Abstract
The cholinergic system has a crucial role to play in visual function. Although cholinergic drugs have been a focus of attention as glaucoma medications for reducing eye pressure, little is known about the potential modality for neuronal survival and/or enhancement in visual impairments. Citicoline, a naturally occurring compound and FDA approved dietary supplement, is a nootropic agent that is recently demonstrated to be effective in ameliorating ischemic stroke, traumatic brain injury, Parkinson’s disease, Alzheimer’s disease, cerebrovascular diseases, memory disorders and attention-deficit/hyperactivity disorder in both humans and animal models. The mechanisms of its action appear to be multifarious including (i) preservation of cardiolipin, sphingomyelin, arachidonic acid content of phosphatidylcholine and phosphatidylethanolamine, (ii) restoration of phosphatidylcholine, (iii) stimulation of glutathione synthesis, (iv) lowering glutamate concentrations and preventing glutamate excitotoxicity, (v) rescuing mitochondrial function thereby preventing oxidative damage and onset of neuronal apoptosis, (vi) synthesis of myelin leading to improvement in neuronal membrane integrity, (vii) improving acetylcholine synthesis and thereby reducing the effects of mental stress and (viii) preventing endothelial dysfunction. Such effects have vouched for citicoline as a neuroprotective, neurorestorative and neuroregenerative agent. Retinal ganglion cells are neurons with long myelinated axons which provide a strong rationale for citicoline use in visual pathway disorders. Since glaucoma is a form of neurodegeneration involving retinal ganglion cells, citicoline may help ameliorate glaucomatous damages in multiple facets. Additionally, trans-synaptic degeneration has been identified in humans and experimental models of glaucoma suggesting the cholinergic system as a new brain target for glaucoma management and therapy.
Keywords: Acetylcholine, Citicoline, Glaucoma, Neurodegeneration, Neuroprotection, Retinal ganglion cell
1. Introduction
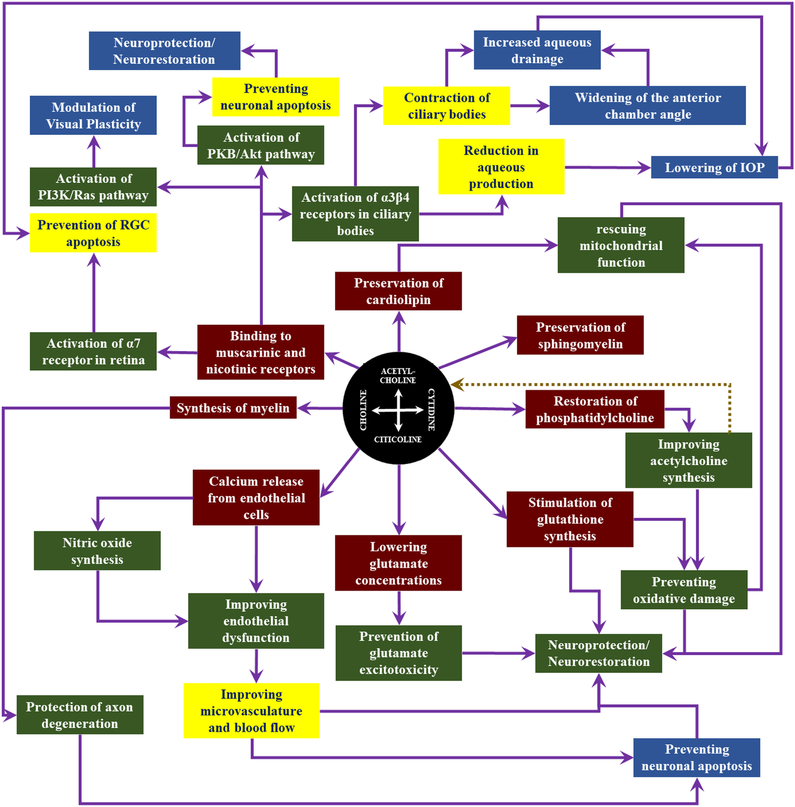
Key hypotheses of glaucoma pathogenesis include chronically elevated intraocular pressure (Bonomi et al., 1998; Chan et al., 2017b; Choi and Kook, 2015; Coleman and Kodjebacheva, 2009; Hayreh et al., 1999; Leske et al., 1997), glutamate excitotoxicity (Dreyer, 1998; Lotery, 2005; Osborne et al., 2006), oxidative stress (Dada et al., 2018; Izzotti et al., 2006; Kimura et al., 2017), failure in axonal transport (Chidlow et al., 2011; Crish et al., 2013; Fahy et al., 2016), neurotrophic factor deprivation (Ghaffariyeh et al., 2011; Harvey et al., 2012; Johnson et al., 2011), mitochondrial dysfunction (Ito and Di Polo, 2017; Kong et al., 2009; Kumar et al., 2013b; Lee et al., 2011b), autoimmune dysregulation (Bell et al., 2013) and central insulin signaling deficit (Faiq et al., 2014b; Faiq and Dada, 2017), though other mechanisms have also been indicated (Burgoyne et al., 2005; Dai et al., 2012; Faiq, 2016, 2018; Faiq et al., 2016b; Faiq et al., 2015; Faiq et al., 2014c; Fry et al., 2018; Gruntzig and Hollmann, 2019; Hasnain, 2006; Janssen et al., 2013; Morrison et al., 2011; Rieck, 2013; Sun et al., 2017; Tamm et al., 2017; Wostyn et al., 2017; Wostyn et al., 2018). Citicoline, a precursor for neurotransmitter acetylcholine and other neuronal membrane components including phosphatidylcholine and sphingomyelin, also mediates neurodegenerative events through reducing glutamate excitotoxicity (Mir et al., 2003), reducing oxidative stress (Qian et al., 2014), elevating neurotrophin level, ameliorating axonal transport deficits (Grieb et al., 2016), improving mitochondrial function (including cardiolipin synthesis) (Zazueta et al., 2018), restoring membrane integrity (Yildirim et al., 2015) and modulating insulin signaling (Krupinski et al., 2012). Since glaucoma is a neurodegenerative disease of the visual system, this puts forth an imperative justification that citicoline can be employed as a potential candidate for glaucoma prevention and treatment via protecting, rescuing/restoring or regenerating neurons (van der Merwe et al., 2016). Meticulous studies on the etiopathogenesis of glaucoma and citicoline actions are important to evaluate the mechanisms, efficacy and safety of neurotherapeutics as a treatment modality for neurodegenerative diseases of the visual system including glaucoma. Here we provide a conceptual outlook of the cholinergic system in the brain and retina followed by arguments and studies substantiating the rationale for using citicoline in glaucoma. Then we go on to discuss about the future of citicoline based treatments as neuroprotective, neurorestorative and neuroregenerative regimens in degenerative diseases afflicting the central nervous system (CNS) and its extended parts including the retina. As a conceptual navigation, Figure 1 guides through the key messages from each part of the paper.
Figure 1: Conceptual guide of the cholinergic system in vision.
This figure highlights the key messages of this paper that are important to the understanding of the cholinergic system and the therapeutic effects of cholinergic drugs including citicoline on the visual system.
2.1. Choline in the Brain
Despite being the first neurotransmitter identified, our understanding of acetylcholine (ACh) and the cholinergic networks remains relatively poor. For example, a PubMed search of the term “acetylcholine” returned 92530 entries on 16th January, 2019 whilst the term “glutamate” returned 155272 entries. A part of the reason is that the cholinergic system is complex and this intricacy increases as the level of inquiry deepens. For instance, distribution of the ACh receptors (AChRs) (Albuquerque et al., 2009; Dani, 2015) along with their tissue specific category differentiate and regulate their inter- and intra-neuron localization for multiscale (temporal and topological) neuromodulation with cumulative complexity at each level. This makes the cholinergic system capable of regulating both short-term and long-term circuit modulation in the CNS (Fagen et al., 2003; Mansvelder and McGehee, 2000; Picciotto et al., 2012). Differences in the expression of various cholinergic moieties by the CNS, the presence of several subtypes of cholinergic neurons [e.g. nicotinic/ionotropic AChRs (nAChRs) and muscarinic AChRs (mAChRs)] and their interactions with other neurons also provide a platform for neuromodulation at somatic, dendritic and synaptic levels. A depiction of the in situ cholinergic system in and around a synapse during an action potential is given in Figure 2.
Figure 2: Representative portrayal of the micro-anatomy and molecular biology of the cholinergic synapse.
This illustration gives an overview of the molecular processes, proteins, receptors and pathways of the cholinergic synapses and their locale in and outside of the neurons. The timeline of the synaptic function runs from left to right. In the presynaptic neuron, ACh is synthesized from the building blocks in the mitochondria, and is transported by the vesicles and released in the synapse. ACh signaling occurs through binding with the muscarinic and nicotinic receptors on the postsynaptic membrane. Such signaling leads to important molecular processes including neuronal plasticity, regulation of apoptosis and other cellular functions. Visual plasticity and perception are relevant to cholinergic signaling in the visual cortex, whereas RGC survival/apoptosis is imperative in glaucoma. This figure also depicts the subtle differences between central cholinergic synapse and the cholinergic synapse in the RGCs. Specifically, the ChAT in RGCs is in an alternative spliced form called pChAT. In case of insufficient production of acetylcholine in the presynaptic neuron, choline is taken back from the synapse in an autoregulatory attempt to the presynaptic membrane thereby rescuing the cellular reservoir of choline for other functions like vesicle formation and membrane component synthesis. ACh also interacts between neurons (including RGCs) and glia, which helps maintain their proper functioning and calcium uptake for the prevention and mediation of hyperactivation.
Differential expression of various subtypes of the cholinergic receptors, their expression in multiple neuronal types within a region, and the varying locations within a neuron (i.e., somatic, dendritic, synaptic etc.) orchestrate a manifold symphony of neuromodulation. A representative example of this intricacy and the differential functional geography in the brain can be found when examining and comparing the olfactory system (Bohnen et al., 2010; D’Souza and Vijayaraghavan, 2014; Hellier et al., 2010), visual system (Bouskila et al., 2016; Groleau et al., 2015; Yi et al., 2015) and hippocampus (Alger et al., 2014; Frotscher et al., 2000; McQuiston, 2014; Yi et al., 2015) in light of cholinergic signaling (Vijayaraghavan and Sharma, 2015). In the olfactory system, nAChR activation has the capability to screen signals of odor in such a way that weak inputs stand rejected while the strong signals pass through the abstract threshold, giving rise to the gain of function phenomenon in the olfactory circuit (D’Souza and Vijayaraghavan, 2014; Spindle et al., 2018) with presumable function in odor discrimination (D’Souza and Vijayaraghavan, 2014; Hellier et al., 2010; Spindle et al., 2018), whereas in the visual cortex, differential functional expression of mAChRs has a role to play in neuronal synchrony and gamma oscillations to modulate the network output during perceptual learning (Groleau et al., 2015). The hippocampus interestingly displays a different pattern where mAChRs control the release of endocannabinoids (Kano, 2014; Zhao and Tzounopoulos, 2011) thereby giving rise to intricate mechanisms involving higher-order primate function and behavioral regulation through cholinergic signaling (Alger et al., 2014; Zou and Kumar, 2018). Apart from the above, cholinergic receptors have been implicated in addictive mechanisms involving interactions of cocaine and nAChRs (Acevedo-Rodriguez et al., 2014). The behavioral changes underlying neurological and psychiatric ailments such as Alzheimer’s disease, Parkinson’s disease, schizophrenia, and autism are also thought to be the resultant phenotypes of cholinergic disturbances (Amodeo et al., 2014; Bohnen et al., 2010; Oddo and LaFerla, 2006; Wallace and Bertrand, 2013) apart from other non-cholinergic mechanisms (Kumar et al., 2017; McCoy et al., 2019; Saboory et al., 2019).
Although maps of brain ACh network have been recently constructed (Guo et al., 2015; Hoover et al., 1978; Li et al., 2018; Sugiura et al., 2012), the mechanisms of ACh mediated signaling remain largely unknown. Anatomically speaking, the cholinergic system emerges in the CNS (Kasa, 1986; McCorry, 2007) from the basal forebrain and the pendunculo-pontine nucleus (Figure 3). The basal forebrain is a collection of structures located to the front of and below the striatum including the nucleus accumbens, nucleus basalis, diagonal band of Broca, substantia innominata, and the medial septal nucleus. The pendunculo-pontine nucleus is a collection of cholinergic neurons located in the brainstem, caudal to the substantia nigra and adjacent to the superior cerebellar peduncle. The pendunculo-pontine nucleus comprises two major divisions, one containing cholinergic neurons in the pars compacta (Gorbachevskaia and Chivileva, 2005), and one containing mostly glutamatergic neurons in the pars dissipata (Fraigne et al., 2015). An important point to note is that a subset of neurons from these structures send out a sparse network of cholinergic neurons to the target sites which become difficult to study in isolation. Hence the source analysis of traditional electrophysiological approaches has not been successful in identifying the exact mechanisms through which the brain cholinergic system works.
Figure 3: The cholinergic system in the eyes and brains of humans and rodents.
This figure illustrates the cholinergic mapping of the human and mouse brains. Note that the labels for the human brain also apply to the mouse brain. Humans and rodents share several similarities in the central cholinergic system. For example, the cholinergic neurons in the visual pathway mainly originate from the basal forebrain which may play a role in glaucoma in terms of visual plasticity, visual perception and regulation of intracranial pressure. The pedunculopontine-lateral dorsal tegmental projections have also been depicted in both human and mouse brains. Apart from the cholinergic innervations within the brain, the eye is sensitive to cholinergic function. Cholinergic modulation of ocular structures can help regulate the intraocular pressure (the only modifiable risk factor in glaucoma). Cholinomimetics cause contraction of ciliary bodies and widening of anterior chamber angle leading to higher rate of aqueous clearance. The muscarinic cholinergic activation decreases the aqueous production thereby leading to lowering intraocular pressure. In addition to aqueous humor dynamics, activation of α−7 nicotinic ACh receptors in the eye induces neuroprotection of retinal ganglion cells (Linn, 2016).
Donald Hebb in his 1949 book, The Organization of Behavior, had given an important idea to decipher the working of the complex wiring of the brain. He suggested an approach to activate or deactivate one type of neurons in the brain while keeping the other types unaltered. Optogenetics (Liu and Tonegawa, 2010; Miller, 2006; Sidor et al., 2015) and presumably chemogenetics (Vlasov et al., 2018), radiogenetics (Leibiger and Berggren, 2015) and magnetogenetics (Nimpf and Keays, 2017) have now enabled such facility and have advantages over other electrophysiological approaches (Gilbert et al., 2003). By employing selective manipulation of the excitability of ACh neurons, optogenetic approaches have explicated the role of ACh in modulation of various brain structures involved in visual processing (Luchicchi et al., 2014; Pinto et al., 2013). Some of the relevant areas that need to be further deliberated include the signaling mechanisms, the trans-synaptic and asynaptic modulation of neuronal activity, the synthesis, distribution and modes of action of ACh metabolizing enzymes and the interaction and competition arena with the co-release of other neurotransmitters around the cholinergic sites.
2.2. Choline in the Visual Brain
Visual stimulation can trigger the release of ACh in the primary visual cortex (Collier and Mitchell, 1966; Laplante et al., 2005) located in and around the calcarine fissure of the occipital lobe (Figure 3). The primary visual cortex is the first stage of cortical visual processing receiving information from the lateral geniculate nucleus (LGN) in the thalamus and encompasses the whole map of the visual field covered by the eyes (Felleman and Van Essen, 1991; Maunsell and Newsome, 1987). Certain novel visual demands also lead to release of ACh in the primary visual cortex (Herrero et al., 2008). In light of these facts, the cholinergic innervation of the primary visual cortex and associated areas presents important candidature for biological and clinical investigations. The basal forebrain provides cholinergic innervations to the primary visual cortex via topographical projections (Carey and Rieck, 1987) and may play a role in visual perception, visual attention and cortical plasticity (Kang et al., 2014a). It has also been shown in rodent studies that cholinergic corticopetal projections meet their termination at the visual cortex in a medio-lateral configuration (Carey and Rieck, 1987). The horizontal limb of the diagonal band of Broca, a structure derived from the ventral telencephalon during development, also supplies cholinergic innervations to the primary visual cortex (Gaykema et al., 1990; Laplante et al., 2005). Metabotropic muscarinic receptors (mAChRs) and the ionotropic nicotinic receptors (nAChRs) are the two major classes of receptors being acted upon by ACh to bring about modulation of the visual cortex (Disney et al., 2007; Prusky et al., 1987; Thiele, 2013; Volpicelli and Levey, 2004). They can be identified within every level of the primary visual cortex including the thalamic projections (layer IV), the lateral projections and the vertical intracortical connections that relay signals to the supragranular (layer I/II/III) and infragranular (layer V/VI) regions (Burkhalter, 1989; Van Hooser, 2007). Neurons arising from the thalamus, cortex and basocortical structures, the pyramidal excitatory neurons and the inhibitory GABAergic interneurons branch out axons that display the expression of these receptors (Burkhalter, 1989; Hashimoto et al., 1994; Mrzljak et al., 1993; Thiele, 2013; Van Hooser, 2007; Zilles et al., 1989).
The microcircuitry of the primary visual cortex is immensely complex and comprehensive but physiologically a few basic circuits can be identified. Previous cortical circuit models were based on rudimentary ‘feedforward’ circuits but now recurrent cortical circuits have been proposed - an enormous theoretical leap intended to explain actual circuits with realistic representation and computational precision (Martin, 2002). Being horizontally as well as vertically organized, the microcircuitry of the primary visual cortex presents as an essential organizational model to establish the anatomical structure to account for various aspects of the visual field including binocularity (Drager and Olsen, 1980; Grieve, 2005), ocular dominance (Cynader et al., 1987; LeVay et al., 1978), orientation (Grinvald et al., 1986), and contrast (Levitt and Lund, 1997). These properties of the neurons entangled in circuits work in different combinations and permutations to give rise to intricate microcircuitry. Development of these properties in a neuron may be thought to be a consequence of continuous adaptations to the input signals that a neuron receives throughout the time course. The strength of the response of these neurons is a primary factor for the organizational characteristics of higher-order cognitive functions. In this way, the primary visual cortex is the first level of the organization of complex visual functions in terms of the integration of visual stimuli. In this context ACh becomes crucial as it determines the strength and temporality of the stimuli imminent from the retina through the LGN. ACh concentration and biochemistry are pivotal in determining and modulating the strength and specificity in response to stimuli in the visual field thereby giving rise to conscious visual perception (Kang et al., 2014b; Levitt and Lund, 1997). As the old adage goes, neurons that fire together, wire together; constant firing in synchrony leads to the formation of new circuits which makes the ACh-mediated visual cortex the center of neural circuit dynamics. This has important role to play in vision, perception, memory, learning and attention. Each stimulus augments its small share towards the fine tuning of the visual circuit. This is also the basis of the hypothesis evident in recent reports of vision restoration using electrical brain stimulation in glaucoma and other neurodegenerative diseases (Gall et al., 2016; Henrich-Noack et al., 2017b), whereby regular and synchronized electric pulses are applied to modify neuronal function by modulation of spontaneous activity and excitability (Antal et al., 2001; Antal et al., 2004b; Fedorov et al., 2011; Fritsch et al., 2017; Gall et al., 2016; Henrich-Noack et al., 2017a; Henrich-Noack et al., 2017b; Sehic et al., 2016; Simonsmeier et al., 2018; Sun et al., 2018; Yavari et al., 2017). In the visual system, application of these electrical pulses induces changes in phosphene, contrast and motion perception as well as modification of amplitude of the visual evoked potential (Gall et al., 2011; Sabel et al., 2011a; Sun et al., 2018) indicating that these stimulations can alter excitability of the visual cortex and other vision related areas in the brain, optic nerve and the retina (Antal et al., 2004a; Antal et al., 2003; Antal and Paulus, 2013; Antal et al., 2004b; Fedorov et al., 2011; Khan et al., 1992; Vosskuhl et al., 2018; Zoefel and Davis, 2017). The efficacy of these interventions for vision restoration appears dependent on the individual’s residual capacity (Sabel, 2008; Sabel et al., 2011b) and requires further studies.
2.3. Cholinergic Interneurons
In addition to the above imperative sites of cholinergic signaling, certain areas of cortex possess cholinergic interneurons (Eckenstein and Thoenen, 1983; Houser et al., 1985; Huppe-Gourgues et al., 2018; Jones, 2004; Scarr et al., 2018; von Engelhardt et al., 2007). The striatum harbors significant levels of ACh (Abudukeyoumu et al., 2018; Calabresi et al., 2000; Grady et al., 2007), nicotinic (Salminen et al., 2004; Wonnacott et al., 2000) and muscarinic receptors (Brann et al., 1988; Howe and Surmeier, 1995; Huff et al., 1994). Striatal ACh is primarily produced by cholinergic interneurons which are approximately 1–2% of all striatal cells (Lim et al., 2014). A subset of such interneurons are also suggested to be involved in Parkinson’s disease.
Interneurons give rise to neural circuits (Dehorter et al., 2017; English et al., 2011) thereby making communications between various parts of CNS possible. Interneurons display important roles in reflexes (Burrows and Siegler, 1982; Cleary et al., 1995), neuronal oscillations (Bartos et al., 2007; Buzsaki and Draguhn, 2004; Wang and Buzsaki, 1996) and neurogenesis (Li et al., 2009; Masiulis et al., 2011; Rymar et al., 2004; Song et al., 2013) in the adult mammalian brain giving rise to optimism about the exploitation of the cholinergic system in neurodegenerative diseases including those affecting vision (e.g. glaucoma). Cholinergic interneurons are not restricted to the striatum, but also identified in the hippocampus (Frotscher and Leranth, 1985; Frotscher et al., 2000; Pitler and Alger, 1992). Lack of the availability of effective probes to the interneurons had given rise to wide gaps in our knowledge. As a result, no function was previously attributed to them. Nowadays, increasing studies indicate that these interneurons are not just vestigial cellular moieties (Kepecs and Fishell, 2014) but have rather important roles to play. Studies by Yi and colleagues (Yi et al., 2015) are an important leap ahead in this direction. They examined hippocampal structures in the transgenic mice ChAT-tauGFP (Choline acetyltransferase-tau Green fluorescent protein) and ChAT-CRE/Rosa26YFP, and demonstrated that the hippocampus of ChAT-tauGFP was densely innervated with GFP-positive axons and that in ChAT-CRE/Rosa26YFP mice ChAT-YFP (Choline acetyltransferase-Yellow fluorescent protein) positive cells were more densely present in the Cornu Ammonis 3 (CA3) and dentate gyrus than the CA1 with partial overlaps with calretinin and vasoactive intestinal polypeptide. Since GFP and YFP expression was driven by the ChAT promoter, it could be concluded that these areas were rich in cholinergic interneurons. Their studies investigated the anatomical distribution, membrane properties, neurochemical characteristics, and role in cholinergic modulation of these interneurons.
Approximately 2% of the neurons in the cerebral structures including caudate, putamen, striatum, neostriatum and nucleus accumbens are cholinergic. A composite structure of the caudate (a component of the visual corticostriatal loop) (Seger, 2013) and putamen makes the neostriatum. Distinct from the other parts of CNS where cholinergic neurons generate diffuse and sparse neuronal networks spreading over relatively larger areas, the striatal cholinergic interneurons are present as dense innervations. Cholinergic interneuron system gives rise to perpetual ACh signals mediated through action potentials tonically at approximately 5Hz. Striatum contains high proportions of acetylcholinesterase thereby immediately ending the ACh signal. This phenomenon suppresses the desensitization of nicotinic AChRs (Zhou et al., 2002). Striatal nicotinic activity accelerates dopamine release which conjoins the local arbors of the cholinergic interneurons and afferent fibers of the dopaminergic system. This combination plays important roles in sensorimotor planning and learning processes (Zhou et al., 2002).
