36.Smagula SF, Beach S, Rosso AL, et al. : Brain structural markers and caregiving characteristics as interacting correlates of caregiving strain. AmJ Geriatr Psychiatry 2017; 25:582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serretti A, Kato M, De Ronchi D, et al. : Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 2007; 12:247–257 [DOI] [PubMed] [Google Scholar]
- 38.Lwi SJ, Ford BQ, Casey JJ, et al. : Poor caregiver mental health predicts mortality of patients with neurodegenerative disease. Proc Natl Acad SciUSA 2017; 114:7319–7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long AD, Langley CH: The power of association studies to detect the contribution of candidate genetic loci to variation in complextraits. Genome Res 1999; 9:720–731 [PMC free article] [PubMed] [Google Scholar]
- 40.Landefeld CC, Hodgkinson CA, Spagnolo PA, et al. : Effects on gene expression and behavior of untagged short tandem repeats: the case of arginine vasopressin receptor 1a (AVPRla) and externalizing behaviors. Transl Psychiatry 2018; 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