3.1. Acetylcholine Signaling and Retinal Ganglion Cells
The RGCs are one of the five neuronal cell types found in the vertebrate retina. They express NMDA as well as non-NMDA ionotropic glutamate receptors (GluRs) (Goebel et al., 1998; Hamassaki-Britto et al., 1993; Lin et al., 2002; Watanabe et al., 1994). These receptors play important roles in excitotoxic cell death thereby precipitating glaucoma. Hence there is a need to identify compounds that would break this continuum of NMDA/non-NMDA mediated excitotoxicity (Mosinger et al., 1991). Latest peer reviewed literature has identified that neuronal nAChRs modulate many processes of the CNS in addition to rapid cholinergic transmission. One key function is that alpha-7 nAChR is involved in neuroprotection precipitated by glutamate-induced excitotoxicity (Dajas-Bailador et al., 2000; Kaneko et al., 1997; Marin et al., 1994; Shimohama et al., 1996) (Figure 3). Since the retina is the extension of the diencephalon of the brain, this function is likely to be valid in the retina also. Although the neuroprotective role of ACh in the retina has not been comprehensively studied, it is known that cholinergic neurons comprise amacrine cells (Famiglietti, 1983; Masland et al., 1984) that are evenly distributed in the retina. These cells, which are alternatively described as starbursts, are arranged as one distinct group in the inner nuclear layer and the other in the RGC layer (Mariani and Hersh, 1988; Masland et al., 1984). They are also found to be sensitive to ocular hypertension even before RGC or optic nerve degeneration (Gunn et al., 2011; Moon et al., 2005; Pang et al., 2015). It has been reported that activation of nicotinic AChRs in pig RGCs leads to neuroprotective effects against glutamate-induced excitotoxicity (Wehrwein et al., 2004). A comprehensive overview of these receptors, the associated molecular signaling pathways and cellular processes in the cholinergic synapse is depicted in Figure 2. This, however, leaves an open question if the RGCs and their optic nerve axons possess the molecular machinery for synthesis and metabolism of ACh. Next section deals with this aspect in detail.
3.2. ChAT System in RGCs and Optic Nerve
The cholinergic essence of RGCs was suggested decades ago (Oswald and Freeman, 1980) but the notion of probing ACh functioning in RGCs was understudied as the concept of glutamate-mediated neurotoxicity came to forefront. Glutamate excitotoxicity initially seemed to explain most aspects of neurodegeneration but later research identified the need for additional mechanisms to explain the experimental observations. This gave rise to recent research revisiting cholinergic mechanisms. The neurotransmitter spectrum of RGCs is, in large part, unknown due to many reasons. RGCs have been verified to be immunoreactive to glutamate by many studies signifying its positive signals in the cell bodies (Crooks and Kolb, 1992; Davanger et al., 1991; Jojich and Pourcho, 1996; Kalloniatis and Fletcher, 1993; Sun and Crossland, 2000) as well as axon terminals (Beaudet et al., 1981; Ehinger, 1981; Mize and Butler, 1996; Montero, 1994; Ortega et al., 1995). These observations have strengthened the indication of glutamate excitotoxicity-mediated mechanism of glaucoma, despite the fact that the exact role of glutamate signaling in RGCs is still elusive. Positive labelling of (3H)-D-aspartate is indicative of the employment of glutamate as a neurotransmitter by a neuron (Beaudet et al., 1981; Ehinger, 1981). Since only 5–10% of all the cells in the ganglion cell layer are reported to be stained positively for [3H]-D-aspartate, it follows that majority of RGCs may be utilizing other neurotransmitters for signal transmission, among which ACh is a good candidate. Also, glutamate is not released in a calcium-dependent manner from the optic nerve terminals (Sandberg and Corazzi, 1983; Tsai et al., 1990). Dipeptide-N-acetylaspartylglutamate is thought to play a role as a neurotransmitter in RGCs but there is no conclusive evidence (Anderson et al., 1987; Tieman and Tieman, 1996; Tsai et al., 1990). It is known that some ganglion cells are able to synthesize a variety of neuropeptides that are not fully elucidated (Cuenca and Kolb, 1989; Kuljis et al., 1984). Although speculations can be drawn, more definite molecular evidence is important to determine ACh as a candidate neurotransmitter in RGCs.
To view acetylcholine as a neurotransmitter in the retina, it is important to demonstrate that the retina contains not only ACh but also the enzymes required for its biosynthesis. The lack of identification and detection of ACh in the retina in past decades can partially be attributed to the lack of reliable histochemical approaches to detect ACh. Also, using immunohistochemistry, several studies have shown that ChAT antibodies stain amacrine cells specifically but not RGCs (Eckenstein and Thoenen, 1982; Pourcho and Osman, 1986; Schmidt et al., 1985; Tumosa et al., 1984; Tumosa and Stell, 1986; Voigt, 1986). Despite the negative affirmation, there is still a possibility that RGCs may utilize a different form of ChAT to synthesize ACh. Such form had been successfully cloned from the cDNA of the rat pterygopalatine ganglion as demonstrated by Tooyama and Kimura, indicating that the presence of enzyme machinery to synthesize ACh in the ganglion cells is confirmable (Nakajima et al., 2000; Nakanishi et al., 1999; Tooyama and Kimura, 2000). This alternative form of ChAT did not contain Exon-6, Exon-7, Exon 8 or Exon-9. Hence Exon-5 and Exon-10 are joined through the alternative splicing activity. This implies that an antibody against the Exon5:Exon10 junction should be used to identify this alternative form of ChAT (Nakajima et al., 2000; Nakanishi et al., 1999; Tooyama and Kimura, 2000). It is important to note that this novel ChAT was detected in the peripheral neurons (thus termed pChAT) but not the brain in these studies. Yashuhara et al. attempted to identify this alternative form of ChAT in the RGCs using pChAT antibodies for immunohistochemistry and western blot analysis (Yasuhara et al., 2003). They also used real-time polymer chain reaction analysis to check the expression of pChAT at the mRNA level. With these techniques, the investigators were able to demonstrate the presence of pChAT in the rat retina and optic nerve. Additionally, they examined the effects of light exposure on pChAT expression and reported the presence of pChAT in the retina, optic nerve and optic tract. This indicates that the RGCs possess a viable ChAT system which may help modulate ACh synthesis and function. This is relevant because exposure to light induces the expression of Fos in retina (Koistinaho and Sagar, 1995; Sagar and Sharp, 1990). The Fos gene family comprises 4 members namely FOS, FOSB, FOSL1 and FOSL2 which code for leucine zipper proteins for dimerizing with proteins of the JUN family. The FOS group proteins can regulate cell proliferation, differentiation, and transformation as well as apoptotic cell demise. In the present context, Fos is a transcription factor for ACh synthesis (Koistinaho and Sagar, 1995). Taken together, these studies suggest that there is a Fos mediated regulation and expression of ChAT and ACh function in the RGCs. Whether this could be exploited as a mechanism to treat retinal disorders including glaucoma is a question that remains to be answered.
Apart from genetic studies in the retina, identification of genetics (Borras, 2017; Budde, 2000; Feng and Xu, 2019; Gobeil et al., 2006; Gong et al., 2004; Kanagavalli et al., 2004; Minegishi et al., 2016; Rozsa et al., 1998; Tamm, 2002; Wiggs and Pasquale, 2017) and gene/protein expression profiles (Feng and Xu, 2019; Funke et al., 2019; Gagrani et al., 2018; Hubens et al., 2019; Jakobs, 2014; Johnson et al., 2007; Oliver et al., 2019; Seet et al., 2016; Wang et al., 2017b) from the peripheral blood may serve as surrogate markers of pre-glaucoma as well as targets for therapeutic intervention. A landmark study in this direction found 28 moieties that might have a potential in treatment for primary congenital glaucoma (Faiq et al., 2016a). In addition, the genome wide association studies (GWAS) have been looking into important loci that might play a role in glaucoma pathogenesis. Interestingly, one of the recently discovered loci for primary angle closure glaucoma on chromosome 10 is involved in synthesis of ACh via ChAT (Khor et al., 2016). Other genes such as CYP1B1 have also been implicated in a significant portion of glaucoma cases and may be involved in endothelial function (Faiq et al., 2013a; Faiq et al., 2013b; Faiq et al., 2014a; Faiq et al., 2015; Faiq et al., 2014c; Rosen et al., 2015; Smith et al., 2011). However, functional studies on CYP1B1 mutations and their effects on the ACh metabolism were lacking due to difficulties in heterologous expression of unmodified human CYP1B1. This problem was, however, solved recently with a novel protocol for enhanced expression of unmodified CYP1B1 in heterologous hosts (Faiq et al., 2014a). This line of research can likely help further understand the role of CYP1B1 in ACh mediated endothelial function.
3.3. Cholinergic Innervations and Rheology
The rheology of ocular structures and the CNS is gaining attention (Carreon et al., 2017; Flammer and Orgul, 1998; Harris et al., 1999; Yamamoto and Kitazawa, 1998) with new reports claiming that the eye is not only affected by the intraocular pressure (IOP) but also intracranial pressure (ICP) (Wang et al., 2017a). This has particular relevance to glaucoma as both pressure systems meet at the optic nerve head and interact with one another at the lamina cribrosa (Johannesson et al., 2018; McMonnies, 2016). It is becoming increasingly evident that translaminar pressure difference, or the difference in the pressure components of IOP and ICP at the lamina cribrosa, may be more important than IOP and ICP taken individually (McMonnies, 2016). Hence, interventions based on modulating translaminar pressure-mediated optic nerve deformation may be pivotal to glaucoma management (Siaudvytyte et al., 2015; Siaudvytyte et al., 2014; Wostyn et al., 2016). Currently, IOP is the only clinically modifiable risk factor for glaucoma while the ICP has been ignored at large. It is essential to identify the factors that regulate ICP as well as their relations to IOP. Among these factors, the cholinergic basal forebrain has been shown to take part in controlling the ICP and the cerebrovascular volume via ACh-mediated decrease in vasoconstriction (Maeda and Miyazaki, 1998) (Figure 3). Within the cortex, cerebrovasomotor reactions and ICP modulation can also be brought about by ACh release via activation of the cholinergic fibers in the nucleus basilis of Meynert (Sato et al., 2001). Although cerebrovascular factors have been implicated in rapid disease progression especially in normal tension glaucoma (Chen et al., 2016; Gungor et al., 2011; Lee et al., 2017), how these physiological factors are regulated in normal conditions and altered in glaucoma remain unclear and require further investigations. It is pertinent to mention that cholinergic medication as neurotherapeutics of choice has not been recognized by researchers. The main reason for this seems to be the lack of carefully drafted studies and the limited number of randomized clinical trials conducted. The following section adds an anecdote on cholinergic medication in visual disorders with glaucoma as a representative disease.
3.4. Cholinergic Medication in Glaucoma
The American Academy of Ophthalmology guidelines for diagnosis and treatment do not specify any preferred ophthalmic medication for primary open angle glaucoma. A part of the reason may be that the treatment of glaucoma is often tailored as per the individual’s conditions, compliance and response to therapy, and may change from time-to-time during the course of the disease. Nowadays, the most commonly used glaucoma medications include prostaglandins and β-adrenergic blockers due to their high tolerance index and availability of generic formulations. Cholinergic agonists can also lower IOP by pupil constriction, or miosis, which decreases resistance to the aqueous humor outflow. These miotic ophthalmic drugs can act on the iris sphincter and ciliary muscles directly (e.g. ACh, pilocarpine, and carbacol) or indirectly (e.g. echothiophate) via the parasympathetic nervous system (Cekic and Batman, 1999; Laranjeira and Buzard, 1996; Shaikh and Mars, 2001; Solomon et al., 1998; Wutthiphan et al., 2000) and may cause cytoskeletal changes in the trabecular meshwork (Yamagishi-Kimura et al., 2018). However, parasympathomimetic medications are presently considered as the third-line treatment for glaucoma (Lee and Higginbotham, 2005), partly because of the reported side effects from ophthalmic pilocarpine use including irritation and surgical difficulties from miosis and headaches. This inevitably slows down the studies of the systemic effects of cholinergic drugs. In addition to acting through the aqueous-outflow pathway, pilocarpine has been shown to ensue protection against glutamate-induced apoptosis of the neurons via activation of muscarinic ACh receptor M1 (Tan et al., 2014; Zhou et al., 2008). Targeting NLRP3 inflammasome by activation of α7 nicotinic ACh receptor or by scutellarin that enhances ACh levels can also offer the antioxidant and antiapoptotic properties in experimental glaucoma and other neurodegenerative disorders (Hu et al., 2018; Zhu et al., 2018). Due to limited randomized controlled trials on the systemic use of cholinergic agonists, it is premature to draw any conclusions about their efficacy when it comes to their use in glaucoma. Among the available cholinergic drugs, it is important to note that citicoline has physiologically useful bioavailability through many routes of administration, while reports on the use of citicoline have shown improvement in visual evoked potential, pattern electroretinogram and visual field function. This indicates that citicoline might work through neuroprotective, neurorestorative and neuroregenerative paradigms, though its effect on IOP cannot be ignored as it also has cholinergic components in structure and activities akin to cholinergic function. There has been no clinical trial on the effect of citicoline on IOP as a primary outcome. In the following sections we discuss the biochemical, physiological and clinical effects of citicoline followed by its role in ameliorating vision loss in general and glaucoma in particular.
4.1. Citicoline: A Physiological Choline Representative
Citicoline, also known by other names as CDP-choline, CDPCho and cytidine-5’-diphosphocholine, is a nootropic agent, a central stimulant and is a member of the drug class oral nutritional supplements (Colucci et al., 2012; Secades, 2011, 2016; Secades and Lorenzo, 2006). It has a molecular weight of 488 g/mol and is chemically recognized as cytidine 5’-(trihydrogen diphosphate), mono[2-(trimetylammonio)ethyl] ester hydroxide inner salt with chemical formula C14-H26-N4-O11-P2. The use of citicoline arose in the early 1970s with a view that it might be a substance for treating drug abuse (Wignall and Brown, 2014). Then the first medical use of citicoline came from reports about its beneficial effects in Parkinson’s disease (Obara, 1974). Citicoline has important roles to play in the biosynthesis of phospholipids and their precursors such as phosphatidylcholine (Grieb, 2014; Zweifler, 2002). Owing to the high turnover rate, cell membranes require an uninterrupted supply of phospholipids for proper maintenance, and citicoline metabolism is a rate limiting step in this process (Jackowski, 1994). Also, citicoline is a nucleotide (Zweifler, 2002) with structural similarities with the building blocks of nucleic acids. For example, citicoline is a monomer with three distinct structures including ribose, cytosine and choline (Figure 4). Ribose is a pentose monosaccharide found in RNA while its 2-deoxy form is found in DNA also. Cytosine is one of the four main bases found in DNA and RNA. Choline is a water soluble vitamin-like essential nutrient which is a basic constituent of lecithin. Ribose and cytose combine to form the nucleoside cytidine, and choline is attached to the cytidine by means of a pyrophosphate bridge. When citicoline enters the body through oral or parenteral route, a quick metabolic process in the order of minutes follows (Grieb, 2014). The immediate catabolism of citicoline leads to the formation of pyramidine and choline derivatives, both of which are important bioactive substances and can be naturally present (Agut et al., 1983; Andersen et al., 1999; Grieb, 2014; Marti Masso and Urtasun, 1991). Thus citicoline is thought to be relatively benign and free of side effects, and is a safe moiety for potential clinical use, though few reports of mild digestive intolerance have been published. The therapeutic dosage of citicoline in humans is 500–2000 mg/day which amounts to 7 to 29 mg/kg body weight/day (Grieb, 2014).
Figure 4: Chemical structure of citicoline.
The chemical name of citicoline is 5’-O-[hydroxy({hydroxy[2-(trimethylammonio)ethoxy]phosphoryl}Moxy)phosphoryl]cytidine. It contains two major structural components, choline and cytidine. Choline is bound to the ribose ring through a pyrophosphate bond. This chemical bridge between ribose and choline gives citicoline the chemical property to be easily broken down and readily resynthesized given the favorable conditions or the presence of relevant enzymes. This property is important to the delivery of citicoline to the CNS, as citicoline cannot cross the blood-brain barrier while choline and cytidine can, hence citicoline has to be hydrolyzed to cytidine and choline in the liver and resynthesized in the brain via the pyrophosphate bridge. Ribose and cytosine make citicoline a component important for RNA biology, though the exact role of which has not yet been deciphered. It is speculated that nucleic acid synthesis may be one of the roles of citicoline in the light of its chemical composition.
4.2. Citicoline Metabolism
A summary of the metabolism of citicoline is illustrated in Figure 5. In brief, phosphatidylcholine is synthesized in a three-step enzymatic process from choline and cytidine (DeLong et al., 1999; Moessinger et al., 2014). In the first step, choline originates from the phosphatidylcholine metabolism and is phosphorylated by cytidine kinase utilizing one molecule of ATP into choline-phosphate. Choline phosphate is then converted to citicoline by combining with cytidine phosphate that is derived from cytidine. The enzyme that catalyzes this reaction is called choline phosphate cytidilyltransferase. Citicoline on the other hand leads to the formation of phosphaptidylcholine by an enzymatic process mediated through CDP-choline:1,2-diacylglicerol choline phosphotransferase. In the penultimate step of this metabolic pathway, citicoline is synthesized which then serves as the requisite element for phosphatidylcholine synthesis, thereby providing justification for the role of citicoline in membrane function and integrity. This pathway is vital to neuronal tissues to maintain the electrochemical gradient for proper action potential generation. The profound similarities in ACh synthesis and localization in and outside the cell in many species indicate that the ACh mechanism is relatively conserved in evolution, upholding the rationale that rodents and zebrafish are appropriate models to investigate this system and the diseases thereof.
Figure 5: Citicoline synthesis and metabolism.
Citicoline and choline are closely related metabolically and are involved in the synthesis of a variety of active biochemical moieties that have widespread roles to play in membrane biology, neurotransmission, apoptosis and bioenergetics in the visual system. When being acted upon by the enzyme CDP-choline 1,2,-diacylglycerol cholinephosohotransferase, citicoline leads to the formation of phosphatidylcholine, which is an important component of neuronal membranes and is imperative to the membrane integrity of the retinal ganglion cells. Phosphatidylcholine can be converted to sphingomyelin and subsequently to myelin, a major white matter component in the brain. Phosphatidylcholine can also be converted to choline, which forms betaine upon catalytic reaction by choline oxidase, and subsequently to serine, which modulates the non-NMDA ionotropic glutamate receptors expressed by inner retinal neurons. By a variety of enzymes including choline acetyltransferase, choline is converted into ACh, which acts as a neurotransmitter and modulates aqueous humor production through parasympathetic activity. ACh can act as a substrate for the synthesis of choline, a process mediated by acetylcholinesterate.
A schematic outlook of the bioavailability and breakdown of citicoline through various routes of administration, different compartments of the body and different metabolic routes is given in Figure 6. Citicoline can be administered by multiple routes but oral administration remains to be the most common because of several reasons. Citicoline is well tolerated orally and does not show adverse effects at the effective dosages. It also has remarkable bioavailability with negligible loss to metabolic processes. Oral and intramuscular administration of citicoline do not show apparent difference in the metabolism and bioavailability (Adhi and Duker, 2013; Clark and Clark, 2012; Fresta et al., 1994). After administration, citicoline is immediately metabolized by the liver into cytidine and choline in the circulation (Galletti et al., 1991; Galletti et al., 1985), and after 30 minutes the resultant metabolites can be observed in liver, kidneys, and brain in rodents (Galletti et al., 1991; Martynov and Gusev, 2015). Cytidine is transformed into uridine which converts to uridine phosphate in the CNS. At the cellular level in the brain, this moiety is then converted into cytidine triphosphate. All the three major routes (urinary, fecal and respiratory) are used for excretion of citicoline (Dinsdale et al., 1983). Since the brain has been indicated a major target for vision loss (Faiq, 2016, 2018; Faiq et al., 2016b), in the following section we explore citicoline with respect to its availability and effects in the brain.
Figure 6: Citicoline bioavailability and pharmacokinetics in different body compartments.
This schematic diagram outlines how citicoline behaves as an exogenous agent (a drug or a supplement) in the mammalian biological system and how this external agent enters the brain. After being administered via oral, intramuscular, ocular, intraperitoneal or intravenous route, citicoline enters the organ of first pass, followed by the systemic circulation and the liver. Since citicoline cannot cross the blood-brain barrier, it needs to be hydrolyzed into choline and cytidine in the liver, which readily cross the blood-brain barrier. Once choline and cytidine enter the brain via the systemic circulation, they recombine to form citicoline which can be used up for various cholinergic functions including neurotransmission, myelin regulation, neuronal membrane rescue and regeneration.
4.3. Citicoline in the Brain
As explained in section 4.1 cytidine and choline are two major constituents of citicoline which are bound by a pyrophosphate bridge. This bridge is broken down during hepatic metabolism and the same bridge can be synthesized again by rephosphorylation leading to the reformation of citicoline. Such breakdown and reformation processes are particularly important to citicoline supply to the brain because citicoline does not cross the blood-brain barrier whereas the circulating cytidine and choline broken down from citicoline can (Grieb, 2014). Upon entering the brain, citicoline can give rise to phosphatidylcholine, ACh, sphingomyelin and cardiolipin (Adibhatla and Hatcher, 2002; Adibhatla et al., 2001, 2002), which play roles in neuronal membrane function, neurotransmission, axonal integrity, myelin homeostasis and inner mitochondrial membrane viability among others (Araki and Wurtman, 1997; Blusztajn et al., 1987; Galvan et al., 2005; Harel and Futerman, 1993; Kirkland et al., 2002; Posse de Chaves and Sipione, 2010; Schwarz et al., 1995). Phosphatidylcholine synthesis has an additional advantage to the formation of cytidine 5’–monophosphate which helps the synthesis of nucleic acids (DNA and RNA). The synthesis of cytidine 5’–monophosphate takes place when choline monophosphate binds with phosphatidylcholine. ACh is formed when choline from citicoline is acetylated.
Cholinergic neurons utilize choline in a dual manner namely the synthesis of the membrane structure phosphatidylcholine and biosynthesis of the neurotransmitter ACh. This signifies the usefulness of citicoline at both structural and functional levels. These two pathways simultaneously compete for choline for binding to cytidine monophosphate and for acetylation respectively (Farber et al., 1996; Ulus et al., 2006). Since brain function is an immediate requirement in most cases, acetylation is often the dominant pathway (Iulia et al., 2017). Thus, when choline supply is restricted or choline is depleted, phosphatidylcholine and other phospholipids are often broken down by hydrolyzation to salvage the shortage of choline levels. In other words, this network suggests that citicoline works via two vital mechanisms by first, serving as a source of choline to produce ACh and second, serving as a rescue recourse for breakdown of phosphatidylcholine and other membrane components. This way citicoline may avoid membrane breakdown in the neurons and may prevent apoptosis during neurodegenerative processes thus ensuring the functional viability of the neurons in question. Such mechanisms also identify the neuroprotective properties of citicoline.
4.4. Why Citicoline in Neurodegeneration?
The neurotherapeutic effects of citicoline appear to be multifarious. Regarding the structure, composition and functional integrity, citicoline serves as a precursor for phosphatidylcholine, phosphatidylethanolamine and sphingomyelin, which are important structural and functional components of cell membranes (Marcucci et al., 2010; Skripuletz et al., 2015). They ensure proper enzymatic viability for the transport of substances across the membrane (Lagace, 2015; van Meer et al., 2008). In addition, they are indispensable in signal transduction (Exton, 1990, 1994) thereby governing numerous cellular processes and maintaining cellular communication with its environment. Most of the neurodegenerative diseases have their etiology mediated through neuronal membrane integrity (Chitnis and Weiner, 2017; de Groot and Burgas, 2015; Sonnino et al., 2014) which, in turn, is linked to these phospholipids. It is important to mention that membrane integrity is also a potential factor of axonal degeneration in glaucoma (Almasieh et al., 2017; Buys et al., 2014; Howell et al., 2013; Petty, 2018) be it the RGC membrane (Osborne et al., 1999; Risner et al., 2018) or the mitochondrial membrane (Munemasa et al., 2010; Osborne et al., 1999; Tatton et al., 2001). To this effect, cholinergic signaling in glaucoma becomes a potent candidate for therapeutic moieties addressing issues in membrane integrity.
On the other hand, the brain is generally devoid of resident endogenous antioxidant mechanisms in order to maintain proper electrophyisiological function (Deisseroth and Dounce, 1970; Kang et al., 1996; Shingu et al., 1985). Therefore, for neurodegenerative diseases involving oxidative stress, there is a need for antioxidants that penetrate the blood-brain barrier (Gilgun-Sherki et al., 2001). Glutathione is a metabolic product of choline that can bring down lipid peroxidation in the CNS. Since glutathione can also come from citicoline, it seems reasonable to view citicoline as a potent therapeutic substance to treat various oxidative stress-induced neurological diseases including, but not limited to, Alzheimer’s disease (Gareri et al., 2017), Parkinson’s disease (Kashkin et al., 2017), glaucoma (Iulia et al., 2017), and ischemic neuropathies (Parisi et al., 2008a; Parisi et al., 2008b).
Citicoline has been reported to inhibit β-amyloid deposition, which makes it a therapeutic candidate for amyloidopathies like Alzheimer’s disease and glaucoma (Cacabelos et al., 1996; Yan et al., 2017). Beta amyloid deposits elicit inflammation (Gorevic, 2013; Ruan et al., 2009) and lead to disintegration of membrane phospholipids (Lau et al., 2006; McLaurin and Chakrabartty, 1996). In two classical studies, the brain electrical activity and cognitive profiles of Alzheimer’s disease patients were reported to be improved after citicoline treatment as compared to controls (Alvarez et al., 1999; Franco-Maside et al., 1994). In particular, patients with mild dementia presented more profound improvements. This indicates that citicoline treatment may be more effective in early cases as compared to late presentation where larger amount of damage has already occurred. Some initial studies on stroke models also reported the protective effects of citicoline as a single or combined therapy with some efficacy in reducing the infarct size and consequent improvement in neurological deficit (Cacabelos et al., 1996). This effect was more profound in clinical trials particularly if citicoline was administered immediately after injury. Later studies, however, show conflicting results and the reproducibility remains to be confirmed (Cheng et al., 2004; Clark and Clark, 2012).
In addition to the above, ACh can be synthesized from citicoline and has an important role to play in the dopaminergic and GABAergic system (Secades, 2011). Citicoline has been suggested to ameliorate neurobehavioral changes in humans and experimental models of Parkinson’s disease via the dopaminergic pathway (Agnoli et al., 1982; Saligaut et al., 1987). Choline and ACh from citicoline can also mediate endothelial viability, nitric oxide production, tissue perfusion and mitochondrial integrity via upregulation of intracellular calcium concentrations and releases in the endothelial cells (Li and Wang, 2006; Zhang et al., 2017), and thereby preventing hypoxia-induced endothelial cell damage (Alkon and Rasmussen, 1988; Asaoka et al., 1992; Rasmussen et al., 1995; Tran et al., 2000; Zhang et al., 2017). Taken together, these results indicate a multipathway mechanism of citicoline action in amelioration of neurodegenerative conditions, which may include glaucoma and other vision-related diseases. Hence, we describe this aspect in the following section.
5.1. Citicoline and Vision
Citicoline is effective in stimulating the dopaminergic system in the visual pathways including the retinal and post-retinal structures (Iulia et al., 2017; Rejdak et al., 2002). By doing so, citicoline improves the visual acuity, visual evoked responses, contrast sensitivity and outcomes of patching treatment in amblyopia (Campos et al., 1996; Fresina et al., 2008; Pawar et al., 2014; Porciatti et al., 1998). In another clinical trial involving patients with non-arteritic ischemic optic neuropathy, 60 days of oral citicoline treatment also showed beneficial effects on the visual acuity, visual evoked potential and pattern electroretinogram (Parisi et al., 2008a; Parisi et al., 2008b). These investigators reported persistent improvements even after the washout period, suggestive of the neuroprotective or the long-lasting neurorestorative effects of citicoline on visual function.
5.2. Citicoline and Retinal Ganglion Cells
The molecular, cellular and physiological interphases between citicoline and RGCs appear tightly linked. Citicoline is involved in the proper maintenance of sphingomyelin and cardiolipin levels (Adibhatla and Hatcher, 2002; Adibhatla et al., 2001, 2002; Gareri et al., 2017; Gareri et al., 2015), whereas the RGCs are rich in myelin in their axons and are the primary site of glaucomatous injury (FitzGibbon and Nestorovski, 2013; Giacci et al., 2018; Yalcin et al., 2013). While sphingomyelin is a sphingolipid in the myelin sheath that surrounds the nerve cell axons, cardiolipin accounts for 20% of the total lipid composition in the inner mitochondrial membrane (Paradies et al., 2014) and is involved in maintaining optimal enzymatic activity in energy metabolism. Since neurons are energetically the most expensive cells of the body (Munzberg et al., 2016; Niven, 2016; Qadri et al., 2018), any hindrance in the maintenance of cardiolipin and sphingomyelin is likely to affect the neurons, especially those with long myelinated axons. RGCs are, for this reason, favorable candidates for such degenerative and proapoptotic insults. If sphingomyelin and cardiolipin damages are involved in glaucoma directly or indirectly, citicoline administration may become one of the potential treatments for the prevention of cellular death in glaucoma. With this premise, we will describe the various aspects of possible citicoline use in glaucoma in the next section.
5.3. Citicoline and Glaucoma
Citicoline appears to possess the potentials for ameliorating glaucomatous damages or vision loss in a number of in vitro and in vivo studies of retinal cell cultures, experimental animal models and clinical trials (Table 1). Using mouse retinal explants, the number of regenerating neurites was found to be higher in damaged RGCs that were treated with citicoline as compared to the control retina (Oshitari et al., 2002). On the other hand, glutamate excitotoxicity has been postulated to be a major factor of glaucoma onset and progression (Dreyer, 1998; Osborne et al., 1999; Salt and Cordeiro, 2006). Interestingly, citicoline was shown to counteract neuronal cell damage in glutamate-treated rat primary retinal cultures via decreasing proapoptotic effects and contrasting synapse loss (Matteucci et al., 2014). Kainic acid is a potent neuroexcitatory amino acid agonist that mediates its neurotoxic effects through activating glutamate receptors. In a rat model of kainic acid-induced retinal damage, animals receiving prolonged citicoline treatment appeared to show less profound retinal thinning and less attenuated immunoreactivities of ChAT as compared to the control (Park et al., 2005). Using experimental glaucoma models, adult rats with optic nerve crush presented higher RGC density after intraperitoneal citicoline treatment than vehicle treatment (Schuettauf et al., 2006). While citicoline did not appear to alter IOP in experimental glaucoma (van der Merwe et al., 2016), the above experimental studies provide direct evidence of the protective effects of citicoline on RGCs and an inference of the specific role of citicoline in alleviating glaucomatous damages. The biochemical mechanisms underlying such effects appear similar to citicoline actions on other neurodegenerative diseases (Faiq, 2016, 2018; Faiq et al., 2016b; Faiq et al., 2014b; Faiq and Dada, 2017).
Table 1:
Summary of the use of citicoline as a therapeutic agent in various experimental models of glaucoma and clinical trials. The details of this table bolster the working hypothesis that citicoline has neuroprotective, neurorestorative and neuroregenerative effects on the visual system.
| Model/Organi sm/Indication | Study design | Sample size | Route of administration | Dosage | Outcome measures | Techniques used | Reference | Results | Implications |
|---|---|---|---|---|---|---|---|---|---|
| RETNAL CELL CULTURES | |||||||||
| Tissue culture of mouse retinal explants | Case-control study | --- | Media supplementation in retinal cultures | 0.1–10 mmol/l citicoline | Regenerating neurite density | TUNEL assay and the assessment of regenerating neurites | (Oshitari et al., 2002) | The number of regenerating neurites was higher in the citicoline treated cultures as compared to the control retinal cultures | Citicoline protects damaged RGCs in retinal tissue cultures |
| Cultures from embryonic rat retina | Case-control study | --- | Media supplementation | 10, 100 and 1000 μM citicoline for 96 hours | Effects of citicoline on cell survival in primary retinal cultures and neuroprotective activity in conditions akin to retinal neurodegeneration | Apoptotic analysis, immunocytochemistry, morphometric analysis, electrophoresis, western blot | (Matteucci et al., 2014) | At the concentration of 100 μM, citicoline blocked neuronal cell damage both in glutamate- and high glucose-treated retinal cultures | Citicoline has potent neuroprotective activity |
| EXPERIMENTAL ANIMAL MODELS | |||||||||
| Adult male Sprague– Dawley rats |
Case-control study | n=15 and 5 for experimental and control groups | Intraperitoneal | 500 mg/kg/twice daily for 1, 3 and 7 days following intraocular kainic acid injection | Neuroprotective effect of citicoline on kainic acid-induced retinal damage | Retinal thickness and immunoreactivities of ChAT and tyrosine hydroxylase | (Park et al., 2005) | Citicoline significantly attenuated the reduction in retinal thickness and immunoreactivities of ChAT and Tyrosine hydroxylase. | Citicoline shows neuroprotective effects on retinal damage due to kainic acid-induced neurotoxicity. |
| Adult female Brown Norway rats | Case-control study | n=24 and 15 for experimental and control groups | Intraperitoneal | 1 g/kg daily for up to 7 days and 300 mg/kg daily afterwards | Effect of citicoline on glaucomatous degeneration of RGC as measure through RGC density and immunoreactivity to BCL2 | Fluorescence microscopy to count the labelled RGCs and immunohistochemistry for BCL2 expression | (Schuettauf et al., 2006) | Citicoline was associated with higher RGC density and expression of BCL2 in optic nerve crush. | Citicoline protects RGCs and their axons in vivo against optic nerve crush mediated degeneration and the effect may be mediated through BCL2 expression |
| CLINICAL TRIALS | |||||||||
| Open angle glaucoma | Cohort study | 30 patients (47 eyes): 17 males and 13 females | Intramuscular | 1 g/day for 10 consecutive days | Behavior of scotomatous area | Central perimetry, automated perimetry, applanation tonometry | (Pecori Giraldi et al., 1989) | Significant improvement in perimetry in 75 percent of the eyes with stability of the effects for at least 3 months. Same results were obtained when the treatment was repeated after 4 months | Citicoline has the potential to complement the conventional ocular hypotensive therapy |
| Open angle glaucoma | Double-blinded placebocontrolled trial | 40 patients (n=25 and 15 for intervention and control groups) | Intramuscular | 1000 mg/day for 60 days | VEP (P100 latency and N75-P100 amplitude), PERG (P50 latency and P50-N95 amplitude), and IOP | VEP, PERG and tonometry | (Parisi et al., 1999) | Citicoline treatment was associated with significant improvements of VEP and PERG parameters | Citicoline induces improvement of the retinal and visual pathway function in glaucoma patients |
| Open angle glaucoma | Case-control study | 23 participants (n=11 and 12 for interventional and control groups) | Intramuscular | 1000 mg/day for 15 days | Visual field parameters | Perimetry | (Virno et al., 2000) | A stable visual field improvement was observed in glaucoma patients and this improvement persisted for 9 years | Citicoline leads to sustained improvement in visual fields in glaucoma patients |
| Open angle glaucoma | Cohort study | 21 glaucomatous eyes | Oral | 1000 mg/day for 15 days | VEP latency and VEP amplitude | Pattern-reversal visual evoked potentials | (Rejdak et al., 2003) | VEP latency reduced and VEP amplitude increased significantly with citicoline treatment | Oral administration of citicoline improves visual evoked potentials in glaucoma patients |
| Open angle glaucoma | Double-blinded placebocontrolled trial | 30 patients (n= 15 and 15 for citicoline and control groups) | Intramuscular | 1000 mg/day for 60 days. | VEP and PERG parameters | VEP and PERG | (Parisi, 2005) | Citicoline significantly improves retinal and cortical bioelectrical responses in glaucoma patients | Citicoline can be used in glaucoma treatment as a complement to ocular hypotensive therapy |
| Open angle glaucoma | Case-control study | 60 patients (70 eyes) | Oral/Intramuscular | 1000–1600 mg/day for 60 days | VEP and PERG parameters | VEP and PERG | (Parisi et al., 2008a; Parisi et al., 2008b) | Improvement of retinal function and neural conduction along visual pathways | Citicoline has neuroprotective effects in glaucoma |
| Open angle glaucoma | Cohort study in retrospect | 41 patients | Oral | 500 mg/day for 2 years | Visual field parameters | Perimetry | (Ottobelli et al., 2013) | The rate of visual field progression significantly changed with citicoline treatment | Citicoline supplementation may be useful in slowing down glaucoma progression |
| Open angle glaucoma | Double-blinded placebocontrolled trial | 34 patients (n= 16 and 18 for citicoline and control groups) | Intraocular (Topical eye drops) | 3 drops/day for two months | VEP and PERG parameters | VEP and PERG | (Roberti et al., 2014) | Improvement in RGC function shown by reduced P50 latency and increased P50-N95 amplitude of pattern electroretinogram | Topical citicoline has neuroprotective effect independent of hypotensive action of ocular hypotensive eye drops |
| Open angle glaucoma | Case-control study | 56 patients (n= 28 and 28 for intervention and control groups) | Intraocular (Topical eye drops) | 3 drops/day for four months | VEP and PERG parameters | VEP and PERG | (Parisi et al., 2015) | Citicoline treatment was associated with shortening of VEP P100 implicit time, which was correlated significantly with the increase of PERG P50-N95 amplitude | Topical citicoline treatment in glaucoma induces enhancement of retinal bioelectrical responses with improvement in bioelectrical activity of the visual cortex |
With regard to the metabolome across the spectrum of neurodegeneration, citicoline may crosstalk with glucose metabolism and may protect neurons from hyperglycemic conditions (Gao et al., 2017; Matteucci et al., 2014) thereby lowering the risk of neurodegeneration in hyperglycemia and diabetes. While initial evidence also suggests that IOP elevation is associated with insulin resistance (Chun et al., 2015; Fujiwara et al., 2015; Oh et al., 2005), its implications in the development of glaucomatous neurodegeneration remain largely unexplored. Faiq et al. recently hypothesized the “brain diabetes theory of glaucoma” (Faiq et al., 2014b; Faiq and Dada, 2017) whereby insulin signaling dysfunction may be implicated in the glaucomatous visual system (Agostinone et al., 2018; Faiq et al., 2017; Hou et al., 2018) similar to other CNS disorders (Datusalia et al., 2018; Montgomery and Turner, 2015; Najem et al., 2014; Schubert et al., 2004; Stewart and Clearkin, 2008). Citicoline and its associated choline-containing components have been suggested to counter the effects of insulin resistance (Gao et al., 2017). Citicoline has also been shown to induce angiogenesis thereby improving the survival of human brain microvessel endothelial cells through insulin receptor substrate-1 mediation (Krupinski et al., 2012). Even though not all glaucoma patients have diabetes and not all diabetics have glaucoma, this line of research may point the way to a new glaucoma phenotype involving insulin resistance and may implicate a potential role of citicoline action on this population (Faiq, 2018).
With regard to clinical evidence, it is important to note that the effectiveness of any treatment has to be proven by standard objective parameters and validated by well-accepted measuring techniques. Several gold standards that are important in the evaluation of neuroprotection and visual function in glaucoma include retinal electrophysiology via visual evoked potential and pattern electroretinogram, and visual field perimetry via Humphrey Field Analyzer. These tools have been used in the investigations of citicoline as a therapeutic modality for glaucoma (Table 1). For example, one of the earliest clinical trials evaluating the efficacy of oral citicoline in glaucoma demonstrated improvement in visual evoked potentials after administrating 500 mg citicoline tablets twice a day (/approximately 14 mg/kg body weight/day) to the glaucoma patients (Rejdak et al., 2003). In another 2 randomized placebo-controlled studies involving intramuscular injection of citicoline, improvements in visual evoked potential and pattern electroretinogram were observed in the citicoline group as against the placebo group (Parisi, 2005; Parisi et al., 1999). Importantly, these effects maintained even after the washout period over the experimental period of up to 8 years. These results indicate that citicoline sustainably improves retinal and cortical bioelectrical responses in glaucoma patients. When evaluating the efficacy of oral suspension citicoline against intramuscular injection (Parisi et al., 2008a), no apparent difference in visual evoked potential or pattern electroretinogram was observed in glaucoma patients with moderate visual field defects.
Since glaucoma involves selective loss of RGCs whose cell bodies are located in the inner layer of the retina, they are therapeutically easy to approach through the ocular route as compared to oral and intramuscular routes. It has also been observed that medication in the form of eye drops has better compliance and adherence than oral and intramuscular medication (Witticke et al., 2012). Thus, it becomes essential to evaluate if citicoline eye drops can cross the cornea and provide sufficient bioavailability at the site of action to the retina. In a study by Roberti et al., citicoline was detected in the vitreous of murine models when given as topical eye drops (Roberti et al., 2014). With a dosage of 1% and 2% citicoline eye drop suspension at the frequency of twice daily, citicoline was detected in the vitreous. 2% administration was also associated with systemic absorption of citicoline. The investigators then moved on to clinical studies and added citicoline eye drops to the regular ocular hypotensive medication in glaucoma patients for 2 months followed by 1 month washout period. Although patients showed improvements in the electrophysiological function of the retina, such effects regressed after 30 days of washout. Parisi et al., carried out a similar trial but with increased citicoline dosage and treatment duration to test if this shows any sustained effects (Parisi et al., 2015). They enrolled patients on β-blocker monotherapy in the trial with the intervention group taking an additional citicoline eye drop regimen at three times a day for 4 months followed by a washout period of 2 months. Results showed a significant improvement in visual evoked potential and pattern electroretinograms after 4 months of citicoline treatment. However, these observations also normalized to baseline levels after medication was stopped. These transient visual restoration effects suggest a constant loss or sub-optimal availability of choline upon topical citicoline treatment, indicating the need of further optimization for effective topical citicoline administration on glaucoma. We presume that early glaucoma treatment might show better and more long-lasting effects than treatment to more severe glaucoma, though practically most cases of glaucoma have already experienced severe RGC damage by the time they are diagnosed. In this regard, pilot studies aiming at detecting glaucoma early via advanced imaging and quality-of-life assessments may be helpful.
6. Brain Imaging in Glaucoma
Since glaucoma is an irreversible disease, it is important to identify early glaucomatous changes and slow down the disease progression effectively. Recent advancements in imaging modalities have begun to shed light on this. For example, within the eye, substantial structural loss in terms of retinal nerve fiber layer thinning appears to be necessary before functional visual field defects become detectable in open-angle glaucoma (Alasil et al., 2014; Wollstein et al., 2012). On the other hand, increasing evidence suggests the involvements of trans-synaptic deteriorations of post-retinal structures along the central visual pathway in glaucoma (Gupta et al., 2006), yet the majority of studies focus only on subjects with glaucoma approaching advanced stages (Lawlor et al., 2018). Longitudinal evaluation of brain changes along the entire spectrum of the disease severity is essential to determine the temporal characteristics and causal relationships between biomarkers in the eye and the brain. Such studies and the testing of targeted neurotherapeutics have been performed preclinically using rodent glaucoma models (Chan et al., 2019; van der Merwe et al., 2016; Yang et al., 2018b) while clinical neuroimaging studies have also been initiated in subjects at different stages of glaucoma. Specifically, initial evidence from Murphy et al. showed that glaucoma deterioration is already present in the brain before vision loss can be detected clinically by conventional visual field tests (Murphy et al., 2016). This observation has been reproduced independently in another laboratory along the optic radiation (Wang et al., 2018), whilst demyelination appears to precede axonal loss in the trans-synaptic spread of human glaucoma, suggesting that the mechanism of trans-synaptic damage may be at least partially mediated by glial components at the cellular level (You et al., 2019). Within the visual cortex, it is also reported that cortical cholinergic and glutamatergic abnormalities are associated with other conventional glaucoma biomarkers in subjects with varying glaucoma severity (Aksoy et al., 2018; Chan et al., 2009; Guo et al., 2018; Murphy et al., 2016)(Figure 7). In this context, citicoline can be evaluated as a therapeutic option apart from ocular hypertensives who are at high risk of glaucoma but have not developed glaucoma as yet. Positive family history can also be considered as the initial stage of glaucoma continuum.
Figure 7: In vivo metabolic assessments of the brain in glaucoma.
Using magnetic resonance spectroscopy, the neurochemistry of the visual cortex in glaucoma can be evaluated non-invasively and can be compared across species spanning from the conventional experimental rat model of unilateral chronic ocular hypertension (A) to glaucoma patients (B). It is important to note that both humans and rodents show a lower choline (Cho) level in the glaucomatous visual cortex relative to the control visual cortex, whereas the creatine (Cr) level appears relatively comparable between glaucoma and control visual cortices. This suggests the reduction of choline-containing compounds in the glaucomatous visual cortex during trans-synaptic degeneration. The volumes of interests sampled are shown in the purple (A) and white boxes (B) in the multiplanar magnetic resonance brain images on the left for references. (Reproduced with permission from (Chan et al., 2009) and (Murphy et al., 2016))
As mentioned in the preceding section, glaucoma damage can be identified by optical coherence tomography (Adhi and Duker, 2013; Mwanza and Budenz, 2018; Schuman, 2008; Schuman et al., 1995) and magnetic resonance imaging prior to detectable clinical vision loss (Murphy et al., 2016; Wang et al., 2018). Though such a statement does not endorse the absence of early functional deterioration, the underlying premise is that vision loss cannot be detected by clinical perimetry as early as the changes in the central visual pathway are picked up by imaging modalities. Latest studies support the inference that glaucoma is a neurodegenerative disease with signs of early deterioration in brain (Chan et al., 2008; Chan et al., 2009; Crish et al., 2010; Faiq et al., 2016b; Gupta and Yucel, 2007; Reilly et al., 2015; Risner et al., 2018; Sponsel et al., 2014). Such a notion has numerous implications with respect to the brain as an investigative, diagnostic, therapeutic and prognostic target for glaucoma from genetic, biochemical, molecular, physiological, and pharmacological and imaging points of view (Faiq et al., 2013a; Faiq et al., 2013b; Faiq, 2018; Faiq et al., 2016b; Faiq et al., 2015; Faiq et al., 2014c; Kasi et al., 2019). This is where neuroimaging comes into picture with the prospect of different MRI techniques including the standard anatomical MRI, diffusion tensor imaging of structural integrity of brain connectivity (Ho et al., 2015; V et al., 2018; Yang et al., 2018a), manganese-enhanced MRI of physiological anterograde axonal transport and neuronal activity (Calkins et al., 2008; Chan et al., 2008; Chan et al., 2017a; Ho et al., 2015; Yang et al., 2018a), functional MRI of hemodynamic brain activity (Murphy et al., 2016; Zhou et al., 2017), and magnetic resonance spectroscopy (MRS) of biochemicals and metabolic processes in the brain (Chan et al., 2009; Chow et al., 2011; Murphy et al., 2016). In particular, choline concentrations in cerebral white matter and grey matter can be identified with proton magnetic resonance spectroscopy (1H MRS) in vivo (Ross and Bluml, 2001). Also, radiolabeled choline (e.g. 11C and 18F) can be effectively employed (Calabria et al., 2018). Citicoline is known to be involved in myelin and acetylcholine synthesis through choline as a metabolite. Since a variety of neurodegenerative diseases and their cardinal features like inflammation are associated with change in choline concentration, an integrative metabolic map of the status of neural tissues may be useful in identifying brain abnormalities early in both glaucoma patients and experimental glaucoma models (Figure 7). By this approach, the effects of drug interventions can be monitored in both clinical trials and preclinical models (Babb et al., 2004). Figure 8 depicts a classical trial of neuroprotection, neurorestoration and neuroregeneration that may represent the possible neurotherapeutic strategies for future glaucoma management. Figure 9 illustrates a schematic diagram of normal, early and advanced stages of glaucoma and the potential neurotherapeutic approaches in correspondence to visual field loss, retinal nerve fiber layer thinning, and brain damages in humans via perimetry, optical coherence tomography and MRI. Such structural and functional imaging paradigms can also be used to explore the neurobehavioral effects of citicoline as demonstrated initially in novel rodent glaucoma models (Chan et al., 2018; Chan et al., 2019; Harwerth et al., 2010; van der Merwe et al., 2016; Yang et al., 2018b).
Figure 8: The classical triad of citicoline actions on neurodegeneration.
This figure summarizes the biochemical and biological activities of citicoline into the triad of pharmacodynamics for treating neurodegeneration. Citicoline protects undamaged axons and hence is neuroprotective (Adibhatla et al., 2002; Bogdanov et al., 2018; Grieb, 2014; Hurtado et al., 2005; Parisi et al., 2018). It rescues the partially damaged neurons presumably through membrane re-integration and therefore is neurorestorative (Saver, 2008). The regenerative function of citicoline arises from the initial in vitro evidence for the drug to regenerate neuronal cells (Ozay et al., 2007; Skripuletz et al., 2015).
Figure 9: Representation of various severity of ocular and central vision loss in glaucoma and the candidate plan for neurotherapeutic intervention.
This figure explicates the idea of glaucoma being a neurodegenerative disorder with definite ocular and brain manifestations. (A) portrays a schematic representation of healthy (1st column), partially lost (2nd column) and completely lost visual function (3rd column). The corresponding clinical manifestations are illustrated in terms of peripapillary retinal nerve fiber layer (RNFL) thickness by optical coherence tomography (B) and visual field perimetry (C). The concomitant structural and functional brain changes in diffusion tensor MRI (D) and functional MRI (E) across increasing extents of vision loss bolsters the notion of glaucoma being a neurodegenerative disease of the visual system. This figure also considers the candidature of each condition for neurotherapeutic intervention. Since citicoline is neuroprotective, a healthy visual field (in high risk individuals) is a candidate for neuroprotection. The neurorestorative and neuroregenerative properties of citicoline make it a candidate for partially damaged and completely damaged visual field (A). Color representations for the principal diffusion directions in (D): blue, caudal-rostral; red, left-right; green, dorsal-ventral. (RNFL: retinal nerve fiber layer; FA: fractional anisotropy; OT: optic tract; VC: visual cortex; BOLD fMRI: blood-oxygenation-level-dependent functional MRI)
7. Psychosomatic Correlates
A major comorbidity of glaucoma is the patients’ fear of blindness over time, which is thought to be far beyond the actual risk (Janz et al., 2007). At the same time, cholinergic neurons are known to be involved in regulating cognitive functions including fear (Boskovic et al., 2018; Wilson and Fadel, 2017). To date, whether such cholinergic neuromodulatory drive can directly affect visual function remains unclear (Chen et al., 2015). In a study assessing the psychological impact of glaucoma, it was found that 80% of the 589 enrolled patients suffered from negative emotional reactions after knowing that they had glaucoma, among which nearly one third had apprehension of developing blindness (Odberg et al., 2001). This is in line with later cross-sectional studies that observed higher anxiety levels (Bechetoille et al., 2008; Hamelin et al., 2002) and depression prevalence in patients with increasing glaucoma severity (Bramley et al., 2008; Skalicky and Goldberg, 2008). Longitudinally, visual field loss in glaucoma (Artes and Chauhan, 2005) appears to progress at a faster rate if the patient is presented with depression-like symptoms (Diniz-Filho et al., 2016). Whilst mental stress can modify cholinergic neurotransmission in the brain (Caspi et al., 2003; Meerson et al., 2010), malfunction of the ACh system can also lead to stress and anxiety (Kumar et al., 2013a; Mark et al., 1996; Mineur et al., 2013; Picciotto et al., 2012; Picciotto et al., 2015) as well as elevation of cortisol (Walker et al., 1990), which in turn is associated with IOP elevation (Schwartz and Seddon, 1981) and vascular dysregulation with relevance to Flammer syndrome or endothelial dysfunction-mediated glaucoma (Buckley et al., 2002; Bukhari et al., 2016; Flammer and Konieczka, 2017; Konieczka et al., 2017; Konieczka et al., 2014; Liu et al., 2016; Resch et al., 2009). Collectively, this overview presents a potential vicious circle whereby vision loss in glaucoma elicits psychological stress and anxiety, which then exert influence on the brain cholinergic system to cause further vision loss. From another viewpoint, this information also provides opportunities to address the pathogenic features of glaucoma at multiple levels, as ameliorating ACh metabolism dysfunction or severity of depression and psychological stress may help to pacify the risks for glaucoma (Chamoun et al., 2017; Dada et al., 2018; Faiq, 2016, 2018; Gagrani et al., 2018; Sabel et al., 2018b). Even though the primary outcome measure of most clinical trials was IOP which is currently the only modifiable risk factor for glaucoma, these studies lend support to the notion that interventions based on eliciting relaxation response may be helpful in ameliorating glaucoma-related symptoms as well as positive changes in gene expression and other markers of stress and wellbeing (Sankalp et al., 2018).
Additionally, glaucoma patients often present visual attention deficits (Rosen et al., 2015; Smith et al., 2011). Visually impaired individuals typically possess a reduced visual span as compared to the normally sighted counterparts. This necessitates the glaucomatous individuals to distribute attention between functioning and deficient visual fields, which in turn elevates the burden on attention reserves for cognitive tasks (Swenor et al., 2017). Damage to the retinal nerve fiber layer or post-retinal visual pathway in glaucoma may also reduce the efficiency of the visual system to execute immediate target detection in the visual field (Loughman et al., 2007). Using advanced neuroimaging, widespread structural and functional brain changes within and beyond the central visual pathway have been found in glaucoma, and these brain regions are often involved in high-order cognitive functions (Murphy et al., 2016; Nuzzi et al., 2018; Song et al., 2014; Wang et al., 2016). It is known that the cholinergic systems may be altered in attention–deficit/hyperactivity disorder (Demeter and Sarter, 2013; Luchicchi et al., 2014; Sarter and Paolone, 2011), whereas nicotine alleviates symptoms due to attention deficit (Conners et al., 1996; Sahakian et al., 1989; Wignall and de Wit, 2011). This brings about two questions of whether the central nicotinic cholinergic function may augment the attention deficits in glaucoma, and if it can be a target similar to attention-deficit/hyperactivity disorder (Potter et al., 2006). Citicoline supplementation has been shown to improve attention, working memory and performance speed (Bruce et al., 2014; McGlade et al., 2019) which suggests that citicoline may work as a therapeutic measure to improve glaucoma outcomes through similar pathways. Since citicoline is a precursor of choline-related compounds, it seems apparent that citicoline may aid in addressing the cholinergic glaucoma-stress relationship in addition to other neuroprotective and neurorestorative realms. More studies are necessary to confirm how specifically cholinergic dysfunctions are involved in glaucoma and whether the multifarious factors aforementioned are indeed effective targets for citicoline therapy. With this note, the final section deals with the status quo and the future prospectus of ACh modulation and citicoline therapy in glaucomatous vision loss.
8. Future Directions and Conclusions
In this paper, we put forth the hypothesis of the cholinergic nervous system as a mechanistic and therapeutic modality in vision and behavior, and exploit the evidence of its role in the etiopathogenesis of glaucoma. Figure 10 elucidates the above with cross references to citicoline biology. These preclinical and clinical studies justify citicoline supplementation by various routes including oral administration, intramuscular injection, intraperitoneal injection and eye drops across different neurodegenerative diseases and species. Various concentrations of citicoline have been used from 50 mg/kg body weight to 1g per day. Outcome measures like retinal catecholamine levels, thickness of retinal layers, expression of choline acetyl transferase and tyrosine hydroxylase, RGC density, expression of anti-apoptotic BCL-2, apoptosis evaluation by TUNEL assays, Caspase 3, Caspase 9 activity, structural and functional brain imaging, pattern electroretinogram, visual evoked potential measurements, and optokinetic behavioral assessments have been investigated with study designs ranging from animal model studies to case control and randomized controlled trials. In light of the above overview, it seems that citicoline holds a strong promise to be a future treatment modality for glaucoma and other neurodegenerative diseases. It is not clear whether citicoline affects IOP or ICP, but citicoline may act through the neurodegenerative, neuropathic and psychosomatic paradigms in glaucoma.
Figure 10: Overview of the involvements of cholinergic metabolism in neuroprotection, neurorestoration and vision rehabilitation in both basic and clinical domains.
ACh and citicoline are reciprocal precursors and are interconvertible through various enzymatic systems. Choline and cytidine are also the metabolites within this network. The reported effects of this pool of moieties (citicoline, ACh, choline and cytidine) include: (1) Preservation of cardiolipin for rescuing mitochondrial function and consequently aiding in neuroprotection and neurorestoration; (2) Preservation of sphingomyelin for myelin formation and thereby protection of neurons and assurance of proper membrane function; (3) Restoration of phosphatidylcholine for improved ACh synthesis. This may help prevent oxidative stress and is important in neuroprotective and neurorestorative processes by maintaining mitochondrial function and viability and preventing mitochondrial genome instability; (4) Stimulation of glutathione synthesis works through two main mechanisms including prevention of oxidative stress and direct neuroprotection. Prevention of oxidative stress, in particular, ensures better bioenergetics and prevents neuronal apoptosis; (5) Lowering of glutamate concentration. This primarily prevents glutamate excitotoxicity thereby promotes neuroprotection and neurorestoration; (6) Releasing calcium from endothelial cells for modulating nitric oxide and improving endothelial function. Proper functioning of the microvasculature and proper tissue perfusion is important for neuronal viability. Ameliorating endothelial dysfunction leads to improved microvasculature and better blood flow, which, in turn, prevents neuronal apoptosis; (7) Myelin synthesis for axon protection and prevention of the axonal degeneration; and (8) Binding to muscarinic and nicotinic receptors for activating molecular pathways that are involved in the prevention of RGC death and neuronal apoptosis, neurorestoration, modulation of neuroplasticity, contraction of ciliary bodies, and widening of the anterior chamber angle with increased aqueous outflow and consequent drop in IOP.
Two obvious approaches towards citicoline use emerge from the studies reported to date. One is aimed at exploring the therapeutic mechanism by employing animal and clinical studies, and the other is focused on the safety and efficacy by animal studies and randomized controlled trials. While citicoline has been found safe at the therapeutic doses being currently administered, an ideal clinical study to evaluate the neurotherapeutic effects of citicoline in glaucoma may be difficult to perform owing to the inherent issues in the natural history of glaucoma such as a long term and rather variable progression curve, the difficulty to predict disease severity and glaucoma risks in terms of elevated IOP, variable and unclear age of onset, involvements of the brain apart from the eye, the lack of consensus in cupping paradigm, the potential role of endothelial dysfunction, the systemic effects on disease onset and progression, role of stress and anxiety, and other psychosomatic involvements (Weinreb, 2007). To circumvent this difficulty, a pre-glaucomatous state identification protocol and long-term follow up would be advantageous but that would require meticulous study design and long periods of monitoring. We explicate on this issue in the following paragraphs on the potential clinical trial settings and their feasibility in both early and advanced glaucoma (Guymer et al., 2019; Levin et al., 2017; Quigley, 2012).
The mechanistic assembly of cholinergic system-glaucoma relation can be broadly classified into 3 domains: protection of undamaged RGCs and axons, rescue of minimally damaged RGCs and axons, and regeneration of damaged RGCs and axons (Figure 8). In terms of conceptual neurobiology, there is no clear boundary between these three processes and the therapeutic effects of citicoline are presumably mediated through the combination and permutation of all three contributions. It is assumed that citicoline may mainly act through the first and second mechanisms (i.e. neuroprotection and neurorestoration) if glaucoma can be diagnosed early. These RGCs and axons often appear in the transition zones of the visual field and are often undetected or ignored clinically. Figure 9 demonstrates this scenario both schematically and from actual data obtained under clinical and experimental neuroimaging settings. Ophthalmologists generally examine visual field results in terms of “black-and-white” where black means lost vision and white means intact vision. The often ignored grey areas may be from a mix of healthy and damaged RGCs or axons given the limited resolution of perimetry. Alternatively, there is initial evidence that corresponds these regions to the minimally damaged cells or axons in early apoptosis stage, whereby the most reservoir for vision rescue and regeneration can be potentiated (Fedorov et al., 2011; Gall et al., 2016; Henrich-Noack et al., 2017b; Kasten et al., 1998; Sabel et al., 2018a; Sabel et al., 2011b). Citicoline might act in the window between cellular/axonal dysfunction and death which offers a pragmatic approach from the end-user point of view. Preclinically, optic nerve crush models may be useful in investigating the neurorestorative/neuroregenerative potentials of citicoline while ocular hypertension models are likely helpful to further our understanding of the neuroprotective/neurorestorative mechanisms thereof. Also, animal models that represent the normotensive subset of glaucoma such as impaired glutamate transporters and transgenic optineurin E50K or TBK1 mice may help in understanding neuroprotective and neurorestorative processes upon IOP-independent mechanisms of RGC apoptosis (Harada et al., 2019). In animal models, the outcome measures can be pattern electroretinogram, visual evoked potential, IOP, ocular and cerebral integrity, protein expression profiling, and gene expression profiling. Visual behavioral assessments are often ignored in preclinical studies but are important to evaluate any overall improvement in functional vision after neurotherapeutics to the the eye and the brain’s visual system. Designing rigorous experiments in this direction may provide answers to the roles of the cholinergic system in glaucoma, and at the same time give rise to novel and relevant questions. Furthermore, detecting the disease early and tracing disease progression in the same subjects are important to research fields to minimize biovariability and develop better understanding of causality, whereas direct detection of the cholinergic signaling in vivo could help improve specificity of our investigative experiments and for personalized medicine.
With regards to clinical studies, various study designs like cohort, case control and retrospective cohort have been investigated (Ottobelli et al., 2013; Parisi, 2005; Parisi et al., 2015; Parisi et al., 2008a; Parisi et al., 2008b; Parisi et al., 1999; Pecori Giraldi et al., 1989; Rejdak et al., 2003; Roberti et al., 2014; Virno et al., 2000) (Table 1). Given the safety of citicoline and the absence of serious adverse events reported, this compound has entered clinical trials for a variety of neurodegenerative diseases including glaucoma. There is, however, a caveat on the sustainability of the therapeutics as the additional beneficial effects supplemented by citicoline eye drops appear to fall back to baseline after the washout period. This indicates the need for further optimization of the pharmacokinetics of different administrative routes and treatment paradigm for improved long-lasting effects. On the other hand, there have been no clinical trials in the use of citicoline treatment on congenital glaucoma, juvenile onset glaucoma, developmental glaucoma or angle closure glaucoma. It is either unclear whether citicoline acts on secondary glaucoma including steroid-induced glaucoma or neovascular glaucoma. Since outcomes measures such as changes in scotomatous area, visual evoked potential, pattern electroretinogram, and visual field have been initially evaluated in citicoline supplementation, a more well-structured clinical trial makes the next coherent rationale. This may include a randomized controlled trial with four arms instead of two: (i) ocular hypertension with no glaucoma, (ii) high-tension glaucoma, (iii) normotensive glaucoma and (iv) healthy controls. Furthermore, the “visual quality of life” assessment has been ignored by the majority of clinical trials. The European Glaucoma Society guidelines emphasize on “incorporation of quality of life measure in the outcome of treatment” (Terminology and Guidelines for Glaucoma, 4th Edition, Clause B3). A general quality-of-life (e.g. WHO-BREF) or glaucoma-specific quality-of-life (e.g. GQL-15 or NEIVFQ25) assessment should be one of the outcome measures in the clinical trials to be carried out in future. Combining the clinical outcomes with exploratory studies like gene expression pattern, reactive oxygen species markers, aging markers, inflammatory markers, apoptosis markers, psychological personality evaluation, and stress level evaluation will reveal a wealth of information to determine the clinical, biological, genetic and psychosocial elements in glaucoma and citicoline treatment.
As citicoline addresses many aspects of neurodegeneration (Adibhatla et al., 2002; Bogdanov et al., 2018; Grieb, 2014; Hurtado et al., 2005; Matteucci et al., 2014; Parisi et al., 2018), clinical trials of citicoline-based intervention in glaucoma may be guided by trials already reported on other neurodegenerative diseases (Cesareo et al., 2015; Ghiso et al., 2013; Mancino et al., 2018; Nucci et al., 2015; Ou et al., 2012). However, since glaucoma is a slowly progressing disease, clinical trials with long-term monitoring are often preferred yet remain challenging (Leske et al., 2003; Parisi et al., 2008a; Parisi et al., 1999; Virno et al., 2000; Weinreb et al., 2018) considering the large sample size needed for deriving statistically and clinically meaningful results from the small effect size, the high cost of the studies, the potential patient dropout or loss of adherence, and the compromise in drawing robust conclusions from incomplete data (McGhee et al., 2016; Stanzione and Tropepi, 2011). For example, if visual field tests are performed every 3 months for 2 years, a sample size of 495 patients per group would be necessary to detect 30 percent reduction in the mean deviation rate of change (De Moraes et al., 2017). There is, however, some hope regarding the design of smaller scale trials that may still provide useful and reliable results (Quigley, 2012). For instance, the United Kingdom Treatment Study employed a novel method of clustered testing paradigm combined with point-wise event based approach to investigate visual field progression upon IOP lowering treatment in a trial spanning less than 2 years with 258 participants per arm (Garway-Heath et al., 2015; Wu et al., 2019). In most clinical trials for glaucoma, IOP is the primary outcome. Recently, a growing number of trials begin to use other outcome measures such as visual field, retinal nerve fiber layer thickness, and electroretinogram (De Moraes et al., 2017; Quigley, 2012; Quigley, 2019; Weinreb, 2007; Weinreb et al., 2018; Wu et al., 2019). Since citicoline is expected to act on glaucomatous neurodegenerative events rather than IOP (Adibhatla et al., 2002; Grieb, 2014; Hurtado et al., 2005; Matteucci et al., 2014; Ou et al., 2012; Parisi et al., 2018), visual field, pattern electroretinography, structural/functional imaging or quality of life may be considered as the primary/secondary outcomes for citicoline-based glaucoma trials (Quigley, 2012; Quigley, 2019; Wu et al., 2019). Citicoline trials should also take IOP lowering into consideration since halting the regular anti-glaucoma medication may pose risks to the patients in addition to other ethical concerns. In such case, the milestone of effective citicoline intervention should be set above the effects from IOP lowering medication alone. Inter-observer variability and time of IOP measurements should be properly accounted for as spontaneous IOP fluctuations and other systematic errors could potentially mask the IOP-independent neurotherapeutic effects and render the data inconclusive or underpowered (Quigley, 2012; Quigley, 2019). In terms of dosage, citicoline does not pose major side effects up to 1600 mg/day (Grieb et al., 2016; Grieb and Rejdak, 2002; Parisi et al., 2008a; Parisi et al., 1999; Parisi et al., 2018; Rejdak et al., 2003; Virno et al., 2000). However, its short- and long-term dose-dependency remains to be systematically evaluated such as using futility trials (Levin, 2015; Schwid and Cutter, 2006; Tilley et al., 2006). To improve adherence, electronic reminders can be used (Boland et al., 2014) whereby different user-friendly and customizable mobile apps have been recently developed for this purpose. Caregivers can also be trained to ensure adherence. Last but not least, future studies can take into consideration more sensitive methods to monitor disease progression and detect therapeutic effects using smaller sample sizes as technology advances (Quigley, 2012; Quigley, 2019; Wu et al., 2019).
Increasing numbers of molecular, in vitro and in vivo studies have revealed that cholinergic signaling is closely related to various neurocognitive functions including visual information processing and RGC viability. In light of the above overview, glaucoma may also be understood in terms of cholinergic dysfunction. Such a conceptual outlook provide the premise to subject cholinergic drugs to experimentation. To further bolster this, cholinergic drugs were one of the earliest therapeutic modalities for glaucoma. A search for novel cholinergic moieties is imperative in this connection. A naturally occurring, inexpensive and relatively harmless compound with good bioavailability and no adverse side effects would be an ideal candidate for glaucoma therapy. Citicoline is a natural product approved as a food supplement. It is safe at the currently approved dosage in clinical trials, and can be metabolized in the body into cytidine and choline which then reassembles in the brain to form citicoline. Citicoline is the source of phosphatidylcholine, ACh, sphingomyelin and cardiolipin and hence is important in maintaining the structural and functional viability of various neurons including RGCs. Citicoline also prevents glutamate excitotoxicity and improves dopamine signaling. This indicates that citicoline may be a potential drug to treat neurodegenerative diseases including glaucoma. It is also pertinent to examine if citicoline can be combined with other substances [e.g. scutellarin (Hu et al., 2018; Zhu et al., 2018) and quercetin (Lee et al., 2016; Lee et al., 2010; Lee et al., 2011a)] and treatments that in part regulate ACh or ACh receptor levels for further improving the antioxidant and antiapoptotic properties in glaucoma and other neurodegenerative disorders. Taken together, intensified research efforts targeting the cholinergic system as glaucoma neurotherapeutic sites are warranted to help reduce the global prevalence and burden of the disease. Next decade is likely to shed light on this issue.
Highlights.
The cholinergic nervous system has a crucial role to play in visual function.
Choline metabolites are imperative for mitochondria, myelin and neuronal functions.
Neurodegenerative events have been identified in human and experimental glaucoma.
Cholinergic system can be an effective target for glaucoma management and therapy.
Acknowledgements
This work is supported in part by the National Institutes of Health R01-EY028125 (Bethesda, Maryland); BrightFocus Foundation G2013077, G2016030 and G20190103 (Clarksburg, Maryland); and Research to Prevent Blindness/Stavros Niarchos Foundation International Research Collaborators Award (New York, New York).
Abbreviations
- ACh
Acetylcholine
- AChE
Acetylcholine esterase
- AChR
Acetylcholine receptor
- Akt
AKT Serine/Threonine Kinase
- ATP
Adenosine triphosphate
- BCL2
B cell lymphoma gene 2
- CDP
Cytidine-5’-diphosphocholine
- ChAT
Choline acetyltransferase
- CMP
Cytidine monophosphate
- CNS
Central nervous system
- DNA
Deoxyribonucleic acid
- GABA
Gamma aminobutyric acid
- GFP
Green fluorescent protein
- ICP
Intracranial pressure
- IOP
Intraocular pressure
- JAK2
Janus Kinase 2
- Kir3
Inwardly Rectifier K+ Channel 3
- Kv7
Voltage-Gated Potassium Channel Subunit Kv7
- mAChR
Muscarinic acetylcholine receptor
- MRI
Magnetic resonance imaging
- mRNA
Messenger RNA
- MRS
Magnetic resonance spectroscopy
- nAChR
Nicotinic acetylcholine receptor
- NMDA
N-methyl-D-aspartate
- PCYTA
Choline-phosphate cytidylyltransferase A
- PDHA
Pyruvate dehydrogenase
- PEMT
Phosphatidylethanolamine n-methyltransferase
- PERG
Pattern electroretinogram
- PI3K
Phosphoinositide-3-Kinase
- PKC
Protein kinase C
- PLC
Phospholipase C
- RGC
Retinal ganglion cell
- RNA
Ribonucleic acid
- ROS
Reactive oxygen species
- VEP
Visual evoked potential
- VGCC
Voltage gated calcium channel
- YFP
Yellow fluorescent protein
Footnotes
Conflicts of interest: The author(s) have made the following disclosure(s): J.S.S.: Royalties e Zeiss, Dublin, CA (for intellectual property licensed by the Massachusetts Institute of Technology and Massachusetts Eye and Ear Infirmary)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M, Arbuthnott GW, 2018. Cholinergic modulation of striatal microcircuits. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Rodriguez A, Zhang L, Zhou F, Gong S, Gu H, De Biasi M, Zhou FM, Dani JA, 2014. Cocaine inhibition of nicotinic acetylcholine receptors influences dopamine release. Front Synaptic Neurosci 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhi M, Duker JS, 2013. Optical coherence tomography--current and future applications. Curr Opin Ophthalmol 24, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, 2002. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res 70, 133–139. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Dempsey RJ, 2001. Effects of citicoline on phospholipid and glutathione levels in transient cerebral ischemia. Stroke 32, 2376–2381. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Dempsey RJ, 2002. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem 80, 12–23. [DOI] [PubMed] [Google Scholar]
- Agnoli A, Ruggieri S, Denaro A, Bruno G, 1982. New strategies in the management of Parkinson’s disease: a biological approach using a phospholipid precursor (CDP-choline). Neuropsychobiology 8, 289–296. [DOI] [PubMed] [Google Scholar]
- Agostinone J, Alarcon-Martinez L, Gamlin C, Yu WQ, Wong ROL, Di Polo A, 2018. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 141, 1963–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agut J, Font E, Sacristan A, Ortiz JA, 1983. Dissimilar effects in acute toxicity studies of CDP-choline and choline. Arzneimittelforschung 33, 1016–1018. [PubMed] [Google Scholar]
- Aksoy DO, Umurhan Akkan JC, Alkan A, Aralasmak A, Otcu Temur H, Yurtsever I, 2018. Magnetic Resonance Spectroscopy Features of the Visual Pathways in Patients with Glaucoma. Clin Neuroradiol. [DOI] [PubMed] [Google Scholar]
- Alasil T, Wang K, Yu F, Field MG, Lee H, Baniasadi N, de Boer JF, Coleman AL, Chen TC, 2014. Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am J Ophthalmol 157, 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW, 2009. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE, Nagode DA, Tang AH, 2014. Muscarinic cholinergic receptors modulate inhibitory synaptic rhythms in hippocampus and neocortex. Front Synaptic Neurosci 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon DL, Rasmussen H, 1988. A spatial-temporal model of cell activation. Science 239, 998–1005. [DOI] [PubMed] [Google Scholar]
- Almasieh M, Catrinescu MM, Binan L, Costantino S, Levin LA, 2017. Axonal Degeneration in Retinal Ganglion Cells Is Associated with a Membrane Polarity-Sensitive Redox Process. J Neurosci 37, 3824–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez XA, Mouzo R, Pichel V, Perez P, Laredo M, Fernandez-Novoa L, Corzo L, Zas R, Alcaraz M, Secades JJ, Lozano R, Cacabelos R, 1999. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find Exp Clin Pharmacol 21, 633–644. [PubMed] [Google Scholar]
- Amodeo DA, Yi J, Sweeney JA, Ragozzino ME, 2014. Oxotremorine treatment reduces repetitive behaviors in BTBR T+ tf/J mice. Front Synaptic Neurosci 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen M, Overgaard K, Meden P, Boysen G, Choi SC, 1999. Effects of citicoline combined with thrombolytic therapy in a rat embolic stroke model. Stroke 30, 1464–1471. [DOI] [PubMed] [Google Scholar]
- Anderson KJ, Borja MA, Cotman CW, Moffett JR, Namboodiri MA, Neale JH, 1987. N-acetylaspartylglutamate identified in the rat retinal ganglion cells and their projections in the brain. Brain Res 411, 172–177. [DOI] [PubMed] [Google Scholar]
- Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W, 2004a. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci 45, 702–707. [DOI] [PubMed] [Google Scholar]
- Antal A, Kincses TZ, Nitsche MA, Paulus W, 2003. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia 41, 1802–1807. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Paulus W, 2001. External modulation of visual perception in humans. Neuroreport 12, 3553–3555. [DOI] [PubMed] [Google Scholar]
- Antal A, Paulus W, 2013. Transcranial alternating current stimulation (tACS). Front Hum Neurosci 7, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Varga ET, Kincses TZ, Nitsche MA, Paulus W, 2004b. Oscillatory brain activity and transcranial direct current stimulation in humans. Neuroreport 15, 1307–1310. [DOI] [PubMed] [Google Scholar]
- Araki W, Wurtman RJ, 1997. Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc Natl Acad Sci U S A 94, 11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artes PH, Chauhan BC, 2005. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res 24, 333–354. [DOI] [PubMed] [Google Scholar]
- Asaoka Y, Nakamura S, Yoshida K, Nishizuka Y, 1992. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci 17, 414–417. [DOI] [PubMed] [Google Scholar]
- Babb SM, Ke Y, Lange N, Kaufman MJ, Renshaw PF, Cohen BM, 2004. Oral choline increases choline metabolites in human brain. Psychiatry Res 130, 1–9. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P, 2007. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8, 45–56. [DOI] [PubMed] [Google Scholar]
- Beaudet A, Cuenod M, Reubi JC, Cuenod M, 1981. Selective bidirectional transport of [3H]d-aspartate in the pigeon retino-tectal pathway. Neuroscience 6, 2021–2034. [DOI] [PubMed] [Google Scholar]
- Bechetoille A, Arnould B, Bron A, Baudouin C, Renard JP, Sellem E, Brouquet Y, Denis P, Nordmann JP, Rigeade MC, Bassols A, Benmedjahed K, Guillemin I, Rouland JF, 2008. Measurement of health-related quality of life with glaucoma: validation of the Glau-QoL 36-item questionnaire. Acta Ophthalmol 86, 71–80. [DOI] [PubMed] [Google Scholar]
- Bell K, Gramlich OW, Von Thun Und Hohenstein-Blaul N, Beck S, Funke S, Wilding C, Pfeiffer N, Grus FH, 2013. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retin Eye Res 36, 199–216. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Liscovitch M, Richardson UI, 1987. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci U S A 84, 5474–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov P, Sampedro J, Sola-Adell C, Simo-Servat O, Russo C, Varela-Sende L, Simo R, Hernandez C, 2018. Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA, 2010. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain 133, 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland MV, Chang DS, Frazier T, Plyler R, Jefferys JL, Friedman DS, 2014. Automated telecommunication-based reminders and adherence with once-daily glaucoma medication dosing: the automated dosing reminder study. JAMA Ophthalmol 132, 845–850. [DOI] [PubMed] [Google Scholar]
- Bonomi L, Marchini G, Marraffa M, Bernardi P, De Franco I, Perfetti S, Varotto A, Tenna V, 1998. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology 105, 209–215. [DOI] [PubMed] [Google Scholar]
- Borras T, 2017. The Pathway From Genes to Gene Therapy in Glaucoma: A Review of Possibilities for Using Genes as Glaucoma Drugs. Asia Pac J Ophthalmol (Phila) 6, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskovic Z, Milne MR, Qian L, Clifton HD, McGovern AE, Turnbull MT, Mazzone SB, Coulson EJ, 2018. Cholinergic basal forebrain neurons regulate fear extinction consolidation through p75 neurotrophin receptor signaling. Transl Psychiatry 8, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskila J, Javadi P, Elkrief L, Casanova C, Bouchard JF, Ptito M, 2016. A Comparative Analysis of the Endocannabinoid System in the Retina of Mice, Tree Shrews, and Monkeys. Neural Plast 2016, 3127658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley T, Peeples P, Walt JG, Juhasz M, Hansen JE, 2008. Impact of vision loss on costs and outcomes in medicare beneficiaries with glaucoma. Arch Ophthalmol 126, 849–856. [DOI] [PubMed] [Google Scholar]
- Brann MR, Buckley NJ, Bonner TI, 1988. The striatum and cerebral cortex express different muscarinic receptor mRNAs. FEBS Lett 230, 90–94. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Werner KB, Preston BF, Baker LM, 2014. Improvements in concentration, working memory and sustained attention following consumption of a natural citicoline-caffeine beverage. Int J Food Sci Nutr 65, 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley C, Hadoke PW, Henry E, O’Brien C, 2002. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br J Ophthalmol 86, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde WM, 2000. Heredity in primary open-angle glaucoma. Curr Opin Ophthalmol 11, 101–106. [DOI] [PubMed] [Google Scholar]
- Bukhari SM, Kiu KY, Thambiraja R, Sulong S, Rasool AH, Liza-Sharmini AT, 2016. Microvascular endothelial function and severity of primary open angle glaucoma. Eye (Lond) 30, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT, 2005. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 24, 39–73. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, 1989. Intrinsic connections of rat primary visual cortex: laminar organization of axonal projections. J Comp Neurol 279, 171–186. [DOI] [PubMed] [Google Scholar]
- Burrows M, Siegler MV, 1982. Spiking local interneurons mediate local reflexes. Science 217, 650–652. [DOI] [PubMed] [Google Scholar]
- Buys ES, Potter LR, Pasquale LR, Ksander BR, 2014. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front Mol Neurosci 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A, 2004. Neuronal oscillations in cortical networks. Science 304, 1926–1929. [DOI] [PubMed] [Google Scholar]
- Cacabelos R, Caamano J, Gomez MJ, Fernandez-Novoa L, Franco-Maside A, Alvarez XA, 1996. Therapeutic effects of CDP-choline in Alzheimer’s disease. Cognition, brain mapping, cerebrovascular hemodynamics, and immune factors. Ann N Y Acad Sci 777, 399–403. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G, 2000. Acetylcholine-mediated modulation of striatal function. Trends Neurosci 23, 120–126. [DOI] [PubMed] [Google Scholar]
- Calabria FF, Barbarisi M, Gangemi V, Grillea G, Cascini GL, 2018. Molecular imaging of brain tumors with radiolabeled choline PET. Neurosurg Rev 41, 67–76. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Horner PJ, Roberts R, Gradianu M, Berkowitz BA, 2008. Manganeseenhanced MRI of the DBA/2J mouse model of hereditary glaucoma. Invest Ophthalmol Vis Sci 49, 5083–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos EC, Bolzani R, Schiavi C, Baldi A, Porciatti V, 1996. Cytidin-5’-diphosphocholine enhances the effect of part-time occlusion in amblyopia. Doc Ophthalmol 93, 247–263. [DOI] [PubMed] [Google Scholar]
- Carey RG, Rieck RW, 1987. Topographic projections to the visual cortex from the basal forebrain in the rat. Brain Res 424, 205–215. [DOI] [PubMed] [Google Scholar]
- Carreon T, van der Merwe E, Fellman RL, Johnstone M, Bhattacharya SK, 2017. Aqueous outflow - A continuum from trabecular meshwork to episcleral veins. Prog Retin Eye Res 57, 108–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R, 2003. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389. [DOI] [PubMed] [Google Scholar]
- Cekic O, Batman C, 1999. Effect of intracameral carbachol on intraocular pressure following clear corneal phacoemulsification. Eye (Lond) 13 ( Pt 2), 209–211. [DOI] [PubMed] [Google Scholar]
- Cesareo M, Martucci A, Ciuffoletti E, Mancino R, Cerulli A, Sorge RP, Martorana A, Sancesario G, Nucci C, 2015. Association Between Alzheimer’s Disease and Glaucoma: A Study Based on Heidelberg Retinal Tomography and Frequency Doubling Technology Perimetry. Front Neurosci 9, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun M, Sergeeva EG, Henrich-Noack P, Jia S, Grigartzik L, Ma J, You Q, Huppe-Gourgues F, Sabel BA, Vaucher E, 2017. Cholinergic Potentiation of Restoration of Visual Function after Optic Nerve Damage in Rats. Neural Plast 2017, 6928489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KC, Fu QL, Hui ES, So KF, Wu EX, 2008. Evaluation of the retina and optic nerve in a rat model of chronic glaucoma using in vivo manganese-enhanced magnetic resonance imaging. Neuroimage 40, 1166–1174. [DOI] [PubMed] [Google Scholar]
- Chan KC, So KF, Wu EX, 2009. Proton magnetic resonance spectroscopy revealed choline reduction in the visual cortex in an experimental model of chronic glaucoma. Exp Eye Res 88, 65–70. [DOI] [PubMed] [Google Scholar]
- Chan KC, van der Merwe Y, Murphy MC, Ho LC, Yang XL, Yu Y, Chau Y, Leung CK, Wollstein G, Schuman JS, 2018. Citicoline ameliorates the effect of elevated intraocular pressure on functional connectivity in the visual pathway, ARVO Investigative Ophthalmology & Visual Science, Seattle, Washington, pp. 6115–6115. [Google Scholar]
- Chan KC, Yu Y, Han Ng S, Mak HK, Yip YWY, van der Merwe Y, Ren T, Yung JSY, Biswas S, Cao X, Chau Y, Leung CKS, 2019. Intracameral Injection of a Chemically Cross-Linked Hydrogel to Study Chronic Neurodegeneration in Glaucoma. Acta Biomater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KC, Zhou IY, Liu SS, van der Merwe Y, Fan SJ, Hung VK, Chung SK, Wu WT, So KF, Wu EX, 2017a. Longitudinal Assessments of Normal and Perilesional Tissues in Focal Brain Ischemia and Partial Optic Nerve Injury with Manganese-enhanced MRI. Sci Rep 7, 43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MPY, Broadway DC, Khawaja AP, Yip JLY, Garway-Heath DF, Burr JM, Luben R, Hayat S, Dalzell N, Khaw KT, Foster PJ, 2017b. Glaucoma and intraocular pressure in EPIC-Norfolk Eye Study: cross sectional study. BMJ 358, j3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sur M, 2015. An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat Neurosci 18, 892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Hu HY, Chu D, Chen HH, Chang CK, Chou P, 2016. Patients with Primary Open-Angle Glaucoma May Develop Ischemic Heart Disease More Often than Those without Glaucoma: An 11-Year Population-Based Cohort Study. PLoS One 11, e0163210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YD, Al-Khoury L, Zivin JA, 2004. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx 1, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, Ebneter A, Wood JP, Casson RJ, 2011. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol 121, 737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis T, Weiner HL, 2017. CNS inflammation and neurodegeneration. J Clin Invest 127, 3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kook MS, 2015. Systemic and Ocular Hemodynamic Risk Factors in Glaucoma. Biomed Res Int 2015, 141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Zhou IY, Fan SJ, Chan KW, Chan KC, Wu EX, 2011. Metabolic changes in visual cortex of neonatal monocular enucleated rat: a proton magnetic resonance spectroscopy study. Int J Dev Neurosci 29, 25–30. [DOI] [PubMed] [Google Scholar]
- Chun YH, Han K, Park SH, Park KM, Yim HW, Lee WC, Park YG, Park YM, 2015. Insulin resistance is associated with intraocular pressure elevation in a non-obese Korean population. PLoS One 10, e112929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WM, Clark TD, 2012. Stroke: Treatment for acute stroke--the end of the citicoline saga. Nat Rev Neurol 8, 484–485. [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Byrne JH, Frost WN, 1995. Role of interneurons in defensive withdrawal reflexes in Aplysia. Learn Mem 2, 133–151. [DOI] [PubMed] [Google Scholar]
- Coleman AL, Kodjebacheva G, 2009. Risk factors for glaucoma needing more attention. Open Ophthalmol J 3, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B, Mitchell JF, 1966. The central release of acetylcholine during stimulation of the visual pathway. J Physiol 184, 239–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci L, Bosco M, Rosario Ziello A, Rea R, Amenta F, Fasanaro AM, 2012. Effectiveness of nootropic drugs with cholinergic activity in treatment of cognitive deficit: a review. J Exp Pharmacol 4, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J, 1996. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD). Psychopharmacol Bull 32, 67–73. [PubMed] [Google Scholar]
- Crish SD, Dapper JD, MacNamee SE, Balaram P, Sidorova TN, Lambert WS, Calkins DJ, 2013. Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience 229, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ, 2010. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A 107, 5196–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks J, Kolb H, 1992. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol 315, 287–302. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Kolb H, 1989. Morphology and distribution of neurons immunoreactive for substance P in the turtle retina. J Comp Neurol 290, 391–411. [DOI] [PubMed] [Google Scholar]
- Cynader MS, Swindale NV, Matsubara JA, 1987. Functional topography in cat area 18. J Neurosci 7, 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza RD, Vijayaraghavan S, 2014. Paying attention to smell: cholinergic signaling in the olfactory bulb. Front Synaptic Neurosci 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada T, Mittal D, Mohanty K, Faiq MA, Bhat MA, Yadav RK, Sihota R, Sidhu T, Velpandian T, Kalaivani M, Pandey RM, Gao Y, Sabel BA, Dada R, 2018. Mindfulness Meditation Reduces Intraocular Pressure, Lowers Stress Biomarkers and Modulates Gene Expression in Glaucoma: A Randomized Controlled Trial. J Glaucoma 27, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y, 2012. Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia 60, 13–28. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Lima PA, Wonnacott S, 2000. The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology 39, 2799–2807. [DOI] [PubMed] [Google Scholar]
- Dani JA, 2015. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. Int Rev Neurobiol 124, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datusalia AK, Agarwal P, Singh JN, Sharma SS, 2018. Hyper-insulinemia increases the glutamate-excitotoxicity in cortical neurons: A mechanistic study. Eur J Pharmacol 833, 524–530. [DOI] [PubMed] [Google Scholar]
- Davanger S, Ottersen OP, Storm-Mathisen J, 1991. Glutamate, GABA, and glycine in the human retina: an immunocytochemical investigation. J Comp Neurol 311, 483–494. [DOI] [PubMed] [Google Scholar]
- de Groot NS, Burgas MT, 2015. Is membrane homeostasis the missing link between inflammation and neurodegenerative diseases? Cell Mol Life Sci 72, 4795–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CG, Liebmann JM, Levin LA, 2017. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog Retin Eye Res 56, 107–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehorter N, Marichal N, Marin O, Berninger B, 2017. Tuning neural circuits by turning the interneuron knob. Curr Opin Neurobiol 42, 144–151. [DOI] [PubMed] [Google Scholar]
- Deisseroth A, Dounce AL, 1970. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev 50, 319–375. [DOI] [PubMed] [Google Scholar]
- DeLong CJ, Shen YJ, Thomas MJ, Cui Z, 1999. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 274, 29683–29688. [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M, 2013. Leveraging the cortical cholinergic system to enhance attention. Neuropharmacology 64, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz-Filho A, Abe RY, Cho HJ, Baig S, Gracitelli CP, Medeiros FA, 2016. Fast Visual Field Progression Is Associated with Depressive Symptoms in Patients with Glaucoma. Ophthalmology 123, 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale JR, Griffiths GK, Rowlands C, Castello J, Ortiz JA, Maddock J, Aylward M, 1983. Pharmacokinetics of 14C CDP-choline. Arzneimittelforschung 33, 1066–1070. [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ, 2007. Gain modulation by nicotine in macaque v1. Neuron 56, 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager UC, Olsen JF, 1980. Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J Comp Neurol 191, 383–412. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, 1998. A proposed role for excitotoxicity in glaucoma. J Glaucoma 7, 62–67. [PubMed] [Google Scholar]
- Eckenstein F, Thoenen H, 1982. Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J 1, 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenstein F, Thoenen H, 1983. Cholinergic neurons in the rat cerebral cortex demonstrated by immunohistochemical localization of choline acetyltransferase. Neurosci Lett 36, 211–215. [DOI] [PubMed] [Google Scholar]
- Ehinger B, 1981. [3H]-D-aspartate accumulation in the retina of pigeon, guinea-pig and rabbit. Exp Eye Res 33, 381–391. [DOI] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM, Koos T, 2011. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci 15, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH, 1990. Signaling through phosphatidylcholine breakdown. J Biol Chem 265, 1–4. [PubMed] [Google Scholar]
- Exton JH, 1994. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta 1212, 26–42. [DOI] [PubMed] [Google Scholar]
- Fagen ZM, Mansvelder HD, Keath JR, McGehee DS, 2003. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann N Y Acad Sci 1003, 185–195. [DOI] [PubMed] [Google Scholar]
- Fahy ET, Chrysostomou V, Crowston JG, 2016. Mini-Review: Impaired Axonal Transport and Glaucoma. Curr Eye Res 41, 273–283. [DOI] [PubMed] [Google Scholar]
- Faiq M, Dada R, Singh H, Dada T, 2017. Comprehensive genomic/transcriptomic architecture of glaucoma in CYP1B1 and MYOC knockouts of primary rat TM and RGCs using CRISPR/Cas9 system, 7th World Glaucoma Congress World Glaucoma Association, Helsinki, Finland, p. 55. [Google Scholar]
- Faiq M, Mohanty K, Dada R, Dada T, 2013a. Molecular Diagnostics and Genetic Counseling in Primary Congenital Glaucoma. J Curr Glaucoma Pract 7, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiq M, Saluja D, Dada R, Mohanty K, Dada T, 2016a. Comprehensive Genomewide Functional Characterization of Etiopathogenesis of Primary Congenital Glaucoma for Identification of Potential Therapeutic Agents and Targets, ARVO Investigative Ophthalmology & Visual Science, Seattle, Washington, p. 4799. [Google Scholar]
- Faiq M, Sharma R, Dada R, Mohanty K, Saluja D, Dada T, 2013b. Genetic, Biochemical and Clinical Insights into Primary Congenital Glaucoma. J Curr Glaucoma Pract 7, 66–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiq MA, 2016. Vision restoration in glaucoma: Nihilism and optimism at the crossroads. Oman J Ophthalmol 9, 123–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiq MA, 2018. The Dark Matter of Vision: In search of a Grand Unifying Theory for Glaucoma. Oman J Ophthalmol 11, 101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiq MA, Ali M, Dada T, Dada R, Saluja D, 2014a. A novel methodology for enhanced and consistent heterologous expression of unmodified human cytochrome P450 1B1 (CYP1B1). PLoS One 9, e110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiq MA, Dada R, Kumar A, Saluja D, Dada T, 2016b. Brain: The Potential Diagnostic and Therapeutic Target for Glaucoma. CNS Neurol Disord Drug Targets 15, 839–844. [DOI] [PubMed] [Google Scholar]
- Faiq MA, Dada R, Qadri R, Dada T, 2015. CYP1B1-mediated Pathobiology of Primary Congenital Glaucoma. J Curr Glaucoma Pract 9, 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiq MA, Dada R, Saluja D, Dada T, 2014b. Glaucoma--diabetes of the brain: a radical hypothesis about its nature and pathogenesis. Med Hypotheses 82, 535–546. [DOI] [PubMed] [Google Scholar]
- Faiq MA, Dada R, Sharma R, Saluja D, Dada T, 2014c. CYP1B1: a unique gene with unique characteristics. Curr Drug Metab 15, 893–914. [DOI] [PubMed] [Google Scholar]
- Faiq MA, Dada T, 2017. Diabetes Type 4: A Paradigm Shift in the Understanding of Glaucoma, the Brain Specific Diabetes and the Candidature of Insulin as a Therapeutic Agent. Curr Mol Med 17, 46–59. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV Jr., 1983. ‘Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res 261, 138–144. [DOI] [PubMed] [Google Scholar]
- Farber SA, Savci V, Wei A, Slack BE, Wurtman RJ, 1996. Choline’s phosphorylation in rat striatal slices is regulated by the activity of cholinergic neurons. Brain Res 723, 90–99. [DOI] [PubMed] [Google Scholar]
- Fedorov A, Jobke S, Bersnev V, Chibisova A, Chibisova Y, Gall C, Sabel BA, 2011. Restoration of vision after optic nerve lesions with noninvasive transorbital alternating current stimulation: a clinical observational study. Brain Stimul 4, 189–201. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC, 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1, 1–47. [DOI] [PubMed] [Google Scholar]
- Feng J, Xu J, 2019. Identification of pathogenic genes and transcription factors in glaucoma. Mol Med Rep 20, 216–224. [DOI] [PubMed] [Google Scholar]
- FitzGibbon T, Nestorovski Z, 2013. Human intraretinal myelination: axon diameters and axon/myelin thickness ratios. Indian J Ophthalmol 61, 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer J, Konieczka K, 2017. The discovery of the Flammer syndrome: a historical and personal perspective. EPMA J 8, 75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer J, Orgul S, 1998. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res 17, 267–289. [DOI] [PubMed] [Google Scholar]
- Fraigne JJ, Torontali ZA, Snow MB, Peever JH, 2015. REM Sleep at its Core - Circuits, Neurotransmitters, and Pathophysiology. Front Neurol 6, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Maside A, Caamano J, Gomez MJ, Cacabelos R, 1994. Brain mapping activity and mental performance after chronic treatment with CDP-choline in Alzheimer’s disease. Methods Find Exp Clin Pharmacol 16, 597–607. [PubMed] [Google Scholar]
- Fresina M, Dickmann A, Salerni A, De Gregorio F, Campos EC, 2008. Effect of oral CDP-choline on visual function in young amblyopic patients. Graefes Arch Clin Exp Ophthalmol 246, 143–150. [DOI] [PubMed] [Google Scholar]
- Fresta M, Puglisi G, Di Giacomo C, Russo A, 1994. Liposomes as in-vivo carriers for citicoline: effects on rat cerebral post-ischaemic reperfusion. J Pharm Pharmacol 46, 974–981. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Gellner AK, Reis J, 2017. Transcranial Electrical Brain Stimulation in Alert Rodents. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Leranth C, 1985. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol 239, 237–246. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Vida I, Bender R, 2000. Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience 96, 27–31. [DOI] [PubMed] [Google Scholar]
- Fry LE, Fahy E, Chrysostomou V, Hui F, Tang J, van Wijngaarden P, Petrou S, Crowston JG, 2018. The coma in glaucoma: Retinal ganglion cell dysfunction and recovery. Prog Retin Eye Res 65, 77–92. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Yasuda M, Ninomiya T, Hata J, Hashimoto S, Yoshitomi T, Kiyohara Y, Ishibashi T, 2015. Insulin Resistance Is a Risk Factor for Increased Intraocular Pressure: The Hisayama Study. Invest Ophthalmol Vis Sci 56, 7983–7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke S, Beutgen VM, Bechter L, Schmelter C, Zurawski V, Perumal N, Pfeiffer N, Grus FH, 2019. An In-Depth View of the Porcine Trabecular Meshwork Proteome. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagrani M, Faiq MA, Sidhu T, Dada R, Yadav RK, Sihota R, Kochhar KP, Verma R, Dada T, 2018. Meditation enhances brain oxygenation, upregulates BDNF and improves quality of life in patients with primary open angle glaucoma: A randomized controlled trial. Restor Neurol Neurosci 36, 741–753. [DOI] [PubMed] [Google Scholar]
- Gall C, Schmidt S, Schittkowski MP, Antal A, Ambrus GG, Paulus W, Dannhauer M, Michalik R, Mante A, Bola M, Lux A, Kropf S, Brandt SA, Sabel BA, 2016. Alternating Current Stimulation for Vision Restoration after Optic Nerve Damage: A Randomized Clinical Trial. PLoS One 11, e0156134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall C, Sgorzaly S, Schmidt S, Brandt S, Fedorov A, Sabel BA, 2011. Noninvasive transorbital alternating current stimulation improves subjective visual functioning and vision-related quality of life in optic neuropathy. Brain Stimul 4, 175–188. [DOI] [PubMed] [Google Scholar]
- Galletti P, De Rosa M, Cotticelli MG, Morana A, Vaccaro R, Zappia V, 1991. Biochemical rationale for the use of CDPcholine in traumatic brain injury: pharmacokinetics of the orally administered drug. J Neurol Sci 103 Suppl, S19–25. [DOI] [PubMed] [Google Scholar]
- Galletti P, De Rosa M, Nappi MA, Pontoni G, del Piano L, Salluzzo A, Zappia V, 1985. Transport and metabolism of double-labelled CDPcholine in mammalian tissues. Biochem Pharmacol 34, 4121–4130. [DOI] [PubMed] [Google Scholar]
- Galvan C, Camoletto PG, Dotti CG, Aguzzi A, Ledesma MD, 2005. Proper axonal distribution of PrP(C) depends on cholesterol-sphingomyelin-enriched membrane domains and is developmentally regulated in hippocampal neurons. Mol Cell Neurosci 30, 304–315. [DOI] [PubMed] [Google Scholar]
- Gao X, Wang Y, Sun G, 2017. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition 33, 28–34. [DOI] [PubMed] [Google Scholar]
- Gareri P, Castagna A, Cotroneo AM, Putignano D, Conforti R, Santamaria F, Marino S, Putignano S, 2017. The Citicholinage Study: Citicoline Plus Cholinesterase Inhibitors in Aged Patients Affected with Alzheimer’s Disease Study. J Alzheimers Dis 56, 557–565. [DOI] [PubMed] [Google Scholar]
- Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC, 2015. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging 10, 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, Diamond JP, Fraser SG, Ho TA, Martin KR, McNaught AI, Negi A, Patel K, Russell RA, Shah A, Spry PG, Suzuki K, White ET, Wormald RP, Xing W, Zeyen TG, 2015. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 385, 1295–1304. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Luiten PG, Nyakas C, Traber J, 1990. Cortical projection patterns of the medial septum-diagonal band complex. J Comp Neurol 293, 103–124. [DOI] [PubMed] [Google Scholar]
- Ghaffariyeh A, Honarpisheh N, Heidari MH, Puyan S, Abasov F, 2011. Brain-derived neurotrophic factor as a biomarker in primary open-angle glaucoma. Optom Vis Sci 88, 80–85. [DOI] [PubMed] [Google Scholar]
- Ghiso JA, Doudevski I, Ritch R, Rostagno AA, 2013. Alzheimer’s disease and glaucoma: mechanistic similarities and differences. J Glaucoma 22 Suppl 5, S36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacci MK, Bartlett CA, Huynh M, Kilburn MR, Dunlop SA, Fitzgerald M, 2018. Three dimensional electron microscopy reveals changing axonal and myelin morphology along normal and partially injured optic nerves. Sci Rep 8, 3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Sethuraman G, Kotagal U, Buncher CR, 2003. Meta-analysis of EEG test performance shows wide variation among studies. Neurology 60, 564–570. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D, 2001. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 40, 959–975. [DOI] [PubMed] [Google Scholar]
- Gobeil S, Letartre L, Raymond V, 2006. Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res 82, 1017–1029. [DOI] [PubMed] [Google Scholar]
- Goebel DJ, Aurelia JL, Tai Q, Jojich L, Poosch MS, 1998. Immunocytochemical localization of the NMDA-R2A receptor subunit in the cat retina. Brain Res 808, 141–154. [DOI] [PubMed] [Google Scholar]
- Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR, 2004. Genetic dissection of myocilin glaucoma. Hum Mol Genet 13 Spec No 1, R91–102. [DOI] [PubMed] [Google Scholar]
- Gorbachevskaia AI, Chivileva OG, 2005. [The organization of the efferent projections of the pedunculo-pontine tegmental nucleus to the dog pallidum ]. Morfologiia 127, 19–23. [PubMed] [Google Scholar]
- Gorevic PD, 2013. Amyloid and inflammation. Proc Natl Acad Sci U S A 110, 16291–16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ, 2007. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol 74, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb P, 2014. Neuroprotective properties of citicoline: facts, doubts and unresolved issues. CNS Drugs 28, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb P, Junemann A, Rekas M, Rejdak R, 2016. Citicoline: A Food Beneficial for Patients Suffering from or Threated with Glaucoma. Front Aging Neurosci 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb P, Rejdak R, 2002. Pharmacodynamics of citicoline relevant to the treatment of glaucoma. J Neurosci Res 67, 143–148. [DOI] [PubMed] [Google Scholar]
- Grieve KL, 2005. Binocular visual responses in cells of the rat dLGN. J Physiol 566, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN, 1986. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324, 361–364. [DOI] [PubMed] [Google Scholar]
- Groleau M, Kang JI, Huppe-Gourgues F, Vaucher E, 2015. Distribution and effects of the muscarinic receptor subtypes in the primary visual cortex. Front Synaptic Neurosci 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruntzig J, Hollmann F, 2019. Lymphatic vessels of the eye - old questions - new insights. Ann Anat 221, 1–16. [DOI] [PubMed] [Google Scholar]
- Gungor IU, Gungor L, Ozarslan Y, Ariturk N, Beden U, Erkan D, Onar MK, Oge I, 2011. Is symptomatic atherosclerotic cerebrovascular disease a risk factor for normal-tension glaucoma? Med Princ Pract 20, 220–224. [DOI] [PubMed] [Google Scholar]
- Gunn DJ, Gole GA, Barnett NL, 2011. Specific amacrine cell changes in an induced mouse model of glaucoma. Clin Exp Ophthalmol 39, 555–563. [DOI] [PubMed] [Google Scholar]
- Guo L, Wang R, Tang Z, Sun X, Wu L, Wang J, Zhong Y, Xiao Z, Zhang Z, 2018. Metabolic Alterations Within the Primary Visual Cortex in Early Open-angle Glaucoma Patients: A Proton Magnetic Resonance Spectroscopy Study. J Glaucoma 27, 1046–1051. [DOI] [PubMed] [Google Scholar]
- Guo Q, Wang D, He X, Feng Q, Lin R, Xu F, Fu L, Luo M, 2015. Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLoS One 10, e0123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH, 2006. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol 90, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Yucel YH, 2007. What changes can we expect in the brain of glaucoma patients? Surv Ophthalmol 52 Suppl 2, S122–126. [DOI] [PubMed] [Google Scholar]
- Guymer C, Wood JP, Chidlow G, Casson RJ, 2019. Neuroprotection in glaucoma: recent advances and clinical translation. Clin Exp Ophthalmol 47, 88–105. [DOI] [PubMed] [Google Scholar]
- Hamassaki-Britto DE, Hermans-Borgmeyer I, Heinemann S, Hughes TE, 1993. Expression of glutamate receptor genes in the mammalian retina: the localization of GluR1 through GluR7 mRNAs. J Neurosci 13, 1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin N, Blatrix C, Brion F, Mathieu C, Goemaere I, Nordmann JP, 2002. [How patients react when glaucoma is diagnosed?]. J Fr Ophtalmol 25, 795–798. [PubMed] [Google Scholar]
- Harada C, Kimura A, Guo X, Namekata K, Harada T, 2019. Recent advances in genetically modified animal models of glaucoma and their roles in drug repositioning. Br J Ophthalmol 103, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel R, Futerman AH, 1993. Inhibition of sphingolipid synthesis affects axonal outgrowth in cultured hippocampal neurons. J Biol Chem 268, 14476–14481. [PubMed] [Google Scholar]
- Harris A, Chung HS, Ciulla TA, Kagemann L, 1999. Progress in measurement of ocular blood flow and relevance to our understanding of glaucoma and age-related macular degeneration. Prog Retin Eye Res 18, 669–687. [DOI] [PubMed] [Google Scholar]
- Harvey AR, Ooi JW, Rodger J, 2012. Neurotrophic factors and the regeneration of adult retinal ganglion cell axons. Int Rev Neurobiol 106, 1–33. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Wheat JL, Fredette MJ, Anderson DR, 2010. Linking structure and function in glaucoma. Prog Retin Eye Res 29, 249–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Shu H, Kuriyama K, 1994. Muscarinic M1 receptor mediated inhibition of GABA release from rat cerebral cortex. Neurochem Int 24, 389–394. [DOI] [PubMed] [Google Scholar]
- Hasnain SS, 2006. Scleral edge, not optic disc or retina, is the primary site of injury in chronic glaucoma. Med Hypotheses 67, 1320–1325. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Pe’er J, Zimmerman MB, 1999. Morphologic changes in chronic high-pressure experimental glaucoma in rhesus monkeys. J Glaucoma 8, 56–71. [PubMed] [Google Scholar]
- Hellier JL, Arevalo NL, Blatner MJ, Dang AK, Clevenger AC, Adams CE, Restrepo D, 2010. Olfactory discrimination varies in mice with different levels of alpha7-nicotinic acetylcholine receptor expression. Brain Res 1358, 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich-Noack P, Sergeeva EG, Eber T, You Q, Voigt N, Kohler J, Wagner S, Lazik S, Mawrin C, Xu G, Biswas S, Sabel BA, Leung CK, 2017a. Electrical brain stimulation induces dendritic stripping but improves survival of silent neurons after optic nerve damage. Sci Rep 7, 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich-Noack P, Sergeeva EG, Sabel BA, 2017b. Non-invasive electrical brain stimulation: from acute to late-stage treatment of central nervous system damage. Neural Regen Res 12, 1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A, 2008. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454, 1110–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LC, Wang B, Conner IP, van der Merwe Y, Bilonick RA, Kim SG, Wu EX, Sigal IA, Wollstein G, Schuman JS, Chan KC, 2015. In Vivo Evaluation of White Matter Integrity and Anterograde Transport in Visual System After Excitotoxic Retinal Injury With Multimodal MRI and OCT. Invest Ophthalmol Vis Sci 56, 3788–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB, Muth EA, Jacobowitz DM, 1978. A mapping of the distribution of acetycholine, choline acetyltransferase and acetylcholinesterase in discrete areas of rat brain. Brain Res 153, 295–306. [DOI] [PubMed] [Google Scholar]
- Hou H, Shoji T, Zangwill LM, Moghimi S, Saunders LJ, Hasenstab K, Ghahari E, Manalastas PIC, Akagi T, Christopher M, Penteado RC, Weinreb RN, 2018. Progression of Primary Open-Angle Glaucoma in Diabetic and Nondiabetic Patients. Am J Ophthalmol 189, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Salvaterra PM, Vaughn JE, 1985. Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: a study of cholinergic neurons and synapses. J Comp Neurol 234, 17–34. [DOI] [PubMed] [Google Scholar]
- Howe AR, Surmeier DJ, 1995. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci 15, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Libby RT, John SW, 2013. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol 246, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Teng S, He J, Sun X, Du M, Kou L, Wang X, 2018. Pharmacological basis for application of scutellarin in Alzheimer’s disease: Antioxidation and antiapoptosis. Mol Med Rep 18, 4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubens WHG, Breddels EM, Walid Y, Ramdas WD, Webers CAB, Gorgels T, 2019. Mapping mRNA Expression of Glaucoma Genes in the Healthy Mouse Eye. Curr Eye Res, 1–12. [DOI] [PubMed] [Google Scholar]
- Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB, 1994. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther 269, 329–335. [PubMed] [Google Scholar]
- Huppe-Gourgues F, Jegouic K, Vaucher E, 2018. Topographic Organization of Cholinergic Innervation From the Basal Forebrain to the Visual Cortex in the Rat. Front Neural Circuits 12, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado O, Moro MA, Cardenas A, Sanchez V, Fernandez-Tome P, Leza JC, Lorenzo P, Secades JJ, Lozano R, Davalos A, Castillo J, Lizasoain I, 2005. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport. Neurobiol Dis 18, 336–345. [DOI] [PubMed] [Google Scholar]
- Ito YA, Di Polo A, 2017. Mitochondrial dynamics, transport, and quality control: A bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion 36, 186–192. [DOI] [PubMed] [Google Scholar]
- Iulia C, Ruxandra T, Costin LB, Liliana-Mary V, 2017. Citicoline - a neuroprotector with proven effects on glaucomatous disease. Rom J Ophthalmol 61, 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Bagnis A, Sacca SC, 2006. The role of oxidative stress in glaucoma. Mutat Res 612, 105–114. [DOI] [PubMed] [Google Scholar]
- Jackowski S, 1994. Coordination of membrane phospholipid synthesis with the cell cycle. J Biol Chem 269, 3858–3867. [PubMed] [Google Scholar]
- Jakobs TC, 2014. Differential gene expression in glaucoma. Cold Spring Harb Perspect Med 4, a020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SF, Gorgels TG, Ramdas WD, Klaver CC, van Duijn CM, Jansonius NM, Bergen AA, 2013. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog Retin Eye Res 37, 31–67. [DOI] [PubMed] [Google Scholar]
- Janz NK, Wren PA, Guire KE, Musch DC, Gillespie BW, Lichter PR, Collaborative Initial Glaucoma Treatment, S., 2007. Fear of blindness in the Collaborative Initial Glaucoma Treatment Study: patterns and correlates over time. Ophthalmology 114, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Johannesson G, Eklund A, Linden C, 2018. Intracranial and Intraocular Pressure at the Lamina Cribrosa: Gradient Effects. Curr Neurol Neurosci Rep 18, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC, 2007. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci 48, 3161–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Martin KR, 2011. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp Eye Res 93, 196–203. [DOI] [PubMed] [Google Scholar]
- Jojich L, Pourcho RG, 1996. Glutamate immunoreactivity in the cat retina: a quantitative study. Vis Neurosci 13, 117–133. [DOI] [PubMed] [Google Scholar]
- Jones BE, 2004. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res 145, 157–169. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Fletcher EL, 1993. Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. J Comp Neurol 336, 174–193. [DOI] [PubMed] [Google Scholar]
- Kanagavalli J, Pandaranayaka E, Krishnadas SR, Krishnaswamy S, Sundaresan P, 2004. A review of genetic and structural understanding of the role of myocilin in primary open angle glaucoma. Indian J Ophthalmol 52, 271–280. [PubMed] [Google Scholar]
- Kaneko S, Maeda T, Kume T, Kochiyama H, Akaike A, Shimohama S, Kimura J, 1997. Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via alpha7-neuronal receptors and neuronal CNS receptors. Brain Res 765, 135–140. [DOI] [PubMed] [Google Scholar]
- Kang JI, Groleau M, Dotigny F, Giguere H, Vaucher E, 2014a. Visual training paired with electrical stimulation of the basal forebrain improves orientation-selective visual acuity in the rat. Brain Struct Funct 219, 1493–1507. [DOI] [PubMed] [Google Scholar]
- Kang JI, Huppe-Gourgues F, Vaucher E, 2014b. Boosting visual cortex function and plasticity with acetylcholine to enhance visual perception. Front Syst Neurosci 8, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Chen Y, Epstein PN, 1996. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem 271, 12610–12616. [DOI] [PubMed] [Google Scholar]
- Kano M, 2014. Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc Jpn Acad Ser B Phys Biol Sci 90, 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa P, 1986. The cholinergic systems in brain and spinal cord. Prog Neurobiol 26, 211–272. [DOI] [PubMed] [Google Scholar]
- Kashkin VA, Shekunova EV, Makarova MN, Makarov VG, 2017. [A study of combination treatment with nacom (levodopa + carbodope) and citicoline in the model of Parkinson disease in rats]. Zh Nevrol Psikhiatr Im S S Korsakova 117, 59–63. [DOI] [PubMed] [Google Scholar]
- Kasi A, Faiq MA, Chan KC, 2019. In vivo imaging of structural, metabolic and functional brain changes in glaucoma. Neural Regen Res 14, 446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten E, Wust S, Behrens-Baumann W, Sabel BA, 1998. Computer-based training for the treatment of partial blindness. Nat Med 4, 1083–1087. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Fishell G, 2014. Interneuron cell types are fit to function. Nature 505, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Teerds K, Dorrington J, 1992. Steroidogenesis-inducing protein promotes deoxyribonucleic acid synthesis in Leydig cells from immature rats. Endocrinology 130, 599–606. [DOI] [PubMed] [Google Scholar]
- Khor CC, Do T, Jia H, Nakano M, George R, Abu-Amero K, Duvesh R, Chen LJ, Li Z, Nongpiur ME, Perera SA, Qiao C, Wong HT, Sakai H, Barbosa de Melo M, Lee MC, Chan AS, Azhany Y, Dao TL, Ikeda Y, Perez-Grossmann RA, Zarnowski T, Day AC, Jonas JB, Tam PO, Tran TA, Ayub H, Akhtar F, Micheal S, Chew PT, Aljasim LA, Dada T, Luu TT, Awadalla MS, Kitnarong N, Wanichwecharungruang B, Aung YY, Mohamed-Noor J, Vijayan S, Sarangapani S, Husain R, Jap A, Baskaran M, Goh D, Su DH, Wang H, Yong VK, Yip LW, Trinh TB, Makornwattana M, Nguyen TT, Leuenberger EU, Park KH, Wiyogo WA, Kumar RS, Tello C, Kurimoto Y, Thapa SS, Pathanapitoon K, Salmon JF, Sohn YH, Fea A, Ozaki M, Lai JS, Tantisevi V, Khaing CC, Mizoguchi T, Nakano S, Kim CY, Tang G, Fan S, Wu R, Meng H, Nguyen TT, Tran TD, Ueno M, Martinez JM, Ramli N, Aung YM, Reyes RD, Vernon SA, Fang SK, Xie Z, Chen XY, Foo JN, Sim KS, Wong TT, Quek DT, Venkatesh R, Kavitha S, Krishnadas SR, Soumittra N, Shantha B, Lim BA, Ogle J, de Vasconcellos JP, Costa VP, Abe RY, de Souza BB, Sng CC, Aquino MC, Kosior-Jarecka E, Fong GB, Tamanaja VC, Fujita R, Jiang Y, Waseem N, Low S, Pham HN, Al-Shahwan S, Craven ER, Khan MI, Dada R, Mohanty K, Faiq MA, Hewitt AW, Burdon KP, Gan EH, Prutthipongsit A, Patthanathamrongkasem T, Catacutan MA, Felarca IR, Liao CS, Rusmayani E, Istiantoro VW, Consolandi G, Pignata G, Lavia C, Rojanapongpun P, Mangkornkanokpong L, Chansangpetch S, Chan JC, Choy BN, Shum JW, Than HM, Oo KT, Han AT, Yong VH, Ng XY, Goh SR, Chong YF, Hibberd ML, Seielstad M, Png E, Dunstan SJ, Chau NV, Bei J, Zeng YX, Karkey A, Basnyat B, Pasutto F, Paoli D, Frezzotti P, Wang JJ, Mitchell P, Fingert JH, Allingham RR, Hauser MA, Lim ST, Chew SH, Ebstein RP, Sakuntabhai A, Park KH, Ahn J, Boland G, Snippe H, Stead R, Quino R, Zaw SN, Lukasik U, Shetty R, Zahari M, Bae HW, Oo NL, Kubota T, Manassakorn A, Ho WL, Dallorto L, Hwang YH, Kiire CA, Kuroda M, Djamal ZE, Peregrino JI, Ghosh A, Jeoung JW, Hoan TS, Srisamran N, Sandragasu T, Set SH, Doan VH, Bhattacharya SS, Ho CL, Tan DT, Sihota R, Loon SC, Mori K, Kinoshita S, Hollander AI, Qamar R, Wang YX, Teo YY, Tai ES, Hartleben-Matkin C, Lozano-Giral D, Saw SM, Cheng CY, Zenteno JC, Pang CP, Bui HT, Hee O, Craig JE, Edward DP, Yonahara M, Neto JM, Guevara-Fujita ML, Xu L, Ritch R, Liza-Sharmini AT, Wong TY, Al-Obeidan S, Do NH, Sundaresan P, Tham CC, Foster PJ, Vijaya L, Tashiro K, Vithana EN, Wang N, Aung T, 2016. Genome-wide association study identifies five new susceptibility loci for primary angle closure glaucoma. Nat Genet 48, 556–562. [DOI] [PubMed] [Google Scholar]
- Kimura A, Namekata K, Guo X, Noro T, Harada C, Harada T, 2017. Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid Med Cell Longev 2017, 2817252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Adibhatla RM, Hatcher JF, Franklin JL, 2002. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience 115, 587–602. [DOI] [PubMed] [Google Scholar]
- Koistinaho J, Sagar SM, 1995. Light-induced c-fos expression in amacrine cells in the rabbit retina. Brain Res Mol Brain Res 29, 53–63. [DOI] [PubMed] [Google Scholar]
- Kong GY, Van Bergen NJ, Trounce IA, Crowston JG, 2009. Mitochondrial dysfunction and glaucoma. J Glaucoma 18, 93–100. [DOI] [PubMed] [Google Scholar]
- Konieczka K, Choi HJ, Koch S, Fankhauser F, Schoetzau A, Kim DM, 2017. Relationship between normal tension glaucoma and Flammer syndrome. EPMA J 8, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczka K, Ritch R, Traverso CE, Kim DM, Kook MS, Gallino A, Golubnitschaja O, Erb C, Reitsamer HA, Kida T, Kurysheva N, Yao K, 2014. Flammer syndrome. EPMA J 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Abudawood M, Matou-Nasri S, Al-Baradie R, Petcu EB, Justicia C, Planas A, Liu D, Rovira N, Grau-Slevin M, Secades J, Slevin M, 2012. Citicoline induces angiogenesis improving survival of vascular/human brain microvessel endothelial cells through pathways involving ERK½ and insulin receptor substrate-1. Vasc Cell 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis RO, Krause JE, Karten HJ, 1984. Peptide-like immunoreactivity in anuran optic nerve fibers. J Comp Neurol 226, 222–237. [DOI] [PubMed] [Google Scholar]
- Kumar A, Pareek V, Faiq MA, Kumar P, Raza K, Prasoon P, Dantham S, Mochan S, 2017. Regulatory role of NGFs in neurocognitive functions. Rev Neurosci 28, 649–673. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rinwa P, Kaur G, Machawal L, 2013a. Stress: Neurobiology, consequences and management. J Pharm Bioallied Sci 5, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Tanwar M, Faiq MA, Pani J, Shamsi MB, Dada T, Dada R, 2013b. Mitochondrial DNA nucleotide changes in primary congenital glaucoma patients. Mol Vis 19, 220–230. [PMC free article] [PubMed] [Google Scholar]
- Lagace TA, 2015. Phosphatidylcholine: Greasing the Cholesterol Transport Machinery. Lipid Insights 8, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante F, Morin Y, Quirion R, Vaucher E, 2005. Acetylcholine release is elicited in the visual cortex, but not in the prefrontal cortex, by patterned visual stimulation: a dual in vivo microdialysis study with functional correlates in the rat brain. Neuroscience 132, 501–510. [DOI] [PubMed] [Google Scholar]
- Laranjeira E, Buzard KA, 1996. Pilocarpine in the management of overcorrection after radial keratotomy. J Refract Surg 12, 382–390. [DOI] [PubMed] [Google Scholar]
- Lau TL, Ambroggio EE, Tew DJ, Cappai R, Masters CL, Fidelio GD, Barnham KJ, Separovic F, 2006. Amyloid-beta peptide disruption of lipid membranes and the effect of metal ions. J Mol Biol 356, 759–770. [DOI] [PubMed] [Google Scholar]
- Lawlor M, Danesh-Meyer H, Levin LA, Davagnanam I, De Vita E, Plant GT, 2018. Glaucoma and the brain: Trans-synaptic degeneration, structural change, and implications for neuroprotection. Surv Ophthalmol 63, 296–306. [DOI] [PubMed] [Google Scholar]
- Lee BH, Choi SH, Kim HJ, Jung SW, Hwang SH, Pyo MK, Rhim H, Kim HC, Kim HK, Lee SM, Nah SY, 2016. Differential Effects of Quercetin and Quercetin Glycosides on Human alpha7 Nicotinic Acetylcholine Receptor-Mediated Ion Currents. Biomol Ther (Seoul) 24, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Kim BR, Lee SM, Lee JH, Kim HC, Park HY, Rhim H, Nah SY, 2010. Quercetin enhances human alpha7 nicotinic acetylcholine receptor-mediated ion current through interactions with Ca(2+) binding sites. Mol Cells 30, 245–253. [DOI] [PubMed] [Google Scholar]
- Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Lee SM, Paik HD, Kim HC, Nah SY, 2011a. Effects of quercetin on alpha9alpha10 nicotinic acetylcholine receptor-mediated ion currents. Eur J Pharmacol 650, 79–85. [DOI] [PubMed] [Google Scholar]
- Lee DA, Higginbotham EJ, 2005. Glaucoma and its treatment: a review. Am J Health Syst Pharm 62, 691–699. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kuo LL, Tan EC, Lee OK, 2017. Is normal-tension glaucoma a risk factor for stroke?-A 10-year follow-up study. PLoS One 12, e0179307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Van Bergen NJ, Kong GY, Chrysostomou V, Waugh HS, O’Neill EC, Crowston JG, Trounce IA, 2011b. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res 93, 204–212. [DOI] [PubMed] [Google Scholar]
- Leibiger IB, Berggren PO, 2015. Regulation of glucose homeostasis using radiogenetics and magnetogenetics in mice. Nat Med 21, 14–16. [DOI] [PubMed] [Google Scholar]
- Leske MC, Connell AM, Wu SY, Hyman L, Schachat AP, 1997. Distribution of intraocular pressure. The Barbados Eye Study. Arch Ophthalmol 115, 1051–1057. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, Early Manifest Glaucoma Trial, G., 2003. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 121, 48–56. [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, Shatz CJ, 1978. Ocular dominance columns and their development in layer IV of the cat’s visual cortex: a quantitative study. J Comp Neurol 179, 223–244. [DOI] [PubMed] [Google Scholar]
- Levin B, 2015. The futility study--Progress over the last decade. Contemp Clin Trials 45, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LA, Crowe ME, Quigley HA, Lasker I.I.o.A., Glaucomatous Neurodegeneration, P., 2017. Neuroprotection for glaucoma: Requirements for clinical translation. Exp Eye Res 157, 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JB, Lund JS, 1997. Contrast dependence of contextual effects in primate visual cortex. Nature 387, 73–76. [DOI] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y, 2009. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 5, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu B, Sun Q, Zhang Y, Ren M, Zhang X, Li A, Yuan J, Madisen L, Luo Q, Zeng H, Gong H, Qiu Z, 2018. Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc Natl Acad Sci U S A 115, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XW, Wang H, 2006. Non-neuronal nicotinic alpha 7 receptor, a new endothelial target for revascularization. Life Sci 78, 1863–1870. [DOI] [PubMed] [Google Scholar]
- Lim SA, Kang UJ, McGehee DS, 2014. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Martin PR, Grunert U, 2002. Expression and distribution of ionotropic glutamate receptor subunits on parasol ganglion cells in the primate retina. Vis Neurosci 19, 453–465. [DOI] [PubMed] [Google Scholar]
- Linn CL, 2016. Retinal ganglion cell neuroprotection induced by activation of alpha7 nicotinic acetylcholine receptors. Neural Regen Res 11, 918–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Su WW, Shie SS, Cheng ST, Su CW, Ho WJ, 2016. Association Between Peripheral Vascular Endothelial Function and Progression of Open-Angle Glaucoma. Medicine (Baltimore) 95, e3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tonegawa S, 2010. Optogenetics 3.0. Cell 141, 22–24. [DOI] [PubMed] [Google Scholar]
- Lotery AJ, 2005. Glutamate excitotoxicity in glaucoma: truth or fiction? Eye (Lond) 19, 369–370. [DOI] [PubMed] [Google Scholar]
- Loughman J, Davison P, Flitcroft I, 2007. Open angle glaucoma effects on preattentive visual search efficiency for flicker, motion displacement and orientation pop-out tasks. Br J Ophthalmol 91, 1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A, Bloem B, Viana JN, Mansvelder HD, Role LW, 2014. Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors. Front Synaptic Neurosci 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Miyazaki M, 1998. Control of ICP and the cerebrovascular bed by the cholinergic basal forebrain. Acta Neurochir Suppl 71, 293–296. [DOI] [PubMed] [Google Scholar]
- Mancino R, Martucci A, Cesareo M, Giannini C, Corasaniti MT, Bagetta G, Nucci C, 2018. Glaucoma and Alzheimer Disease: One Age-Related Neurodegenerative Disease of the Brain. Curr Neuropharmacol 16, 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS, 2000. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27, 349–357. [DOI] [PubMed] [Google Scholar]
- Marcucci H, Paoletti L, Jackowski S, Banchio C, 2010. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J Biol Chem 285, 25382–25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AP, Hersh LB, 1988. Synaptic organization of cholinergic amacrine cells in the rhesus monkey retina. J Comp Neurol 267, 269–280. [DOI] [PubMed] [Google Scholar]
- Marin P, Maus M, Desagher S, Glowinski J, Premont J, 1994. Nicotine protects cultured striatal neurones against N-methyl-D-aspartate receptor-mediated neurotoxicity. Neuroreport 5, 1977–1980. [DOI] [PubMed] [Google Scholar]
- Mark GP, Rada PV, Shors TJ, 1996. Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience 74, 767–774. [DOI] [PubMed] [Google Scholar]
- Marti Masso JF, Urtasun M, 1991. Citicoline in the treatment of Parkinson’s disease. Clin Ther 13, 239–242. [PubMed] [Google Scholar]
- Martin KA, 2002. Microcircuits in visual cortex. Curr Opin Neurobiol 12, 418–425. [DOI] [PubMed] [Google Scholar]
- Martynov MY, Gusev EI, 2015. Current knowledge on the neuroprotective and neuroregenerative properties of citicoline in acute ischemic stroke. J Exp Pharmacol 7, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiulis I, Yun S, Eisch AJ, 2011. The interesting interplay between interneurons and adult hippocampal neurogenesis. Mol Neurobiol 44, 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Mills JW, Hayden SA, 1984. Acetylcholine-synthesizing amacrine cells: identification and selective staining by using radioautography and fluorescent markers. Proc R Soc Lond B Biol Sci 223, 79–100. [DOI] [PubMed] [Google Scholar]
- Matteucci A, Varano M, Gaddini L, Mallozzi C, Villa M, Pricci F, Malchiodi-Albedi F, 2014. Neuroprotective effects of citicoline in in vitro models of retinal neurodegeneration. Int J Mol Sci 15, 6286–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, Newsome WT, 1987. Visual processing in monkey extrastriate cortex. Annu Rev Neurosci 10, 363–401. [DOI] [PubMed] [Google Scholar]
- McCorry LK, 2007. Physiology of the autonomic nervous system. Am J Pharm Educ 71, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy CR, Glover ME, Flynn LT, Simmons RK, Cohen JL, Ptacek T, Lefkowitz EJ, Jackson NL, Akil H, Wu X, Clinton SM, 2019. Altered DNA Methylation in the Developing Brains of Rats Genetically Prone to High versus Low Anxiety. J Neurosci 39, 3144–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee DJ, Ritchie CW, Zajicek JP, Counsell CE, 2016. A review of clinical trial designs used to detect a disease-modifying effect of drug therapy in Alzheimer’s disease and Parkinson’s disease. BMC Neurol 16, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade E, Agoston AM, DiMuzio J, Kizaki M, Nakazaki E, Kamiya T, Yurgelun-Todd D, 2019. The Effect of Citicoline Supplementation on Motor Speed and Attention in Adolescent Males. J Atten Disord 23, 121–134. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A, 1996. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem 271, 26482–26489. [DOI] [PubMed] [Google Scholar]
- McMonnies CW, 2016. The interaction between intracranial pressure, intraocular pressure and lamina cribrosal compression in glaucoma. Clin Exp Optom 99, 219–226. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, 2014. Acetylcholine release and inhibitory interneuron activity in hippocampal CA1. Front Synaptic Neurosci 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D, 2010. Changes in brain MicroRNAs contribute to cholinergic stress reactions. J Mol Neurosci 40, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, 2006. Optogenetics. Shining new light on neural circuits. Science 314, 1674–1676. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Nakayama M, Iejima D, Kawase K, Iwata T, 2016. Significance of optineurin mutations in glaucoma and other diseases. Prog Retin Eye Res 55, 149–181. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR, 2013. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A 110, 3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir C, Clotet J, Aledo R, Durany N, Argemi J, Lozano R, Cervos-Navarro J, Casals N, 2003. CDP-choline prevents glutamate-mediated cell death in cerebellar granule neurons. J Mol Neurosci 20, 53–60. [DOI] [PubMed] [Google Scholar]
- Mize RR, Butler GD, 1996. Postembedding immunocytochemistry demonstrates directly that both retinal and cortical terminals in the cat superior colliculus are glutamate immunoreactive. J Comp Neurol 371, 633–648. [DOI] [PubMed] [Google Scholar]
- Moessinger C, Klizaite K, Steinhagen A, Philippou-Massier J, Shevchenko A, Hoch M, Ejsing CS, Thiele C, 2014. Two different pathways of phosphatidylcholine synthesis, the Kennedy Pathway and the Lands Cycle, differentially regulate cellular triacylglycerol storage. BMC Cell Biol 15, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero VM, 1994. Quantitative immunogold evidence for enrichment of glutamate but not aspartate in synaptic terminals of retino-geniculate, geniculo-cortical, and cortico-geniculate axons in the cat. Vis Neurosci 11, 675–681. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Turner N, 2015. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 4, R1–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JI, Kim IB, Gwon JS, Park MH, Kang TH, Lim EJ, Choi KR, Chun MH, 2005. Changes in retinal neuronal populations in the DBA/2J mouse. Cell Tissue Res 320, 51–59. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Cepurna Ying Guo WO, Johnson EC, 2011. Pathophysiology of human glaucomatous optic nerve damage: insights from rodent models of glaucoma. Exp Eye Res 93, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosinger JL, Price MT, Bai HY, Xiao H, Wozniak DF, Olney JW, 1991. Blockade of both NMDA and non-NMDA receptors is required for optimal protection against ischemic neuronal degeneration in the in vivo adult mammalian retina. Exp Neurol 113, 10–17. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS, 1993. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 90, 5194–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa Y, Kitaoka Y, Kuribayashi J, Ueno S, 2010. Modulation of mitochondria in the axon and soma of retinal ganglion cells in a rat glaucoma model. J Neurochem 115, 1508–1519. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Qualls-Creekmore E, Berthoud HR, Morrison CD, Yu S, 2016. Neural Control of Energy Expenditure. Handb Exp Pharmacol 233, 173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Conner IP, Teng CY, Lawrence JD, Safiullah Z, Wang B, Bilonick RA, Kim SG, Wollstein G, Schuman JS, Chan KC, 2016. Retinal Structures and Visual Cortex Activity are Impaired Prior to Clinical Vision Loss in Glaucoma. Sci Rep 6, 31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanza JC, Budenz DL, 2018. New developments in optical coherence tomography imaging for glaucoma. Curr Opin Ophthalmol 29, 121–129. [DOI] [PubMed] [Google Scholar]
- Najem D, Bamji-Mirza M, Chang N, Liu QY, Zhang W, 2014. Insulin resistance, neuroinflammation, and Alzheimer’s disease. Rev Neurosci 25, 509–525. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H, 2000. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J Chem Neuroanat 18, 31–40. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Tooyama I, Yasuhara O, Aimi Y, Kitajima K, Kimura H, 1999. Immunohistochemical localization of choline acetyltransferase of a peripheral type in the rat larynx. J Chem Neuroanat 17, 21–32. [DOI] [PubMed] [Google Scholar]
- Nimpf S, Keays DA, 2017. Is magnetogenetics the new optogenetics? EMBO J 36, 1643–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven JE, 2016. Neuronal energy consumption: biophysics, efficiency and evolution. Curr Opin Neurobiol 41, 129–135. [DOI] [PubMed] [Google Scholar]
- Nucci C, Martucci A, Cesareo M, Garaci F, Morrone LA, Russo R, Corasaniti MT, Bagetta G, Mancino R, 2015. Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog Brain Res 221, 49–65. [DOI] [PubMed] [Google Scholar]
- Nuzzi R, Dallorto L, Rolle T, 2018. Changes of Visual Pathway and Brain Connectivity in Glaucoma: A Systematic Review. Front Neurosci 12, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara M, 1974. [Prolongation of the viability of the preserved lung used in experimental lung transplantation]. Nihon Kyobu Geka Gakkai Zasshi 22, 1059–1070. [PubMed] [Google Scholar]
- Odberg T, Jakobsen JE, Hultgren SJ, Halseide R, 2001. The impact of glaucoma on the quality of life of patients in Norway. II. Patient response correlated to objective data. Acta Ophthalmol Scand 79, 121–124. [DOI] [PubMed] [Google Scholar]
- Oddo S, LaFerla FM, 2006. The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J Physiol Paris 99, 172–179. [DOI] [PubMed] [Google Scholar]
- Oh SW, Lee S, Park C, Kim DJ, 2005. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab Res Rev 21, 434–440. [DOI] [PubMed] [Google Scholar]
- Oliver JAC, Ricketts SL, Kuehn MH, Mellersh CS, 2019. Primary closed angle glaucoma in the Basset Hound: Genetic investigations using genome-wide association and RNA sequencing strategies. Mol Vis 25, 93–105. [PMC free article] [PubMed] [Google Scholar]
- Ortega F, Hennequet L, Sarria R, Streit P, Grandes P, 1995. Changes in the pattern of glutamate-like immunoreactivity in rat superior colliculus following retinal and visual cortical lesions. Neuroscience 67, 125–134. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Chidlow G, Wood JP, 2006. Glutamate excitotoxicity in glaucoma: truth or fiction? By AJ Lotery. Eye (Lond) 20, 1392–1394. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS, 1999. Ganglion cell death in glaucoma: what do we really know? Br J Ophthalmol 83, 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshitari T, Fujimoto N, Adachi-Usami E, 2002. Citicoline has a protective effect on damaged retinal ganglion cells in mouse culture retina. Neuroreport 13, 2109–2111. [DOI] [PubMed] [Google Scholar]
- Oswald RE, Freeman JA, 1980. Optic nerve transmitters in lower vertebrate species. Life Sci 27, 527–533. [DOI] [PubMed] [Google Scholar]
- Ottobelli L, Manni GL, Centofanti M, Iester M, Allevena F, Rossetti L, 2013. Citicoline oral solution in glaucoma: is there a role in slowing disease progression? Ophthalmologica 229, 219–226. [DOI] [PubMed] [Google Scholar]
- Ou Y, Grossman DS, Lee PP, Sloan FA, 2012. Glaucoma, Alzheimer disease and other dementia: a longitudinal analysis. Ophthalmic Epidemiol 19, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozay R, Bekar A, Kocaeli H, Karli N, Filiz G, Ulus IH, 2007. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol 68, 615–622. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Frankfort BJ, Gross RL, Wu SM, 2015. Elevated intraocular pressure decreases response sensitivity of inner retinal neurons in experimental glaucoma mice. Proc Natl Acad Sci U S A 112, 2593–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G, 2014. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837, 408–417. [DOI] [PubMed] [Google Scholar]
- Parisi V, 2005. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5’-diphosphocholine (citicoline): a study of 8 years of follow-up. Doc Ophthalmol 110, 91–102. [DOI] [PubMed] [Google Scholar]
- Parisi V, Centofanti M, Ziccardi L, Tanga L, Michelessi M, Roberti G, Manni G, 2015. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefes Arch Clin Exp Ophthalmol 253, 1327–1340. [DOI] [PubMed] [Google Scholar]
- Parisi V, Coppola G, Centofanti M, Oddone F, Angrisani AM, Ziccardi L, Ricci B, Quaranta L, Manni G, 2008a. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog Brain Res 173, 541–554. [DOI] [PubMed] [Google Scholar]
- Parisi V, Coppola G, Ziccardi L, Gallinaro G, Falsini B, 2008b. Cytidine-5’-diphosphocholine (Citicoline): a pilot study in patients with non-arteritic ischaemic optic neuropathy. Eur J Neurol 15, 465–474. [DOI] [PubMed] [Google Scholar]
- Parisi V, Manni G, Colacino G, Bucci MG, 1999. Cytidine-5’-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology 106, 1126–1134. [DOI] [PubMed] [Google Scholar]
- Parisi V, Oddone F, Ziccardi L, Roberti G, Coppola G, Manni G, 2018. Citicoline and Retinal Ganglion Cells: Effects on Morphology and Function. Curr Neuropharmacol 16, 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Kim YS, Noh HS, Cheon EW, Yang YA, Yoo JM, Choi WS, Cho GJ, 2005. Neuroprotective effect of citicoline against KA-induced neurotoxicity in the rat retina. Exp Eye Res 81, 350–358. [DOI] [PubMed] [Google Scholar]
- Pawar PV, Mumbare SS, Patil MS, Ramakrishnan S, 2014. Effectiveness of the addition of citicoline to patching in the treatment of amblyopia around visual maturity: a randomized controlled trial. Indian J Ophthalmol 62, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecori Giraldi J, Virno M, Covelli G, Grechi G, De Gregorio F, 1989. Therapeutic value of citicoline in the treatment of glaucoma (computerized and automated perimetric investigation). Int Ophthalmol 13, 109–112. [DOI] [PubMed] [Google Scholar]
- Petty HR, 2018. Frontiers of Complex Disease Mechanisms: Membrane Surface Tension May Link Genotype to Phenotype in Glaucoma. Front Cell Dev Biol 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS, 2012. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS, 2015. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology 96, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y, 2013. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci 16, 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE, 1992. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J Physiol 450, 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Schiavi C, Benedetti P, Baldi A, Campos EC, 1998. Cytidine-5’-diphosphocholine improves visual acuity, contrast sensitivity and visually-evoked potentials of amblyopic subjects. Curr Eye Res 17, 141–148. [DOI] [PubMed] [Google Scholar]
- Posse de Chaves E, Sipione S, 2010. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett 584, 1748–1759. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA, Bucci DJ, 2006. Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res 175, 201–211. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Osman K, 1986. Cytochemical identification of cholinergic amacrine cells in cat retina. J Comp Neurol 247, 497–504. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Shaw C, Cynader MS, 1987. Nicotine receptors are located on lateral geniculate nucleus terminals in cat visual cortex. Brain Res 412, 131–138. [DOI] [PubMed] [Google Scholar]
- Qadri R, Namdeo M, Behari M, Goyal V, Sharma S, Mukhopadhyay AK, 2018. Alterations in mitochondrial membrane potential in peripheral blood mononuclear cells in Parkinson’s Disease: Potential for a novel biomarker. Restor Neurol Neurosci 36, 719–727. [DOI] [PubMed] [Google Scholar]
- Qian K, Gu Y, Zhao Y, Li Z, Sun M, 2014. Citicoline protects brain against closed head injury in rats through suppressing oxidative stress and calpain over-activation. Neurochem Res 39, 1206–1218. [DOI] [PubMed] [Google Scholar]
- Quigley HA, 2012. Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol 23, 144–154. [DOI] [PubMed] [Google Scholar]
- Quigley HA, 2019. Glaucoma Neuroprotection Trials Are Practical Using Visual Field Outcomes. Glaucoma 2, 69–71 [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Isales CM, Calle R, Throckmorton D, Anderson M, Gasalla-Herraiz J, McCarthy R, 1995. Diacylglycerol production, Ca2+ influx, and protein kinase C activation in sustained cellular responses. Endocr Rev 16, 649–681. [DOI] [PubMed] [Google Scholar]
- Reilly MA, Villarreal A, Maddess T, Sponsel WE, 2015. Refined Frequency Doubling Perimetry Analysis Reaffirms Central Nervous System Control of Chronic Glaucomatous Neurodegeneration. Transl Vis Sci Technol 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejdak R, Toczolowski J, Kurkowski J, Kaminski ML, Rejdak K, Stelmasiak Z, Grieb P, 2003. Oral citicoline treatment improves visual pathway function in glaucoma. Med Sci Monit 9, PI24–28. [PubMed] [Google Scholar]
- Rejdak R, Toczolowski J, Solski J, Duma D, Grieb P, 2002. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res 34, 146–149. [DOI] [PubMed] [Google Scholar]
- Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L, 2009. Endothelial dysfunction in glaucoma. Acta Ophthalmol 87, 4–12. [DOI] [PubMed] [Google Scholar]
- Rieck J, 2013. The pathogenesis of glaucoma in the interplay with the immune system. Invest Ophthalmol Vis Sci 54, 2393–2409. [DOI] [PubMed] [Google Scholar]
- Risner ML, Pasini S, Cooper ML, Lambert WS, Calkins DJ, 2018. Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma. Proc Natl Acad Sci U S A 115, E2393–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberti G, Tanga L, Parisi V, Sampalmieri M, Centofanti M, Manni G, 2014. A preliminary study of the neuroprotective role of citicoline eye drops in glaucomatous optic neuropathy. Indian J Ophthalmol 62, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen PN, Boer ER, Gracitelli CP, Abe RY, Diniz-Filho A, Marvasti AH, Medeiros FA, 2015. A Portable Platform for Evaluation of Visual Performance in Glaucoma Patients. PLoS One 10, e0139426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Bluml S, 2001. Magnetic resonance spectroscopy of the human brain. Anat Rec 265, 54–84. [DOI] [PubMed] [Google Scholar]
- Rozsa FW, Shimizu S, Lichter PR, Johnson AT, Othman MI, Scott K, Downs CA, Nguyen TD, Polansky J, Richards JE, 1998. GLC1A mutations point to regions of potential functional importance on the TIGR/MYOC protein. Mol Vis 4, 20. [PubMed] [Google Scholar]
- Ruan L, Kang Z, Pei G, Le Y, 2009. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Res 6, 531–540. [DOI] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, Sadikot AF, 2004. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol 469, 325–339. [DOI] [PubMed] [Google Scholar]
- Sabel BA, 2008. Plasticity and restoration of vision after visual system damage: an update. Restor Neurol Neurosci 26, 243–247. [PubMed] [Google Scholar]
- Sabel BA, Cardenas-Morales L, Gao Y, 2018a. Vision Restoration in Glaucoma by Activating Residual Vision with a Holistic, Clinical Approach: A Review. J Curr Glaucoma Pract 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel BA, Fedorov AB, Naue N, Borrmann A, Herrmann C, Gall C, 2011a. Noninvasive alternating current stimulation improves vision in optic neuropathy. Restor Neurol Neurosci 29, 493–505. [DOI] [PubMed] [Google Scholar]
- Sabel BA, Henrich-Noack P, Fedorov A, Gall C, 2011b. Vision restoration after brain and retina damage: the “residual vision activation theory”. Prog Brain Res 192, 199–262. [DOI] [PubMed] [Google Scholar]
- Sabel BA, Wang J, Cardenas-Morales L, Faiq M, Heim C, 2018b. Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J 9, 133–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboory E, Mohammadi S, Dindarian S, Mohammadi H, 2019. Prenatal stress and elevated seizure susceptibility: Molecular inheritable changes. Epilepsy Behav 96, 122–131. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, 1990. Light induces a Fos-like nuclear antigen in retinal neurons. Brain Res Mol Brain Res 7, 17–21. [DOI] [PubMed] [Google Scholar]
- Sahakian B, Jones G, Levy R, Gray J, Warburton D, 1989. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry 154, 797–800. [DOI] [PubMed] [Google Scholar]
- Saligaut C, Daoust M, Moore N, Boismare F, 1987. Circling behaviour in rats with unilateral lesions of the nigrostriatum induced by 6-hydroxydopamine: changes induced by oral administration of cytidine-5’-diphosphocholine. Neuropharmacology 26, 1315–1319. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR, 2004. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65, 1526–1535. [DOI] [PubMed] [Google Scholar]
- Salt TE, Cordeiro MF, 2006. Glutamate excitotoxicity in glaucoma: throwing the baby out with the bathwater? Eye (Lond) 20, 730–731; author reply 731–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M, Corazzi L, 1983. Release of endogenous amino acids from superior colliculus of the rabbit: in vitro studies after retinal ablation. J Neurochem 40, 917–921. [DOI] [PubMed] [Google Scholar]
- Sankalp, Dada T, Yadav RK, Faiq MA, 2018. Effect of Yoga-Based Ocular Exercises in Lowering of Intraocular Pressure in Glaucoma Patients: An Affirmative Proposition. Int J Yoga 11, 239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Paolone G, 2011. Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci 125, 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Sato Y, Uchida S, 2001. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Int J Dev Neurosci 19, 327–337. [DOI] [PubMed] [Google Scholar]
- Saver JL, 2008. Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair. Rev Neurol Dis 5, 167–177. [PubMed] [Google Scholar]
- Scarr E, Hopper S, Vos V, Seo MS, Everall IP, Aumann TD, Chana G, Dean B, 2018. Low levels of muscarinic M1 receptor-positive neurons in cortical layers III and V in Brodmann areas 9 and 17 from individuals with schizophrenia. J Psychiatry Neurosci 43, 170202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Wassle H, Humphrey M, 1985. Number and distribution of putative cholinergic neurons in the cat retina. Neurosci Lett 59, 235–240. [DOI] [PubMed] [Google Scholar]
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC, 2004. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A 101, 3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettauf F, Rejdak R, Thaler S, Bolz S, Lehaci C, Mankowska A, Zarnowski T, Junemann A, Zagorski Z, Zrenner E, Grieb P, 2006. Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat. Exp Eye Res 83, 1128–1134. [DOI] [PubMed] [Google Scholar]
- Schuman JS, 2008. Spectral domain optical coherence tomography for glaucoma (an AOS thesis). Trans Am Ophthalmol Soc 106, 426–458. [PMC free article] [PubMed] [Google Scholar]
- Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP, Hertzmark E, Izatt JA, Swanson EA, Fujimoto JG, 1995. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol 113, 586–596. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Seddon JM, 1981. Increased plasma cortisol levels in ocular hypertension. Arch Ophthalmol 99, 1791–1794. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Rapaport E, Hirschberg K, Futerman AH, 1995. A regulatory role for sphingolipids in neuronal growth. Inhibition of sphingolipid synthesis and degradation have opposite effects on axonal branching. J Biol Chem 270, 10990–10998. [DOI] [PubMed] [Google Scholar]
- Schwid SR, Cutter GR, 2006. Futility studies: spending a little to save a lot. Neurology 66, 626–627. [DOI] [PubMed] [Google Scholar]
- Secades JJ, 2011. Citicoline: pharmacological and clinical review, 2010 update. Rev Neurol 52 Suppl 2, S1–S62. [PubMed] [Google Scholar]
- Secades JJ, 2016. Citicoline: pharmacological and clinical review, 2016 update. Rev Neurol 63, S1–S73. [PubMed] [Google Scholar]
- Secades JJ, Lorenzo JL, 2006. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol 28 Suppl B, 1–56. [PubMed] [Google Scholar]
- Seet LF, Narayanaswamy A, Finger SN, Htoon HM, Nongpiur ME, Toh LZ, Ho H, Perera SA, Wong TT, 2016. Distinct iris gene expression profiles of primary angle closure glaucoma and primary open angle glaucoma and their interaction with ocular biometric parameters. Clin Exp Ophthalmol 44, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, 2013. The visual corticostriatal loop through the tail of the caudate: circuitry and function. Front Syst Neurosci 7, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehic A, Guo S, Cho KS, Corraya RM, Chen DF, Utheim TP, 2016. Electrical Stimulation as a Means for Improving Vision. Am J Pathol 186, 2783–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh MH, Mars JS, 2001. The acute effect of pilocarpine on pulsatile ocular blood flow in ocular hypertension. Eye (Lond) 15, 63–66. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Akaike A, Kimura J, 1996. Nicotine-induced protection against glutamate cytotoxicity. Nicotinic cholinergic receptor-mediated inhibition of nitric oxide formation. Ann N Y Acad Sci 777, 356–361. [DOI] [PubMed] [Google Scholar]
- Shingu M, Yoshioka K, Nobunaga M, Yoshida K, 1985. Human vascular smooth muscle cells and endothelial cells lack catalase activity and are susceptible to hydrogen peroxide. Inflammation 9, 309–320. [DOI] [PubMed] [Google Scholar]
- Siaudvytyte L, Januleviciene I, Daveckaite A, Ragauskas A, Bartusis L, Kucinoviene J, Siesky B, Harris A, 2015. Literature review and meta-analysis of translaminar pressure difference in open-angle glaucoma. Eye (Lond) 29, 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siaudvytyte L, Januleviciene I, Ragauskas A, Bartusis L, Meiliuniene I, Siesky B, Harris A, 2014. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J Ophthalmol 2014, 937360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidor MM, Davidson TJ, Tye KM, Warden MR, Diesseroth K, McClung CA, 2015. In vivo optogenetic stimulation of the rodent central nervous system. J Vis Exp, 51483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsmeier BA, Grabner RH, Hein J, Krenz U, Schneider M, 2018. Electrical brain stimulation (tES) improves learning more than performance: A meta-analysis. Neurosci Biobehav Rev 84, 171–181. [DOI] [PubMed] [Google Scholar]
- Skalicky S, Goldberg I, 2008. Depression and quality of life in patients with glaucoma: a cross-sectional analysis using the Geriatric Depression Scale-15, assessment of function related to vision, and the Glaucoma Quality of Life-15. J Glaucoma 17, 546–551. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Manzel A, Gropengiesser K, Schafer N, Gudi V, Singh V, Salinas Tejedor L, Jorg S, Hammer A, Voss E, Vulinovic F, Degen D, Wolf R, Lee DH, Pul R, Moharregh-Khiabani D, Baumgartner W, Gold R, Linker RA, Stangel M, 2015. Pivotal role of choline metabolites in remyelination. Brain 138, 398–413. [DOI] [PubMed] [Google Scholar]
- Smith ND, Crabb DP, Garway-Heath DF, 2011. An exploratory study of visual search performance in glaucoma. Ophthalmic Physiol Opt 31, 225–232. [DOI] [PubMed] [Google Scholar]
- Solomon KD, Stewart WC, Hunt HH, Stewart JA, Cate EA, 1998. Intraoperative intracameral carbachol in phacoemulsification and posterior chamber lens implantation. Am J Ophthalmol 125, 36–43. [DOI] [PubMed] [Google Scholar]
- Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, Ming GL, Song H, 2013. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci 16, 1728–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Mu K, Wang J, Lin F, Chen Z, Yan X, Hao Y, Zhu W, Zhang H, 2014. Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One 9, e89493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnino S, Aureli M, Grassi S, Mauri L, Prioni S, Prinetti A, 2014. Lipid rafts in neurodegeneration and neuroprotection. Mol Neurobiol 50, 130–148. [DOI] [PubMed] [Google Scholar]
- Spindle MS, Parsa PV, Bowles SG, D’Souza RD, Vijayaraghavan S, 2018. A dominant role for the beta 4 nicotinic receptor subunit in nicotinic modulation of glomerular microcircuits in the mouse olfactory bulb. J Neurophysiol 120, 2036–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponsel WE, Groth SL, Satsangi N, Maddess T, Reilly MA, 2014. Refined Data Analysis Provides Clinical Evidence for Central Nervous System Control of Chronic Glaucomatous Neurodegeneration. Transl Vis Sci Technol 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzione P, Tropepi D, 2011. Drugs and clinical trials in neurodegenerative diseases. Ann Ist Super Sanita 47, 49–54. [DOI] [PubMed] [Google Scholar]
- Stewart RM, Clearkin LG, 2008. Insulin resistance and autoregulatory dysfunction in glaucoma and retinal vein occlusion. Am J Ophthalmol 145, 394–396. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Zaima N, Setou M, Ito S, Yao I, 2012. Visualization of acetylcholine distribution in central nervous system tissue sections by tandem imaging mass spectrometry. Anal Bioanal Chem 403, 1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Crossland WJ, 2000. Quantitative assessment of localization and colocalization of glutamate, aspartate, glycine, and GABA immunoreactivity in the chick retina. Anat Rec 260, 158–179. [DOI] [PubMed] [Google Scholar]
- Sun P, Li H, Lu Z, Su X, Ma Z, Chen J, Li L, Zhou C, Chen Y, Chai X, 2018. Comparison of cortical responses to the activation of retina by visual stimulation and transcorneal electrical stimulation. Brain Stimul 11, 667–675. [DOI] [PubMed] [Google Scholar]
- Sun X, Dai Y, Chen Y, Yu DY, Cringle SJ, Chen J, Kong X, Wang X, Jiang C, 2017. Primary angle closure glaucoma: What we know and what we don’t know. Prog Retin Eye Res 57, 26–45. [DOI] [PubMed] [Google Scholar]
- Swenor BK, Varadaraj V, Dave P, West SK, Rubin GS, Ramulu PY, 2017. Impact of the Ability to Divide Attention on Reading Performance in Glaucoma. Invest Ophthalmol Vis Sci 58, 2456–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER, 2002. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res 21, 395–428. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Ethier CR, Lasker I.I.o.A., Glaucomatous Neurodegeneration, P., 2017. Biological aspects of axonal damage in glaucoma: A brief review. Exp Eye Res 157, 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PP, Yuan HH, Zhu X, Cui YY, Li H, Feng XM, Qiu Y, Chen HZ, Zhou W, 2014. Activation of muscarinic receptors protects against retinal neurons damage and optic nerve degeneration in vitro and in vivo models. CNS Neurosci Ther 20, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RM, Sud A, Podos SM, Mittag TW, 2001. Maintaining mitochondrial membrane impermeability. an opportunity for new therapy in glaucoma? Surv Ophthalmol 45 Suppl 3, S277–283; discussuin S295–276. [DOI] [PubMed] [Google Scholar]
- Thiele A, 2013. Muscarinic signaling in the brain. Annu Rev Neurosci 36, 271–294. [DOI] [PubMed] [Google Scholar]
- Tieman SB, Tieman DG, 1996. N-acetylaspartylglutamate immunoreactivity in human retina. Vision Res 36, 941–947. [DOI] [PubMed] [Google Scholar]
- Tilley BC, Palesch YY, Kieburtz K, Ravina B, Huang P, Elm JJ, Shannon K, Wooten GF, Tanner CM, Goetz GC, Investigators N-P, 2006. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology 66, 628–633. [DOI] [PubMed] [Google Scholar]
- Tooyama I, Kimura H, 2000. A protein encoded by an alternative splice variant of choline acetyltransferase mRNA is localized preferentially in peripheral nerve cells and fibers. J Chem Neuroanat 17, 217–226. [DOI] [PubMed] [Google Scholar]
- Tran QK, Ohashi K, Watanabe H, 2000. Calcium signalling in endothelial cells. Cardiovasc Res 48, 13–22. [DOI] [PubMed] [Google Scholar]
- Tsai G, Stauch BL, Vornov JJ, Deshpande JK, Coyle JT, 1990. Selective release of N-acetylaspartylglutamate from rat optic nerve terminals in vivo. Brain Res 518, 313–316. [DOI] [PubMed] [Google Scholar]
- Tumosa N, Eckenstein F, Stell WK, 1984. Immunocytochemical localization of putative cholinergic neurons in the goldfish retina. Neurosci Lett 48, 255–259. [DOI] [PubMed] [Google Scholar]
- Tumosa N, Stell WK, 1986. Choline acetyltransferase immunoreactivity suggests that ganglion cells in the goldfish retina are not cholinergic. J Comp Neurol 244, 267–275. [DOI] [PubMed] [Google Scholar]
- Ulus IH, Watkins CJ, Cansev M, Wurtman RJ, 2006. Cytidine and uridine increase striatal CDP-choline levels without decreasing acetylcholine synthesis or release. Cell Mol Neurobiol 26, 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V, T, Y, C, C, P, A, A, JW, B, M, W, I, C, G, W, J, S, KC., C, 2018. Evaluation of volumetric and diffusional brain changes and their associations with retinal structures and visual field function in glaucoma using MRI, OCT and perimetry, ARVO Investigative Ophthalmology & Visual Science, Hawaii, USA, pp. 3502–3502. [Google Scholar]
- van der Merwe Y, Yang X, Ho LC, Yu Y, Chau Y, Leung CK, Conner IP, Wollstein G, Schuman JS, Chan KC, 2016. Citicoline preserves optic nerve integrity and visuomotor function following chronic intraocular pressure elevation, Invest Ophthalmol Vis Sci, pp. 3788–3800. [Google Scholar]
- Van Hooser SD, 2007. Similarity and diversity in visual cortex: is there a unifying theory of cortical computation? Neuroscientist 13, 639–656. [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW, 2008. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Sharma G, 2015. Editorial: Brain cholinergic mechanisms. Front Synaptic Neurosci 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virno M, Pecori-Giraldi J, Liguori A, De Gregorio F, 2000. The protective effect of citicoline on the progression of the perimetric defects in glaucomatous patients (perimetric study with a 10-year follow-up). Acta Ophthalmol Scand Suppl, 56–57. [DOI] [PubMed] [Google Scholar]
- Vlasov K, Van Dort CJ, Solt K, 2018. Optogenetics and Chemogenetics. Methods Enzymol 603, 181–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T, 1986. Cholinergic amacrine cells in the rat retina. J Comp Neurol 248, 19–35. [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, Levey AI, 2004. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res 145, 59–66. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H, 2007. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci 27, 5633–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosskuhl J, Struber D, Herrmann CS, 2018. Non-invasive Brain Stimulation: A Paradigm Shift in Understanding Brain Oscillations. Front Hum Neurosci 12, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SW, Strachan MW, Lightly ER, Williams BC, Bird IM, 1990. Acetylcholine stimulates cortisol secretion through the M3 muscarinic receptor linked to a polyphosphoinositide-specific phospholipase C in bovine adrenal fasciculata/reticularis cells. Mol Cell Endocrinol 72, 227–238. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D, 2013. Alpha7 neuronal nicotinic receptors as a drug target in schizophrenia. Expert Opin Ther Targets 17, 139–155. [DOI] [PubMed] [Google Scholar]
- Wang B, Tran H, Smith MA, Kostanyan T, Schmitt SE, Bilonick RA, Jan NJ, Kagemann L, Tyler-Kabara EC, Ishikawa H, Schuman JS, Sigal IA, Wollstein G, 2017a. In-vivo effects of intraocular and intracranial pressures on the lamina cribrosa microstructure. PLoS One 12, e0188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li T, Sabel BA, Chen Z, Wen H, Li J, Xie X, Yang D, Chen W, Wang N, Xian J, He H, 2016. Structural brain alterations in primary open angle glaucoma: a 3T MRI study. Sci Rep 6, 18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qu D, An J, Yuan G, Liu Y, 2017b. Integrated microarray analysis provided novel insights to the pathogenesis of glaucoma. Mol Med Rep 16, 8735–8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Tang Z, Sun X, Wu L, Wang J, Zhong Y, Xiao Z, 2018. White Matter Abnormalities and Correlation With Severity in Normal Tension Glaucoma: A Whole Brain Atlas-Based Diffusion Tensor Study. Invest Ophthalmol Vis Sci 59, 1313–1322. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G, 1996. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16, 6402–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y, 1994. Distinct spatiotemporal distributions of the Nmethyl-D-aspartate receptor channel subunit mRNAs in the mouse cervical cord. J Comp Neurol 345, 314–319. [DOI] [PubMed] [Google Scholar]
- Wehrwein E, Thompson SA, Coulibaly SF, Linn DM, Linn CL, 2004. Acetylcholine protection of adult pig retinal ganglion cells from glutamate-induced excitotoxicity. Invest Ophthalmol Vis Sci 45, 1531–1543. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, 2007. Glaucoma neuroprotection: What is it? Why is it needed? Can J Ophthalmol 42, 396–398. [PubMed] [Google Scholar]
- Weinreb RN, Liebmann JM, Cioffi GA, Goldberg I, Brandt JD, Johnson CA, Zangwill LM, Schneider S, Badger H, Bejanian M, 2018. Oral Memantine for the Treatment of Glaucoma: Design and Results of 2 Randomized, Placebo-Controlled, Phase 3 Studies. Ophthalmology 125, 1874–1885. [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Pasquale LR, 2017. Genetics of glaucoma. Hum Mol Genet 26, R21–R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall ND, Brown ES, 2014. Citicoline in addictive disorders: a review of the literature. Am J Drug Alcohol Abuse 40, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall ND, de Wit H, 2011. Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Exp Clin Psychopharmacol 19, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Fadel JR, 2017. Cholinergic regulation of fear learning and extinction. J Neurosci Res 95, 836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witticke D, Seidling HM, Klimm HD, Haefeli WE, 2012. Do we prescribe what patients prefer? Pilot study to assess patient preferences for medication regimen characteristics. Patient Prefer Adherence 6, 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollstein G, Kagemann L, Bilonick RA, Ishikawa H, Folio LS, Gabriele ML, Ungar AK, Duker JS, Fujimoto JG, Schuman JS, 2012. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol 96, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW, 2000. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol 393, 51–58. [DOI] [PubMed] [Google Scholar]
- Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP, 2016. The translaminar pressure difference as an index for neurotoxic burden in the anterior part of the optic nerve. Eye (Lond) 30, 1146–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostyn P, De Groot V, Van Dam D, Audenaert K, Killer HE, De Deyn PP, 2017. The Glymphatic Hypothesis of Glaucoma: A Unifying Concept Incorporating Vascular, Biomechanical, and Biochemical Aspects of the Disease. Biomed Res Int 2017, 5123148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostyn P, Van Dam D, De Deyn PP, 2018. Intracranial pressure and glaucoma: Is there a new therapeutic perspective on the horizon? Med Hypotheses 118, 98–102. [DOI] [PubMed] [Google Scholar]
- Wu Z, DPC C, BC, h., JG, C, FA, M, 2019. Improving the feasibility of glaucoma clinical trials using trend-based visual field progression end points. Ophthalmology Glaucoma 2, 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutthiphan S, Hanutsaha P, Jenchitr W, 2000. Intracameral pilocarpine in topical phacoemulsification. J Med Assoc Thai 83, 1452–1457. [PubMed] [Google Scholar]
- Yalcin E, Balci O, Akingol Z, 2013. Association of extensive myelinated nerve fibers and high degree myopia: case report. Indian J Ophthalmol 61, 606–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi-Kimura R, Honjo M, Komizo T, Ono T, Yagi A, Lee J, Miyata K, Fujimoto T, Inoue T, Tanihara H, Nishida J, Uchida T, Araki Y, Aihara M, 2018. Interaction Between Pilocarpine and Ripasudil on Intraocular Pressure, Pupil Diameter, and the Aqueous-Outflow Pathway. Invest Ophthalmol Vis Sci 59, 1844–1854. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kitazawa Y, 1998. Vascular pathogenesis of normal-tension glaucoma: a possible pathogenetic factor, other than intraocular pressure, of glaucomatous optic neuropathy. Prog Retin Eye Res 17, 127–143. [DOI] [PubMed] [Google Scholar]
- Yan Z, Liao H, Chen H, Deng S, Jia Y, Deng C, Lin J, Ge J, Zhuo Y, 2017. Elevated Intraocular Pressure Induces Amyloid-beta Deposition and Tauopathy in the Lateral Geniculate Nucleus in a Monkey Model of Glaucoma. Invest Ophthalmol Vis Sci 58, 5434–5443. [DOI] [PubMed] [Google Scholar]
- Yang X, van der Merwe Y, Ho LC, Sims J, Parra C, Schuman JS, Wollstein G, Lothrop KL, Chan KC, 2018a. Age-related Changes in Eye, Brain and Visuomotor Behavior in the DBA/2J Mouse Model of Chronic Glaucoma. Sci Rep in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, van der Merwe Y, Sims J, Parra C, Ho LC, Schuman JS, Wollstein G, Lathrop KL, Chan KC, 2018b. Age-related Changes in Eye, Brain and Visuomotor Behavior in the DBA/2J Mouse Model of Chronic Glaucoma. Sci Rep 8, 4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara O, Tooyama I, Aimi Y, Bellier JP, Hisano T, Matsuo A, Park M, Kimura H, 2003. Demonstration of cholinergic ganglion cells in rat retina: expression of an alternative splice variant of choline acetyltransferase. J Neurosci 23, 2872–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari F, Nitsche MA, Ekhtiari H, 2017. Transcranial Electric Stimulation for Precision Medicine: A Spatiomechanistic Framework. Front Hum Neurosci 11, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Catudio-Garrett E, Gabriel R, Wilhelm M, Erdelyi F, Szabo G, Deisseroth K, Lawrence J, 2015. Hippocampal “cholinergic interneurons” visualized with the choline acetyltransferase promoter: anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Front Synaptic Neurosci 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim T, Eylen A, Lule S, Erdener SE, Vural A, Karatas H, Ozveren MF, Dalkara T, Gursoy-Ozdemir Y, 2015. Poloxamer-188 and citicoline provide neuronal membrane integrity and protect membrane stability in cortical spreading depression. Int J Neurosci 125, 941–946. [DOI] [PubMed] [Google Scholar]
- You Y, Joseph C, Wang C, Gupta V, Liu S, Yiannikas C, Chua BE, Chitranshi N, Shen T, Dheer Y, Invernizzi A, Borotkanics R, Barnett M, Graham SL, Klistorner A, 2019. Demyelination precedes axonal loss in the transneuronal spread of human neurodegenerative disease. Brain. [DOI] [PubMed] [Google Scholar]
- Zazueta C, Buelna-Chontal M, Macias-Lopez A, Roman-Anguiano NG, Gonzalez-Pacheco H, Pavon N, Springall R, Aranda-Frausto A, Bojalil R, Silva-Palacios A, Velazquez-Espejel R, Galvan Arzate S, Correa F, 2018. Cytidine-5’-Diphosphocholine Protects the Liver From Ischemia/Reperfusion Injury Preserving Mitochondrial Function and Reducing Oxidative Stress. Liver Transpl 24, 1070–1083. [DOI] [PubMed] [Google Scholar]
- Zhang LC, Jin X, Huang Z, Yan ZN, Li PB, Duan RF, Feng H, Jiang JH, Peng H, Liu W, 2017. Protective effects of choline against hypoxia-induced injuries of vessels and endothelial cells. Exp Ther Med 13, 2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tzounopoulos T, 2011. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci 31, 3158–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA, 2002. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol 53, 590–605. [DOI] [PubMed] [Google Scholar]
- Zhou W, Muir ER, Nagi KS, Chalfin S, Rodriguez P, Duong TQ, 2017. Retinotopic fMRI Reveals Visual Dysfunction and Functional Reorganization in the Visual Cortex of Mild to Moderate Glaucoma Patients. J Glaucoma 26, 430–437. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhu X, Zhu L, Cui YY, Wang H, Qi H, Ren QS, Chen HZ, 2008. Neuroprotection of muscarinic receptor agonist pilocarpine against glutamate-induced apoptosis in retinal neurons. Cell Mol Neurobiol 28, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen L, Qi Y, Feng J, Zhu L, Bai Y, Wu H, 2018. Protective effects of Erigeron breviscapus Hand.- Mazz. (EBHM) extract in retinal neurodegeneration models. Mol Vis 24, 315–325. [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schroder H, Schroder U, Horvath E, Werner L, Luiten PG, Maelicke A, Strosberg AD, 1989. Distribution of cholinergic receptors in the rat and human neocortex. EXS 57, 212–228. [DOI] [PubMed] [Google Scholar]
- Zoefel B, Davis MH, 2017. Transcranial electric stimulation for the investigation of speech perception and comprehension. Lang Cogn Neurosci 32, 910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Kumar U, 2018. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifler RM, 2002. Membrane stabilizer: citicoline. Curr Med Res Opin 18 Suppl 2, s14–17. [DOI] [PubMed] [Google Scholar]